- 1Department of Electronic Engineering, Sir Syed University of Engineering & Technology, Karachi, Pakistan
- 2Department of Computer Science, Faculty of Engineering Science and Technology, Iqra University, Karachi, Pakistan
- 3Department of Biomedical Engineering, Salim Habib University (Formerly Barrett Hodgson University), Karachi, Pakistan
- 4Department of Information Systems, College of Computer and Information Sciences, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 5Department of Informatics and Computer Systems, College of Computer Science, King Khalid University, Abha, Saudi Arabia
- 6Department of Informatics, School of Business, Örebro Universitet, Örebro, Sweden
Introduction: Precisely segmenting lung nodules in CT scans is essential for diagnosing lung cancer, though it is challenging due to the small size and intricate shapes of these nodules.
Methods: This study presents Trans RCED-UNet3+, an enhanced version of the RCED-UNet3+ framework designed to address these challenges. The model features a transformer-based bottleneck that captures global context and long-range dependencies, along with residual connections that facilitate efficient feature flow and prevent gradient loss. To improve boundary accuracy, we employ a hybrid loss function that combines Dice loss with Binary Cross-Entropy, enhancing the clarity of nodule edges.
Results: Evaluation on the LIDC-IDRI dataset demonstrates a notable advancement, as Trans RCED-UNet3+ achieves a Dice score of 0.990, exceeding the original model’s score of 0.984.
Discussion: These findings underscore the value of merging convolutional and transformer architectures, delivering a robust approach for precise segmentation in medical imaging. This model enhances the detection of subtle and irregular structures, enabling more accurate lung cancer diagnoses in clinical environments.
1 Introduction
Lung cancer ranks among the deadliest and most frequently diagnosed cancers worldwide. Detecting it at an early stage is crucial, as timely intervention significantly improves survival chances. Often, its initial signs appear as small nodular formations in the lungs, making accurate identification and delineation essential for distinguishing between benign and malignant lesions (1). Computed Tomography (CT) scans remain the standard imaging technique for screening purposes, ability to produce detailed cross-sectional images of lung anatomy (2). However, the large number of CT scans generated in medical practice (3), along with the wide variation in nodule characteristics ranging in shape, size, surface texture, and internal composition, such as ground-glass opacity or calcifications, poses substantial difficulties for radiologists (4). Manually analyzing medical image slices by is not only a time-consuming but also subject to inconsistencies across different specialists. These challenges highlight the growing need for dependable automated segmentation methods, which can assist clinicians by facilitating faster analysis, enhanced consistency, and improved diagnostic accuracy.
Lung nodule segmentation methods can be grouped into three main categories: conventional machine learning, deep learning models, and more recent Transformer-based techniques. The earlier, conventional strategies rely heavily on manually defined characteristics like texture cues or shape-related details and tend to perform practically well when working with small datasets (5).Nonetheless, they often lack flexibility and struggle when applied to diverse or unseen data, partly due to their dependence on extensive preprocessing steps. Convolutional neural networks (CNNs), a core component of deep learning, have significantly advanced the field by recognizing diverse lung nodule characteristics (6). However, CNNs have a notable limitation: they concentrate mainly on nearby pixel patterns, which can limit their ability to capture the bigger picture or the spatial context that’s vital for precise segmentation. Transformer-based architectures aim to overcome this issue by using self-attention mechanisms, enabling them to account for distant spatial relationships within the image ultimately leading to more accurate results (7). However, these models typically demand large-scale datasets and considerable computing resources to achieve their best performance. To bridge this gap, new hybrid designs that fuse CNNs with Transformer elements are gaining traction, offering a practical mix of reliability and efficiency.
Transformer-driven architectures have made significant strides image processing, especially due to their capacity to model distant relationships in image data. Vision transformer ViT (8) is enhanced into CRViT to recognize images through random fourier features and inductive bias using causal relationships and convolutional down-sampling. The method has lower parameter sensitivity, and it performs better than baseline ViT and CNNs on small and medium datasets. DeiT (9) introduces a distillation technique to enable more efficient Transformer training. DETR (10) redefines object detection through set prediction using Transformer backbones. In the realm of medical imaging. Furthermore, TransUNet (11) has successfully integrated ViT into segmentation tasks, paving the way for further innovations in automated diagnostic tools. A number of hybrid CNN-Transformer-based models have been suggested to enhance lung nodule segmentation. SSLKD-UNet (12) is a semi-supervised teacher student approach. It utilizes coarse and fine annotations to assist in resolving the issue of data scarcity, though, further training makes it more complex. S3TU-Net (13) is a convolution system designed with a non-residual superPixel vision transformer and it scores high on both LIDC-IDRI and EPDB data sets. Nonetheless, it has a complicated multi-modular design. SW-UNet (14) uses a sliding-window attention to support global context modelling at computational efficiency. It generalizes to tumor datasets and is better at LUNA16. It does not expressly enhance feature propagation along the encoder-decoder path.
RCED-UNet3+ effectively overcomes various shortcomings of segmentation methods by incorporating dense connectivity and refined feature aggregation mechanisms (15). Although the model demonstrated enhanced performance, its convolutional structure remained limited in effectively capturing broader contextual dependencies, particularly when handling complex lung nodules such as small (<3mm) lesions or diffuse ground-glass opacities. To overcome these challenges, the Trans RCED-UNet3+ architecture was introduced, embedding Transformer modules within the RCED-UNet3+ framework to strengthen global feature representation.
The defining innovation of Trans RCED-UNet3+ is its hybrid design, which effectively merges the benefits of CNNs and transformers. Unlike TransUNet, which replaces the encoder with a transformer-based module (11), our approach embeds transformer-driven bottleneck connections within the encoder-decoder structure. This offers three major advantages: (1) it retains the CNN’s strength in local feature extraction, (2) enhances global contextual understanding through well-placed self-attention mechanisms, and (3) optimizes computational efficiency by restricting transformer operations to bottleneck layers. Additionally, the model employs residual connections to improve gradient propagation and mitigate information loss in deeper layers. This design fosters superior feature fusion and enhances the detection of small nodules. By addressing existing gaps, the proposed model aims to improve segmentation accuracy and reliability, making it more aligned with clinical requirements.
This study contributes the following:
1. This work enhances the RCED-UNet3+ architecture by incorporating transformer-based bottlenecks and residual connections, resulting in the Trans RCED-UNet3+ model. The proposed model improves segmentation precision and reliability by combining the convolutional neural networks’ capacity for extracting detailed local features with the transformer architecture’s strength in modeling global context and long-range relationships.
2. The study thoroughly explores how transformers contribute to medical image segmentation, particularly in detecting lung nodules. Robust experiments have been conducted to evaluate how effectively they capture long-distance dependencies, enhance feature quality, and mitigate performance degradation within the network. The impact of transformer integration is assessed in terms of segmentation accuracy, adaptability across different nodule sizes, and robustness to variations in medical imaging datasets.
3. To overcome the issue of limited data in transformer-based models, we implemented data augmentation as a fundamental approach. Since transformers require extensive datasets to effectively learn spatial relationships, augmentation was applied to synthetically enlarge the dataset and strengthen the model’s ability to generalize.
4. To demonstrate the effectiveness of Trans-RCEDUNet3+, an extensive evaluation is conducted against leading segmentation approaches. Various benchmark datasets are used to measure performance based on Dice Score and IoU. The findings highlight notable improvements in detecting small and complex lung nodules, confirming the benefits of integrating transformers into RCED-UNet3+.
2 Literature review
Medical image segmentation has undergone substantial advancement, progressing from traditional methods to advanced frameworks driven by deep learning techniques. Early segmentation strategies included thresholding, region-growing (16), edge detection, iterative local thresholding, active contour models, and clustering algorithms (17). Although these methods required significant manual effort for parameter tuning and preprocessing. Consequently, they often lacked the precision and flexibility necessary to manage the intricate anatomical details observed in medical scans.
Deep learning techniques have increasingly demonstrated superior performance in medical image analysis, offering improved accuracy, robustness, automated feature extraction, and greater generalization across various imaging scenarios. The fundamental tools for semantic segmentation is 2D segmentation networks, which is structures with an encoder to capture feature extraction and decoder to reconstruct the pixel wise classification maps (18). Long et al. (19) introduces Fully Conventional Network (FCN) which enables direct pixel-level prediction in an end-to-end learning framework. Ronneberger et al. (20) proposed U-Net architecture by integrating skip connections between corresponding encoder and decoder layers to address the loss of spatial resolution. While the U-Net architecture achieved considerable success, it faced limitations in accurately segmenting very small or indistinct structures, which led to the development of more advanced variants. To address these challenges, Huang et al. in 2020 (21) proposed UNet3+, which integrates full-scale skip connections and multi-scale feature fusion to improve spatial precision. Subsequently, Zhou et al. (22) extended this idea through UNet++, introducing densely nested skip connections aimed at minimizing the semantic gap between encoder and decoder representations. Building upon these improvements, Xiao et al. (23) presented SAUNet++, combining Squeeze-and-Excitation Residual (SER) modules with Atrous Spatial Pyramid Pooling (ASPP) to suppress irrelevant background noise and enable rich multi-scale feature extraction. More recently, Yao et al. (24) further refined the design by incorporating attention mechanisms and residual blocks, thereby enhancing deeper feature learning and achieving better segmentation of fine-grained structures. Wu et al. (25) brings forward a set of architectural improvements for segmentation tasks. The conventional U-Net encoder is substituted with a residual framework inspired by ResNet, followed by the integration of an atrous spatial pyramid pooling (ASPP) unit to capture multi-scale context. Additionally, the decoder is refined by incorporating a cross-fusion module that leverages both spatial and channel-level attention mechanisms to enhance feature representation.
