- 1Department of Radiation Oncology, Dartmouth Hitchcock Medical Center, Hanover, NH, United States
- 2Boston Scientific, Marlborough, MA, United States
- 3Department of Radiation Oncology, University of Washington School of Medicine, Seattle, WA, United States
- 4Balor College of Medicine, Radiation Oncology, Houston, TX, United States
Introduction: Rectal spacers (RS), when used in prostate cancer (PCa) patients treated with radiotherapy (RT), reduce radiation dose to the rectum. While RS incur additional upfront cost, they may result in long-term cost-savings by reducing toxicity-related adverse events and associated medical costs. This study examined long-term pattern of insurer-paid healthcare costs among patients with and without polyethylene glycol hydrogel RS use.
Methods: Men with PCa who received RT during 2015–2020 were identified from Medicare 5% and Merative™ MarketScan Commercial data. Multivariable generalized linear models assessed the association between RS utilization and total costs from 1-year prior to RT to 4- years after RT, controlling for age, comorbidity, RT modality, secondary cancer, baseline dysfunction, data source, year of RT, and state. Analyses were stratified by payer type (Medicare, commercial) and cost type (overall, those for specific conditions).
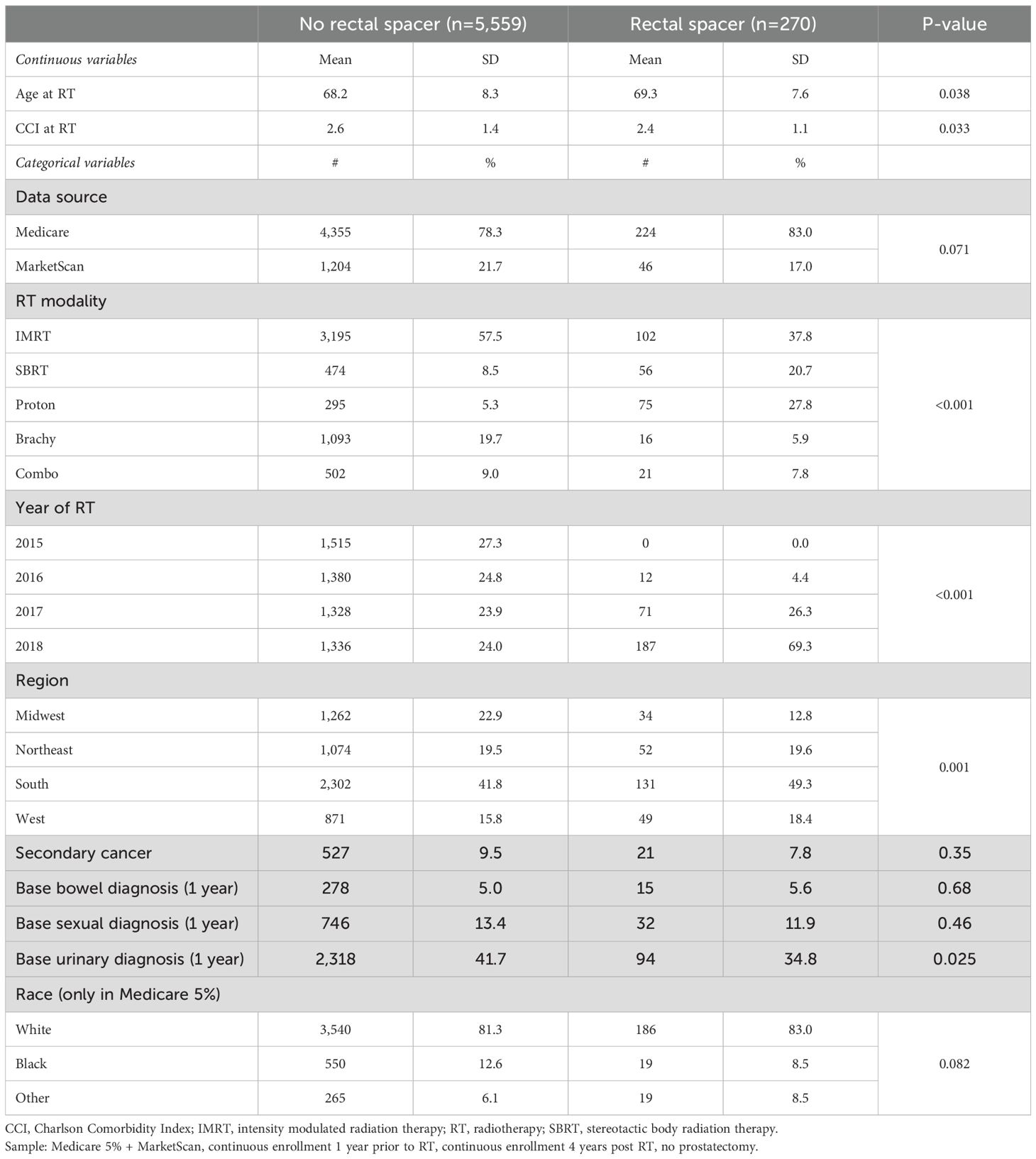
Results: The analysis included 5,829 individuals, 270 (4.6%) of whom received RS. After controlling for covariates, costs 1-year pre-RT were significantly higher for RS patients by +$1,811 ($17,378 vs. $15,567, p=0.023), as were costs for RT (including RS) at the time of treatment by +$3,949 ($31,712 vs. $27,763, p<0.001). However, total insurer paid costs over the following 4 years post-RT were significantly lower for RS patients by $8,095 ($52,345 vs. $60,440, p=0.011). Similar patterns were observed when examining costs related to bowel, sexual, or urinary dysfunction separately.
Conclusions: Patients with RS use undergoing PCa RT had significantly lower long-term overall healthcare costs despite incurring higher initial costs prior to and during RT, suggesting that upfront investment in RS may be offset by long-term savings for insurers.
1 Introduction
Radiotherapy (RT) is a common and effective treatment for localized prostate cancer (PCa) (1, 2) but it is known to potentially cause long-term rectal, urinary, and sexual toxicity (3, 4); including diarrhea, rectal bleeding, proctitis, urinary obstruction, incontinence, urethral strictures, and erectile dysfunction (5–8). Phase III trials have indicated similar toxicity profiles across common types of external beam radiation therapy including intensity-modulated RT (IMRT), stereotactic body RT (SBRT), and proton beam RT (9, 10). Attempts to mitigate rectal toxicity include image guidance, more precise RT techniques (11), and/or using temporary rectal spacers (RS) to increase the distance between the rectum and the prostate during RT (12–15). The use of RS among PCa patients has surged in recent years, rising from 4% in 2017 to 28% in 2021, and reaching 47% among SBRT patients in 2021 (16).
RS may reduce overall long-term healthcare costs by reducing toxicity-related complications (13, 17–19). The use of RS may also provide benefits beyond toxicity-related cost reduction, potentially influencing healthcare utilization and minimizing declines in quality of life (QOL) (13, 20, 21). The use of RS does incur additional cost at the time of RT delivery. Some studies have also reported that the use of RS may result in rare but severe toxicities, potentially leading to higher costs (22, 23). Economic evaluations of the use of RS to date have involved simulated models and focused solely on toxicity-related cost projections (24–26). Given the potential for RS to reduce healthcare costs through reduced toxicity and via other benefits, a more thorough economic analysis of healthcare utilization and costs using real-world data is needed. Therefore, this study evaluated patterns of overall long-term healthcare costs among patients with and without polyethylene glycol (PEG) hydrogel RS use from a payer perspective.
