Abstract
This paper reported a 64-year-old, secondary central nervous system lymphoma (sCNSL) patient with COVID-19 infection during CD19 chimeric antigen receptor T-cell (CAR-T) therapy following autologous stem cell transplantation (ASCT). Our findings demonstrate that the combination of ASCT and CAR-T for sCNSL may be feasible, even for SARS-CoV-2-positive immunocompromised lymphoma patients.
Introduction
Patients with secondary central nervous system lymphoma (sCNSL) have a very poor prognosis and have an urgent need for effective treatment. Only few reports support the use of autologous stem cell transplantation (ASCT) for sCNSL, owing to unsuccessful stem cell harvest and the high incidence of relapse after ASCT. CD19 chimeric antigen receptor T-cell (CAR-T) therapy is a novel treatment strategy for relapsed or refractory B-cell malignancies. Patients with central nervous lymphoma were excluded from most trials due to concerns for cytokine release syndrome (CRS) of the central nervous system (1–3).With the improvement of CAR-T production and complication management, CAR T-cell immunotherapy following ASCT for central nervous system lymphoma has been reported recently. However, very few subjects were included (4, 5).
COVID-19 infection had a dramatic impact on the mortality of patients with hematological malignancies, particularly for patients who underwent ASCT or CAR-T therapy. Mortality rates may escalate up to 40% (6). This raised concerns about initiating ASCT or CAR-T therapy in patients with COVID-19 infection, due to the potential for development of severe pneumonia or acute respiratory distress syndrome. The present study first reports the treatment of ASCT and CAR-T therapy in an sCNSL patient with concurrent COVID-19 infection and makes a literature review. We aimed to provide insights into the therapeutic strategy for sCNSL patients with COVID-19 infection.
Case report
A 64-year-old man complaining of pain in the upper abdomen was admitted to the Department of Hematology, the First Affiliated Hospital of Xi’an Jiaotong University on 6 February 2023. The patient had no history of specific disease, family disease, or genetic disease. The complete blood count test showed the following results: white blood cell count, 7.68 × 109/L; hemoglobin level, 140 g/L; and platelet count, 445 × 109/L. Additional laboratory tests showed that the serum lactic dehydrogenase level was 539 U/L (upper limit of normal: 250 U/L). Positron emission tomography/computed tomography (PET/CT) revealed multiple enlarged lymph nodes in the right axillary, chest wall muscle spaces, and retroperitoneum. The patient was diagnosed as having diffuse large B-cell lymphoma (DLBCL) by right axillary lymph node biopsy. The immunohistochemistry showed CD20(+), CD19(+), CD3(−), CD5(−), Bcl2(+80%), C-myc(+70%), CD10(−), Bcl6(+), Mum1(+), CyclinD1 (−), CD30(−), P53(+80%), and Ki67(+80%) and that the patient had a 46, XY(20) karyotype.
