- 12ndDepartment of Oncology, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia
- 2Department of Clinical Pathophysiology, Faculty of Medicine, Comenius University, Bratislava, Slovakia
Introduction: Testicular germ cell tumors (TGCT) are highly curable malignancies, with excellent survival rates largely attributable to advances in cancer treatment. Consequently, there is a growing population of long-term TGCT survivors whose life expectancy approaches that of the general population. However, these survivors may experience acute and late adverse effects of cancer treatment, with cardiovascular toxicity being among the most serious and potentially life-threatening.
Methods: This narrative review synthesizes current evidence on cardiovascular toxicity in testicular cancer survivors, including clinical manifestations, pathophysiology of cisplatin-induced cardiovascular damage, additional adverse effects of radiotherapy, and prevalence of traditional cardiovascular risk factors. Key clinical guidelines, observational studies, and experimental findings were analyzed to identify trends, knowledge gaps, and opportunities for improving survivorship care.
Results: Multiple studies consistently demonstrate an increased risk of cardiovascular disease (CVD) among TGCT survivors, particularly following cisplatin-based chemotherapy. Common clinical manifestations include myocardial infarction, angina pectoris, cerebrovascular events, thromboembolism, and heart failure. The highest risk occurs within the first year post-treatment but may persist or recur even after a decade. Cisplatin-induced cardiovascular toxicity involves vascular injury - characterized by endothelial dysfunction, oxidative stress, and prothrombotic state - and myocardial damage driven by oxidative stress, inflammation, and apoptosis. Furthermore, TGCT survivors exhibit a higher prevalence of traditional cardiovascular risk factors, such as smoking, hypertension, dyslipidemia, diabetes, and obesity, contributing to the overall elevated CVD risk.
Discussion: There is an urgent need for a structured, long-term survivorship care model for TGCT survivors. Cardiovascular risk assessment and prevention should be central components, especially in survivors treated with cisplatin-based chemotherapy. Early detection of treatment-related toxicities, combined with lifestyle interventions and regular monitoring, is essential. Future research should focus on elucidating molecular mechanisms of cardiovascular toxicity, validating TGCT survivor-specific screening tools, identifying early biomarkers of cardiac injury, and exploring pharmacologic and behavioral interventions.
Conclusion: Protecting cardiovascular health in TGCT survivors requires a proactive, personalized, and multidisciplinary approach. Integrating cardiometabolic monitoring, risk factor modification, and tailored follow-up strategies into survivorship care is vital. Focused research and clinical attention are needed to ensure that the long-term success of cancer treatment is not compromised by preventable cardiovascular disease.
1 Introduction
In both Europe and United States, cardiovascular disease (CVD) and cancer are among the leading causes of morbidity and mortality (1, 2). Current evidence indicates a bidirectional relationship between these conditions, driven by shared risk factors and overlapping pathophysiological mechanisms (3).
Testicular germ cell tumors (TGCT) are the most common solid malignancies in men aged 20 to 40 years, with a globally rising incidence in recent decades (4, 5). TGCTs are highly curable, with an outstanding > 95% 5-year survival rate, largely attributable to advances in cancer treatment, especially surgery and cisplatin-based chemotherapy (6–8).
Successful cancer treatment has led to a growing number of long-term TGCT survivors whose life expectancy is comparable to those of the healthy population (9). However, these survivors may experience a range of acute and late adverse effects from cancer treatment, including secondary malignancies, an increased risk of cardiovascular disease, pulmonary toxicity, nephrotoxicity, ototoxicity and neurotoxicity, hypogonadism, infertility, and sexual dysfunction. In addition, TGCT survivors may experience psychosocial problems such as depression, anxiety, sleeping disturbances, post-traumatic stress disorder (PTSD), and cognitive dysfunction. All of these adverse effects can contribute to a reduced quality of life. Secondary malignancies and cardiovascular toxicity are the most serious, and potentially life-threatening consequences, as well as the most common causes of mortality in testicular cancer survivors (10).
Cardiovascular toxicity may occur at any time, from days after the first dose of cisplatin to several years following the completion of treatment. Acute cardiovascular events (such as acute vasospasm, thromboembolism, arrhythmias) typically manifest within the first year after exposure to chemotherapy. Late cisplatin-related cardiovascular toxicity is diagnosed beyond 12 months after completing the cardiotoxic treatment, and includes mainly ischemia, myocardial infarction, cardiac dysfunction/heart failure, hypertension, and hyperlipidemia.
The increased risk of CVD in this population may result not only from the adverse cardiovascular effects of anticancer therapies, but also from preexisting cardiovascular risk factors and to advanced cancer itself (11–14).
In this review, we summarize current evidence on cardiovascular toxicity in testicular cancer survivors, including its clinical manifestations, the pathophysiology of cisplatin-related cardiovascular toxicity, and the prevalence of cardiovascular risk factors. We conducted a narrative review of the literature, focusing on clinical and epidemiological studies published in peer-reviewed journals. Our aim is to highlight key findings and emphasize the critical need for increased awareness and integration of cardiovascular health into long-term survivorship care. A comprehensive literature search was conducted using PubMed and related databases up to June 2025. Keywords included “testicular cancer,” “germ cell tumor,” “cancer treatment,” “cisplatin,” “cardiovascular toxicity,” and “cardiovascular disease.” Studies were selected based on their relevance to cardiovascular outcomes in testicular cancer survivors, focusing on clinical manifestations, risk factors, and population-based data. Both original research articles and relevant reviews published in English were included. Articles not available in English or lacking sufficient clinical data were excluded.
2 Cardiovascular disease in testicular cancer survivors
2.1 Clinical manifestations of cardiovascular toxicity
Cardiovascular toxicity in TGCT survivors encompasses a spectrum of clinical manifestations of cardiac and vascular damage, ranging from asymptomatic ECG changes, cardiac conduction system abnormalities, and alterations in cardiac function or structure, to life-threatening cardiovascular events (CVE) such as myocardial infarction, heart failure, thromboembolism, and cerebrovascular accidents. However, subclinical electrophysiological, structural, and functional cardiovascular abnormalities may not necessarily progress to clinical CVE (15, 16).
Although cardiac toxicity associated with cisplatin is relatively rare, some reports have described cardiac events suggestive of vasospasm, ischemia, hypertension, decreased diastolic and/or systolic function, myocarditis, pericarditis, myocardial infarction, stroke, and heart failure (15, 17, 26, 27). In addition, cisplatin has occasionally been reported to cause cardiac arrhythmias, including supraventricular tachycardia, atrial fibrillation, ventricular arrhythmias, sinus bradycardia, left bundle branch block, atrioventricular block, and, infrequently, complete atrioventricular block (28). Cisplatin is also associated with an increased risk of thromboembolic events, such as arterial thrombosis, deep venous thrombosis, and pulmonary embolism (16, 29).
2.2 Risk of cardiovascular disease
Over the years, multiple studies have demonstrated increased risk of CVD among survivors treated for testicular cancer, particularly those who received cisplatin-based chemotherapy (16–24). In contrast, the risk of CVD and CVD-related mortality in patients with clinical stage I disease treated with orchiectomy alone appears to be comparable to that of the general population (25). Studies reporting the risk of CVD in TGCT survivors are summarized in Table 1.
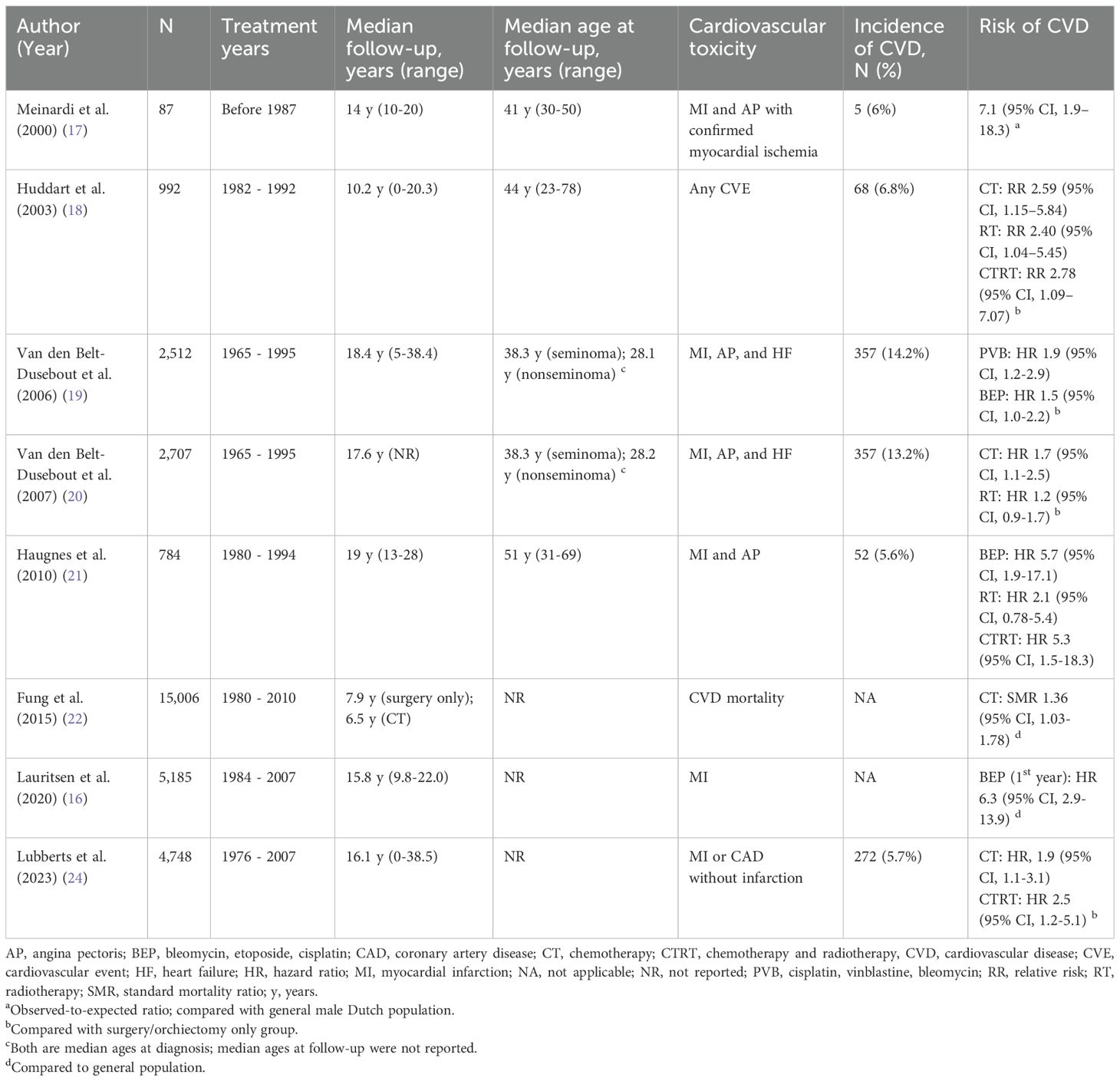
Table 1. Summary of studies reporting cardiovascular disease risk in testicular germ cell tumor survivors.
