- Department of Pathology, Chair of Oncology, Medical University of Lodz, Lodz, Poland
Background: Fibroblast growth factor receptor 2 (FGFR2) is an oncogenic driver in luminal breast cancer (BCa), with emerging evidence linking it to tumour immune microenvironment (TIME) modulation. While FGFR2’s role in endocrine resistance is established, its potential involvement in shaping immune infiltration—particularly in the transition from ductal carcinoma in situ (DCIS) to invasive ductal carcinoma (IDC)—remains underexplored.
Methods: This retrospective study analysed 99 BCa specimens collected between 2004–2019. Immunohistochemistry was used to assess FGFR2 expression and immune markers (CD8, CD68, CD163, FOXP3). Clinical and pathological variables were evaluated, and immune cell densities were compared across disease stages and BCa subtypes (luminal vs. non-luminal). Correlations between FGFR2 expression and immune markers were assessed using non-parametric statistical tests.
Results: Progression from DCIS to IDC was associated with increased infiltration by CD8+ T cells and CD68+ macrophages. FGFR2 expression showed differences between DCIS and IDC with an extensive DCIS component and was positively correlated with CD8+, CD163+, and FOXP3+ cell densities. The latter associations were exclusive to luminal A tumours, with no such correlations observed in non-luminal subtypes.
Conclusions: FGFR2 expression in luminal A BCa correlates with markers of immunosuppressive TIME, particularly CD163+ macrophages and FOXP3+ T cells. These subtype-specific interactions suggest a synergistic role of FGFR2 and estrogen receptor signalling in immune evasion and tumour progression, warranting further mechanistic and therapeutic investigation. However, the small number of cases in certain subgroups, particularly DCIS and non-luminal tumours, limits the generalizability of these findings and warrants cautious interpretation.
1 Background
Intercellular communication with the tumour microenvironment (TME) mediated by the family of highly conserved transmembrane tyrosine kinase receptors (FGFR1-4), is a well-recognized mechanism of breast cancer (BCa) progression. In particular, the FGFR2 has recently emerged as a potent oncogenic driver in the luminal BCa (1, 2), and its activation by its cognate ligands, the TME-derived fibroblast growth factors (FGFs), was shown to affect the function of steroid receptors and to promote development of resistance to endocrine therapy (3–6).
There is some pre-clinical evidence to suggest that the involvement of FGFR signalling in BCa biology might not be restricted to their FGF-induced effect on BCa cells, and that modulation of FGFRs by the stimuli derived from the tumour immune microenvironment (TIME), particularly, tumour-infiltrating lymphocytes (TILs) and tumour-associated macrophages (TAMs), contributes to tumour immune evasion and invasive progression (7–9). Reported associations of FGFR expression level with several features of an immunosuppressive microenvironment, such as decrease of T-cell infiltration, enhanced Treg survival, M2-like polarization of TAMs, and downregulating MHC expression on tumour cells, seem to support this notion (7–11).
Breast cancer progression from ductal carcinoma in situ (DCIS) to invasive ductal carcinoma (IDC) is accompanied by dynamic changes in TIME (12). While DCIS is typically characterized by low immune infiltration, a gradual increase in immune cell presence is observed during the transition to IDC. These TIME alterations are also subtype-specific: luminal breast cancers (BCa), although generally considered less immunogenic than HER2-positive or triple-negative subtypes (13–15), demonstrate progressive immune activation during IDC progression. This suggest that this increase in immunogenicity in luminal BCa may not be incidental, but rather influenced by FGFR2-mediated modulation of TIME. In this context, FGFR2 may play an active, subtype-specific role in shaping the immune landscape during tumour evolution, contributing to the transition from DCIS to IDC even in tumours with initially low baseline immune activity.
2 Methods
2.1 Patient selection and samples
This study included 99 breast cancer (BCa) specimens collected from patients treated at the Regional Oncologic Centre of the Copernicus Memorial Hospital in Łódź and the Holy Cross Cancer Centre in Kielce, Poland, between 2004 and 2019. The cohort consisted of 27 pure DCIS cases (without an invasive component, hereafter called DCIS), including 23 luminal and 4 non-luminal subtypes, as well as 72 cases with invasive ductal carcinoma with co-existing DCIS (hereafter called IDC-DCIS), comprising 47 luminal and 25 non-luminal BCa specimens. Flow chart of patient recruitment is presented on Supplementary Figure 1. Clinicopathological characterization of the cohort is presented in Supplementary Table 1. The study was approved by the Local Ethics Committee (Approval No. KE/16/21 and RNN/284/13/KE).
2.2 Collection of the clinical and pathological data
For each patient, clinical data were collected, including age at diagnosis, menopausal status, type of treatment, occurrence of relapse, disease progression, and survival outcomes (date of relapse, disease progression, death, or last follow-up). The diagnosis was established according to the 2012 World Health Organization (WHO) Classification of Breast Tumours, and the disease stage was recorded.
Pathological data included nuclear grade, mitotic index, hormonal receptor status, tumour size, surgical margin status and breast cancer phenotype. Additionally, the morphological characteristics of DCIS were obtained, including histoarchitectural pattern, nuclear grade, presence of necrosis.