The patient received two courses of R-CHOP (rituximab 700 mg on day 0, vindesine 4 mg on day 1, ifosfamide 1.5 g on day 1, doxorubicin liposome 40 mg on day 1, 20 mg on day 2, and prednisone 60 mg on days 1–5). Twenty-one days after the last round of therapy, PET/CT showed that the lymph nodes of the right axillary, chest wall muscle spaces, and retroperitoneum were enlarged. Therefore, the patient was given six cycles of R-Pola-Gemox therapy (rituximab 700 mg on day 1, pola 131 mg on day 1, gemcitabine 1,870 mg on day 2, and oxaliplatin 187 mg on day 2) and two cycles of rituximab 700 mg and gemcitabine 1,870 mg and then chemotherapy was stopped. After four cycles of R-Pola-Gemox therapy, the status of the disease was complete metabolic remission evaluated by PET/CT. Nine months after the treatment ended, the patient had a headache. Cranial magnetic resonance imaging (MRI) showed a left frontal lobe mass with peripheral edema. PET/CT showed new soft tissue nodules in the deep left frontal lobe and basal ganglia area with increased glucose metabolism, which indicated relapse of the disease. Robot-assisted stereotactic biopsy under general anesthesia was done and the pathology showed “left frontal” diffuse large B-cell lymphoma (germinal center type). The immunohistochemistry of biopsy showed the following: LCA(+), CD20(+), CD19(+), CD3(−), CD5(−), CyclinD1(−), CD30(−), Bcl-2(−), Bcl-6(40%+), C-myc(10%+), MUM1(+), CD10(+), Ki67 (70%+), P53 (wild type), and ALK-p80(−). The patient received two cycles of rituximab 600 mg on day 0, methotrexate 5,500 mg on day 1, and temozolomide 150 mg on day 3 and 200 mg on days 4–7. Then, lymphocytes were collected, and 1 month later, the peripheral hematopoietic stem cells were harvested. The conditioning regimen for the patient undergoing ASCT was TEAM (thiotepa, etoposide, cytarabine, and melphalan), which included thiotepa (5 mg/kg) on day −7, cytarabine (200 mg/m2) every 12 h, etoposide (200 mg/m2) on days −6 to −3, and melphalan (140 mg/m2) on day −2. During the conditioning, the patient developed a fever (37.8 °C) and nasal congestion on day −4; the COVID-19 polymerase chain reaction (PCR) on the nasal swab sample was positive, which resulted in a negative nasal swab after molnupiravir treatment. Autologous hematopoietic stem cells were infused on day 0 with a mononuclear cell dose of 6.44 × 108/kg and a CD34+ cell dose of 6.79 × 106/kg. On day +1, he developed a fever (38 °C) due to agranulocytosis; imipenem cilastatin in combination with caspofungin was thus given but the patient still had a low grade fever. The COVID-19 PCR test on the nasal swab sample was again positive and became negative after 5 days’ treatment of nirmatrelvir/ritonavir. Concurrent medications of nirmatrelvir/ritonavir included levetiracetam, carbopenem, imipenem cilastatin, calcium leucovorin, ursodeoxycholic acid, prostaglandin E1, SMZ-TMP, and acyclovir; dose adjustments of these drugs were not required.
Additionally, 2 × 106/kg CD19-CAR-T cells were infused 8 days after ASCT. There were no manifestations of CRS and neurotoxicity in this patient. The engraftment times of neutrophil and platelet were 10 days and 10 days after ASCT, respectively. The cytokine levels after CAR-T therapy are shown in Figure 1. The patient achieved complete remission and received zanubrutinib maintenance therapy from 3 months after CAR-T therapy and was still in complete remission for 6 months. The follow-up is still ongoing, the blood routine was normal, and the patient had no infection with B-cell aplasia during the follow-up. The treatment regimen of the patient is depicted in Figure 2. The in vivo expansion and persistence of CAR-T cells were monitored by reverse transcription polymerase chain reaction (RT-PCR) and a flow cytometer (Figures 3, 4). The copies of CAR-T cells reached their first peak 7 days after infusion and then dropped.