The risk of cisplatin-induced CVD appears to be highest within the first year after treatment but may persist or increase again after several years, suggesting a biphasic pattern of cardiovascular risk (16, 19, 21). Late cisplatin-related cardiovascular toxicity can occur more than ten years post-treatment, leading to atherogenesis, thrombosis, and premature vascular aging (30–32).
It remains unclear whether cardiovascular events in TGCT survivors are solely attributable to cisplatin, other anticancer agents or the underlying malignancy itself (11). Current evidence suggests that testicular cancer, compared to other malignancies such as pancreatic, gastric, or lung cancer, carries a relatively low intrinsic risk of cancer-associated thrombosis. Rather, the increased cardiovascular risk appears to result from cancer treatments, treatment-induced metabolic changes, and both preexisting and therapy-related cardiovascular risk factors (13). Importantly, thrombotic risk is also influenced by disease stage and patients with advanced TGCT, where orchiectomy alone is insufficient, exhibit a higher incidence of thrombotic and cardiovascular events (12–14). This helps explain the apparent discrepancy: while orchiectomy in early-stage TGCT does not increase CVD risk, the need for systemic treatment in advanced-stage disease introduces additional cardiovascular burden.
2.3 Population-based studies
A Dutch study (17) reported major CVE in 5 (6%) of 87 TGCT survivors (aged 30–42 years; 9–16 years post-chemotherapy). Two experienced myocardial infarctions (one of which was fatal), and three developed angina pectoris with confirmed myocardial ischemia. Compared to the general Dutch male population, the observed-to-expected ratio for coronary artery disease was 7.1 (95% CI, 1.9–18.3). In addition, one patient aged 41 experienced a cerebrovascular accident 11 years after chemotherapy. Huddart et al. (18) observed 68 CVE (including 18 deaths) among 992 TGCT patients after a median follow-up of 10.2 years. Compared to the orchiectomy-only group, the risk of developing CVD was more than two-fold higher following chemotherapy (relative risk [RR] 2.59; 95% CI, 1.15–5.84; p = 0.022), radiotherapy (RR 2.40; 95% CI, 1.04–5.45; p = 0.036), and chemotherapy + radiotherapy (RR 2.78; 95% CI, 1.09–7.07; p = 0.032).
A large retrospective study from the Netherlands (19) reported 694 cases of CVD among 2,512 TGCT survivors after a median follow-up of 18.4 years (range 5–38.4). The most prevalent diagnoses were coronary artery disease - including 141 cases of myocardial infarction (20.3%) and 150 cases of angina pectoris (21.6%) - followed by peripheral vascular disease (79; 11.4%), heart failure (66; 9.5%), and cerebrovascular accidents (55; 7.9%). Compared to age-matched data from the general Dutch male population, the standardized incidence ratio (SIR) for coronary artery disease was 1.17 (95% CI, 1.04–1.31). The risk of myocardial infarction was significantly higher in nonseminoma survivors under 45 years and those aged 45–54 years (SIR 2.06 and 1.86, respectively), compared to the age-matched general population. An almost two-fold increased risk was also observed across all age groups within 5 to 9 years after treatment (SIR 1.91). However, the risk in this study declined in survivors aged > 54 years and in those followed for 10 years or more. In a subsequent study (20), a higher risk of coronary artery disease was observed in patients who received subdiaphragmatic radiotherapy combined with chemotherapy compared to those treated with chemotherapy alone (SIR 2.3; 95% CI, 1.6–3.1 vs. SIR 1.4; 95% CI, 1.0–1.8).
Most data on the incidence of cardiovascular late effects in testicular cancer survivors have been derived from epidemiological studies with follow-up periods of up to 20 years post-treatment. Consequently, limited information is available on the health status of TGCT survivors beyond 20 years after the completion of cancer treatment (15, 26, 31).
A Norwegian study (N = 990) with a median follow-up of 19 years (range, 13-28) (21) demonstrated increased risks of atherosclerotic disease in all treatment groups compared to the surgery only: radiotherapy (hazard ratio [HR] 2.3; 95% CI, 1.03–5.3), BEP (bleomycin, etoposide, and cisplatin) chemotherapy (HR 4.7; 95% CI, 1.8–12.2), and radiotherapy + chemotherapy (HR 4.7; 95% CI, 1.6–14.1). Furthermore, BEP chemotherapy was associated with a 3.1-fold increased risk of myocardial infarction (95% CI, 1.2–7.7) compared to age-matched healthy male controls, with an even higher risk observed in the radiotherapy + chemotherapy group (HR 4.8; 95% CI, 1.6–13.9). Notably, the risk of incident stroke was significantly elevated in survivors treated with radiotherapy alone (HR 4.0; 95% CI, 1.4–11.7), while no significant increase was observed in the other treatment groups compared to healthy controls.
A population-based study (22) evaluated short- and long-term CVD mortality in nonseminoma patients treated with chemotherapy (n = 6,909) or surgery alone (n = 8,097). CVD mortality was significantly increased in the chemotherapy group (standardized mortality ratio [SMR] 1.36; 95% CI, 1.03–1.78), but not in the surgery-only group (SMR 0.81; 95% CI, 0.60–1.07). The excess CVD mortality following chemotherapy was primarily confined to the first year after diagnosis (SMR 5.31; absolute excess risk [AER] 13.90 per 10,000 person-years), and was mainly attributable to cerebrovascular disease (SMR 21.72; AER 7.43) and heart disease (SMR 3.45; AER 6.64).
Consistent with previous findings, Lauritsen et al. (16) reported that among 1,819 patients treated with BEP chemotherapy, there were significantly increased risks for myocardial infarction (HR 6.3; 95% CI, 2.9–13.9), cerebrovascular accident (HR 6.0; 95% CI, 2.6–14.1), and venous thromboembolism (HR 24.7; 95% CI, 14.0–43.6) during the first year after treatment initiation. One year after completing BEP treatment, the risk of CVD returned to levels comparable to the general population. However, after 10 years, an increased risk re-emerged for myocardial infarction (HR 1.4; 95% CI, 1.0–2.0) and cardiovascular-related mortality (HR 1.6; 95% CI, 1.0–2.5).
In a recent study (24) with a median follow-up of 16.1 years (range, 0–38.5), 272 out of 4,748 TGCT patients developed CVD. Among them, 64% experienced a myocardial infarction, and 28% had coronary artery disease without infarction. Subsequently, 16% of these patients developed heart failure. In 6% of cases CVE were fatal (n = 16). Compared to orchiectomy alone, cisplatin-based chemotherapy was associated with an increased risk of CVD (HR 1.9; 95% CI, 1.1–3.1). Additionally, survivors who developed CVD after treatment reported significantly lower quality of life across multiple domains, including physical and social functioning, role limitations due to physical health, less energy and vitality, and had lower general health score compared to survivors without CVD (all p ≤ 0.01).
In summary, evidence from multiple studies demonstrates a consistently increased risk of CVD among testicular cancer survivors, particularly following cisplatin-based chemotherapy and radiotherapy. Combined modality treatment appears to be associated with a higher cardiovascular risk than chemotherapy alone. The most commonly reported clinical manifestations include myocardial infarction, angina pectoris, cerebrovascular accident, venous thromboembolism, and heart failure. The risk is highest in the first year after treatment but can persist or recur even after a decade. Furthermore, TGCT survivors who develop CVD report significantly lower quality of life.
3 Pathophysiology of cisplatin-related cardiovascular toxicity
Cisplatin-induced CVD involves both vascular and heart damage through several distinct but interconnected mechanisms. Three key hypotheses have been proposed to explain the pathophysiology of CVD in testicular cancer survivors treated with cisplatin-based chemotherapy (33, 34). The direct vascular damage hypothesis postulates that cisplatin directly injures the vascular endothelium, initiating early vascular toxicity. The indirect hypothesis proposes that cisplatin-based chemotherapy increases the prevalence of traditional cardiovascular risk factors - such as hypertension, dyslipidemia, and insulin resistance - which then contribute to long-term cardiovascular morbidity (34). Finally, the multiple-hit hypothesis suggests that combination of direct endothelial injury and chemotherapy-induced risk factor accumulation acts synergistically to increase the overall risk of CVD in this population (33).
3.1 Mechanisms of cisplatin-induced vascular toxicity
The mechanisms by which cisplatin may cause arrhythmias and heart failure are not yet completely understood. However, the mechanisms of acute and late vascular toxicity have been more clearly explained. Cisplatin-based regimens used in the treatment of TGCT primarily drive vascular toxicity by endothelial dysfunction and prothrombotic state (30, 31). Platinum-therapy related damage of vasculature may be mediated also through inflammatory response to cytokine release, oxidative stress and electrolyte dysbalance (35).
3.1.1 Endothelial dysfunction
Endothelial dysfunction is characterized by an imbalance between vasodilation and vasoconstriction, shifting toward reduced vasodilation and a proinflammatory, prothrombotic state. In patients receiving cisplatin, impaired endothelium-dependent vasodilation may be attributed to reduced AKT–endothelial nitric oxide synthase (eNOS) signaling. Endothelial dysfunction plays a central role in the cascade of events leading to atherosclerosis and subsequent CVD and cerebrovascular accidents. In addition to classical cardiovascular risk factors - such as dyslipidemia, arterial hypertension, hyperglycemia, smoking, and diabetes mellitus - vascular toxicity is further influenced by chronic inflammation, oxidative stress, and shear stress, all of which reduce the bioavailability of nitric oxide. These mechanisms not only contribute to endothelial dysfunction and vascular injury but may also accelerate vascular aging and increase long-term cardiovascular risk (30–32, 36).
Cisplatin has a direct cytotoxic effect on endothelial cells. Acute and late vascular toxicity induced by cisplatin therapy appear to have distinct pathogenic mechanisms.
In the early phase, vascular toxicity is mainly caused by oxidative stress, endothelial apoptosis, platelet activation, and thrombus formation. Cisplatin-induced oxidative stress is primarily driven by mitochondrial reactive oxygen species (ROS). This oxidative stress leads to mitochondrial damage, including membrane depolarization and ultrastructural abnormalities. It is characterized by excessive ROS generation, lipid peroxidation, depletion of antioxidant defenses, and activation of inflammatory and apoptotic signaling pathways that contribute to adverse effects, such as thromboembolism, myocardial infarction, stroke, and newly diagnosed hypertension (29, 30).