In 2021, Wang et al. (26) introduced an improved U-Net variant by embedding Squeeze-and-Excitation (SE) blocks (27) at each decoder stage. These SE modules calculated attention weights across feature channels, amplifying the most informative signals and significantly improving the segmentation of small areas. Although convolutional neural networks excel in various image analysis applications, they often struggle to effectively capture long-range interactions and manage complex, high-dimensional feature representations (11).
Recent years have seen the emergence of Transformer-based architectures (28). Vision transformer-based models have been found to provide better lung segmentation of CXR images by effectively extracting both global and local features that allow it to outperform U-Net in accuracy and robustness (29). This has led to hybrid models that combine convolutional network local feature extraction with Transformer global contextual understanding. In 2021, Chen et al. (11) proposed TransUNet, a hybrid architecture that embeds Transformer modules within the U-Net framework. Specifically, it integrates multiple Transformer blocks between the encoder and decoder stages. Nonetheless, naively stacking several Transformer layers post-encoder often results in overfitting, which is especially detrimental when working with limited datasets. To overcome this limitation, TransFuse (30) presents a dual-path architecture that operates in parallel one branch based on CNNs and the other on Transformers combining their respective features via a dedicated fusion mechanism. Chen et al. (31) conducted a comprehensive comparison of eight state-of-the-art neural network models UNet, SegNet, GCN, FCN, DeepLabV3+, PSPNet, TransUNet, and SwinUNet for lung nodule segmentation in CT scans, using the LIDC-IDRI dataset. The study also examined the influence of preprocessing techniques, including region-of-interest (ROI) cropping and lung contour overlays, on segmentation accuracy. Among the tested models, Transformer-based architectures, particularly TransUNet, achieved the highest performance, recording a Dice score of 0.871 across four experimental settings and outperforming conventional CNN-based approaches. UCTransNet (32) addresses semantic inconsistencies between encoder and decoder by substituting conventional skip connections with Transformer-based modules that leverage channel-wise self-attention. UTNet (33) retains the encoder-decoder structure of the U-shaped architecture but inserts self-attention layers after each module pair; however, the proliferation of these attention layers can degrade performance on small-scale datasets. ResT (34) introduces a multi-scale Transformer design that enhances inter-head communication among self-attention modules using lightweight convolutional blocks. MCTrans (35) combines both self-attention and cross-attention techniques to model semantic correlations and enrich feature maps, enabling effective multi-scale dependency capture across different feature levels.
Table 1 presents a comparative analysis of these contributions, outlining the strengths and limitations of each approach and demonstrating the progression of segmentation models over time. This review underscores the necessity for continued research to refine segmentation frameworks, ensuring an optimal balance between computational efficiency, accuracy, and reliability for real-world clinical applications.
Although remarkable steps have been made in medical image segmentation, a number of difficulties still remain. These include reliably detecting very small, managing variability across different datasets, and addressing the tendency of complex models to overfit. Overcoming such issues requires ongoing refinement, particularly through the development of more effective hybrid modeling strategies. In this study, we aim to contribute to that effort by investigating new ways to combine architectural components in order to enhance segmentation outcomes. Our focus lies in improving accuracy particularly in identifying small lung nodules within CT scans. To this end, we introduce a architecture that merges the RCED-UNet3+ framework with Transformer-based elements. This combination is intended to better capture wide-ranging contextual information while minimizing performance degradation across layers.
3 Materials and methods
As discussed in the introduction, we have adopted the RCED-UNet3+ framework and further extended it by integrating a Transformer component, resulting in the Trans RCED-UNet3+ architecture, as depicted in Figure 1. This improved design maintains the core strength of residual connections, which support the encoder in extracting high-level features. At the same time, it introduces a Transformer-based bottleneck layer designed to recognize global spatial patterns and interpret contextual information throughout the image. While residual pathways play a key role in preventing performance degradation during deep learning, the Transformer component enhances the model’s ability to generalize when faced with unfamiliar data. To further support accurate segmentation, a hybrid loss function has been incorporated, aimed at minimizing pixel-level classification errors and improving the distinction between foreground and background regions. The following sections delve into the architectural improvements and technical implementation details of these modifications.
3.1 Dataset
This study utilizes LIDC-IDRI, a database of lung CT scans which frequently used in medical imaging studies. This dataset comprises 1,018 CT scans collected from 1,010 unique patients, each of which was annotated by four certified radiologists. It provides chest CT images stored in DICOM format, accompanied by XML files. All scans are standardized to a resolution of 512 × 512 pixels, formatted in three channels, and normalized using Hounsfield Units (HU) to ensure consistency in intensity values. The LIDC-IDRI dataset is used for developing and testing deep learning models due to its comprehensive annotations and uniform imaging standards designed for lung nodule segmentation and the early diagnosis of lung cancer.
3.2 Preprocessing
The preprocessing stage begins by isolating the lung parenchyma from each CT scan, ensuring that unrelated anatomical structures such as bones and soft tissue are excluded. Because lung nodules are embedded within this parenchymal tissue, accurately identifying and extracting this region is critical for improving segmentation accuracy and reducing the likelihood of false positives. In CT images, the parenchyma appears as a darker, low-density zone, distinctly contrasted by the lighter shades representing surrounding musculature. The segmentation process initiates with a thresholding technique that converts the grayscale scan into a binary image displaying the lung regions in black and the rest of the anatomy in white. To address interruptions caused by dense regions within the lungs, morphological dilation is subsequently applied. This step broadens the segmented lung areas and bridges any disjointed sections, ultimately creating a more complete and continuous mask of the lung parenchyma. The overview of lung region segmentation in CT imaging is shown in Figure 2 (15).
3.3 Data augmentation
To overcome the limitation of small training dataset inherent in transformer-based segmentation models, data augmentation serves as a critical preprocessing step. Transformers demand substantial datasets to effectively learn complex spatial hierarchies; therefore, augmentation plays a key role in synthetically increasing dataset diversity and enhancing model generalization. This study employs three augmentation techniques horizontal flip, vertical flip, and rotation resulting in a fourfold expansion of each input sample and generating a total of 62,200 augmented instances. These transformations introduce spatial perturbations that enhance the model perform well on data not included in training while minimizing the chances of over fitting. Experimental evidence from prior research supports the efficiency of such approaches; for example, Ahmed et al. (36) present an ecologically valid augmentation method by integrating real pathological stroke lesions into healthy brain MRI scans, demonstrating notable improvements in segmentation accuracy for transformer-based networks. Shah et al. (37) highlighted that various data augmentation techniques have the potential to substantially enhance the resilience and functionality of Vision Transformer models in medical image segmentation to adversarial risk and scant data challenges, among others. These studies highlight the effectiveness of augmentation techniques in optimizing transformer-based segmentation models, aligning with the approach used in this work. The augmentation methods, horizontal flipping, vertical flipping, and rotation are implemented to achieve geometric transformation invariance, as depicted in Figure 3 (15).
3.4 Proposed methodology
This section presents the architectural advancements introduced in the Trans RCED-UNet3+ model, an evolution of the previously established RCED-UNet3+ (15). The proposed model integrates a bottleneck transformer module aimed at enhancing segmentation performance by effectively modeling long range spatial dependencies and global contextual information, while preserving the strength of the original model’s residual connectivity. The underlying framework of Trans RCED-UNet3+ builds upon the RCED-UNet3+ structure, which is characterized by the strategic incorporation of residual networks within both the encoder and decoder pathways. These residual connections facilitate efficient gradient flow, address vanishing gradient challenges. Figure 4 presents the proposed model architecture, which facilitates effective feature flow across the entire network while employing multi-head self-attention to capture global contextual relationships.