Figure 1
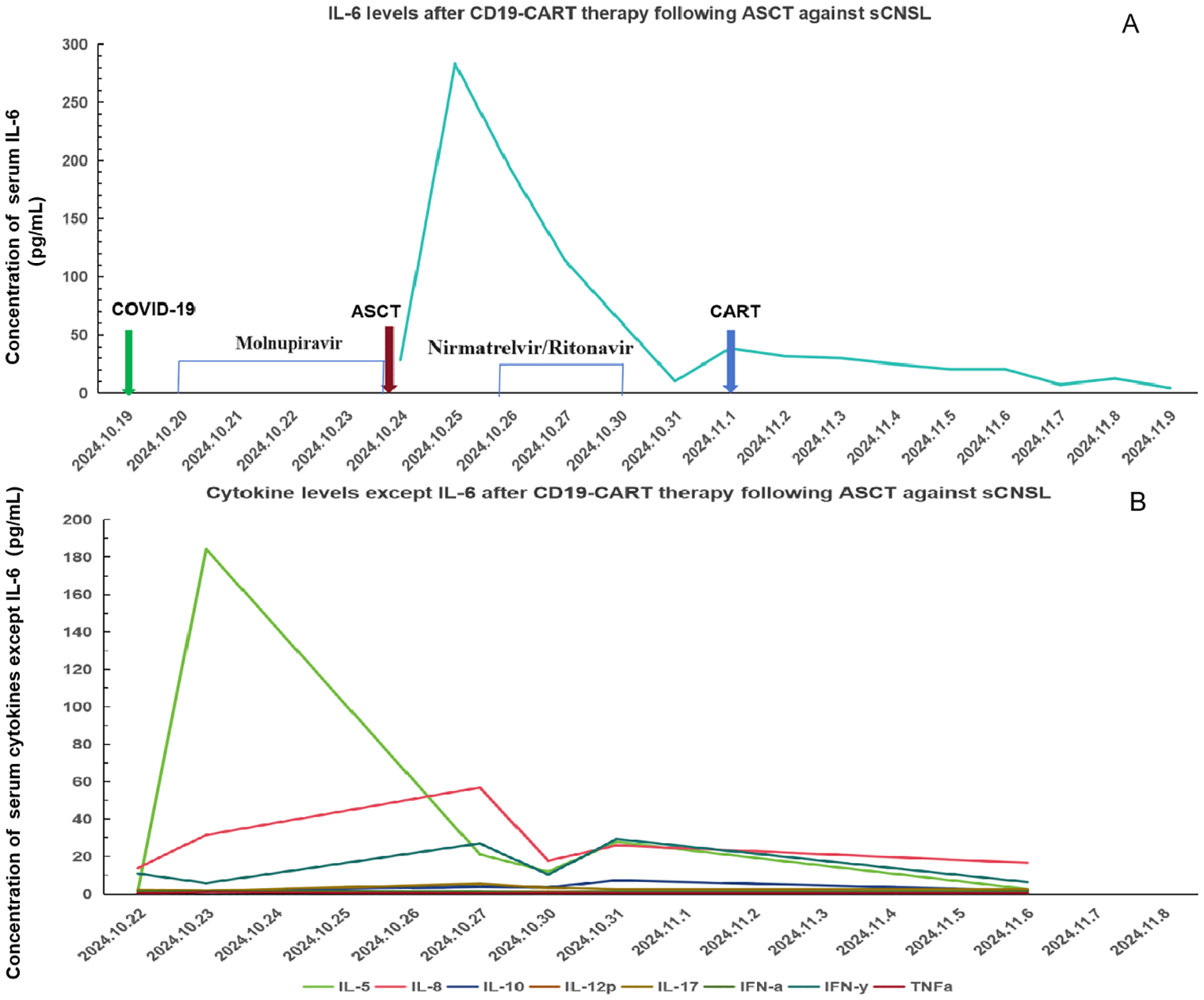
Cytokine levels after CD19-CAR-T therapy following ASCT against sCNSL (A) IL-6; levels after CD19-CART therapy following ASCT against sCNSL, peaking on October 25, 2024, and declining after. (B) Levels of other cytokines, excluding IL-6, over the same period.