2 Materials and methods
2.1 Study type and patient population
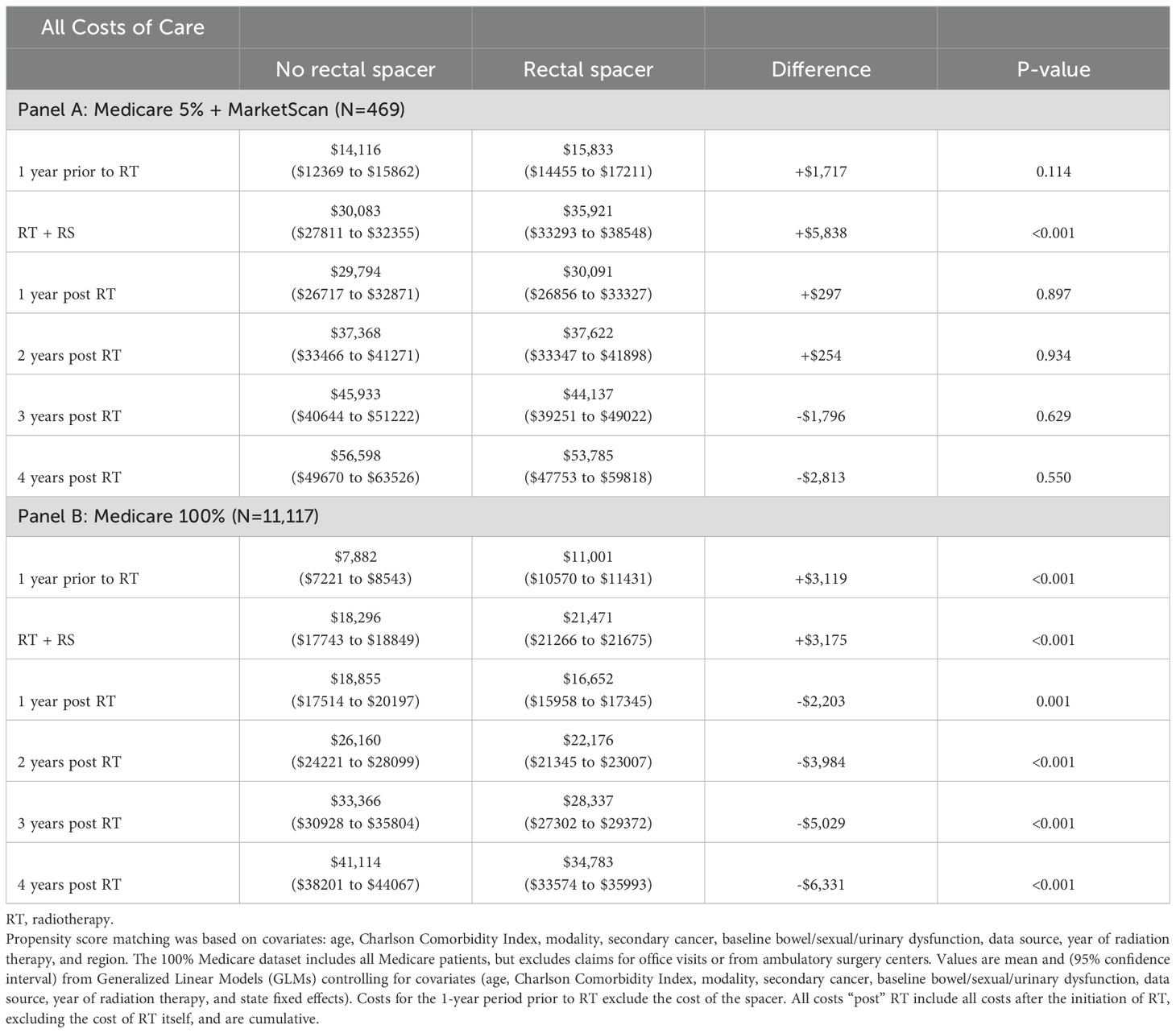
This was a retrospective cohort study that utilized the Medicare 5% Standard Analytic Files (SAF) and the Merative™ MarketScan® Commercial Database. Included were men with PCa who received RT (IMRT, SBRT, proton beam RT, brachytherapy, or any combination of these) between 2015 and 2020. Patients were required to be aged 65+ years if they were from the Medicare data and 18–64 years if they were from the MarketScan data. Only those with continuous enrollment from one year prior to RT to four years after RT were included; those undergoing prostatectomy were excluded. Medicare 100% SAF data were used for robustness analyses. While the 5% Medicare dataset used in the main analysis randomly samples five percent of all Medicare patients for all care settings, the 100% Medicare dataset includes all Medicare patients. However, the 100% Medicare sample excludes claims for office visits or from ambulatory surgery centers. Because of the retrospective nature and the use of de-identified claims data, consent and IRB approval were not required.
2.2 Outcomes and covariates
The primary outcome was total insurer-paid healthcare costs 1 year prior to RT, during RT (including costs associated with RS use), and up to 4 years after RT, comparing patients with and without RS. The measurement of costs was consistent across different datasets and was inflation-adjusted for 2023. Costs specifically related to bowel, sexual, or urinary dysfunction were also examined using diagnosis and procedure codes to identify them (9). A secondary outcome was the average number of medical visits (clinic visits) per year overall and specifically for bowel/sexual/urinary dysfunction.
Measures used as covariates in adjusted models included patient age, Charlson Comorbidity Index (CCI) (27) based on claims prior to RT, RT modality (IMRT, SBRT, proton beam RT, brachytherapy, or combination), the year of RT, the presence of secondary cancers (colon, rectal, bladder), bowel/sexual/urinary dysfunction at baseline (one year prior to RT), data source (Medicare 5% vs. MarketScan), and state fixed effect.
The clinical benefit of RS placement may vary by RT modality due to differences in dose distribution, treatment geometry, and proximity of adjacent organs. For example, SBRT delivers higher dose per fraction and may benefit more from RS in reducing rectal toxicity, whereas brachytherapy’s confined dose delivery might reduce the relative incremental value of RS. To account for these differences, RT modality was included as a covariate in all models.