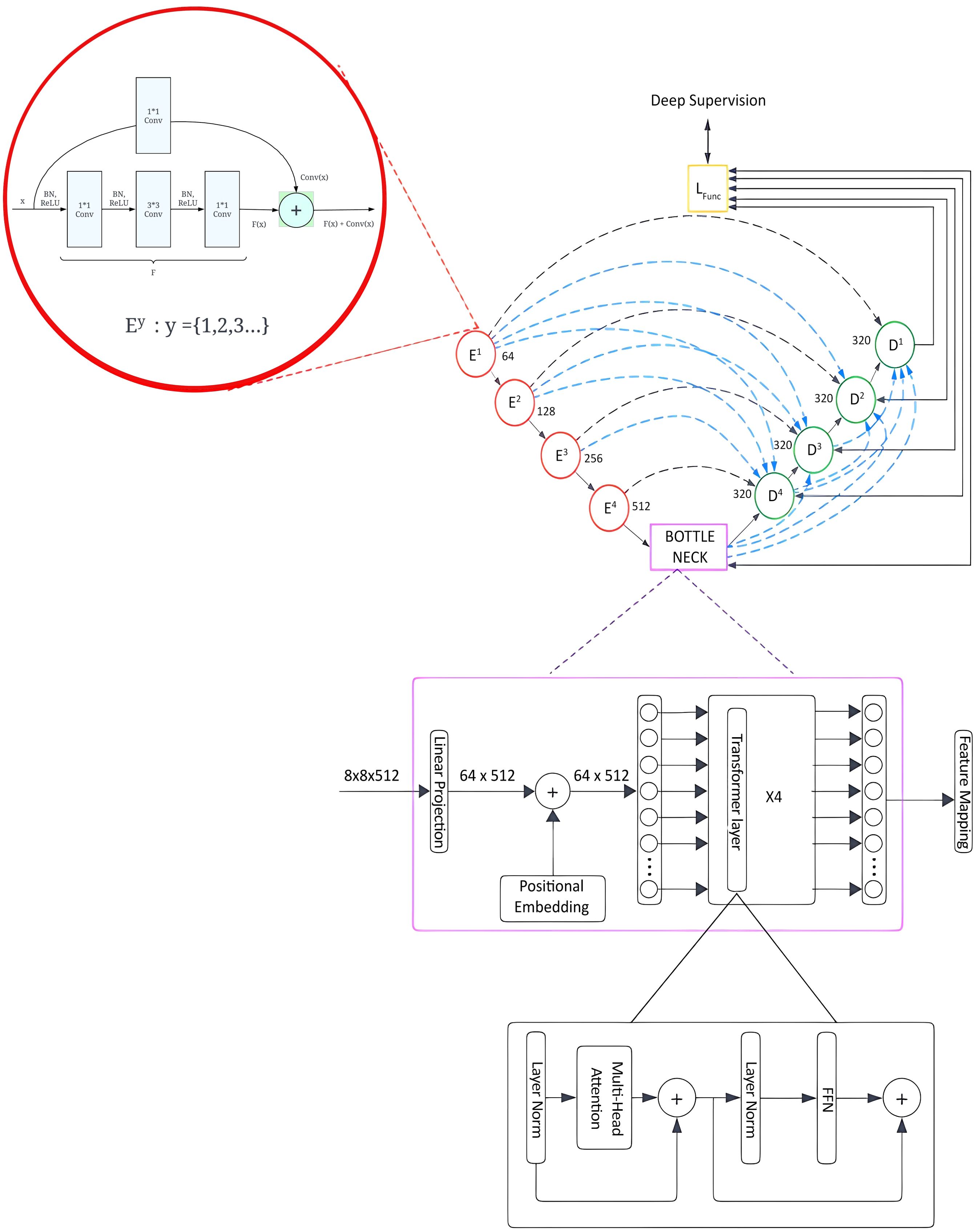
Figure 4. Trans RCED-UNet3+ architecture with a transformer bottleneck for global context modeling, integrating residual dense blocks, deep supervision, and multi-head self-attention.
3.4.1 Residual connection networks
The RCED-UNet3+ architecture leverages residual connections to facilitate stable and efficient information flow across network layers, addressing the degradation problem commonly observed in deep convolutional architectures. These residual pathways ensure uninterrupted gradient propagation during both forward and backward passes, thereby enhancing training stability and convergence. Each residual block consists of convolutional structure employing kernel sizes of 1 1, 3 3, and 1 1, respectively, which collectively optimize local and cross-channel feature extraction. To ensure stable training and improve regularization, batch normalization is performed before convolution, followed by the application of a ReLU activation function. This integration standardizes intermediate outputs, minimizes internal covariate shifts, and reduces overfitting throughout the training process. The residual connection can be mathematically formulated as shown in Equation (1).
where denotes the input feature and represents the non-linear transformation learned by the convolutional subnetwork. To ensure element-wise addition, a 1 1 convolutional projection with zero-padding is applied to , the residual computation is therefore defined in Equation (2).
Structural consistency within the residual block is achieved by aligning both spatial and channel dimensions. By incorporating residual connections, the model improves feature representation, which enables gradients to flow more effectively during training. Moreover, they reduce the loss of features in deeper layers, ultimately leading to higher segmentation accuracy.
Transformer-based bottleneck is added in the Trans RCED-UNet3+ to improve its ability to grasp long-range spatial dependencies and global contextual information. Conventional convolutional layers, in contrast, are limited to localized feature extraction and are unable to capture global contextual information. By integrating these global contextual representations with detailed local information, the architecture can achieve more robust and precise segmentation results.
3.4.2 Transformer-driven bottleneck
The integration of a transformer-based bottleneck into the Trans RCED-UNet3+ architecture improves the segmentation accuracy, which recognizes long-range spatial dependencies and global contextual information in lung CT images. Conventional convolutional neural networks (CNNs) are limited to investigating local regions, but the transformer module overcomes this by using self-attention throughout the image. This component is based on Vision Transformer (ViT), where feature maps from the encoder are segmented into uniform patches, which are converted into embeddings and processed through multi-head self-attention (MHSA). This process enables the network to model complex, non-local dependencies and enhance its understanding of spatial relationships across the medical scan. The fundamental components of the architecture include:
3.4.2.1 Patch tokenization
O Feature maps from the encoder are divided into smaller patches and transformed into sequential 1D tokens.
O The segmented patches are transformed into a higher-dimensional representation through a linear projection layer.
3.4.2.2 Spatial encoding
O Since transformers do not inherently capture spatial structures, positional encoding is added to retain location-based information.
O This ensures the model can distinguish between different regions within the image.
3.4.2.3 Multi-head self-attention
O The multi-head self-attention mechanism enables the model to focus on multiple regions of the image simultaneously.
O Each attention head generates a feature representation by computing a weighted combination of the input features, as defined in Equation (3).
Here, Q, K, and V represent the query, key, and value matrices derived from input embedding.
This mechanism allows the model to highlight relevant lung regions while reducing interference from background noise.
3.4.2.4 Feed-forward network & residual links
o After self-attention, the extracted representations are passed through a fully connected feed-forward module (FFN).
O Residual connections are included to stabilize gradient flow and improve training efficiency.
The output from the transformer is up sampled and processed through convolutional layers to produce refined feature maps that incorporate improved global contextual information.
3.4.3 Comparison with traditional CNN-based methods
3.4.3.1 Improved global feature capture
O CNNs are primarily designed to extract localized spatial details, which may limit their ability to detect relationships between distant lung nodules.
O Transformers employ self-attention to capture global relationships, which contributes to more precise and reliable segmentation outcomes.
3.4.3.2 Computational complexity
O The processing demand of the self-attention component in transformer architectures grows quadratically with sequence length O(n2), making it more resource-intensive than CNNs, which operate at However, by incorporating a transformer bottleneck instead of a full transformer framework, a balance between computational efficiency and segmentation performance is achieved.
4 Experimental setup
The proposed model was trained and tested using the LIDC-IDRI dataset, a reputable and extensively used resource in lung nodule segmentation research. Preprocessing steps included isolating the lung parenchyma through binarization and morphological operations. Since transformers require large datasets to learn spatial dependencies effectively, augmentation helps artificially increase the dataset size and improve model generalization, augmentation techniques such as vertical and horizontal flipping, as well as rotation, were applied.
4.1 Training configuration
The model was trained using the Stochastic Gradient Descent (SGD) optimizer, configured with a learning rate of 1e-3, a dropout rate of 0.15, and a batch size of 32. To improve segmentation accuracy, the hybrid loss function from the RCED-UNet3+ model was retained, which penalizes inaccuracies in both foreground and background classification. This hybrid loss function combines dice Loss and binary cross-entropy Loss. Dice loss is particularly effective for assessing the overlap between predicted segmentation and ground truth, while BCE loss penalizes incorrect classifications.
Let denotes the ground truth mask, the predicted segmentation. The dice loss is defined in Equation (4)
The binary-cross entropy loss is denoted as and is expressed in Equation (5):
The hybrid loss function which combines both Dice and BCE losses, is expressed in Equation (6).
4.2 Transformer-based enhancements
The Trans-RCEDUNet3+ model introduces a transformer-based bottleneck to improve segmentation accuracy. One enhancement explored in this study is an attention-weighted loss function, where Dice and BCE losses are adjusted using an attention score derived from the transformer module. This prioritization aids in refining segmentation, particularly for small and complex lung nodules. The attention-weighted Dice loss function is given in Equation (7)
Where:
Ai represents the attention weight assigned to each pixel
Pi and Gi are the predicted and ground truth values respectively.
Є is small constant to prevent division by zero.
4.3 Hyper parameter selection and optimization
To ensure optimal performance of Trans RCED-UNet3+, a systematic hyperparameter tuning process was conducted, focusing on parameters critical for both CNN-based feature extraction and transformer-based self-attention mechanisms.
4.3.1 Tuning of optimizer and learning rate parameters
O The learning rate was tuned using a grid search over the range {1e-4, 5e-4, 1e-3, 5e-3, 1e-2} with 1e-3 providing the best balance between convergence speed and stability.
O The Stochastic Gradient Descent (SGD) optimizer with momentum (0.9) and weight decay (1e-4) was used.
O AdamW was also tested, showing similar performance but with slightly higher memory usage, making SGD the preferred choice.
4.3.2 Transformer-specific hyperparameter tuning
O Number of Attention Heads: We tested {4, 8, 12} attention heads, with 8 heads yielding the best trade-off between accuracy and computational efficiency.
