- 1Department of Surgery, Asan Medical Center, University of Ulsan, College of Medicine, Seoul, Republic of Korea
- 2Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, Seoul, Republic of Korea
Introduction: Breast cancer (BC) treatments can impair fertility in young women, causing considerable distress and potentially influencing treatment decisions, yet comprehensive real-world data on pregnancy outcomes after BC remain limited. This study aims to provide comprehensive real-world data on pregnancy following BC treatment to guide clinical practice and patient counseling.
Methods: We conducted a retrospective cohort study using medical records from a single tertiary medical center in South Korea. The study included 995 premenopausal women aged 18 to 40 years who were diagnosed with stage 0–III BC between December 2010 and September 2020. The primary outcomes included post-treatment pregnancy rates, factors associated with subsequent pregnancy, timing of conception, pregnancy outcomes, and oncologic outcomes among those who conceived.
Results: The median age was 32 years (interquartile range [IQR], 30–34 years). Of 995 patients, 115 had at least one pregnancy after their BC diagnosis. Significant differences in pregnancy rates and the interval from BC treatment to pregnancy were observed according to hormone receptor status and pregnancy history prior to BC diagnosis. Among those who conceived, 46.1% discontinued endocrine therapy (ET) to achieve pregnancy. Following BC treatment, pregnancies were observed in 7.8% of women who were >35 years old at diagnosis, 17.8% of women who were unmarried at diagnosis, and 6.8% of women who already had children. Of the 76 patients who discontinued ET to attempt pregnancy, 53 (69.7%) successfully conceived. Among those who achieved pregnancy after ET discontinuation, four patients (7.5%) experienced cancer recurrence.
Discussion: Effective fertility preservation counseling is necessary for patients of reproductive age with BC, regardless of age, marital status, or whether they had children before BC diagnosis. This study can be referenced to appropriately address and manage the impact of chemotherapy and ET on pregnancy after BC treatment.
Introduction
Breast cancer (BC) remains the most prevalent malignancy among women of reproductive age globally, with Asian regions demonstrating particularly high proportions of young-onset cases (1, 2). While improvements in early detection and treatment have increased survival rates, these advances have raised fertility considerations among young survivors (3). BC treatments, particularly endocrine therapy and chemotherapy, are known to potentially impair fertility, causing considerable distress among these young survivors (4). Approximately 63% of young women of childbearing age diagnosed with BC have concerns about their fertility post-treatment, and 39% report that these concerns influence their treatment decision-making (5–7). Notably, 2% of patients may decline recommended chemotherapy or endocrine therapy (ET) owing to fertility concerns, potentially compromising their oncologic outcomes (7). BC survivors exhibit significantly lower pregnancy rates (RR, 0.40; 95% confidence interval [CI], 0.32–0.49) compared to the general population, substantiating these fertility concerns (8). Prior studies have demonstrated the safety of pregnancy and fertility preservation in BC survivors (9, 10). A recent prospective study, the POSITIVE trial, demonstrated the safety of interrupting hormonal therapy to achieve pregnancy (11), as well as the safety of using assisted reproductive techniques in BC survivors (12). Nevertheless, concerns persist regarding potential treatment delays, increased recurrence rates, and decreased treatment adherence (4, 13). Moreover, while fertility preservation strategies exist, there remains uncertainty regarding their utilization and efficacy in patients with BC (14).
This study aimed to provide comprehensive real-world data on pregnancy following BC treatment, with the goal of informing clinical practice and patient counseling regarding this critical aspect of survivorship.
Materials and methods
Study population
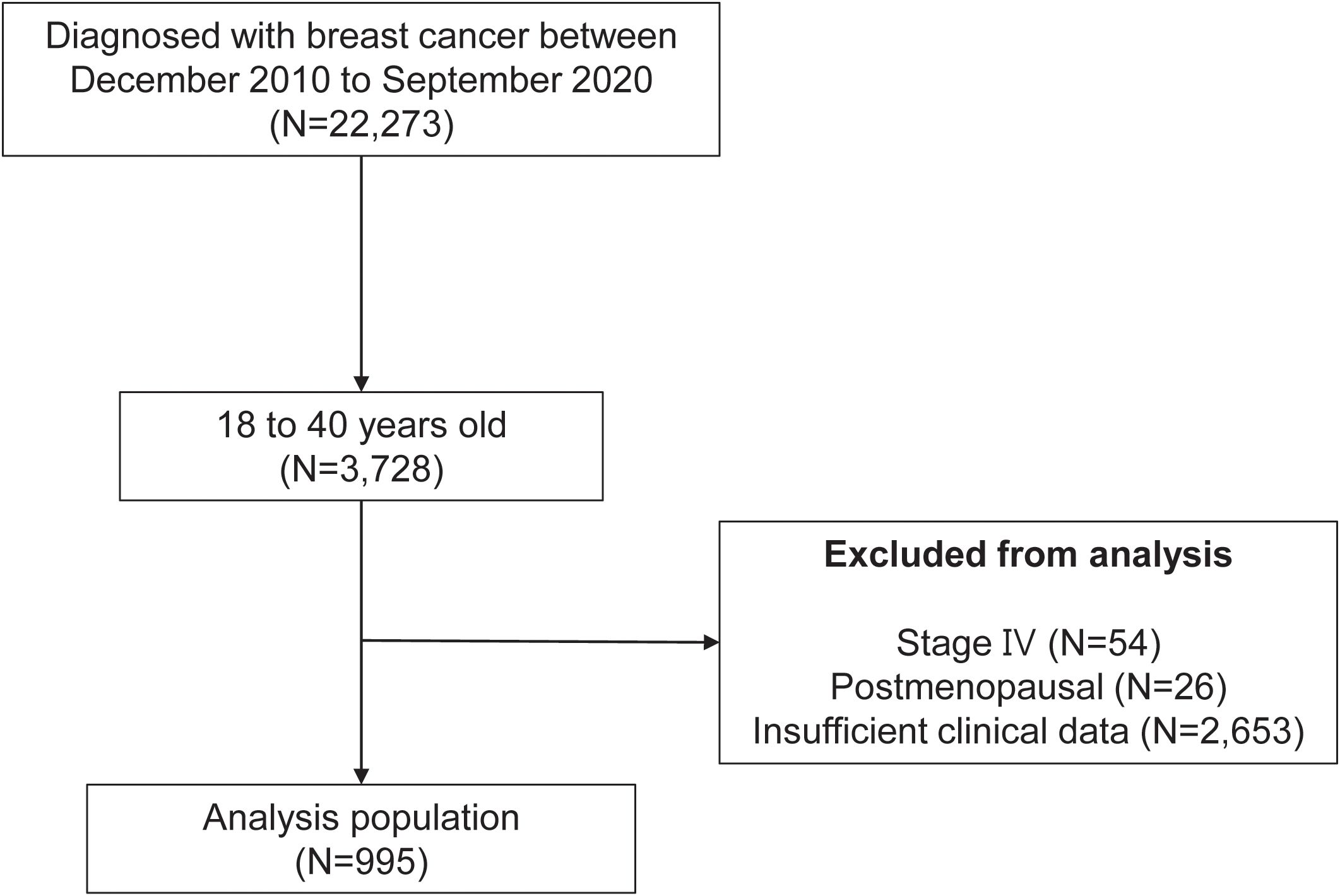