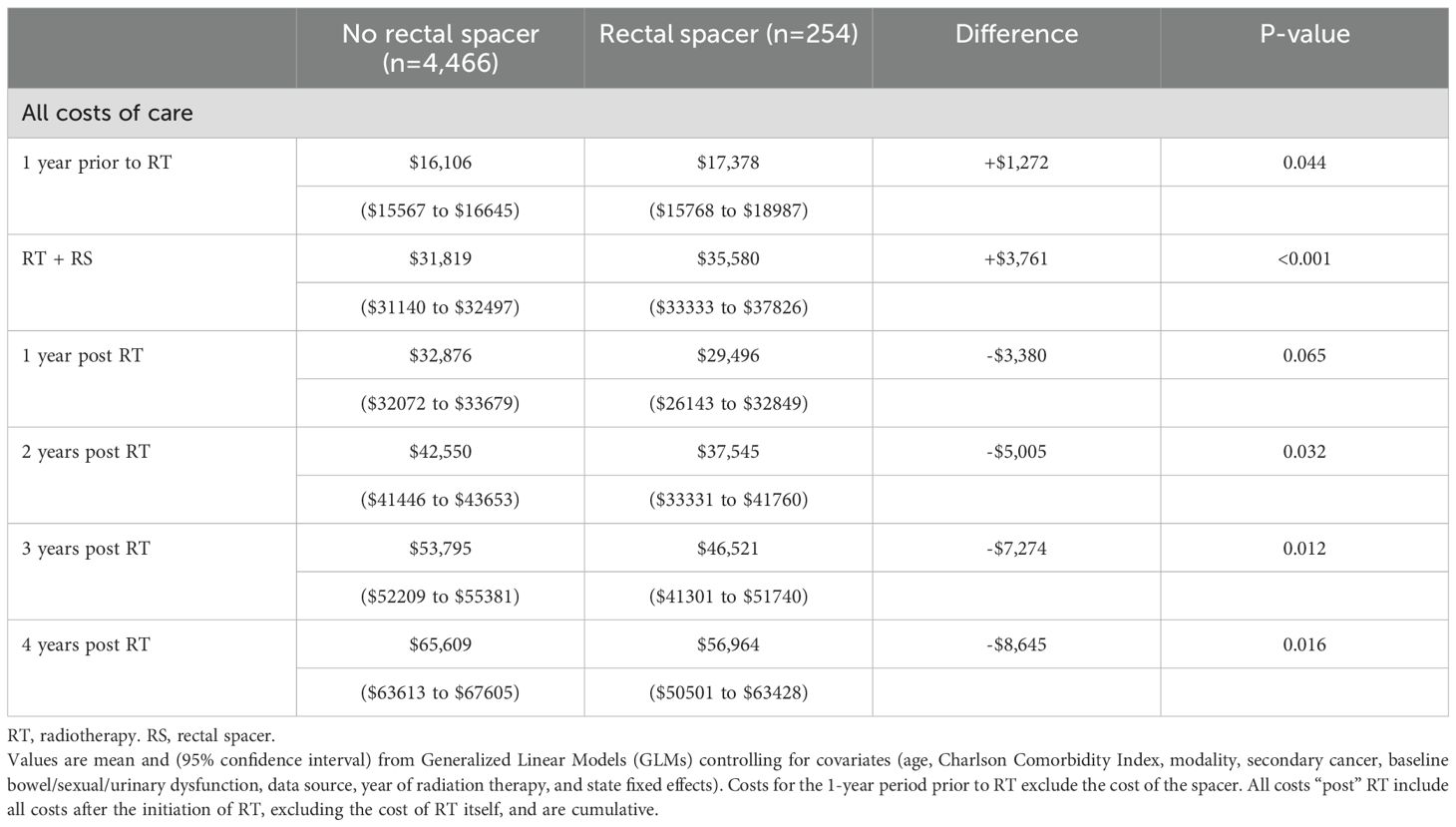
Two sets of robustness analyses were conducted. The first robustness analysis excluded patients who underwent brachytherapy, as they were less likely to receive RS and may have higher catheter use, which could influence overall costs. The second robustness check was conducted with an additional covariate to indicate the use of androgen deprivation therapy (ADT) after PCa diagnosis as a surrogate for cancer risk, since higher-risk patients may incur greater costs and may be less likely to use a rectal spacer, as they were not included in the phase III trials. This information was available only in MarketScan data.
2.3 Statistical analyses
A Generalized Linear Model (GLM) with gamma distribution and log-link assessed cost differences, adjusting for covariates mentioned above. GLM regressions were also performed using propensity score–matched (PSM) samples, with patients matched on the same set of covariates described above. Results using PSM is supplemental to the main results as the number of observations included in the analysis drops substantially. Post-hoc robustness analyses were performed using a sample from the Medicare 100% SAF to examine whether the lack of significance found in certain differences in the original analysis was due to small sample sizes. Poisson regression was used to evaluate the number of medical visits, adjusting for the same covariates. All analyses were performed using the Instant Health Data (IHD) software (Panalgo, Boston, MA, USA) and Stata 18.0 (StataCorp LLC, Texas, USA).
3 Results
3.1 Patient population