2.3 Tissue specimen, immunohistochemistry
Formalin-fixed paraffin embedded (FFPE) tissue blocks with BCa postoperative specimens were collected. Each paraffin-embedded sample was sectioned into 3.5-μm-thick slices and stained with haematoxylin and eosin (H&E) for histopathological evaluation. Immunohistochemical staining for a panel of selected biomarkers (Supplementary Table 2) was performed using a protocol recommended by the manufacturer.
2.4 Evaluation of TIL, TAM and FGFR2 expression
Morphological and semi-quantitative analysis of stromal TIICs was carried out on H&E and IHC preparations using an UltraFast Scanner (Philips) with DigiPath™ software (Xerox), following the International Guidelines on TIL Assessment in Breast Cancer. The ‘hot-spots’ was defined as areas rich in TIICs adjacent to tumour cells were selected for further phenotypical analysis, i.e. cells positive for CD4, CD8, CD68, CD163, FOXP3 were counted for each tumour in four representative areas of 0.25 mm2 under magnification of 200×. Additionally, CD4/CD8 and CD68/CD163 ratios were calculated. Counting was conducted independently by two researchers (JS and AZ) and supervised by the pathologist (MB). In case of significant disparities between the scores (20 or 5 cells/mm2 for TILs or TAMs, respectively, or difference >20% of the mean value), the case was additionally reassessed by another pathologist (HRK).
FGFR2 levels were quantified according to the semiquantitative H-score approach by two independent pathologists (JS,AZ). The data were presented in 0–300 scale resulting from multiplication of percentage of positive cells by intensity of staining: 0—no staining, 1–3—increased intensity of both cytoplasmic and membrane staining (subgroups by H-score: 0–75 for negative/weak; 76–150 for moderate; 151–225 for strong; 226–300 for very strong expression). Cases from 1st tercile of H-score were regarded as FGFR2low and cases from 2nd and 3rd terciles were classified as FGFR2high.
2.5 Statistical analysis
Continuous variables were presented as medians with interquartile ranges (IQR), and categorical variables as counts with percentages in parentheses. The Shapiro–Wilk test was used to assess normality of distribution. As data presented non-normal distribution, comparisons between two groups were made using the Mann–Whitney U test, and across multiple groups using the Kruskal–Wallis test with Conover–Inman post hoc analysis. Associations between categorical variables were assessed using Pearson’s chi-squared test. Correlations between continuous variables were evaluated using Spearman’s rank correlation coefficient. Statistical analyses were performed using Statistica version 13.1 (Dell Inc., Round Rock, TX, USA). A p-value below 0.05 was considered statistically significant.
3 Results
3.1 Histopathological and immune features of DCIS and DCIS with invasive component
Of the 99 patients included, 27 (27.3%) had DCIS, and 72 (72.7%) had IDC-DCIS. There were no significant differences between DCIS and IDC-DCIS in terms of age, grade and menopausal status (Supplementary Table 1). In terms of tumour size, all cases DCIS were diagnosed as pTis, whereas in the IDC-DCIS group, there was equal distribution between pT1 and pT2 (p>0.05, Supplementary Table 1). The estrogen receptor (ER) positivity rate did not differ significantly between the groups (p=0.0862, Supplementary Table 1). In contrast, HER2 protein was more frequently detectable and showed higher abundance in DCIS compared to IDC-DCIS (p<0.0001, Supplementary Table 1) tumours. Infiltration by total TILs and TAMs, as assessed on H&E-stained sections, was higher in IDC-DCIS compared to DCIS lesions (p=0.0001 and p=0.0002, respectively, Table 1). Consistently, immunohistochemical evaluation showed increased infiltration of both CD8+ T cells and CD68+ macrophages in IDC-DCIS compared to DCIS groups (p<0.0001, Table 1). Moreover, CD68/CD163 ratio was also increased in IDC-DCIS tumours (p=0.0004, Table 1). No significant differences were observed in densities of CD163+ or FOXP3+ cells (p=0.4925 and p=0.8717, respectively, Table 1).

Table 1. Characteristics of tumour-infiltrating immune cells, FGFR2 expression and ER and HER2 status in the study group.
3.2 Relationship between immune markers and extent of DCIS compartment
Previous studies suggest that IDC with an extensive DCIS component (extensive intraductal component- EIC) is associated with a more favourable prognosis than IDC with a less extensive DCIS involvement (restricted intraductal component – RIC), supposedly representing an intermediate stage between DCIS and fully invasive carcinoma (16). Based on the extent of DCIS component within the tumour, IDC-DCIS cases were sub-grouped into two categories: (1) IDC with EIC (IDC-EIC), defined as IDC with ≥30% DCIS (n = 48), and (2) IDC without EIC (IDC-RIC), defined as IDC with <30% DCIS (n = 16). Results showed that, when this stratification of the cohort was applied, a stepwise increase of infiltration by TILs, TAMs, CD8+ T cells, and CD68+ macrophages from DCIS via IDC-EIC to IDC-RIC was observed (AKW p < 0.0001; Figures 1, 2A, B, Supplementary Table 3, Supplementary Figure 2). Interestingly, the CD68/CD163 ratio peaked in the IDC- EIC group (AKW p = 0.0026; Figure 2C, Supplementary Table 3), but neither densities of CD4+, CD163+, or FOXP3+ cells nor the CD4/CD8 ratio differed between the subsets (Supplementary Table 3). A similar association with a gradual increase of infiltration by TILs, TAMs, CD8, CD68 as well as the CD68/CD163 ratio was observed for tumour size (pTis →pT1→pT2). (Supplementary Figure 3, Supplementary Table 4).
