- 1School of Clinical Medicine, Chengdu Medical College, Chengdu, Sichuan, China
- 2Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Chengdu Medical College, Chengdu, Sichuan, China
- 3Key Laboratory of Geriatric Respiratory Diseases of Sichuan Higher Education Institutes, Chengdu, Sichuan, China
- 4General Practice Department, The First Affiliated Hospital of Chengdu Medical College, Chengdu, Sichuan, China
- 5Department of Pulmonary and Critical Care Medicine, The First People’s Hospital of Chengdu City, Chengdu, Sichuan, China
Background: Sarcopenia is prevalent among patients undergoing pancreaticoduodenectomy (PD). However, the effect of sarcopenia on postoperative complications and the prognosis of patients undergoing PD remain controversial. This meta-analysis aimed to evaluate the potential use of sarcopenia as a prognostic indicator in patients undergoing PD.
Methods: A systematic search was conducted using the databases of Web of Science, EMBASE, China National Knowledge Infrastructure, Cochrane Library, and PubMed from inception to March 14, 2025, to identify studies on sarcopenia in patients undergoing PD. The pooled prevalence of sarcopenia and its 95% confidence interval (CI) were calculated, and heterogeneity was assessed using the I² test. Associations between sarcopenia and major postoperative complications, postoperative pancreatic fistula (POPF), postoperative biliary fistula (POBF), mortality, disease-free survival (DFS), and overall survival (OS) were expressed as odds ratios (ORs) or hazard ratios (HRs) with 95% CIs. Statistical analyses were performed using Stata version 11.0.
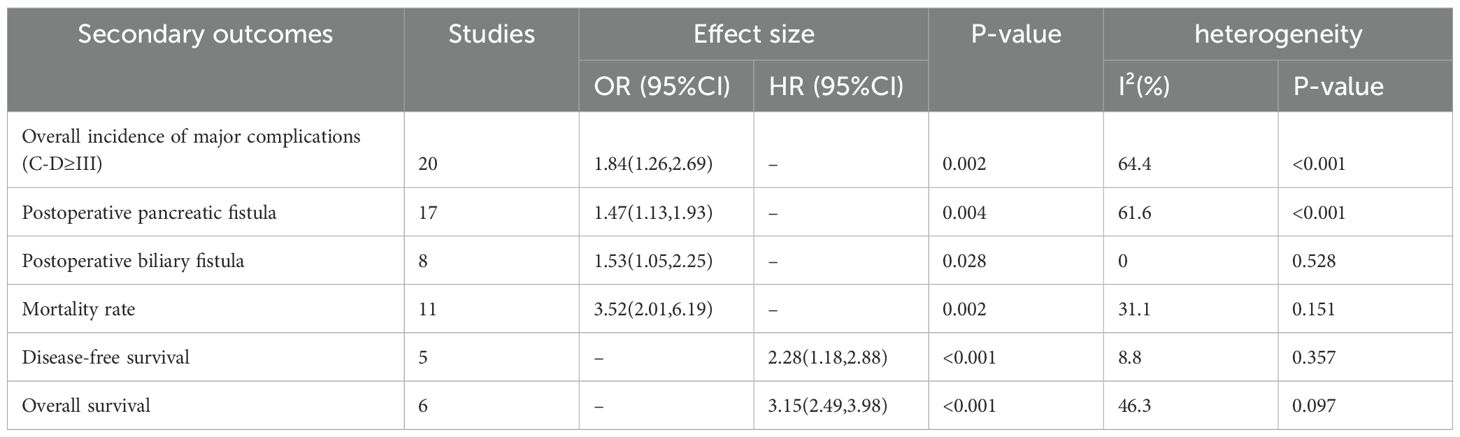
Results: This meta-analysis included 30 articles involving 5,323 participants. The prevalence of sarcopenia before PD was 35%. Patients with sarcopenia exhibited a significantly higher risk of major complications (Clavien–Dindo [CD] grade ≥ III) (OR = 1.84, 95% CI = 1.26–2.69, P = 0.002), POPF (OR = 1.47, 95% CI = 1.13–1.93, P = 0.004), and POBF (OR = 1.53, 95% CI = 1.05–2.25, P = 0.028) than those without sarcopenia. In addition, postoperative mortality was higher in patients with sarcopenia (OR = 3.52, 95% CI = 2.01–6.19, P = 0.002). Patients without sarcopenia exhibited better DFS and OS after PD than those with sarcopenia (DFS: HR = 2.28, 95% CI = 1.18–2.88, P < 0.001; OS: HR = 3.15, 95% CI = 2.49–3.98, P < 0.001).
Conclusion: A high proportion of patients presented with sarcopenia before undergoing PD. Patients undergoing PD with sarcopenia face a higher risk of overall incidence of major complications (CD grade ≥ III), POPF, POBF, and mortality, and they exhibit worse DFS and OS than those without sarcopenia. Future studies should adopt stricter definitions of sarcopenia to further validate these findings.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42025635939, identifier CRD42025635939.
1 Introduction
Pancreaticoduodenectomy (PD) is a complex surgical procedure for treating benign and malignant diseases in the pancreatic head, periampullary region, and distal common bile duct (1). The procedure involves the resection of the affected pancreatic tissue, along with segments of the duodenum, common bile duct, gallbladder, and portions of the stomach (2). Despite advancements in surgical approaches and perioperative management, PD remains a technically challenging and high-risk procedure. The postoperative complication rates of PD range from 30%–50% (3), emphasizing the necessity of identifying key risk factors.
Recent studies have highlighted the significant effect of sarcopenia on the clinical outcomes and prognosis of patients undergoing major surgeries (4). Sarcopenia is characterized by the progressive loss of skeletal muscle mass and is often accompanied by diminished muscle strength and an impaired capacity to perform daily activities (5, 6). Affected individuals typically experience reduced mobility, lower quality of life, and higher risk of adverse outcomes such as falls and mortality (7, 8). Contributing factors to sarcopenia include malnutrition, hormonal changes, chronic inflammation, alteration in gut microbiota, physical inactivity, and genetic and psychosocial influences (9–11). This condition is prevalent among older patients (12, 13) and is associated with a poor prognosis across various cancer types (14, 15). Sarcopenia is more common in patients undergoing PD. Balcer (16) reported that 49% of patients undergoing PD exhibited sarcopenia, with 10% diagnosed with sarcopenic obesity. Patients with sarcopenia often present with low body mass index (BMI), low skeletal muscle index (SMI), and reduced subcutaneous fat. The SMI at the third lumbar vertebra, derived from computed tomography (CT), is a reliable indicator of sarcopenia (17). For patients undergoing PD, routine CT scans are valuable for assessing tumor lesions and monitoring metastasis and for evaluating skeletal muscle mass without the need for additional radiation exposure.
However, the effect of comorbid sarcopenia on clinical outcomes and prognosis after PD remains unclear. Previous meta-analyses have identified sarcopenia as a prevalent comorbidity in patients undergoing PD, with those exhibiting preoperative sarcopenia experiencing higher morbidity, higher mortality, and poorer prognosis (18). Although several studies have investigated the association between sarcopenia and complications in patients undergoing PD, their findings remain inconclusive. This study aimed to evaluate the effect of sarcopenia on postoperative outcomes in patients undergoing PD and to provide a robust evidence base to inform perioperative management strategies.
2 Methods
2.1 Literature search strategy
This study adhered to the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (2020) guidelines, and the protocol was registered with PROSPERO (CRD42025635939). The literature search, conducted by Jie He and Jia Liu, utilized the PubMed, Web of Science, Cochrane Library, China National Knowledge Infrastructure, WanFang, and Embase databases. The search spanned from the inception of the databases to March 14, 2025, and included only articles published in Chinese and English. Key search terms included “sarcopenia,” “frailty,” “muscle weakness,” “muscle atrophy,” “pancreaticoduodenectomy,” “Whipple procedure,” “pancreaticoduodenectomies,” “duodenopancreatectomy,” and “pancreatoduodenectomy.” Additionally, the references cited within the identified articles were reviewed. The search strategies employed across all databases were outlined.
2.2 Eligibility criteria
The inclusion criteria were as follows: (1) observational design, including cross-sectional, case–control, and cohort studies, regardless of sample size; (2) studies that diagnosed sarcopenia and PD using validated methods, defining sarcopenia as reduced muscle mass and strength with low physical performance; and (3) a study population comprising individuals who underwent PD. Included studies were required to provide access to the full text and allow for accurate data extraction. The exclusion criteria encompassed reviews, systematic reviews, case reports, commentaries, non-clinical trials, and duplicate publications based on the same cohort. Furthermore, studies lacking critical clinical data or outcome measures, or those exhibiting substantial risk of bias, were excluded.
2.3 Data extraction
The study data were independently extracted by two authors (Jie He and Jia Liu), and discrepancies were resolved through discussions. If consensus could not be reached, a third investigator adjudicated the issue. Key extracted parameters included baseline information (first author, country, publication date, study duration, study design, sample size, mean age, disease type, BMI, diagnostic criteria, and sarcopenia prevalence) and clinical outcome measures (Clavien–Dindo [CD] grade ≥ III complications, grade B/C postoperative pancreatic fistula [POPF], postoperative biliary fistula [POBF], mortality, disease-free survival [DFS], and overall survival [OS]) (19). Continuous variables were summarized as means and standard deviations (SDs); for studies reporting medians or ranges, means and SDs deviations were estimated using Hozo’s method (20).
2.4 Literature quality assessment
Study quality was independently assessed by at least two authors (Meng Liu and Jie He) by using standardized assessment tools. The risk of bias in the included studies was assessed with the Joanna Briggs Institute’s critical appraisal checklist (Supplementary Table 1). Prognostic studies were assessed using the Quality in Prognostic Studies (QUIPS) tool (21), which evaluates risk of bias across six key areas: selection bias, attrition bias, measurement bias of prognostic factors, measurement bias of outcomes, confounding factors, and bias related to statistical analysis and result presentation. The QUIPS tool was selected as the most suitable method for assessing the quality of the studies under review. We slightly modified the original tool by introducing the “not applicable” option for rating items in the bias domains. We employed three rating levels, namely, high, moderate, and low, to evaluate the risk of bias in each domain. A study was deemed to have a high or moderate risk of bias if any domain received a high or moderate rating. Conversely, a study was considered to have a low risk of bias if all six domains were rated as low risk. Disagreements during quality assessment were addressed through discussions by the reviewers (Jia Li and Jia Liu) or resolved by expert arbitration (Jiaqing Jiang) when necessary.
2.5 Outcome measures
The study aimed to: (1) examine the sarcopenia prevalence in patients undergoing PD; (2) examine the association between sarcopenia and key complications, including pancreatic fistula, biliary fistula, and mortality in patients undergoing PD; (3) investigate the effect of sarcopenia on the prognosis of patients undergoing PD.
2.6 Statistical analysis
RevMan version 5.3.5 and Stata version 11.0 (Cochrane Collaboration, Oxford, UK) were utilized for the meta-analysis. Sarcopenia prevalence was calculated using raw data or reported prevalence (%). In longitudinal studies reporting prevalence at multiple time points, the overall prevalence for a specific period was used. A meta-analysis of prevalence was conducted using a generalized linear mixed model with a logit transformation and a fixed or random effects model. The relationships between sarcopenia occurrence and PD, and its effects on mortality and complications, were evaluated using adjusted odds ratios (ORs) with 95% confidence intervals (CIs) and adjusted hazard ratios (HRs) with 95% CIs, respectively. Heterogeneity was assessed using the I² statistic and Cochran’s Q test within random effects models. Intra-study heterogeneity was estimated via restricted maximum likelihood estimation, with significance determined by the Q value, which indicates whether moderator exploration is required, and the I² statistic, which quantifies the percentage of total variability attributable to heterogeneity (none: < 25%; low: 25%–50%; moderate: 51%–75%; high: ≥ 75%).