A total of 5,829 PCa patients were identified as undergoing RT, including 270 (4.6%) who received RS. Those receiving RS were slightly older (69.3vs. 68.2, p=0.038) with a lower CCI (2.4 vs. 2.6, p=0.03) than those treated with RT without RS. The race distribution among the Medicare population (the only patients for whom race data were available) was not statistically significantly different between groups. Patients who received RS were more likely to undergo SBRT (20.7% vs. 8.5%, p<0.001) and less likely to receive IMRT (37.8% vs. 57.5%, p<0.001, Table 1) than those treated without RS. Patients who underwent brachytherapy had the lowest likelihood of receiving RS at 1.4% (table not shown).
3.2 Costs
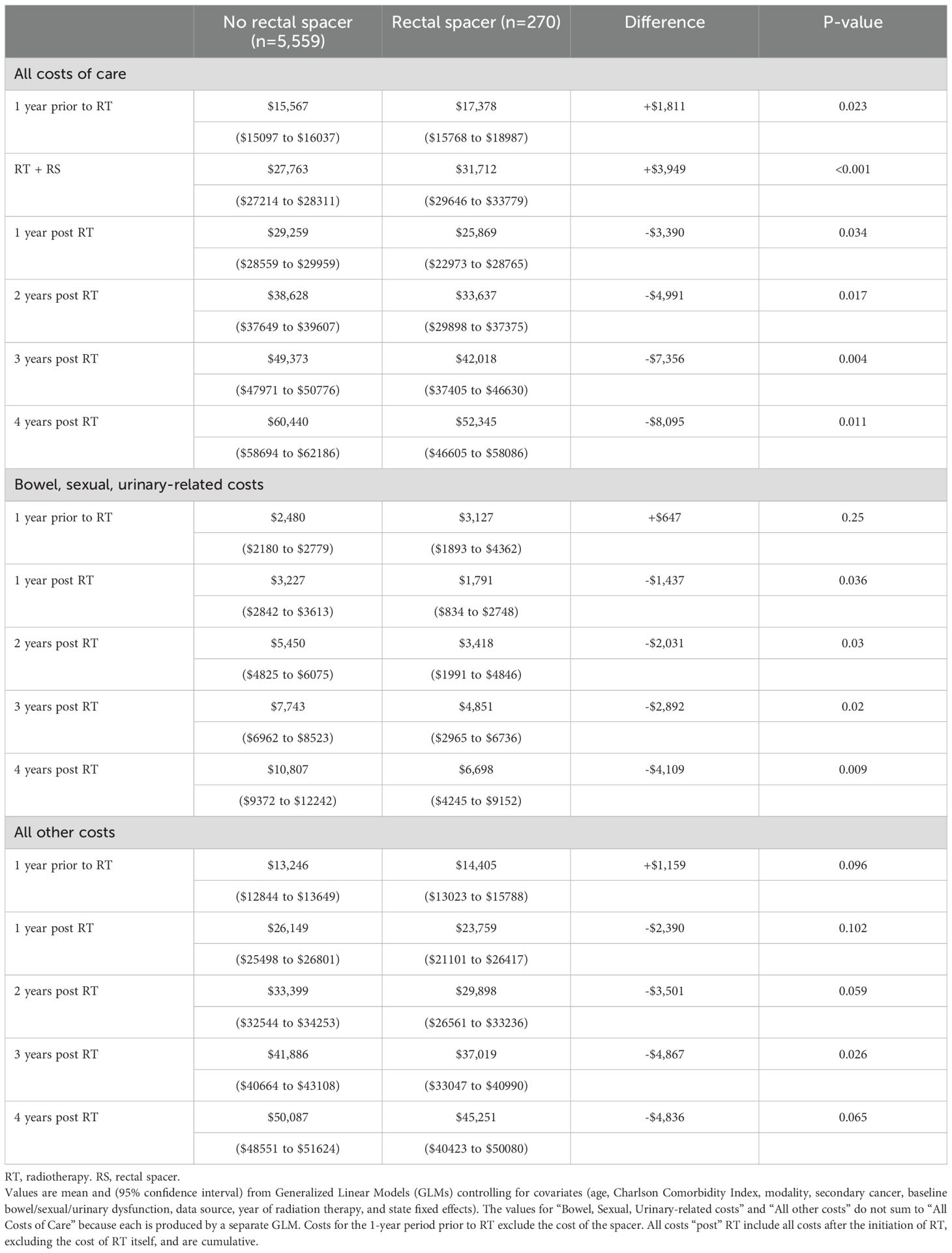
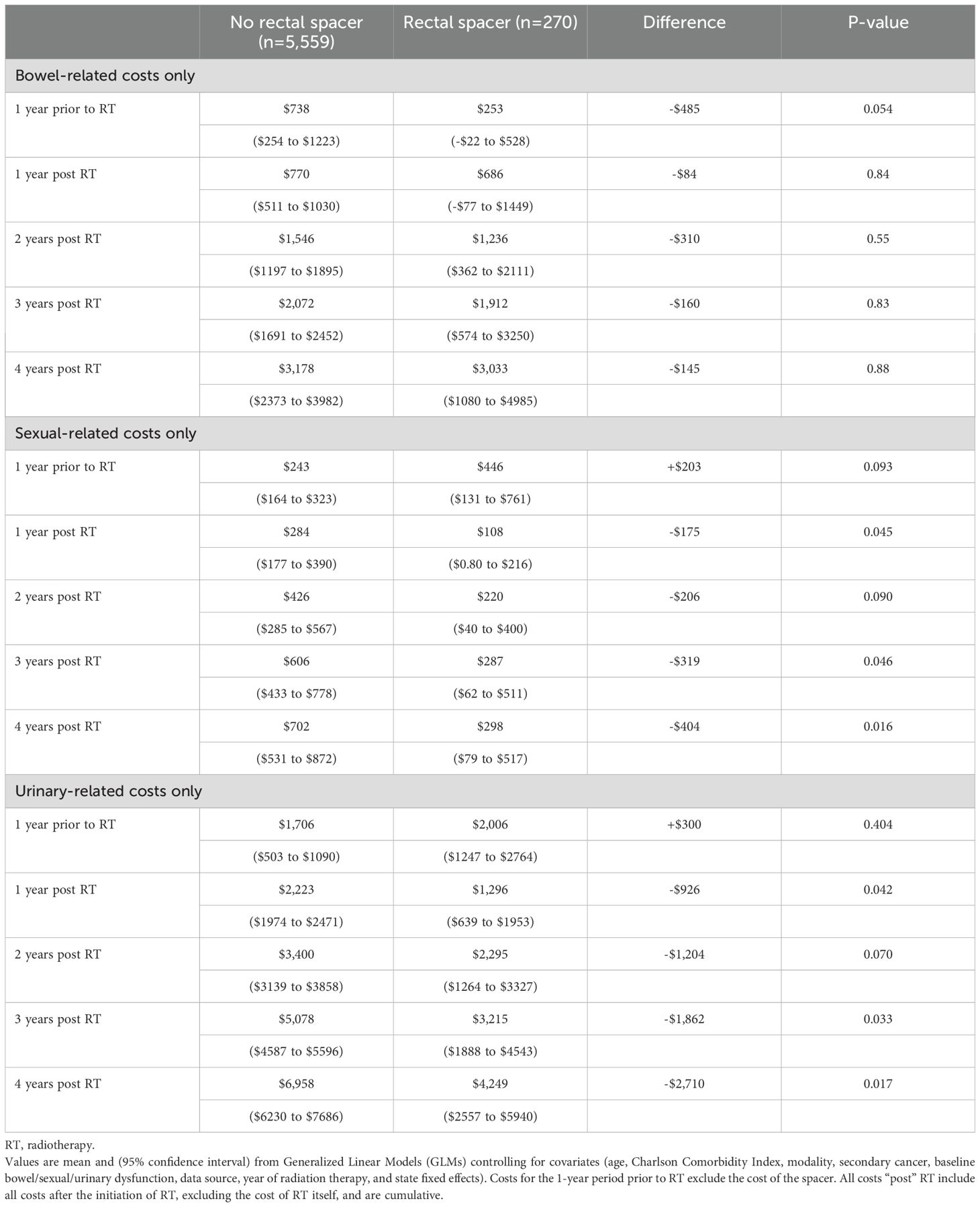
After controlling for covariates in the regression, estimated costs during the 1-year period pre-RT were significantly higher for RS patients by +$1,811 ($17,378 vs. $15,567, p=0.023), as were costs for RT, including costs associated with RS use, by +$3,949 ($31,712 vs. $27,763, p<0.001). However, over four years post-RT total insurer-paid costs were significantly lower for RS patients by -$8,095 ($52,345 vs. $60,440, p=0.011, Table 2, Figure 1 Panel A). The pattern of lower costs for those treated with RS was consistent after applying PSM. However, the results were not statistically significant in the Medicare 5% and MarketScan datasets, likely due to the small sample size (Table 3). Alternatively, the findings were both consistent and statistically significant when using the Medicare 100% dataset. Given that RS use was lowest among patients who underwent brachytherapy, Table 4 presents total cost of care results excluding these patients, demonstrating the consistency of the findings. Furthermore, the result was robust and consistent even after the use of ADT was controlled for in the regression analyses in MarketScan data (Supplementary Table 1). Costs specific to bowel/sexual/urinary-related conditions were -$4,109 lower at 4-years post-RT in those with RS as compared to those without ($10,807 versus $6,698, p=0.009, Table 2).