Figure 2
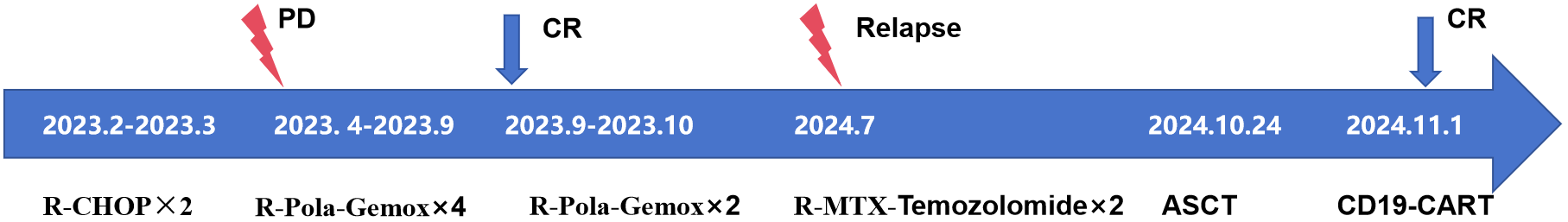
Timeline diagram showing treatment progression.
Figure 3
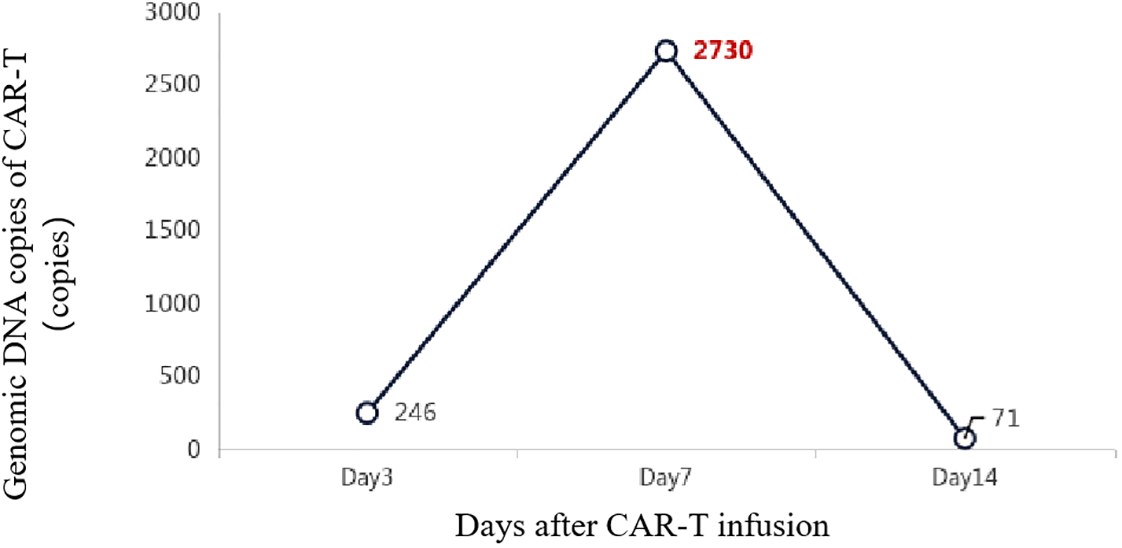
The in vivo expansion and persistence of CAR-T cells were monitored by RT-PCR.