In addition to oxidative stress, inflammation also plays a crucial role in cisplatin-induced endothelial dysfunction. This dysfunction is a complex process involving cytokines such as TNF-α, IL-1β, IL-6, IL-8, IL-18, IL-4, and IL-13; chemokines like RANTES; cell adhesion molecules; transcription factors including NF-κB and STAT6; the NLRP3 inflammasome; endothelins; and other inflammatory mediators.
Long-lasting degenerative changes in vasculature initiated by platinum-based chemotherapy are believed to be key pathogenic drivers of late vascular toxicity (31, 32). Late toxicity is associated with persistent and irreversible endothelial dysfunction, vascular remodeling, and chronic degenerative processes. This contributes to an elevated risk of cardiovascular death, coronary artery disease, myocardial infarction, hypertension, hyperlipidemia, diabetes mellitus, and metabolic syndrome (16, 30–32, 36, 37). Studies in long-term testicular cancer survivors have also demonstrated elevated levels of circulating endothelial cells detectable even two decades after the completion of cancer therapy (38). Notably, circulating cisplatin levels can also remain detectable for many years following treatment (39).
Recent preclinical research has provided new insights into endothelial alterations induced by cisplatin treatment at the molecular level. Using single-cell sequencing, specific gene expression changes were identified, revealing significant upregulation of pathways involved in DNA damage (Ddit4, Acer2), hypoxia (Phlda3, Mt1, Slc3a2, Ier3, Klf9, Adipor2, UCP2), inflammatory responses (Timp4, Tns1, Gdf15, Neat1), cell cycle arrest (Trp53inp1), intrinsic and extrinsic apoptosis (Fas, Bax, Ei24, Tgm2), blood vessel remodeling (Pim3), angiogenesis (Timp3, Flt1), and cellular senescence (Cdkn1a) (40).
3.1.2 Prothrombotic state
Several pathogenetic mechanisms have been proposed to explain how cisplatin-based chemotherapy may contribute to a prothrombotic state, such as:
a. direct cytotoxic effect of cisplatin on endothelium;
b. the release of procoagulant factors and cytokines from damaged cancer cells;
c. decreased production of anticoagulants due to liver damage - either from cancer itself or from chemotherapy-induced hepatotoxicity.
Multiple studies have demonstrated that cisplatin can cause hepatotoxicity in both in vitro and in vivo models. Regarding the specific impact on production of procoagulant and anticoagulant molecules, there is no direct evidence or clinical studies explicitly demonstrating effects of cisplatin on these pathways in the liver.
Acute thrombosis has been reported during cisplatin therapy even in the absence of atherosclerotic plaque rupture or clinically manifested atherosclerosis (37).
In recent years, superficial erosion has gained attention as a patomechanism underlying acute coronary syndromes in patients treated with cisplatin chemotherapy (32). Moreover, blood flow perturbations may induce endothelial activation and the recruitment of inflammatory cells, particularly neutrophils. Early atherosclerosis is characterized by the formation of fatty streaks, during which oxidized low-density lipoprotein (LDL) particles accumulate in the arterial intima. This triggers a cascade of endothelial inflammation and dysfunction, accompanied by the secretion of chemoattractant molecules that facilitate the recruitment of monocytes and lymphocytes. Concurrently, upregulation of adhesion molecules on endothelial cells promotes the adherence of monocytes and T lymphocytes to the intimal surface, contributing to lesion progression and endothelial apoptosis (41–44). Furthermore, von Willebrand factor (vWF) is released from damaged endothelial cells and secreted into the circulation or subendothelial space. This molecule stimulates platelet adhesion, activation and aggregation (45).
The risk of arterial thromboembolic events appears to be highest during the first month after starting chemotherapy and remains significantly elevated throughout the first year. In addition to acute arterial thromboembolism, both the venous and arterial compartments of the vascular system are involved in thrombotic complications in patients with TGCT (37, 43, 46, 47). Cisplatin may also increase levels of vWF in circulation. Elevated circulating vWF serves as a biomarker of endothelial injury. Nuver et al. (48) were among the first to observe an increase in vWF levels during chemotherapy in patients with TGCT, supporting the hypothesis that chemotherapy induces endothelial damage. Patients with preexisting elevated vWF levels may be at higher risk of cardiovascular toxicity. In addition, the persistent elevation of vWF after treatment suggests ongoing endothelial stimulation or damage following cisplatin-based chemotherapy (37).
3.2 Mechanisms of cisplatin-induced cardiac toxicity
Pathogenesis of cisplatin-induced cardiac toxicity is complex and not yet fully understood. However, findings from preclinical studies suggest multiple mechanisms including oxidative stress, mitochondrial damage, inflammatory process, apoptosis, and alterations in cardiac proteins and hemodynamics.
Cisplatin-induced cardiac damage is thought to be primarily exacerbated by oxidative stress. Cisplatin causes excessive production of ROS in cardiac tissue, overwhelming the antioxidant defense systems such as glutathione and superoxide dismutase. This oxidative stress can lead to mitochondrial DNA damage, mitochondrial membrane depolarization, and ultrastructural abnormalities in the mitochondria (28, 44). Cisplatin may accumulate in the mitochondrial matrix, disrupting mitochondrial respiration and depleting intracellular energy levels. Moreover, it may activate signaling pathways such as MAPKs and PI3K/Akt, which are involved in apoptosis in cardiac cells (49).
Cisplatin- related cardiac damage may be mediated through an inflammatory process, Accumulating evidence indicates that cisplatin increases secretion of several pro-inflammatory cytokines and chemokines (such as interleukin-1 and -6, tumor necrosis factor alpha (TNF-α) The translocation of the transcription factor nuclear factor kappa B (NF-κB) from the cytosol to the nucleus promotes the production pro-inflammatory TNF-α in cardiomyocytes (28, 50, 51).
In addition to oxidative stress and inflammation, apoptosis plays a crucial role in cisplatin-induced cardiac toxicity. It has been demonstrated in both in vivo and in vitro models. Cisplatin induces the release of pro-apoptotic factors such as cytochrome c, endonuclease G, and apoptosis-inducing factor (AIF) from the mitochondria into the cytosol. Cytochrome c activates caspase-9, which in turn triggers a cascade of downstream caspase activation, leading to apoptosis in a caspase-dependent manner. Conversely, endonuclease G and AIF translocate to and accumulate in the nucleus after their mitochondrial release, inducing apoptosis via a caspase-independent pathway (51). Furthermore, cisplatin induces alterations in renal tubular cells functions, which leads to impaired magnesium reabsorption. Hypomagnesemia is associated with an increased incidence or aggravation of hypertension, heart failure, serious cardiac arrhythmias, including torsades de points (52).
Myocardial toxicity is primarily mediated by oxidative stress, inflammation, and apoptosis, resulting in mitochondrial dysfunction and cardiac cell injury. Together, these processes can contribute to the increased cardiovascular risk seen in long-term survivors of testicular cancer treated with cisplatin. Mechanisms of cisplatin-induced cardiovascular toxicity, along with the most common clinical manifestations, are illustrated in Figure 1.
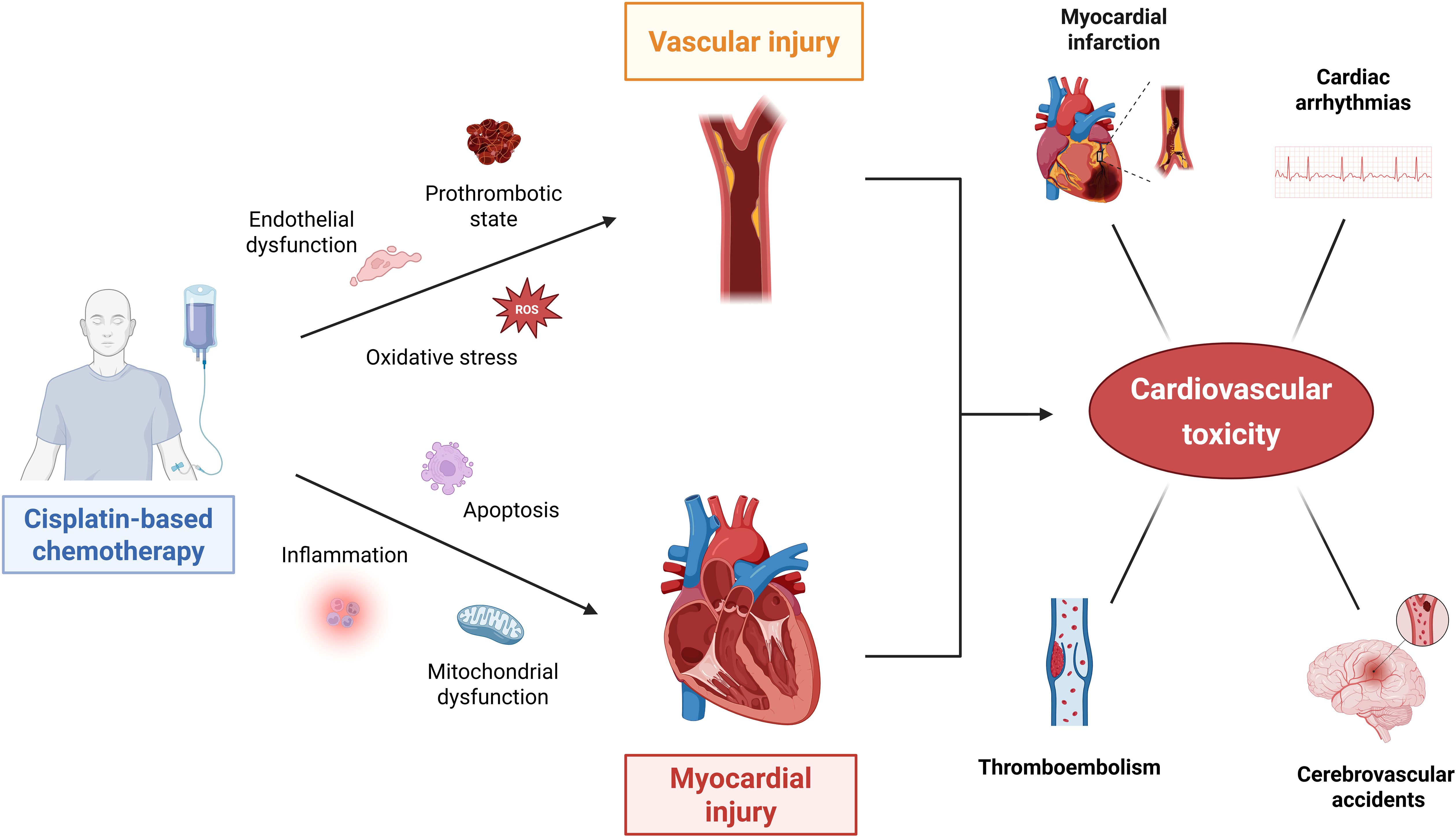
Figure 1. Molecular mechanisms of cisplatin-induced cardiovascular toxicity in testicular germ cell tumor survivors. Cisplatin-based chemotherapy contributes to cardiovascular toxicity through two interconnected pathways: vascular injury and myocardial injury. In the vascular compartment, cisplatin promotes endothelial dysfunction, oxidative stress, and a prothrombotic state, leading to early thrombotic events and long-term development of atherosclerosis. Concurrently, myocardial toxicity is mediated by inflammation, oxidative stress, apoptosis, and mitochondrial dysfunction, resulting in direct cardiomyocyte injury. These processes collectively contribute to increased risks of CVD, such as myocardial infarction, heart failure, arrhythmias, cerebrovascular events, and thromboembolism in testicular cancer survivors. This figure was created using BioRender.com.
