- 1Department of Oncology, Southwest Hospital, Army Medical University, Chongqing, China
- 2Department of Clinical Laboratory, The 990 Hospital of the Joint Logistics Support Force of the Chinese People’s Liberation Army, Zhumadian, China
- 3Department of Intensive Care Unit (ICU), The 942 Hospital of the Joint Logistics Support Force of the Chinese People’s Liberation Army, Yinchuan, China
- 4Department of General Surgery, Xin Qiao Hospital, The Second Affiliated Hospital, Army Medical University, Chongqing, China
Introduction: Early stage breast cancer treated with adjuvant radiotherapy with two different techniques, tomotherapy (TOMO) and intensity-modulated radiation therapy (IMRT), and their acute adverse events in terms of skin toxicity, localized edema, sore throat, tracheal mucositis, nausea, oral mucositis, esophagitis, and pneumonitis outcomes are compared.
Materials/methods: A retrospective cohort study was conducted to compare the adverse events of IMRT and TOMO in early stage breast cancer. We reviewed the data of female patients who underwent lumpectomy or mastectomy for breast cancer at the Oncology Department of the First Affiliated Hospital, Army Medical University, from September 2021 to February 2024. A total of 315 female patients were enrolled in this study, including 130 and 185 in the TOMO and IMRT groups, respectively. In this study, the adverse events in the two groups of patients were compared and analyzed.
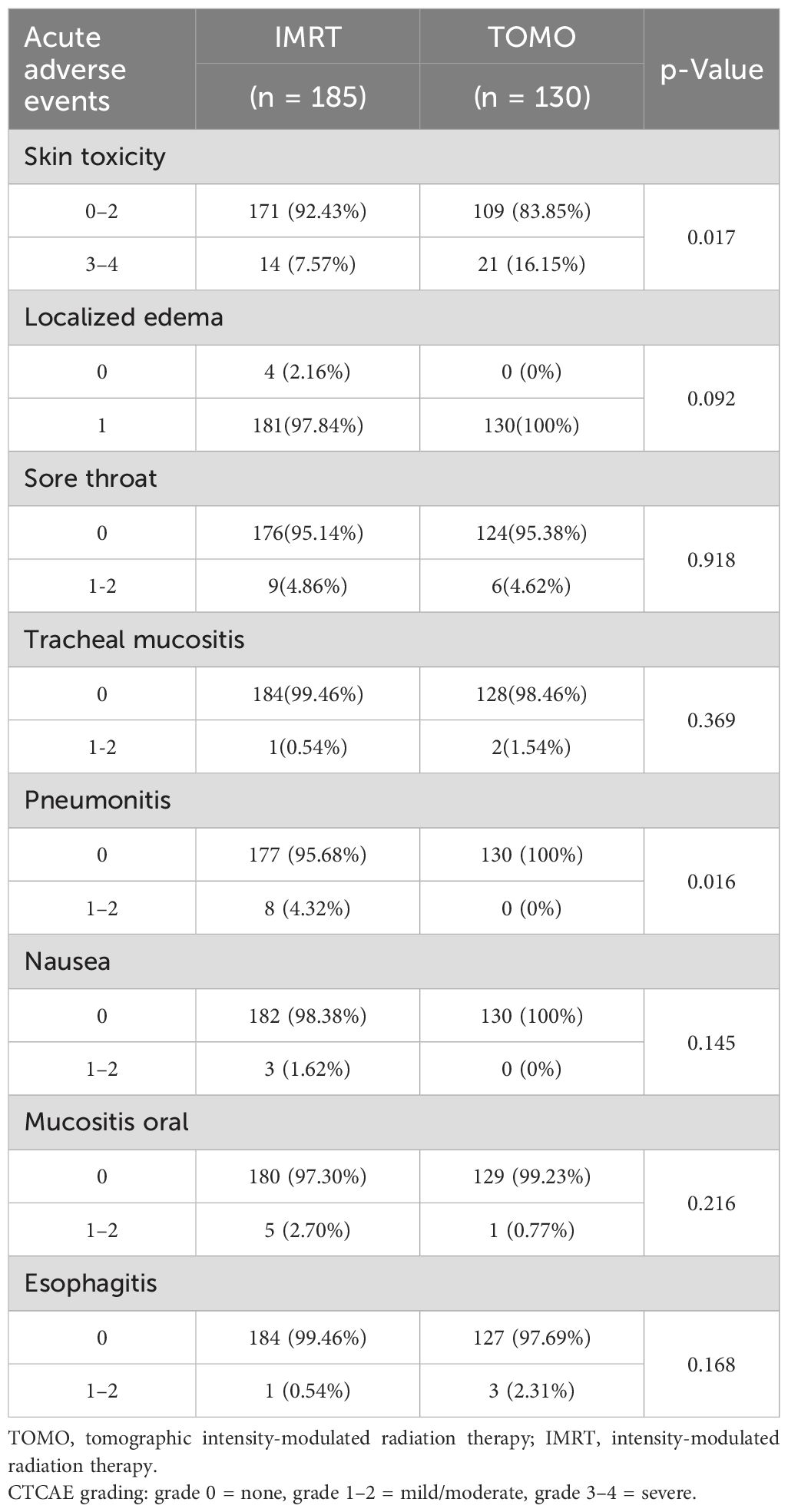
Results: The median age of the patients in this retrospective cohort was 47 years (range, 20–74 years). The follow-up period was 3 months. A total of 185 patients (59%) received IMRT and 130 (41%) underwent TOMO. No significant differences were observed in terms of menopausal status, laterality, pathology, estrogen receptor status, progesterone receptor status, triple negative, clinical T stage, clinical N stage, or surgical methods. Negative HER-2 overexpression was found in 38% and 51% of the TOMO and IMRT groups, respectively (relative risk [RR], 0.63; 95% CI 0.40 –0.99; P = 0.053).With regard to the degree of tumor differentiation, poor- moderate differentiation was 69% in the TOMO group and 81% in the IMRT group (RR 0.53; 95% CI, 0.31 –0.89; P = 0.052). In the TOMO and IMRT groups, 66% and 55% of the patients received hormone therapy, respectively (RR 1.59; 95% CI 1.00 –2.53; P = 0.5). However, there were no statistical differences in the demographic and tumor characteristics between the TOMO and IMRT groups. A comparison of adverse events between the TOMO and IMRT groups showed no significant differences in localized edema, sore throat, tracheal mucositis, nausea, oral mucositis, and the IMRT groups. Compared to the IMRT group, the TOMO group had a higher proportion of grade 3–4 skin toxicity [16.2% (TOMO) versus 7.6% (IMRT), (RR 2.13; 95% CI 1.04 –4.37; P = 0.017)]. Pneumonitis was lower in the TOMO group than in the IMRT group [0.0% (TOMO) versus 4.3% (IMRT), (RR 1.05; 95% CI 1.01 –1.08; P = 0.016].