In this retrospective study, we examined the medical records of patients diagnosed with BC at a single tertiary medical center between December 2010 and September 2020 (Figure 1). The inclusion criteria were as follows: female BC patients aged between 18 and 40 years who were diagnosed with BC during the study period. A total of 3,728 patients met these criteria from an initial cohort of 22,273 female BC patients. We then applied exclusion criteria to ensure the reliability and validity of the study. First, we excluded patients with stage IV BC owing to the distinct clinical course and prognosis associated with this advanced disease stage. Next, postmenopausal women were excluded, as the study was focused on fertility concerns relevant to premenopausal women. Lastly, patients with insufficient clinical data were also excluded to ensure comprehensive and accurate analysis. After applying these exclusion criteria, the final analysis population consisted of 995 patients. These patients formed the basis of the study and all subsequent analysis.
Variables and definitions
The treatment administered to patients was in accordance with national guidelines. Patient and disease characteristics were retrieved from digital medical records. During their initial consultation after referral to Asan Medical Center, patients’ marital status (married, single, or divorced) and reproductive history (number of children, year of birth) were documented. Information on pregnancies following breast cancer treatment was obtained from standardized medical record forms for young patients, which physicians are required to complete during follow-up outpatient visits conducted at six-month intervals. These forms include data on pregnancy status and the expected or actual date of delivery, and were used for analysis in this study.
Statistical analysis
Data analysis was performed using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org). Pregnancy curves were generated using the Kaplan–Meier method, and the significance of pregnancy rate differences among selected variables was verified using the log-rank test. Hazard ratios were estimated using univariate Cox regression analysis. Further, multivariate Cox regression analysis, implemented with a backward elimination approach, was used to estimate both hazard ratios and p-values, aiding in the identification of independent prognostic indicators. Any unknown groups within each variable were excluded before initiating the Cox analysis. All reported p-values are two-sided, with statistical significance attributed to p-values < 0.05.
Ethics approval
All actions performed in the research involving human participants complied with the ethical guidelines of the institutional and national research committees. In addition, the study adhered to the principles of the Helsinki Declaration of 1964, along with its subsequent updates, or met similar ethical criteria.
The study was reviewed and approved by the Institutional Review Board of Asan Medical Center (approval number 2021-1382). The authors had access to anonymized participant data. Informed consent was waived as the study used retrospective clinical data.
Results
Patient characteristics of the overall cohort and pregnancy group
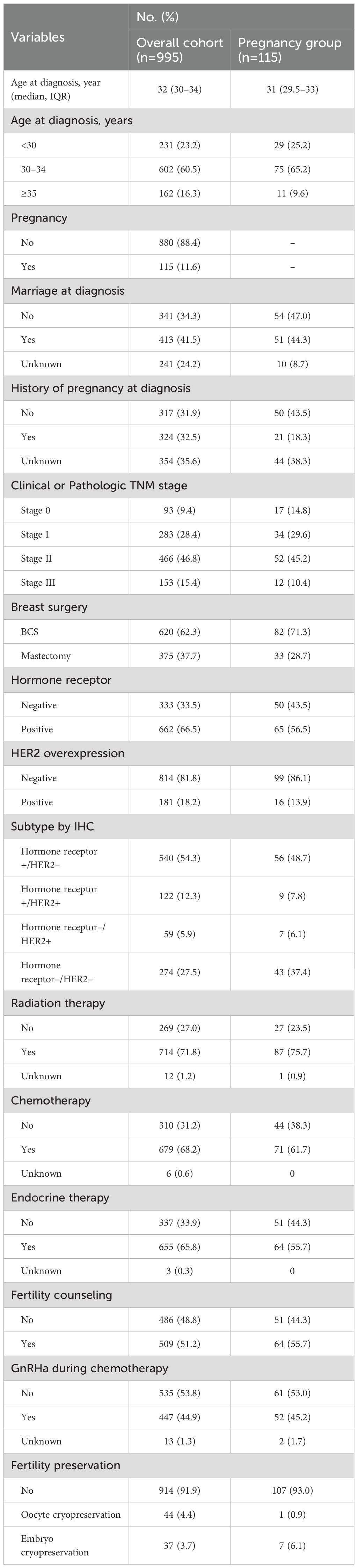
The overall cohort included 995 young women diagnosed with BC, of which 115 experienced a pregnancy after treatment during the median follow-up period of 63 months (Table 1). The mean age at diagnosis for the overall cohort was 32 years (interquartile range [IQR], 30–34 years), whereas for those who experienced a pregnancy, the mean age was 31 years (IQR, 29.5–33 years). Among patients who became pregnant after BC treatment, 47.0% were unmarried, and 18.3% had children at the time of diagnosis. Concerning the TNM stage distribution in the pregnancy group, 10.4% of the cases were stage III. Hormone receptor status was positive in 66.5% of the overall cohort and 56.5% of the pregnancy group. Chemotherapy was received by 61.7% of the pregnancy group. ET was administered to 55.7% of the pregnancy group. Fertility counseling was provided to 51.2% of the overall cohort and 55.7% of the pregnancy group. Oocyte/embryo cryopreservation as a fertility preservation procedure was utilized by 8.1% of the overall cohort and 7.0% of the pregnancy group. The implementation of educational material was associated with higher fertility preservation rates compared to pre-implementation (65.5% vs. 34.5%, respectively) (Supplementary Figure 1).