4 Cardiovascular risk factors
The increased CVD risk observed in TGCT survivors cannot be attributed solely to the direct toxicity of chemotherapy or radiotherapy. Growing evidence indicates a higher prevalence of cardiovascular risk factors - such as smoking, hypertension, dyslipidemia, diabetes mellitus, obesity, and metabolic syndrome - among this population (16–18, 21, 24, 26, 53–61). Selected studies from the past 15 years evaluating cardiovascular risk factors among TGCT survivors treated with cisplatin-based chemotherapy are summarized in Table 2.
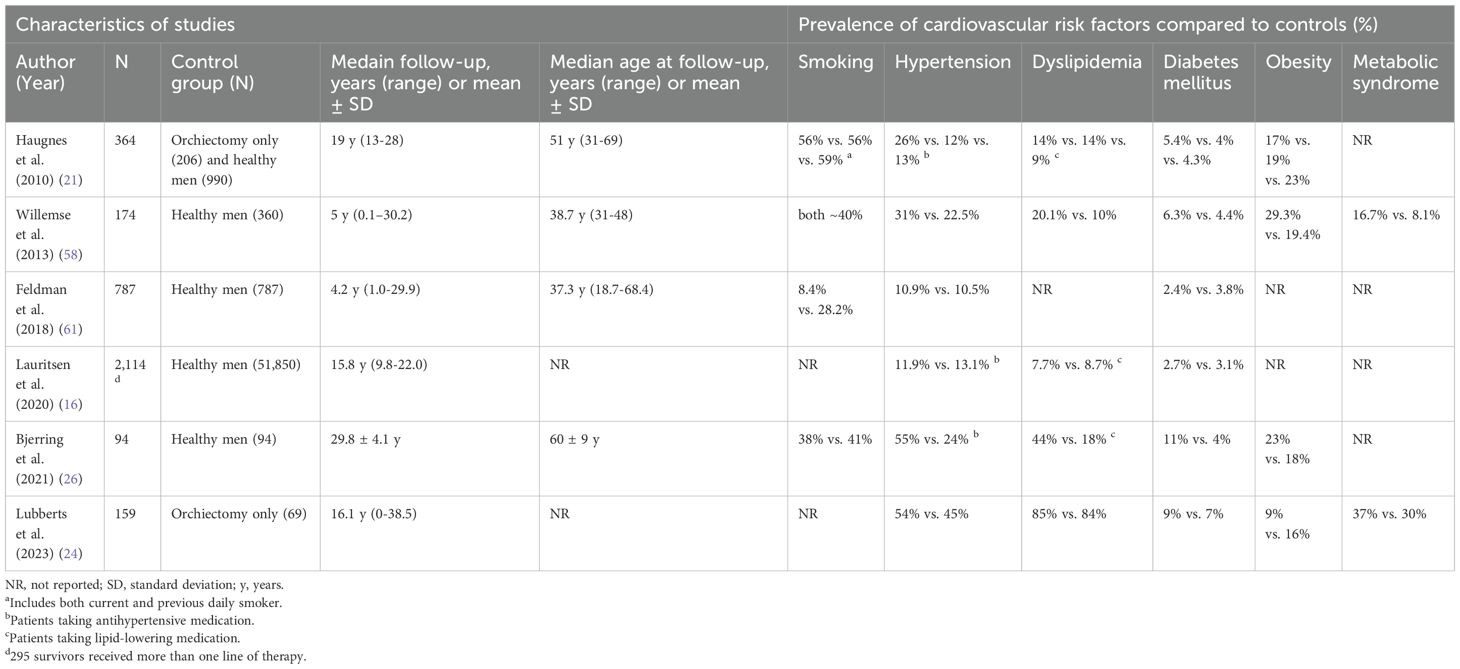
Table 2. Summary of selected studies evaluating cardiovascular risk factors in testicular germ cell tumor survivors treated with cisplatin-based chemotherapy (since 2010).
4.1 Smoking
Smoking is a well-known risk factor for CVD and many types of cancer, and remains the leading preventable cause of disease, death, and disability in the United States (62). According to data from the 1992–2023 National Health Interview Survey, 11.4% of cancer survivors aged 18 and older reported current cigarette smoking (63). In TGCT patients, two studies have demonstrated that smoking is associated with worse outcomes and survival in both stage I and metastatic disease (64, 65). The reported prevalence of smoking among TGCT survivors varies in the literature, ranging from 8.4% to 40% (18, 21, 26, 56, 58, 61). In most studies, no significant differences in smoking status were observed between TGCT survivors and the general population, or between different treatment groups (17, 18, 26, 58).
4.2 Hypertension
Although the definition of arterial hypertension varied across studies, its high prevalence among TGCT survivors appears indisputable. Over 25 years ago, Meinardi et al. (17) reported a hypertension prevalence of 39% in survivors treated with cisplatin-based chemotherapy, significantly higher than in those with stage I disease treated with orchiectomy alone (13%). A large study (56) evaluating blood pressure in 1,289 TGCT survivors treated with different modalities found a higher prevalence of hypertension in patients who received chemotherapy or radiotherapy compared to those who underwent surgery alone, after a median follow-up of 11.2 years (range, 5–22). Moreover, hypertension rates increased with cumulative cisplatin dose: 39% in the surgery group, 50% in those receiving ≤ 850 mg of cisplatin, and 53% in those receiving > 850 mg (p < 0.001). Compared to healthy controls, TGCT survivors who received more than 850 mg of cisplatin had over twice the risk of hypertension (age-adjusted OR 2.3; 95% CI, 1.5–3.7). Notably, the prevalence of hypertension was also high among patients treated with radiotherapy, reaching 54%. The increased odds of hypertension with higher cumulative doses of cisplatin have been confirmed by other studies (16, 57). Furthermore, Beitzen-Heineke et al. (15) demonstrated that a cumulative cisplatin dose ≥ 200 mg/m² was associated with attenuated biventricular systolic function and myocardial tissue alterations in asymptomatic long-term TGCT survivors. Lauritsen et al. (16) reported a persistently elevated risk of hypertension in patients treated with BEP and those receiving more than one line of therapy (HR 1.4, 95% CI, 1.3–1.6; and HR 1.7, 95% CI, 1.3–2.3, respectively), from treatment initiation through the end of follow-up, compared with the general population. In contrast, radiotherapy was not associated with a significantly increased risk of hypertension. Another study (21) found no significant differences in systolic or diastolic blood pressure between treatment groups. However, the use of antihypertensive medication was highest among patients treated with chemotherapy alone (OR 3.1; 95% CI, 1.9–5.2) and with combined chemotherapy and radiotherapy (OR 3.7; 95% CI, 1.6–8.9). In contrast, the radiotherapy-only group did not differ significantly from the surgery group (OR 1.5; 95% CI, 0.9–2.5). Additionally, all cytotoxic treatment groups showed significantly higher prevalences of antihypertensive medication use compared to the general population.
4.3 Dyslipidemia
Dyslipidemia is defined as abnormalities in serum lipid levels, including elevated total cholesterol (TC), high triglycerides (TG), low high-density lipoprotein cholesterol (HDL), and elevated low-density lipoprotein cholesterol (LDL) (66). A growing number of studies have reported a high prevalence of lipid abnormalities and the use of lipid-lowering medications among TGCT survivors (17, 21, 24, 26, 53, 55, 58). Haugnes et al. reported that all treatment groups had increased odds of using lipid-lowering medications compared to the healthy male population, with the highest odds observed in the chemotherapy plus radiotherapy group (odds ratio [OR] = 2.59) and the chemotherapy group (OR = 2.07) (21). Another more recent study also showed that the prevalence of lipid-lowering medication use was significantly higher in TGCT survivors compared to healthy controls (44% vs. 18%) (26). Interestingly, in a large study by Lubberts et al., the prevalence of dyslipidemia ranged from 84% to 88% and did not significantly differ among the chemotherapy, radiotherapy, and orchiectomy-only groups (p = 0.507) (24). However, Willemse et al. reported a higher prevalence of dyslipidemia among patients treated with combination chemotherapy compared to those who underwent surgery alone and to healthy controls (20.1% vs. 12.3% vs. 10%) (58). In our recent study (67) of a Slovak population of long-term TGCT survivors (N = 154) with a median follow-up of 10 years (range, 4–32), 57.8% had elevated total cholesterol, 42.9% had elevated triglycerides, 10.4% had suboptimal HDL, 72.1% had elevated LDL, and 42.9% had elevated VLDL levels. While lipid profiles did not significantly differ across treatment groups, patients treated with chemotherapy had the highest levels of total cholesterol, triglycerides, LDL, and VLDL, as well as the lowest HDL levels. Moreover, survivors who received cumulative cisplatin doses ≥ 400 mg/m² demonstrated higher total cholesterol, triglycerides, LDL, and VLDL levels, and lower HDL compared to the surveillance group. Despite the lipid abnormalities, the use of lipid-lowering medications in our population was surprisingly low (only 3.9%).
4.4 Diabetes mellitus
Diabetes mellitus has been observed particularly in long-term TGCT survivors treated with radiotherapy to the retroperitoneal lymph nodes (16, 21). In a study by Haugnes et al. (21), the overall prevalence of diabetes was 7.3% at a median follow-up of 19 years (range, 13–28), with the highest rates seen in the radiotherapy group (10.2%) and the combined chemotherapy plus radiotherapy group (15.6%). Compared to the surgery-only group, the odds ratios were 2.3 (95% CI, 1.5–3.7) for radiotherapy alone and 3.9 (95% CI, 1.4–10.9) for combined treatment. The authors noted that standard dog-leg and para-aortic radiation fields often include most of the pancreatic gland, suggesting that radiation-induced pancreatic dysfunction, including diabetes, may be a long-term complication of infradiaphragmatic radiotherapy (68, 69). Lauritsen et al. (16) reported an increased risk of diabetes more than 10 years after radiotherapy, with HR of 1.4 (95% CI, 1.0–2.0).