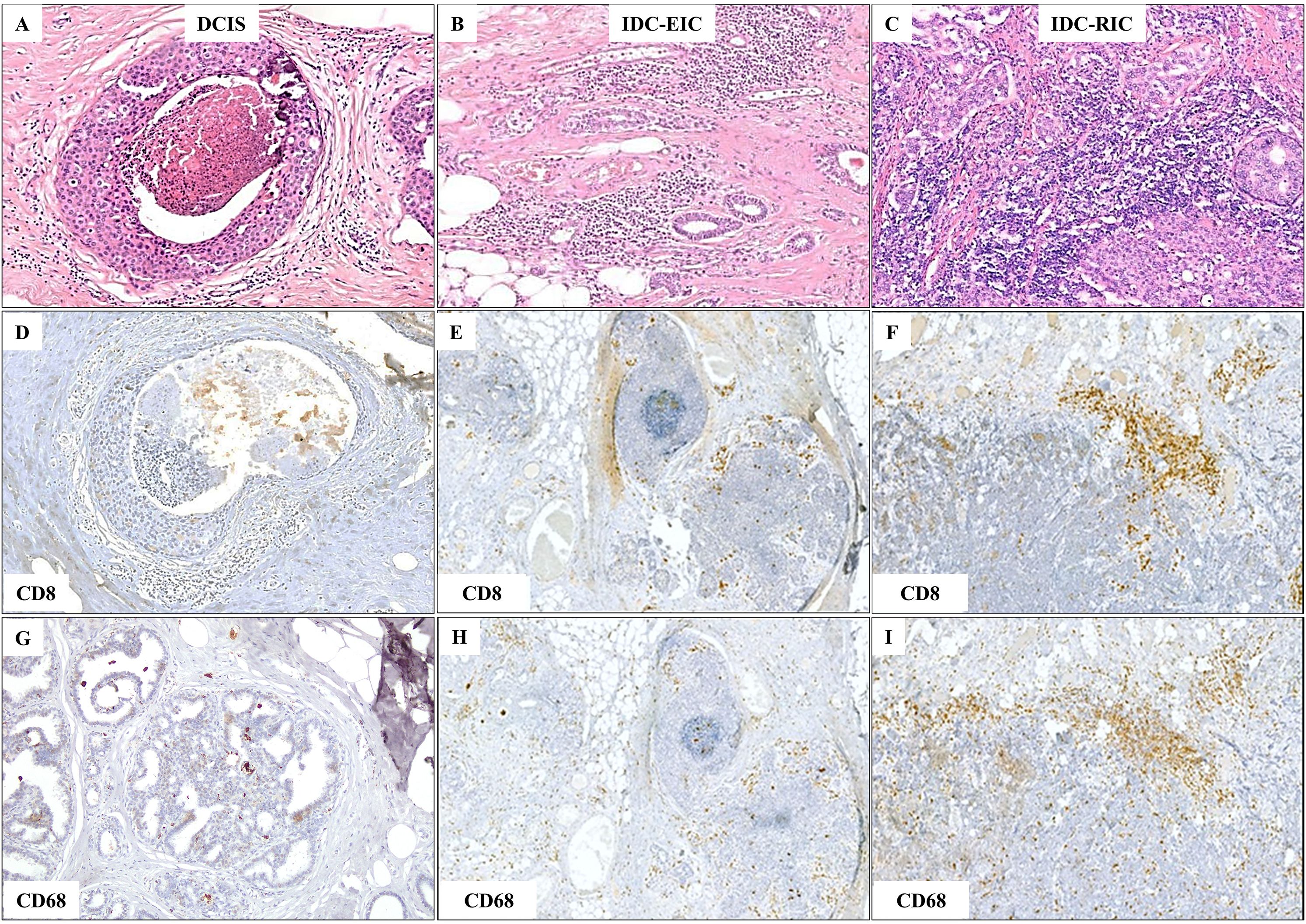
Figure 1. Gradual increase in TIL and TAM density observed in representative areas of DCIS, invasive ductal carcinoma with an extensive intraductal component (IDC-EIC), and IDC without EIC (IDC-RIC). (A–C) TILs and TAMs on H&E-stained sections in DCIS (A), IDC-EIC (B), and IDC-RIC (C). (D–F) CD8 expression in DCIS (D), IDC-EIC (E), and IDC-RIC (F). (G–I) CD68 expression in DCIS (G), IDC-EIC (H), and IDC-RIC (I).
