- 1Department of Radiation Oncology, Corewell Health William Beaumont University Hospital, Royal Oak, MI, United States
- 2Department of Radiation Oncology, Oakland University William Beaumont School of Medicine, Rochester, MI, United States
- 3Department of Breast Surgery, Corewell Health William Beaumont University Hospital, Royal Oak, MI, United States
- 4Department of Medical Oncology, Corewell Health William Beaumont University Hospital, Royal Oak, MI, United States
- 5Department of Medical Oncology and Cancer Genetics, Corewell Health William Beaumont University Hospital, Royal Oak, MI, United States
- 6Department of Endocrinology, Henry Ford Health System, Detroit, MI, United States
- 7Department of Radiology, Corewell Health William Beaumont University Hospital, Royal Oak, MI, United States
- 8Department of Plastic Surgery, Somerset Plastic Surgery, Troy, MI, United States
The role of androgen receptor (AR) signaling in breast cancer is underexplored and may be particularly important in the treatment of patients with higher levels of circulating androgens. We discuss the management of a 70-year-old, postmenopausal transgender man with a six-and-one-half-year history of testosterone therapy, who presented with locoregionally advanced, invasive lobular carcinoma with apocrine features that was estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, human epidermal growth factor receptor 2 (HER2)-positive, and AR-positive. The approach to discontinue his testosterone indefinitely upon diagnosis was determined through shared-decision making with the patient. He received neoadjuvant HER2-directed chemotherapy and achieved a complete metabolic response. He underwent bilateral total mastectomies with left targeted axillary lymph node dissection. Final pathology showed a near complete pathologic response in the breast and a pathologic complete response in three sentinel lymph nodes. He completed a course of conventionally fractionated left chest wall and regional nodal proton beam irradiation and received adjuvant HER-2 directed therapy. He tolerated treatment well and remains disease-free two years since diagnosis. This case report and review underscore the importance of a multi-disciplinary and nuanced approach to personalized management of breast cancer in a gender-diverse patient population. Continued characterization of the AR as a potential therapeutic target in patients with breast cancer is warranted
Introduction
The majority (70-80%) of breast cancer is estrogen receptor (ER)-positive, and blocking the ER with endocrine therapy is a cornerstone of the treatment of all stages of ER-positive disease (1). The androgen receptor (AR) is also expressed in the majority (~75%) of all breast cancer with variable expression among biological subtypes (2–6). The impact that AR expression has on breast cancer progression depends on the co-expression or absence of ER and human epidermal growth factor receptor 2 (HER2) and on the balance of circulating estrogens and androgens (7–15). With relatively limited clinical data characterizing the therapeutic benefit of systemic agents that either inhibit or potentiate AR signaling, AR expression is not routinely determined (16).
AR expression may be particularly relevant for patients with high levels of circulating androgens (either because of high endogenous production or receipt of androgen therapy). Cisgender men and transgender men with breast cancer constitute an underrepresented and understudied patient population, yet these patients may derive considerable benefit from therapies that modulate the AR. Here we discuss the management of a 70-year-old postmenopausal, transgender man who presented with ER/progesterone receptor (PR)-negative, HER2-positive, AR-positive locoregionally advanced breast cancer. We provide a review of the AR as an emerging therapeutic target in breast cancer and an update of breast cancer management in the transgender patient population.
Case description
Our patient provided written, informed consent for this case report. He self-identifies as a Non-Hispanic White, transgender man with pronouns he/him/his. He underwent menopause at age 52 and received routine cervical cancer screening until age 50 and routine screening mammography until presentation at age 70. At age 64, he began weekly testosterone injections (50 mg) as part of his gender-affirming hormone therapy, and his serum total testosterone measured 709 ng/dL three months prior to his diagnosis of breast cancer.
The patient presented in September 2023 with pain and “swelling” in his left underarm. Diagnostic imaging identified a 2.7 cm mass in the central portion of his left breast and abnormal-appearing lymph nodes in the ipsilateral axilla. Review of the core needle biopsy of the breast and an axillary lymph node confirmed grade 2 invasive lobular carcinoma with apocrine features. The carcinoma was ER-negative (0% staining), PR-negative (0% staining), HER2-positive (2+ staining by immunohistochemistry and positive on in situ hybridization with a ratio of 2.70), and AR-positive (diffuse, strong staining) (Figure 1). Fluorodeoxyglucose-positron emission tomography (FDG-PET) revealed glucose uptake in the central left breast mass and in multiple ipsilateral axillary (levels I, II, and III) lymph nodes but no distant metastasis (Figure 2). The patient was assigned with anatomic/clinical prognostic stage IIIC/IIIB (cT2, cN3a(f), cM0) disease. Germline genetic testing did not reveal any pathogenic or likely pathogenic variants.
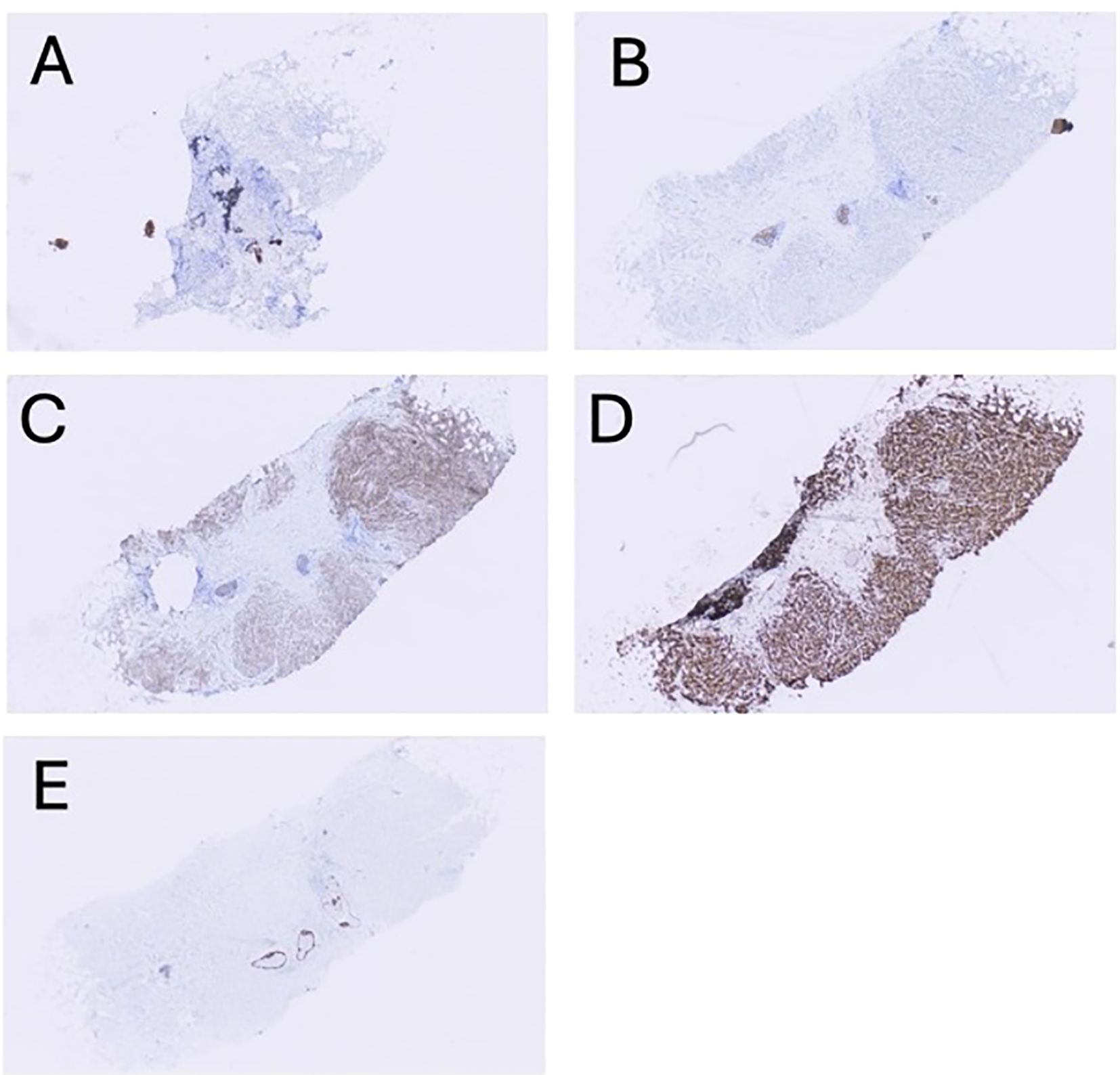
Figure 1. Representative, low-magnification (4x) images showing immunohistochemical staining results for hormonal and adhesion markers: (A) ER (estrogen receptor), (B) PR (progesterone receptor), (C) HER2 (human epidermal growth factor receptor 2), (D) AR (androgen receptor), and (E) E-cadherin (ECAD).