Figure 4
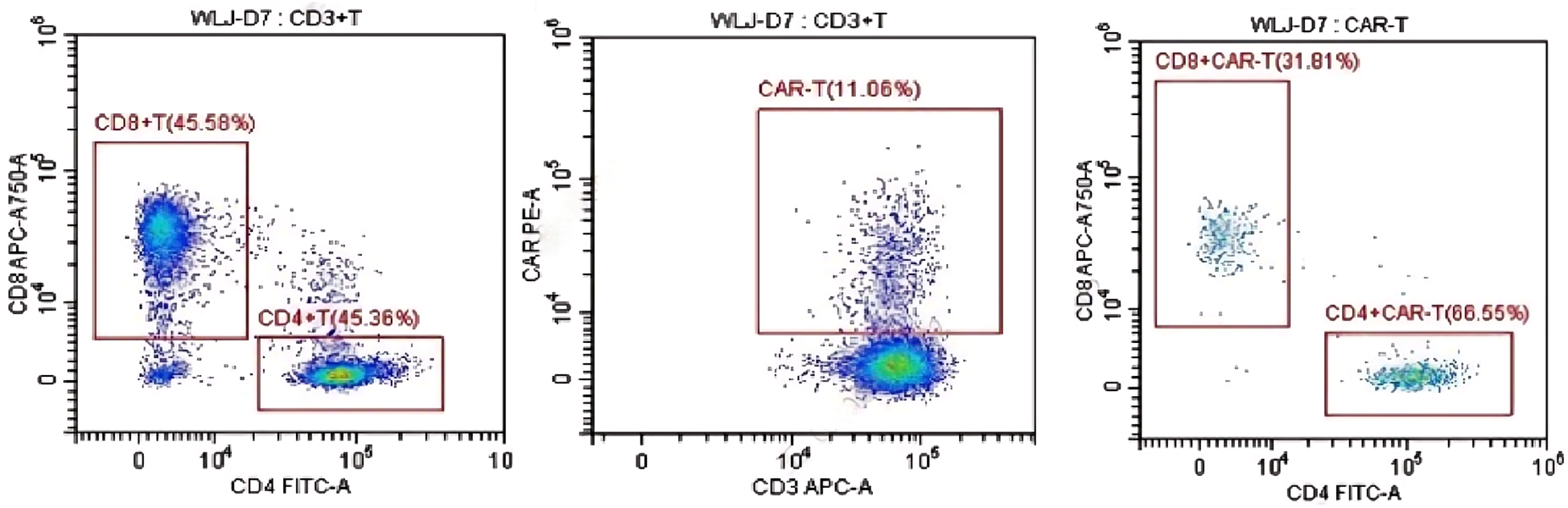
The in vivo persistence of CAR-T cells 7 days after infusion was monitored by a flow cytometer.
Discussion
Central nervous system involvement in DLBCL accounts for 5%–10% of DLBCL cases and is associated with very poor prognosis, with a median survival of less than 6 months. Traditional treatment approaches included high-dose methotrexate-based chemotherapy combined with whole-brain radiotherapy, but long-term survival rates remained below 30%. ASCT as consolidation therapy following high-dose chemotherapy can improve survival in some patients. However, because of the blood–brain barrier, drug penetration is limited, and central nervous relapse rates after ASCT remained high. Targeted drugs such as Bruton’s tyrosine kinase (BTK) inhibitors, interleukin-1 receptor-associated kinase-4 inhibitors, and immunomodulators including lenalidomide and pomalidomide had also show certain efficacy in central nervous system lymphoma. In order to further improve the efficacy, most of these drugs were combined with other chemotherapy regimens or used for maintenance therapy. In recent years, CD19-CAR-T therapy has demonstrated significant efficacy in relapsed/refractory DLBCL, particularly showing some penetration into central nervous system lesions, offering new hope for DLBCL patients with central nervous system infiltration (7–10). Nevertheless, neurotoxicity following CAR-T therapy [e.g., immune effector cell-associated neurotoxicity syndrome (ICANS)] and the impact of the central nervous system-specific microenvironment required further investigation.
Currently, there is no standard approach for the optimal sequencing of ASCT and CAR-T therapy. Clinical decision-making must consider the patient’s disease status, prior treatment response, and tolerability. Existing research supports two primary strategies: For chemotherapy-sensitive patients eligible for transplantation, ASCT first can maximally reduce tumor burden and enhance the efficacy of subsequent CAR-T therapy. Table 1 shows several reports about the strategy of ASCT prior to CAR-T therapy. Patients achieving partial remission or with minimal residual disease positive after ASCT may experience prolonged progression-free survival with CAR-T therapy. Additionally, the lymphocyte-depleting effect of ASCT may enhance CAR-T cell expansion and persistence. However, delayed immune recovery after ASCT may increase infection risk, necessitating careful evaluation. For patients with primary resistance or early relapse after ASCT, prioritizing CAR-T therapy may be more appropriate. CAR-T can induce deep remission, followed by ASCT to eliminate residual disease, particularly in high-risk genetic subgroups (e.g., double-hit or triple-hit lymphomas). However, long-term remission rates after CAR-T therapy remained limited.