Conclusions: Compared with IMRT, TOMO decreases the incidence of radiation pneumonitis but fails to improve acute skin toxicity. Based on our research, TOMO may contribute to higher odds of acute skin toxicity, which should be considered by clinicians.
Introduction
Breast cancer is a major disease that threatens women’s health (1). Breast cancer is the fourth leading cause of cancer mortality worldwide and the leading cause of cancer-related deaths among women (2, 3).
Radiotherapy has become one of the main methods used for adjuvant treatment of breast cancer (4, 5). Female patients with early stage breast cancer (stages 0 –II) commonly receive adjuvant radiation therapy after lumpectomy with or without (stage 0) sentinel node biopsy (6, 7). Adjuvant radiation showed a significant improvement in the local recurrence rate and overall survival rates in female patients with early stage breast cancer (8). However, female patients who received radiotherapy for breast cancer experienced different degrees of acute toxicity, such as tenderness or swelling of the chest wall, radiation dermatitis, radiation pneumonitis, and fatigue (9, 10).
In the past few years, toxic acute adverse events after radiotherapy for early stage breast cancer have been reported as an important issue (11, 12). According to the literature, tomotherapy (TOMO) has many advantages over precision radiotherapy, which contributes to the development and improvement of clinical treatment for breast cancer and minimizes the toxicity during radiotherapy. Precision radiotherapy strategies, including traditional intensity-modulated radiotherapy (IMRT) and TOMO, are the two main treatments for those female patients with early stage breast cancer after lumpectomy (13, 14).
Recently, many studies have revealed the toxicity of IMRT or TOMO radiotherapy for breast cancer (15, 16), but few studies have focused on the comparison of toxic side effects between IMRT and TOMO for early stage breast cancer. In particular, owing to the lack of direct comparative evidence on acute toxicity between TOMO and IMRT in early stage, node-negative breast cancer, it is challenging for clinicians to conduct a comprehensive comparison of the treatment effects and side effects between the two regimens. Therefore, physicians and patients urgently require more experiential support and evidence-based medical references for the selection of clinical treatment strategies for breast cancer radiotherapy. In this study, we aimed to evaluate the incidence of adverse events in the TOMO schedule compared with the IMRT schedule for early stage, node-negative breast cancer.
For this reason, we present our clinical experience using TOMO as an adjuvant radiation strategy for the early-stage breast cancer, and hope that would help improving the treatment strategies based on the results of this retrospective study. This study is also expected to provide a more optimized radiation strategy to decrease adverse events in female patients with early-stage breast cancer after lumpectomy or mastectomy.
Materials and methods
A retrospective cohort study was conducted to compare the adverse events of TOMO and IMRT in early stage breast cancer. We reviewed the data of female patients who underwent lumpectomy or mastectomy for breast cancer at the Oncology Department of the First Affiliated Hospital, Army Medical University (Chongqing, China) from September 2021 to February 2024. A total of 315 female patients were enrolled in this study, including 130 in the TOMO group and 185 in the IMRT group. In this study, the adverse events of the two groups of patients were compared and analyzed. The study was approved by the internal ethics committee, and patient consent was obtained.
Patient selection
In our clinic, patients who received adjuvant radiotherapy with TOMO or IMRT after surgery for early stage breast cancer in the Oncology Department of the First Affiliated Hospital, Army Medical University were evaluated retrospectively. Patient characteristics, treatment details, and acute adverse event data were obtained from the electronic medical records system, patient interview notes, and patient follow-up records. Acute adverse events in patients were evaluated by medical oncologists, radiation oncologists, and surgeons.
Grouping methods
This study was designed as a single- center retrospective cohort analysis. All patients with early stage breast cancer who underwent lumpectomy or mastectomy between September 2021 and February 2024 were included in the primary analyses. According to the inclusion criteria, the patients were divided into two groups, the IMRT and TOMO groups, based on the radiotherapy regimens they received. After screening with the exclusion criteria, patients who met the above two criteria were included in the final analyses.
