- 1Research Unit of Epidemiology and Prevention, IRCCS Istituto Neurologico Mediterraneo Neuromed, Pozzilli, Italy
- 2Istituto di Radiologia, Università Cattolica S. Cuore, Rome, Italy
- 3Radiotherapy Unit, Responsible Research Hospital, Campobasso, Italy
- 4Medical Physic Unit, Responsible Research Hospital, Campobasso, Italy
- 5Radiation Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 6Metabolomic Unit, Research Innovation Centre, Fondazione Edmund Mach, San Michele all’Adige, Italy
- 7Department of Biosciences, Università degli Studi di Milano, Milano, Italy
- 8Department of Medicine and Surgery, LUM University, Casamassima, Italy
Background/Objectives: Little is known regarding the influence of circulating plasma branched-chain amino acids (BCAAs) such as leucine, isoleucine, and valine on acute skin toxicity (AST) after breast cancer (BC) radiotherapy. Hence, this study examined the association between circulating plasma BCAAs and the risk of ≥ grade 2 AST post-radiotherapy among BC patients.
Methods: An observational study was conducted among 161 BC patients treated with radiotherapy within the ATHENA project in Italy. Plasma BCAAs were measured at 2-time points: at baseline (T0) and at the end of radiotherapy (T1) (after 3 or 5 weeks), and were ascertained using a validated method based on tandem mass spectrometry. AST was measured at T1 and defined according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment Cancer (RTOG/EORTC) criteria. Analysis was conducted in two parts with separate study designs using multivariable-adjusted logistic regression models: 1) A cross-sectional analysis explored the association between plasma BCAAs at T1 and odds of AST post-radiotherapy; 2) A prospective analysis examined the association between plasma BCAAs at T0 and odds of AST post-radiotherapy.
Results: AST post-radiotherapy was observed in 45 (28%) patients. In the cross-sectional analysis, at T1, plasma isoleucine (1-SD increment) was associated with 43% reduced odds of ≥ grade 2 AST post-radiotherapy (OR = 0.57;95% CI 0.36 to 0.91). A similar trend was observed in the prospective analysis at T0 (OR = 0.65;95% CI 0.42 to 1.02). There was no evidence of an association between plasma leucine and valine with AST post-radiotherapy, either at T0 or T1. Plasma isoleucine was associated with lower odds of AST post-radiotherapy in BC patients.
Conclusions: The findings highlight that plasma isoleucine is associated with a low risk of ≥ grade 2 AST post-radiotherapy among BC patients. However, further studies such as isoleucine supplementation trials are needed to validate these findings.
1 Introduction
Breast cancer (BC) is the most commonly diagnosed type of cancer in women, with an estimated 2.3 million new cases in 2022, thus posing a major burden on public health (1). Surgery, chemotherapy, and radiotherapy are the most common modalities for BC treatment. Among these, radiotherapy remains a highly cost-effective single mode of treatment, accounting for only 5% of the overall cancer care costs. It is estimated that approximately 80% of BC patients must receive radiotherapy at some point in their treatment, hence highlighting its essential role in BC recovery (2, 3). Although widely used, radiotherapy also damages healthy tissues in the irradiation field. The patients mostly develop skin damage, and it is usually dependent on patient-related factors (age, hemoglobin levels, smoking habits, comorbidities including cardiovascular disease, diabetes mellitus, obesity), and the location and duration of the breast organ area exposed in the radiotherapy (4, 5).
Nearly 85-95% of patients with all types of cancer report different degrees of skin damage induced by radiotherapy, known as radiation-induced skin injury. These are of two types: (1) acute skin toxicity (AST) involving dry and wet desquamation and skin ulcers, and (2) chronic skin toxicity changes including chronic ulcers, keratosis due to radiation and fibrosis (6). In the Athena Study (N = 161), around 62% of patients experienced acute skin toxicity, according to a recent publication (7). Plasma-free amino acids are either ingested or endogenously synthesized, circulate abundantly, and are metabolic regulators in humans (8). Among them, branched-chain amino acids (BCAAs) such as leucine, isoleucine, and valine are essential amino acids that the body cannot synthesize and must be introduced through the diet to support healthy protein synthesis. Studies have suggested that red meat, fish, dairy products, and eggs are rich sources of BCAAs (9–12). The key functions of BCAAs are as follows: (1) activate the mammalian target of rapamycin (mTOR) signaling pathway required for protein synthesis, especially leucine; (2) enhance immunity and glucose consumption, especially isoleucine; (3) prevent cellular damage caused due to oxidative stress, especially valine; (4) insufficient or excess BCAAs levels enhance lipolysis (13–15).
Studies have suggested that both higher dietary and elevated circulating BCAAs levels were associated with a lower severity of BC (16, 17) by suppressing tumor growth and metastasis (17, 18). Interestingly, there is sparse evidence on the influence of amino acids, especially BCAAs, on the risk of radiation-induced skin injury in cancer, specifically BC.
However, it is not known whether BCAAs such as leucine, isoleucine, and valine influence the risk of AST after radiotherapy among BC women. Therefore, the main objective of this study was to examine the association between circulating plasma BCAA levels and the risk of ≥ grade (G) 2 (moderate/severe) AST among 161 BC patients’ post-radiotherapy within the ATHENA project in Italy.
2 Materials and methods
2.1 Trial design and participants
We used data from the ATHENA project for the current observational study. The ATHENA project was a double-blind, randomized, placebo-controlled trial designed to evaluate the impact of anthocyanin supplementation derived from purple corn cobs on radiation-induced skin toxicity in women with BC undergoing radiotherapy.