4.5 Overweight and obesity
People living with overweight or obesity are at increased risk for cardiovascular morbidity and mortality (70). In a recent study (24) of 304 TGCT survivors with a median follow-up of 21.8 years (range, 7.9–39.0), the prevalence of overweight and obesity was 64.5% and 11.5%, respectively. Furthermore, obesity at the time of testicular cancer diagnosis was identified as a significant risk factor for developing CVD after treatment (HR 4.7; 95% CI, 2.4–9.3). The authors noted that, in addition to being an established independent cardiovascular risk factor, adipose tissue may serve as a long-term reservoir for platinum. This suggests that obesity at diagnosis could be associated with prolonged circulating platinum levels following chemotherapy (24). Willemse et al. (58) reported that TGCT survivors had a significantly higher prevalence of obesity and dyslipidemia compared with age-matched healthy controls. Notably, those treated with combination chemotherapy had the highest prevalence (OR 1.7; 95% CI, 1.1–2.6). Interestingly, in a cohort of 455 TGCT survivors treated with cisplatin (median follow-up 26 months; IQR 16–59 months), a higher visceral-to-subcutaneous fat ratio - measured using pre-chemotherapy CT scans - was significantly associated with an increased risk of developing cardiometabolic conditions such as hypertension, dyslipidemia, or diabetes among obese men (age-adjusted HR 3.14; 95% CI, 1.02–9.71). The study also found that post-chemotherapy weight gain was primarily due to visceral fat accumulation, which correlated with higher estimated cardiovascular risk, especially in younger men. These findings support central obesity as a key predictor of cardiometabolic complications in this population (71).
4.6 Metabolic syndrome
Metabolic syndrome is defined as a combination of abdominal obesity, hypertension, dyslipidemia, and insulin resistance that together significantly increase the risk of CVD (72–75). Over the past decades, several studies have evaluated the risk of metabolic syndrome among TGCT survivors (21, 55, 57, 58, 60, 76–78). Furthermore, several studies have reported an association between low testosterone levels and the development of metabolic syndrome in this population (55, 60, 76). However, reported prevalence rates vary due to differences in the definition of metabolic syndrome, characteristics of control groups, and length of follow-up. While many studies have demonstrated an increased prevalence of individual components of metabolic syndrome in TGCT survivors, the overall evidence remains inconclusive regarding an increased prevalence of metabolic syndrome itself after cancer treatment. Some studies have found an association with cisplatin-based chemotherapy (55, 58, 76), whereas others have not (57, 60, 78).
4.7 Hypogonadism
In aging men, hypogonadism, or testosterone deficiency, is associated with a range of complications, including an increased risk of osteoporosis, metabolic syndrome, obesity, diabetes, and CVD. In addition, it is also associated with decreased quality of life (79, 80). In testicular cancer patients, hypogonadism may develop following any treatment modality (10). There is strong evidence that chemotherapy, higher cumulative doses of cisplatin, infradiaphragmatic radiotherapy, and combination of chemotherapy and radiotherapy are associated with an increased risk of testosterone deficiency in TGCT patients compared to those treated with orchiectomy alone. The risk appears to be highest among patients who received higher doses of cisplatin and combination of chemotherapy and radiotherapy (81). A recent study by Fosså et al. reported that 40% of TGCT survivors over the age of 60 had low testosterone levels, compared to 10% of age-matched healthy controls, after a median follow-up of 27 years (range 24–31 years). Nearly three decades after testicular cancer diagnosis, the probability of biochemical hypogonadism was significantly associated with advancing age and higher treatment intensity (82). As mentioned earlier, several studies have identified low testosterone levels a potential risk factor of metabolic syndrome in TGCT survivors (55, 60, 76). Bogefors et al. reported that testicular cancer survivors with hypogonadism had significantly higher insulin levels compared to eugonadal patients. Additionally, the hypogonadal group had an increased risk of metabolic syndrome (OR = 4.4; p = 0.01) (60). Results from the Platinum Study (83) showed that among 491 testicular cancer survivors with median age 38.2 years (range 18.7 - 68.4 years), 38.5% had hypogonadism. Compared to survivors without hypogonadism, those with hypogonadism were more likely to use lipid-lowering medications (20.1% vs. 6.0%; p < 0.001) or antihypertensive medications (18.5% vs. 10.6%; p = 0.013). A marginally significant trend was also observed for increased use of medications for diabetes (5.8% vs. 2.6%; p = 0.07).
To summarize, the available evidence indicates that testicular cancer survivors, especially those treated with cisplatin-based chemotherapy or radiotherapy, show a consistently higher prevalence of cardiovascular risk factors. These include hypertension, dyslipidemia, diabetes, and obesity. Hypertension and lipid abnormalities are among the most frequent findings, often linked to higher cumulative cisplatin doses. Diabetes is more common in survivors treated with retroperitoneal radiotherapy, likely due to pancreatic exposure. Obesity - particularly central obesity - and weight gain after treatment are also key contributors and may be related to persistent platinum exposure. Importantly, hypogonadism has emerged as a significant and prevalent long-term complication, affecting over a third of survivors, and is strongly associated with many of the adverse health outcomes. Testosterone deficiency contributes to the development of metabolic syndrome, insulin resistance, obesity, and dyslipidemia, and it may further exacerbate CVD risk in this population.
5 Discussion
This review synthesizes current evidence on cardiovascular toxicity in testicular cancer survivors, emphasizing the complexity of cardiovascular disease in this unique population. While the cardiotoxic effects of cisplatin-based chemotherapy and retroperitoneal radiotherapy are established contributors to increased CVD risk, it is clear that treatment-related toxicity alone does not fully explain the elevated incidence of cardiovascular morbidity. The high prevalence of traditional cardiovascular risk factors - such as hypertension, dyslipidemia, diabetes mellitus, obesity, and metabolic syndrome - among TGCT survivors underscores a multifactorial etiology involving both direct treatment effects and lifestyle or metabolic influences.
With the growing number of testicular cancer survivors worldwide, there is an increasing need for a structured approach to survivorship care. Given the high prevalence of cardiovascular risk factors among this population, effective long-term strategies for monitoring and prevention of CVD are essential. Early identification of treatment-related toxicities and integration of preventive care along with surveillance for recurrence should be central components of survivorship management (84).
Cardiovascular complications may develop years or even decades after cancer treatment, often progressing silently before becoming clinically apparent. Therefore, long-term follow-up is critical to identify early signs of cardiometabolic dysfunction. Based on current evidence, regular assessment of blood pressure, lipid profiles, hormone and plasma glucose levels, and body composition should be integrated into survivorship care plans - especially for testicular cancer survivors who have received cisplatin-based chemotherapy or radiotherapy to the retroperitoneum (85). All cancer survivors, including those with a history of testicular cancer, are advised to undergo annual clinical evaluations and optimization of cardiovascular risk factors. Individuals at higher risk should receive additional cardiovascular assessments, such as cardiac imaging. Routine testing for biomarkers to detect early cardiovascular damage is not currently recommended (86).
Effective prevention and management of CVD in testicular cancer survivors require a coordinated, multidisciplinary approach. Oncologists play a central role in identifying patients at risk based on treatment history - particularly those who had advanced disease and those treated with more than one line of conventional-dose chemotherapy and/or high-dose chemotherapy followed by autologous stem cell transplantation. Primary care providers and cardiologists are essential for long-term follow-up, cardiovascular screening, and risk management. Involving other specialists, such as dietitians and physiotherapists, can further support patients in managing metabolic complications and maintaining a healthy lifestyle. Establishing survivorship clinics or care pathways that facilitate communication among specialists can enhance care coordination and improve long-term outcomes (87).
According to the 2022 ESC Guidelines on cardio-oncology, testicular cancer survivors treated with platinum-based chemotherapy should have their cardiovascular risk factors closely monitored and be educated to promptly report any new cardiac symptoms to their healthcare provider. However, the role of screening for coronary artery disease in these patients remains unknown, and predicting individual cardiovascular risk continues to be challenging (88).
One of the most effective strategies for preventing CVD in cancer survivors is regular physical exercise. Over the past decade, numerous studies in testicular cancer survivors have reported the benefits of physical activity in mitigating cancer treatment-related toxicities, improving overall health and quality of life, and even reducing mortality risk (89–94). In addition, structured exercise programs tailored to the needs of cancer survivors can improve functional capacity, muscle strength, and psychological well-being. Given these benefits, incorporating physical activity into survivorship care plans is strongly recommended as part of a comprehensive approach to reduce long-term cardiovascular complications and promote healthy aging in this population (84, 95, 96). Alongside regular physical activity and dietary strategies, 2025 NCCN Survivorship Guidelines recommend counseling cancer survivors on smoking cessation, alcohol moderation, sleep hygiene, and stress management (97).
Despite advances in the field of survivorship care, significant gaps remain in our understanding of cardiovascular toxicity among TGCT survivors:
1. The molecular mechanisms underlying cisplatin-induced vascular and cardiac toxicity are complex and not yet fully understood.
2. The majority of available clinical studies are observational and vary widely in design, treatment exposures, follow-up duration, and outcome definitions, limiting both comparability and the ability to perform robust meta-analyses.
3. The predictive value of existing cardiovascular risk assessment tools, primarily developed for the general population, is unclear in TGCT survivors, who may have unique risk profiles influenced by treatment-related factors.
4. The optimal type, frequency, and intensity of cardiovascular screening, as well as its cost-effectiveness in survivors treated with platinum-based chemotherapy, remain unknown.
5. There is a lack of validated biomarkers and imaging modalities for the early detection of subclinical cardiac injury, hindering opportunities for early intervention.
6. Psychological and socioeconomic determinants that affect adherence to cardiovascular preventive strategies in this population are underexplored.
To address these research gaps, we propose several future research directions to advance the understanding and management of cardiovascular toxicity in TGCT survivors. Preclinical and translational studies are needed to clarify the molecular and cellular mechanisms of cisplatin-induced vascular and myocardial damage. To improve comparability and facilitate synthesis of findings, future clinical research should prioritize prospective, multicenter cohort studies with standardized protocols for treatment classification, cardiovascular outcome definitions, and follow-up intervals. Collaborative efforts to harmonize data collection would further support pooled analyses and meta-analyses.
There is a pressing need to develop and validate cardiovascular risk assessment models specifically tailored to TGCT survivors, in order to enhance risk stratification and guide personalized follow-up strategies. A proposed algorithm for cardiovascular risk assessment and follow-up in testicular cancer survivors is outlined in Figure 2. Randomized trials or modeling studies should evaluate the effectiveness, optimal timing, and cost-effectiveness of various cardiovascular screening modalities (e.g., echocardiography, arterial stiffness measurements, coronary calcium scoring) in this population. Research should also investigate whether intensified surveillance improves long-term cardiovascular outcomes in high-risk subgroups. There is also a need for clinical trials evaluating interventions such as statins or ACE inhibitors in high-risk survivors. High-risk subgroups can be defined as patients treated with cisplatin-based chemotherapy, receiving a cumulative cisplatin dose of ≥ 400 mg/m² (i.e., undergoing more than one line of chemotherapy), patients treated with both chemotherapy and radiotherapy, and individuals with preexisting cardiovascular risk factors (e.g., hypertension, dyslipidemia, smoking, obesity, diabetes) or diagnosed at an older age. These patients might be more likely to benefit from targeted cardiovascular screening and early lifestyle or pharmacologic interventions.