The eligibility criteria were as follows: age >18 years, invasive cancer, American Joint Committee on Cancer AJCC Stage I to II, lumpectomy or mastectomy, and TOMO or IMRT radiotherapy. The main exclusion criteria were extensive intraductal carcinoma, multiple foci of cancer, final surgical margins < 5 mm, lack of clinical data, vital organ failure, and failure to complete radiotherapy.
Treatment planning
Radiation therapy was systematically prescribed following our institutional policy. Radiotherapy treatment was started 30 days and within 60 days from the surgery; if adjuvant chemotherapy was performed, radiotherapy was postponed until 4 weeks after the last chemotherapy cycle. Patients in different groups received radiation of the whole breast and/or surgical bed using two different devices, TOMO or IMRT. The treatment procedure followed institutional rules, which have been described in detail elsewhere (17, 18).
TOMO planning
Treatment planning for TOMO was performed using the Accuray® Planning Station System (TomoHDTM version 2.1.9, Inc., Sunnyvale, CA, USA). The Monte Carlo algorithm is used for dose calculation, treatment planning, and quality assurance. The grid size is 3 mm. In line with the internal irradiation regimes, the dose prescribed to the planning target volume (PTV) varied from 40 Gy to 60 Gy, with a median dose of 50 Gy for the PTV. Before radiation treatment, the patient’s positioning in each automatic registration was executed by experienced staff members (see Supplementary File 1, detailed protocols of treatment planning).
As a newly introduced therapy equipment, TOMO is more expensive than that of IMRT. TOMO was chosen for some patients mainly based on their economic conditions and fully complied with the patient’s voluntary choice.
IMRT planning
We utilized the Eclipse version 16.1 (Varian Medical Systems Inc., Palo Alto, USA) to create IMRT treatment plans. Patients undergoing IMRT were administered a cumulative dose of 50 Gy in 25 fractions. Subsequently, a radiation therapy boost of 10 Gy was administered in five weekly fractions to the surgical bed. The dose was delivered through wedged photon tangential fields, and the boost was treated using an electron direct field. The organs at risk (OARs) were contoured according to internal guidelines. The constraints specified that 5% of the heart and 20% of the lung should receive a dose of less than 20 Gy (see Supplementary File 1, detailed protocols for treatment planning).
Follow-up
The follow-up period was 3 months. After completion of TOMO or IMRT radiotherapy, according to the research plan, the follow-up schedule was as follows: we followed up all patients weekly for 3 months. The start date of follow-up for each patient was the radiation therapy start date, and the end date was 3 months after the last radiation therapy date. Follow-up mainly depended on the outpatient department, and telephone contact was reserved as an auxiliary method. Clinical examinations were performed by clinicians at each follow-up visit, and other examinations, such as hematologic or endoscopic examinations, were performed depending on the patients’ suspected symptoms. Adverse events were diagnosed by clinicians according to objective clinical and physical examinations after radiotherapy during treatment and follow-up. This study was completed in February 2024.
Outcomes
The clinical endpoint of this study was acute adverse events immediately following the completion of radiation therapy. Patients’ acute adverse events were prospectively recorded for a period of 3 months and were evaluated by medical oncologists, radiation oncologists and clinicians in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) (Version 5.0) (19). In this study, acute adverse events were defined as those first observed and diagnosed within 90 days of the latest radiotherapy session. We recorded acute skin toxicity (erythema, epilation, desquamation, decreased sweating, edema, ulceration, hemorrhage, and necrosis), localized edema, sore throat, tracheal mucositis, nausea, oral mucositis, esophagitis, and pneumonitis. Adverse events were recorded weekly during TOMO or IMRT treatment and then repeated until 3 months after the last radiotherapy. Particularly, before the implementation of treatment, clinicians assessed the skin condition to ensure that the skin was clear, normal, and with out lesions. Patients were also excluded from the study if they had any risk factors (such as comorbidities or concurrent medications) for vulnerable skin.
Statistical methods
This study was designed to compare the toxicity rates of IMRT and TOMO in patients with early stage breast cancer after lumpectomy or mastectomy. Statistical analyses were performed using the SPSS Statistics software (version 26; SPSS Statistics, IBM Corporation, Armonk, NY, USA). Independent t-tests, chi-square tests, and Fisher’s exact tests were used to compare the statistical differences between the two groups. Econometric data that conformed to normal distribution with homogeneous variances were expressed as mean ± standard deviation () and subjected to t-tests. Count data were expressed as the number of cases (percentage) N (%), and intergroup comparisons were performed using the X2-test or Fisher’s exact test. All 2-sided P values <0.05 were considered significant.
Results
Between September 2021 and February 2024, 394 patients with early stage breast cancer who underwent lumpectomy or mastectomy were included in the primary analyses. After screening patients with the exclusion criteria, 79 patients were excluded, and 315 patients were enrolled in this study. The TOMO group and IMRT groups comprised 130 and 185 patients, respectively (Figure 1).