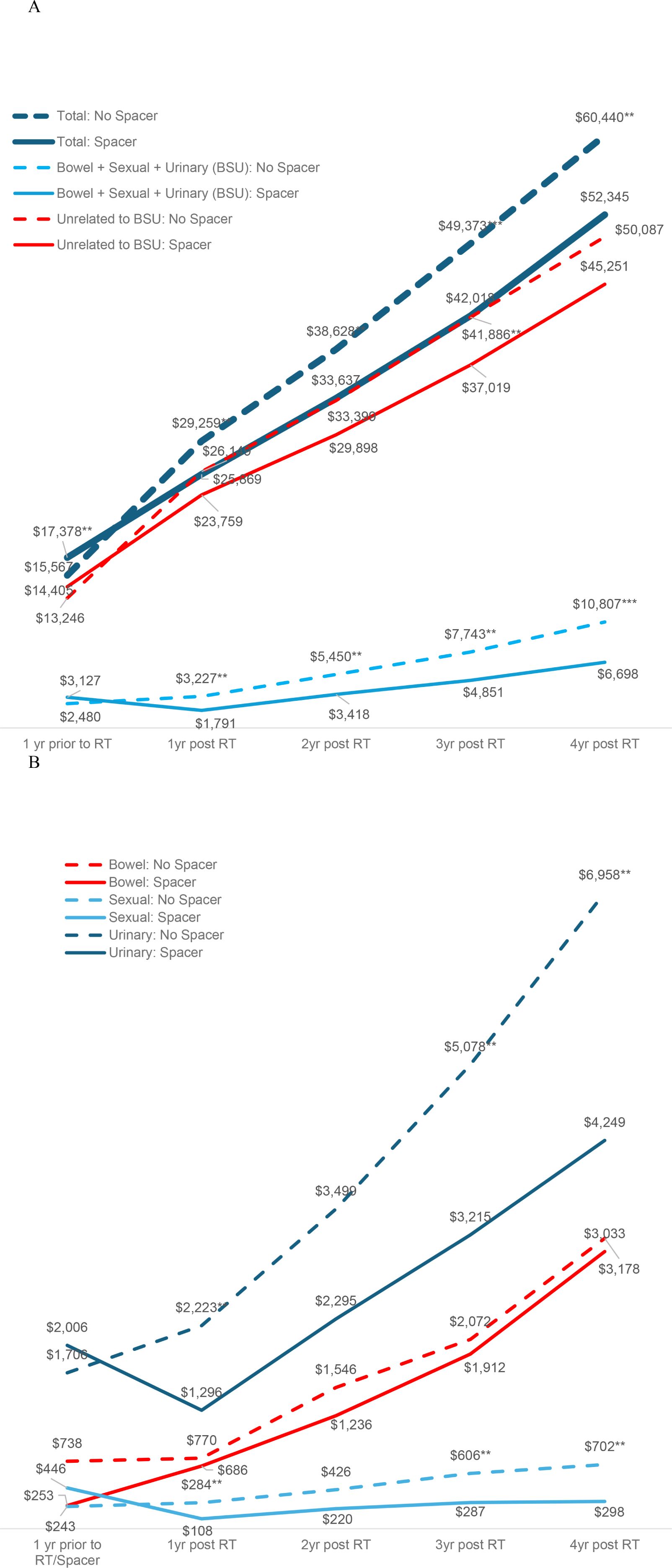
Figure 1. Costs of care over time by use of a rectal spacer during radiotherapy. Panel (A) Total costs, costs related to bowel/sexual/urinary dysfunction, and other unrelated costs. Panel (B) Costs of Care for Bowel, Sexual, or Urinary Dysfunction.
The pattern in bowel/sexual/urinary-related costs was primarily driven by urinary-related costs that were insignificantly higher among patients with RS prior to RT ($2,006 vs. $1,706, p=0.404) but significantly lower at 3- and 4-years post RT ($3,215 vs. $5,078, p=0.033 at 3-years; $4,249 vs. $6,958, p=0.017 at 4-years, Table 5, Figure 1 Panel B). Sexual-related costs followed a similar pattern but with a smaller cost difference; there were higher costs among RS patients prior to RT ($446 vs. $243, p=0.093) but lower costs post RT ($287 vs. $606, p=0.046 at 3-years; $298 vs. $702, p=0.016 at 4-years). Bowel-related costs were also lower among RS patients post RT, but not significantly different from non-RS patients ($3,033 vs. $3,178, p=0.884 at 4-years). However, when costs were examined using the larger Medicare 100% sample, which included 114,777 PCa patients treated without RS and 8,740 treated with RS, total costs were significantly lower at 4-years in those treated with RS by -$3,092 ($38,523 vs. $35,431, p<0.001 at 4-years), and bowel-related costs were also significantly lower at 2- and 4-years post RT among patients who received RS ($617 vs. $823, p=0.022 at 2-years; $1,345 vs. $1,649, p=0.030 at 4-years, Supplementary Table 2).
When analyzed separately for each RT modality, patients who received RS generally had higher costs one year prior to RT and during RT (including the cost of the RS). However, four-year post-RT costs were lower, although the difference was not statistically significant when broken down by individual RT modality (Supplementary Figure 1, Panel A). Given the modest sample sizes, the analysis was repeated using Medicare 100% data and patients with RS use had significantly lower costs four years post-RT among those who underwent SBRT, IMRT, or a combination of multiple RT modalities (Supplementary Figure 1, Panel B).
The overall cost difference was most pronounced among commercially-insured (MarketScan) patients. The cost difference at 4 years among MarketScan patients was -$34,995 ($48,210 for patients with RS vs. $83,205 for those without, p<0.001, Supplementary Table 3), while a similar but not significant difference was observed among Medicare patients (-$4,284 lower for patients with RS, p=0.17). Within MarketScan patients, the cost savings associated with the use of RS occurred at each of the 4 years post-RT (1-year post-RT = -$22,477, p=0.001; 2-years post-RT = -$27,055, p<0.001; 3-years post-RT = -$31,813, p<0.001; 4-years post-RT = -$34,995, p<0.001; Supplementary Table 3). It should be noted that in general, costs for each time period and group were typically 1.5 to 3 times higher in MarketScan patients than in Medicare patients.
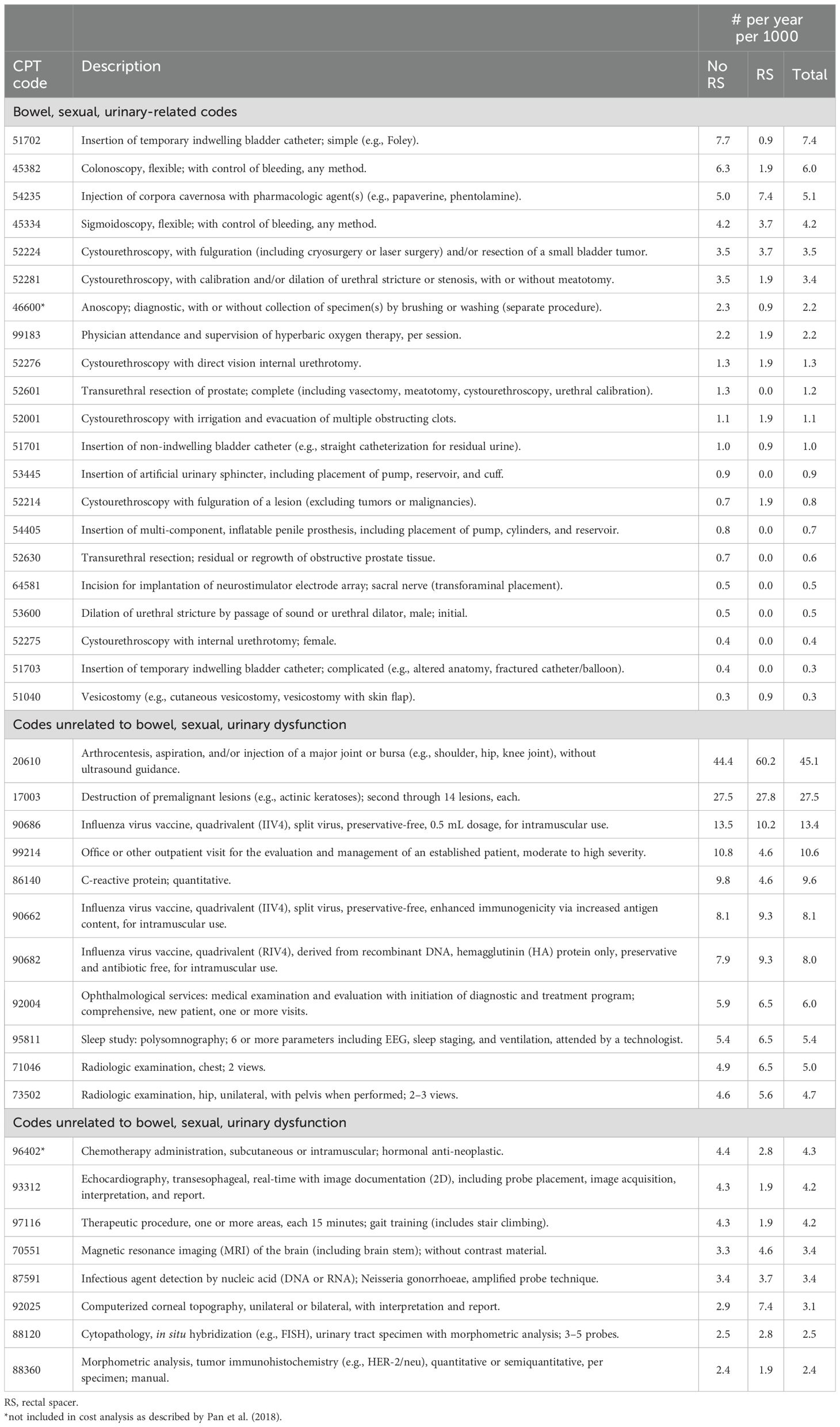
3.3 Procedures
The common procedures related to bowel/sexual/urinary dysfunction during the 4-year period post-RT are enumerated in Table 6 and Supplementary Table 4, with the most common procedures with the greatest differences noted for “Insertion of temporary indwelling bladder catheter” (7.7 without RS vs. 0.9 with RS per 1000 person-years), “Colonoscopy, flexible; with control of bleeding, any method” (6.3 without RS vs. 1.9 with RS per 1000 person-years), and “Injection of corpora cavernosa with pharmacologic agent(s)” (5.0 without RS vs. 7.4 with RS per 1000 person-years). Overall, there were fewer bowel- and urinary-related procedures observed among RS patients during the 4-year post-RT period: 10.2 with RS vs 14.9 without RS per 1000 person-years for bowel-related procedures (difference -4.7 per 1000 person-years); 17.6 with RS vs 27.5 without RS per 1000 person-years for urinary-related procedures (difference -9.9 per 1000 person-years), while the number of sexual-related procedures was low for both RS and non-RS patients: 7.4 with RS vs 6.8 without RS per 1000 person/years (difference +0.6 per 1000 person-years) (Supplementary Table 4b). Two CPT codes presented in Table 6—46600 and 96402—were not included in the primary cost analysis, which strictly adhered to the categorization defined by Pan et al. (2018), and were therefore initially classified as “codes unrelated to bowel, sexual, or urinary dysfunction.” However, code 46600 was subsequently reclassified as a “bowel, sexual, urinary-related code,” specifically within the bowel-related domain, based on clinical judgment. In contrast, code 96402 was retained in the “unrelated” category despite its potential relevance to the sexual domain, as it represents part of the initial treatment rather than an intervention addressing post-radiotherapy dysfunction.
3.4 Clinic visits