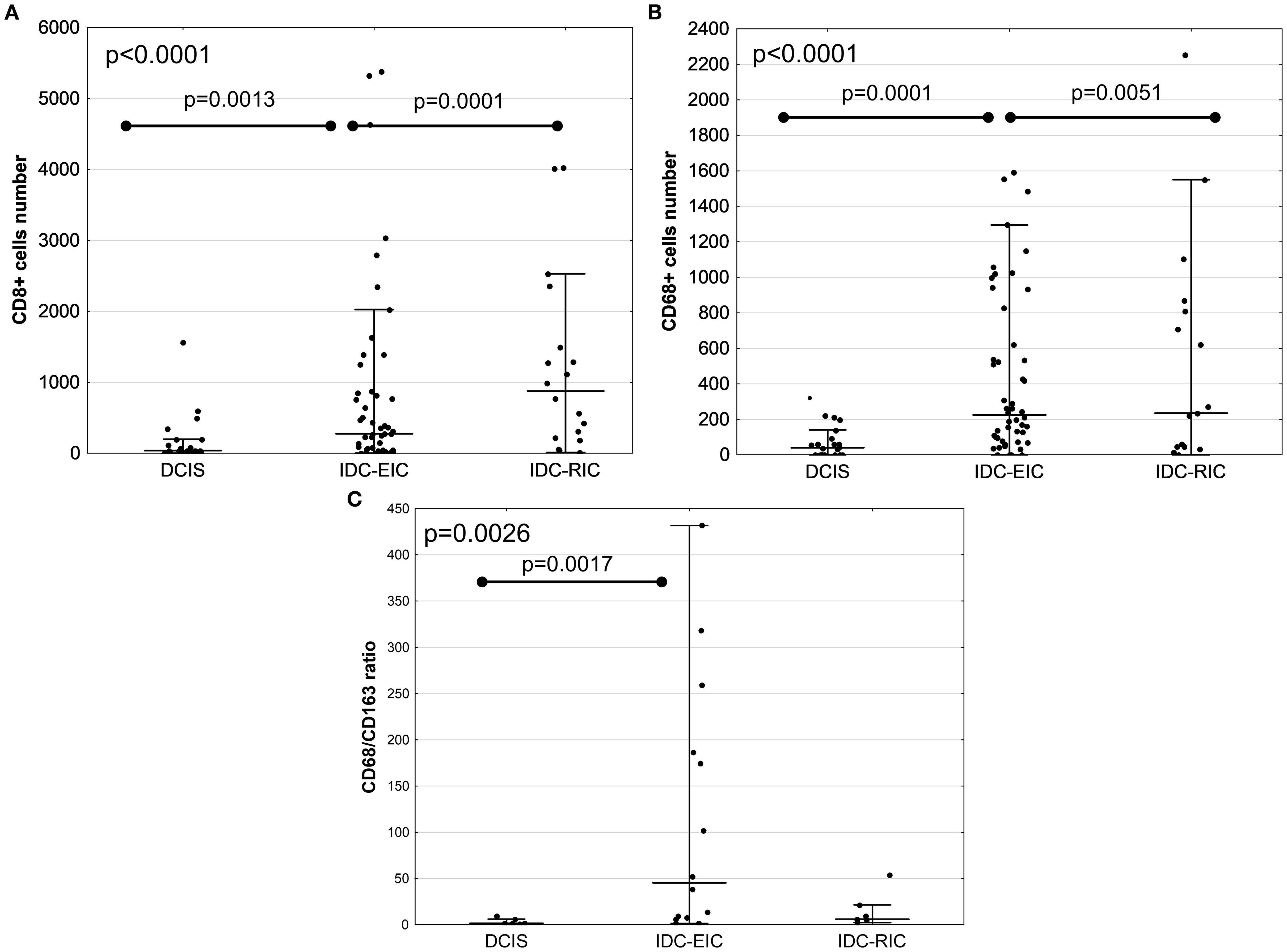
Figure 2. Comparison of immune marker infiltration between DCIS, IDC with EIC (IDC-EIC), and IDC without EIC (IDC-RIC). Infiltration by CD8+ T cells, CD68+ macrophages, and the CD68/CD163 ratio (A–C, respectively). Groups were compared using Kruskal–Wallis ANOVA test.
3.3 Correlations between immune markers and FGFR2 expression
As FGFR2 is thought to be involved in BCa progression, FGFR2 expression at specific stages of BCa evolution was investigated. FGFR2 expression in BCa cells did not differ between DCIS and IDC-DCIS tumours (p=0.8448, Table 1). However, in the subgroup analysis, the FGFR2 H-score was significantly higher in IDC-EIC cases compared to pure DCIS (p = 0.0154, Supplementary Figure 4). Next, taking into account the possible association between the FGFR2-TIME crosstalk and BCa evolution, the relationship between expression of FGFR2 on tumour cells and markers of TIME’s features was analysed in all cases (N=99) (7, 17). Although FGFR2 expression (H-score) did not correlate with total numbers of either TILs or TAMs, as measured on HE-stained sections (p=0.2067 and p=0.6086, respectively, Supplementary Table 5), when analysed in relation to specific phenotypes of infiltrating cells, positive correlations with densities CD8+ T-cells (p=0.0491, R=0.21), CD163+ macrophages (p=0.0161, R=0.27) and FOXP3+ T-cells (p=0.0233, R=0.25) (Figure 3, Supplementary Figure 5, Supplementary Table 5) were noticed. There was no association of FGFR2 with either CD4+ T-cells, CD68+ macrophages, CD68/CD163 or CD4/CD8 ratios (Supplementary Table 5).