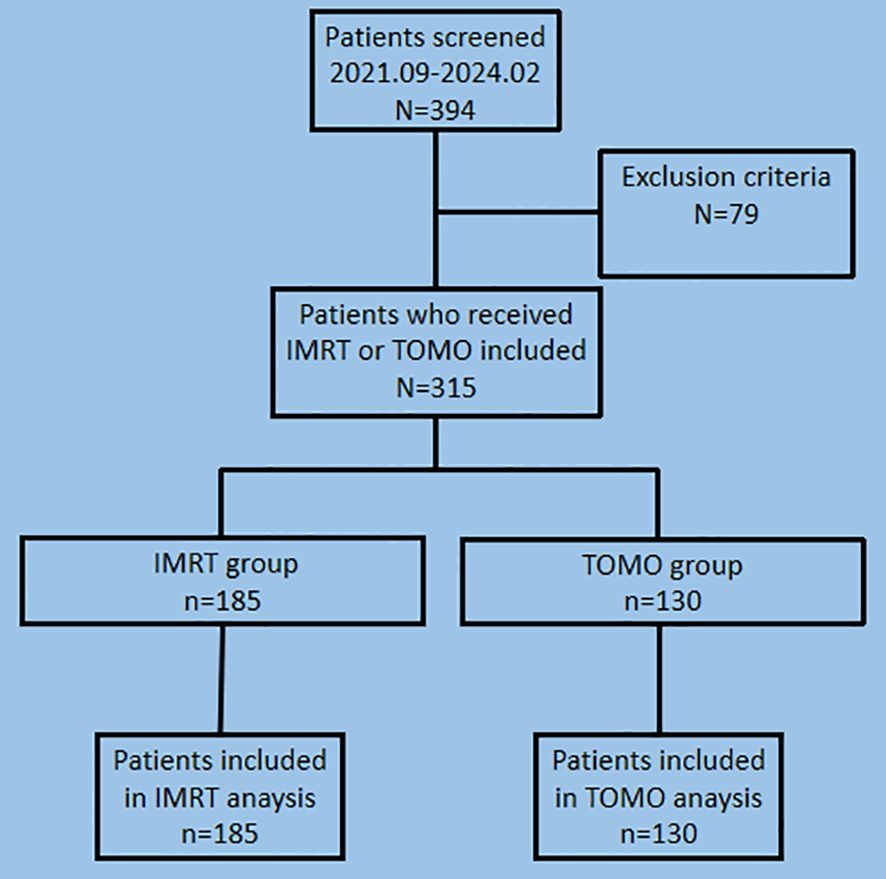
Figure 1. Screening and patient flow in the study of adverse events comparison between TOMO and IMRT for early stage breast cancer. TOMO, tomographic intensity-modulated radiation therapy; IMRT, intensity-modulated radiation therapy.
Clinical characteristics
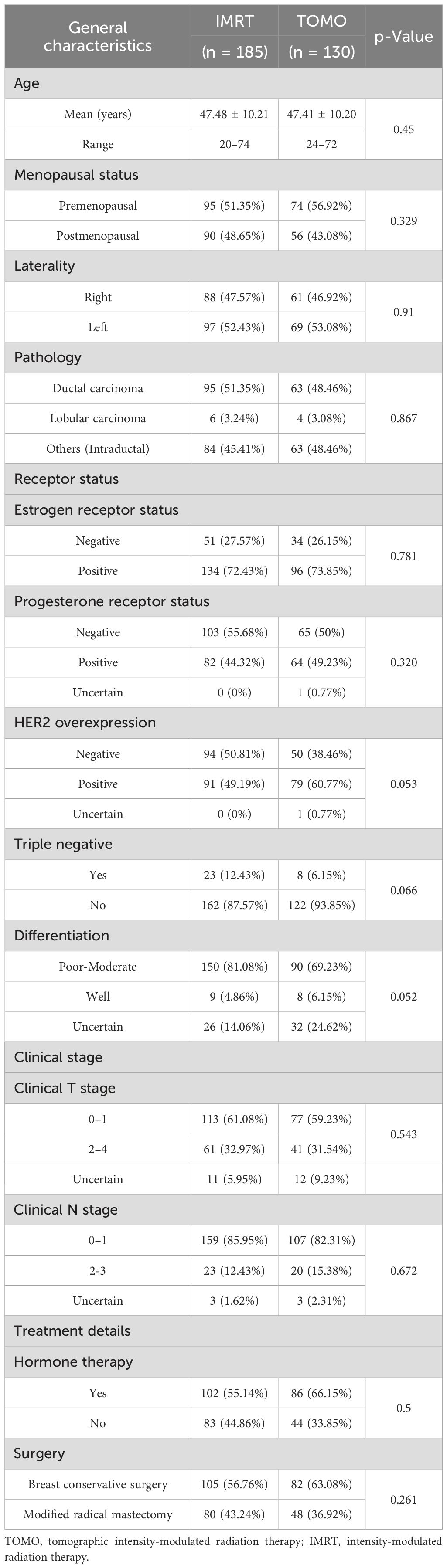
The median age of the patients in this retrospective cohort was 47 years (range, 20–74 years). Table 2 summarizes the clinical characteristics of the 315 patients, divided by planning method into IMRT and TOMO. The length of follow-up was 3 months. 185 patients (59%) received IMRT and 130 patients (41%) underwent TOMO. Negative HER-2 overexpression was found in 38% and 51% of the TOMO and IMRT groups, respectively (RR 0.63; 95% CI 0.40 –0.99; P = 0.053).With regard to the degree of tumor differentiation, poor-moderate differentiation was 69% in the TOMO group and 81% in the IMRT group (RR 0.53; 95% CI 0.31 –0.89; P = 0.052). There were 66% and 55% of TOMO and IMRT groups, respectively, receiving hormone therapy (RR 1.59; 95% CI 1.00 –2.53; P = 0.5). However, there were no statistical differences in the demographic and tumor characteristics between the TOMO and IMRT groups.