O Feed-Forward Network (FFN) Dimensionality: Evaluated {512, 1024, 2048}, with 1024 providing optimal performance.
O Dropout Rate: A dropout rate of 0.15 was retained, as it offered a good balance between generalization and overfitting prevention. Lower dropout values (0.05, 0.1) led to overfitting, while higher dropout values (0.2, 0.3) negatively impacted convergence.
4.3.3 Batch size and training stability
O To accommodate the memory constraints of the NVIDIA RTX 3090 GPU, a batch size of 32 was selected.
O Increasing the batch size to 64 led to unstable training behavior, attributed to greater variability in gradient updates.
4.3.4 Grid search vs. random search for optimization
O A grid search was initially conducted for learning rate, optimizer, and dropout rate.
O A random search was used for transformer-specific parameters (attention heads, FFN size) due to the high-dimensional search space (Table 2).
4.4 Evaluation metrics
To further investigate the model’s performance, we calculated Accuracy, Precision, and Recall using standard classification outcomes: true positives (TP), true negatives (TN), false positives (FP), and false negatives (FN).
• Accuracy, which measures the proportion of correctly classified pixels relative to the total pixel count in the segmentation output, is defined in Equation (8)
• Precision, which reflects the proportion of lung nodule pixels accurately identified by the model out of all pixels classified as nodules, is defined in Equation (9).
• Recall, which calculates the fraction of pixels correctly recognized as belonging to a lung nodule compared to the total number of lung nodule pixels, is defined in Equation (10).
These metrics, along with the Intersection over Union (IoU) and the Dice coefficient, provided a comprehensive assessment of the model’s segmentation accuracy and effectiveness.
5 Results and discussion
The performance of the Trans-RCEDUNet3+ model is evaluated using segmentation metrics: the Dice Similarity Coefficient (DSC) and Intersection over Union (IoU). These metrics were selected for their ability to examine how accurately the model segments lung nodules within CT scan images.
5.1 Ablation study
The addition of residual connections and transformer modules to UNet3+ architecture systematically over each of the proposed building blocks provided a more detailed analysis of the contribution of each element to the proposed framework, realized through an ablation study. The analysis shows the contribution of individual modules and explains how they contributed to the further development of the segmentation of the lung nodules. Table 3 presents the results of four different versions, namely (i) UNet3+ (baseline), (ii) RCED-UNet3+ with residual connections between encoder and decoder, (iii) TransUNet3+ with a transformer bottleneck, and (iv) Trans-RCEDUNet3+. The results show that the residual connections added to UNet3+ regularize the training procedure and solve the vanishing gradient problem to get improved Dice and IoU scores. Even within the context of the transformer bottleneck in UNet3+, the model can capture long-range relationships and global contextual information, which further improves the quality of the segmentation. Finally, the combination of two residual connections and transformer modules in Trans-RCEDUNet3+ provides the best overall performance.
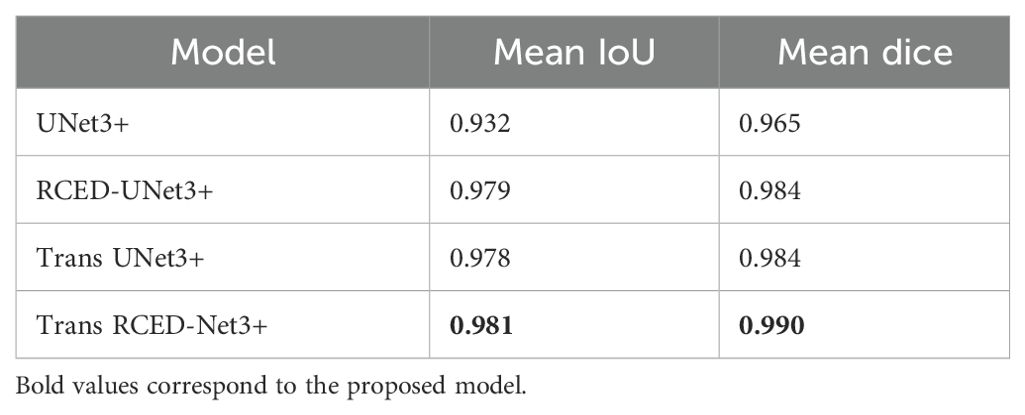
Table 3. Quantitative Assessment of Segmentation Accuracy for Baseline UNet3+, RCED-UNet3+, Trans UNet3+ and Trans RCED-UNet3+.
5.2 Comparative analysis
The comparative analysis is based on the ablation study and offers a comprehensive assessment of the four model variants, namely UNet3+, RCED-UNet3+, Trans-UNet3+, and Trans-RCEDUNet3+. Table 3 shows a quantitative evaluation and Table 4 shows the computational performance and efficiency of these models. Based on the findings, Trans RCED-UNet3+ has superior performance in segmentation than baseline models. Despite the practicality of the performance improvement brought by utilizing residual connections in combination with transformer modules, the complexity of the model prevents its application in resource-limited settings as the hyperparameters need to be carefully fine-tuned and the training process takes more time. It was also evaluated using a single dataset, other studies will be conducted in future to evaluate performance on diverse datasets using different scanners, institutions and imaging protocols to ascertain additional generalizability and clinical applicability.
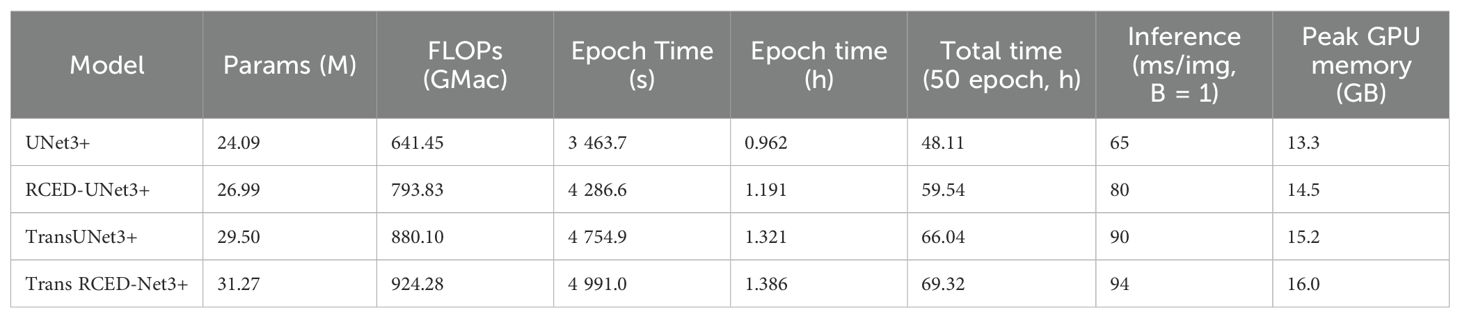
Table 4. Computational analysis and performance metrics for baseline UNet3+, RCED-UNet3+ model, Trans UNet3+, and Trans RCED-UNet3+.
To support these findings, pair wise t-tests were conducted on values of Dice Score between the four models. The t -test in Table 5 indicates that most of the model comparison provided statistically significant differences (p < 0.001), and this justified the performance improvement strength in architecture. Specifically, a significant improvement over the baseline UNet3+ was observed in RCED-UNet3 +, Trans UNet3+ and Trans RCED-UNet3 +. Also, Trans RCED-UNet3+ has continuously performed better than RCED-UNet3+ and Trans UNet3+, indicating its superiority in segmentation. The comparison that was not statistically significant was that between RCED-UNet3+ and Trans UNet3+ (p = 0.43); consequently, both shows indicated the similarity in performances. Overall, these findings verify that integration of residual connections and transformer modules into the UNet3+ architecture leads to large performance gains and that Trans RCED-UNet3+ offers the most consistent and informative advancement.
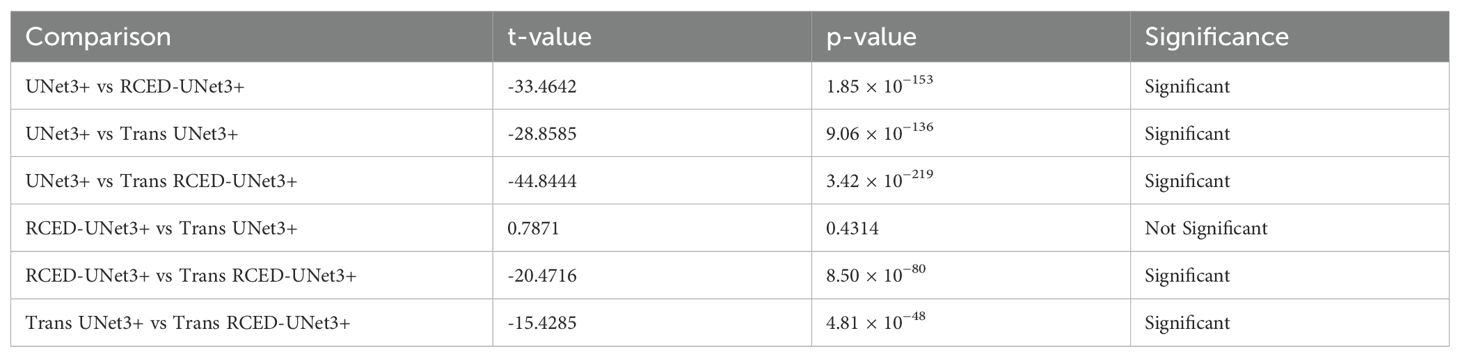
Table 5. Statistical significance t-test of dice scores between UNet3+, RCED-UNet3+, Trans UNet3+, and Trans RCED-UNet3+ models.