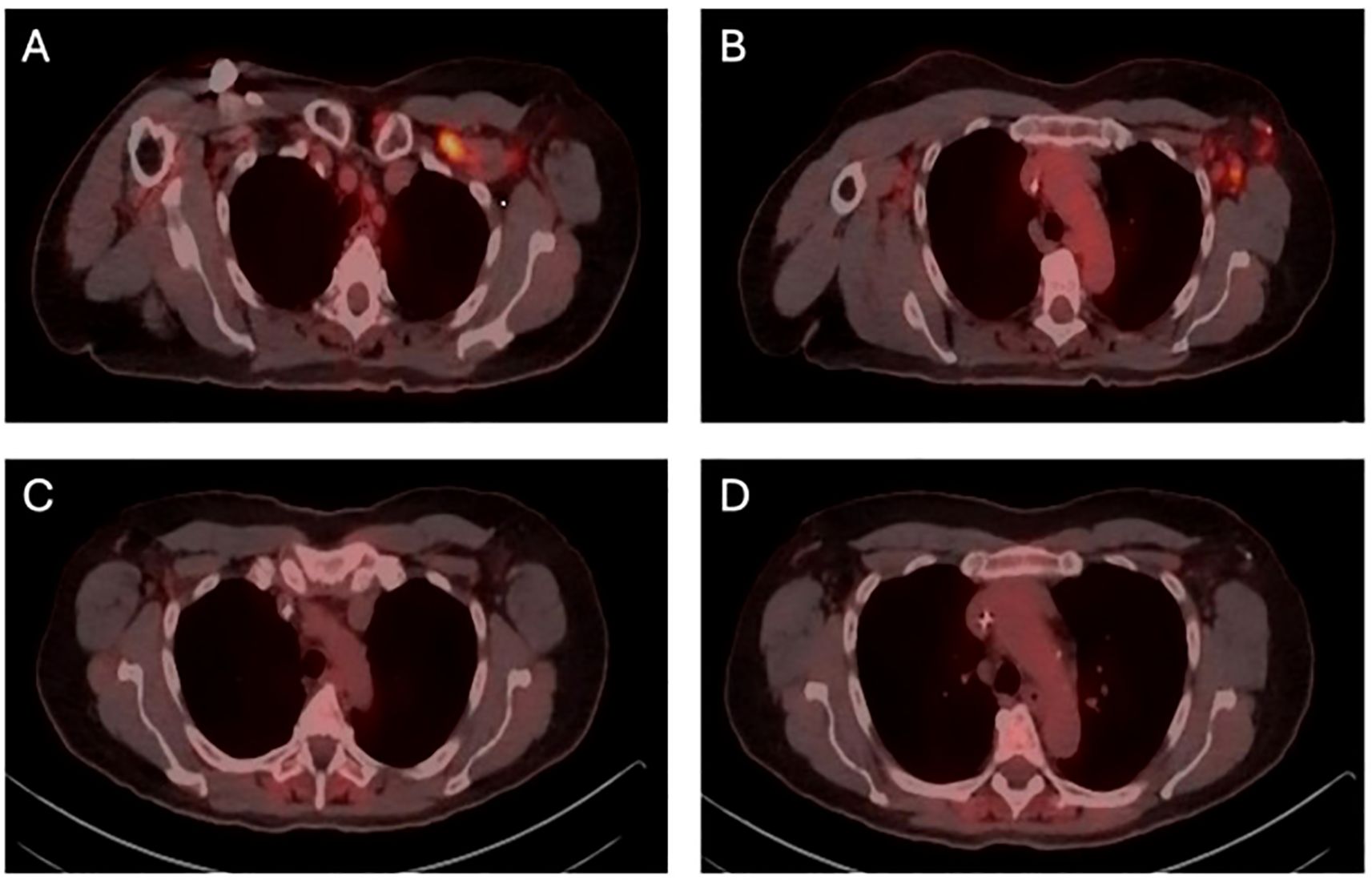
Figure 2. FDG-PET (fluorodeoxyglucose-positron emission tomography) demonstrating metabolic response to systemic therapy. (A) initial FDG-PET prior to neoadjuvant chemotherapy, showing uptake in superior axillary (levels I, II, and III) lymph nodes (B) initial FDG-PET prior to neoadjuvant chemotherapy, showing uptake in inferior axillary (level I and II) lymph nodes. (C) re-staging FDG-PET following neoadjuvant chemotherapy, showing metabolic complete response in superior axillary lymph nodes. (D) re-staging FDG-PET following neoadjuvant chemotherapy, showing metabolic complete response in inferior axillary (levels I and II) lymph nodes.
The patient received neoadjuvant chemotherapy with 6 cycles of docetaxel, carboplatin, trastuzumab, and pertuzumab. FDG-PET following neoadjuvant therapy supported a complete metabolic response. He underwent bilateral total mastectomies with a targeted axillary dissection on the left. Pathology revealed a 2.5 mm residual focus of grade 3 invasive lobular carcinoma in the left breast. All surgical margins were clear by at least 2 mm. There was a complete pathologic response in three recovered axillary lymph nodes, including the biopsied lymph node. He completed a course of conventionally fractionated, intensity modulated proton beam irradiation directed to the left chest wall and axillary (levels I, II, and III), internal mammary, supraclavicular, and posterior cervical lymph nodes. He received adjuvant trastuzumab emtansine.
The conversation surrounding the patient’s gender-affirming hormone therapy was compassionate, nuanced, and non-dogmatic. The potential oncological risk of continued testosterone therapy was weighed against the profound impact that withdrawal of this treatment might have on the patient’s gender identity and mental health. Ultimately, the treatment approach was determined through shared decision-making with the patient. A dialogue between the patient, his cancer treatment providers (including his breast surgeon, medical oncologist, and radiation oncologist), his endocrinologist (also the prescriber of his testosterone), and his counselor was initiated at the time of diagnosis, maintained throughout his cancer treatment, and continues into his survivorship care.
It was explained to the patient that there was a potential role of continued high levels of circulating androgens in promoting the growth of androgen receptor-positive cancer cells, which may increase the risk of cancer recurrence. While the clinical benefits of estrogen blockade in cisgender women with ER-positive breast cancer and of androgen blockade in select cisgender men with prostate cancer are well established, we explained to the patient that the role of the AR in cisgender women with breast cancer is still unclear—and that the role of the AR in transgender men taking testosterone is even less well studied. The consensus recommendation from his treating physicians and from our institution’s multi-disciplinary breast tumor board was for him to consider discontinuing his testosterone therapy. The patient was asked where he was on the spectrum between being strongly opposed and strongly motivated to discontinue his testosterone. We discussed his feelings toward discontinuing this treatment indefinitely, resuming the treatment after a certain period of cancer-free survival, continuing on a reduced dose of testosterone, and continuing on his current dosage. Ultimately, the patient decided that “any risk” that testosterone therapy might have on increasing cancer recurrence made it worth seeing how he would tolerate stopping this treatment.