Factors associated with pregnancy
Age at diagnosis, marital status, previous pregnancies, TNM stage, hormone receptor status, and chemotherapy were evaluated for their association with pregnancy rates and interval after BC treatment (Table 2). Overall, the cumulative incidence of pregnancy at 10 years was 14.2%, with a median time from BC to pregnancy of 3 years (IQR, 2–4 years) (Figure 2A). In patients with hormone receptor-positive and negative BC, the cumulative incidence of pregnancy at 10 years was 13.5% (65 out of 662 hormone receptor-positive patients) and 18.1% (50 out of 333 hormone receptor-negative patients), respectively (HR = 0.60, 95% CI: 0.41–0.87, P = 0.006) (Figure 2B). The median time from BC to pregnancy was significantly longer in patients with hormone receptor-positive (3 years, IQR 2–5 years) than patients with hormone receptor-negative (2 years, IQR 2–3 years) status.
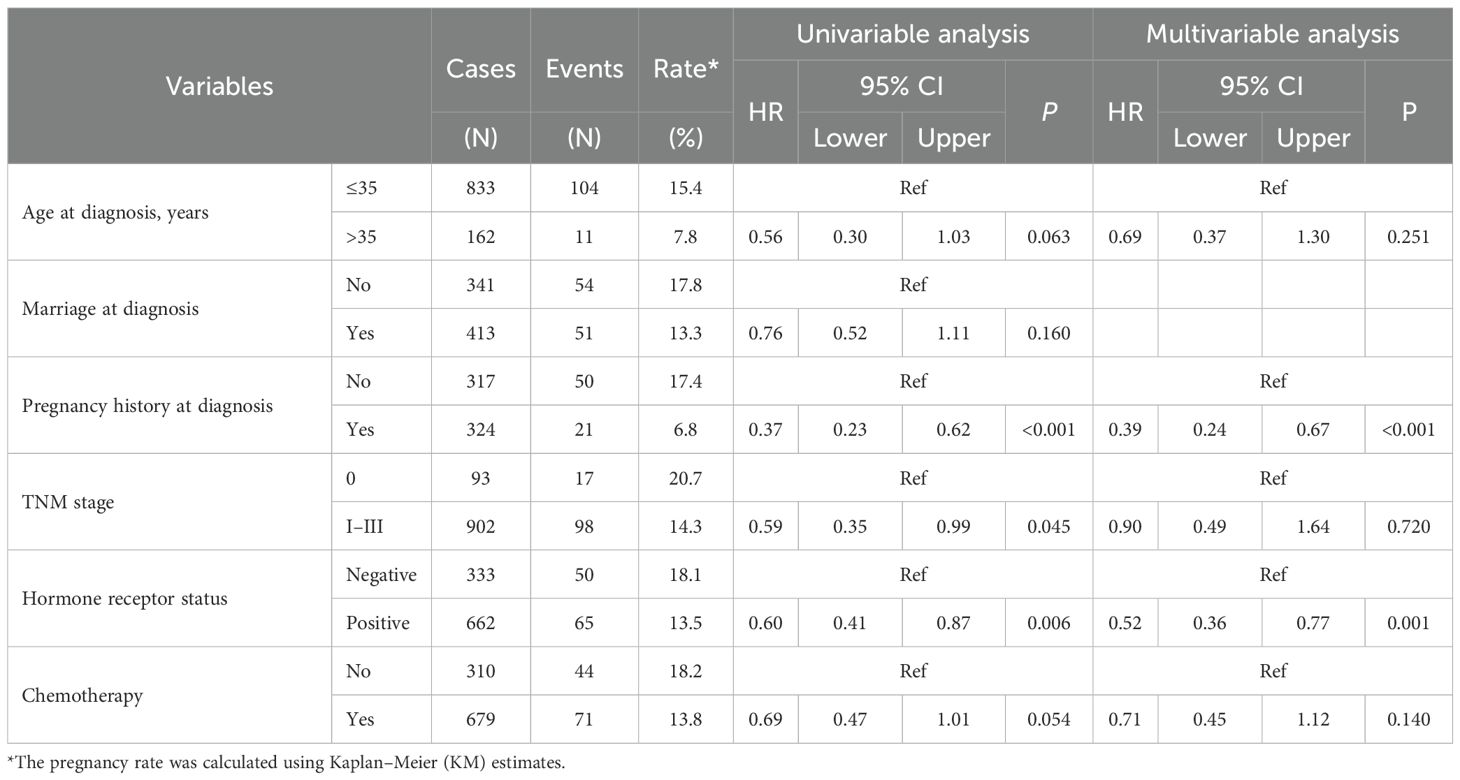
Table 2. Results of uni- and multi-variable cox proportional hazards model evaluating time to pregnancy.