Figure 5 presents the progression of Dice and IoU metrics of UNet3+, RCED-UNet3+, Trans UNet3+, and Trans RCED-UNet3+ across training epochs. The UNet3+ model shows the steady but plateaus at a comparatively lower accuracy. By enhancing the UNet3+ model with residual connections in the encoder and decoder, the RCED-UNet3+ architecture stabilizes more quickly and achieves higher results. The Trans UNet3+ converges more quickly and achieves higher accuracy by incorporating a transformer in the UNet3+ model, which recognizes long-range spatial dependencies and global contextual information. The combination of RCED-UNet3+ and Trans UNet3+ architecture, the Trans RCED-UNet3+ exhibits both faster convergence and greater stability, maintaining a consistent upward trend throughout training.
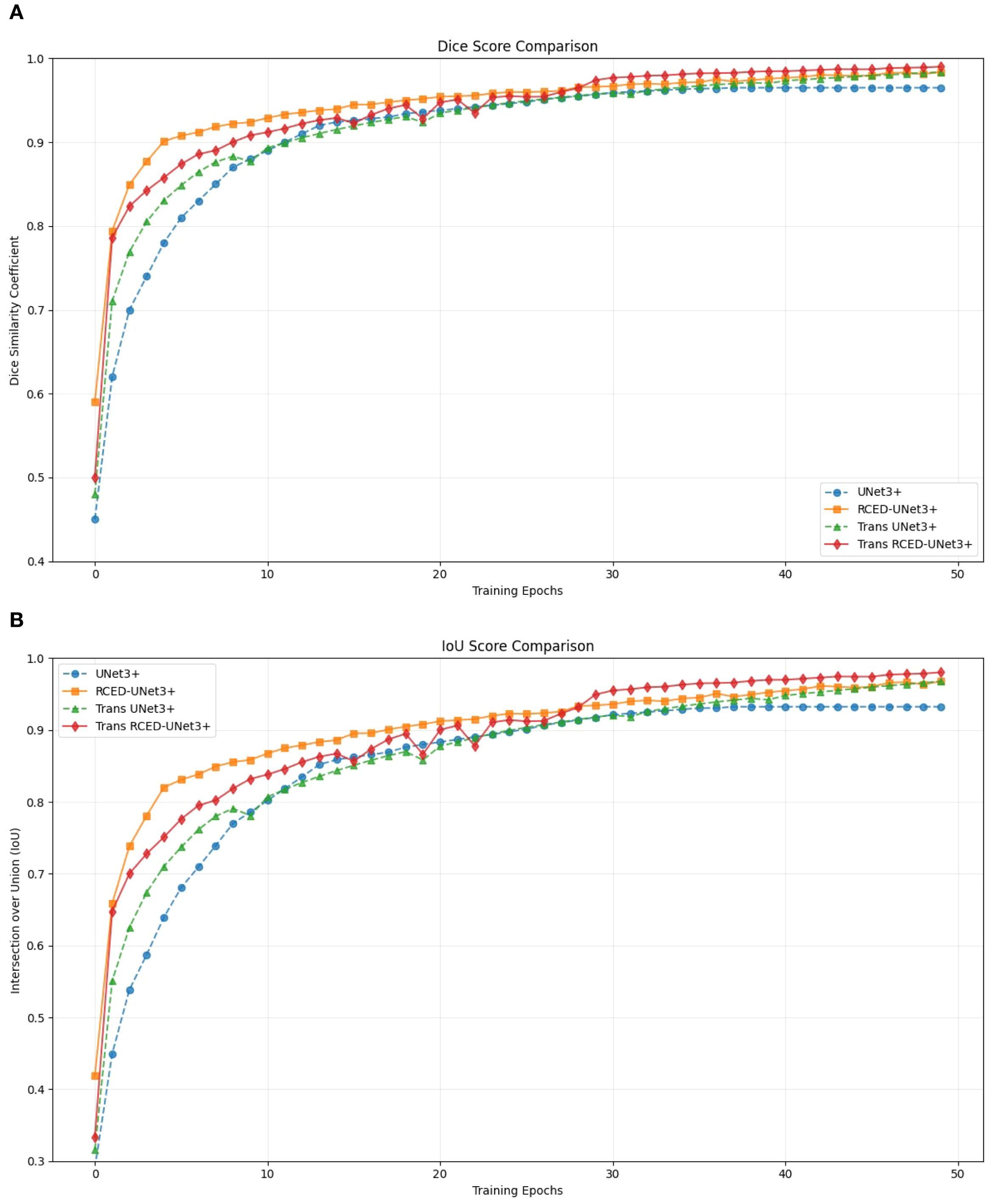
Figure 5. Segmentation performance progression (A) Dice score of UNet3+, RCED-UNet3+, Trans UNet3+, and Trans RCED-UNet3+ (B) Intersection over Union (IoU) of UNet3+, RCED-UNet3+, Trans UNet3+, and Trans RCED-UNet3+.
Figure 6 presents the segmentation results across nodules of various sizes, revealing that Trans RCED- UNet3+ achieves dice score values ranging from 0.988 to 0.994. This shows that the model effectively segments irregular, heterogeneous, spiculated nodules and additionally shows high accuracy for smaller homogeneous cases. This is due to the enhancement of the residual connection in the encoder and decoder, and the bottleneck transformer.
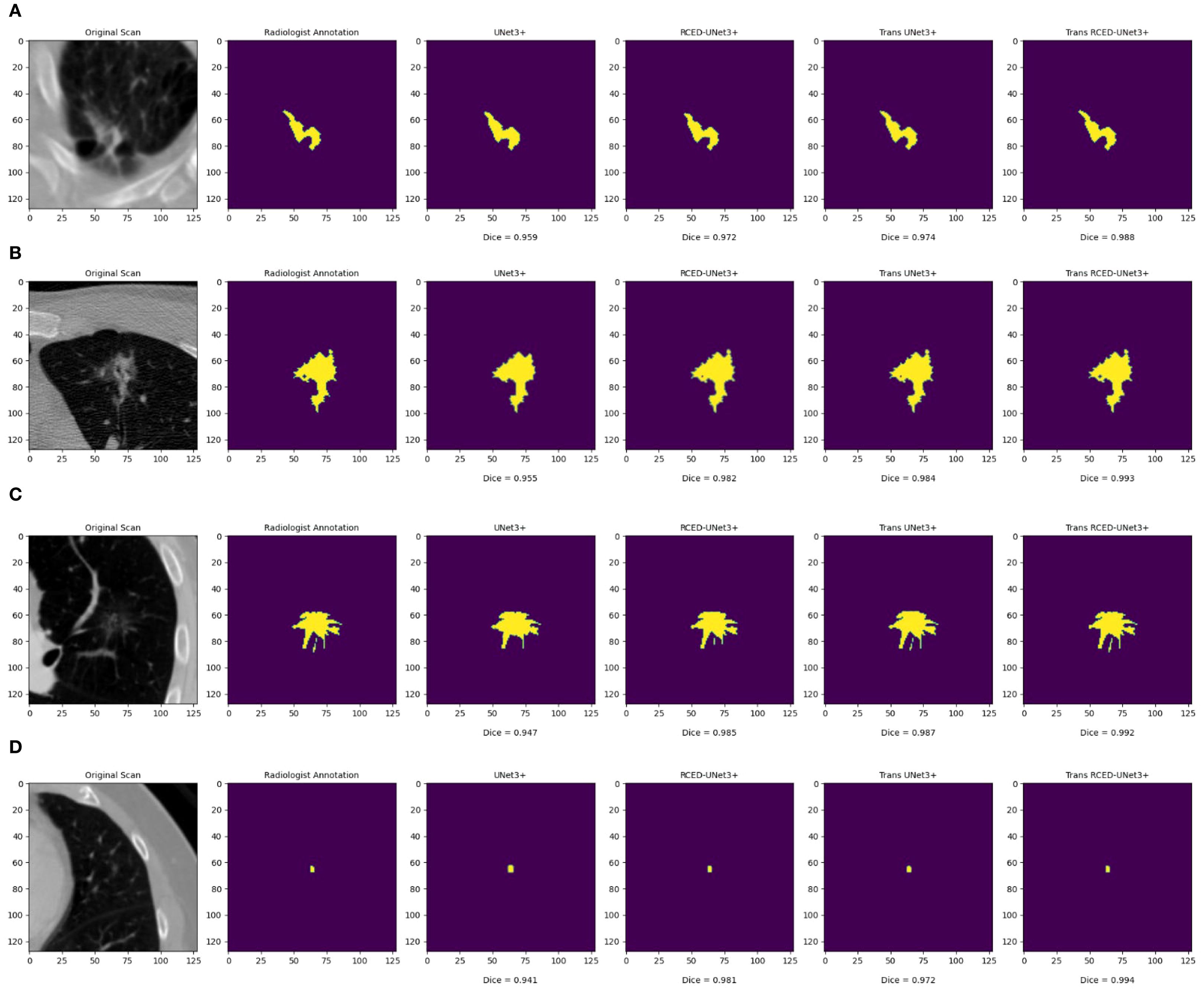
Figure 6. Segmentation results across nodules of varying sizes: (A) medium irregular lesion, (B) large heterogeneous mass, (C) medium spiculated lesion, and (D) small homogeneous nodule.