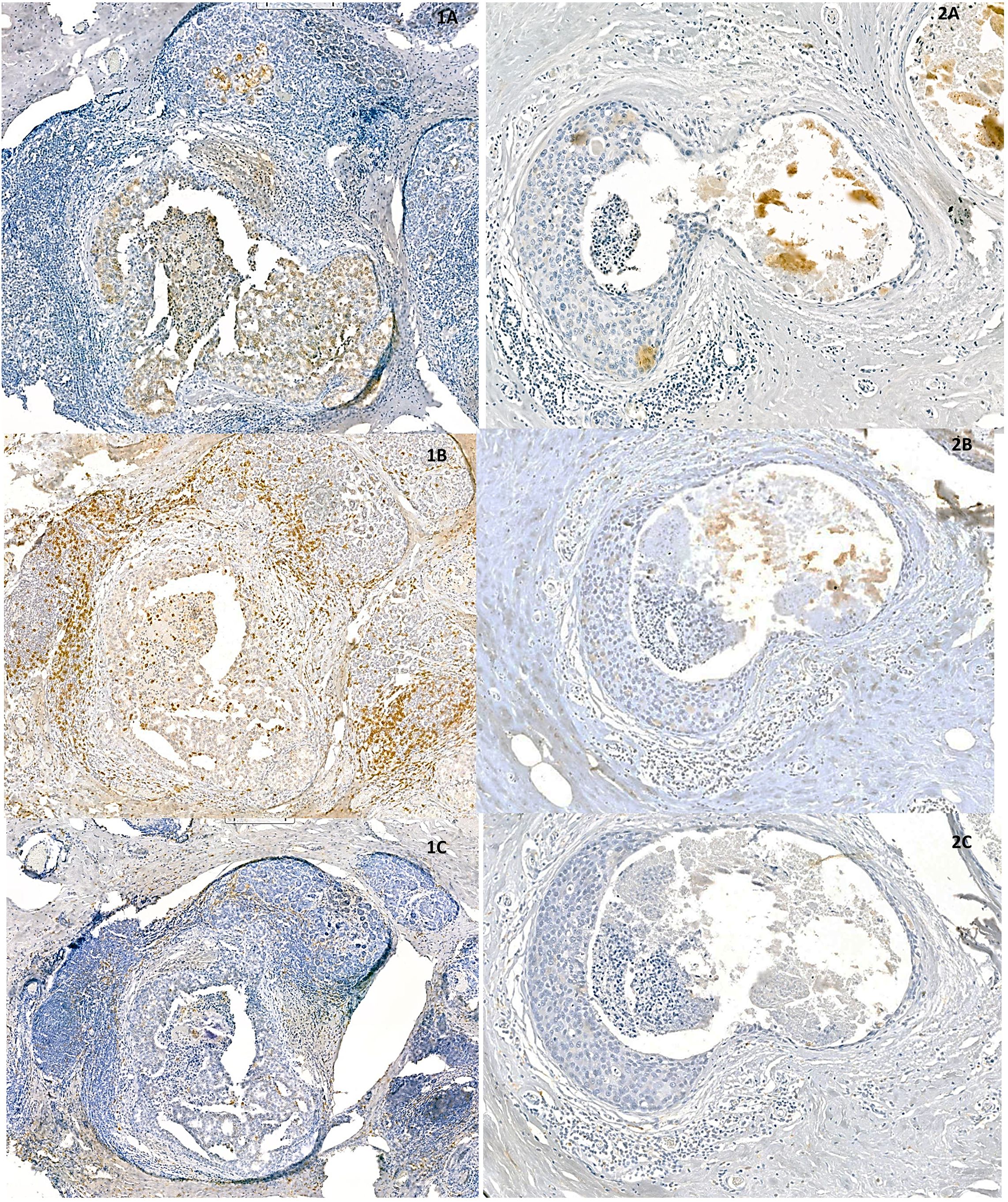
Figure 3. Representative areas of two, densely (1A–1C) and sparsely (2A–2C) infiltrated DCIS samples show positive correlation between expression of FGFR2 (1A, 2A) and densities of CD8 - positive (1B, 2B) and CD163 – positive cells (1C, 2C).
3.4 FGFR2 correlation with immune markers is restricted to ER positive patients
Taking into account the reported oncogenic role of FGFR2 in luminal A BCa (3–5), we then evaluated the relationship between FGFR2 and immune markers in relation to the ER status, i.e. in luminal A (n=70) versus non-luminal A tumours (n=29). The data showed that the correlations between FGFR2 expression and infiltration by both CD163+ TAMs (p = 0.0027, R = 0.38) and FOXP3+ regulatory TILs (p = 0.0031, R = 0.37) were observed in patients with the luminal A subtype but not in patients with non-luminal BCa (Table 2, Supplementary Figure 6). Moreover, the correlations between CD163, FOXP3 and FGFR2 (previously found in whole group -Supplementary Table 5) maintained exclusively in patients with the luminal A subtype (Table 2). These findings suggest that the immunomodulatory role of FGFR2 supporting BCa evolution may indeed be restricted to the luminal A subtype.