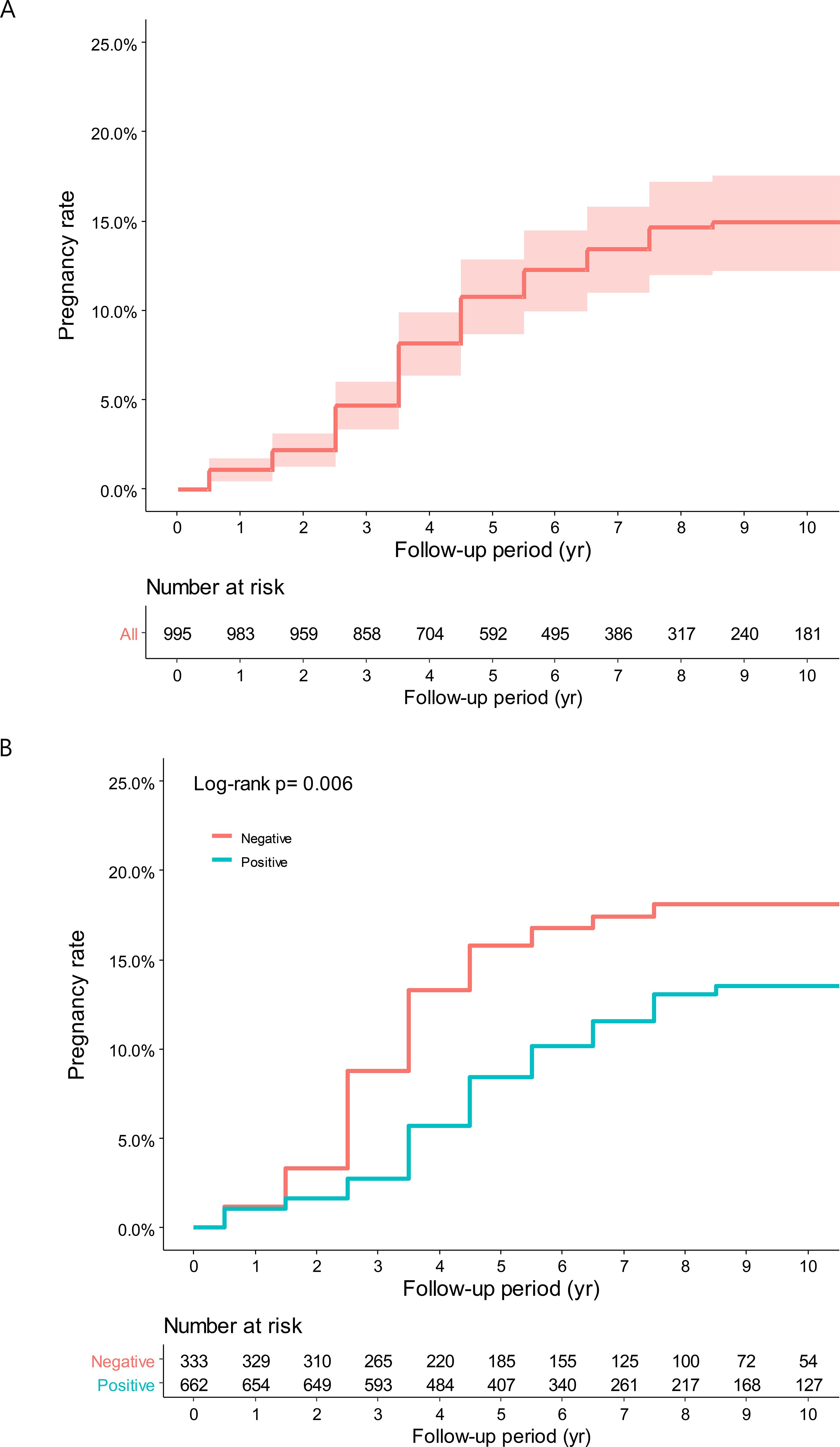
Figure 2. (A) Cumulative incidence of overall pregnancy. (B) Cumulative incidence of pregnancy according to hormone receptor status.
The pregnancy history before diagnosis proved to be significant in pregnancy after treatment. For those with no history of pregnancy, cumulative incidence of pregnancy at 10 years was 17.4% (50 out of 317 patients), compared to 6.8% (21 out of 324 patients) (HR = 0.37, 95% CI: 0.23–0.62, P < 0.001). The TNM stage at diagnosis showed a similar trend. Among stage 0 patients, the cumulative incidence of pregnancy at 10 years was 20.7% (17 out of 93 patients), compared to 14.3% (98 out of 902 patients) with stages I–III (HR = 0.59, 95% CI: 0.35–0.99, P = 0.045). Age above 35 years and chemotherapy showed a trend toward lower pregnancy rates.
Multi-variable analysis was carried out to adjust for potential confounders. The hormone receptor-positive status continued to be associated with lower pregnancy rates (HR = 0.52, 95% CI: 0.36–0.77, P = 0.001). A history of pregnancy remained a significant predictor, with patients who had been pregnant before being less likely to become pregnant after treatment (HR = 0.39, 95% CI: 0.24–0.67, P < 0.001).
Outcomes in pregnancy after BC treatment
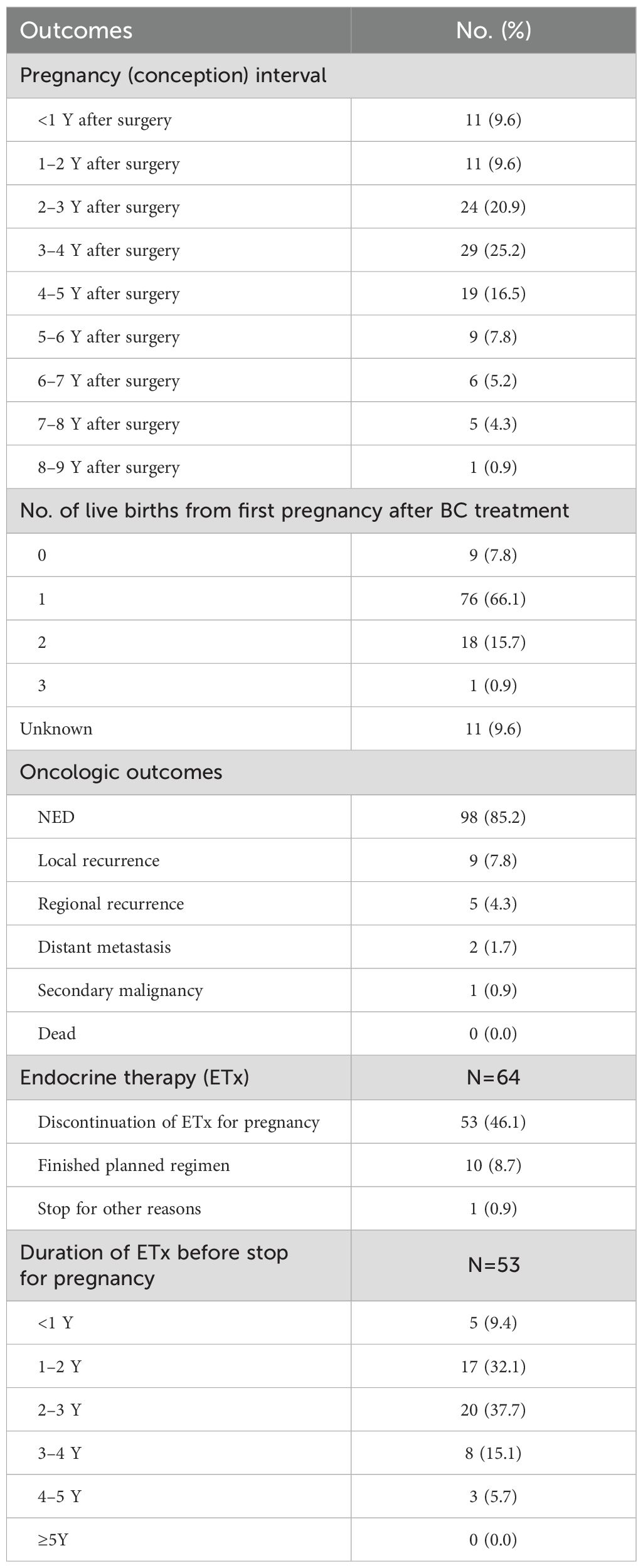
The outcomes for the 115 patients who experienced a pregnancy after treatment are detailed in Table 3. The interval between surgery and conception varied, with 9.6% conceiving within 1 year after surgery, and the highest number (25.2%) of pregnancies occurred between 3 and 4 years post-surgery. Among first pregnancies after BC treatment, 7.8% resulted in no live births, 66.1% in one live birth, 15.7% in two live births, and 0.9% in three live births. The oncologic outcomes showed that 85.2% of the patients who became pregnant were without evidence of disease (NED). Local recurrence occurred in 7.8% of the patients, regional recurrence in 4.3%, and distant metastasis in 1.7%. There was one case (0.9%) of secondary malignancy, and no patients had died at the time of the study.
ET was discontinued for pregnancy in 46.1% of the patients, whereas 8.7% finished their planned regimen and 0.9% stopped for other reasons. Among those who discontinued ET for pregnancy, 9.4% stopped within the first year, 32.1% after 1–2 years, 37.7% after 2–3 years, 15.1% after 3–4 years, and 5.7% after 4–5 years. No patients continued ET for ≥5 years. We analyzed the relationship between the duration of ET and subsequent pregnancy rates upon its discontinuation because of pregnancy (Supplementary Table 2). Patients who received treatment for a duration of 1–2 years demonstrated the highest pregnancy rate of 85.0% (17 out of 20). In the overall cohort, 76 patients discontinued ET to attempt pregnancy, and among them, 17% experienced a recurrence of BC (Supplementary Figure 2).
Discussion
This large-scale retrospective study provides real-world data on pregnancy outcomes among young BC survivors. Patients with hormone receptor-positive BC had significantly lower pregnancy rates and longer pregnancy intervals after treatment. Outcomes for the 115 patients who conceived post-BC treatment showed varied intervals between surgery and conception, with the highest number of pregnancies occurring 3–4 years post-surgery. During the follow-up period, 82.7% of the conceptions resulted in one or more live births, and 85.2% were without evidence of disease, with no patient deaths reported. Among the patients who became pregnant, 46.1% had discontinued hormone therapy for pregnancy.