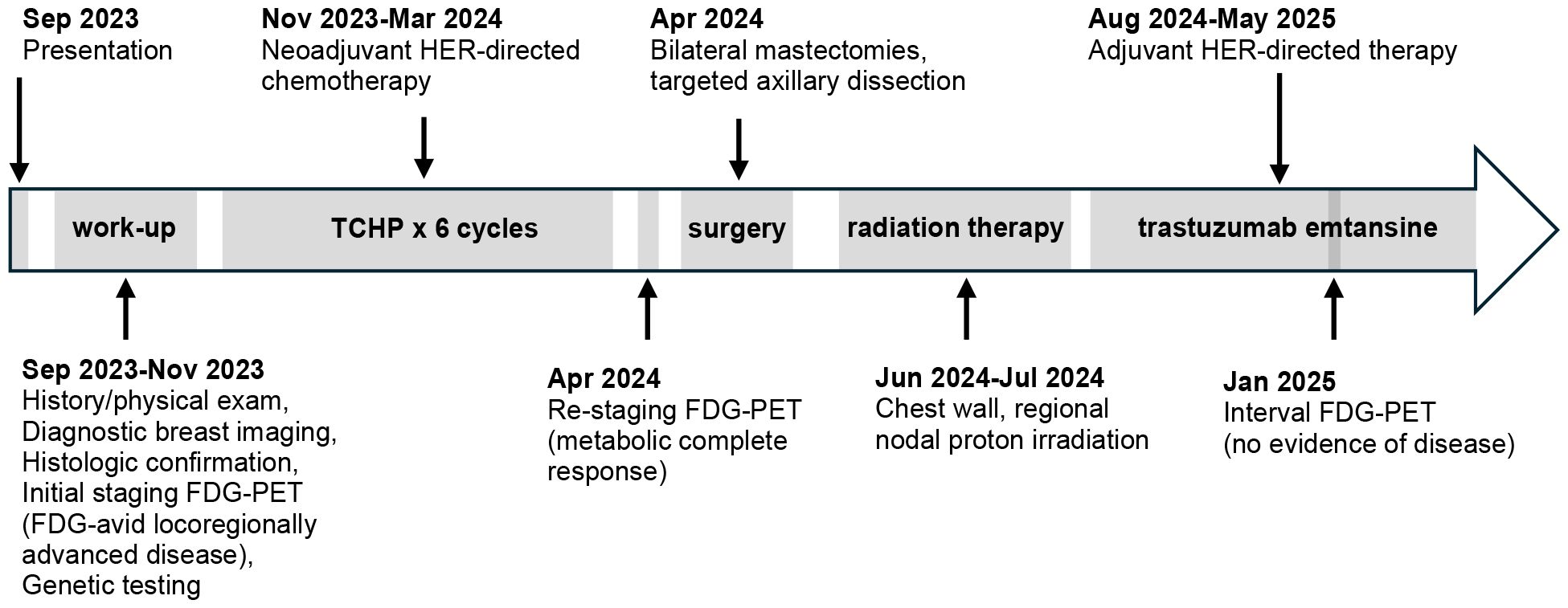
With discontinuation of testosterone, he experienced decreased energy, arthralgias, and insomnia. He also experienced changes that worsened his gender dysphoria, including redistribution of fat to his face, hips, and buttocks, decreased density of his beard, and increase pitch of his voice. At the patient’s last follow-up nearly two years after diagnosis, the patient stated he still “misses his thick beard, his energy, and his narrow hips” but that he remains motivated to not re-initiate his testosterone therapy. He manages his symptoms with diet, exercise (biking, swimming, and walking), meditation, and regular visits with his counselor. He remains disease free at the time of this publication. Figure 3 shows the patient’s treatment timeline.
Discussion
Epidemiology
Breast cancer is the most common non-cutaneous cancer diagnosed in cisgender women, affecting approximately 1 in 8 (~143 cases per 100,000 person years) (17). Breast cancer in cisgender men accounts for less than 1% of all breast cancer diagnoses and affects approximately 1 in 833 (~1 case per 100,000 person years) (17). Transgender individuals carry an intermediate risk of breast cancer. One study reported a collective ~43 cases per 100,000 person years for a cohort of 2,260 transgender women and 1,229 transgender men (18). The rate of breast cancer in the transgender population, however, is highly variable and dependent upon the type and duration of gender-affirming hormone therapy these individuals receive (19–22). Estrogens promote development of mammary tissue in transgender women. These changes, including the formation of ducts, lobules, and acini, may become histologically and radiographically indistinguishable from cisgender women (23–26). Receipt of feminizing hormones also leads to a higher frequency of benign breast processes (such as cysts and fibroadenomas) and malignant transformation of breast tissue. Conversely, testosterone causes atrophy of breast tissue in transgender men, which may lower the risk of malignant transformation (18).
Screening
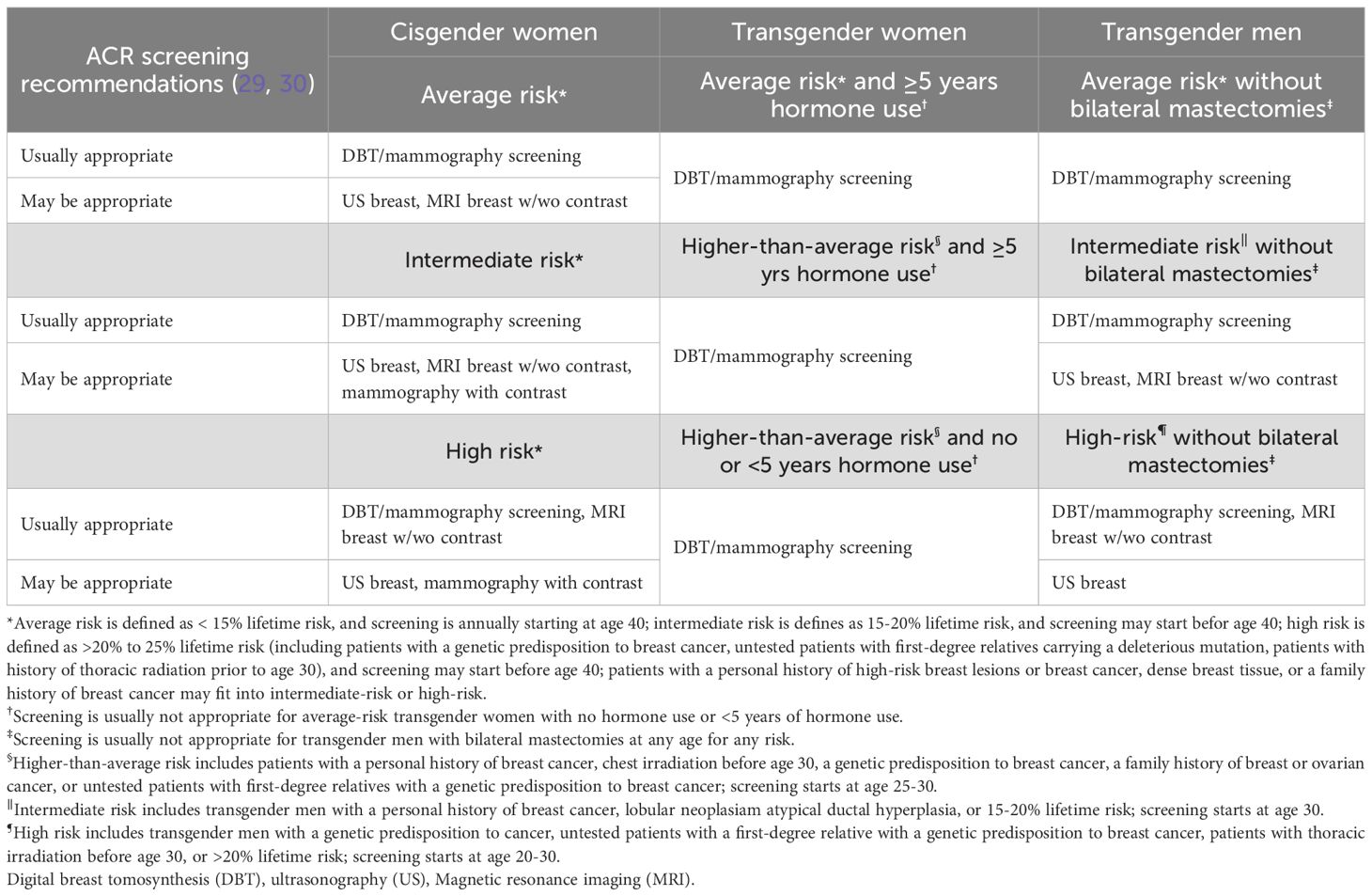
Transgender individuals face significant healthcare disparities and limited access to appropriate breast cancer screening; this contributes to delayed diagnosis and suboptimal care (27, 28). Progress has been made, however, to standardize screening practices for the gender-diverse patient population. The American College of Radiology published a comprehensive list of Appropriateness Criteria for transgender breast cancer screening in 2021 and an update of Appropriateness Criteria for cisgender female breast cancer screening in 2024 (29, 30). Table 1 summarizes the organization’s recommendations, including which imaging modalities are “usually appropriate” or “may be appropriate,” depending on risk classification, age, gender identity, hormone therapy use, and if the patient underwent bilateral mastectomies. Annual screening breast imaging (including mammography and digital breast tomosynthesis) starting at age 40 is offered to all average-risk cisgender women, average-risk transgender women with at least five years of hormone use, and average-risk transgender men who have not undergone bilateral mastectomies.