Figure 7 illustrates the box plot comparison of UNet3+, RCED-UNet3+, Trans UNet3+ and Trans RCED-UNet3+ shows clear performance differences. The findings reveal that UNet3+ exhibited the lowest performance, followed by RCED-UNet3+ and Trans UNet3+ while Trans-RCED-UNet3+ achieved the best results. This demonstrates that incorporating residual connections into UNet3+ and integrating a transformer within the bottleneck of RCED-UNet3+ effectively enhanced the model’s performance.
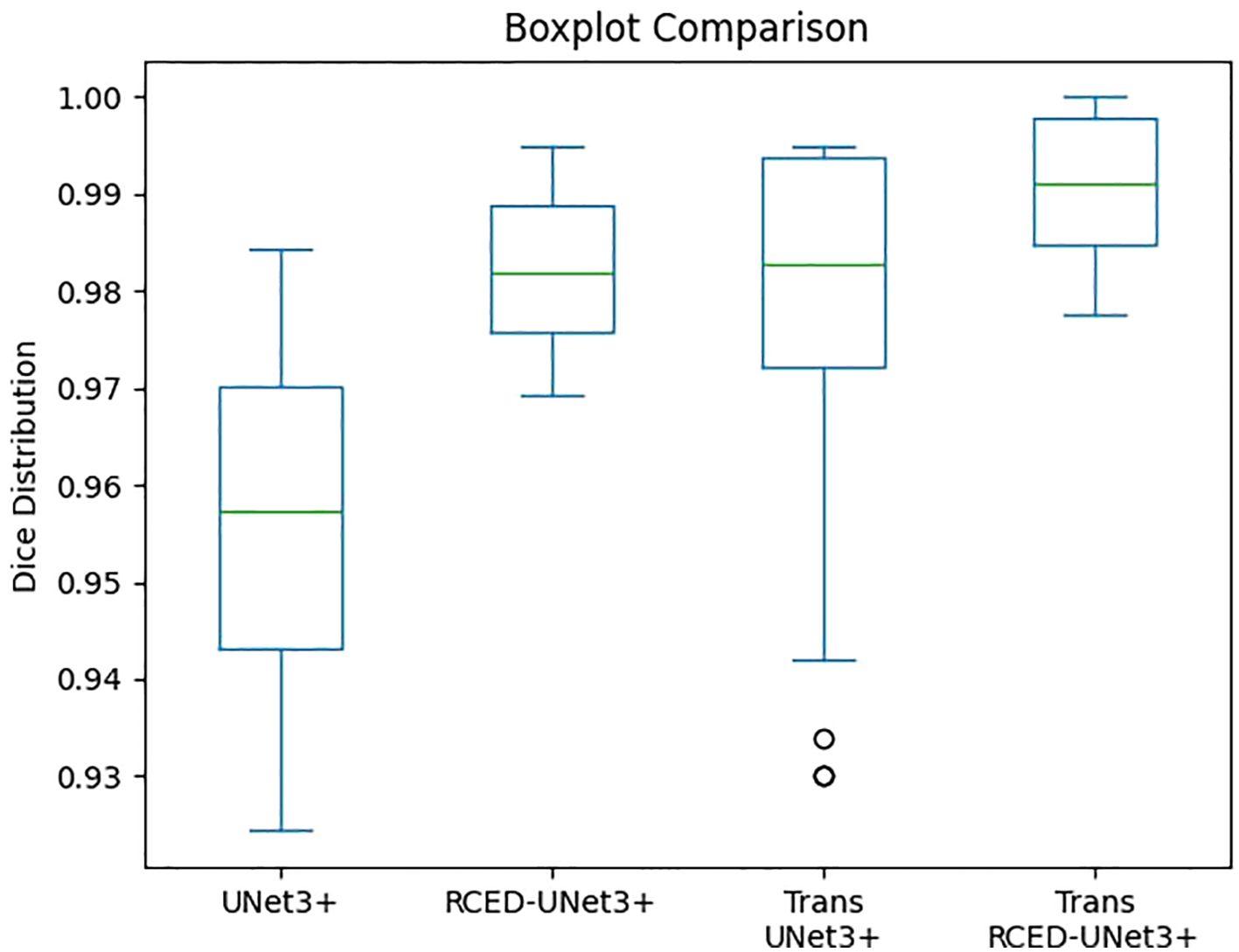
Figure 7. Comparison of the segmentation performance of UNet3+, RCED-UNet3+, Trans UNet3+ and Trans RCED-UNet3+ using the boxplot.
5.3 Loss function and hyperparameter optimization
The hybrid loss function used in RCED-UNet3+ has been retained and proven effective for lung nodule segmentation. However, with the integration of transformer-based self-attention, an attention-weighted loss function could further enhance performance. While the current loss function ensures strong overlap accuracy (Dice = 0.990, IoU = 0.981), an attention-guided approach might:
● Prioritize high-attention regions, improving the segmentation of small and complex lung nodules.
● Adapt dynamically to feature importance, reducing misclassification in challenging cases.
● Better integrate transformer capabilities by reinforcing areas with strong feature responses.
The performance improvements of Trans-RCEDUNet3+ are also partly due to the careful tuning of hyperparameters for the transformer module:
● Fine-tuning the number of attention heads and FFN size significantly improved segmentation accuracy, especially for small lung nodules.
● A dropout rate of 0.15 effectively prevented overfitting, enabling the model to generalize well across the LIDC-IDRI dataset.
● Grid search and random search methods ensured that the model avoided unnecessary computational overhead while maintaining optimal performance.
Figure 8 presents the changes in the hybrid loss throughout the training epochs, a gradual decrease in the loss value reflects the model’s improved learning efficiency and its increasing effectiveness in tackling the challenges associated with lung nodule segmentation.
5.4 Performance evaluation and comparative analysis
The Trans-RCEDUNet3+ model was compared with nine other state-of-the-art lung nodule segmentation models, as shown in Table 6. The results highlight the superior performance of Trans-RCEDUNet3+, which achieves a Dice score of 0.990, outperforming all other models.
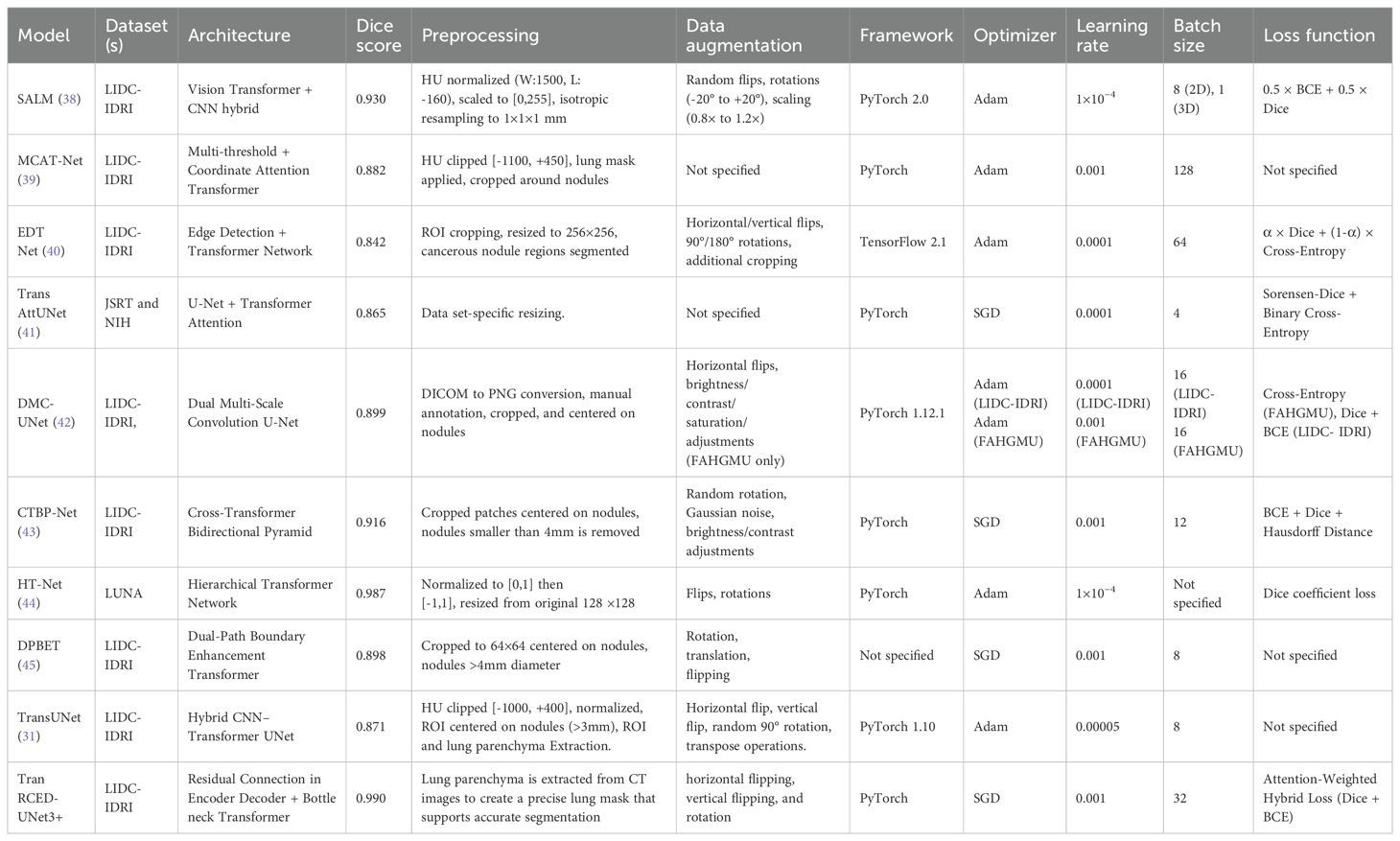
Table 6. Comparison of Trans RCED-UNet architecture with 09 other state-of-the-art lung nodule segmentation architectures.