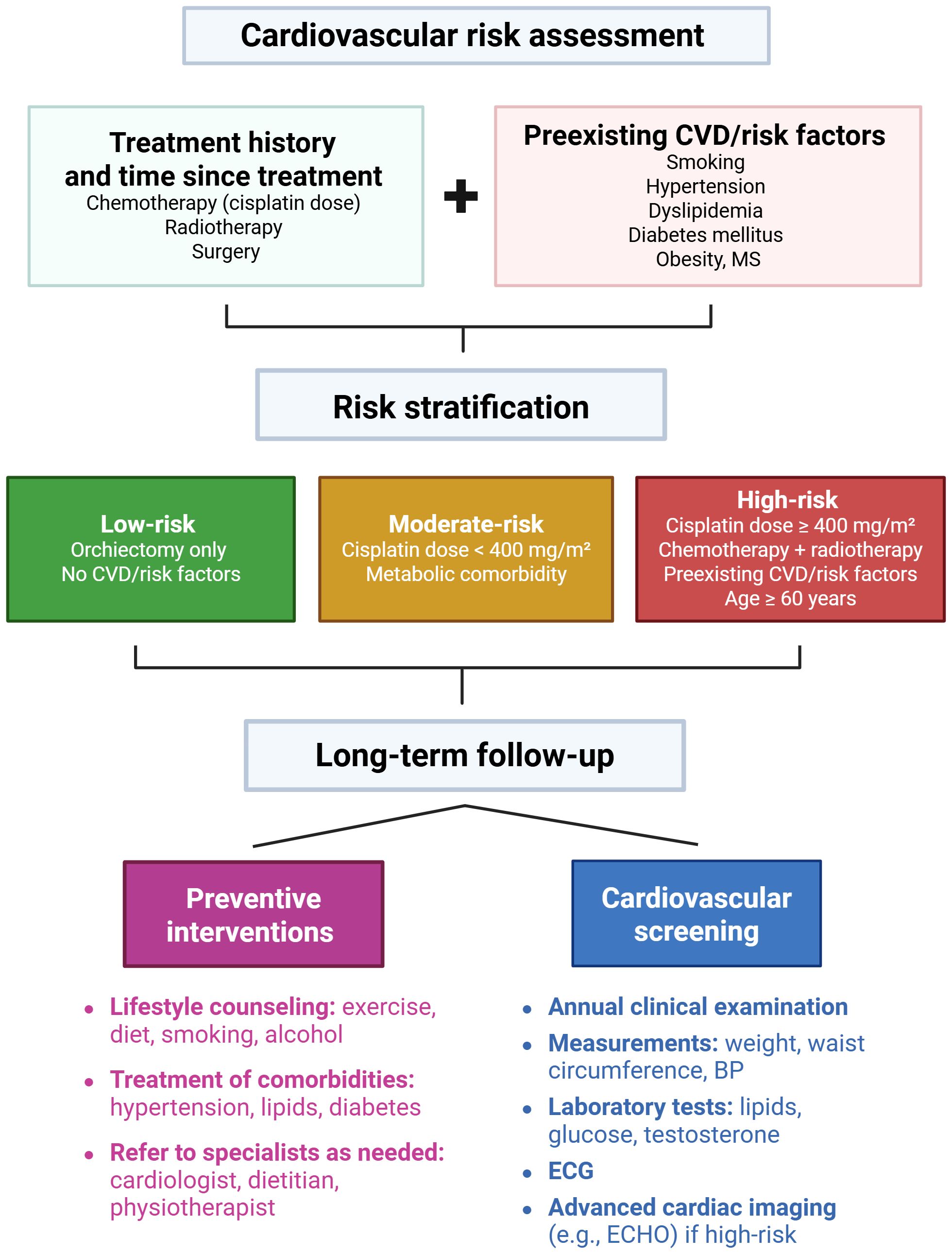
Figure 2. Proposed algorithm for cardiovascular risk assessment and follow-up in testicular cancer survivors. Cardiovascular risk assessment is based on patients’ treatment history, time since treatment completion, and presence of preexisting cardiovascular disease or risk factors. Patients are stratified into low, moderate, and high-risk groups. All patients receive long-term follow-up that includes: (1) preventive interventions such as lifestyle counseling, management of comorbidities, and referral to specialists as needed; and (2) cardiovascular screening, comprising annual clinical examinations, measurements of weight, waist circumference, blood pressure, laboratory tests, ECG, and advanced cardiac imaging when indicated. BP, blood pressure; CVD, cardiovascular disease; ECG, electrocardiogram; ECHO, echocardiography; MS, metabolic syndrome. This figure was created using BioRender.com.
Furthermore, studies aimed at identifying reliable circulating biomarkers (e.g., cardiac troponins, natriuretic peptides, inflammatory markers, endothelial dysfunction markers) and advanced imaging techniques (e.g., cardiac MRI, strain echocardiography) for the early detection of subclinical cardiac injury are essential. Finally, future research should explore the psychological, social, and economic barriers that influence adherence to cardiovascular prevention guidelines among TGCT survivors, to inform the development of targeted behavioral interventions.
6 Conclusion
Testicular cancer survivors face a substantially increased risk of cardiovascular disease due to the combined effects of cancer therapy, particularly cisplatin-based chemotherapy, and modifiable cardiovascular risk factors. Protecting their long-term cardiovascular health requires a proactive, individualized, and multidisciplinary approach. While survival rates are excellent, the risk of late-onset cardiovascular complications remains a significant concern. Integrating cardiometabolic monitoring, lifestyle interventions, and personalized risk assessment into survivorship care is essential. Addressing current evidence gaps through high-quality research will be key to refining screening strategies and improving outcomes. As a young and growing survivor population, testicular cancer survivors deserve focused, sustained efforts to ensure that the success of cancer treatment is not compromised by preventable cardiovascular disease.
Author contributions
ZO: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. BM: Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MM: Supervision, Writing – review & editing. MC: Funding acquisition, Resources, Validation, Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Slovak Research and Development Agency (APVV-15-0086, APVV-19-0411); the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic (VEGA 1/0327/19, VEGA 2/0144/23); and an R4 Fellowship for Excellence in Research (09I03-03-V04-00248).
Acknowledgments
We would like to acknowledge our colleagues, patients and their families.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. European Commission, Eurostat. (2023). Causes of death – deaths by country of residence and occurrence (dataset?code: hlth_cd_aro). Available at: https://ec.europa.eu/eurostat/databrowser/product/page/HLTH_CD_ARO (Accessed June 22, 2025).
2. Kochanek KDM, Sherry L, Xu J, and Arias E. Mortality in the United States, 2022. Hyattsville, MD: National Center for Health Statistics (2024). doi: 10.15620/cdc:135850
3. Wilcox NS, Amit U, Reibel JB, Berlin E, Howell K, Ky B, et al. Cardiovascular disease and cancer: shared risk factors and mechanisms. Nat Rev Cardiol. (2024) 21:617–31. doi: 10.1038/s41569-024-01017-x
4. Znaor A, Skakkebaek NE, Rajpert-De Meyts E, Laversanne M, Kulis T, Gurney J, et al. Testicular cancer incidence predictions in Europe 2010-2035: A rising burden despite population ageing. Int J Cancer. (2020) 147:820–8. doi: 10.1002/ijc.32810
5. National Cancer Institute. SEER Cancer Stat Facts: Testicular Cancer. Bethesda, MD Bethesda, MD: National Cancer Institute (2025). Available online at: https://seer.cancer.gov/statfacts/html/testis.html (Accessed May 31, 2025).
6. Einhorn LH. Testicular cancer as a model for a curable neoplasm: The Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. (1981) 41:3275–80.