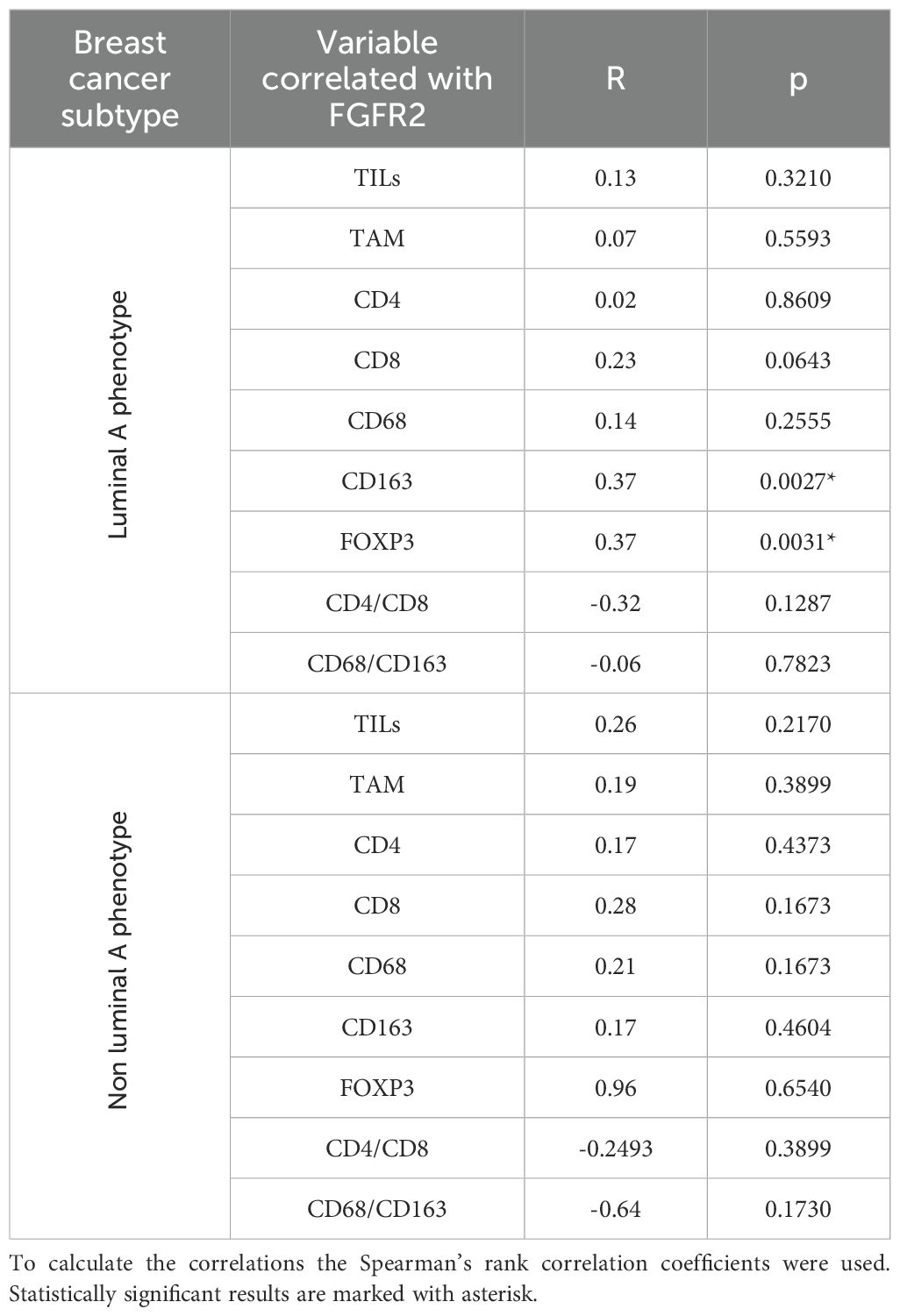
Table 2. Correlations between FGFR2 expression (H score) and immune markers in patients with and without luminal A phenotype.
4 Discussion
The present study provides an insight into a novel aspect of FGFR2 involvement in the progression of breast cancer (BCa). The analyses reveal direct correlations between FGFR2 expression and immune cell infiltration in early-stage luminal BCa specimens, that are subtype- specific and relevant particularly to estrogen receptor-positive (ER+) disease. These findings build on the established role of FGFR2 as an oncogenic driver in luminal BCa and offer new perspectives on how it may facilitate immune escape during tumour evolution.
In this context, breast cancer progression from ductal carcinoma in situ (DCIS) to invasive ductal carcinoma (IDC) was accompanied by a gradual increase in immune infiltration, particularly involving CD8+ T cells and CD68+ macrophages. These changes were consistent with previous reports and underline the stepwise immunological evolution of BCa (18). Our findings extend this knowledge by demonstrating that FGFR2 expression is positively correlated with markers of an immunosuppressive TIME, namely CD163+ macrophages and FOXP3+ regulatory T cells, and that these associations are particularly evident in luminal A tumours. Interestingly, FGFR2 expression did not correlate with the overall density of immune infiltrates but showed specificity for these key immunosuppressive cell subsets. This suggests that FGFR2 may influence (or be influenced by) the qualitative composition of the TIME rather than its overall magnitude.
Several studies have explored the immunological consequences of FGFR signalling, mainly in triple-negative breast cancer (TNBC), where FGFR activity has been linked to immune exclusion and resistance to immune checkpoint inhibitors (8, 10, 19, 20). The current work shifts the focus to luminal BCa, where the biological context in ER+ tumours differs substantially, both in terms of baseline immunogenicity and the role of FGFR2 in BCa signalling network. Here, we show that the immune effects of FGFR2 are detectable at the earliest stages of tumour development and appear to be restricted to the ER+ subtype. This supports the idea that the subtype-specific immune environment provides the setting favourable for the crosstalk between FGFR2-mediated and ER pathways to promote tumour evolution.