The annual average number of clinic visits was similar during the 1-year period prior to RT (32.97 vs 32.98, p=0.99), but over the 4 years of follow-up, patients with RS averaged 0.87 fewer visits per year than patients without RS (29.6 versus 28.8, p=0.018, Supplementary Table 5). This difference included significantly fewer urinary-related visits (1.42 versus 1.27, p=0.036) and visits unrelated to bowel/sexual/urinary conditions (27.7 versus 27.0, p=0.042).
4 Discussion
This analysis of real-world data from 1 year prior to and 4 years after RT with or without RS provides a robust sample to analyze health-related costs from the payer perspective. To date, three different RS devices have been approved by the FDA for use during prostate RT, with available phase III trials data limited to follow-up durations of 33, 6, and 6 months, respectively. All 3 devices demonstrated substantial reductions in radiation dose delivered to critical normal structures in the pelvis with a focus on rectal sparing (13–15). However, the long-term cost-benefit of these devices has been less robustly analyzed. Of note, the primary analysis group presented in this study – consisting of 270 patients treated with RT and RS and followed for 4 years – provides twice the cumulative follow-up of all three prospective phase III trials combined for patients with RS. Furthermore, the expanded analysis using the Medicare 100% population, which includes 8,740 patients treated with RT and RS and followed for 4-years, represents a >60-fold increased cumulative follow-up.
In this observational analysis of Medicare and commercial insurance data from 2015 to 2020, patients who received rectal spacers (RS) had lower overall healthcare costs over the 4-year period following RT. This was observed despite showing that the costs of RT (including RS) as well as costs during the 1-year period prior to RT were significantly higher among patients who received RS. Lower overall healthcare costs following RT among the RS cohort were consistently observed across multiple analyses (1): using PSM to reduce selection bias in RS use (2), excluding patients who underwent brachytherapy—who were least likely to receive RS and may have higher catheter use—and (3) controlling for ADT use as a proxy for cancer severity in the MarketScan sample. Cost differences were observed in both commercial and Medicare populations; however, they were larger in commercial populations, which may be partly due to higher costs generally in commercial versus Medicare plans (28, 29). It is notable that, despite the high costs billed to commercial insurance, commercially insured RS patients incurred post-RT expenses comparable to those of Medicare RS patients (e.g., $40,268 for Medicare vs. $39,478 for MarketScan over three years post-RT; $50,692 for Medicare vs. $48,210 for MarketScan over four years post-RT). However, the number of observations for MarketScan RS patients was small (n=46), requiring cautious interpretation. As such, these findings should be considered exploratory and not definitive.
Other studies have attempted to estimate the cost-effectiveness of the use of RS, but all used models or simulation instead of real-world data and focused solely on toxicity-related costs without assessing overall costs. Studies by Levy et al. and by Venneste et al. used Markov Models with 5-year horizons. These studies estimated higher incremental costs for patients using RS over the 5-year period ($3578 and €1540, respectively) (24, 26). However, the costs considered post-RT were limited. For example, Venneste et al. considered lab tests, medication for diarrhea, transfusions, sigmoidoscopies, and specialist consultations only, depending on level of toxicity (26). Levy et al. considered post-RT costs associated with remission, intestinal/urinal toxicity, and erectile dysfunction (24). In an additional analysis, Hutchinson et al. estimated an incremental cost of RS of only $518 over 10-years following IMRT using a decision tree model, but also considered only costs related to toxicity (25). It is notable that even without considering all costs, Hutchinson et al. found the use of RS for patients receiving dose escalated SBRT to be immediately cost-effective due to savings from reduced toxicity. In contrast, the current study provides real-world evidence from national Medicare and commercial claims databases, encompassing all-cause insurer-paid costs over a 5-year window (1 year pre-RT and 4 years post-RT). This broader scope includes not only bowel, urinary, and sexual dysfunction-related costs, but also general medical expenses that may reflect downstream effects of treatment-related morbidity or patient frailty. While prior models assumed a constrained cost impact from RS based on toxicity outcomes alone, our findings demonstrate that RS use was consistently associated with lower long-term healthcare costs—particularly among commercially insured patients—even when accounting for the higher upfront cost of the device and RT delivery. This comprehensive cost accounting underscores the potential for RS to yield economic value beyond toxicity mitigation, a contribution that simulation models to date have not been able to fully capture.