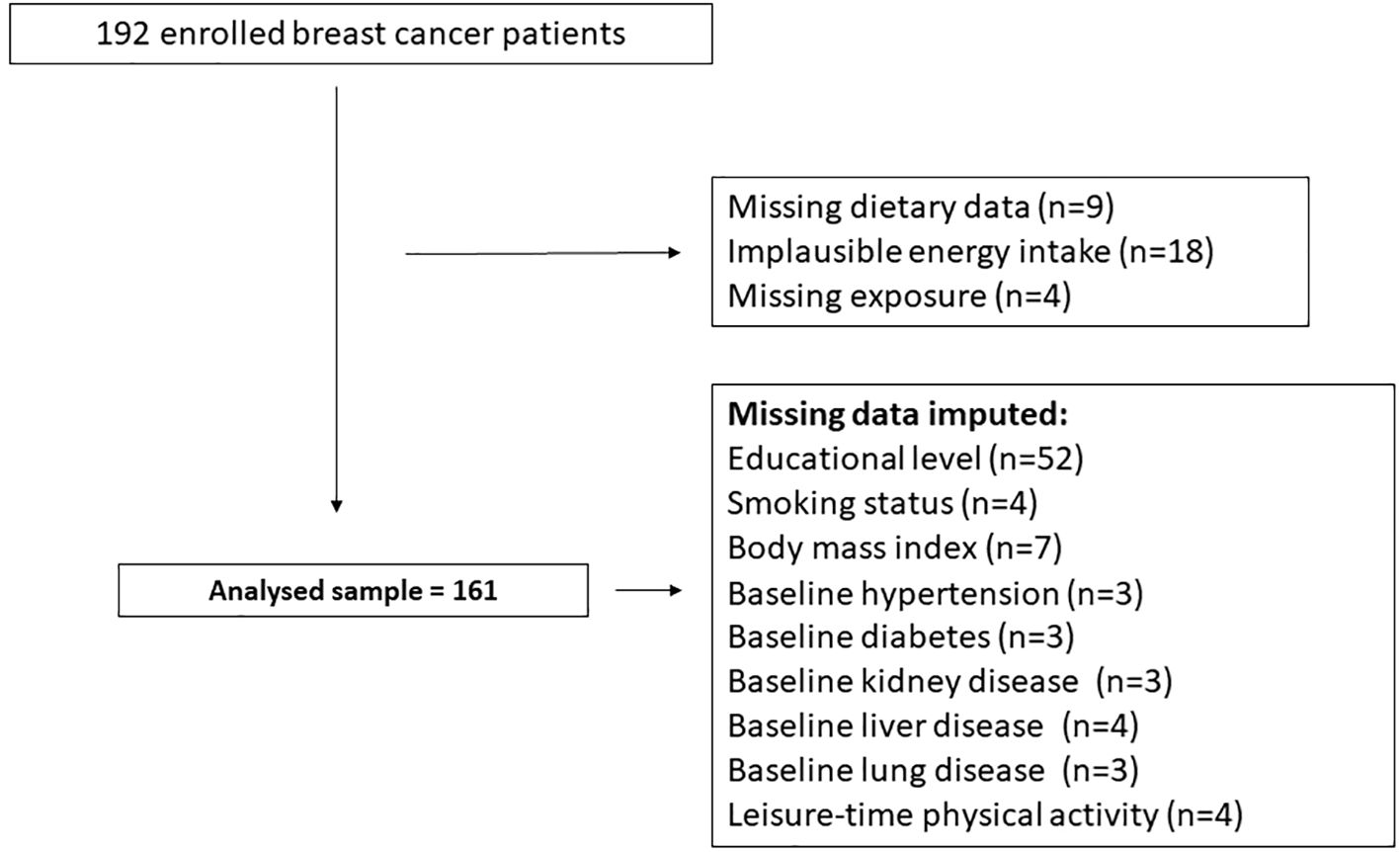
For the purposes of the present analyses, data from 161 women with BC who received radiotherapy were included. Comprehensive methodological details regarding the ATHENA randomized controlled trial (RCT) are available in previously published reports (19). Participants’ flow chart is provided in Figure 1.
2.2 Eligibility criteria
Women aged 18 years or older with a confirmed diagnosis of BC and deemed suitable for radiotherapy were considered for inclusion in the current analysis. Participant screening and selection were carried out by clinical staff based on inclusion and exclusion criteria established by the ATHENA RCT protocol (19).
Inclusion criteria encompassed patients with invasive breast carcinoma who had undergone breast-conserving surgery (lumpectomy or quadrantectomy) along with axillary staging procedures. Exclusion criteria included pregnancy or lactation at the time of recruitment, documented psychiatric or substance use disorders, as well as a history of non-invasive BC, synchronous bilateral invasive disease, non-epithelial breast tumors, multicentric carcinoma, or any prior radiotherapy to the breast or thoracic region for any indication (19). The ATHENA RCT was performed at the Gemelli Molise Hospital Radiotherapy Unit in Campobasso, Italy, and was conducted according to the guidelines of the Declaration of Helsinki.
Recruitment occurred between June 9, 2014, and June 26, 2017, with study follow-up concluding on October 10, 2018. The study received approval from the Ethics Committees of both the Catholic University of Rome and the Regional Health Authority of Molise (ASReM). All participants provided written informed consent. The trial was registered on ClinicalTrials.gov (Identifier: NCT02195960) and was conducted in compliance with Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines (20).
2.3 Radiotherapy treatment
Within the ATHENA RCT, radiotherapy protocols were tailored based on individual recurrence risk profiles (low or moderate to high).
Participants classified as low risk underwent a hypofractionated radiotherapy regimen over a three-week period, receiving a total dose of 40 Gray (Gy) to the remaining breast tissue, with a simultaneous integrated boost of 4 Gy directed to the tumor bed. Those at moderate to high risk were administered a 5-week treatment with standard doses of radiotherapy, consisting of 50 Gy to the residual breast and a 10 Gy boost to the tumor bed.
All patients (3- and 5-week schedules) were treated with forward-planned intensity-modulated radiation therapy (IMRT) and were instructed to apply a topical cream (Atonderma Radiomed®) to the irradiated site approximately 2–3 hours before and after each session, starting from the first day of treatment (19).
For the present analysis, data from the entire cohort receiving radiotherapy were included, regardless of the specific treatment schedule or risk stratification.
