- 1Department of Haematology, University College London (UCL) Cancer Institute, London, United Kingdom
- 2Department of Haematology, University College London Hospitals National Health Service (NHS) Foundation Trust, London, United Kingdom
As multiple myeloma (MM) patients live longer, maintaining quality of life (QOL) has become an important treatment goal. However, the commonly used general quality of life questionnaires (QOLQs) were developed over 20 years ago. In this survey, 224 MM patients and 48 healthcare professionals (HCPs) were asked to grade the relevance and importance of QOL items from 9 validated, frequently used MM QOLQs. The results from this survey highlighted significant discrepancy between MM patients’ and HCPs’ perception of important and relevant QOL issues. Whilst MM HCPs found all QOL items relevant, the patients reported a proportion of these items being relevant. These were mainly related to physical functioning, social/family wellbeing, pain and fatigue. This real-world survey stressed the need for the development of an updated QOLQ that is relevant to patients and current MM therapies.
Introduction
Multiple myeloma (MM) is the second most common haematological malignancy characterised by bone marrow proliferation of clonal plasma cells, resulting in anaemia, hypercalcaemia, renal failure and bone destruction. MM patients often experience more symptom burden than patients with some other cancers and have reduced health-related QOL (1). Several validated QOLQs are available to assess QOL in MM patients. Moreover, large clinical trials and real-world studies are increasingly incorporating QOL assessments into their study protocols (2). However, most of these QOLQs such as EORTC-QLQ-C30 and FACT-G are general cancer questionnaires that were designed over 20 years ago, and the MM treatment landscape has since changed dramatically. Aside from newer agents with specific toxicities, the increasing use of consolidation and maintenance combination therapies means that patients often have no treatment-free period. The aim of this survey was to investigate in real-world how relevant and important items from the most commonly used validated QOLQs were for MM patients and MM HCPs.
Methods