Subgroup analyses were conducted to identify the factors contributing to heterogeneity, including race and sarcopenia definition criteria. Publication bias was assessed using Egger’s test, Begg’s test, and funnel plots. A sensitivity analysis based on the leave-one-out approach was planned if a sufficient number of studies were available for evaluating the robustness of the findings. Statistical significance was set at a two-tailed P < 0.05.
3 Results
3.1 Eligible studies
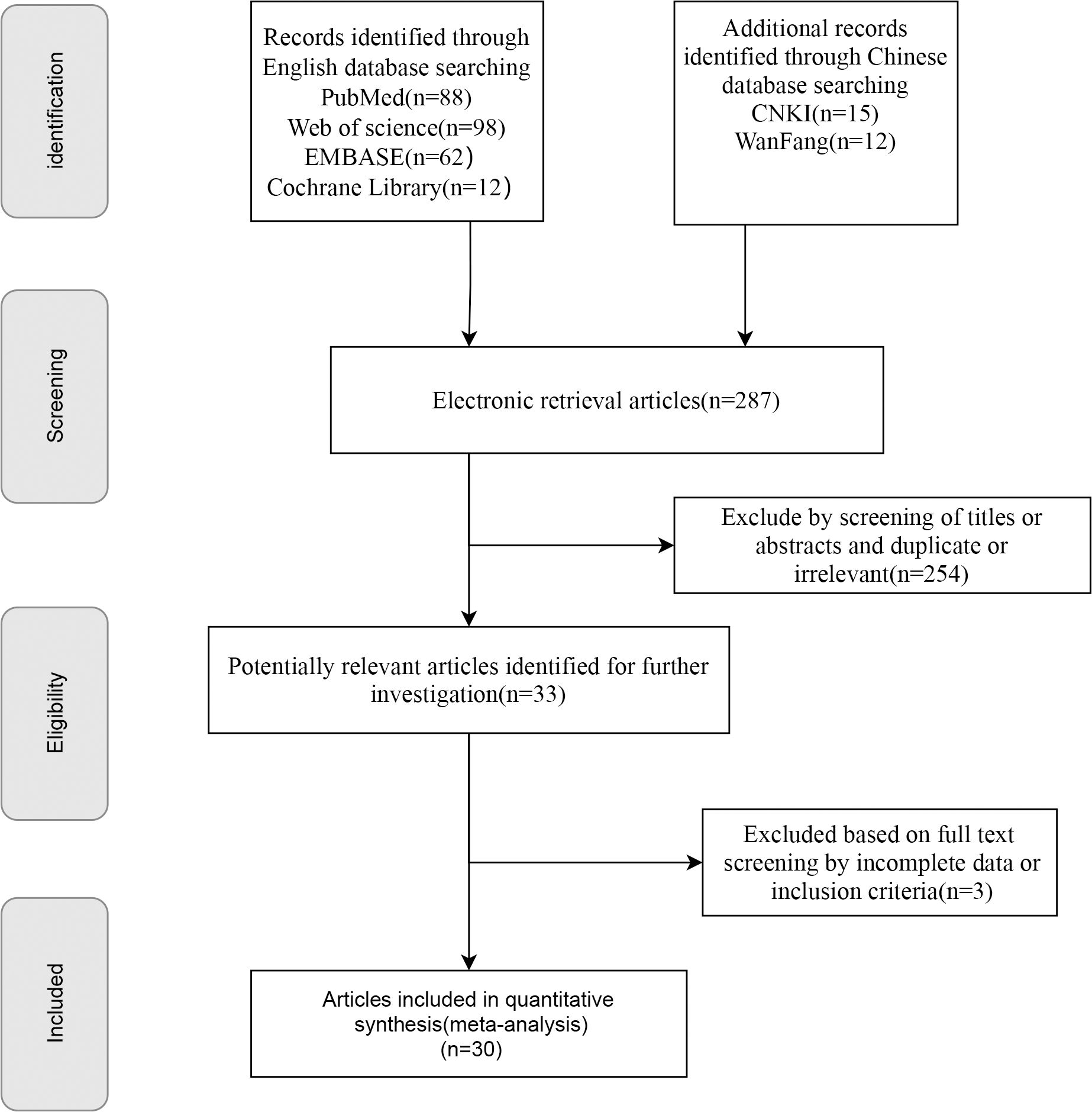
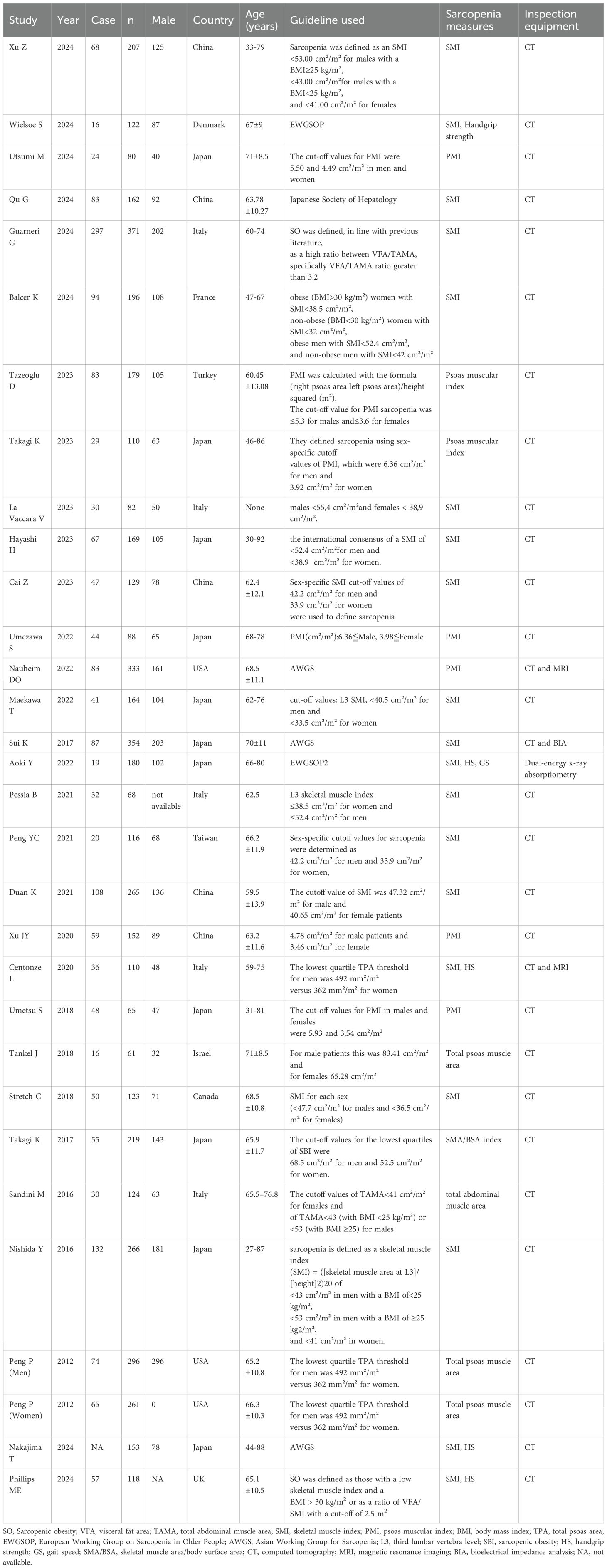
A total of 287 publications were retrieved. After multiple rounds of screening, 30 studies were included. The initial search yielded 287 articles, which were narrowed to 254 articles after removing duplicates; among these, 33 were selected for further analysis based on their titles and abstracts. The full texts of 33 articles were reviewed, resulting in the exclusion of three articles for the reasons outlined in Figure 1. Additional irrelevant or duplicate studies were excluded, leaving 30 articles that met the inclusion criteria (16, 22–50); among these, 28 examined the prevalence of sarcopenia in patients undergoing PD (16, 22–26, 29–45, 47–51), five investigated sarcopenic obesity (16, 27, 29, 33, 40), 19 focused on major complications (16, 23–27, 29–31, 34–36, 38–40, 44–46, 49), 11 addressed postoperative mortality (16, 23, 24, 30, 31, 36, 41, 42, 44, 47, 49), 17 explored POPF (26–32, 34, 36, 38, 41, 43–45, 47–49), three studies reported the differences in SMI between patients with and without POPF (28, 33, 34), eight examined POBF (26, 29, 30, 32, 34, 41, 43, 48), five reported on the relationship between sarcopenia and DFS (16, 25, 26, 30, 38), and six analyzed the association between sarcopenia and OS in patients undergoing PD (16, 25, 26, 30, 38, 40). All included studies were cohort studies. The screening details are presented in Figure 1, basic information on the included studies is presented in Table 1 and Supplementary Table 2, and the quality assessment is provided in Supplementary Tables 3 and 4.
3.2 Characteristics of the included articles
Table 1 and Supplementary Table 2 present the characteristics of the included articles. A total of 30 studies involving 5,323 patients were included. The age of the participants ranged from 27 to 88 years. Geographically, 18 studies were conducted in Asia, 8 in Europe, and 3 in North America. Eighteen articles used SMI to define sarcopenia, seven articles used the psoas muscle index (PMI)A to define sarcopenia, and five articles employed other indicators to define sarcopenia. Among these studies, 2 were prospective, and 26 were retrospective. Muscle mass was assessed using dual-energy X-ray absorptiometry, bioelectrical impedance analysis, or CT, whereas muscle strength was measured using a hand dynamometer (Table 1). Physical performance was evaluated based on gait speed, measured through 4-, 5-, and 6-minute walk tests. The quality assessment is presented in Supplementary Tables 3 and 4.
3.3 Meta-analysis results
3.3.1 Overall sarcopenia prevalence in patients undergoing PD (primary outcome)
The study indicated a preoperative sarcopenia prevalence of 35% (95% CI = 29%–41%) in patients undergoing PD (Figure 2A) with notable heterogeneity (P < 0.001; I² = 95%). When SMI was used as the detection indicator, the incidence of sarcopenia was 36% (95% CI = 27%–45%, I² = 96.0%); when PMI was used, the incidence was 41% (95% CI = 29%–54%, I², 92.0%). The results additionally revealed a 36% sarcopenia prevalence in Asian patients undergoing PD (95% CI = 28%–43%, I² = 94.3%, P < 0.001), which is lower than the 40% prevalence observed in Caucasian patients undergoing PD (95% CI = 24%–56%, I² = 98.6%, P < 0.001) (Table 2). Regarding age, the prevalence in patients undergoing PD aged < 65 years (37%, 95% CI = 26%–48%, I² = 98.1%, P < 0.001) was lower than those aged > 65 years (39%, 95% CI = 34%–45%, I² = 79.7%, P < 0.001) (Table 2).