2.4 Study exposure
2.4.1 Determination of plasma branched-chain amino acids
Plasma BCAAs (leucine, isoleucine, and valine) were extracted and analyzed according to the protocol by Anesi et al. (21). Briefly, the plasma samples stored in the Neuromed Biobanking Centre were thawed on ice, and 25 µl aliquots were loaded onto Ostro plates (Waters, Milan, Italy) together with 25 µl of deuterated internal standards in methanol. Protein precipitation and metabolite extraction were achieved by loading 75 µl of ice-cold acetonitrile containing 1% formic acid; plates were covered and shaken on an orbital shaker for 5 min at 600 rpm. Subsequently, plates were filtered for 5 min using a positive pressure manifold with nitrogen at 4 psi. Extraction was repeated by adding 75 µl of ice-cold acetonitrile containing 1% formic acid. Filtrates were dried down using nitrogen at 37 °C and re-constituted in 200 µl of water containing 0.5% formic acid, and 1 mM ammonium formate. Samples were randomized prior to extraction. Quality control (QC) samples were created by pooling together 10 µl of each plasma sample and were extracted as described above. 2 µl of each sample were injected and analyzed by ultrahigh-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) by using Multiple Reaction Monitoring (MRM) on a ABSciex 6500+ triple quadrupole connected to a Shimadzu LC-30 pump (ABSciex, Milan, Italy). Metabolites were separated on a Waters HSST3 column (100 x 2.1 mm, 1.8 µm) by using water 0.1% formic acid (A) and acetonitrile 0.1% formic acid (B) as mobile phases. Chromatographically resolved isoleucine (RT: 2.00 min) and leucine (RT: 2.20 min) were quantified by using MRM 132.1>86.2 in positive ion mode, valine (RT 1.20 min) by MRM 118.1>72.0. QC samples were injected at the beginning of the analytical sequence to condition UHPLC-MS/MS and at fixed intervals during the sequence to check the performance of our methodology. We ran 400–500 samples in one batch, each run lasting for approximately 10 minutes. QC was then injected at specific time points during acquisition to ensure stable instrument response (<20% indicated good stability throughout the acquisition). All the QC were prepared by pooling together equal amount of samples and were extracted in the same manner. Finally, samples were randomized prior to extraction. However, T0 and T1 samples from the same subject were acquired consecutively or vice versa to get the same MS response.
Finally, plasma BCAAs (leucine, isoleucine, and valine) were measured twice, at T0 (baseline) and again at T1 (after 3 or 5 weeks of undergoing radiotherapy).
2.5 Definition of study outcomes
The primary objective of this analysis was to assess the likelihood of experiencing AST greater than Grade 2. Skin reactions were evaluated by clinical staff during follow-up visits after completion of radiotherapy, using the RTOG/EORTC criteria for both acute and late toxicities (22).
AST was assessed at a follow-up time point (T1), corresponding to either 3- or 5-weeks post-treatment, depending on the radiotherapy schedule.
The irradiated area was examined for specific skin changes and reactions. Skin toxicity was dichotomized for analysis: a score of ‘0’ included Grade 0 (no visible skin changes) and Grade 1 (symptoms such as follicular, faint or dull erythema/epilation/dry desquamation/decreased sweating), while a score of ‘1’ included Grade 2 and above, indicating more pronounced effects such as tender or bright erythema, patchy moist desquamation/moderate edema (Grade 2); confluent, moist desquamation other than skin folds, pitting edema (Grade 3); and ulceration, hemorrhage, necrosis (Grade 4) (22, 23).
2.6 Assessment of covariates
At the baseline assessment (T0), data were collected on each participant’s medical history, anthropometric and clinical parameters, as well as dietary and lifestyle behaviors.
The following variables were included as covariates in the analysis: age (in years), body mass index (calculated as kilograms/meters square), C-reactive protein (CRP) levels at T0, total energy intake (calculated as kilocalorie [kcal] per day), education (0= none; 1= primary school diploma and secondary school diploma; 2= high school diploma; 3= bachelor’s degree and master’s degree and master/doctorate/post-doctorate), physical activity levels (0= no activity or sedentary; 1= moderate intensity; 2=vigorous-intensity), hypertension (systolic blood pressure [SBP] >140 mmHg and/or diastolic [DBP] >90 mmHg or anti-hypertensive treatment), smoking habits (1=yes, 0=no or 2=former), treatment classification (B=treatment [anthocyanin supplementation]/A=placebo) recorded at baseline (T0), and weeks of radiotherapy (3 or 5 weeks). The sensitivity analysis was adjusted for chemotherapy treatment (1=yes or 0=no).
We used directed acyclic graphs (DAGs) (24, 25) to identify the covariates to then include in the analyses as they provide a straightforward and visual presentation for identifying and testing assumptions about causal relationships between variables by deducing an algorithm, thus providing an adjustment set of covariates for estimating causal effects.
2.7 Statistical analyses
Analyses were performed in two parts: (1) a cross-sectional analysis to study the association between plasma BCAAs at T1 and the odds of ≥ G2 AST at T1 at the end of radiotherapy (3 or 5 weeks); (2) a prospective analysis for the association between plasma BCAAs at T0 and the odds of ≥ G2 AST at T1 at the end of radiotherapy (3 or 5 weeks).
Data for categorical variables are represented as numbers and percentages, and for continuous variables represented as mean and standard deviation (SD). For the association between plasma BCAAs and the odds of ≥ G2 AST amongst post-radiation BC patients, we used multivariable-adjusted logistic regression models to derive odds ratios (ORs) and corresponding 95% confidence intervals (CI).
For logistic regression, AST was coded as a binary outcome variable (‘0’ denoted the absence of ≥ G2 AST, and ‘0’ denoted the presence of ≥ G2 AST). Plasma BCAAs (leucine, isoleucine, and valine) data were recorded as continuous variables, and for interpretation, the ORs were computed for 1-SD increments in plasma BCAAs.
Analyses were conducted constructing four models: (i) Model 1: unadjusted logistic regression presented for each plasma BCAA and ≥ G2 AST; (ii) Model 2: ORs separately presented for individual associations between each plasma BCAA and ≥ G2 AST, and minimally adjusted for age, BMI, weeks of radiotherapy (3 or 5 weeks) and treatment group (treatment/placebo); (iii) Model 3: Additionally adjusted for smoking habits, CRP-levels at baseline, education, physical activity levels, hypertension, and total energy intakes; (iv) Model 4: Mutually adjusted for other plasma BCAAs in the model, and fully adjusted for age, body mass index, CRP levels at baseline, smoking habits, total energy intake (kcal), education, physical activity level, hypertension, weeks of radiotherapy (3 or 5 weeks) and treatment classification (treatment/placebo).
In a sensitivity analysis, Model 4 was further adjusted for chemotherapy treatment (yes or no) to examine the robustness of associations.
Missing data on covariates are listed in Figure 1. To maximize data availability, missing data were handled using single imputation with the PROC MI procedure in SAS. A regression-based imputation method was employed to estimate the missing values.
For analysis, we used two-sided statistical tests, and the significance was set at 95% CI. We used STATA/SE software version 18.0 (StataCorp, College Station, TX, USA) and SAS/STAT software, Version 9.4.