7. Gillessen S, Sauve N, Collette L, Daugaard G, de Wit R, Albany C, et al. Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): results from the IGCCCG update consortium. J Clin Oncol. (2021) 39:1563–74. doi: 10.1200/JCO.20.03296
8. Beyer J, Collette L, Sauve N, Daugaard G, Feldman DR, Tandstad T, et al. Survival and new prognosticators in metastatic seminoma: results from the IGCCCG-update consortium. J Clin Oncol. (2021) 39:1553–62. doi: 10.1200/JCO.20.03292
9. Capocaccia R, Gatta G, and Dal Maso L. Life expectancy of colon, breast, and testicular cancer patients: an analysis of US-SEER population-based data. Ann Oncol. (2015) 26:1263–8. doi: 10.1093/annonc/mdv131
10. Chovanec M, Lauritsen J, Bandak M, Oing C, Kier GG, Kreiberg M, et al. Late adverse effects and quality of life in survivors of testicular germ cell tumour. Nat Rev Urol. (2021) 18:227–45. doi: 10.1038/s41585-021-00440-w
11. Terwoord JD, Beyer AM, and Gutterman DD. Endothelial dysfunction as a complication of anti-cancer therapy. Pharmacol Ther. (2022) 237:108116. doi: 10.1016/j.pharmthera.2022.108116
12. Abdel-Razeq H, Tamimi F, Abdel-Razeq R, Salah S, Omari Z, Salama O, et al. Predictors of venous thromboembolism in patients with testicular germ cell tumors: A retrospective study. Clin Appl Thromb Hemost. (2021) 27:10760296211024756. doi: 10.1177/10760296211024756
13. Paffenholz P, Grein K, Heidegger I, Nestler T, Grabbert M, Salem J, et al. Predictors of thrombosis in testicular cancer during platinum-based chemotherapy. World J Urol. (2019) 37:1907–16. doi: 10.1007/s00345-018-2598-7
14. Shields LBE, Daniels MW, Mar N, and Rezazadeh Kalebasty A. Thromboembolic events in metastatic testicular cancer treated with cisplatin-based chemotherapy. World J Clin Oncol. (2021) 12:183–94. doi: 10.5306/wjco.v12.i3.183
15. Beitzen-Heineke A, Rolling CC, Seidel C, Erley J, Molwitz I, Muellerleile K, et al. Long-term cardiotoxicity in germ cell cancer survivors after platinum-based chemotherapy: cardiac MR shows impaired systolic function and tissue alterations. Eur Radiol. (2024) 34:4102–12. doi: 10.1007/s00330-023-10420-w
16. Lauritsen J, Hansen MK, Bandak M, Kreiberg MB, Skott JW, Wagner T, et al. Cardiovascular risk factors and disease after male germ cell cancer. J Clin Oncol. (2020) 38:584–92. doi: 10.1200/JCO.19.01180
17. Bjerring AW, Fossa SD, Haugnes HS, Nome R, Stokke TM, Haugaa KH, et al. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J Clin Oncol. (2000) 18:1725–32. doi: 10.1200/JCO.2000.18.8.1725
18. Meinardi MT, Gietema JA, van der Graaf WT, van Veldhuisen DJ, Runne MA, Sluiter WJ, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. (2003) 21:1513–23. doi: 10.1200/JCO.2003.04.173
19. Ozben B, Kurt R, Oflaz H, Sezer M, Basaran M, Goren T, et al. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. (2006) 24:467–75. doi: 10.1200/JCO.2005.02.7193
20. El-Awady el SE, Moustafa YM, Abo-Elmatty DM, and Radwan A. Treatment-specific risks of second Malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. (2007) 25:4370–8. doi: 10.1200/JCO.2006.10.5296
21. Dieckmann KP, Marghawal D, Pichlmeier U, and Wulfing C. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol. (2010) 28:4649–57. doi: 10.1200/JCO.2010.29.9362
22. Huddart RA, Norman A, Shahidi M, Horwich A, Coward D, Nicholls J, et al. Cardiovascular disease mortality after chemotherapy or surgery for testicular nonseminoma: A population-based study. J Clin Oncol. (2015) 33:3105–15. doi: 10.1200/JCO.2014.60.3654
23. van den Belt-Dusebout AW, Nuver J, de Wit R, Gietema JA, ten Bokkel Huinink WW, Rodrigus PT, et al. Cumulative burden of morbidity among testicular cancer survivors after standard cisplatin-based chemotherapy: A multi-institutional study. J Clin Oncol. (2018) 36:1505–12. doi: 10.1200/JCO.2017.77.0735
24. van den Belt-Dusebout AW, de Wit R, Gietema JA, Horenblas S, Louwman MW, Ribot JG, et al. Cardiovascular disease in testicular cancer survivors: identification of risk factors and impact on quality of life. J Clin Oncol. (2023) 41:3512–22. doi: 10.1200/JCO.22.01016
25. Haugnes HS, Wethal T, Aass N, Dahl O, Klepp O, Langberg CW, et al. Outcomes in stage I testicular seminoma: a population-based study of 9193 patients. Cancer. (2013) 119:2771–7. doi: 10.1002/cncr.28086
26. Fung C, Fossa SD, Milano MT, Sahasrabudhe DM, Peterson DR, Travis LB, et al. The cardiac impact of cisplatin-based chemotherapy in survivors of testicular cancer: a 30-year follow-up. Eur Heart J Cardiovasc Imaging. (2021) 22:443–50. doi: 10.1093/ehjci/jeaa289
27. Kerns SL, Fung C, Monahan PO, Ardeshir-Rouhani-Fard S, Abu Zaid MI, Williams AM, et al. Acute anterior myocardial infarction after chemotherapy for testicular seminoma in a young patient. Clin Appl Thromb Hemost. (2007) 13:439–42. doi: 10.1177/1076029607303334
28. Lubberts S, Groot HJ, de Wit R, Mulder S, Witjes JA, Kerst JM, et al. Cisplatin-induced cardiotoxicity: Mechanisms and cardioprotective strategies. Eur J Pharmacol. (2011) 650:335–41. doi: 10.1016/j.ejphar.2010.09.085
29. Beard CJ, Travis LB, Chen MH, Arvold ND, Nguyen PL, Martin NE, et al. Thromboembolic events in patients with testicular germ cell tumours are predominantly triggered by advanced disease and by central venous access systems. Urol Int. (2021) 05:257–63. doi: 10.1159/000512055
30. Clasen SC, Dinh PC, Hou L, Fung C, Sesso HD, Travis LB, et al. Cisplatin, environmental metals, and cardiovascular disease: an urgent need to understand underlying mechanisms. Cardiooncology. (2021) 7:34. doi: 10.1186/s40959-021-00120-z
31. Stelwagen J, Lubberts S, Steggink LC, Steursma G, Kruyt LM, Donkerbroek JW, et al. Vascular aging in long-term survivors of testicular cancer more than 20 years after treatment with cisplatin-based chemotherapy. Br J Cancer. (2020) 123:1599–607. doi: 10.1038/s41416-020-01049-3
32. Herrmann J. Vascular toxic effects of cancer therapies. Nat Rev Cardiol. (2020) 17:503–22. doi: 10.1038/s41569-020-0347-2
33. Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, and Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. (2007) 50:1435–41. doi: 10.1016/j.jacc.2007.06.037
34. Feldman DR, Schaffer WL, and Steingart RM. Late cardiovascular toxicity following chemotherapy for germ cell tumors. J Natl Compr Canc Netw. (2012) 10:537–44. doi: 10.6004/jnccn.2012.0051
35. Travis LB, Beard C, Allan JM, Dahl AA, Feldman DR, Oldenburg J, et al. Testicular cancer survivorship: research strategies and recommendations. JNCI: J Natl Cancer Institute. (2010) 102:1114–30. doi: 10.1093/jnci/djq216
36. Morelli MB, Bongiovanni C, Da Pra S, Miano C, Sacchi F, Lauriola M, et al. Cardiotoxicity of anticancer drugs: molecular mechanisms and strategies for cardioprotection. Front Cardiovasc Med. (2022) 9:847012. doi: 10.3389/fcvm.2022.847012
37. Dieckmann KP, Struss WJ, and Budde U. Evidence for acute vascular toxicity of cisplatin-based chemotherapy in patients with germ cell tumour. Anticancer Res. (2011) 31:4501–5.
38. Vaughn DJ, Palmer SC, Carver JR, Jacobs LA, and Mohler ER. Cardiovascular risk in long-term survivors of testicular cancer. Cancer. (2008) 112:1949–53. doi: 10.1002/cncr.23389
39. Gietema JA, Meinardi MT, Messerschmidt J, Gelevert T, Alt F, Uges DR, et al. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet. (2000) 355:1075–6. doi: 10.1016/S0140-6736(00)02044-4
40. Pan H, Song X, Rajewski A, and Wickline SA. Single cell sequencing unveils endothelial alterations after cisplatin treatment. Eur Heart J. (2022) 43(2). doi: 10.1093/eurheartj/ehac544.3046
41. Pepin ME and Gupta RM. The role of endothelial cells in atherosclerosis: insights from genetic association studies. Am J Pathol. (2024) 194:499–509. doi: 10.1016/j.ajpath.2023.09.012
42. Yau JW, Teoh H, and Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. (2015) 15:130. doi: 10.1186/s12872-015-0124-z
43. Akinbo DB and Ajayi OI. Thrombotic pathogenesis and laboratory diagnosis in cancer patients, an update. Int J Gen Med. (2023) 16:259–72. doi: 10.2147/IJGM.S385772
44. Koukorava C, Ahmed K, Almaghrabi S, Pointon A, Haddrick M, Cross MJ, et al. Anticancer drugs and cardiotoxicity: the role of cardiomyocyte and non-cardiomyocyte cells. Front Cardiovasc Med. (2024) 11:1372817. doi: 10.3389/fcvm.2024.1372817
45. Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. (2003) 1:1335–42. doi: 10.1046/j.1538-7836.2003.00260.x
46. Weijl NI, Rutten MF, Zwinderman AH, Keizer HJ, Nooy MA, Rosendaal FR, et al. Thromboembolic events during chemotherapy for germ cell cancer: a cohort study and review of the literature. J Clin Oncol. (2000) 18:2169–78. doi: 10.1200/JCO.2000.18.10.2169
47. Cameron AC, McMahon K, Hall M, Neves KB, Rios FJ, Montezano AC, et al. Comprehensive characterization of the vascular effects of cisplatin-based chemotherapy in patients with testicular cancer. JACC CardioOncol. (2020) 2:443–55. doi: 10.1016/j.jaccao.2020.06.004
48. Nuver J, Smit AJ, van der Meer J, van den Berg MP, van der Graaf WT, Meinardi MT, et al. Acute chemotherapy-induced cardiovascular changes in patients with testicular cancer. J Clin Oncol. (2005) 23:9130–7. doi: 10.1200/JCO.2005.01.4092
49. Achkar IW, Abdulrahman N, Al-Sulaiti H, Joseph JM, Uddin S, Mraiche F, et al. Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J Transl Med. (2018) 16:96. doi: 10.1186/s12967-018-1471-1
50. El-Sawalhi MM and Ahmed LA. Exploring the protective role of apocynin, a specific NADPH oxidase inhibitor, in cisplatin-induced cardiotoxicity in rats. Chem Biol Interact. (2014) 207:58–66. doi: 10.1016/j.cbi.2013.11.008
51. Dugbartey GJ, Peppone LJ, and de Graaf IA. An integrative view of cisplatin-induced renal and cardiac toxicities: Molecular mechanisms, current treatment challenges and potential protective measures. Toxicology. (2016) 371:58–66. doi: 10.1016/j.tox.2016.10.001
52. Chrysant SG and Chrysant GS. Adverse cardiovascular and blood pressure effects of drug-induced hypomagnesemia. Expert Opin Drug Saf. (2020) 19:59–67. doi: 10.1080/14740338.2020.1700228
53. Strumberg D, Brugge S, Korn MW, Koeppen S, Ranft J, Scheiber G, et al. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann Oncol. (2002) 13:229–36. doi: 10.1093/annonc/mdf058
54. Nuver J, Smit AJ, Sleijfer DT, van Gessel AI, van Roon AM, van der Meer J, et al. Microalbuminuria, decreased fibrinolysis, and inflammation as early signs of atherosclerosis in long-term survivors of disseminated testicular cancer. Eur J Cancer. (2004) 40:701–6. doi: 10.1016/j.ejca.2003.12.012
55. Nuver J, Smit AJ, Wolffenbuttel BH, Sluiter WJ, Hoekstra HJ, Sleijfer DT, et al. The metabolic syndrome and disturbances in hormone levels in long-term survivors of disseminated testicular cancer. J Clin Oncol. (2005) 23:3718–25. doi: 10.1200/JCO.2005.02.176
56. Sagstuen H, Aass N, Fossa SD, Dahl O, Klepp O, Wist EA, et al. Blood pressure and body mass index in long-term survivors of testicular cancer. J Clin Oncol. (2005) 23:4980–90. doi: 10.1200/JCO.2005.06.882
57. Haugnes HS, Aass N, Fossa SD, Dahl O, Klepp O, Wist EA, et al. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann Oncol. (2007) 18:241–8. doi: 10.1093/annonc/mdl372
58. Willemse PM, Burggraaf J, Hamdy NA, Weijl NI, Vossen CY, van Wulften L, et al. Prevalence of the metabolic syndrome and cardiovascular disease risk in chemotherapy-treated testicular germ cell tumour survivors. Br J Cancer. (2013) 109:60–7. doi: 10.1038/bjc.2013.226
59. Lubberts S, Boer H, Altena R, Meijer C, van Roon AM, Zwart N, et al. Vascular fingerprint and vascular damage markers associated with vascular events in testicular cancer patients during and after chemotherapy. Eur J Cancer. (2016) 63:180–8. doi: 10.1016/j.ejca.2016.05.022
60. Bogefors C, Isaksson S, Bobjer J, Kitlinski M, Leijonhufvud I, Link K, et al. Hypogonadism in testicular cancer patients is associated with risk factors of cardiovascular disease and the metabolic syndrome. Andrology. (2017) 5:711–7. doi: 10.1111/andr.12354
61. Feldman DR, Ardeshir-Rouhani-Fard S, Monahan P, Sesso HD, Fung C, Williams AM, et al. Predicting cardiovascular disease among testicular cancer survivors after modern cisplatin-based chemotherapy: application of the framingham risk score. Clin Genitourin Cancer. (2018) 16:e761–e9. doi: 10.1016/j.clgc.2018.01.011
62. NIH. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA: Centers for Disease Control and Prevention (US (2014).
63. National Cancer Institute. Cancer Trends Progress Report. U.S. Department of Health and Human Services, National Institutes of Health. Bethesda, MD (2025). Available online at: https://progressreport.cancer.gov (Accessed June 23, 2025).