The primary analysis performed here analyzed all health care costs for those treated with RT with and without RS to provide the most comprehensive and unbiased approach. Planned secondary analyses broke down by costs most likely to be related to prostate cancer and/or treatment (bowel, urinary, or sexual events) as compared to other costs less likely to be directly related to prostate cancer or treatment. Interestingly, and somewhat unexpectedly, the greatest difference in bowel, urinary, and sexual costs was for urinary costs. In the Medicare 5% samples (which is a much smaller population of only 5,839 men but which covers all billing and coding costs), total costs, costs related to urinary/bowel/sexual side effects, and unrelated costs were all lower in those with RS (although non-related costs were only lower through 3 years). When broken down further, both urinary and sexual costs were lower in those with RS while bowel costs were not. We hypothesized that one reason that bowel costs were not significantly lower could be the much smaller sample size in the Medicare 5% random sample as compared to the 100% Medicare data set. The larger Medicare 100% dataset includes 114,777 patients with RT without RS and 8,744 patients with RS, but this dataset has less complete coverage of billing codes as neither office visits nor surgical center codes are captured (Supplementary Table 2). In this larger Medicare data, all costs, bowel costs, and urinary costs were all lower with the use of RS, with the total cost difference after 4-years (-$3,092) comparable to the added cost of RS at the time of RT (+$3,131). The observed lower total cost over 4-years in the Medicare 100% data set (-$3,092) is also notable in that this group of patients who later were treated with RS and RT had utilized substantially greater resources in the year prior to RT (+$2,998), as such, the reduction at 4 years may actually have been significantly greater than the observed reduction by almost 50% (-$3,092 reduced cost at 4 years and +$2,998 greater cost prior to RT = difference -$6,090).
The current study adds to the literature in this space by using real-world data and considering all costs, in addition to examining costs reasonably attributed to RT and/or RS toxicity. This study observed differences not just in costs attributable to dysfunction that may be related to bowel/sexual/urinary toxicity, but in other and unrelated costs as well as overall costs. It is unclear why costs for services not directly related to bowel/urinary/sexual dysfunction were higher following RT in those who did not have RS as compared to those with had RT with RS. One possibility is that greater treatment-related side-effects such as those related to bowel/urinary/sexual dysfunction post-RT led to more frequent medical visits and a corresponding increase in other costs not typically associated with PCa or the toxicity directly attributable to treatment. Another explanation for higher costs in non-RS patients is that despite adjustment by age and comorbid conditions, that the non-RS cohort was enriched with patients who were more likely to fit a “high need” Medicare phenotype (i.e., those with heart disease, skilled nursing facility or home care, hospitalizations, and Alzheimer’s disease and related dementias with functional dependency) (30). However, the finding of lower healthcare costs for patients without RS placement in the year prior to RT somewhat refutes that explanation. One would expect a “high need” phenotype to have had higher healthcare costs in the year prior to RT in addition to the years after.
Beyond cost considerations, the use of rectal spacers has been associated with improvements in patient-reported quality of life (QoL). Phase III trial data and pooled prospective studies have demonstrated that RS placement reduces bowel toxicity, which translates into meaningful long-term QoL benefits, particularly in bowel and urinary domains. For example, Hamstra et al. reported improved sexual QoL outcomes in patients receiving intensity-modulated radiation therapy (IMRT) with a rectal spacer compared to those without (21). Similarly, Pham et al. showed favorable long-term patient-reported QoL metrics following SBRT in patients who received hydrogel spacers (20). While QoL outcomes were not directly measured in our claims-based study, these prior findings suggest that the reduction in toxicity-related complications observed here likely confers parallel benefits in patient experience and daily functioning. Future real-world studies integrating clinical and patient-reported outcomes would help validate these relationships more directly.
The strengths of this study include the use of two large national datasets that cover all ages 18 years and over and that provide real-world data with 4 years of follow-up. Also, in addition to considering costs specific to rectal, urinary, and sexual toxicity, this study examines overall costs. Limitations of this study include those inherent to the nature of claims-based analyses. Namely, claims reflect billing and are subject to human error in coding and/or completeness. And, while these data allow for the examination of association, inferences on causation cannot be made. In particular, there is the potential for unmeasured confounding and selection bias, as the decision to use a rectal spacer was not randomized and may have been influenced by clinical judgment, patient preferences, tumor characteristics, provider experience, or institutional protocols. These factors - many of which are not captured in claims data - may correlate with both the likelihood of RS utilization and downstream healthcare utilization or cost. Although measurable confounders were adjusted including age, comorbidity, baseline dysfunction, and RT modality, and conducted propensity score matching and sensitivity analyses, residual confounding cannot be excluded. Future studies with more granular clinical data or randomized designs would be needed to better isolate the causal impact of RS use on long-term outcomes. Finally, the retrospective design may introduce selection bias, as patients were not randomly assigned to receive RS.
Despite these limitations, this study found that although patients undergoing PCa RT with RS had higher baseline healthcare costs, they subsequently experienced significantly lower overall healthcare costs over the 4 years after RT, even after accounting for the added cost of the RS. Some of these differences may be due to underlying patient selection factors that could not be captured in claims data and thus not controlled in the analyses. In addition, this claims-based analysis cannot directly account for reduced toxicity and improved QOL that has been demonstrated with the use of RS. Nonetheless, these findings highlight the potential beneficial economic impact of RS placement from a payer perspective.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Data are not available for sharing because of data-use agreements with CMS and Merative. However, data can be made available to reasonable requests upon the approval of both entities. Requests to access these datasets should be directed to Research Data Assistance Center (ResDAC), cmVzZGFjQHVtbi5lZHU=; Merative MarketScan Research databases, aW5mb0BtZXJhdGl2ZS5jb20=.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
JY: Writing – review & editing, Supervision, Validation. RS: Formal Analysis, Project administration, Data curation, Methodology, Visualization, Conceptualization, Writing – original draft, Investigation. MF: Supervision, Writing – review & editing, Validation. SB: Writing – review & editing. EE: Writing – review & editing, Validation. DH: Writing – review & editing, Validation, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Boston Scientific.
Acknowledgments
The authors would like to acknowledge Craig Solid of Solid Research Group, LLC, for assistance in developing this manuscript.