3 Results
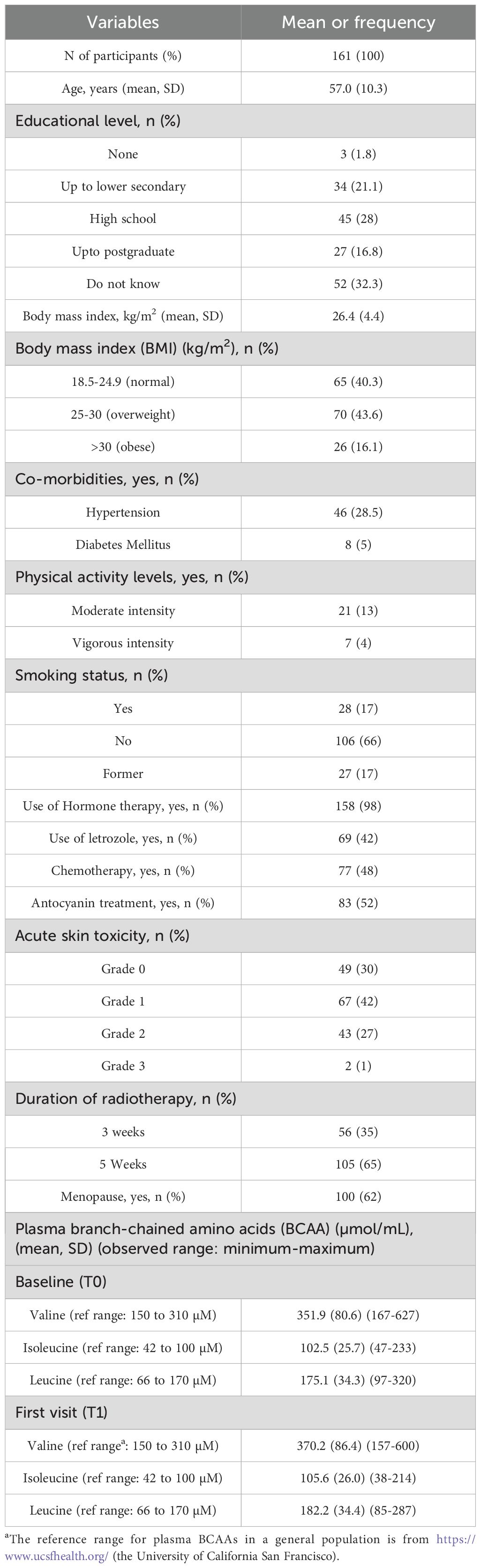
The sample size of the current study was 161 women with a mean age of 57 years (SD ± 10.3). Refer to Table 1 for the characteristics of the ATHENA participants. Around 28% (n=45) of women reported AST ≥G2 of the total sample. 28.5% (n=46) were hypertensive, and 98% (n=158) reportedly used prescribed hormone therapy, particularly letrozole (42%, n=69). Further, based on the BMI (kg/m2), 43.4% (n=70) participants were overweight and 16.1% (n=26) as obese.
Finally, the plasma BCAAs (leucine, isoleucine, and valine) measurements conducted at T0 and T1 remained mostly similar before and after radiotherapy (T0 and T1).
In the fully adjusted multivariable logistic regression model (Model 4) of the cross-sectional analysis (refer to Table 2), of the three plasma BCAAs measured at T1, plasma isoleucine (1-SD increment) was associated with 43% reduced odds of ≥ G2 AST post-radiotherapy (OR = 0.57; 95% CI 0.36 to 0.91; p = 0.01).
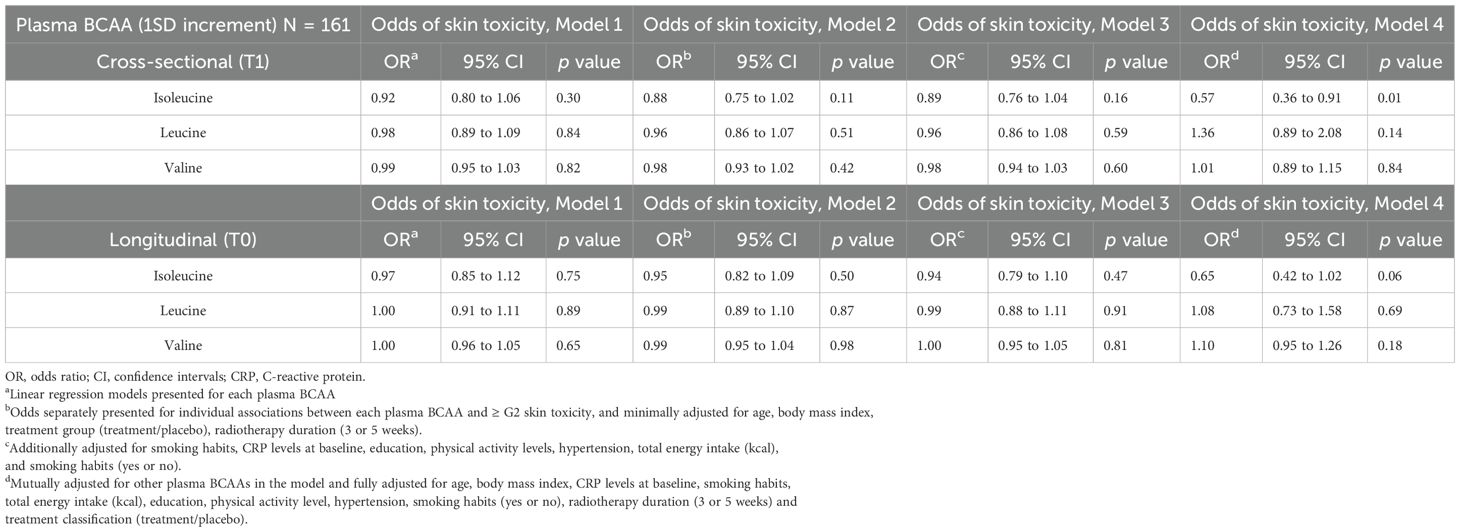
Table 2. Association between plasma branch chain amino acids (BCAA) levels and the odds of post-radiation ≥G2 acute skin toxicity in cross-sectional (T1) and longitudinal analysis (T0) in ATHENA project.
Further, in Model 4 of the prospective analysis, an inverse association was observed between plasma isoleucine (1-SD increment) and the odds of ≥G2 AST post-radiotherapy (OR = 0.65; 95% CI: 0.42 to 1.02; p = 0.06), although the estimate did not reach conventional levels of statistical significance and should be interpreted with caution due to limited precision. However, when valine and leucine BCAA were individually or together analyzed along with other covariates in the models, there was no evidence of an association with ≥ G2 AST post-radiotherapy (refer to Models 1–4 in Table 2). Finally, in a sensitivity analysis, the association between plasma isoleucine and odds of ≥ G2 AST post-radiotherapy resulted statistically significant after an additional adjustment for chemotherapy treatment (refer to Table 3).