An interesting finding from this study is that even among patients who were >35 years old, unmarried at diagnosis, or had children, 7.8%, 17.8%, and 6.8%, respectively, became pregnant after BC treatment. These figures are noteworthy when compared to the overall cohort’s pregnancy rate of 14.2%. Therefore, adequate counseling and planning for having children should be conducted before starting treatment, even for BC patients who are older, unmarried, or have not previously given birth. In this analysis, a history of pregnancy before BC diagnosis significantly reduced the chances of conception after BC treatment. This might be attributed to the fact that women with one or more children are less motivated to have a baby after overcoming BC. Additionally, the vague fear among patients and physicians that pregnancy could negatively impact the prognosis of BC could contribute to low motivation. Survey studies conducted among BC specialists revealed that 20–30% of physicians incorrectly held the belief that pregnancy adversely affects BC prognosis (15, 16). However, a prior survey study on BC survivors revealed that 56% of young women who had one or more children before their diagnosis wished to have more children after their BC treatment (5). Our analysis revealed that subsequent pregnancies occurred even among patients who already had children. Among patients with one child, 11% (n=18/163) achieved an additional pregnancy, and notably, 1.9% (n=3/161) of patients with two or more children conceived after BC treatment (Supplementary Table 1). These findings suggest that pre-existing parenthood status should not be a limiting factor when considering fertility preservation options for patients, and fertility preservation counseling should be offered to all patients regardless of their parental status.
Another significant factor influencing pregnancy outcomes after BC treatment was hormone receptor status. Our findings suggested that hormone receptor-positive patients were less likely to conceive post-treatment compared to their hormone receptor-negative counterparts. This could be attributed, first, to concerns shared by patients and physicians that increased estrogen levels during pregnancy may have negative effects on the prognosis of hormone receptor-positive BC (17). However, previous studies have shown that pregnancy and breastfeeding do not have a negative impact on the prognosis of hormone receptor-positive BC (18). Second, the fear of discontinuing treatment owing to pregnancy as a contraindication during hormone therapy could lead to the decision to forego pregnancy (19). Our analysis revealed that more than half of the patients received fertility preservation counseling prior to treatment, indicating their desire for future pregnancies. However, among patients receiving ET, only 7.6% discontinued treatment because of pregnancy. This suggests a tendency among younger patients to give up having a baby and opt for treatment continuation. In the POSITIVE trial, women with hormone receptor-positive early BC who temporarily stopped ET because of pregnancy had an 8.9% incidence of BC events (including distant recurrence) over 3 years, which did not show an increased risk compared to the external control group (11). Similarly, in the retrospective analysis of this study, the cumulative incidence of BC events over 10 years was 10.1%. These findings, from both the POSITIVE trial and our study, may provide reassuring evidence for patients who consider discontinuing ET to pursue pregnancy. Additionally, the results of this study revealed that the majority of pregnant patients discontinued their ET after 1–3 years (Supplementary Table 2). Previously, there was no consensus on the duration of ET maintenance before attempting pregnancy. However, it is considered safe to interrupt ET after maintaining it for a minimum of one and a half years, based on the findings of the POSITIVE trial (11). Among participants in the POSITIVE trial who discontinued ET to pursue pregnancy, 74% achieved at least one successful conception, with the highest success rates documented in patients who underwent embryo/oocyte cryopreservation before initiating treatment (12). Therefore, comprehensive fertility and pregnancy counseling should be integrated into the initial BC management plan at the time of diagnosis.