Table 6 presents a comprehensive comparison between the proposed Trans RCED-UNet3+ and nine advanced lung nodule segmentation models. The analysis includes the datasets, architectural structures, preprocessing techniques, augmentation methods, implementation platforms, optimization methods, and loss functions. With a Dice score of 0.990, Trans RCED-UNet3+ outperforms ten leading architectures, showcasing its superior segmentation capability. It exceeds SALM (0.930), which incorporates Vision Transformers and utilizes a refined positional encoding strategy. It also surpasses MCAT (0.882), a model that integrates a Multi-threshold Feature Separation Module for texture enhancement, a Coordinate Attention Mechanism for spatial precision, and Transformers for improved global feature learning. Additionally, it outperforms EDTNet (0.842), which employs successive transformer blocks, patch-expanding layers, and both down-sampling and up-sampling mechanisms to refine segmentation performance. Furthermore, HT-Net (0.987), known for its multi-scale and hierarchical context modules, falls short in comparison. Trans RCED-UNet3+ also delivers superior results over CTBP-Net (0.916), both of which integrate convolution-transformer techniques and cross-transformer mechanisms. It also surpasses DMC-UNet (0.899), which leverages a compact residual framework with multiscale aggregation, and TransAttUnet (0.865), which utilizes self-aware attention inspired by transformers along with multi-scale skip connections. The approach ultimately yields superior segmentation accuracy over DPBET (0.898), a method that utilizes a dual-path architecture in combination with a hybrid transformer. The Trans RCED-UNet3+ model achieves high dice score is due to its transformer-based bottleneck neck, which recognizes long-range spatial dependencies and global contextual information along with a residual connection, which solves the problem of vanishing gradient.
5.5 Assessment using additional metrics
Table 7 presents assessment of the model using Accuracy, Precision, and Recall, which prvides more detailed evaluation of the model’s segmentation effectiveness.
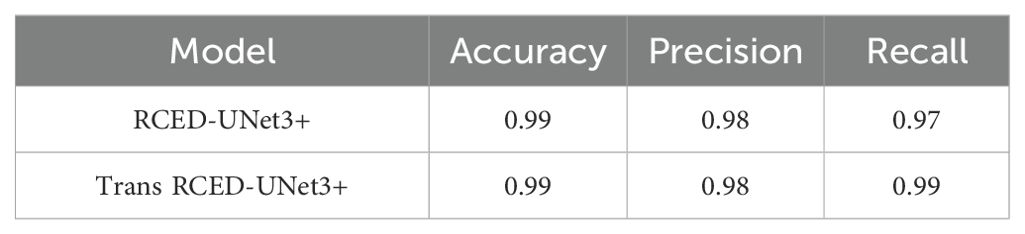
Table 7. Evaluation of accuracy, precision, and recall for RCED-UNet3+ and Trans-RCED-UNet3+ models trained on augmented datasets.
6 Conclusion
The Trans RCED-UNet3+ architecture achieves high segmentation accuracy compared to the RCED-UNet3+ by incorporating a transformer-based bottleneck, which recognizes long-range spatial dependencies and global contextual information across the image. The model achieved a Dice score of 0.990, surpassing the accuracy of 0.984. These results are attributed to the combination of convolutional structures with transformer components to address the challenges of medical image segmentation, particularly to accurately detect nodules, including those attached to the lung walls. Future research will explore the application of Trans RCED-UNet3+ across a variety of medical imaging datasets to assess its robustness under different conditions. Enhancing transformer modules and attention mechanisms is also expected to further improve accuracy and computational efficiency. The findings demonstrate promising potential for advancing automated medical image analysis toward greater accuracy and reliability.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for this study because it utilized a publicly available dataset. The data was fully anonymized and did not contain any personally identifiable information. No direct interaction with human participants occurred, and no intervention or identifiable private data was involved. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was not required for this study because it was conducted using a publicly available and fully anonymized dataset. The dataset does not contain any personal identifiers, and no direct contact with participants occurred. Written informed consent for publication was not obtained because the study used a publicly available and fully anonymized dataset. The data does not contain any identifiable information or images of individuals, and no direct interaction with human subjects occurred.
Author contributions
SR: Validation, Data curation, Methodology, Conceptualization, Formal analysis, Investigation, Writing – review & editing, Writing – original draft, Software. RZ: Writing – review & editing, Methodology, Supervision, Conceptualization. IU: Methodology, Writing – review & editing, Validation, Conceptualization. NoA: Conceptualization, Methodology, Data curation, Writing – review & editing, Software, Resources. NaA: Writing – review & editing, Conceptualization, Resources, Validation, Methodology. MH: Resources, Software, Data curation, Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a Large Group Research Project under grant number RGP2/324/46.
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R410), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guessous I, Cornuz J, and Paccaud F. Lung cancer screening: current situation and perspective. Swiss Med weekly. (2007) 137:304–11. doi: 10.4414/smw.2007.11993
2. Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumor phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. (2014) 5:4006. doi: 10.1038/ncomms5006
3. El-Baz A, Gimel’farb G, Falk R, and El-Ghar MA. A new CAD system for early diagnosis of detected lung nodules. In: 2007 IEEE international conference on image processing, vol. 2. Antonio, TX, USA: IEEE (2007). p. II–461. doi: 10.1109/ICIP.2007.4379192
4. Way T, Chan HP, Hadjiiski L, Sahiner B, Chughtai A, Song TK, et al. Computer-aided diagnosis of lung nodules on CT scans:: ROC study of its effect on radiologists’ performance. Acad Radiol. (2010) 17:323–32. doi: 10.1016/j.acra.2009.10.016
5. Liu H, Geng F, Guo Q, Zhang C, and Zhang C. A fast weak-supervised pulmonary nodule segmentation method based on modified self-adaptive FCM algorithm. Soft computing. (2018) 22:3983–95. doi: 10.1007/s00500-017-2608-5
6. Amorim PH, de Moraes TF, da Silva JV, and Pedrini H. Lung nodule segmentation based on convolutional neural networks using multi-orientation and patchwise mechanisms. In: ECCOMAS thematic conference on computational vision and medical image processing. Springer International Publishing, Cham (2019). p. 286–95. doi: 10.1007/978-3-030-32040-9_30
7. Cao H, Wang Y, Chen J, Jiang D, Zhang X, Tian Q, et al. Swin-unet: Unet-like pure transformer for medical image segmentation. In: European conference on computer vision. Springer Nature Switzerland, Cham (2022). p. 205–18. doi: 10.1007/978-3-031-25066-8_9
8. Lu F, Jia K, Zhang X, and Sun L. CRViT: Vision transformer advanced by causality and inductive bias for image recognition. Appl Intell. (2025) 55:68. doi: 10.1007/s10489-024-05910-3
9. Touvron H, Cord M, Douze M, Massa F, Sablayrolles A, and Jégou H. Training data-efficient image transformers & distillation through attention. In: International conference on machine learning. PMLR (2021). p. 10347–57. Available online at: https://proceedings.mlr.press/v139/touvron21a.html (Accessed July 2021).