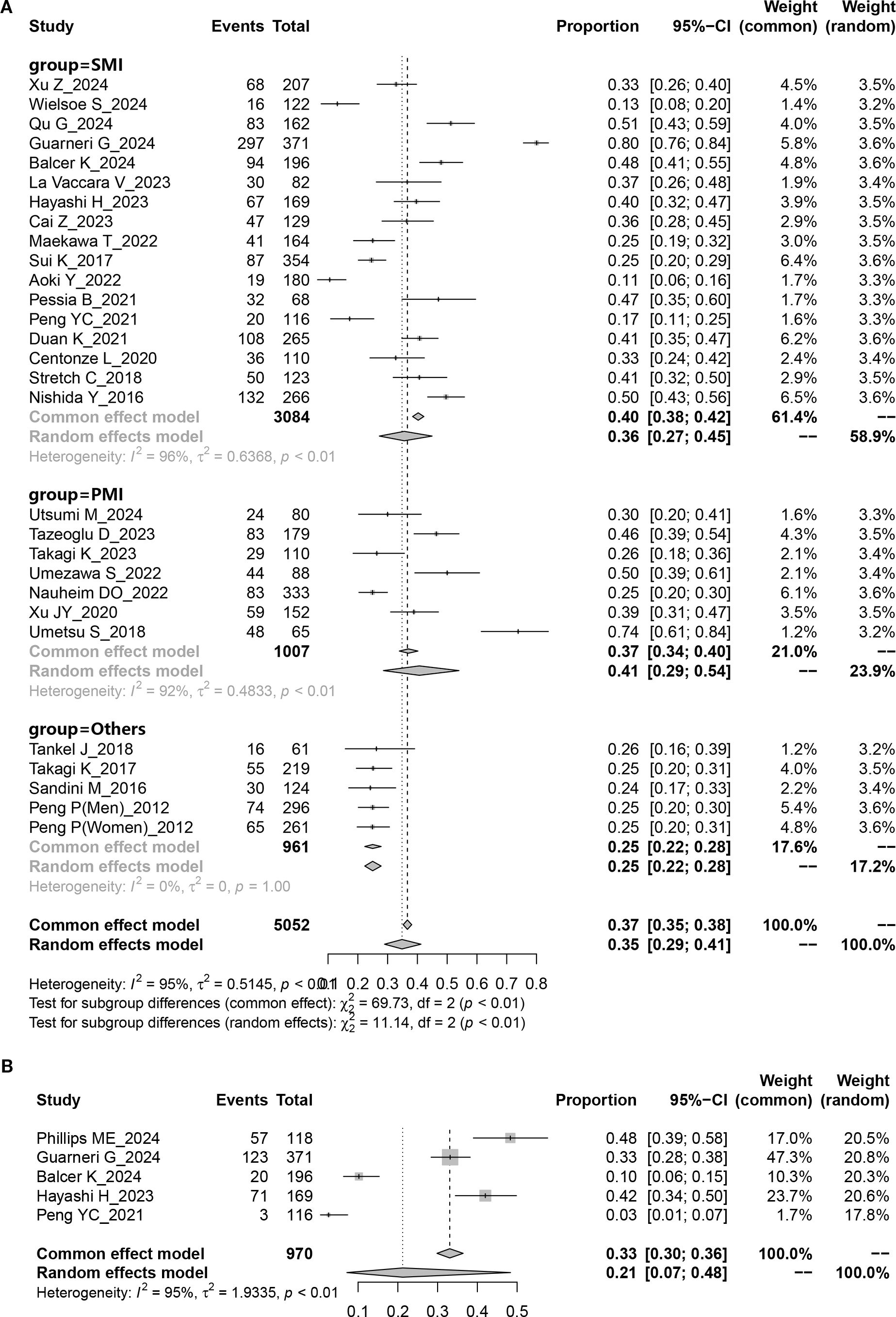
Figure 2. Pooled overall prevalence of sarcopenia and sarcopenic obesity in patients undergoing pancreaticoduodenectomy. (A) sarcopenia; (B) sarcopenic obesity.
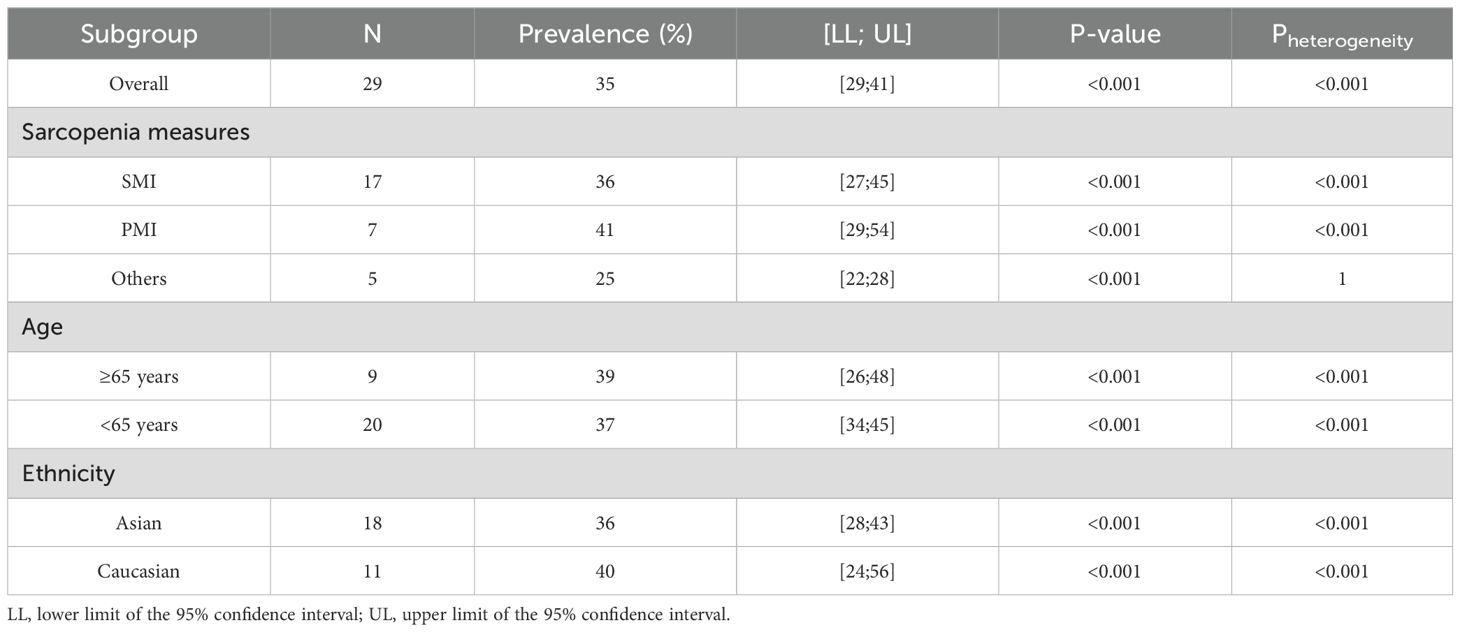
Table 2. Comparison of sarcopenia prevalence in patients undergoing pancreaticoduodenectomy regarding age, ethnicity, and sarcopenia assessments.
3.3.1.1 Publication bias and sensitivity analysis
Funnel plots and Egger’s and Begg’s tests were used to assess potential biases in the literature inclusion process. The funnel plot shows a symmetrical inverted funnel shape. Statistical tests showed no significant bias, with Egger’s and Begg’s tests yielding P = 0.583 and P = 0.103, respectively. These results suggest the absence of publication bias. A sensitivity analysis was subsequently conducted by sequentially excluding individual studies. No statistically significant variations were observed in the results, thus reinforcing the robustness of our findings (Supplementary Figures 1A, B).
3.3.1.2 Overall sarcopenic obesity prevalence in patients undergoing PD (primary outcome)
Five studies provided data on the prevalence of preoperative sarcopenic obesity in patients undergoing PD. The results showed that the overall preoperative sarcopenic obesity prevalence was 21% (95% CI = 0.07%–48%) (Figure 2B), with substantial heterogeneity (P < 0.001, I2 = 95.0%).
3.3.1.3 Publication bias and sensitivity analysis
The funnel plot was symmetrical, and both Egger’s test (P = 0.291) and Begg’s test (P = 0.260) yielded non-significant results, indicating the absence of publication bias. Sensitivity analysis, excluding one study at a time, showed no significant differences in outcomes, thus further supporting its robustness (Supplementary Figures 2A, B).
3.3.2 Secondary outcomes
3.3.2.1 Overall incidence of major complications (CD grade ≥ III)
Twenty studies reported the incidence of major postoperative complications (CD grade ≥ III) in patients with sarcopenia and matched controls. Most studies indicated a significantly higher incidence of major complications in patients with sarcopenia than in the controls, with an overall rate 1.84 times higher (OR = 1.84, 95% CI = 1.26–2.69, P = 0.002) (Figure 3, Table 3).
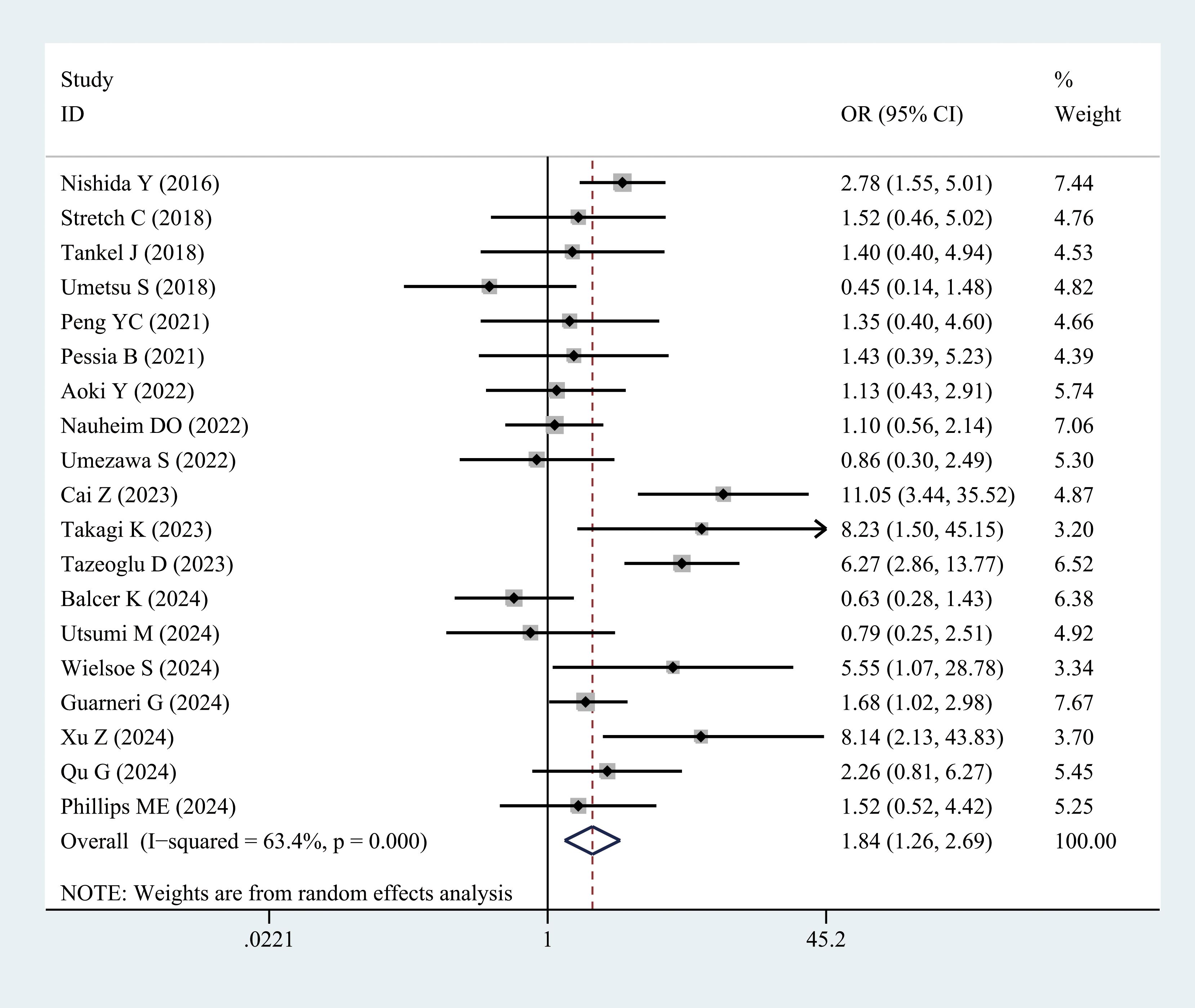
Figure 3. Comparison of the overall rate of major complications (Clavien–Dindo grade ≥ III) between the sarcopenia and non-sarcopenia groups.