64. O'Donnell E, Markt SC, Miller R, Bernard B, Albiges L, Beard C, et al. Smoking and disease outcomes in patients with Malignant germ cell tumors. Clin Genitourin Cancer. (2017), S1558-7673(17)30234-3. doi: 10.1016/j.clgc.2017.07.024
65. Bandak M, Nielsen KS, Kreiberg M, Wagner T, Rosenvilde J, Pissinger C, et al. Smoking as a prognostic factor for survival in patients with disseminated germ cell cancer. J Natl Cancer Inst. (2023) 115:753–6. doi: 10.1093/jnci/djad039
66. NIH and NCBI. Dyslipidemias - meSH (2025). Available online at: https://www.ncbi.nlm.nih.gov/mesh/?term=dyslipidemia.
67. Orszaghova Z, Alzeer R, Kalavska K, Lesko P, Obertova J, Palacka P, et al. Dyslipidemia in long-term survivors of testicular germ cell tumors. J Clin Oncol. (2025) 43:e17017. doi: 10.1200/JCO.2025.43.16_suppl.e17017
68. Levy P, Menzelxhiu A, Paillot B, Bretagne JF, Flejou JF, Bernades P, et al. Abdominal radiotherapy is a cause for chronic pancreatitis. Gastroenterology. (1993) 105:905–9. doi: 10.1016/0016-5085(93)90911-U
69. Groot HJ, Gietema JA, Aleman BMP, Incrocci L, de Wit R, Witjes JA, et al. Risk of diabetes after para-aortic radiation for testicular cancer. Br J Cancer. (2018) 119:901–7. doi: 10.1038/s41416-018-0248-x
70. Lopez-Jimenez F, Almahmeed W, Bays H, Cuevas A, Di Angelantonio E, le Roux CW, et al. Obesity and cardiovascular disease: mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur J Prev Cardiol. (2022) 29:2218–37. doi: 10.1093/eurjpc/zwac187
71. Wibmer AG, Dinh PC, Travis LB, Chen C, Bromberg M, Zheng J, et al. Associations of body fat distribution and cardiometabolic risk of testicular cancer survivors after cisplatin-based chemotherapy. JNCI Cancer Spectr. (2022) 6(4):pkac030. doi: 10.1093/jncics/pkac030
72. Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. (2001) 24:683–9. doi: 10.2337/diacare.24.4.683
73. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. (2002) 288:2709–16. doi: 10.1001/jama.288.21.2709
74. Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. (2004) 89:2595–600. doi: 10.1210/jc.2004-0372
75. Alberti KG, Zimmet P, Shaw J, et al. The metabolic syndrome–a new worldwide definition. Lancet. (2005) 366:1059–62. doi: 10.1016/S0140-6736(05)67402-8
76. de Haas EC, Altena R, Boezen HM, Zwart N, Smit AJ, Bakker SJ, et al. Early development of the metabolic syndrome after chemotherapy for testicular cancer. Ann Oncol. (2013) 24:749–55. doi: 10.1093/annonc/mds527
77. Wethal T, Kjekshus J, Roislien J, Ueland T, Andreassen AK, Wergeland R, et al. Treatment-related differences in cardiovascular risk factors in long-term survivors of testicular cancer. J Cancer Surviv. (2007) 1:8–16. doi: 10.1007/s11764-007-0012-3
78. Zaid MA, Gathirua-Mwangi WG, Fung C, Monahan PO, El-Charif O, Williams AM, et al. Clinical and genetic risk factors for adverse metabolic outcomes in north american testicular cancer survivors. J Natl Compr Canc Netw. (2018) 16:257–65. doi: 10.6004/jnccn.2017.7046
79. Yeap BB. Testosterone and ill-health in aging men. Nat Clin Pract Endocrinol Metab. (2009) 5:113–21. doi: 10.1038/ncpendmet1050
80. Traish AM and Zitzmann M. The complex and multifactorial relationship between testosterone deficiency (TD), obesity and vascular disease. Rev Endocr Metab Disord. (2015) 16:249–68. doi: 10.1007/s11154-015-9323-2
81. Bandak M, Jorgensen N, Juul A, Vogelius IR, Lauritsen J, Kier MG, et al. Testosterone deficiency in testicular cancer survivors - a systematic review and meta-analysis. Andrology. (2016) 4:382–8. doi: 10.1111/andr.12177
82. Fossa SD, Bjerner LJ, Tandstad T, Brydoy M, Dahl AA, Nome RV, et al. Biochemical hypogonadism in aging testicular cancer survivors: A clinical challenge. Eur Urol Open Sci. (2025) 72:10–6. doi: 10.1016/j.euros.2024.12.010
83. Abu Zaid M, Dinh PC, Monahan PO, Fung C, El-Charif O, Feldman DR, et al. Adverse health outcomes in relationship to hypogonadism after chemotherapy: A multicenter study of testicular cancer survivors. J Natl Compr Canc Netw. (2019) 17:459–68. doi: 10.6004/jnccn.2018.7109
84. Bagrodia A, Haugnes HS, Hellesnes R, Dabbas M, Millard F, Nappi L, et al. Key updates in testicular cancer: optimizing survivorship and survival. Am Soc Clin Oncol Educ Book. (2025) 45:e472654. doi: 10.1200/EDBK-25-472654
85. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: american society of clinical oncology clinical practice guideline. J Clin Oncol. (2017) 35:893–911. doi: 10.1200/JCO.2016.70.5400
86. Blaes A, Nohria A, Armenian S, Bergom C, Thavendiranathan P, Barac A, et al. Cardiovascular considerations after cancer therapy: gaps in evidence and JACC: cardioOncology expert panel recommendations. JACC CardioOncol. (2025) 7:1–19. doi: 10.1016/j.jaccao.2024.06.006
87. Kadambi S, Clasen SC, and Fung C. How to manage cisplatin-based chemotherapy-related cardiovascular disease in patients with testicular cancer. JACC CardioOncol. (2022) 4:409–12. doi: 10.1016/j.jaccao.2022.06.007
88. Lyon AR, Lopez-Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. (2022) 43:4229–361. doi: 10.1093/eurheartj/ehac244
89. Rovito MJ, Brazendale K, Gibson S, Martinez S, Fairman C, Badolato C, et al. Physical activity and testicular cancer survivorship health-related quality of life: a scoping review. Ther Adv Urol. (2025) 17:17562872251322658. doi: 10.1177/17562872251322658
90. Amiri A, Krumpolec P, Mego M, Ukropcova B, Chovanec M, Ukropec J, et al. Habitual physical activity modulates cardiometabolic health in long-term testicular cancer survivors. Support Care Cancer. (2023) 31:539. doi: 10.1007/s00520-023-08000-1
91. Amiri A, Chovanec M, Oliva V, Sedliak M, Mego M, Ukropec J, et al. Chemotherapy-induced toxicity in patients with testicular germ cell tumors: The impact of physical fitness and regular exercise. Andrology. (2021) 9:1879–92. doi: 10.1111/andr.13078
92. Thorsen L, Courneya KS, Steene-Johannessen J, Gran JM, Haugnes HS, Negaard HFS, et al. Association of physical activity with overall mortality among long-term testicular cancer survivors: A longitudinal study. Int J Cancer. (2023) 153:1512–9. doi: 10.1002/ijc.34625
93. Adams SC, DeLorey DS, Davenport MH, Stickland MK, Fairey AS, North S, et al. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: A phase 2 randomized controlled trial. Cancer. (2017) 123:4057–65. doi: 10.1002/cncr.30859
94. Christensen JF, Bandak M, Campbell A, Jones LW, and Hojman P. Treatment-related cardiovascular late effects and exercise training countermeasures in testicular germ cell cancer survivorship. Acta Oncol. (2015) 54:592–9. doi: 10.3109/0284186X.2014.995776
95. Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, et al. American college of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. (2019) 51:2391–402. doi: 10.1249/MSS.0000000000002117
96. Jung W, Cho IY, Jung J, Cho MH, Koo HY, Park YM, et al. Changes in physical activity and cardiovascular disease risk in cancer survivors: A nationwide cohort study. JACC CardioOncol. (2024) 6:879–89. doi: 10.1016/j.jaccao.2024.09.013
Keywords: cardiotoxicity, cancer treatment, late toxicity, testicular cancer, germ cell tumor, survivorship
Citation: Orszaghova Z, Mladosievicova B, Mego M and Chovanec M (2025) Cardiovascular toxicity in testicular germ cell tumor survivors. Front. Oncol. 15:1654063. doi: 10.3389/fonc.2025.1654063
Received: 25 June 2025; Accepted: 27 July 2025;
Published: 13 August 2025.
Edited by:
Anna Borowiec, Maria Sklodowska-Curie National Research Institute of Oncology, PolandReviewed by:
M. Raheel Khan, St. James’s Hospital, IrelandAnjali Rajpoot, Banasthali University, India
Copyright © 2025 Orszaghova, Mladosievicova, Mego and Chovanec. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zuzana Orszaghova, b3JzemFnaG92YS56dXprYUBnbWFpbC5jb20=
 Zuzana Orszaghova
Zuzana Orszaghova Beata Mladosievicova
Beata Mladosievicova Michal Mego
Michal Mego Michal Chovanec
Michal Chovanec