In the results of this study, chemotherapy did not show a significant impact on pregnancy rates. This finding differs from the conventional belief that chemotherapy compromises ovarian function (20–22). GnRHa can be used concurrently with chemotherapy for ovarian protection (23). In our study, GnRHa was used for fertility preservation in 447 out of 679 patients (65.8%). The PROMISE-GIM6 study conducted by Lambertini et al. demonstrated a higher frequency of menstrual resumption in the GnRHa group (24), and the 2015 POEMS study by Moore et al. reported that GnRHa use, compared to chemotherapy alone, prevented ovarian failure, reduced premature ovarian failure, and showed higher pregnancy rates (25). However, as the primary outcome in most clinical trials using GnRHa was menstrual resumption rather than fertility, evidence verification is needed regarding its use for the purpose of fertility preservation.
Oocyte or embryo cryopreservation represents an established method of fertility preservation that can be implemented during chemotherapy treatment (26–28). In a recent international multicenter study (29) conducted on young patients with BC who are BRCA carriers, 659 out of 4,732 BRCA carriers had at least one pregnancy; among these, 8.2% became pregnant using previously cryopreserved oocytes or embryos after BC treatment. In contrast, our study found this rate to be slightly lower at 7%. The lower rate of fertility preservation procedures in this study, compared to that of international BRCA carriers who are at a higher risk for hereditary BC, may indicate that adequate counseling on the effectiveness and side effects of oocyte and embryo cryopreservation is not being provided. Moreover, a secondary analysis of the recently published POSITIVE trial demonstrated that embryo/oocyte cryopreservation followed by embryo transfer is a safe and effective method for achieving pregnancy after BC treatment (12). Furthermore, in contrast to the United States and European countries, South Korea imposes legal restrictions on pre-implantation or prenatal genetic testing (excluding BRCA mutations) (30–32), which may induce hesitancy in women diagnosed with cancer concerning their pregnancy and fertility preservation options. Therefore, the establishment of a comprehensive fertility preservation system that facilitates the dissemination of accurate information and supports informed decision-making is essential.
Fertility preservation counseling is also necessary to improve medication adherence for appropriate breast cancer treatment. Despite the beneficial impact on survival associated with tamoxifen use, a recent study revealed that 13.4% of women choose not to start taking tamoxifen, and an additional 15.5% discontinue its use before completing the recommended duration of 5 years (33). In this study, fertility emerged as a significant factor influencing early discontinuation of tamoxifen among young patients with BC. Alongside side effects, it is considered one of the most crucial causes for not initiating or discontinuing ET. According to another research finding, individuals who used tamoxifen did not exhibit a decrease in ovarian reserve compared to those who did not use tamoxifen (34). Therefore, providing education on these pieces of evidence to patients in the reproductive age group is necessary.
According to several survey studies conducted among healthcare providers treating breast cancer, there are significant barriers to implementing fertility preservation. These barriers include lack of knowledge (35) and insufficient system resources, such as time and human resources (36, 37). Consequently, there is a growing need for comprehensive multidisciplinary counseling programs that address both fertility preservation and BC treatment (38). Various research initiatives are currently underway to address these challenges. The PREFER study in Italy is collecting prospective data, including outcomes from patients who have successfully conceived, to improve oncofertility counseling (39). The MYBC trial in Korea is working to establish evidence for an effective multidisciplinary shared decision-making program for fertility preservation (40). The results from these trials, combined with real-world data, are expected to contribute to improving both treatment approaches and fertility preservation options for young patients with BC.
This study has some limitations. First, as a retrospective single-center study, it inherently carries the limitations associated with this study design, including potential selection bias and the inability to control for all confounding variables. Because randomized trials on pregnancy-related topics may raise ethical concerns, we are currently collecting prospective pregnancy data through an ongoing trial focusing on multidisciplinary decision-making in young breast cancer patients (40), and we plan to conduct future research based on these data. Second, decisions and outcomes related to pregnancy are significantly influenced by national policies, cultural factors, and societal norms, which may limit the generalizability of our findings to other populations and healthcare settings. Despite these limitations, our study has a notable strength. It represents one of the largest long-term follow-up studies conducted in a conservative Asian setting, providing valuable insights into pregnancy outcomes and fertility preservation in patients with BC within this cultural context. This unique perspective contributes significantly to the existing body of literature on fertility and pregnancy after BC treatment.