3.3.2.2 POPF
Grades B and C fistulas were defined as clinically relevant POPF. Seventeen studies examined the incidence of POPF in patients with sarcopenia compared with the controls. Meta-analysis results revealed a higher incidence of POPF in patients with sarcopenia (OR = 1.47, 95% CI = 1.13–1.93, P = 0.004) (Figure 4, Table 3). POPF is a major complication of PD. Three studies compared the differences in SMI between patients with and without POPF. Nakajima et al. (28) and Hayashi et al. (33) reported that SMI values in patients with POPF were slightly higher than those in patients without POPF. However, Cai et al. (34) demonstrated that the SMI values were lower in patients with POPF, as presented in Supplementary Figure 3.
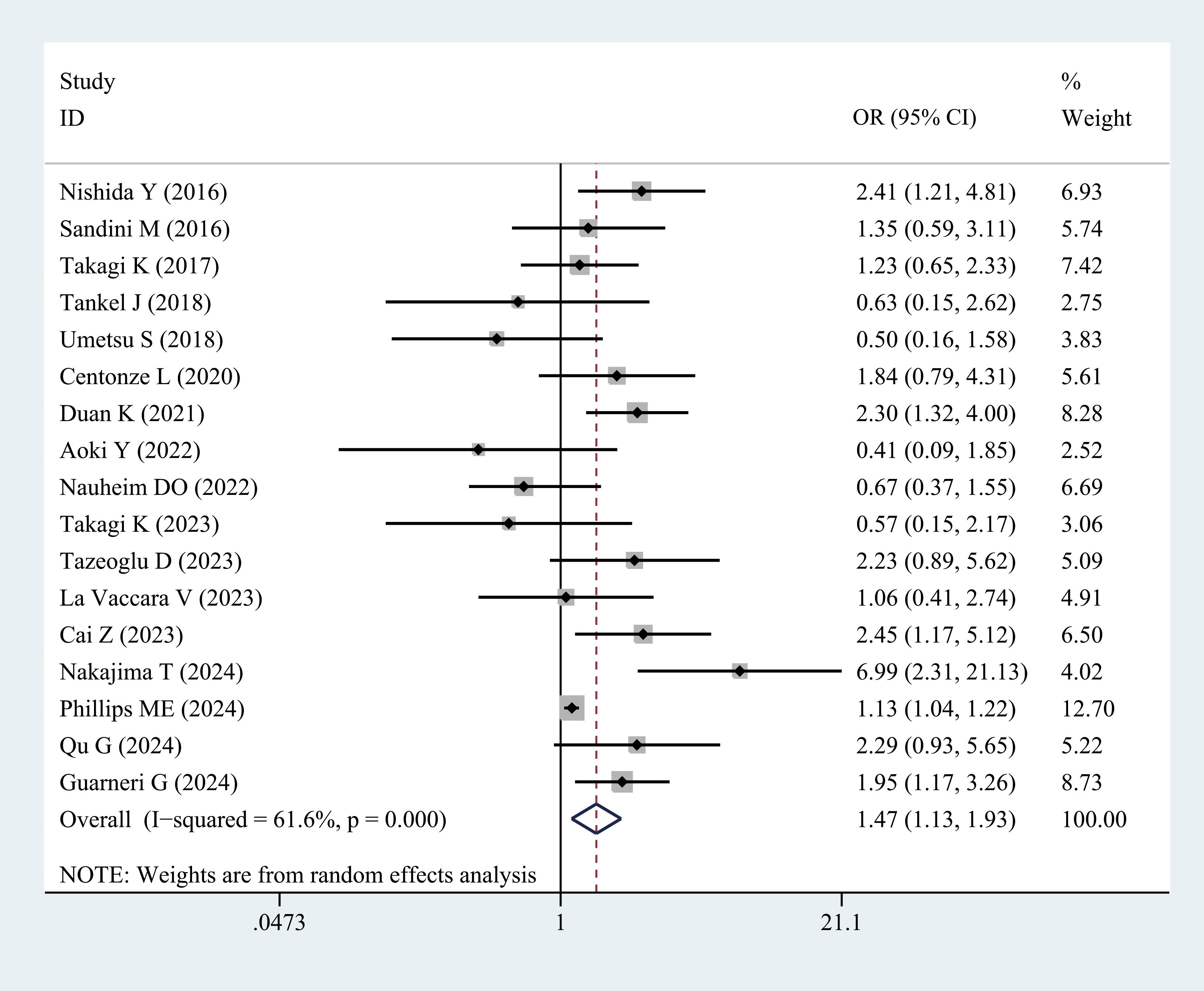
Figure 4. Comparison of the overall rate of postoperative pancreatic fistula between the sarcopenia and non-sarcopenia groups.
3.3.2.3 POBF
Eight studies reported the incidence of POBF in patients with sarcopenia and controls. The results demonstrated a significantly higher incidence of POBF in patients with sarcopenia (OR = 1.53, 95% CI = 1.05–2.25, P = 0.028) (Figure 5, Table 3).
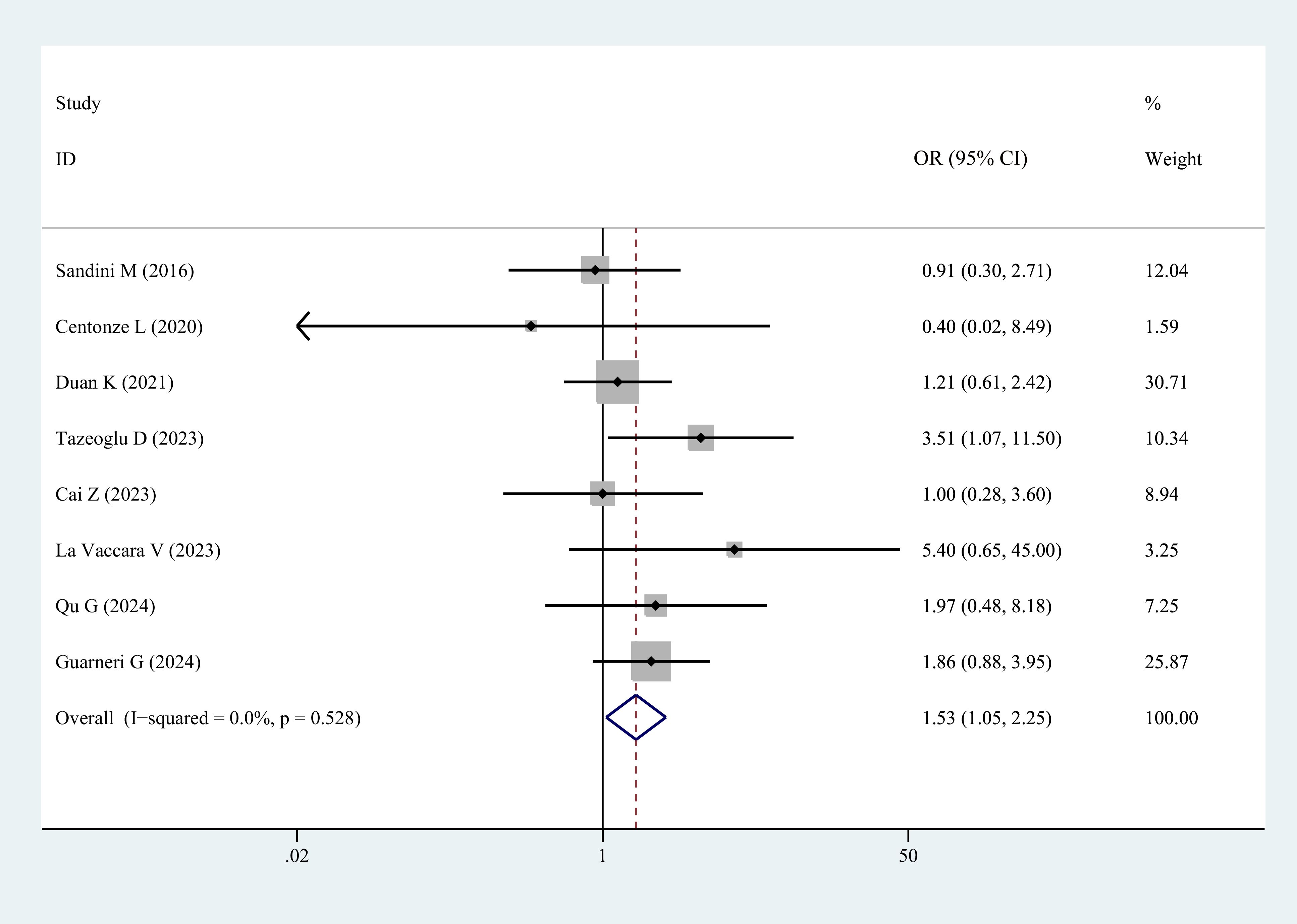
Figure 5. Comparison of the overall rate of postoperative biliary fistula between the sarcopenia and non-sarcopenia groups.
3.3.2.4 Mortality rate
Eleven studies reported on postoperative mortality. The results demonstrated that patients with sarcopenia exhibited a higher mortality rate (OR = 3.52, 95% CI = 2.01–6.19, P = 0.002) (Figure 6, Table 3).
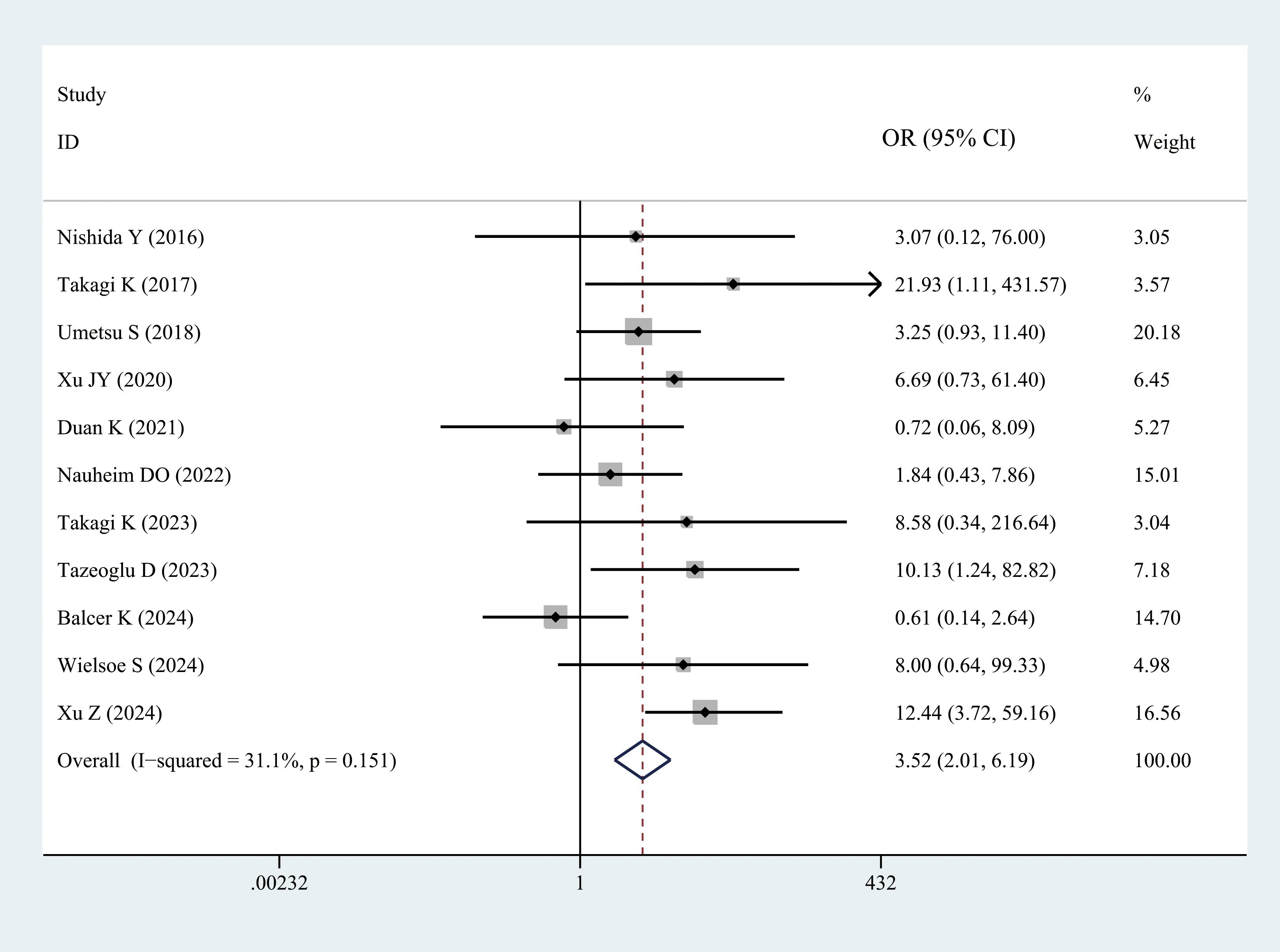
Figure 6. Comparison of the overall mortality rate between the sarcopenia and non-sarcopenia groups.