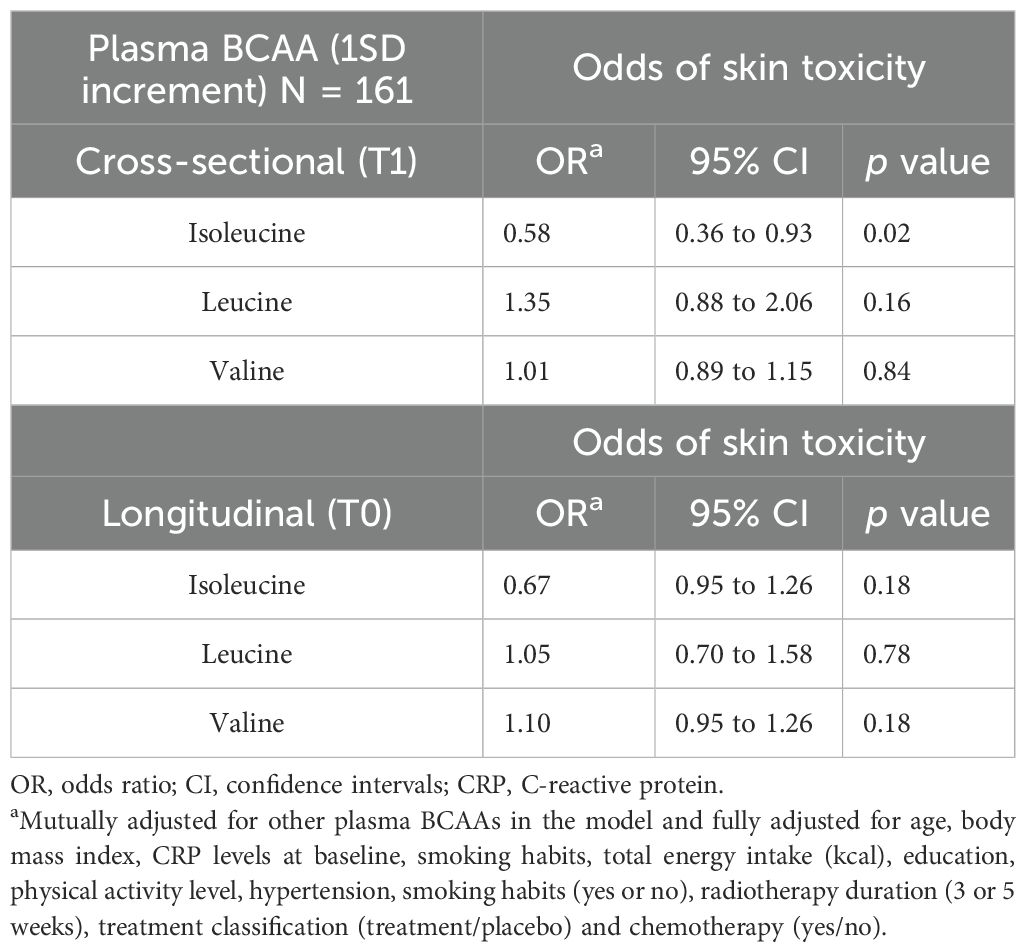
Table 3. Sensitivity analysis: Association between plasma branch chain amino acids (BCAA) levels and the odds of post-radiation ≥G2 acute skin toxicity in cross-sectional (T1) and longitudinal analysis (T0) in ATHENA project.
4 Discussion
The current study examined the influence of plasma BCAAs (leucine, isoleucine, and valine) on post-radiation ≥ G2 AST in a BC cohort. Findings in the cross-sectional analysis indicated that among plasma BCAAs measured at T1, plasma isoleucine was associated with lower odds of ≥ G2 AST post-radiotherapy. Meanwhile, the prospective analysis findings suggested similar trend in the statistical significance for the results between plasma isoleucine measured at baseline (T0) and the odds of ≥ G2 AST post-radiotherapy. To the best of our knowledge, this is the first study investigating the association between circulating plasma BCAAs (leucine, isoleucine, and valine) and the odds of ≥ G2 AST amongst BC patients who underwent radiotherapy.
The association of isoleucine levels with lower odds of ≥ G2 AST after the radiotherapy at T1 could indicate the protective short-term effects of isoleucine after radiotherapy in BC patients. Indeed, radiotherapy triggers a series of inflammatory responses in the normal tissue. Oxidative stress causes cell injury, producing a reaction of lymphocytes and macrophages, subsequently releasing pro-inflammatory cytokines and fibroblast stimulation, releasing reactive oxygen species (ROS) (4).
A study explored isoleucine’s immunological response in relation to immunotherapy for tuberculosis and suggested that isoleucine was strongly correlated with beta-defensin, predominantly secreted from leukocytes and epithelial cells (26). To elaborate, beta-defensin are small peptides (15–20 residues) that have antimicrobial defense properties by penetrating the microbe’s cell membrane and causing microbial death in a method similar to antibiotics. Therefore, this mechanism could potentially be postulated in the context of AST post-radiotherapy in BC. However, further studies are warranted to precisely identify this effect.
In the past, few studies only suggested the use of glutamine (in enteral form) to improve wound matrix formation in patients with hypercatabolic conditions, including cancers requiring radiotherapy and its side effects, such as mucositis and radiodermitis (27–29). Further, in relation to radiation-induced skin injury in BC, glutamine-treated BC patients post-radiotherapy demonstrated a lower rate of AST as compared to the corresponding placebo group (9% developed grade I AST vs. 80% developed grade II AST)—based on the RTOG/EORTC criteria (22) for AST (29).
Additionally, previous nutritional studies evidenced that high-protein diets might lower the risk of AST but not the protective effect of circulating plasma BCAAs in the context of radiotherapy treatment-related skin injuries (6). In recent years, the potential role of dietary BCAAs has been explored in relation to the risk of BC. To elaborate, one animal model study explored the impact of BCAAs in their dietary form on the risk of breast metastasis among mice. The findings demonstrated that high BCAA concentration impaired the ability of tumor cells to invade and migrate due to the downregulation of N-cadherin (18). In contrast, another study examined the long-term dietary intakes of BCAA and reported no evidence of an association with invasive BC risk (30). However, interestingly, there is a dearth of evidence for BCAAs in relation to radiotherapy treatment-related side effects, such as AST affecting BC patients.