In conclusion, our study highlights that effective fertility preservation counseling is necessary for all patients with BC of reproductive age, regardless of age, marital status, or whether they had children before BC diagnosis. It provides real-world data that can be referenced to appropriately address and manage the impact of chemotherapy and ET on pregnancy after BC treatment. These findings can be valuable knowledge for clinical decision-making and counseling.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: For external transfer of the dataset, approval from the institutional IRB is required. Requests to access these datasets should be directed to Young-Jin Lee, aW1hcmN0aWFAbmF2ZXIuY29t.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Asan Medical Center (approval number 2021-1382). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
Y-JL: Validation, Writing – review & editing, Conceptualization, Investigation, Methodology, Writing – original draft, Data curation, Formal analysis, Visualization. T-KY: Investigation, Writing – review & editing, Supervision, Validation. SL: Writing – review & editing, Validation, Supervision. JK: Writing – review & editing, Supervision. IC: Supervision, Writing – review & editing, Conceptualization, Investigation. BK: Writing – review & editing, Supervision, Conceptualization. JL: Supervision, Writing – review & editing. BS: Writing – review & editing, Supervision, Conceptualization. SK: Methodology, Data curation, Visualization, Validation, Software, Formal analysis, Writing – review & editing. HK: Conceptualization, Writing – review & editing, Funding acquisition, Investigation, Supervision, Validation, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Korea-US Collaborative Cancer R&D Program funded by the Ministry of Health & Welfare, Republic of Korea (RS-2025-02233001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1656429/full#supplementary-material
References
1. Sopik V. International variation in breast cancer incidence and mortality in young women. Breast Cancer Res Treat. (2021) 186:497–507. doi: 10.1007/s10549-020-06003-8
2. Cha CD, Park CS, Shin HC, Han J, Choi JE, Kim JH, et al. Breast cancer statistics in Korea, 2021. J Breast Cancer. (2024) 27:351–61. doi: 10.4048/jbc.2024.0213
3. Din HN, Singh-Carlson S, Corliss HL, Hartman SJ, Strong D, Madanat H, et al. Perceived and objective fertility risk among female survivors of adolescent and young adult cancer. JAMA Netw Open. (2023) 6:e2337245. doi: 10.1001/jamanetworkopen.2023.37245
4. Hong YH, Park C, Paik H, Lee KH, Lee JR, Han W, et al. Fertility preservation in Young women with breast cancer: A review. J Breast Cancer. (2023) 26:221–42. doi: 10.4048/jbc.2023.26.e28
5. Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. (2004) 22:4174–83. doi: 10.1200/JCO.2004.01.159
6. Ruddy KJ, Gelber SI, Tamimi RM, Ginsburg ES, Schapira L, Come SE, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. (2014) 32:1151–6. doi: 10.1200/JCO.2013.52.8877
7. Ruggeri M, Pagan E, Bagnardi V, Bianco N, Gallerani E, Buser K, et al. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: Baseline results from an ongoing prospective cohort study in selected European Centers. Breast. (2019) 47:85–92. doi: 10.1016/j.breast.2019.07.001
8. Lambertini M, Blondeaux E, Bruzzone M, Perachino M, Anderson RA, de Azambuja E, et al. Pregnancy after breast cancer: A systematic review and meta-analysis. J Clin Oncol. (2021) 39:3293–305. doi: 10.1200/JCO.21.00535
9. Arecco L, Blondeaux E, Bruzzone M, Latocca MM, Mariamidze E, Begijanashvili S, et al. Safety of pregnancy after breast cancer in young women with hormone receptor-positive disease: A systematic review and meta-analysis. ESMO Open. (2023) 8:102031. doi: 10.1016/j.esmoop.2023.102031
10. Kasum M, von Wolff M, Franulić D, Čehić E, Klepac-Pulanić T, Orešković S, et al. Fertility preservation options in breast cancer patients. Gynecol Endocrinol. (2015) 31:846–51. doi: 10.3109/09513590.2015.1081684
11. Partridge AH, SM N, Ruggeri M, Peccatori FA, Azim HA Jr, Colleoni M, et al. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. (2023) 388:1645–56. doi: 10.1056/NEJMoa2212856
12. Azim HA Jr., Niman SM, Partridge AH, Demeestere I, Ruggeri M, Colleoni M, et al. Fertility preservation and assisted reproduction in patients with breast cancer interrupting adjuvant endocrine therapy to attempt pregnancy. J Clin Oncol. (2024) 42:2822–32. doi: 10.1200/JCO.23.02292
13. Baynosa J, Westphal LM, Madrigrano A, and Wapnir I. Timing of breast cancer treatments with oocyte retrieval and embryo cryopreservation. J Am Coll Surg. (2009) 209:603–7. doi: 10.1016/j.jamcollsurg.2009.08.006
14. Crown A, Muhsen S, Sevilimedu V, Kelvin J, Goldfarb SB, and Gemignani ML. Fertility preservation in Young women with breast cancer: Impact on treatment and outcomes. Ann Surg Oncol. (2022) 29:5786–96. doi: 10.1245/s10434-022-11910-9
15. Lambertini M, Di Maio M, Pagani O, Curigliano G, Poggio F, Del Mastro L, et al. The BCY3/BCC 2017 survey on physicians’ knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast. (2018) 42:41–9. doi: 10.1016/j.breast.2018.08.099
16. Villarreal-Garza C, Martinez-Cannon BA, Barragan-Carrillo R, Bargallo-Rocha JE, Platas A, Peña-Curiel O, et al. Physicians’ attitudes, knowledge, and perceived barriers toward fertility preservation in Young breast cancer patients in a developing country. Rev Invest Clin. (2021) 73:347–53. doi: 10.24875/RIC.20000064
17. Senkus E, Gomez H, Dirix L, Jerusalem G, Murray E, Van Tienhoven G, et al. Attitudes of young patients with breast cancer toward fertility loss related to adjuvant systemic therapies. EORTC study 10002 BIG 3-98. PsychoOncology. (2014) 23:173–82. doi: 10.1002/pon.3384
18. de Bree E, Makrigiannakis A, Askoxylakis J, Melissas J, and Tsiftsis DD. Pregnancy after breast cancer. A comprehensive review. J Surg Oncol. (2010) 101:534–42. doi: 10.1002/jso.21514