3.3.2.5 DFS
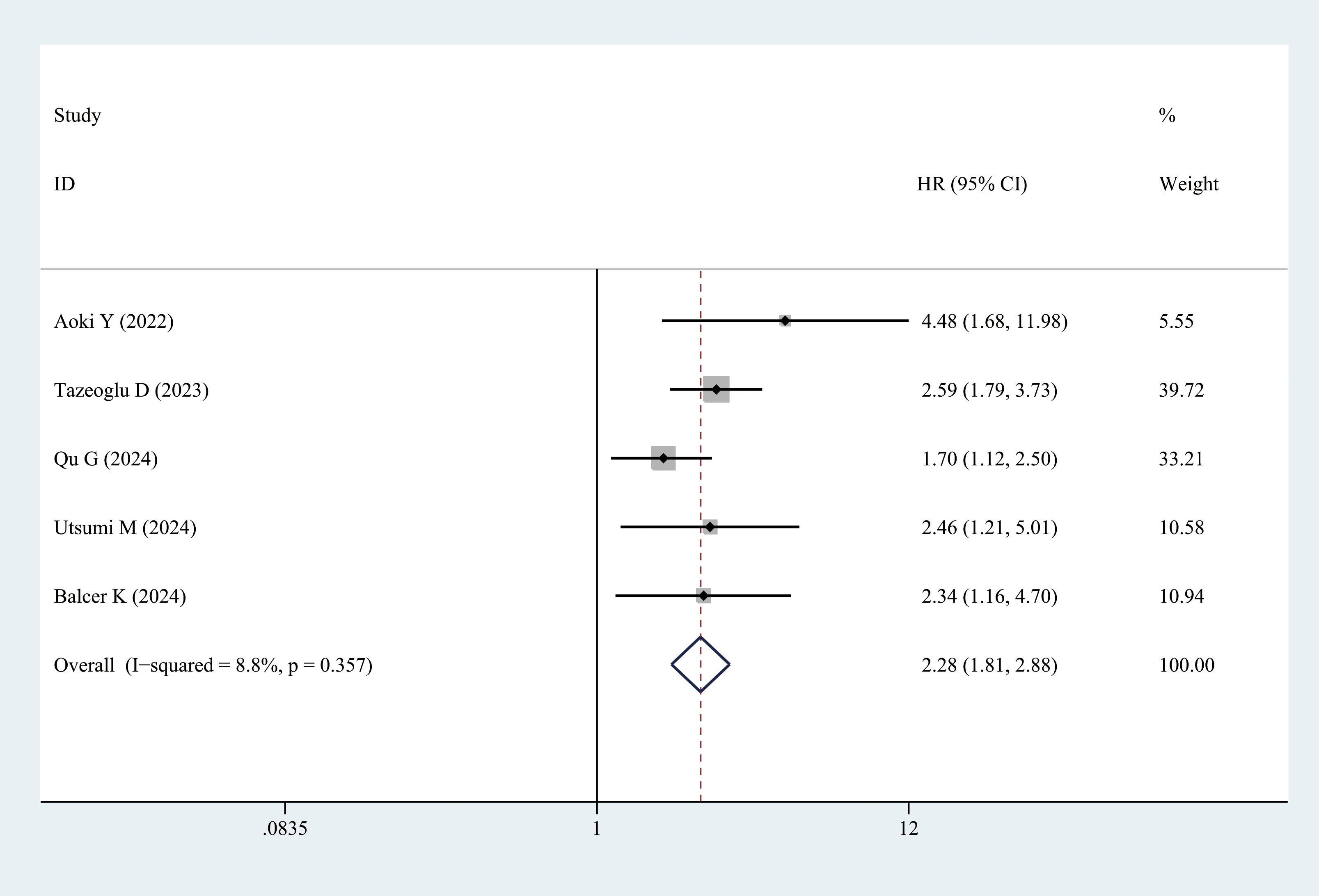
Five studies provided DFS data. Patients with sarcopenia exhibited significantly lower DFS than those without sarcopenia (multivariate analysis: HR = 2.28, 95% CI = 1.18–2.88, P < 0.001) (Figure 7, Table 3).
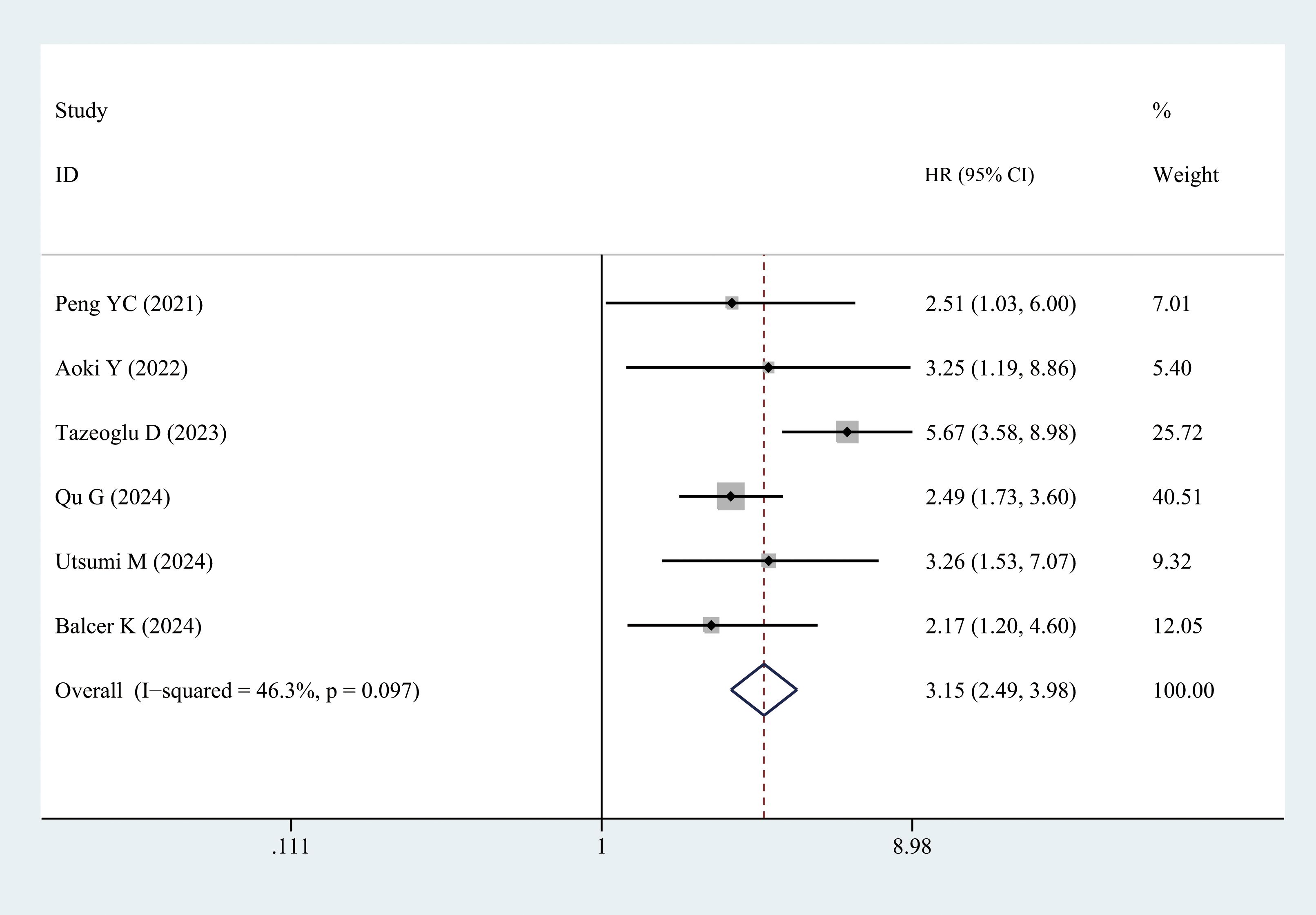
3.3.2.6 OS
Six studies reported the OS data. Patients with sarcopenia exhibited significantly worse OS than those without sarcopenia (multivariate analysis: HR = 3.15, 95% CI = 2.49–3.98, P < 0.001) (Figure 8, Table 3).
3.3.2.7 Publication bias
A funnel plot of publication bias across all secondary outcomes is presented in Supplementary Figure 4. The evaluation indicated that all the inverted funnel plots were roughly symmetric, thus suggesting a low risk of publication bias.
4 Discussion
Sarcopenia is characterized by a gradual decline in both muscle mass and function and is primarily driven by aging, lifestyle factors, and underlying pathological conditions (52). It is prevalent among older adults, with a reported incidence of up to 29%, and contributes significantly to increased disability and mortality (53). The progressive loss of skeletal muscle is a hallmark of sarcopenia, with studies indicating that muscle mass may decrease by as much as 6% annually after middle age (54). Recent studies have indicated a higher incidence of sarcopenia among individuals undergoing surgical interventions, particularly among those with malignancies. The incidence of sarcopenia in patients with liver cancer ranges from 11% to 45% (55). Similarly, sarcopenia affects 33% of patients with cholangiocarcinoma and gallbladder cancer (56), whereas the incidence in patients undergoing surgery for pancreatic cancer varies from 17% to 62% (57). Pancreaticobiliary tumors, which are often associated with obstructive jaundice, malnutrition, compromised intestinal mucosal integrity, and dysbiosis, are key contributors to preoperative sarcopenia (58). Consistent with these reports, the current meta-analysis revealed that 35% of patients undergoing PD presented with sarcopenia preoperatively, whereas 21% exhibited sarcopenic obesity. Consequently, the preoperative assessment of muscle mass and strength in patients undergoing PD is critical because sarcopenia may negatively influence clinical outcomes.
This study examined the prevalence of preoperative comorbid sarcopenia in patients undergoing PD and evaluated the effects of race, age, and diagnostic criteria on sarcopenia rates. These findings indicated significant racial variations in the prevalence of sarcopenia, which were probably due to differences in body composition, lifestyle factors, muscle mass, and strength assessments across geographic populations. Subgroup analysis further revealed a higher prevalence of comorbid sarcopenia in patients aged > 65 years than in those aged < 65 years, thus highlighting the strong association between aging and skeletal muscle loss. Sarcopenia is prevalent among older adults, with epidemiological studies in China reporting rates of 12.9% and 11.2% in community-dwelling men and women, respectively (59). Sarcopenia is characterized by age-related reduction in muscle mass and strength. A Japanese study found that 11.5% of men and 16.7% of women experienced varying degrees of skeletal muscle loss and hypofunction, with prevalence rates exceeding 50% in individuals > 80 years of age (60). Sarcopenia results from a combination of internal and external factors, and aging is a significant contributor. Age-related changes include substantial reductions in skeletal muscle mass, fiber size, strength, and endurance (61). Furthermore, aging is associated with increased systemic inflammation, which may lead to the overactivation of the ubiquitin–proteasome system (UPS). Protein degradation in skeletal muscles is primarily mediated by the UPS and the autophagy–lysosomal system pathways (62). Aging disrupts physiological homeostasis, thus leading to multiorgan dysfunction and frailty, particularly mitochondrial dysfunction; furthermore, aging plays a central role in the onset of sarcopenia (63, 64).