Surgical management
In the non-oncologic setting, the goal of gender-affirming mastectomies for transgender men is to achieve a flat, masculine chest contour through glandular excision, skin recontouring, and modification of the nipple-areolar complex (31, 32). Incorporating chest masculinization into the surgical management of cisgender men and transgender men with breast cancer is appropriate when desired by the patient and safe from an oncologic standpoint (33). The choice of mastectomy incision, flap, and closure technique depends on a patient’s anatomy and aesthetic expectation. The double incision with free nipple graft provides broad exposure for glandular excision and masculinized contouring, making it well-suited for moderate to large chests (33, 34). Periareolar incision techniques may be appropriate for smaller chests but may leave residual retroareolar tissue, potentially limiting oncologic adequacy (31, 32). The wide-base bipedicled (WIBB) flap, utilized in nipple-sparing mastectomy, incorporates a mastopexy design to improve contour and closure during oncologic and gender-affirming procedures (35). The Angel Wing technique, developed for aesthetic flat closure following mastectomy, extends the incision laterally and superiorly to excise excess tissue in an elliptical fashion, improving lateral chest wall contour and symmetry by eliminating dog-ears or lateral adiposity. This approach is especially useful in patients not pursuing breast mound reconstruction (36). Adjunctive procedures such as pectoral implants that enhance chest projection may further support masculinization, particularly in delayed reconstruction (37). The Goldilocks procedure, which utilizes deepithelialized local tissue for volume restoration, may benefit patients who decline prosthetics but still desire chest projection in a non-delayed fashion (38). The patient in our case study underwent flat closure following his mastectomies and is considering delayed masculinization surgery.
Following an oncologic mastectomy, cisgender women and transgender women may be candidates for implant-based reconstruction, using either a two-stage approach with placement of a tissue expander followed by an exchange for a permanent implant, or a single-stage, direct-to-implant approach (39). Alternatively, autologous reconstruction uses the patient’s own tissue—most commonly from the abdomen, back, or thighs—to recreate a natural breast mound. Combination techniques may integrate implants, fat grafting, or autologous reconstruction to enhance contour, symmetry, or volume.
Radiation oncological management
The administration of radiation therapy with respect to target volumes and organs at risk are similar among patients, regardless of gender identity, and depend on a patient’s anatomy and cancer stage. A discussion regarding potential radiation related toxic effects and their management is important and includes potential treatment related complications regarding breast/chest wall reconstruction (40–42). The patient in this case report had a pathologic complete response in the recovered lymph nodes, including the biopsied lymph node. A recent randomized clinical trial showed that regional nodal irradiation did not improve the invasive breast cancer recurrence-free interval at 5 years for select patients with clinical T1-3 and clinical N1 disease who convert to ypN0 disease following neoadjuvant systemic therapy (43). This trial, however, specifically excluded those with cN2 and cN3 disease. This trial’s design was consistent with previous data showing a significant disease-free survival benefit and overall survival benefit conferred by post-mastectomy irradiation for patients with clinical stage III disease (but not clinical stage I or II disease) after achieving a pathologic complete response to neoadjuvant chemotherapy (44). Many stage III patients in this study harbored cN3 disease. Considering the patient’s high axillary burden at diagnosis (including cN3 involvement), that only 3 sentinel lymph nodes were recovered, and the patient’s preference, his radiation oncologist recommended treatment. He received left chest wall and regional nodal irradiation with inclusion of the internal mammary chain. He received intensity modulated proton beam irradiation, which achieved significant reductions in integral dose to normal tissues, including the heart, left anterior descending artery, lung, and contralateral chest wall, compared to a rival photon plan. While modern techniques (such as deep inspiration breath hold, prone positioning, intensity-modulated radiation therapy, and volumetric modulate arc therapy) ensure that photon therapy is very safe for the majority of patients (45–48), proton therapy typically results in further reductions in dose to normal tissues. For select patients, this dosimetric advantage may confer a clinically meaningful decrease in the risk of pulmonary or cardiac toxic effects. Patients most likely to benefit from proton therapy include those who have challenging anatomy, a history of previous radiation therapy to the breast/thorax, a pathogenic germline variant in a cancer susceptibility gene (in which case sparing of non-target tissue decreases the risk for second malignancy), and those who require left-sided or bilateral regional nodal treatment (49–55). Multiple trials investigating the safety of de-escalating radiation therapy in select patients who respond well to neoadjuvant systemic therapy are ongoing and will help guide treatment recommendations for future patients.
Biological subtyping and medical management
Breast cancer is classified based on tumor gene expression and molecular characteristics, particularly the expression of ER, PR, and HER2 (3, 56, 57). Four main biological subtypes are used in clinical practice: Luminal A, Luminal B, HER2-enriched, and basal-like (which overlaps considerably with triple negative breast cancer). These subtypes carry prognostic value, predict therapy response, and guide systemic therapy recommendations. ER signaling, for example, is targeted with endocrine therapy (e.g., selective estrogen receptor modulators/degraders and aromatase inhibitors), while HER2 signaling is targeted with monoclonal antibodies and antibody-drug conjugates. Specific regimens of cytotoxic chemotherapy, immunotherapy, and a variety of small molecule inhibitors are also administered depending on cancer subtype (58–60).
Breast tissue normally expresses the AR, which binds primarily testosterone and 5α-dihydrotestosterone. While generally growth inhibitory to mammary epithelial cells, androgens are also precursors to estrogens, which in turn may drive the development of ER-positive breast cancer (61, 62). Androgens have also been associated with HER2 overexpression and the activation of the epidermal growth factor receptor; this explains how androgens may act to drive ER-negative breast cancer (63, 64). The AR is expressed in ~75% of all breast cancers but more commonly in ER-positive disease (80-90%), compared to ER-negative disease (30-50%), HER2-positive disease (50-60%, regardless of ER status), and triple negative breast cancer (20-40%) (2–6, 65). Higher levels of circulating androgens, either because of higher endogenous production or exogenous testosterone use, may amplify signaling crosstalk among the AR, ER, and HER2.