The baseline clinical characteristics were well-balanced between the TOMO and IMRT groups (Table 1), and no significant differences were observed in terms of age, menopausal status, laterality, pathology, estrogen receptor status, progesterone receptor status, triple negative, clinical T stage, clinical N stage, or surgical methods.
Acute adverse events evaluation
The clinical results of adverse responses and comparisons between TOMO and IMRT are summarized in Table 3. There was no significant relationship between observed localized edema (RR 1.02; 95% CI 1.00 –1.04; P = 0.092); sore throat (RR 0.95; 95% CI 0.33 –2.73; P = 0.918); tracheal mucositis (RR 2.88; 95% CI 0.26 –32.04; P = 0.369); nausea (RR 1.02; 95% CI 1.00 –1.04; P = 0.145); Oral mucositis (RR 0.28; 95% CI 0.03 –2.42; P = 0.216); esophagitis (RR 4.35; 95% CI 0.45 –42.26; P = 0.168). Compared to the IMRT group, the TOMO group had a higher proportion of grade 3–4 grade skin toxicity [16.2% (TOMO) versus 7.6% (IMRT), (RR 2.13; 95% CI 1.04 –4.37; P = 0.017)]. Pneumonitis was lower in the TOMO group than in the IMRT group [0.0% (TOMO) vs. 4.3% (IMRT), (RR 1.05; 95% CI 1.01 –1.08; P = 0.016]]. No fair or poor judgments were recorded in the 315 patients during the follow-up period. No other adverse events or toxicities were observed during the follow-up period. The clinical results are summarized in Table 1.
On comparing adverse events, we found a statistically significant difference between the TOMO and IMRT groups in the presence of acute skin toxicity (P = 0.017) and pneumonitis (P = 0.016). Compared with the IMRT group, the TOMO group seemed to have a lower incidence rate of pneumonitis. However, the incidence of acute skin toxicity was higher in the TOMO group was higher than that in the IMRT group, especially for grade 3–4 skin toxicity. The results suggest that TOMO could decrease the incidence rate of pneumonitis but increase the risk of acute skin toxicity.
Discussion
In the present retrospective, single-center study of 315 patients treated for breast cancer with IMRT or TOMO, we found that adverse events occurred very commonly (observed in 98.7% of the patients), and a considerable number of patients in this study suffered at least one (mainly mild) toxicity adverse event. Our study showed a notable improvement in reducing the incidence of radiation pneumonitis in the TOMO group. For the IMRT group, 4.3% of all patients developed radiation-related pneumonitis, but it was not severe (with only eight events of grade 2 or lower), while the incidence in the TOMO group was 0%. Similarly, studies (20, 21) have revealed that TOMO could decrease unnecessary breast overdose in breast-conserving treatment of breast cancer; as a result, TOMO decreased adverse events in some critical organs, such as the lungs, by optimizing ipsilateral lung dosimetry (21, 22).
In addition, in a single -center retrospective study, Felix et al. (23) discovered that TOMO presented low rates of acute toxicity in critical organs. Pneumonitis was observed in 1.8% of patients who received treatment (23). During the follow -up period, none of the patients experienced toxicities higher than grade 3. A recent retrospective study that investigated the clinical outcomes and adverse events associated with adjuvant radiotherapy using TOMO after breast -conserving surgery disclosed that the adverse events were mild, and there was no occurrence of pneumonitis in the observed patients (24).
A similar result was also obtained in our study, and we also found no pneumonitis in patients after TOMO. As mentioned above, TOMO improved the critical organ risk, especially for the lungs, during radiotherapy by optimizing treatment planning.
However, that study showed no improvement in other acute adverse events related to radiotherapy, such as localized edema, sore throat, tracheal mucositis, nausea, oral mucositis, and esophagitis. Notably, acute skin toxicity appeared to be more severe in the TOMO group. Although there was no statistical difference between the two groups in the incidence of skin toxicity (grade 0–4), unfortunately, skin toxicity grade 3–4 was significantly increased in the TOMO group. In the TOMO and IMRT groups, 16.2% and 7% of all patients had acute skin toxicity grade 3–4, respectively. Simon et al. (25) explained that if the skin surface is set as a radiation therapy optimization target, tangential beam segments would concentrate on the skin surface as a result of inverse planning, which would increase acute skin toxicity. The flexibility of TOMO in delivering doses to the tumor bed makes it easier to accumulate high doses to superficial targets, such as the skin, resulting in significant acute skin toxicity (26, 27). Theoretically, if the “hot-spot” (>10% of prescribed dose) of TOMO delivers an overdose on the skin surface, an abnormally high incidence of acute skin toxicity follows (26).
Moreover, according to the results previously reported in the literature, factors such as the TOMO planning system (27–29), patient positioning (27, 30), breast size variation (31), treatment delivery time (27), and edema or breath variation (32) contribute to the incidence of skin toxicity. Clinically, it is difficult to diminish the impact of these risk factors. For example, a patient positioning shift of 5 mm during TOMO may induce an extra dose variation of 3% –9% (30). In addition, different systems of TOMO planning software may contribute 3%–13% of overdose to skin tissue (29).