Conflict of interest
RS, SB, and EE are employees of Boston Scientific. MF has received travel reimbursement from Varian and Boston Scientific, and his affiliated institutions have received funding for clinical trials from Boston Scientific. JY reports consulting fees from Boston Scientific, Teleflex, and AlphaSights, research support from Pfizer/Myovant/Sumitomo Pharma, and is a stockholder of Modifi Bio. DH is a paid consultant for Boston Scientific.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1654925/full#supplementary-material
References
1. Bekelman JE, Rumble RB, Chen RC, Pisansky TM, Finelli A, Feifer A, et al. Clinically localized prostate cancer: ASCO clinical practice guideline endorsement of an american urological association/american society for radiation oncology/society of urologic oncology guideline. J Clin Oncol. (2018) 36:JCO.18.00606. doi: 10.1200/JCO.18.00606
2. Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, et al. Prostate cancer, version 4.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2023) 21:1067–96. doi: 10.6004/jnccn.2023.0050
3. Brand DH, Tree AC, Ostler P, van der Voet H, Loblaw A, Chu W, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. (2019) 20:1531–43. doi: 10.1016/S1470-2045(19)30569-8
4. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. (2016) 17:1047–60. doi: 10.1016/S1470-2045(16)30102-4
5. Budäus L, Bolla M, Bossi A, Cozzarini C, Crook J, Widmark A, et al. Functional outcomes and complications following radiation therapy for prostate cancer: A critical analysis of the literature. Eur Urol. (2012) 61:112–27. doi: 10.1016/j.eururo.2011.09.027
6. Moltzahn F, Pra AD, Furrer M, Thalmann G, and Spahn M. Urethral strictures after radiation therapy for prostate cancer. Investig Clin Urol. (2016) 57:309–15. doi: 10.4111/icu.2016.57.5.309
7. Vanneste BGL, Limbergen EJV, Van Lin EN, van Roermund JGH, and Lambin P. Prostate cancer radiation therapy: what do clinicians have to know? BioMed Res Int. (2016) 2016:6829875. doi: 10.1155/2016/6829875
8. Hasterok M, Szołtysik M, Nowicka Z, Goc B, Gräupner D, Majewski W, et al. Rectum and bladder toxicity in postoperative prostate bed irradiation: dose–volume parameters analysis. Cancers. (2023) 15:5334. doi: 10.3390/cancers15225334
9. Pan HY, Jiang J, Hoffman KE, Tang C, Choi SL, Nguyen QN, et al. Comparative toxicities and cost of intensity-modulated radiotherapy, proton radiation, and stereotactic body radiotherapy among younger men with prostate cancer. J Clin Oncol. (2018) 36:1823–30. doi: 10.1200/JCO.2017.75.5371
10. Yu JB, Cramer LD, Herrin J, Soulos PR, Potosky AL, and Gross CP. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol. (2014) 32:1195–201. doi: 10.1200/JCO.2013.53.8652
11. Tree AC, Alexander EJ, As NJV, Dearnaley DP, and Khoo V. Biological Dose Escalation and Hypofractionation: What is There to be Gained and How Will it Best be Done? Clin Oncol. (2013) 25:483–98. doi: 10.1016/j.clon.2013.05.003
12. Pinkawa M. Current role of spacers for prostate cancer radiotherapy. World J Clin Oncol. (2015) 6:189. doi: 10.5306/wjco.v6.i6.189
13. Hamstra DA, Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat OncolBiolPhys. (2017) 97:976–85. doi: 10.1016/j.ijrobp.2016.12.024
14. Mariados NF, Orio PF, Schiffman Z, Van TJ, Engelman A, Nurani R, et al. Hyaluronic acid spacer for hypofractionated prostate radiation therapy. JAMA Oncol. (2023) 9:511–8. doi: 10.1001/jamaoncol.2022.7592
15. Song D, Dabkowski M, Costa P, Nurani R, Kos M, Vanneste B, et al. Prospective, randomized controlled pivotal trial of biodegradable balloon rectal spacer for prostate radiation therapy. Int J Radiat OncolBiolPhys. (2024) 120:1410–20. doi: 10.1016/j.ijrobp.2024.07.2145
16. Hankins R, Morris K, McGovern A, Collins SP, and Yu JB. Examining rectal spacer use in prostate cancer radiation therapy by treatment modality: insights from medicare claims data. Int J Radiat OncolBiolPhys. (2024) 120:e757–8. doi: 10.1016/j.ijrobp.2024.07.1663
17. Seymour ZA, Hamstra DA, Daignault-Newton S, Bosch W, Michalski J, Gay HA, et al. Long-term follow-up after radiotherapy for prostate cancer with and without rectal hydrogel spacer: a pooled prospective evaluation of bowel-associated quality of life. BJU Int. (2020) 126:367–72. doi: 10.1111/bju.15097
18. Teyateeti A, Grossman C, Kollmeier MA, Fiasconaro M, Hopkins M, McBride S, et al. Influence of hydrogel spacer placement with prostate brachytherapy on rectal and urinary toxicity. BJU Int. (2022) 129:337–44. doi: 10.1111/bju.15572
19. Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, Beyer D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat OncolBiolPhys. (2015) 92:971–7. doi: 10.1016/j.ijrobp.2015.04.030
20. Pham HH, Jean-Baptiste S, Newman NB, Shinohara ET, Shinde A, and Kirschner AN. Long-term patient reported quality of life outcomes after moderately hypofractionated and stereotactic prostate radiation therapy with perirectal hydrogel spacer. Int J Radiat OncolBiolPhys. (2024) 120:S172. doi: 10.1016/j.ijrobp.2024.07.2207
21. Hamstra DA, Mariados N, Sylvester J, Shah D, Gross E, Hudes R, et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: Secondary analysis of a phase 3 trial. Pr Radiat Oncol. (2018) 8:e7–15. doi: 10.1016/j.prro.2017.07.008
22. McLaughlin MF, Folkert MR, Timmerman RD, Hannan R, Garant A, Hudak SJ, et al. Hydrogel spacer rectal wall infiltration associated with severe rectal injury and related complications after dose intensified prostate cancer stereotactic ablative radiation therapy. Adv Radiat Oncol. (2021) 6:100713. doi: 10.1016/j.adro.2021.100713
23. Millot JC, Arenas-Gallo C, Silver E, Goldman M, Picciotto S, Jia AY, et al. Major complications and adverse events related to use of spaceOAR hydrogel for prostate cancer radiotherapy. Urology. (2024) 188:94–100. doi: 10.1016/j.urology.2023.12.034
24. Levy JF, Khairnar R, Louie AV, Showalter TN, Mullins CD, and Mishra MV. Evaluating the cost-effectiveness of hydrogel rectal spacer in prostate cancer radiation therapy. Pr Radiat Oncol. (2019) 9:e172–9. doi: 10.1016/j.prro.2018.10.003
25. Hutchinson RC, Sundaram V, Folkert M, and Lotan Y. Decision analysis model evaluating the cost of a temporary hydrogel rectal spacer before prostate radiation therapy to reduce the incidence of rectal complications. Urol Oncol: Semin Orig Investig. (2016) 34:291.e19–291.e26. doi: 10.1016/j.urolonc.2016.02.024
26. Vanneste BGL, Pijls-Johannesma M, Voorde LVD, van Lin EN, van de Beek K, van Loon J, et al. Spacers in radiotherapy treatment of prostate cancer: Is reduction of toxicity cost-effective? Radiother Oncol. (2015) 114:276–81. doi: 10.1016/j.radonc.2015.01.005
27. Charlson ME, Pompei P, Ales KL, and MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
28. Lopez E, Neuman T, Jacobson G, and Levitt L. How much more than medicare do private insurers pay? A review of the literature. San Francisco, USA: KFF (2020). Available online at: https://www.kff.org/medicare/issue-brief/how-much-more-than-medicare-do-private-insurers-pay-a-review-of-the-literature/ (Accessed June 30, 2025).
29. Meiselbach MK, Wang Y, Xu J, Bai G, and Anderson GF. Hospital prices for commercial plans are twice those for medicare advantage plans when negotiated by the same insurer. Heal Aff. (2023) 42:1110–8. doi: 10.1377/hlthaff.2023.00039
Keywords: prostate cancer, radiotherapy, rectal spacers, healthcare cost, healthcare utilization
Citation: Yu JB, Sato R, Folkert MR, Bhattacharyya S, Ezekekwu E and Hamstra DA (2025) Rectal spacer use and overall long-term healthcare costs: payer perspective. Front. Oncol. 15:1654925. doi: 10.3389/fonc.2025.1654925
Received: 27 June 2025; Accepted: 25 July 2025;
Published: 12 August 2025.
Edited by:
Thomas Kole, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Nima Aghdam, New York University, United StatesVirginie Monceau, Institut de Radioprotection et de Sûreté Nucléaire, France
Copyright © 2025 Yu, Sato, Folkert, Bhattacharyya, Ezekekwu and Hamstra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryoko Sato, cnlva29zMTIyNkBnbWFpbC5jb20=
 James B. Yu
James B. Yu Ryoko Sato
Ryoko Sato Michael R. Folkert3
Michael R. Folkert3 Daniel A. Hamstra
Daniel A. Hamstra