19. Buonomo B, Brunello A, Noli S, Miglietta L, Del Mastro L, Lambertini M, et al. Tamoxifen exposure during pregnancy: A systematic review and three more cases. Breast Care (Basel). (2020) 15:148–56. doi: 10.1159/000501473
20. Ben-Aharon I, Granot T, Meizner I, Hasky N, Tobar A, Rizel S, et al. Long-term follow-up of chemotherapy-induced ovarian failure in Young breast cancer patients: The role of vascular toxicity. Oncologist. (2015) 20:985–91. doi: 10.1634/theoncologist.2015-0044
21. Bedoschi G, Navarro PA, and Oktay K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncol. (2016) 12:2333–44. doi: 10.2217/fon-2016-0176
22. Schuurman T, Song JY, Wolters V, van de Ven M, van Trommel N, Beerendonk I, et al. Effects of chemotherapy on ovaries of pregnant mice. Arch Gynecol Obstet. (2023) 307:1163–76. doi: 10.1007/s00404-022-06793-w
23. Li ZY, Dong YL, Cao XZ, Ren SS, and Zhang Z. Gonadotropin-releasing hormone agonists for ovarian protection during breast cancer chemotherapy: A systematic review and meta-analysis. Menopause. (2022) 29:1093–100. doi: 10.1097/GME.0000000000002019
24. Lambertini M, Boni L, Michelotti A, Gamucci T, Scotto T, Gori S, et al. Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: A randomized clinical trial. JAMA. (2015) 314:2632–40. doi: 10.1001/jama.2015.17291
25. Moore HC, Unger JM, Phillips KA, Boyle F, Hitre E, Porter D, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. (2015) 372:923–32. doi: 10.1056/NEJMoa1413204
26. White R, Wilson A, Bechman N, Keay SD, McAvan L, Quenby S, et al. Fertility preservation, its effectiveness and its impact on disease status in pre-menopausal women with breast cancer: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2023) 287:8–19. doi: 10.1016/j.ejogrb.2023.05.030
27. Practice Committee of the American Society for Reproductive Medicine. Electronic address:YXNybUBhc3JtLm9yZw==. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil Steril. (2019) 112:1022–33. doi: 10.1016/j.fertnstert.2019.09.013
28. Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. (2020) 31:1664–78. doi: 10.1016/j.annonc.2020.09.006
29. Lambertini M, Blondeaux E, Agostinetto E, Hamy AS, Kim HJ, Di Meglio A, et al. Pregnancy after breast cancer in Young BRCA carriers: An international hospital-based cohort study. JAMA. (2024) 331:49–59. doi: 10.1001/jama.2023.25463
30. Ministry of Health and Welfare, Republic of Korea. (2024). Available online at: https://www.mohw.go.kr/board.es?mid=a10501010200&bid=0003&act=view&list_no=1482408 (Accessed December 22, 2024).
31. Practice Committee and Genetic Counseling Professional Group of the American Society for Reproductive Medicine and American Society for Reproductive Medicine, Washington, D.C Indications and management of preimplantation genetic testing for monogenic conditions: A committee opinion. Fertil Steril. (2023) 120:61–71. doi: 10.1016/j.fertnstert.2023.03.003
32. Michaan N, Leshno M, Cohen Y, Safra T, Peleg-Hasson S, Laskov I, et al. Preimplantation genetic testing for BRCA gene mutation carriers: A cost effectiveness analysis. Reprod Biol Endocrinol. (2021) 19:153. doi: 10.1186/s12958-021-00827-9
33. Llarena NC, Estevez SL, Tucker SL, and Jeruss JS. Impact of fertility concerns on tamoxifen initiation and persistence. J Natl Cancer Inst. (2015) 107:djv202. doi: 10.1093/jnci/djv202
34. Shandley LM, Spencer JB, Fothergill A, Mertens AC, Manatunga A, Paplomata E, et al. Impact of tamoxifen therapy on fertility in breast cancer survivors. Fertil Steril. (2017) 107:243–252.e5. doi: 10.1016/j.fertnstert.2016.10.020
35. King JW, Davies MC, Roche N, Abraham JM, and Jones AL. Fertility preservation in women undergoing treatment for breast cancer in the UK: A questionnaire study. Oncologist. (2012) 17:910–6. doi: 10.1634/theoncologist.2012-0064
36. Quinn GP, Vadaparampil ST, Lee JH, Jacobsen PB, Bepler G, Lancaster J, et al. Physician referral for fertility preservation in oncology patients: A national study of practice behaviors. J Clin Oncol. (2009) 27:5952–7. doi: 10.1200/JCO.2009.23.0250
37. Shimizu C, Bando H, Kato T, Mizota Y, Yamamoto S, and Fujiwara Y. Physicians’ knowledge, attitude, and behavior regarding fertility issues for young breast cancer patients: A national survey for breast care specialists. Breast Cancer. (2013) 20:230–40. doi: 10.1007/s12282-011-0328-8
38. Baek SY, Lee KH, Kim SB, Gomez H, Vidaurre T, Park YH, et al. Knowledge, attitudes, and behaviors toward fertility preservation in patients with breast cancer: A cross-sectional survey of physicians. Front Oncol. (2023) 13:1109694. doi: 10.3389/fonc.2023.1109694
39. Lambertini M, Anserini P, Fontana V, Poggio F, Iacono G, Abate A, et al. The PREgnancy and FERtility (PREFER) study: An Italian multicenter prospective cohort study on fertility preservation and pregnancy issues in young breast cancer patients. BMC Cancer. (2017) 17:346. doi: 10.1186/s12885-017-3348-8
Keywords: breast cacner, pregnanacy, fertility preservation, endocrine therapy, young patient
Citation: Lee Y-J, Yoo T-K, Lee SB, Kim J, Chung I-Y, Ko BS, Lee JW, Son BH, Kim S and Kim HJ (2025) Pregnancy after breast cancer treatment in young patients. Front. Oncol. 15:1656429. doi: 10.3389/fonc.2025.1656429
Received: 30 June 2025; Accepted: 31 July 2025;
Published: 25 August 2025.
Edited by:
Ioannis Boutas, National and Kapodistrian University of Athens, GreeceReviewed by:
Marija Ban, University Hospital Split, CroatiaAdana A. M. Llanos, Columbia University, United States
Copyright © 2025 Lee, Yoo, Lee, Kim, Chung, Ko, Lee, Son, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hee Jeong Kim, aGVlamVvbmdraW0uYnJAZ21haWwuY29t
 Young-Jin Lee
Young-Jin Lee Tae-Kyung Yoo
Tae-Kyung Yoo Sae Byul Lee
Sae Byul Lee Jisun Kim
Jisun Kim Il-Yong Chung
Il-Yong Chung Beom Seok Ko1
Beom Seok Ko1 Jong Won Lee
Jong Won Lee Hee Jeong Kim
Hee Jeong Kim