The subtype-specific nature of these correlations may also suggest that the immunomodulatory function of FGFR2 may depend on the functional ER signalling. This would align with previous observations that ER activation can modulate immune cell composition, including suppressing pro-inflammatory macrophage activity and influencing the accumulation of PD-1+ CD8+ T cells (21–23). In luminal tumours, FGFR2 and ER pathways may thus act synergistically to regulate selective immune cell recruitment and establish a microenvironment permissive to immune evasion. Moreover, the differential correlations observed only in luminal A tumours—despite similar levels of FGFR2 expression across subtypes—highlight the importance of hormonal context in modulating FGFR2-immune interactions.
Taken together, although the study is limited to a relatively small cohort, the findings suggest that FGFR2 expression may be associated with features of an immunosuppressive TIME in luminal BCa, and could serve as a potential biomarker for identifying this tumour microenvironment subtype. While the observed correlations between FGFR2 and CD163+ or FOXP3+ immune cells are modest, their selective presence in ER+ tumours is noteworthy and warrants further investigation. Importantly, the current data do not allow us to determine whether FGFR2 actively modulates the immune microenvironment or is itself regulated by it. Future studies will be necessary to elucidate the directionality of this interaction and to explore whether FGFR2 contributes to mechanisms of resistance to endocrine therapy. Prospective studies integrating transcriptomic and spatial profiling approaches will be instrumental in clarifying this crosstalk and may inform rational therapeutic strategies targeting both tumour-intrinsic FGFR2 signalling and the TIME.
5 Limitation of the study
A key limitation of this study is the relatively small sample size in certain subgroups, notably DCIS and non-luminal breast cancers, which reduces statistical power and may affect the robustness and generalizability of the observed associations. These findings should therefore be interpreted with caution and validated in larger, independent cohorts.
Another limitation is the absence of correction for multiple testing, which increases the risk of type I error. Given the exploratory nature of this study and the relatively small sample size, we chose to report unadjusted p-values, prioritizing biological plausibility and consistency across analyses. Nonetheless, these results should be interpreted as hypothesis-generating and validated in larger studies with appropriate statistical corrections.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Local Ethics Committee (Approval No. KE/16/21 and RNN/284/13/KE). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Written informed consent was waived as the study used archival, anonymized tissue samples and involved no risk or harm to patients.
Author contributions
JS: Visualization, Formal Analysis, Writing – review & editing, Investigation, Data curation, Writing – original draft. AZ: Investigation, Visualization, Writing – review & editing, Writing – original draft, Data curation. RK: Writing – review & editing, Writing – original draft, Supervision. HR: Funding acquisition, Supervision, Writing – review & editing, Writing – original draft, Conceptualization. MB: Conceptualization, Supervision, Writing – original draft, Funding acquisition, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Rector’s scientific grant of the Medical University of Łódź under the Internal Grant Program BRaIn, contract No. 1/2024, accounting reference No. 503/1-034-03/503-90-105. This study was also supported by a student research grants from the Medical University of Lodz and Roche, awarded as part of the institutional program promoting scientific development among young researchers.
Acknowledgments
The authors would like to express their sincere gratitude to Mateusz Bobrowski from Sysmex Polska sp. z o.o. for his invaluable assistance in the imaging of specimens using the 3DHISTECH software.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1655438/full#supplementary-material
References
1. Lei H and Deng CX. Fibroblast growth factor receptor 2 signaling in breast cancer. Int J Biol Sci. (2017) 13:1163–71. doi: 10.7150/ijbs.20792
2. Santolla MF and Maggiolini M. The FGF/FGFR system in breast cancer: oncogenic features and therapeutic perspectives. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12103029
3. Piasecka D, Braun M, Kitowska K, Mieczkowski K, Kordek R, Sadej R, et al. FGFs/FGFRs-dependent signalling in regulation of steroid hormone receptors - implications for therapy of luminal breast cancer. J Exp Clin Cancer Res. (2019) 38:230. doi: 10.1186/s13046-019-1236-6
4. Turczyk L, Kitowska K, Mieszkowska M, Mieczkowski K, Czaplinska D, Piasecka D, et al. FGFR2-driven signaling counteracts tamoxifen effect on ERalpha-positive breast cancer cells. Neoplasia. (2017) 19:791–804. doi: 10.1016/j.neo.2017.07.006