Although these risk factors for acute skin toxicity are difficult to overcome, additional care must be taken to ensure patient safety and prevent skin toxicity. According to the literature reported above, when treating breast cancer patients with TOMO, clinicians should pay more attention to ensure that patients are in accurate positioning (27). Meanwhile, optimized measurements or dose recalculation techniques should be applied to the TOMO planning software to ensure adequate dosing for superficial organs, including the skin, during radiation therapy (27, 32). Furthermore, more robust new techniques, including artificial intelligence, should be applied using TOM to reduce skin dose and avoid toxicity (25, 33–35).
In the present study, 21 (16.15%) patients in the TOMO group had severe (grade 3–4) skin lesions, while the data in the IMRT group was 14 (7.57%). Thus, TOMO results in a higher incidence of skin toxicity. When examining the underlying reasons, we are more inclined to attribute the higher incidence of skin toxicity to the unique mechanism of action of TOMO. The reasons are as follows: First, all treatment plans were performed by operators from the same group; therefore, instrument operation-related risk factors, such as patient positioning or treatment planning, should be excluded. Second, in our study, the sample size was relatively large for a single-center study, which could effectively mitigate the impact of patient heterogeneity on clinical treatment responses; consequently, the results in the present study are relatively reliable. Finally, in terms of skin toxicity, our research results were consistent with previous literature reports, which presented an abnormally high incidence of acute skin toxicity in their studies.
Few studies have reported adverse events associated with TOMO vs. IMRT in early stage breast cancer. This study reports our initial experience with postoperative radiotherapy using TOMO in breast cancer. In our experience, although we were not able to optimize the radiation dose on the skin tissue and reduce the incidence of acute skin toxicity, TOMO could still decrease radiation pneumonitis in early stage breast cancer after surgery. Furthermore, other clinical results also showed that the acute adverse events related to radiotherapy in TOMO were not inferior to those in IMRT, which suggested that compared to IMRT, TOMO may achieve similar or superior target coverage and better critical organ sparing.
Limitation
We are aware of the limitations of our study. The retrospective, single-center design of this analysis might affect the interpretation of the data, and the persuasiveness of our conclusions was weaker than that of multicenter prospective studies. Moreover, although we enrolled patients strictly in accordance with the inclusion and exclusion criteria, the absence of randomization in group assignment might have led to potential selection bias.
In the present study, we focused only on eight types of adverse events, with a relatively small number of clinical indicators observed during radiotherapy. Furthermore, the primary goal of this data release is to retain as much clinical data as possible for reference and discussion among physician peers. Based on the above considerations, we did not apply strict multiple test correction methods, such as the Bonferroni correction, to adjust the p-values. Instead, we only performed limited statistical methods, such as t-test and chi-square analysis, which might increase the risk of false positives in the statistical results.
Due to the lack of experience in our work, we failed to precisely match the occurrence of adverse events with the corresponding follow -up periods while implementing the research plan. We only summarized the outcomes after the three-month follow-up was completed, which hindered our understanding of how time influenced the development of adverse events during or after radiotherapy. In other words, we were unable to leverage weekly follow-up data using longitudinal models to evaluate toxicity trajectories over time.
It is worth mentioning that a 3-month follow-up might be too short to capture the late effects of radiation therapy. Because we only focused on short-term complications at the initial stage of the study, a relatively short follow-up period was designed accordingly. Consequently, only acute toxicity complications were reported in our study, while long-term prognosis, including late effects and survival analysis results, were lacking due to the short follow-up duration.
Lastly, the relatively simplistic and limited statistical methods, such as the T-test and Chi-square analysis, employed in the study may compromise the persuasiveness of the results and weaken the robustness of the conclusions.
Future perspectives call for continued efforts to conduct more extensive studies with longer follow -up periods. In the next phase of our study, we will extend the follow-up period to 3 years and shift our focus to late adverse events and survival analysis to gain a better understanding of survival and long -term toxicity outcomes and provide valuable clinical research data and experience for radiotherapy treatment after breast cancer surgery.
Conclusion
Compared with IMRT, TOMO decreases the incidence of radiation pneumonitis but fails to improve acute skin toxicity. The present experience of applying TOMO in radiotherapy for early stage breast cancer suggests that, with the exception of pneumonitis, it may not be conducive to decreasing acute toxicity adverse events in early stage breast cancer after lumpectomy or mastectomy. Based on our research, TOMO may contribute to higher odds of acute skin toxicity, which should be considered by clinicians. Clinicians should consider the balance between the benefits and risks of TOMO . However, long-term follow-up is needed to perform in order to assess chronic toxicity and survival outcomes after TOMO in early stage breast cancer.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Army Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YX: Writing – review & editing, Writing – original draft. Y-CY: Supervision, Writing – review & editing, Software, Investigation. H-QR: Writing – review & editing, Validation, Data curation, Supervision. Y-ZW: Data curation, Writing – review & editing, Investigation. Q-FLi: Writing – review & editing, Investigation, Software. G-RY: Writing – original draft, Methodology, Data curation, Investigation. Y-kL: Writing – original draft, Software, Data curation. K-CJ: Writing – original draft, Data curation, Methodology. Y-YY: Writing – original draft, Methodology, Data curation, Investigation. Q-FLu: Software, Investigation, Data curation, Methodology, Writing – original draft. Z-HB: Supervision, Writing – review & editing. TZ: Writing – review & editing, Validation, Supervision. J-QL: Validation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors acknowledge the support of the Army Medical University Library.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1654609/full#supplementary-material
Supplementary File 1 | Detailed protocols of treatment plan about TOMO and IMRT.