The term “sarcopenia” primarily refers to the loss of muscle mass; however, several international organizations advocate for diagnostic criteria that additionally incorporate reductions in muscle strength and/or physical function alongside muscle mass loss (65). Although this expanded diagnostic framework has gained widespread acceptance in geriatric medicine, cancer research continues to emphasize muscle mass as the primary diagnostic parameter. Most studies included in this analysis relied on a single method for diagnosing sarcopenia, and were predominantly retrospective. Studies that define sarcopenia using only the SMI or PMI lack sufficient rigor. Although CT is considered the gold standard for muscle mass assessment, it does not directly measure muscle strength. Notably, most studies reviewed in this research employed SMI, which was determined by measuring the muscle area on cross-sectional CT scans at the L3 level. However, some studies suggest that skeletal muscle strength and/or physical function may more accurately predict the prognostic relevance of cancer-related sarcopenia, particularly in patients with gastrointestinal tumors (66, 67). Therefore, additional prospective cohort studies are needed to determine whether these markers should be incorporated into sarcopenia diagnostics for patients undergoing PD.
Few comprehensive studies have explored the effect of sarcopenia on the clinical outcomes of patients undergoing PD. To address this gap, a meta-analysis of 30 studies involving 5323 participants was conducted. Six key factors were evaluated, including major complication rates (CD grade ≥ III), pancreatic fistula, biliary fistula, postoperative mortality, DFS, and OS. The analysis revealed a significant association between sarcopenia and several adverse outcomes in patients with sarcopenia compared with those without sarcopenia. Specifically, individuals with sarcopenia exhibited higher rates of major postoperative complications and pancreatic and biliary fistulas, as well as reduced DFS and OS rates. Patients with sarcopenia are often burdened with multiple comorbidities, including osteoporosis, cardiopulmonary insufficiency, and malignancies, and are more prone to malnutrition, skeletal muscle depletion, and fractures (68). Hu et al. (69) reported that sarcopenia was notably linked to diminished lung function and obstructive pulmonary disease, thus suggesting that muscle fiber atrophy associated with sarcopenia could impair respiratory muscle function. Preoperative respiratory insufficiency, prolonged bed rest after surgery, and pain from upper abdominal incisions may further compromise recovery and contribute to complications. In this study, the worse postoperative clinical outcomes and prognoses in patients with sarcopenia could be attributed to preexisting respiratory dysfunction.
Recent studies have demonstrated a strong association between sarcopenia and pancreatic fistula. Nishida et al. (49) evaluated the skeletal muscle area at the L3 level in 266 patients undergoing PD and found that the incidence of POPF was higher in patients with skeletal muscle depletion. Sarcopenia, second only to pancreatic cancer, is a key predictor of POPF complications. Jang et al. (70) similarly identified sarcopenia, particularly sarcopenic obesity, as an independent predictor of POPF complications in patients undergoing PD. This study revealed that the risk of POPF was significantly higher in patients with sarcopenia than in those without sarcopenia. However, whether patients with POPF truly have lower SMI values than those without POPF remains controversial, and this finding may be related to the sample sizes of the included studies. Patients with sarcopenia, particularly those with sarcopenic obesity, often experience systemic malnutrition, which may impair the healing (71). Additionally, a reduction in skeletal muscle and an increase in fat mass, particularly visceral fat, can alter the pancreatic texture, thus complicating pancreaticojejunostomy and increasing the risk of fistula formation (72). Furthermore, visceral fat contributes to surgical complications by releasing proinflammatory cytokines, which may hinder recovery and promote POPF development. Therefore, preoperative sarcopenia assessment should be emphasized in patients undergoing PD, as well as proactive nutritional and exercise interventions, to address malnutrition and muscle wasting. This approach may reduce the incidence of POPF and enhance surgical outcomes.
Sarcopenia significantly affects the perioperative course of PD. A decline in muscle function reduces postoperative mobility, whereas respiratory muscle weakness increases the risk of hypoxia, respiratory complications, and subsequent lung infections (73). Furthermore, as key metabolic organs, the muscles are crucial for the metabolism of proteins, amino acids, and carbohydrates. Loss of muscle mass disrupts the metabolism of these substances, thus predisposing patients to malnutrition before and after surgery. Recent studies (74–76) have further highlighted immune dysfunction, intestinal flora alteration, and elevated inflammatory marker levels (e.g., tumor necrosis factor, interleukin 6, and nuclear factor kappa-light-chain-enhancer of activated B cells) in patients with sarcopenia. These factors collectively impair surgical tolerance and increase perioperative risk. The findings of this meta-analysis emphasized that preoperative comorbid sarcopenia is a significant predictor of poor postoperative outcomes after PD. Studies have demonstrated that hormones secreted by muscle cells inhibit tumor cell growth (77). The reduced expression of these hormones in patients with sarcopenia may contribute to the proliferation and recurrence of tumors post-surgery. Research on patients with gastric cancer, cholangiocarcinoma, and hepatocellular carcinoma undergoing surgery identified sarcopenia as a negative prognostic factor for long-term survival after surgery (51, 78, 79). The clinical relevance of this study lies in its potential to identify patients with sarcopenia through the preoperative screening of patients undergoing PD. Sarcopenia can be evaluated across three domains, namely, muscle strength, muscle mass, and physical status, thus allowing for timely intervention. Nutritional strategies for sarcopenia focus on addressing malnutrition, ensuring adequate protein intake, supplementing with nutrients such as leucine and vitamin D, and modulating the gut microbiota. In addition, personalized exercise regimens, including tailored rehabilitation training at specific times, intensities, and cycles, should be developed based on the patient’s physical condition. A combination of resistance training and aerobic exercises is recommended (80, 81). These strategies offer significant benefits to patients undergoing PD.
Despite these strengths, this study has a few limitations: (1) All included articles are cohort studies and predominantly retrospective, thus necessitating further validation through randomized controlled trials. (2) Only English- and Chinese-language publications were considered, thus potentially introducing language bias and limiting the comprehensiveness of the review. (3) PD is a complicated surgery used to treat both non-cancerous and cancerous conditions in the pancreatic head, periampullary area, and distal common bile duct. The subgroup analysis was not conducted based on disease type. (4) Variations in sarcopenia diagnostic criteria and cutoff values across studies may have influenced the results. (5) POPF is the most common and dreaded complication following PD, with an incidence ranging from 9% to 50% (82–86). The incidence of POBF ranges from 4% to 12% (87–90). However, it can be hypothesized that combined fistulas (POPF/POBF) are associated with higher mortality rates than isolated POPF or POBF. Aghalarov et al. (91) reported that the incidence of POPF/POBF after PD ranges from 1.8% to 7.7%. Analyzing the effect of sarcopenia on POPF/POBF would be highly meaningful. Unfortunately, few studies have reported the simultaneous occurrence of POPF and POBF after PD. The studies included in our review provided separate data on POPF and POBF, making it impossible to extract valid data on combined fistulas from the literature. Therefore, further reports in this emerging research area are anticipated in the near future.
5 Conclusion
In conclusion, the prevalence of comorbid sarcopenia among patients undergoing PD prior to surgery was notably elevated, thus significantly influencing postoperative clinical outcomes. Patients undergoing PD with sarcopenia face a higher risk of major complications, clinically relevant POPF and POBF, increased mortality, and exhibit worse DFS and OS. Future research using a more precise definition of sarcopenia is essential to confirm our results. The preoperative screening and evaluation of sarcopenia should be prioritized, with proactive interventions targeting nutrition and exercise in patients undergoing PD to enhance clinical outcomes and overall prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JH: Software, Writing – original draft, Formal analysis, Writing – review & editing, Methodology, Data curation, Investigation. ML: Validation, Data curation, Writing – review & editing, Conceptualization, Investigation. JLi: Validation, Investigation, Writing – review & editing. JLiu: Validation, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Priming Scientific Research Foundation for the Introduced Talents of The First Affiliated Hospital of Chengdu Medical College (CYFY-GQ59).
Acknowledgments
We would like to thank Bullet Edits Limited for linguistic editing and proofreading of the manuscript. Moreover, the authors would like to thank Jiaqing Jiang, MD, for his efforts in cross-checking the results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1656834/full#supplementary-material
Supplementary Figure 1 | Sensitivity analysis and funnel plots for the prevalence of sarcopenia in patients undergoing pancreaticoduodenectomy. (A) Funnel plots of Begg’s test; (B) Sensitivity analysis.
Supplementary Figure 2 | Sensitivity analysis and funnel plots for the prevalence of sarcopenic obesity in patients undergoing pancreaticoduodenectomy. (A) Funnel plots of Begg’s test; (B) Sensitivity analysis.
Supplementary Figure 3 | Comparison of SMI value between the postoperative pancreatic fistula and non-postoperative biliary fistula groups.
Supplementary Figure 4 | Funnel plots for the secondary outcomes. (A) Major complications (Clavien–Dindo grade ≥ III); (B) Pancreatic fistula; (C) Biliary fistula; (D) Mortality rate; (E) Disease-free survival; (F) Overall survival.