A retrospective review of pathology specimens from female-to-male transgender patients undergoing gender-affirming mastectomies investigated the impact of exogenous androgens on breast tissue (66). This study showed that androgen-exposed breast tissue revealed dense fibrotic stroma, lobular atrophy, thickened lobular basement membranes, and gynecomastoid changes. Longer duration of androgen exposure was associated with more pronounced changes. The study also showed that ER and AR expression were highest in patients with intermediate duration of androgen exposure. Two additional studies compared pathology specimens from gender-affirming chest-contouring surgery in patients who did and did not receive testosterone therapy (67, 68). Longer duration of testosterone was associated with higher degrees of lobular atrophy but not fibrous content, decreasing amounts of epithelium and stroma, and a higher incidence of cysts, fibroadenomas, pseudoangiomatous stromal hyperplasia, and papillomas. For a subset of transmasculine patients who had a portion of the nipple-areolar complex available for evaluation, these specimens were compared to those of cisgender women who underwent a total mastectomy (69). The presence of Toker cell hyperplasia was higher in transmasculine patients than in cisgender women, and in cases of Toker cell hyperplasia, the rate of gland formation was higher in transmasculine patients. Further, the majority (90%) of Toker cells were AR-positive. These data underscore the complexity of extrapolating AR-targeted therapy evidence from cisgender women to gender-diverse populations.
The impact that AR expression has on breast cancer progression and prognosis is variable, depending on the co-expression or absence of ER and HER2 (7–15). A clinical meta-analysis evaluating over 10,000 patients with breast cancer reported a subgroup analysis of those with either ER-positive, ER-negative, ER-negative and HER2-positive, or triple negative disease. AR expression conferred improved disease-free survival and overall survival in all patients with ER-positive breast cancer (9). The same study showed a similar positive association with AR expression and clinical outcomes in patients with triple negative breast cancer. AR expression, however, did not improve clinical outcomes in all patients with ER negative breast cancer and was associated with worse overall survival in patients with ER-negative, HER2-positive breast cancer. These trends have been supported by multiple preclinical and clinical studies (3, 7, 10–13, 65, 70, 71) and have informed efforts to target AR signaling in patients with breast cancer.
While androgen deprivation has been a long-standing treatment approach for other AR-positive cancers—notably prostate cancer—the AR remains an emerging target in the treatment of breast cancer (72, 73). A phase II clinical trial enrolled patients with AR-positive, ER/PR-negative breast cancer and reported that bicalutamide, a nonsteroidal antiandrogen, produced a 19% clinical benefit rate at 6 months (74). Another phase II trial evaluated the efficacy and safety of the second-generation antiandrogen enzalutamide in patients with AR-positive triple negative locally advanced or metastatic breast cancer. This study showed 25% and 33% clinical benefit rates at 16 weeks in the intent-to-treat population and evaluable subgroup, respectively (75). Enobosarm, a novel oral selective androgen receptor modulator (SARM), was recently shown in a randomized, phase 2 clinical trial to have anti-tumor activity in patients with previously treated AR-positive, ER-positive, HER2-negative locally advanced or metastatic breast cancer (76). These and other drugs that modulate AR signaling, including 17 α-hydroxylase/17,20 lyase (CYP17) inhibitors, exogenous androgens (dehydroepiandrosterone, 4-OH-testosterone), androgen synthesis inhibitors, and AR-specific antisense oligonucleotides, are currently being investigated in clinical trials (72, 77).
AR signaling may be particularly relevant in patients with high levels of circulating androgens. Whether data from clinical trials investigating AR modulation (which enroll almost exclusively cisgender women) can be generalized to cisgender men and transgender men should be confirmed. In cisgender men with ER-positive breast cancer, the AR is typically targeted albeit inadvertently. Because of the conversion of androstenediones to estradiol through aromatase, current guidelines support the administration of gonadotropin-releasing hormone agonists with aromatase inhibitors in cisgender men. Tamoxifen is usually recommended first line, however, because of good tolerability and lack of high-level prospective studies in this patient population (78, 79).
Management of symptoms of hypogonadism and gender dysphoria
The positive benefits that gender-affirming hormone therapy (GAHT) provide transgender individuals are well established. GAHT has been shown to improve mental health and quality of life measures, alleviate gender dysphoria by facilitating appearance congruence, and improve body satisfaction and social functioning (80–83). Thus, the significance of withdrawing GAHT should not be taken lightly: the potential oncological risk of continued GAHT should be weighed against the profound impact that withdrawal of this treatment might have on the patient’s gender identity and mental health. Ultimately, the treatment approach should be determined through shared decision-making with the patient. In addition to a multi-disciplinary cancer treatment team, mental health support, including social workers, counselors, family members, and patient interest groups is essential.
Cessation of testosterone in transgender men may result in a decrease in masculinization (e.g., decreased muscle mass, body hair, and libido), a return of feminine features (e.g., fat redistribution to the gluteofemoral region and increased pitch in voice), and mood disturbances related to gender dysphoria. Transgender men who have undergone menopause may experience symptoms related to estrogen deficiency, including vasomotor symptoms, fatigue, arthralgias, cognitive dysfunction, and disturbances in mood, sleep, and sexual function. Hormonal therapies are generally contraindicated in breast cancer survivors. Testosterone is not approved by the U.S. Food and Drug Administration for use in cisgender men with breast cancer (Depo testosterone package insert), and the existing data do not support the use of testosterone in transgender men with breast cancer. Hormone replacement with estrogen and progesterone has been associated with increased risk for breast cancer recurrence in a randomized clinical trial (HABITS) and a meta-analysis, especially in those with ER-positive tumors and cannot be recommended (84, 85). There is a lack of data investigating the impact that GAHT has on breast cancer in the transgender population. Further investigation is needed to determine if resuming GAHT after a period of cancer-free survival or if continuing on a reduced dose of GAHT (provided there are no other contraindications to this treatment) is safe from an oncological standpoint in the transgender population.
Nonhormonal options to manage vasomotor symptoms include selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, gabapentin, oxybutynin, clonidine, and fezolinetant (86, 87). These and other symptoms related to the withdrawal of sex hormones may also be managed with dietary supplements, mind-body techniques (e.g., cognitive behavioral therapy, hypnosis), and various lifestyle modifications, including exercise, weight loss, trigger avoidance, and yoga (88).
Conclusions
AR-targeted therapy in breast cancer is nuanced. Appropriate selection of agents that either block or potentiate AR signaling likely depends on a patient’s cancer biological subtype, levels and balance of circulating estrogens and androgens, and previous or concurrent exposure to ER-directed endocrine therapy. Targeting the AR may represent an underutilized treatment strategy, particularly for patients with high levels of circulating androgens, and should be further investigated. Nonhormonal pharmacologic options, lifestyle modifications, and mind-body techniques may help manage symptoms related to hormone withdrawal, including gender dysphoria in the transgender population.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual for the publication of any potential identifiable images or data included in this article.