Table 1
| Published online | Numbers of patients | Age (years) | Target | CRS | Response | Survival |
|---|---|---|---|---|---|---|
| Sylvain Choquet, 2024 (11) | 14 | 68 (34–76) | CD19 | 92% | 80% | Median OS, 21.2 months |
| Xiaoxi Zhou, 2024 (5) | 29 | 42 (23–66) | CD19+CD22 | 41.4% (20.7% ICANS) | 82.75% | 2-year OS, 72% |
| Fei Xue, 2022 (4) | 8 | 42 (32–66) | CD19/CD20/CD22 | 41% (29% ICANS) | 100% | Median OS, 19.3 months |
| Jiaying Wu, 2021 (12) | 13 | 42 (23–65) | CD19+CD22 | 23% | 84.61% | 1-year OS, 82.5% |
Treatment and outcomes from prior trials of ASCT combined with CAR-T cell therapy for central nervous system lymphoma (literature review).
CRS, cytokine release syndrome. ICANAS, immune effector cell-associated neurotoxicity syndrome; OS, overall survival.
Because of the lymphodepleting chemotherapy, persistent B-cell aplasia, hypogammaglobulinemia, and CRS, patients who received CD19-CAR-T therapy had high risk of infections, especially the viral kind. Early diagnosis and effective antiviral treatment were crucial for reducing mortality. In the post-COVID-19 era, the impact of COVID-19 on CAR-T therapy needed to be taken seriously. It was recommended that nucleic acid testing for COVID-19 was done before CAR-T therapy. For hospitalized COVID-19 patients, concurrent antiviral therapy with corticosteroids may decrease the risk of CRS while reducing viral load (13). The utilization of IL-6 inhibitors and JAK inhibitors in severe COVID-19 cases required careful consideration, especially for lymphoma patients with B-cell aplasia. Controlled trials had demonstrated that early utilization of antiviral drugs such as molnupiravir and nirmatrelvir/ritonavir may significantly reduce mortality. Tixagevimab/cilgavimab was recommended for prophylaxis during the COVID-19 pandemic, particularly for patients within 1 year of CAR-T therapy (13).
This paper is the first to report successful CD19-CAR-T therapy following ASCT in an sCNSL patient with COVID-19 infection. It may indicate the feasibility of ASCT and CAR-T therapy for immunocompromised patients with COVID-19 infection.
For immunocompromised patients with COVID-19 infection during the treatment, they present distinct challenges, characterized by prolonged viral shedding, severe infections being more likely in these patients, and the release of inflammatory factors exacerbating CRS. This was a complex decision-making process for both physicians and the patient. The patient positive for COVID-19 did not pursue CAR-T therapy, especially considering the absence of pulmonary involvement. Early detection and intervention, comprehensive management, and collaborative decision-making were important for achieving positive outcomes for patients who face dual challenges of immunosuppression and COVID-19 infection (14). For this case, the patient did not have COVID-19 infection before admission, and at that time, there was a COVID-19 epidemic in the hospital; thus, the patient becoming COVID-19 positive may be considered as nosocomial infection. When patients develop a fever, they were screened for COVID-19 and received antiviral and anti-infection supportive treatment, so that severe pneumonia and CRS, especially ICANS, may be prevented. This also reminded us about the importance of early diagnosis and treatment; in addition, we needed to enhance the prevention and control of infection during the epidemic period.