5. Piasecka D, Kitowska K, Czaplinska D, Mieczkowski K, Mieszkowska M, Turczyk L, et al. Fibroblast growth factor signalling induces loss of progesterone receptor in breast cancer cells. Oncotarget. (2016) 7:86011–25. doi: 10.18632/oncotarget.13322
6. Cerliani JP, Guillardoy T, Giulianelli S, Vaque JP, Gutkind JS, Vanzulli SI, et al. Interaction between FGFR-2, STAT5, and progesterone receptors in breast cancer. Cancer Res. (2011) 71:3720–31. doi: 10.1158/0008-5472.CAN-10-3074
7. Ruan R, Li L, Li X, Huang C, Zhang Z, Zhong H, et al. Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumour microenvironment. Mol Cancer. (2023) 22:60. doi: 10.1186/s12943-023-01761-7
8. Im JH, Buzzelli JN, Jones K, Franchini F, Gordon-Weeks A, Markelc B, et al. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nat Commun. (2020) 11:4064. doi: 10.1038/s41467-020-17914-x
9. Li C, Jiang P, Wei S, Xu X, and Wang J. Regulatory T cells in tumour microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. (2020) 19:116. doi: 10.1186/s12943-020-01234-1
10. Wu Y, Yi Z, Li J, Wei Y, Feng R, Liu J, et al. FGFR blockade boosts T cell infiltration into triple-negative breast cancer by regulating cancer-associated fibroblasts. Theranostics. (2022) 12:4564–80. doi: 10.7150/thno.68972
11. Akhand SS, Liu Z, Purdy SC, Abdullah A, Lin H, Cresswell GM, et al. Pharmacologic inhibition of FGFR modulates the metastatic immune microenvironment and promotes response to immune checkpoint blockade. Cancer Immunol Res. (2020) 8:1542–53. doi: 10.1158/2326-6066.CIR-20-0235
12. Kim M, Chung YR, Kim HJ, Woo JW, Ahn S, and Park SY. Immune microenvironment in ductal carcinoma in situ: a comparison with invasive carcinoma of the breast. Breast Cancer Res. (2020) 22:32. doi: 10.1186/s13058-020-01267-w
13. Wang Y, Zhu M, Guo F, Song Y, Fan X, and Qin G. Identification of tumour microenvironment-related prognostic biomarkers in luminal breast cancer. Front Genet. (2020) 11:555865. doi: 10.3389/fgene.2020.555865
14. Ciarka A, Piatek M, Peksa R, Kunc M, and Senkus E. Tumour-infiltrating lymphocytes (TILs) in breast cancer: prognostic and predictive significance across molecular subtypes. Biomedicines. (2024) 12(4):763. doi: 10.3390/biomedicines12040763
15. Moura T, Caramelo O, Silva I, Silva S, Goncalo M, Portilha MA, et al. Early-stage luminal B-like breast cancer exhibits a more immunosuppressive tumour microenvironment than luminal A-like breast cancer. Biomolecules. (2025) 15(1):78. doi: 10.3390/biom15010078
16. Corsi F, Albasini S, Ciciriello S, Villani L, Truffi M, Sevieri M, et al. Extensive intraductal component in breast cancer: what role in disease-free survival? J Surg Res. (2023) 283:233–40. doi: 10.1016/j.jss.2022.10.094
17. Shen L, Li Y, and Zhao H. Fibroblast growth factor signaling in macrophage polarization: impact on health and diseases. Front Immunol. (2024) 15:1390453. doi: 10.3389/fimmu.2024.1390453
18. Wang J, Li B, Luo M, Huang J, Zhang K, Zheng S, et al. Progression from ductal carcinoma in situ to invasive breast cancer: molecular features and clinical significance. Signal Transduct Target Ther. (2024) 9:83. doi: 10.1038/s41392-024-01779-3
19. Ye T, Wei X, Yin T, Xia Y, Li D, Shao B, et al. Inhibition of FGFR signaling by PD173074 improves antitumour immunity and impairs breast cancer metastasis. Breast Cancer Res Treat. (2014) 143:435–46. doi: 10.1007/s10549-013-2829-y
20. Roussot N, Lecuelle J, Dalens L, Truntzer C, and Ghiringhelli F. FGF/FGFR genomic amplification as a predictive biomarker for immune checkpoint blockade resistance: a short report. J Immunother Cancer. (2023) 11(10):e007763. doi: 10.1136/jitc-2023-007763
21. McGuinness C and Britt KL. Estrogen receptor regulation of the immune microenvironment in breast cancer. J Steroid Biochem Mol Biol. (2024) 240:106517. doi: 10.1016/j.jsbmb.2024.106517
22. Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. (2015) 21:1688–98. doi: 10.1158/1078-0432.CCR-14-0432
Keywords: breast cancer, ductal carcinoma in situ, invasive breast cancer, tumour immunemicroenvironment, tumour-infiltrating lymphocytes, tumour-associated macrophages, fibroblast growth factor receptors, FGFR2
Citation: Sołek J, Zielińska A, Kordek R, Romańska H and Braun M (2025) FGFR2 expression relates to subtype-specific tumour microenvironment (TIME) during luminal breast cancer evolution. Front. Oncol. 15:1655438. doi: 10.3389/fonc.2025.1655438
Received: 27 June 2025; Accepted: 26 August 2025;
Published: 12 September 2025.
Edited by:
Robert Wieder, Rutgers University, United StatesReviewed by:
Fabiana Napolitano, University of Texas Southwestern Medical Center, United StatesAntonia Syrnioti, Aristotle University of Thessaloniki, Greece
Copyright © 2025 Sołek, Zielińska, Kordek, Romańska and Braun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanna Romańska, aGFubmEucm9tYW5za2FAZ21haWwuY29t; Marcin Braun, YnJhdW5tYXJjaW5AZ21haWwuY29t; bWFyY2luLmJyYXVuQHVtZWQubG9kei5wbA==
†These authors have contributed equally to this work and share first authorship
 Julia Sołek†
Julia Sołek† Hanna Romańska
Hanna Romańska Marcin Braun
Marcin Braun