Author contributions
YR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. CS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. LN: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. DZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. SL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. AB: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. SK: Conceptualization, Formal Analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. CB: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. JD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kim N and Lukong KE. Treating ER-positive breast cancer: a review of the current FDA-approved SERMs and SERDs and their mechanisms of action. Oncol Rev. (2025) 19:1564642. doi: 10.3389/or.2025.1564642
2. McNamara KM, Yoda T, Nurani AM, Shibahara Y, Miki Y, Wang L, et al. Androgenic pathways in the progression of triple-negative breast carcinoma: a comparison between aggressive and non-aggressive subtypes. Breast Cancer Res Treat. (2014) 145:281–93. doi: 10.1007/s10549-014-2942-6
3. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. (2011) 121:2750–67. doi: 10.1172/JCI45014
4. McNamara KM, Moore NL, Hickey TE, Sasano H, and Tilley WD. Complexities of androgen receptor signalling in breast cancer. Endocr Relat Cancer. (2014) 21:T161–81. doi: 10.1530/ERC-14-0243
5. Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, and Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. (2010) 23:205–12. doi: 10.1038/modpathol.2009.159
6. Kensler KH, Regan MM, Heng YJ, Baker GM, Pyle ME, Schnitt SJ, et al. Prognostic and predictive value of androgen receptor expression in postmenopausal women with estrogen receptor-positive breast cancer: results from the Breast International Group Trial 1-98. Breast Cancer Res. (2019) 21:30. doi: 10.1186/s13058-019-1118-z
7. Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, et al. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol. (2011) 22:1755–62. doi: 10.1093/annonc/mdq678
8. Zhau HE, He H, Wang CY, Zayzafoon M, Morrissey C, Vessella RL, et al. Human prostate cancer harbors the stem cell properties of bone marrow mesenchymal stem cells. Clin Cancer Res. (2011) 17:2159–69. doi: 10.1158/1078-0432.CCR-10-2523
9. Bozovic-Spasojevic I, Zardavas D, Brohée S, Ameye L, Fumagalli D, Ades F, et al. The prognostic role of androgen receptor in patients with early-stage breast cancer: A meta-analysis of clinical and gene expression data. Clin Cancer Res. (2017) 23:2702–12. doi: 10.1158/1078-0432.CCR-16-0979
10. Elebro K, Borgquist S, Simonsson M, Markkula A, Jirström K, Ingvar C, et al. Combined androgen and estrogen receptor status in breast cancer: treatment prediction and prognosis in a population-based prospective cohort. Clin Cancer Res. (2015) 21:3640–50. doi: 10.1158/1078-0432.CCR-14-2564
11. Micello D, Marando A, Sahnane N, Riva C, Capella C, and Sessa F. Androgen receptor is frequently expressed in HER2-positive, ER/PR-negative breast cancers. Virchows Arch. (2010) 457:467–76. doi: 10.1007/s00428-010-0964-y
12. Ogawa Y, Hai E, Matsumoto K, Ikeda K, Tokunaga S, Nagahara H, et al. Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol. (2008) 13:431–5. doi: 10.1007/s10147-008-0770-6
13. McNamara KM, Yoda T, Miki Y, Chanplakorn N, Wongwaisayawan S, Incharoen P, et al. Androgenic pathway in triple negative invasive ductal tumors: its correlation with tumor cell proliferation. Cancer Sci. (2013) 104:639–46. doi: 10.1111/cas.12121
14. Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, and Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. (2007) 109:25–32. doi: 10.1002/cncr.22381
15. Sutton LM, Cao D, Sarode V, Molberg KH, Torgbe K, Haley B, et al. Decreased androgen receptor expression is associated with distant metastases in patients with androgen receptor-expressing triple-negative breast carcinoma. Am J Clin Pathol. (2012) 138:511–6. doi: 10.1309/AJCP8AVF8FDPTZLH
16. Kotsopoulos J and Narod SA. Androgens and breast cancer. Steroids. (2012) 77:1–9. doi: 10.1016/j.steroids.2011.10.002
17. Anderson WF, Jatoi I, Tse J, and Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. (2010) 28:232–9. doi: 10.1200/JCO.2009.23.8162
18. de Blok CJM, Wiepjes CM, Nota NM, van Engelen K, Adank MA, Dreijerink KMA, et al. Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in the Netherlands. BMJ. (2019) 365:l1652. doi: 10.1136/bmj.l1652
19. Eismann J, Heng YJ, Fleischmann-Rose K, Tobias AM, Phillips J, Wulf GM, et al. Interdisciplinary management of transgender individuals at risk for breast cancer: case reports and review of the literature. Clin Breast Cancer. (2019) 19:e12–9. doi: 10.1016/j.clbc.2018.11.007
20. Gurrala RR, Kumar T, Yoo A, Mundinger GS, Womac DJ, and Lau FH. The impact of exogenous testosterone on breast cancer risk in transmasculine individuals. Ann Plast Surg. (2023) 90:96–105. doi: 10.1097/SAP.0000000000003321
21. Heng YJ, Zhang KJ, Valero MG, Baker GM, Fein-Zachary VJ, Irwig MS, et al. Invasive ductal carcinoma of the breast in a transgender man: A case report. Case Rep Oncol. (2023) 16:811–7. doi: 10.1159/000529859
22. Sato S, Imada S, Hayami R, Arai K, Kosugi R, Tsuneizumi M, et al. Complexities in adjuvant endocrine therapy for breast cancer in female-to-male transgender patients. Case Rep Oncol. (2024) 17:208–16. doi: 10.1159/000536212
23. Maglione KD, Margolies L, Jaffer S, Szabo J, Schmidt H, Weltz C, et al. Breast cancer in male-to-female transsexuals: use of breast imaging for detection. AJR Am J Roentgenol. (2014) 203:W735–40. doi: 10.2214/AJR.14.12723
24. Sonnenblick EB, Shah AD, Goldstein Z, and Reisman T. Breast imaging of transgender individuals: A review. Curr Radiol Rep. (2018) 6:1. doi: 10.1007/s40134-018-0260-1
25. Reisner SL, Vetters R, Leclerc M, Zaslow S, Wolfrum S, Shumer D, et al. Mental health of transgender youth in care at an adolescent urban community health center: a matched retrospective cohort study. J Adolesc Health. (2015) 56:274–9. doi: 10.1016/j.jadohealth.2014.10.264
26. D’hoore L and T’Sjoen G. Gender-affirming hormone therapy: An updated literature review with an eye on the future. J Intern Med. (2022) 291:574–92. doi: 10.1111/joim.13441
27. Fehl A, Ferrari S, Wecht Z, and Rosenzweig M. Breast cancer in the transgender population. J Adv Pract Oncol. (2019) 10:387–94. doi: 10.6004/jadpro.2019.10.4.6
28. Di Lisa FS, Villa A, Filomeno L, Arcuri T, Chiofalo B, Sanguineti G, et al. Breast and cervical cancer in transgender men: literature review and a case report. Ther Adv Med Oncol. (2024) 16:17588359241259466. doi: 10.1177/17588359241259466
29. Expert Panel on Breast Imaging, Brown A, Lourenco AP, Niell BL, Cronin B, Dibble EH, et al. ACR appropriateness criteria® Transgender breast cancer screening. J Am Coll Radiol. (2021) 18:S502–15. doi: 10.1016/j.jacr.2021.09.005
30. Expert Panel on Breast Imaging, Niell BL, Jochelson MS, Amir T, Brown A, Adamson M, et al. ACR appropriateness criteria® Female breast cancer screening: 2023 update. J Am Coll Radiol. (2024) 21:S126–43. doi: 10.1016/j.jacr.2024.02.019