References
1. Zhang J, Li S, Zhu W, Leng X, Gao J, and Zhang D. Subtotal gastrectomy pancreaticoduodenectomy versus conventional pancreaticoduodenectomy in the incidence of delayed gastric emptying: single-center retrospective cohort study. BMC Surg. (2022) 22:376. doi: 10.1186/s12893-022-01824-4, PMID: 36329420
2. Zafar HB. Surgical management of chronic pancreatitis: A systemic review. Cureus. (2023) 15:e35806. doi: 10.7759/cureus.35806, PMID: 36891174
3. Wang HY, Li MX, and Xiu DR. Shark mouth pancreaticojejunostomy: a new enteric reconstruction procedure of pancreatic stump. Chin Med J (Engl). (2019) 132:1354–8. doi: 10.1097/cm9.0000000000000219, PMID: 30896569
4. Bellos TC, Tzelves LI, Manolitsis IS, Katsimperis SN, Berdempes MV, Skolarikos A, et al. Sarcopenia in urinary bladder cancer: definition, prevalence and prognostic value in survival. Maedica (Bucur). (2022) 17:427–35. doi: 10.26574/maedica.2022.17.2.427, PMID: 36032591
5. Heymsfield SB, Smith B, Chung EA, Watts KL, Gonzalez MC, Yang S, et al. Phenotypic differences between people varying in muscularity. J Cachexia Sarcopenia Muscle. (2022) 13:1100–12. doi: 10.1002/jcsm.12959, PMID: 35170220
6. Milewska M, Przekop Z, Szostak-Węgierek D, Chrzanowska M, Raciborski F, Traczyk I, et al. Prevalence of risk of sarcopenia in polish elderly population-A population study. Nutrients. (2022) 14. doi: 10.3390/nu14173466, PMID: 36079726
7. Cornish SM, Cordingley DM, Shaw KA, Forbes SC, Leonhardt T, Bristol A, et al. Effects of omega-3 supplementation alone and combined with resistance exercise on skeletal muscle in older adults: A systematic review and meta-analysis. Nutrients. (2022) 14. doi: 10.3390/nu14112221, PMID: 35684018
8. Yin G, Qin J, Wang Z, Lv F, and Ye X. A nomogram to predict the risk of sarcopenia in older people. Med (Baltimore). (2023) 102:e33581. doi: 10.1097/md.0000000000033581, PMID: 37083805
9. Ticinesi A, Nouvenne A, Cerundolo N, Parise A, and Meschi T. Accounting gut microbiota as the mediator of beneficial effects of dietary (Poly)phenols on skeletal muscle in aging. Nutrients. (2023) 15. doi: 10.3390/nu15102367, PMID: 37242251
10. Buckinx F and Aubertin-Leheudre M. Sarcopenia in menopausal women: current perspectives. Int J Womens Health. (2022) 14:805–19. doi: 10.2147/ijwh.S340537, PMID: 35769543
11. Jee H, Park SH, and Park S. Evidence-based optimal cutoff values with the validation of criterion-referenced standards for sarcopenic elderly fitness improvement. BioMed Res Int. (2020) 2020:1638082. doi: 10.1155/2020/1638082, PMID: 32382528
12. González-Blanco L, Bermúdez M, Bermejo-Millo JC, Gutiérrez-Rodríguez J, Solano JJ, Antuña E, et al. Cell interactome in sarcopenia during aging. J Cachexia Sarcopenia Muscle. (2022) 13:919–31. doi: 10.1002/jcsm.12937, PMID: 35178901
13. Kumar P, Nayak K, Umakanth S, and Girish N. Effect of targeted intervention on C-terminal agrin fragment and its association with the components of sarcopenia: a scoping review. Aging Clin Exp Res. (2023) 35:1161–86. doi: 10.1007/s40520-023-02396-w, PMID: 36977974
14. Wei J, Xiang W, Wei H, Hu X, Lu Y, and Dong X. Impact of nutrition risk index, prognostic nutritional index and skeletal muscle index on early myelosuppression of first-line chemotherapy in stage IV gastric cancer patients. BMC Gastroenterol. (2024) 24:452. doi: 10.1186/s12876-024-03548-6, PMID: 39695992
15. Zhang Y, Zhang J, Zhan Y, Pan Z, Liu Q, and Yuan W. Sarcopenia is a prognostic factor of adverse effects and mortality in patients with tumour: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2024) 15:2295–310. doi: 10.1002/jcsm.13629, PMID: 39529263
16. Balcer K, Garnier J, Richa Y, Bruneel-Zupanc C, Piessen G, Turrini O, et al. Impact on survival of sarcopenia, systemic inflammatory response and anthropometric factors after pancreatectomy for resectable pancreatic adenocarcinoma. World J Surg Oncol. (2024) 22:232. doi: 10.1186/s12957-024-03510-6, PMID: 39232731
17. Dong D, Shi JY, Shang X, Liu B, Xu WL, Cui GZ, et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma treated with lenvatinib: A retrospective analysis. Med (Baltimore). (2022) 101:e28680. doi: 10.1097/md.0000000000028680, PMID: 35119010
18. Zhang QH, Ma JD, Lu YM, Zhang RN, Zhao ZH, Li YT, et al. Sarcopenia adversely impacts clinical outcomes in patients undergoing pancreaticoduodenectomy: A systematic review and meta-analysis. World J Gastrointest Surg. (2024) 16:1857–70. doi: 10.4240/wjgs.v16.i6.1857, PMID: 38983342
19. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. (2017) 161:584–91. doi: 10.1016/j.surg.2016.11.014, PMID: 28040257
20. Hozo SP, Djulbegovic B, and Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13, PMID: 15840177
21. Hayden JA, van der Windt DA, Cartwright JL, Côté P, and Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009, PMID: 23420236
22. Sui K, Okabayshi T, Iwata J, Morita S, Sumiyoshi T, Iiyama T, et al. Correlation between the skeletal muscle index and surgical outcomes of pancreaticoduodenectomy. Surg Today. (2018) 48:545–51. doi: 10.1007/s00595-017-1622-7, PMID: 29285616
23. Xu Z, Chen J, Mou Y, Li O, and Zhou Y. Effects of nutritional status on short-term prognosis after minimally invasive pancreaticoduodenectom. Sci Rep. (2024) 14:29549. doi: 10.1038/s41598-024-81016-7, PMID: 39609488
24. Wielsøe S, Sundberg A, Kristensen TS, Christensen J, Sillesen M, Hansen CP, et al. Impact of sarcopenia and muscle strength on postoperative complication risk following pancreatic resection. Clin Nutr ESPEN. (2024) 64:263–73. doi: 10.1016/j.clnesp.2024.10.003, PMID: 39395757
25. Utsumi M, Inagaki M, Kitada K, Tokunaga N, Yunoki K, Okabayashi H, et al. Combination of sarcopenia and systemic inflammation-based markers for predicting the prognosis of patients undergoing pancreaticoduodenectomy for pancreatic cancer. PloS One. (2024) 19:e0305844. doi: 10.1371/journal.pone.0305844, PMID: 38913646
26. Qu G, Zhou C, Zhang Y, Lyu SC, and Lang R. Influence of sarcopenia on postoperative complications and long-term survival in pancreatic cancer patients undergone pancreaticoduodenectomy. Front Nutr. (2024) 11:1434630. doi: 10.3389/fnut.2024.1434630, PMID: 39027658
27. Phillips ME, Robertson MD, Bennett-Eastley K, Rowe L, Frampton AE, and Hart KH. Standard nutritional assessment tools are unable to predict loss of muscle mass in patients due to undergo pancreatico-duodenectomy: highlighting the need for detailed nutritional assessment. Nutrients. (2024) 16. doi: 10.3390/nu16091269, PMID: 38732516
28. Nakajima T, Ikuta S, Fujikawa M, Ikuta L, Matsuki G, Ichise N, et al. High hand grip strength is a significant risk factor and a useful predictor of postoperative pancreatic fistula following pancreaticoduodenectomy. Langenbecks Arch Surg. (2024) 409:85. doi: 10.1007/s00423-024-03274-3, PMID: 38438660
29. Guarneri G, Pecorelli N, Bettinelli A, Campisi A, Palumbo D, Genova L, et al. Prognostic value of preoperative CT scan derived body composition measures in resected pancreatic cancer. Eur J Surg Oncol. (2024) 50:106848. doi: 10.1016/j.ejso.2023.02.005, PMID: 36863915
30. Tazeoglu D, Benli S, Colak T, and Apaydin FD. Comparative analysis of the sarcopenia and HALP score on postoperative outcomes in pancreatic cancer patients after pancreatoduodenectomy. Pancreatology. (2023) 23:530–6. doi: 10.1016/j.pan.2023.05.006, PMID: 37210304
31. Takagi K, Inoue Y, Oba A, Ono Y, Sato T, Ito H, et al. Impact of sarcopenia on S1 adjuvant chemotherapy and prognosis in pancreatic cancer patients. Biosci Trends. (2023) 17:310–7. doi: 10.5582/bst.2023.01209, PMID: 37648468
32. La Vaccara V, Cascone C, Coppola A, Farolfi T, Cammarata R, Emerenziani S, et al. Role of preoperative sarcopenia in predicting postoperative complications and survival after pancreatoduodenectomy for pancreatic cancer. Ann Ital Chir. (2023) 94:45–51., PMID: 36810297
33. Hayashi H, Shimizu A, Kubota K, Notake T, Masuo H, Yoshizawa T, et al. A new fistula risk score using sarcopenic obesity and subcutaneous fat area for predicting postoperative pancreatic fistula after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. (2023) 30:792–801. doi: 10.1002/jhbp.1283, PMID: 36448256
34. Cai Z, Yang Y, Fu X, Mao L, and Qiu Y. Predictive value of body composition parameters for postoperative complications in patients who received pancreaticoduodenectomy. Eur Surg Res. (2023) 64:252–60. doi: 10.1159/000529429, PMID: 36754029
35. Umezawa S, Kobayashi S, and Otsubo T. Low preoperative psoas muscle mass index is a risk factor for distal cholangiocarcinoma recurrence after pancreatoduodenectomy: a retrospective analysis. World J Surg Oncol. (2022) 20:176. doi: 10.1186/s12957-022-02627-w, PMID: 35655260
36. Nauheim DO, Hackbart H, Papai E, Moskal D, Yeo CJ, Lavu H, et al. Preoperative sarcopenia is a negative predictor for enhanced postoperative recovery after pancreaticoduodenectomy. Langenbecks Arch Surg. (2022) 407:2355–62. doi: 10.1007/s00423-022-02558-w, PMID: 35593934
37. Maekawa T, Maehira H, Iida H, Mori H, Nitta N, Tokuda A, et al. Impact of preoperative muscle mass maintenance and perioperative muscle mass loss prevention after pancreatectomy: association between perioperative muscle mass and postoperative nutritional status. Pancreas. (2022) 51:1179–85. doi: 10.1097/mpa.0000000000002168, PMID: 37078943
38. Aoki Y, Furukawa K, Suzuki D, Takayashiki T, Kuboki S, Takano S, et al. Influence of sarcopenia as defined by EWGSOP2 on complications after pancreaticoduodenectomy and on the prognosis of pancreatic head cancer: A prospective cohort study. Nutrition. (2022) 99-100:111660. doi: 10.1016/j.nut.2022.111660, PMID: 35576875
39. Pessia B, Giuliani A, Romano L, Bruno F, Carlei F, Vicentini V, et al. The role of sarcopenia in the pancreatic adenocarcinoma. Eur Rev Med Pharmacol Sci. (2021) 25:3670–8. doi: 10.26355/eurrev_202105_25933, PMID: 34109576
40. Peng YC, Wu CH, Tien YW, Lu TP, Wang YH, and Chen BB. Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur Radiol. (2021) 31:2472–81. doi: 10.1007/s00330-020-07294-7, PMID: 32974690
41. Duan K, Gao X, Wei L, Gong M, Feng B, Zhou J, et al. Skeletal muscle depletion and nutrition support affected postoperative complications in patients who underwent pancreatoduodenectomy. Eur J Clin Nutr. (2021) 75:1218–26. doi: 10.1038/s41430-020-00851-9, PMID: 33483631
42. Xu JY, Li C, Zhang H, Liu Y, and Wei JM. Total psoas area index is valuable to assess sarcopenia, sarcopenic overweight/obesity and predict outcomes in patients undergoing open pancreatoduodenectomy. Risk Manag Healthc Policy. (2020) 13:761–70. doi: 10.2147/rmhp.S257677, PMID: 32753989
43. Centonze L, Di Sandro S, Lauterio A, De Carlis R, Botta F, Mariani A, et al. The impact of sarcopenia on postoperative course following pancreatoduodenectomy: single-center experience of 110 consecutive cases. Dig Surg. (2020) 37:312–20. doi: 10.1159/000504703, PMID: 31958796
44. Umetsu S, Wakiya T, Ishido K, Kudo D, Kimura N, Miura T, et al. Effect of sarcopenia on the outcomes after pancreaticoduodenectomy for distal cholangiocarcinoma. ANZ J Surg. (2018) 88:E654–e658. doi: 10.1111/ans.14304, PMID: 29388312