References
1. Bray F, Laversanne M, Weiderpass E, and Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer-Am Cancer Soc. (2021) 127:3029–30. doi: 10.1002/cncr.33587
2. Guida F, Kidman R, Ferlay J, Schuz J, Soerjomataram I, Kithaka B, et al. Global and regional estimates of orphans attributed to maternal cancer mortality in 2020. Nat Med. (2022) 28:2563–72. doi: 10.1038/s41591-022-02109-2
3. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
4. Meattini I, Becherini C, Caini S, Coles CE, Cortes J, Curigliano G, et al. International multidisciplinary consensus on the integration of radiotherapy with new systemic treatments for breast cancer: European Society for Radiotherapy and Oncology (ESTRO)-endorsed recommendations. Lancet Oncol. (2024) 25:e73–83. doi: 10.1016/S1470-2045(23)00534-X
5. De Rose F, Carmen DSM, Lucidi S, Ray CR, Marino L, Cucciarelli F, et al. Dose constraints in breast cancer radiotherapy. A Crit review Radiother Oncol. (2025) 202:110591. doi: 10.1016/j.radonc.2024.110591
6. Bourgier C, Aimard L, Bodez V, Bollet MA, Cutuli B, Franck D, et al. Adjuvant radiotherapy in the management of axillary node negative invasive breast cancer: a qualitative systematic review. Crit Rev Oncol Hemat. (2013) 86:33–41. doi: 10.1016/j.critrevonc.2012.09.010
7. Bathily T, Borget I, Rivin DCE, and Rivera S. Bourgier C. Partial versus whole breast irradiation: Side effects, patient satisfaction and costs. Cancer Radiother. (2019) 23:83–91. doi: 10.1016/j.canrad.2018.06.020
8. Darby SC, McGale P, Taylor CW, and Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. (2005) 6:557–65. doi: 10.1016/S1470-2045(05)70251-5
9. Chadha M, Vongtama D, Friedmann P, Parris C, Boolbol SK, Woode R, et al. Comparative acute toxicity from whole breast irradiation using 3-week accelerated schedule with concomitant boost and the 6.5-week conventional schedule with sequential boost for early-stage breast cancer. Clin Breast CANCER. (2012) 12:57–62. doi: 10.1016/j.clbc.2011.09.002
10. Scorsetti M, Alongi F, Fogliata A, Pentimalli S, Navarria P, Lobefalo F, et al. Phase I-II study of hypofractionated simultaneous integrated boost using volumetric modulated arc therapy for adjuvant radiation therapy in breast cancer patients: a report of feasibility and early toxicity results in the first 50 treatments. Radiat Oncol. (2012) 7:145. doi: 10.1186/1748-717X-7-145
11. Meattini I, Poortmans PM, Aznar MC, Becherini C, Bonzano E, Cardinale D, et al. Association of breast cancer irradiation with cardiac toxic effects: A narrative review. JAMA Oncol. (2021) 7:924–32. doi: 10.1001/jamaoncol.2020.7468
12. Haussmann J, Corradini S, Nestle-Kraemling C, Bolke E, Njanang F, Tamaskovics B, et al. Recent advances in radiotherapy of breast cancer. Radiat Oncol. (2020) 15:71. doi: 10.1186/s13014-020-01501-x
13. Xiang M, Chang DT, and Pollom EL. Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer-Am Cancer Soc. (2020) 126:3560–8. doi: 10.1002/cncr.32938
14. Uhl M, Sterzing F, Habl G, Schubert K, Holger H, Debus J, et al. Breast cancer and funnel chest. Comparing helical tomotherapy and three-dimensional conformal radiotherapy with regard to the shape of pectus excavatum. Strahlenther Onkol. (2012) 188:127–35. doi: 10.1007/s00066-011-0022-y
15. Meattini I, Marrazzo L, Saieva C, Desideri I, Scotti V, Simontacchi G, et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: long-term results of the randomized phase III APBI-IMRT-florence trial. J Clin Oncol. (2020) 38:4175–83. doi: 10.1200/JCO.20.00650
16. Marrazzo L, Meattini I, Simontacchi G, Livi L, and Pallotta S. Updates on the APBI-IMRT-florence trial (NCT02104895) technique: from the intensity modulated radiation therapy trial to the volumetric modulated arc therapy clinical practice. Pract Radiat Oncol. (2023) 13:e28–34. doi: 10.1016/j.prro.2022.05.010
17. Levegrun S, Pottgen C, Jawad JA, Berkovic K, Hepp R, and Stuschke M. Megavoltage computed tomography image guidance with helical tomotherapy in patients with vertebral tumors: analysis of factors influencing interobserver variability. Int J Radiat Oncol. (2013) 85:561–9. doi: 10.1016/j.ijrobp.2012.04.010
18. Crop F, Pasquier D, Baczkiewic A, Dore J, Bequet L, Steux E, et al. Surface imaging, laser positioning or volumetric imaging for breast cancer with nodal involvement treated by helical TomoTherapy. J Appl Clin Med Phys. (2016) 17:200–11. doi: 10.1120/jacmp.v17i5.6041
19. Freites-Martinez A, Santana N, Arias-Santiago S, and Viera A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). (2021) 112:90–2. doi: 10.1016/j.ad.2019.05.009