Our findings showed that plasma leucine and valine were not associated with lower odds of post-radiotherapy ≥ G2 AST in BC patients. This is despite leucine being the most abundant amino acid responsible for muscle repair and maintaining energy homeostasis. This could elucidate the complex interplay between isoleucine and leucine in mammalian epithelial cells, ultimately promoting healthy growth and proliferation of breast cells and increasing longevity. Moreover, in animal studies, both leucine and isoleucine are suggested to improve the fractional protein synthesis rates in bovine mammary glandular cells with phosphorylation of mTOR, a protein kinase responsible for immune response, autophagy, and maintenance of health cellular metabolism—protein degradation is lowered. However, the pathways involving valine are so far unknown (31–33).
From an oncology nutrition standpoint, the current findings could provide the groundwork for dietary BCAA administration for BC patients undergoing radiotherapy. BCAAs account for 30-40% of the essential amino acids and cannot be synthesized in the body. Therefore, they need to be supplied through the diet (34, 35). Moreover, BCAAs are suggested to act as a fuel source to slower protein degradation during catabolic diseases (9, 36). In addition, further studies including isoleucine supplementation trials could validate our study’s findings to increase overall clinical significance.
To compensate for the protein losses the following BCAAs-related clinical strategies could be considered: (1) the time of administration, especially BCAA supply (before or immediately after radiotherapy); and (2) the form of protein (a) BCAA-rich animal or vegetable sources (for example, fish, meat, eggs, and pulses and cereals such as soybean meal, wheat germ, rye, barley, and sorghum) (9–12, 37) and (b) dietary supplementation rich in BCAAs [commercial supplement formulations comprised of protein-rich substrates such as soybean or chicken breast] (38, 39).
Finally, the sensitivity analysis adjusted for chemotherapy resulted statistically significant for BC patients; both those who underwent radiotherapy and those who underwent chemotherapy coupled with radiotherapy (40).
4.1 Strengths and limitations
Our study had some strengths to be considered. The study design and the analysis used in the methodology optimized the study findings. We had the available data on the BC patients’ circulating plasma BCAAs and repeated post-radiation ≥ G2 AST measures recorded over different time points during the follow-up period. Plasma BCAAs were ascertained using a validated method based on tandem mass spectrometry, providing accurate measurements in plasma samples. The AST readings were classified according to the international guidelines (RTOG grading) to make the findings widely applicable and replicable in clinical settings. We note that the clinicians assessing AST were completely blinded to BCAA levels and study hypotheses, since plasma BCAAs were measured retrospectively from samples collected during routine care. This blinding supports the objectivity and strengthens the credibility of our findings. The regression models were carefully adjusted for covariates with possible clinical significance (24, 25). The analyses were adjusted for radiotherapy duration (3 or 5 weeks) and anthocyanin treatment/placebo, thus accounting for the original study design (19) and commonly used cancer treatments, such as chemotherapy, to test their robustness.
There were some limitations to be considered. First, the study had a modest sample size (N = 161), which could justify the lack of statistical associations, and part of the analysis was cross-sectional. Second, the cross-sectional findings captured relatively shorter time points. However, plasma BCAAs represent the real-time status of the BCAA circulation. They are available in abundant quantities sufficient to exhibit any immediate changes in the metabolism post-radiotherapy among women with BC. Therefore, tracking the plasma BCAAs status after each patient’s radiotherapy visit during radiotherapy treatment might assist the doctors in making clinical decisions, such as dietary supplementation. Third, there were no recommendations/guidelines for plasma BCAA reference ranges available for a population with breast cancer. Hence, it was challenging to compare the differences in plasma BCAAs during baseline and post-radiotherapy and make appropriate clinical conclusions. Fourth, we did not apply correction for multiple comparisons. This choice was motivated by the exploratory nature of the study and the relatively small sample size, which may limit statistical power. However, we acknowledge this as a limitation that could increase the likelihood of false-positive findings. Finally, we could not examine the association between dietary BCAAs and radiation-induced AST in BC patients because of the lack of available data. Perhaps future studies could explore the longitudinal associations between dietary BCAAs and the risk of AST in women with BC.
5 Conclusions
Among circulating plasma BCAAs, high plasma isoleucine was associated with lower odds of ≥ G2 AST among BC women who underwent radiotherapy. However, further studies such as isoleucine supplementation trials are needed to validate the protective role of isoleucine thus contributing to evidence-based clinical management strategies for BC women undergoing radiotherapy.
Data availability statement
The data underlying this article will be shared upon reasonable request to the corresponding author. The data are stored in an institutional repository (https://repository.neuromed.it) and access is restricted by the ethics approval and the legislation of the European Union.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by two Ethics Committees: Catholic University, Rome, and the Azienda Sanitaria Regionale del Molise (ASReM) (P/98/CE/2010 and date of approval: 16/02/2010). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SS: Formal analysis, Writing – original draft, Conceptualization, Methodology. FB: Writing – review & editing, Methodology, Data curation. ADiC: Data curation, Methodology, Formal analysis, Investigation, Conceptualization, Writing – review & editing. ER: Formal analysis, Writing – review & editing. ADeC: Data curation, Writing – review & editing, Resources. CC: Writing – review & editing, Data curation. GdG: Writing – review & editing. FD: Writing – review & editing, Investigation, Data curation. GM: Data curation, Investigation, Writing – review & editing. MB (10th Author): Methodology, Writing – review & editing, Investigation. SC: Methodology, Investigation, Writing – review & editing. AM: Investigation, Writing – review & editing, Methodology. FM: Writing – review & editing, Methodology, Investigation. AA: Methodology, Investigation, Writing – review & editing. KP: Writing – review & editing. CT: Writing – review & editing. DM: Writing – review & editing, Resources. LI: Conceptualization, Writing – review & editing. MB (19th Author): Writing – review & editing, Formal Analysis, Methodology, Conceptualization, Data curation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The ATHENA Project was supported by the EU FP7-KBBE-2009-3 ATHENA Project N. 245121. This study was partially supported by the Italian Ministry of Health (Ricerca Corrente 2022-24) and the Autonomous Province of Trento (Italy), ADP 2022. All Authors were and are independent from funders.