45. Tankel J, Dagan A, Vainberg E, Boaz E, Mogilevsky L, Hadas I, et al. Sarcopenia is associated with a greater incidence of delayed gastric emptying following pancreaticoduodenectomy. Clin Nutr ESPEN. (2018) 27:105–9. doi: 10.1016/j.clnesp.2018.05.011, PMID: 30144881
46. Stretch C, Aubin JM, Mickiewicz B, Leugner D, Al-Manasra T, Tobola E, et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PloS One. (2018) 13:e0196235. doi: 10.1371/journal.pone.0196235, PMID: 29723245
47. Takagi K, Yoshida R, Yagi T, Umeda Y, Nobuoka D, Kuise T, et al. Radiographic sarcopenia predicts postoperative infectious complications in patients undergoing pancreaticoduodenectomy. BMC Surg. (2017) 17:64. doi: 10.1186/s12893-017-0261-7, PMID: 28549466
48. Sandini M, Bernasconi DP, Fior D, Molinelli M, Ippolito D, Nespoli L, et al. A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition. (2016) 32:1231–7. doi: 10.1016/j.nut.2016.04.002, PMID: 27261062
49. Nishida Y, Kato Y, Kudo M, Aizawa H, Okubo S, Takahashi D, et al. Preoperative sarcopenia strongly influences the risk of postoperative pancreatic fistula formation after pancreaticoduodenectomy. J Gastrointest Surg. (2016) 20:1586–94. doi: 10.1007/s11605-016-3146-7, PMID: 27126054
50. Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. (2012) 16:1478–86. doi: 10.1007/s11605-012-1923-5, PMID: 22692586
51. Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: A meta-analysis. Ann Surg. (2018) 268:58–69. doi: 10.1097/sla.0000000000002679, PMID: 29373365
52. Cannataro R, Carbone L, Petro JL, Cione E, Vargas S, Angulo H, et al. Sarcopenia: etiology, nutritional approaches, and miRNAs. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22189724, PMID: 34575884
53. Papadopoulou SK. Sarcopenia: A contemporary health problem among older adult populations. Nutrients. (2020) 12. doi: 10.3390/nu12051293, PMID: 32370051
54. Cho MR, Lee S, and Song SK. A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J Korean Med Sci. (2022) 37:e146. doi: 10.3346/jkms.2022.37.e146, PMID: 35535373
55. Takada H, Kurosaki M, Nakanishi H, Takahashi Y, Itakura J, Tsuchiya K, et al. Impact of pre-sarcopenia in sorafenib treatment for advanced hepatocellular carcinoma. PloS One. (2018) 13:e0198812. doi: 10.1371/journal.pone.0198812, PMID: 29912922
56. Chakedis J, Spolverato G, Beal EW, Woelfel I, Bagante F, Merath K, et al. Pre-operative sarcopenia identifies patients at risk for poor survival after resection of biliary tract cancers. J Gastrointest Surg. (2018) 22:1697–708. doi: 10.1007/s11605-018-3802-1, PMID: 29855867
57. Kuan LL, Dennison AR, and Garcea G. Prevalence and impact of sarcopenia in chronic pancreatitis: A review of the literature. World J Surg. (2021) 45:590–7. doi: 10.1007/s00268-020-05828-0, PMID: 33165641
58. Churchward-Venne TA, Breen L, and Phillips SM. Alterations in human muscle protein metabolism with aging: Protein and exercise as countermeasures to offset sarcopenia. Biofactors. (2014) 40:199–205. doi: 10.1002/biof.1138, PMID: 24105883
59. Chen Z, Li WY, Ho M, and Chau PH. The prevalence of sarcopenia in chinese older adults: meta-analysis and meta-regression. Nutrients. (2021) 13. doi: 10.3390/nu13051441, PMID: 33923252
60. Kitamura A, Seino S, Abe T, Nofuji Y, Yokoyama Y, Amano H, et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. (2021) 12:30–8. doi: 10.1002/jcsm.12651, PMID: 33241660
61. Iyer SR, Hsia RC, Folker ES, and Lovering RM. Age-dependent changes in nuclear-cytoplasmic signaling in skeletal muscle. Exp Gerontol. (2021) 150:111338. doi: 10.1016/j.exger.2021.111338, PMID: 33862137
62. Martin-Rincon M, Pérez-López A, Morales-Alamo D, Perez-Suarez I, de Pablos-Velasco P, Perez-Valera M, et al. Exercise mitigates the loss of muscle mass by attenuating the activation of autophagy during severe energy deficit. Nutrients. (2019) 11. doi: 10.3390/nu11112824, PMID: 31752260
63. Rai R, Ghosh AK, Eren M, Mackie AR, Levine DC, Kim SY, et al. Downregulation of the apelinergic axis accelerates aging, whereas its systemic restoration improves the mammalian healthspan. Cell Rep. (2017) 21:1471–80. doi: 10.1016/j.celrep.2017.10.057, PMID: 29117554
64. Romanello V. The interplay between mitochondrial morphology and myomitokines in aging sarcopenia. Int J Mol Sci. (2020) 22. doi: 10.3390/ijms22010091, PMID: 33374852
65. Martin D, Maeder Y, Kobayashi K, Schneider M, Koerfer J, Melloul E, et al. Association between CT-based preoperative sarcopenia and outcomes in patients that underwent liver resections. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14010261, PMID: 35008425
66. Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, et al. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis. (2015) 17:O256–264. doi: 10.1111/codi.13067, PMID: 26194849
67. Wang SL, Zhuang CL, Huang DD, Pang WY, Lou N, Chen FF, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: A prospective study. Ann Surg Oncol. (2016) 23:556–64. doi: 10.1245/s10434-015-4887-3, PMID: 26668085
68. Lena A, Hadzibegovic S, von Haehling S, Springer J, Coats AJ, and Anker MS. Sarcopenia and cachexia in chronic diseases: from mechanisms to treatment. Pol Arch Intern Med. (2021) 131. doi: 10.20452/pamw.16135, PMID: 34775741
69. Hu Z, Tian Y, Song X, Zeng F, and Yang A. Associations between sarcopenia with asthmatic prevalence, lung function and comorbidity. BMC Geriatr. (2022) 22:703. doi: 10.1186/s12877-022-03394-9, PMID: 36002808
70. Jang M, Park HW, Huh J, Lee JH, Jeong YK, Nah YW, et al. Predictive value of sarcopenia and visceral obesity for postoperative pancreatic fistula after pancreaticoduodenectomy analyzed on clinically acquired CT and MRI. Eur Radiol. (2019) 29:2417–25. doi: 10.1007/s00330-018-5790-7, PMID: 30406311
71. Mintziras I, Miligkos M, Wächter S, Manoharan J, Maurer E, and Bartsch DK. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int J Surg. (2018) 59:19–26. doi: 10.1016/j.ijsu.2018.09.014, PMID: 30266663
72. Kirihara Y, Takahashi N, Hashimoto Y, Sclabas GM, Khan S, Moriya T, et al. Prediction of pancreatic anastomotic failure after pancreatoduodenectomy: the use of preoperative, quantitative computed tomography to measure remnant pancreatic volume and body composition. Ann Surg. (2013) 257:512–9. doi: 10.1097/SLA.0b013e31827827d0, PMID: 23241871
73. Deniz O, Coteli S, Karatoprak NB, Pence MC, Varan HD, Kizilarslanoglu MC, et al. Diaphragmatic muscle thickness in older people with and without sarcopenia. Aging Clin Exp Res. (2021) 33:573–80. doi: 10.1007/s40520-020-01565-5, PMID: 32406014
74. Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C, et al. Gut microbiota, muscle mass and function in aging: A focus on physical frailty and sarcopenia. Nutrients. (2019) 11. doi: 10.3390/nu11071633, PMID: 31319564
75. Bilen MA, Martini DJ, Liu Y, Shabto JM, Brown JT, Williams M, et al. Combined effect of sarcopenia and systemic inflammation on survival in patients with advanced stage cancer treated with immunotherapy. Oncologist. (2020) 25:e528–35. doi: 10.1634/theoncologist.2019-0751, PMID: 32162807
76. Shi Y, Zhou L, Yan E, Yang L, Yang C, and Liu C. Sarcopenia and perioperative management of elderly surgical patients. Front Biosci (Landmark Ed). (2021) 26:882–94. doi: 10.52586/4995, PMID: 34719213
77. Yu J and Zhou FX. Sarcopenia and gastrointestinal cancer. Electronic J Metab Nutr Cancer. (2017) 4:483–8. doi: 10.16689/j.cnki.cn11-9349/r.2017.04.020
78. Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. (2015) 261:1173–83. doi: 10.1097/sla.0000000000000743, PMID: 24950264
79. Yu H, Wang M, Wang Y, Yang J, Deng L, Bao W, et al. The prognostic value of sarcopenia combined with preoperative fibrinogen-albumin ratio in patients with intrahepatic cholangiocarcinoma after surgery: A multicenter, prospective study. Cancer Med. (2021) 10:4768–80. doi: 10.1002/cam4.4035, PMID: 34105304
80. Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr. (2021) 40:4745–61. doi: 10.1016/j.clnu.2021.03.031, PMID: 34242915
81. Tang G, Zhang L, Tao J, and Wei Z. Effects of perioperative probiotics and synbiotics on pancreaticoduodenectomy patients: A meta-analysis of randomized controlled trials. Front Nutr. (2021) 8:715788. doi: 10.3389/fnut.2021.715788, PMID: 34485364
82. Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): A systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Med (Baltimore). (2017) 96:e6858. doi: 10.1097/md.0000000000006858, PMID: 28489778
83. Dusch N, Lietzmann A, Barthels F, Niedergethmann M, Rückert F, and Wilhelm TJ. International study group of pancreatic surgery definitions for postpancreatectomy complications: applicability at a high-volume center. Scand J Surg. (2017) 106:216–23. doi: 10.1177/1457496916680944, PMID: 28376656
84. Eshmuminov D, Schneider MA, Tschuor C, Raptis DA, Kambakamba P, Muller X, et al. Systematic review and meta-analysis of postoperative pancreatic fistula rates using the updated 2016 International Study Group Pancreatic Fistula definition in patients undergoing pancreatic resection with soft and hard pancreatic texture. HPB (Oxford). (2018) 20:992–1003. doi: 10.1016/j.hpb.2018.04.003, PMID: 29807807
85. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. (2005) 138:8–13. doi: 10.1016/j.surg.2005.05.001, PMID: 16003309
86. Crippa S, Salvia R, Falconi M, Butturini G, Landoni L, and Bassi C. Anastomotic leakage in pancreatic surgery. HPB (Oxford). (2007) 9:8–15. doi: 10.1080/13651820600641357, PMID: 18333107
87. Antolovic D, Koch M, Galindo L, Wolff S, Music E, Kienle P, et al. Hepaticojejunostomy–analysis of risk factors for postoperative bile leaks and surgical complications. J Gastrointest Surg. (2007) 11:555–61. doi: 10.1007/s11605-007-0166-3, PMID: 17394045
88. Suzuki H, Shimura T, Mochhida Y, Wada S, Araki K, Kubo N, et al. To stent or not to stent hepaticojejunostomy–analysis of risk factors for postoperative bile leaks and surgical complication. Hepatogastroenterology. (2014) 61:920–6.
89. Herzog T, Belyaev O, Hessam S, Uhl W, and Chromik AM. Management of isolated bile leaks after pancreatic resections. J Invest Surg. (2014) 27:273–81. doi: 10.3109/08941939.2014.916368, PMID: 24830477
90. Burkhart RA, Relles D, Pineda DM, Gabale S, Sauter PK, Rosato EL, et al. Defining treatment and outcomes of hepaticojejunostomy failure following pancreaticoduodenectomy. J Gastrointest Surg. (2013) 17:451–60. doi: 10.1007/s11605-012-2118-9, PMID: 23292459
Keywords: pancreaticoduodenectomy, sarcopenia, postoperative complications, disease-free survival, overall survival, meta-analysis
Citation: He J, Li J, Liu J and Liu M (2025) Sarcopenia as a prognostic marker in patients undergoing pancreaticoduodenectomy: an updated meta-analysis. Front. Oncol. 15:1656834. doi: 10.3389/fonc.2025.1656834
Received: 30 June 2025; Accepted: 12 September 2025;
Published: 29 September 2025.
Edited by:
Tamer Saad Kaoud, The University of Texas at Austin, United StatesReviewed by:
Beata Jabłońska, Medical University of Silesia, KatowiceDenіz Tazeoglu, Osmaniye Il Saglik Mudurlugu, Türkiye
Copyright © 2025 He, Li, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie He, MjMyNUBjbWMuZWR1LmNu
†These authors have contributed equally to this work
 Jie He
Jie He Jia Li
Jia Li Jia Liu
Jia Liu Meng Liu
Meng Liu