31. Huber PD, Bittencourt RC, and Jeziorowski A. Masculinizing mammoplasty for female-to-male transgenders: 10 years’ Experience. Aesthetic Plast Surg. (2024) 48:3825–35. doi: 10.1007/s00266-024-03931-4
32. Salibian AA, Axelrod DM, Smith JA, Fischer BA, Agarwal C, and Bluebond-Langner R. Oncologic considerations for safe gender-affirming mastectomy: preoperative imaging, pathologic evaluation, counseling, and long-term screening. Plast Reconstr Surg. (2021) 147:213e–21e. doi: 10.1097/PRS.0000000000007589
33. Panichella JC, Araya S, Nannapaneni S, Robinson SG, You S, Gubara SM, et al. Cancer screening and management in the transgender population: Review of literature and special considerations for gender affirmation surgery. World J Clin Oncol. (2023) 14:265–84. doi: 10.5306/wjco.v14.i7.265
34. Schafer RE, Fodor R, Marlar R, Jensen KK, Meyers A, Isakov R, et al. Nonbinary and transgender male patient preferences for gender-affirming top surgery. Ann Plast Surg. (2024) 93:e36–44. doi: 10.1097/SAP.0000000000004052
35. Cordova A, Rossi M, Roggio T, Cammarata E, Cipolla C, Vieni S, et al. The wide base bipedicled (WIBB) flap in nipple-sparing skin-reducing mastectomy. Sci Rep. (2024) 14:9226. doi: 10.1038/s41598-024-52396-7
36. Klenotic E, Ochoa D, Stephenson K, Croswell C, Sullivan S, Sherman AC, et al. Flat aesthetic mastectomy closure with the angel wing technique to address lateral adiposity: technique and outcome analysis. Breast J. (2024) 2024:7349633. doi: 10.1155/2024/7349633
37. Kääriäinen M, Salonen K, Helminen M, and Karhunen-Enckell U. Chest-wall contouring surgery in female-to-male transgender patients: a one-center retrospective analysis of applied surgical techniques and results. Scand J Surgery. (2017) 106:74–9. doi: 10.1177/1457496916645964
38. Richardson H and Ma G. The goldilocks mastectomy. Int J Surg. (2012) 10:522–6. doi: 10.1016/j.ijsu.2012.08.003
39. Simion L, Petrescu I, Chitoran E, Rotaru V, Cirimbei C, Ionescu SO, et al. Breast reconstruction following mastectomy for breast cancer or prophylactic mastectomy: therapeutic options and results. Life. (2024) 14:138. doi: 10.3390/life14010138
40. Takayesu JSK, Baglien B, Edwards D, Marsh R, Shah J, Pierce L, et al. Effect of prepectoral versus subpectoral implant-based reconstruction on post-mastectomy radiation dosimetry. Ann Surg Oncol. (2025) 32:3705–12. doi: 10.1245/s10434-024-16836-y
41. Park SH, Yang YJ, Sung S, Choi Y, and Yang EJ. Postoperative complications of hypofractionated and conventional fractionated radiation therapy in patients with implant-based breast reconstruction: A systematic review and meta-analysis. Breast. (2024) 77:103782. doi: 10.1016/j.breast.2024.103782
42. Sinik LM and Collins MS. Challenges in autologous breast reconstruction: A review of recommendations. J Clin Med. (2024) 13:971. doi: 10.3390/jcm13040971
43. Mamounas EP, Bandos H, White JR, Julian TB, Khan AJ, Shaitelman SF, et al. Omitting regional nodal irradiation after response to neoadjuvant chemotherapy. N Engl J Med. (2025) 392:2113–24. doi: 10.1056/NEJMoa2414859
44. McGuire SE, Gonzalez-Angulo AM, Huang EH, Tucker SL, Kau SW, Yu TK, et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. (2007) 68:1004–9. doi: 10.1016/j.ijrobp.2007.01.023
45. Song J, Tang T, Caudrelier JM, Bélec J, Chan J, Lacasse P, et al. Dose-sparing effect of deep inspiration breath hold technique on coronary artery and left ventricle segments in treatment of breast cancer. Radiother Oncol. (2021) 154:101–9. doi: 10.1016/j.radonc.2020.09.019
46. Gaál S, Kahán Z, Rárosi F, Fodor GH, Tolnai J, Deák B, et al. Individual benefit in heart sparing during DIBH-supported left breast radiotherapy. Clin Transl Radiat Oncol. (2024) 46:100746. doi: 10.1016/j.ctro.2024.100746
47. Peters GW, Gao SJ, Knowlton C, Zhang A, Evans SB, Higgins S, et al. Benefit of deep inspiratory breath hold for right breast cancer when regional lymph nodes are irradiated. Pract Radiat Oncol. (2022) 12:e7–e12. doi: 10.1016/j.prro.2021.08.010
48. Gough E, Ashworth S, Moodie T, Wang W, Byth K, Beldham-Collins R, et al. DIBH reduces right coronary artery and lung radiation dose in right breast cancer loco-regional radiotherapy. Med Dosim. (2024) 49:307–13. doi: 10.1016/j.meddos.2024.03.002
49. Kirby AM, Haviland JS, Mackenzie M, Fleming H, Anandadas C, Wickers S, et al. Proton beam therapy in breast cancer patients: the UK PARABLE trial is recruiting. Clin Oncol (R Coll Radiol). (2023) 35:347–50. doi: 10.1016/j.clon.2023.02.015
50. Stick LB, Lorenzen EL, Yates ES, Anandadas C, Andersen K, Aristei C, et al. Selection criteria for early breast cancer patients in the DBCG proton trial - The randomised phase III trial strategy. Clin Transl Radiat Oncol. (2021) 27:126–31. doi: 10.1016/j.ctro.2021.01.012
51. Mutter RW, Choi JI, Jimenez RB, Kirova YM, Fagundes M, Haffty BG, et al. Proton therapy for breast cancer: A consensus statement from the particle therapy cooperative group breast cancer subcommittee. Int J Radiat Oncol Biol Phys. (2021) 111:337–59. doi: 10.1016/j.ijrobp.2021.05.110
52. Ranger A, Dunlop A, Hutchinson K, Convery H, Maclennan MK, Chantler H, et al. A dosimetric comparison of breast radiotherapy techniques to treat locoregional lymph nodes including the internal mammary chain. Clin Oncol (R Coll Radiol). (2018) 30:346–53. doi: 10.1016/j.clon.2018.01.017
53. Settatree S, Dunlop A, Mohajer J, Brand D, Mooney L, Ross G, et al. What can proton beam therapy achieve for patients with pectus excavatum requiring left breast, axilla and internal mammary nodal radiotherapy? Clin Oncol (R Coll Radiol). (2021) 33:e570–7. doi: 10.1016/j.clon.2021.06.011
54. Depauw N, Batin E, Johnson A, MacDonald SM, and Jimenez RB. Arms positioning in post-mastectomy proton radiation: Feasibility and development of a new arms down contouring atlas. Phys Imaging Radiat Oncol. (2020) 14:6–11. doi: 10.1016/j.phro.2020.04.003
55. Brooks ED, Mailhot Vega RB, Vivers E, Burchianti T, Liang X, Spiguel LR, et al. Proton therapy for bilateral breast cancer maximizes normal-tissue sparing. Int J Part Ther. (2023) 9:290–301. doi: 10.14338/IJPT-22-00041.1
56. Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. (2009) 27:1160–7. doi: 10.1200/JCO.2008.18.1370
57. Horton JK, Jagsi R, Woodward WA, and Ho A. Breast cancer biology: clinical implications for breast radiation therapy. Int J Radiat Oncol Biol Phys. (2018) 100:23–37. doi: 10.1016/j.ijrobp.2017.08.025
58. Shao T, Grossbard ML, and Klein P. Breast cancer in female-to-male transsexuals: two cases with a review of physiology and management. Clin Breast Cancer. (2011) 11:417–9. doi: 10.1016/j.clbc.2011.06.006
59. Nikolić D, Granić M, Ivanović N, Zdravković D, Nikolić A, Stanimirović V, et al. Breast cancer and its impact in male transsexuals. Breast Cancer Res Treat. (2018) 171:565–9. doi: 10.1007/s10549-018-4875-y
60. Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, and Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. (2011) 9:16–32. doi: 10.1038/nrclinonc.2011.177
61. Missmer SA, Eliassen AH, Barbieri RL, and Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. (2004) 96:1856–65. doi: 10.1093/jnci/djh336
62. Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. (2006) 98:1406–15. doi: 10.1093/jnci/djj376