Acknowledgments
The present study was performed in the context of the Fondazione Umberto Veronesi ETS–IRCCS Neuromed framework agreement. We thank all the patients who had accepted to participate in the ATHENA trial, the radiation oncologists, nurses, radiotherapy technicians of the Radiotherapy Unit, and the physicists of Medical Physics Unit for collaboration, and the personnel of the Laboratory of Analyses (Stefano Papini) at the Responsible Research Hospital, formerly called the Gemelli Molise Hospital, Campobasso, Italy, for the laboratory test analyses. The authors maintain a grateful memory of Cinzia Di Gesù, of the Radiotherapy Unit, Responsible Research Hospital, Campobasso, Italy, who had most valuable contributions to the original ATHENA project but prematurely passed away. SS was supported by the Joint Platform Umberto Veronesi Foundation-Research Unit of Epidemiology and Prevention at IRCCS Neuromed in Pozzilli, Italy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BC, Breast cancer; BCAAs, Branched-chain amino acids; AST, Acute skin toxicity; RTOG, Radiation Therapy Oncology Group; EORTC, European Organization for Research and Treatment Cancer; SD, Standard deviation; OR, Odds ratio; CI, Confidence interval; G, Grade; RCT, Randomized controlled trial; Gy, Gray.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Delaney G, Jacob S, Featherstone C, and Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. (2005) 104:1129–37. doi: 10.1002/cncr.21324
3. Baskar R, Lee KA, Yeo R, and Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. (2012) 9:193–9. doi: 10.7150/ijms.3635
4. Córdoba EE, Lacunza E, and Güerci AM. Clinical factors affecting the determination of radiotherapy-induced skin toxicity in breast cancer. Radiat Oncol J. (2021) 39:315. doi: 10.3857/roj.2020.00395
5. Andersen ER, Eilertsen G, Myklebust AM, and Eriksen S. Women’s experience of acute skin toxicity following radiation therapy in breast cancer. J Multidiscip Healthc. (2018) 11:139. doi: 10.2147/JMDH.S155538
6. Yang X, Ren H, Guo X, Hu C, and Fu J. Radiation-induced skin injury: pathogenesis, treatment, and management. Aging (Albany NY). (2020) 12:23379. doi: 10.18632/aging.103932
7. Cilla S, Romano C, Macchia G, Boccardi M, Pezzulla D, Buwenge M, et al. Machine-learning prediction model for acute skin toxicity after breast radiation therapy using spectrophotometry. Front Oncol. (2023) 12. doi: 10.3389/fonc.2022.1044358
8. Miyagi Y, Higashiyama M, Gochi A, Akaike M, Ishikawa T, Miura T, et al. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS One. (2011) 6:e24143. Available online at: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0024143 (Accessed April 16, 2024).
9. Xu B, Wang M, Pu L, Shu C, Li L, and Han L. Association of dietary intake of branched-chain amino acids with long-term risks of CVD, cancer and all-cause mortality. Public Health Nutr. (2022) 25:3390. doi: 10.1017/S1368980021004948
10. Rousseau M, Guénard F, Garneau V, Allam-Ndoul B, Lemieux S, Pérusse L, et al. Associations between dietary protein sources, plasma BCAA and short-chain acylcarnitine levels in adults. Nutrients. (2019) 11. doi: 10.3390/nu11010173
11. Jennings A, MacGregor A, Pallister T, Spector T, and Cassidy A. Associations between branched chain amino acid intake and biomarkers of adiposity and cardiometabolic health independent of genetic factors: A twin study. Int J Cardiol. (2016) 223:992–8. doi: 10.1016/j.ijcard.2016.08.307
12. Zheng Y, Li Y, Qi Q, Hruby A, Manson JAE, Willett WC, et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol. (2016) 45:1482–92. doi: 10.1093/ije/dyw143
13. Kohlmeier M. Amino acids and nitrogen compounds. Nutrient Metab. (2015), 265–477. doi: 10.1016/B978-0-12-387784-0.00008-0
14. Zhang S, Zeng X, Ren M, Mao X, and Qiao S. Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol. (2017) 8. doi: 10.1186/s40104-016-0139-z
15. Sharma S, Zhang X, Azhar G, Patyal P, Verma A, Grishma KC, et al. Valine improves mitochondrial function and protects against oxidative stress. Biosci Biotechnol Biochem. (2024) 88:168–76. doi: 10.1093/bbb/zbad169
16. Zeleznik OA, Balasubramanian R, Ren Y, Tobias DK, Rosner BA, Peng C, et al. Branched-chain amino acids and risk of breast cancer. JNCI Cancer Spectr. (2021) 5. doi: 10.1093/jncics/pkab059
17. Nouri-Majd S, Salari-Moghaddam A, Benisi-Kohansal S, Azadbakht L, and Esmaillzadeh A. Dietary intake of branched-chain amino acids in relation to the risk of breast cancer. Breast Cancer. (2022) 29:993–1000. Available online at: https://europepmc.org/article/med/35794412 (Accessed April 17, 2024).
18. Xu E, Ji B, Jin K, and Chen Y. Branched-chain amino acids catabolism and cancer progression: focus on therapeutic interventions. Front Oncol. (2023) 13:1220638. doi: 10.3389/fonc.2023.1220638
19. Bracone F, De Curtis A, Di Castelnuovo A, Pilu R, Boccardi M, Cilla S, et al. Skin toxicity following radiotherapy in patients with breast carcinoma: is anthocyanin supplementation beneficial? Clin Nutr. (2021) 40:2068–77. doi: 10.1016/j.clnu.2020.09.030
20. Schulz KF, Altman DG, and Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. (2010) 340:698–702. Available online at: https://www.bmj.com/content/340/bmj.c332 (Accessed April 29, 2024).