Maintenance therapy after sequential treatment may further delay relapse, particularly in sCNSL patients. Potential options include BTK inhibitors (e.g., zanubrutinib and ibrutinib). These agents can penetrate the blood–brain barrier and inhibit B-cell receptor signaling, reducing central nervous system relapse. Immunomodulatory drugs (e.g., lenalidomide) enhance T-cell function and remodel the anti-tumor immune microenvironment. PD-1/PD-L1 inhibitors have shown activity in some central nervous system lymphomas but require caution due to immune-related adverse effects. The indications, duration, and optimal combinations for maintenance therapy still require prospective validation (15–17). The patient in this case report began maintenance treatment with BTK inhibitors approximately 3 months after treatment, and the follow-up time is still short; thus, we need to continue monitoring the changes in efficacy.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of the first affiliated hospital of Xi’an Jiaotong Univeristy. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XW: Data curation, Project administration, Investigation, Writing – original draft. JR: Writing – review & editing, Investigation, Project administration. YW: Investigation, Writing – original draft, Methodology. ML: Writing – review & editing, Data curation. JL: Investigation, Writing – review & editing, Methodology. PH: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. We would like to acknowledge the funding support of the National Key R&D Program (grant no. 2022YFC2502700).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
OrellaOrellana-Noia V Abousaud A . Secondary central nervous system lymphoma: updates in treatment and prophylaxis strategies. Curr Treat Options Oncol. (2022) 23:1443–56. doi: 10.1007/s11864-022-01017-4
2
Epperla N Feng L Shah NN Fitzgerald L Shah H Stephens DM et al . Outcomes of patients with secondary central nervous system lymphoma following CAR T-cell therapy: a multicenter cohort study. J Hematol Oncol. (2023) 16:111. doi: 10.1186/s13045-023-01508-3
3
Asghar N Masood A Dhaliwal A Khurana S Davis J Hashmi H et al . Chimeric antigen receptor T-cell (CAR T-cell) therapy for primary and secondary central nervous system lymphoma: A systematic review of literature. Clin Lymphoma Myeloma Leuk. (2023) 23:15–21. doi: 10.1016/j.clml.2022.09.008
4
Xue F Zheng P Liu R Feng S Guo Y Shi H et al . The autologous hematopoietic stem cells transplantation combination-based chimeric antigen receptor T-cell therapy improves outcomes of relapsed/refractory central nervous system B-cell lymphoma. J Oncol. (2022) 2022:2900310. doi: 10.1155/2022/2900310
5
Zhou X Yu Q Dai Z Wang J Li C Huang L et al . CD19/CD22 CAR-T-cell cocktail therapy following autologous transplantation is an optimizing strategy for treating relapsed/refractory central nervous system lymphoma. Exp Hematol Oncol. (2024) 13:100. doi: 10.1186/s40164-024-00538-y
6
Al-Ramahi JS Shahzad M Li K DeJarnette S Chaudhary SG Lutfi F et al . Lessons learned from COVID-19 pandemic: outcomes after SARS-CoV-2 infection in hematopoietic cell transplant and cell therapy recipients. Leuk Lymphoma. (2023) 64:1981–91. doi: 10.1080/10428194.2023.2243355
7
Wang T Xu L Gao L Tang G Chen L Chen J et al . Chimeric antigen receptor T-cell therapy combined with autologous stem cell transplantation improved progression-free survival of relapsed or refractory diffuse large B-cell lymphoma patients: A single-center, retrospective, cohort study. Hematol Oncol. (2022) 40:637–44. doi: 10.1002/hon.2975
8