20. Hijal T, Fournier-Bidoz N, Castro-Pena P, Kirova YM, Zefkili S, Bollet MA, et al. Simultaneous integrated boost in breast conserving treatment of breast cancer: a dosimetric comparison of helical tomotherapy and three-dimensional conformal radiotherapy. Radiother Oncol. (2010) 94:300–6. doi: 10.1016/j.radonc.2009.12.043
21. Chira C, Kirova YM, Liem X, Campana F, Peurien D, Amessis M, et al. Helical tomotherapy for inoperable breast cancer: a new promising tool. BioMed Res Int. (2013) 2013:264306. doi: 10.1155/2013/264306
22. Franco P, Zeverino M, Migliaccio F, Cante D, Sciacero P, Casanova BV, et al. Intensity-modulated and hypofractionated simultaneous integrated boost adjuvant breast radiation employing statics ports of tomotherapy (TomoDirect): a prospective phase II trial. J Cancer Res Clin. (2014) 140:167–77. doi: 10.1007/s00432-013-1560-8
23. Zwicker F, Klepper R, Hauswald H, Hoefel S, Raether L, Huber PE, et al. Helical tomotherapy of lymph node-negative early-stage breast cancer after breast-conserving surgery: long-term results. Anticancer Res. (2023) 43:2041–53. doi: 10.21873/anticanres.16365
24. Hauswald H, Schempp M, Liebig P, Hoefel S, Debus J, Huber PE, et al. Long-term outcome after helical tomotherapy following breast conserving surgery for ductal carcinoma In Situ. Technol Cancer Res T. (2024) 23:2060960561. doi: 10.1177/15330338241264847
25. Thomas SJ and Hoole AC. The effect of optimization on surface dose in intensity modulated radiotherapy (IMRT). Phys Med Biol. (2004) 49:4919–28. doi: 10.1088/0031-9155/49/21/005
26. Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. (2008) 26:2085–92. doi: 10.1200/JCO.2007.15.2488
27. Wojcieszynski AP, Olson AK, Rong Y, Kimple RJ, and Yadav P. Acute toxicity from breast cancer radiation using helical tomotherapy with a simultaneous integrated boost. Technol Cancer Res T. (2016) 15:257–65. doi: 10.1177/1533034615574387
28. Cheek D, Gibbons JP, Rosen II, and Hogstrom KR. Accuracy of TomoTherapy treatments for superficial target volumes. Med Phys. (2008) 35:3565–73. doi: 10.1118/1.2952362
29. Cherpak A, Studinski RC, and Cygler JE. MOSFET detectors in quality assurance of tomotherapy treatments. Radiother Oncol. (2008) 86:242–50. doi: 10.1016/j.radonc.2007.10.025
30. Tournel K, Verellen D, Duchateau M, Fierens Y, Linthout N, Reynders T, et al. An assessment of the use of skin flashes in helical tomotherapy using phantom and in-vivo dosimetry. Radiother Oncol. (2007) 84:34–9. doi: 10.1016/j.radonc.2007.06.003
31. Klepper R, Hofel S, Botha U, Kohler P, and Zwicker F. Dosimetric effects of swelling or shrinking tissue during helical tomotherapy breast irradiation. A phantom study J Appl Clin Med Phys. (2014) 15:382–91. doi: 10.1120/jacmp.v15i4.4873
32. Giorgia N, Antonella F, Alessandro C, Eugenio V, and Luca C. Planning strategies in volumetric modulated are therapy for breast. Med Phys. (2011) 38:4025–31. doi: 10.1118/1.3598442
33. Saibishkumar EP, MacKenzie MA, Severin D, Mihai A, Hanson J, Daly H, et al. Skin-sparing radiation using intensity-modulated radiotherapy after conservative surgery in early-stage breast cancer: a planning study. Int J Radiat Oncol. (2008) 70:485–91. doi: 10.1016/j.ijrobp.2007.06.049
34. Lee TF, Chang CH, Chi CH, Liu YH, Shao JC, Hsieh YW, et al. Utilizing radiomics and dosiomics with AI for precision prediction of radiation dermatitis in breast cancer patients. BMC Cancer. (2024) 24:965. doi: 10.1186/s12885-024-12753-1
Keywords: breast cancer, toxicity, adverse event, intensity-modulated radiation therapy, tomotherapy
Citation: Xia Y, Yang Y-C, Ren H-Q, Wang Y-Z, Li Q-F, Yu Y-Y, Yang G-R, Li Y-k, Jin K-C, Luo Q-F, Bian Z-H, Zeng T and Li J-Q (2025) Comparison of adverse events between intensity-modulated radiation therapy and tomotherapy for early stage breast cancer: a retrospective cohort study. Front. Oncol. 15:1654609. doi: 10.3389/fonc.2025.1654609
Received: 26 June 2025; Accepted: 29 September 2025;
Published: 21 October 2025.
Edited by:
Jie Hao, Southeast Colorado Hospital, United StatesReviewed by:
Yi Kang, Washington University in St. Louis, United StatesPeng Fan, Northwestern University, United States
Copyright © 2025 Xia, Yang, Ren, Wang, Li, Yu, Yang, Li, Jin, Luo, Bian, Zeng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Qing Li, bGlqdW5xaW5nMjU5Nzc1QHRtbXUuZWR1LmNu
 Yan Xia
Yan Xia Yan-Cheng Yang2
Yan-Cheng Yang2 Qing-Feng Li
Qing-Feng Li Qi-Fa Luo
Qi-Fa Luo