21. Anesi A, Berding K, Clarke G, Stanton C, Cryan JF, Caplice N, et al. Metabolomic workflow for the accurate and high-throughput exploration of the pathways of tryptophan, tyrosine, phenylalanine, and branched-chain amino acids in human biofluids. J Proteome Res. (2022) 21:1262–75. doi: 10.1021/acs.jproteome.1c00946
22. Cox JD, Stetz JA, and Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat OncologyBiologyPhysics. (1995) 31:1341–6. doi: 10.1016/0360-3016(95)00060-C
23. Pastore F, Conson M, D’Avino V, Palma G, Liuzzi R, Solla R, et al. Dose-surface analysis for prediction of severe acute radio-induced skin toxicity in breast cancer patients. Acta Oncol (Madr). (2016) 55:466–73. doi: 10.3109/0284186X.2015.1110253
24. Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, and Ellison GT. Robust causal inference using directed acyclic graphs: the R package “dagitty. Int J Epidemiol. (2016) 45:1887–94. doi: 10.1093/ije/dyw341
25. Tennant PWG, Murray EJ, Arnold KF, Berrie L, Fox MP, Gadd SC, et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: review and recommendations. Int J Epidemiol. (2021) 50:620–32. doi: 10.1093/ije/dyaa213
26. Rivas-Santiago CE, Rivas-Santiago B, León DA, Castañeda-Delgado J, and Hernández Pando R. Induction of β-defensins by l-isoleucine as novel immunotherapy in experimental murine tuberculosis. Clin Exp Immunol. (2011) 164:80–9. doi: 10.1111/j.1365-2249.2010.04313.x
27. Wang ZE, Zheng JJ, Bin Feng J, Wu D, Su S, Yang YJ, et al. Glutamine relieves the hypermetabolic response and reduces organ damage in severe burn patients: A multicenter, randomized controlled clinical trial. Burns. (2022) 48:1606–17. doi: 10.1016/j.burns.2021.12.005
28. Anderson PM and Lalla RV. Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients. (2020) 12:1–15. doi: 10.3390/nu12061675
29. Eda K, Uzer K, Murat T, and Cenk U. The effects of enteral glutamine on radiotherapy induced dermatitis in breast cancer. Clin Nutr. (2016) 35:436–9. doi: 10.1016/j.clnu.2015.03.009
30. Tobias DK, Chai B, Tamimi RM, Manson JAE, Hu FB, Willett WC, et al. Dietary intake of branched chain amino acids and breast cancer risk in the NHS and NHS II prospective cohorts. JNCI Cancer Spectr. (2021) 5. doi: 10.1093/jncics/pkab032
31. Lei J, Feng D, Zhang Y, Zhao FQ, Wu Z, Gabriel AS, et al. Nutritional and regulatory role of branched-chain amino acids in lactation. Front Bioscience. (2011) 17:2725–39. Available online at: https://www.imrpress.com/journal/FBL/17/7/10.2741/4082 (Accessed April 30, 2024).
32. Ranga Niroshan Appuhamy JAD, Knoebel NA, Deepthi Nayananjalie WA, Escobar J, and Hanigan MD. Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. J Nutr. (2012) 142:484–91. doi: 10.3945/jn.111.152595
33. Chi R, Yao C, Chen S, Liu Y, He Y, Zhang J, et al. Elevated BCAA suppresses the development and metastasis of breast cancer. Front Oncol. (2022) 12:887257. Available online at: www.frontiersin.org (Accessed April 23, 2024).
34. Santos C de S and Nascimento FEL. Isolated branched-chain amino acid intake and muscle protein synthesis in humans: a biochemical review. Einstein (Sao Paulo). (2019) 17:eRB4898. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC6718193
35. Kaspy MS, Hannaian SJ, Bell ZW, and Churchward-Venne TA. The effects of branched-chain amino acids on muscle protein synthesis, muscle protein breakdown and associated molecular signalling responses in humans: an update. Nutr Res Rev. (2023), 1–14. Available online at: https://www.cambridge.org/core/journals/nutrition-research-reviews/article/effects-of-branchedchain-amino-acids-on-muscle-protein-synthesis-muscle-protein-breakdown-and-associated-molecular-signalling-responses-in-humans-an-update/9912227DD5144B0F7EB06260029520D7.
36. Børsheim E, Bui QUT, Tissier S, Kobayashi H, Ferrando AA, and Wolfe RR. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr. (2008) 27:189–95. doi: 10.1016/j.clnu.2008.01.001
37. Brestenský M, Nitrayová S, Patráš P, Heger J, and Nitray J. Branched chain amino acids and their importance in nutrition. J microbiology Biotechnol Food Sci. (2015) 5:197–202. Available online at: https://office2.jmbfs.org/index.php/JMBFS/article/view/8394 (Accessed April 30, 2024).
38. Jeon SH, Seong HJ, Kim H, Kim D, Yang KY, and Nam SH. Improvement of branched-chain amino acid production by isolated high-producing protease from Bacillus amyloliquefaciens NY130 on isolated soy/whey proteins and their muscle cell protection. Food Chem. (2024) 450:139327. doi: 10.1016/j.foodchem.2024.139327
39. Jin HM, Im AE, Cho JY, and Nam SH. Production of BCAA fortified soybean powder hydrolysate using enzymes from bacillus amyloliquefaciens and its application in functional protein formula foods. J Korean Soc Food Sci Nutr. (2023) 52:383–93. doi: 10.3746/jkfn.2023.52.4.383
40. Trayes KP and Cokenakes SEH. Breast cancer treatment. Am Fam Physician. (2021) 104:171–8. Available online at: https://www.aafp.org/pubs/afp/issues/2021/0800/p171.html (Accessed April 19, 2024).
Keywords: breast cancer, protein, radiotherapy, skin toxicity, branched-chain amino acid
Citation: Sharma S, Bracone F, Di Castelnuovo A, Ruggiero E, De Curtis A, Cerletti C, de Gaetano G, Deodato F, Macchia G, Boccardi M, Cilla S, Morganti AG, Mattivi F, Anesi A, Petroni K, Tonelli C, Donati MB, Iacoviello L and Bonaccio M (2025) Plasma branched-chain amino acids and risk of radiation-induced acute skin toxicity in women with breast cancer: results from the ATHENA project. Front. Oncol. 15:1653293. doi: 10.3389/fonc.2025.1653293
Received: 24 June 2025; Accepted: 30 September 2025;
Published: 20 October 2025.
Edited by:
Sharon R Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Sebastian Zahnreich, Johannes Gutenberg University Mainz, GermanyAlexandra McMahon, University of Miami, United States
Copyright © 2025 Sharma, Bracone, Di Castelnuovo, Ruggiero, De Curtis, Cerletti, de Gaetano, Deodato, Macchia, Boccardi, Cilla, Morganti, Mattivi, Anesi, Petroni, Tonelli, Donati, Iacoviello and Bonaccio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Licia Iacoviello, bGljaWEuaWFjb3ZpZWxsb0Btb2xpLXNhbmkub3Jn
 Sukshma Sharma
Sukshma Sharma Francesca Bracone
Francesca Bracone Augusto Di Castelnuovo
Augusto Di Castelnuovo Emilia Ruggiero
Emilia Ruggiero Amalia De Curtis
Amalia De Curtis Chiara Cerletti
Chiara Cerletti Giovanni de Gaetano
Giovanni de Gaetano Francesco Deodato
Francesco Deodato Gabriella Macchia
Gabriella Macchia Mariangela Boccardi
Mariangela Boccardi Savino Cilla
Savino Cilla Alessio Giuseppe Morganti
Alessio Giuseppe Morganti Fulvio Mattivi
Fulvio Mattivi Andrea Anesi
Andrea Anesi Katia Petroni
Katia Petroni Chiara Tonelli
Chiara Tonelli Maria Benedetta Donati
Maria Benedetta Donati Licia Iacoviello
Licia Iacoviello Marialaura Bonaccio
Marialaura Bonaccio