Wei J Xiao M Mao Z Wang N Cao Y Xiao Y et al . Outcome of aggressive B-cell lymphoma with TP53 alterations administered with CAR T-cell cocktail alone or in combination with ASCT. Signal Transduct Target Ther. (2022) 7:101. doi: 10.1038/s41392-022-00924-0
9
Li D Liu R Fu Z Yang F Ma L Guo Y et al . Combination autologous stem cell transplantation with chimeric antigen receptor T-cell therapy for refractory/relapsed B-cell lymphoma: a single-arm clinical study. Front Immunol. (2025) 16:1532460. doi: 10.3389/fimmu.2025.1532460
10
Ye M Gao L Wang T Yu J Gui J Yang J . CD19 chimeric antigen receptor T-cell therapy following autologous stem cell transplantation against relapsed or refractory Burkitt lymphoma/leukemia: A case report and literature review. Front Oncol. (2022) 12:932254. doi: 10.3389/fonc.2022.932254
11
Choquet S Soussain C Azar N Morel V Metz C Ursu R et al . CAR T-cell therapy induces a high rate of prolonged remission in relapsed primary CNS lymphoma: Real-life results of the LOC network. Am J Hematol. (2024) 99:1240–9. doi: 10.1002/ajh.27316
12
Wu J Meng F Cao Y Zhang Y Zhu X Wang N et al . Sequential CD19/22 CAR T-cell immunotherapy following autologous stem cell transplantation for central nervous system lymphoma. Blood Cancer J. (2021) 11:131. doi: 10.1038/s41408-021-00523-2
13
Qian H Yang X Zhang T Zou P Zhang Y Tian W et al . Improving the safety of CAR-T-cell therapy: The risk and prevention of viral infection for patients with relapsed or refractory B-cell lymphoma undergoing CAR-T-cell therapy. Am J Hematol. (2024) 99:662–78. doi: 10.1002/ajh.27198
14
Radici V Giagulli C Accorsi Buttini E Farina M Polverelli N Brugnoni D et al . Successful CAR-T cell therapy in a refractory MCL patient with bacterial, fungal and COVID-19 infection: a case report. Front Transplant. (2023) 2, 1238494. doi: 10.3389/frtra.2023.1238494
15
Zou R Zhou X Liu H Wang P Xia F Kang L et al . Long-term complete remission of decitabine-primed tandem CD19/CD22 CAR-T therapy with PD-1 and BTK inhibitors maintenance in a refractory primary central nervous system lymphoma patient. Cancer Res Treat. (2023) 55:1363–8. doi: 10.4143/crt.2023.371
16
Xin X Zhu X Yang Y Wang N Wang J Xu J et al . Efficacy of programmed cell death 1 inhibitor maintenance after chimeric antigen receptor T cells in patients with relapsed/refractory B-cell non-Hodgkin-lymphoma. Cell Oncol (Dordr). (2024) 47:1425–40. doi: 10.1007/s13402-024-00940-y
17
Ping N Qu C Li M Kang L Kong D Chen X et al . Overall survival benefits provided by lenalidomide maintenance after chimeric antigen receptor T cell therapy in patients with refractory/relapsed diffuse large B-cell lymphoma. Ann Transl Med. (2022) 10:298. doi: 10.21037/atm-22-20
Summary
Keywords
diffuse large B-cell lymphoma, secondary central nervous system lymphoma, CD19 chimeric antigen receptor T-cell, autologous stem cell transplantation, COVID-19
Citation
Wang X, Ren J, Wang Y, Luo M, Li J and He P (2025) Successful CD19 chimeric antigen receptor T-cell therapy following autologous stem cell transplantation in a secondary central nervous system lymphoma patient with COVID-19 infection: a case report and literature review. Front. Oncol. 15:1656034. doi: 10.3389/fonc.2025.1656034
Received
29 June 2025
Accepted
11 August 2025
Published
05 September 2025
Volume
15 - 2025
Edited by
Jia Wei, Huazhong University of Science and Technology, China
Reviewed by
Dinesh Pendharkar, Sarvodaya Hospital and Research Centre, India
Mao Zekai, Huazhong University of Science and Technology, China
Weiwei Tian, Shanxi Medical University, China
Updates
Copyright
© 2025 Wang, Ren, Wang, Luo, Li and He.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengcheng He, hepengcheng@xjtufh.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.