63. Secreto G, Girombelli A, and Krogh V. Androgen excess in breast cancer development: implications for prevention and treatment. Endocr Relat Cancer. (2019) 26:R81–94. doi: 10.1530/ERC-18-0429
64. Vranic S, Tawfik O, Palazzo J, Bilalovic N, Eyzaguirre E, Lee LM, et al. EGFR and HER-2/neu expression in invasive apocrine carcinoma of the breast. Mod Pathol. (2010) 23:644–53. doi: 10.1038/modpathol.2010.50
65. Sehested M, Bindslev N, Demant EJ, Skovsgaard T, and Jensen PB. Daunorubicin and vincristine binding to plasma membrane vesicles from daunorubicin-resistant and wild type Ehrlich ascites tumor cells. Biochem Pharmacol. (1989) 38:3017–27. doi: 10.1016/0006-2952(89)90010-5
66. Chaum M, Grossi S, Chen J, Hu V, Ray E, Giuliano A, et al. Masculinizing hormone therapy effect on breast tissue: Changes in estrogen and androgen receptors in transgender female-to-male mastectomies. Breast. (2023) 72:103596. doi: 10.1016/j.breast.2023.103596
67. Baker GM, Guzman-Arocho YD, Bret-Mounet VC, Torous VF, Schnitt SJ, Tobias AM, et al. Testosterone therapy and breast histopathological features in transgender individuals. Mod Pathol. (2021) 34:85–94. doi: 10.1038/s41379-020-00675-9
68. Heng YJ, Baker GM, Fein-Zachary VJ, Guzman-Arocho YD, Bret-Mounet VC, Massicott ES, et al. Effect of testosterone therapy on breast tissue composition and mammographic breast density in trans masculine individuals. medRxiv [Preprint]. 2024 Jan;10:2024.01.09.24300987. doi: 10.1101/2024.01.09.24300987. Breast Cancer Res. (2024) 26:109. doi: 10.1186/s13058-024-01867-w
69. Baker GM, Bret-Mounet VC, Xu J, Fein-Zachary VJ, Tobias AM, Bartlett RA, et al. Toker cell hyperplasia in the nipple-areolar complex of transmasculine individuals. Mod Pathol. (2023) 36:100121. doi: 10.1016/j.modpat.2023.100121
70. Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, et al. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. (2011) 20:119–31. doi: 10.1016/j.ccr.2011.05.026
71. Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. (2013) 19:5533–40. doi: 10.1158/1078-0432.CCR-13-0799
72. Proverbs-Singh T, Feldman JL, Morris MJ, Autio KA, and Traina TA. Targeting the androgen receptor in prostate and breast cancer: several new agents in development. Endocr Relat Cancer. (2015) 22:R87–R106. doi: 10.1530/ERC-14-0543
73. Rahim B and O’Regan R. AR signaling in breast cancer. Cancers (Basel). (2017) 9:21. doi: 10.3390/cancers9030021
74. Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, et al. Translational Breast Cancer Research Consortium (TBCRC 011). Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res. (2013) 19:5505–12. doi: 10.1158/1078-0432.CCR-12-3327
75. Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O’Shaughnessy J, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. (2018) 36:884–90. doi: 10.1200/JCO.2016.71.3495
76. Palmieri C, Linden H, Birrell SN, Wheelwright S, Lim E, Schwartzberg LS, et al. Activity and safety of enobosarm, a novel, oral, selective androgen receptor modulator, in androgen receptor-positive, oestrogen receptor-positive, and HER2-negative advanced breast cancer (Study G200802): a randomised, open-label, multicentre, multinational, parallel design, phase 2 trial. Lancet Oncol. (2024) 25:317–25. doi: 10.1016/S1470-2045(24)00004-4. Erratum in: Lancet Oncol. 2024 Apr;25(4):e137. doi: 10.1016/S1470-2045(24)00144-X. Erratum in: Lancet Oncol. 2024 Jul;25(7):e284. doi: 10.1016/S1470-2045(24)00289-4.
77. Chia K, O’Brien M, Brown M, and Lim E. Targeting the androgen receptor in breast cancer. Curr Oncol Rep. (2015) 17:4. doi: 10.1007/s11912-014-0427-8
79. Hassett MJ, Somerfield MR, and Giordano SH. Management of male breast cancer: ASCO guideline summary. JCO Oncol Pract. (2020) 16:e839–43. doi: 10.1200/JOP.19.00792
80. Powell L, Puebla A, and Lepping RJ. Gender-affirming hormone therapy and impacts on quality of life: a narrative review. medRxiv. (2025) 12:2025.03.11.25323442. doi: 10.1101/2025.03.11.25323442. Preprint.
81. Mazur M and Larionow P. The effects of gender-affirming hormone therapy on quality of life: the importance of research on youth. Healthcare (Basel). (2024) 12:1336. doi: 10.3390/healthcare12131336
82. Morssinkhof MWL, Wiepjes CM, van den Heuvel OA, Kreukels BPC, van der Tuuk K, T’Sjoen G, et al. Changes in depression symptom profile with gender-affirming hormone use in transgender persons. J Affect Disord. (2024) 348:323–32. doi: 10.1016/j.jad.2023.12.056
83. van Leerdam TR, Zajac JD, and Cheung AS. The effect of gender-affirming hormones on gender dysphoria, quality of life, and psychological functioning in transgender individuals: A systematic review. Transgend Health. (2023) 8:6–21. doi: 10.1089/trgh.2020.0094
84. Poggio F, Del Mastro L, Bruzzone M, Ceppi M, Razeti MG, Fregatti P, et al. Safety of systemic hormone replacement therapy in breast cancer survivors: a systematic review and meta-analysis. Breast Cancer Res Treat. (2022) 191:269–75. doi: 10.1007/s10549-021-06436-9
85. Holmberg L, Iversen OE, Rudenstam CM, Hammar M, Kumpulainen E, Jaskiewicz J, et al. Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst. (2008) 100:475–82. doi: 10.1093/jnci/djn058. Erratum in: J Natl Cancer Inst. 2008 May 7;100(9):685. Maenpa, Johanna [corrected to Maenpaa, Johanna].
86. Shufelt CL, Brown V, Carpenter JS, Chism LA, Faubion SS, Joffe H, et al. The 2023 Nonhormone Therapy Position Statement of The North American Menopause Society” Advisory Panel. The 2023 nonhormone therapy position statement of The North American Menopause Society. Menopause. (2023) 30:573–90. doi: 10.1097/GME.0000000000002200
87. Servayge J, Verduyn AC, Page A, Lagaert L, and Tjalma WAA. Clinical guidelines for managing menopausal symptoms in women with (a history of) breast cancer. Facts Views Vis Obgyn. (2023) 15:297–308. doi: 10.52054/FVVO.15.4.102
88. National Comprehensive Cancer Network. Bone Cancer . Available online at: www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf (Accessed May 10, 2025).
Keywords: male breast cancer, transgender, androgen receptor, invasive lobular carcinoma, testosterone therapy, personalized treatment
Citation: Ramdas Y, Reinicke T, Schnurr CA, Nadeau L, Zakalik D, Lahiri SW, Brudvik A, Kiran S, Busuito CM and Dilworth JT (2025) Case Report: Androgen receptor-positive, locoregionally advanced breast cancer in a transgender man and an update on breast cancer management in a gender-diverse patient population. Front. Oncol. 15:1654048. doi: 10.3389/fonc.2025.1654048
Received: 25 June 2025; Accepted: 04 September 2025;
Published: 25 September 2025.
Edited by:
Mandi L Pratt-Chapman, George Washington University, United StatesReviewed by:
Jason Domogauer, New York University, United StatesElizabeth Cathcart-Rake, Mayo Clinic, United States
Copyright © 2025 Ramdas, Reinicke, Schnurr, Nadeau, Zakalik, Lahiri, Brudvik, Kiran, Busuito and Dilworth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joshua T. Dilworth, am9zaHVhLmRpbHdvcnRoQGNvcmV3ZWxsaGVhbHRoLm9yZw==
 Yastira Ramdas1
Yastira Ramdas1 Trenton Reinicke
Trenton Reinicke Joshua T. Dilworth
Joshua T. Dilworth