10. Carion N, Massa F, Synnaeve G, Usunier N, Kirillov A, and Zagoruyko S. End-to-end object detection with transformers. In: European conference on computer vision. Springer International Publishing, Cham (2020). p. 213–29. doi: 10.1007/978-3-030-58452-8_13
11. Chen J, Lu Y, Yu Q, Luo X, Adeli E, Wang Y, et al. TransUNet: Transformers make strong encoders for medical image segmentation. (2021). doi: 10.48550/arXiv.2102.04306
12. Liu W, Zhang L, Li X, Liu H, Feng M, and Li Y. A semisupervised knowledge distillation model for lung nodule segmentation. Sci Rep. (2025) 15:10562. doi: 10.1038/s41598-025-94132-9
13. Wu Y, Liu X, Shi Y, Chen X, Wang Z, Xu Y, et al. S3 TU-Net: Structured convolution and superpixel transformer for lung nodule segmentation. Med Biol Eng Computing. (2025), 1–15. doi: 10.1007/s11517-025-03425-8
14. Ma J, Yuan G, Guo C, Gang X, and Zheng M. SW-UNet: a U-Net fusing sliding window transformer block with CNN for segmentation of lung nodules. Front Med. (2023) 10:1273441. doi: 10.3389/fmed.2023.1273441
15. Raza S, Zia R, Usmani IA, and Waheed A. RCED-UNet3+: unleashing residual connections in encoder-decoder architecture for precise lung nodule segmentation. Traitement du Signal. (2024) 41:3063. doi: 10.18280/ts.410623
16. Dehmeshki J, Amin H, Valdivieso M, and Ye X. Segmentation of pulmonary nodules in thoracic CT scans: a region growing approach. IEEE Trans Med Imaging. (2008) 27:467–80. doi: 10.1109/TMI.2007.907555
17. El-Regaily SA, Salem MAM, Aziz MHA, and Roushdy MI. (2017). Lung nodule segmentation and detection in computed tomography, in: 2017 Eighth International Conference on Intelligent Computing and Information Systems (ICICIS), Cairo, Egypt. pp. 72–8. IEEE. doi: 10.1109/INTELCIS.2017.8260029
18. Ulku I and Akagündüz E. A survey on deep learning-based architectures for semantic segmentation on 2d images. Appl Artif Intell. (2022) 36:2032924. doi: 10.1080/08839514.2022.2032924
19. Long J, Shelhamer E, and Darrell T. (2015). Fully convolutional networks for semantic segmentation, in: Proceedings of the IEEE conference on computer vision and pattern recognition, Boston, MA, USA. pp. 3431–40. doi: 10.1080/08839514.2022.2032924
20. Ronneberger O, Fischer P, and Brox T. U-net: Convolutional networks for biomedical image segmentation. In: International Conference on Medical image computing and computer-assisted intervention. Springer international publishing, Cham (2015). p. 234–41. doi: 10.1007/978-3-319-24574-4_28
21. Huang H, Lin L, Tong R, Hu H, Zhang Q, Iwamoto Y, et al. (2020). Unet 3+: A full-scale connected unet for medical image segmentation, in: ICASSP 2020–2020 IEEE international conference on acoustics, speech and signal processing (ICASSP), Barcelona, Spain. pp. 1055–9. Ieee. doi: 10.1109/ICASSP40776.2020.9053405
22. Zhou Z, Rahman Siddiquee MM, Tajbakhsh N, and Liang J. Unet++: A nested u-net architecture for medical image segmentation. In: International workshop on deep learning in medical image analysis. Springer International Publishing, Cham (2018). p. 3–11. doi: 10.1007/978-3-030-00889-5_1
23. Xiao H, Ran Z, Mabu S, Li Y, and Li L. SAUNet++: an automatic segmentation model of COVID-19 lesion from CT slices. Visual Comput. (2023) 39:2291–304. doi: 10.1007/s00371-022-02414-4
24. Yao C, Tang J, Hu M, Wu Y, Guo W, Li Q, et al. Claw u-net: A unet variant network with deep feature concatenation for scleral blood vessel segmentation. In: CAAI international conference on artificial intelligence. Springer International Publishing, Cham (2021). p. 67–78. doi: 10.1007/978-3-030-93049-3_6
25. Wu Z, Li X, and Zuo J. RAD-UNet: Research on an improved lung nodule semantic segmentation algorithm based on deep learning. Front Oncol. (2023) 13:1084096. doi: 10.3389/fonc.2023.1084096
26. Wang J, Lv P, Wang H, and Shi C. SAR-U-Net: Squeeze-and-excitation block and atrous spatial pyramid pooling based residual U-Net for automatic liver segmentation in Computed Tomography. Comput Methods Programs Biomedicine. (2021) 208:106268. doi: 10.1016/j.cmpb.2021.106268
27. Hu J, Shen L, and Sun G. (2018). Squeeze-and-excitation networks, in: Proceedings of the IEEE conference on computer vision and pattern recognition, Salt Lake City, UT, USA. pp. 7132–41. doi: 10.1109/CVPR.2018.00745
28. Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, et al. Attention is all you need. Adv Neural Inf Process Syst. (2017) 30. doi: 10.5555/3295222.3295349
29. Ghali R and Akhloufi MA. Vision transformers for lung segmentation on CXR images. SN Comput Sci. (2023) 4:414. doi: 10.1007/s42979-023-01848-4
30. Zhang Y, Liu H, and Hu Q. Transfuse: Fusing transformers and cnns for medical image segmentation. In: International conference on medical image computing and computer-assisted intervention. Springer International Publishing, Cham (2021). p. 14–24. doi: 10.1007/978-3-030-87193-2_2
31. Chen W, Wang Y, Tian D, and Yao Y. Ct lung nodule segmentation: A comparative study of data preprocessing and deep learning models. IEEE Access. (2023) 11:34925–31. doi: 10.1109/ACCESS.2023.3265170
32. Wang H, Cao P, Wang J, and Zaiane OR. Uctransnet: rethinking the skip connections in u-net from a channel-wise perspective with transformer. In: Proceedings of the AAAI conference on artificial intelligence, vol. 36. (2022). p. 2441–9. doi: 10.1609/aaai.v36i3.20144
33. Gao Y, Zhou M, and Metaxas DN. UTNet: a hybrid transformer architecture for medical image segmentation. In: International conference on medical image computing and computer-assisted intervention. Springer International Publishing, Cham (2021). p. 61–71. doi: 10.1007/978-3-030-87199-4_6
34. Zhang Q and Yang YB. Rest: An efficient transformer for visual recognition. Adv Neural Inf Process Syst. (2021) 34:15475–85. doi: 10.48550/arXiv.2105.13677
35. Ji Y, Zhang R, Wang H, Li Z, Wu L, Zhang S, et al. Multi-compound transformer for accurate biomedical image segmentation. In: International conference on medical image computing and computer-assisted intervention. Springer International Publishing, Cham (2021). p. 326–36. doi: 10.1007/978-3-030-87193-2_31
36. Ahmed R, Al Shehhi A, Werghi N, and Seghier ML. Segmentation of stroke lesions using transformers-augmented MRI analysis. Hum Brain Mapp. (2024) 45:e26803. doi: 10.1002/hbm.26803
37. Shah OI, Rizvi DR, and Mir AN. Transformer-based innovations in medical image segmentation: A mini review. SN Comput Sci. (2025) 6:375. doi: 10.1007/s42979-025-03929-y
38. Gayap HT and Akhloufi MA. SALM: A unified model for 2D and 3D region of interest segmentation in lung CT scans using vision transformers. Appl Biosci. (2025) 4:11. doi: 10.3390/applbiosci4010011
39. Hu T, Lan Y, Zhang Y, Xu J, Li S, and Hung CC. A lung nodule segmentation model based on the transformer with multiple thresholds and coordinate attention. Sci Rep. (2024) 14:31743. doi: 10.1038/s41598-024-82877-8
40. Yadav DP, Sharma B, Webber JL, Mehbodniya A, and Chauhan S. EDTNet: A spatial aware attention-based transformer for the pulmonary nodule segmentation. PloS One. (2024) 19:e0311080. doi: 10.1371/journal.pone.0311080
41. Chen B, Liu Y, Zhang Z, Lu G, and Kong AWK. Transattunet: Multi-level attention-guided u-net with transformer for medical image segmentation. IEEE Trans Emerging Topics Comput Intell. (2023) 8:55–68. doi: 10.1109/TETCI.2023.3309626
42. Fan X, Lu Y, Hou J, Lin F, Huang Q, and Yan C. DMC-UNet-based segmentation of lung nodules. IEEE Access. (2023) 11:110809–26. doi: 10.1109/ACCESS.2023.3322437
43. Li X, Jiang A, Wang S, Li F, and Yan S. CTBP-Net: Lung nodule segmentation model based on the cross-transformer and bidirectional pyramid. Biomed Signal Process Control. (2023) 82:104528. doi: 10.1016/j.bspc.2022.104528
44. Ma M, Xia H, Tan Y, Li H, and Song S. HT-Net: hierarchical context-attention transformer network for medical ct image segmentation. Appl Intell. (2022) 52:10692–705. doi: 10.1007/s10489-021-03010-0
Keywords: transformer bottleneck, lung nodule, hybrid loss function, RCED-UNet 3+, LIDC-IDRI
Citation: Raza S, Zia R, Usmani IA, Almujally NA, Alasbali N and Hanif M (2025) Trans RCED-UNet3+: a hybrid CNN-transformer model for precise lung nodule segmentation. Front. Oncol. 15:1654466. doi: 10.3389/fonc.2025.1654466
Received: 26 June 2025; Accepted: 23 September 2025;
Published: 16 October 2025.
Edited by:
Yuxia Tang, Nanjing Medical University, ChinaReviewed by:
Yuanpin Zhou, Zhejiang University, ChinaQingnan Huang, Guangxi University of Technology, China
Copyright © 2025 Raza, Zia, Usmani, Almujally, Alasbali and Hanif. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sadaf Raza, c21pbmhhakBzc3VldC5lZHUucGs=; Muhammad Hanif, bXVoYW1tYWQuaGFuaWZAb3J1LnNl
 Sadaf Raza
Sadaf Raza Razia Zia1,2
Razia Zia1,2 Irfan Ahmed Usmani
Irfan Ahmed Usmani Nouf Abdullah Almujally
Nouf Abdullah Almujally Nada Alasbali
Nada Alasbali Muhammad Hanif
Muhammad Hanif