Myeloma patients and HCPs from a single academic MM centre were invited to participate in this specially designed online survey. Demographics, disease characteristics and treatment history were gathered in this anonymised survey. All 172 QOL items from EORTC QLQ-C30, EORTC QLQ-MY20, EORTC QLQ-CIPN20, EQ5D-3L, FACT-G, as well as additional items from the FACT-MM, FACT-N, FACIT-fatigue, FACT-BMT were listed in this survey and participants were asked to grade the relevance of each item in assessing QOL on a five-level Likert scale (not relevant, slightly relevant, relevant, fairly relevant, very relevant). Descriptive statistics (e.g., mean, median, range) were used to summarise the dataset. Weighted mean scores for each item were calculated based on participants’ responses (not relevant scored 1, slight relevant 2, relevant 3, fairly relevant 4, very relevant 5). Depending on where the scores lie in the interval scales, each item was then assigned an overall descriptive equivalent on the Likert scale (3) (See Supplementary Data).
Results
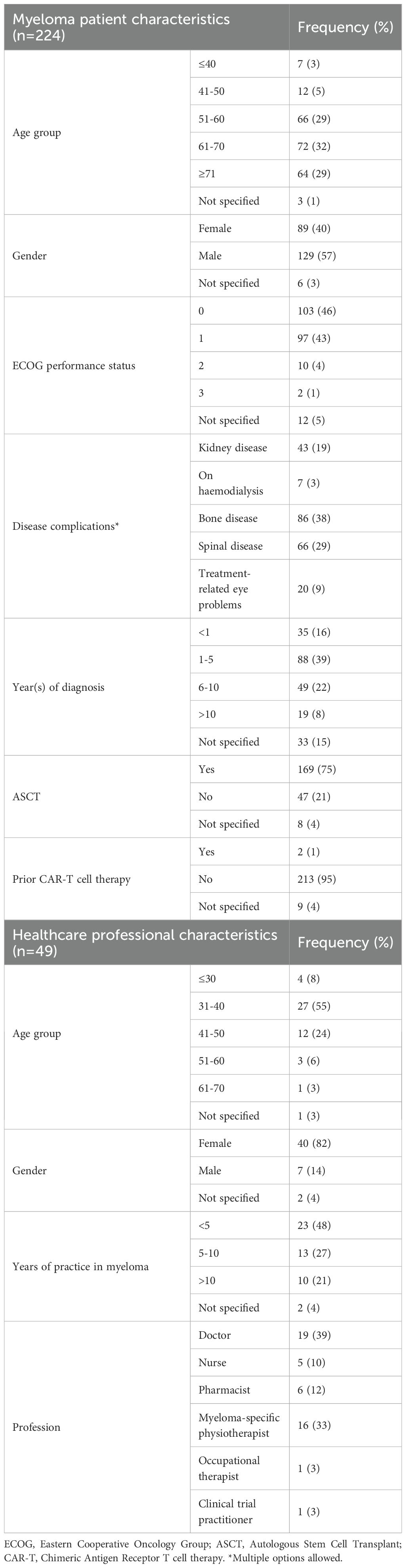
Two hundred- and twenty-four-MM patients (n=224) completed the survey. Their characteristics are shown in Table 1. The median age of MM patients was 64 (range 28-82) and the median number of years from diagnosis was 4 (range 1-22). Regarding symptom burden, 38% had bone disease, 29% had spinal disease, 19% had renal impairment and 9% had treatment-related eye problems. A quarter of the patients had newly diagnosed myeloma (25%, n=56). The median number of MM treatment lines was 1.5 (range 1-6). 21% received radiotherapy, 78% received a stem cell transplantation and 27% participated in a MM clinical trial. Patients completed 84% of all survey items (16% were skipped). Forty-eight HCPs completed the survey, including 19 doctors, 5 nurses, 6 pharmacists, 16 MM physiotherapists, 1 occupational therapist, 1 clinical trial practitioner. Nearly 50% (n=23) had over 5 years of experience in the field of MM.
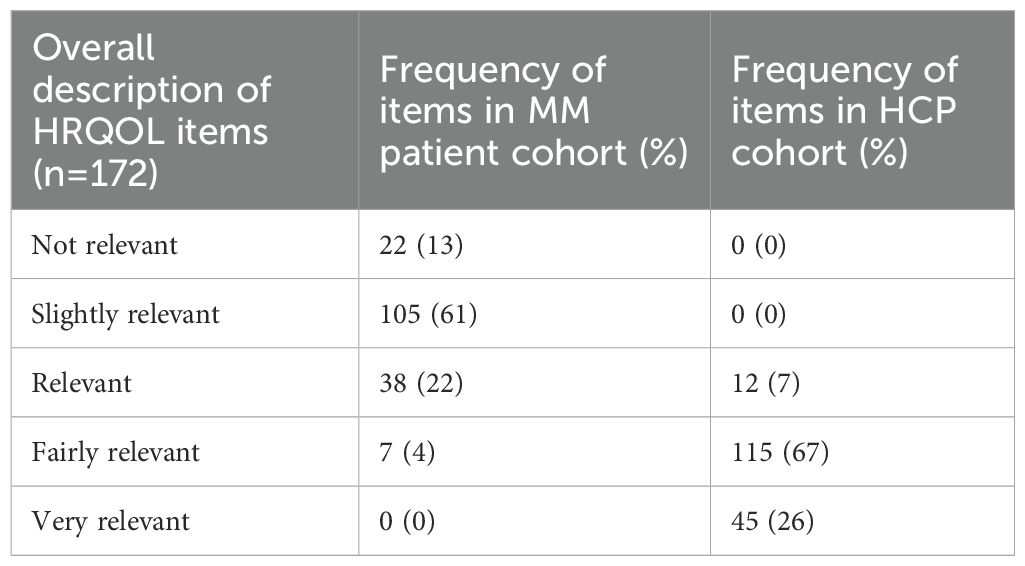
Of the 172 HRQOL items, 22 (13%) had an overall description of ‘not relevant’, 105 (61%) were ‘slightly relevant’, 38 (22%) were ‘relevant’ and 7 (4%) were ‘fairly relevant’ in the MM patient group (Table 2). None were described as ‘very relevant’. Items that were described as ‘relevant’ or above (relevant, fairly relevant, very relevant) included those related to physical functioning (e.g. able to do strenuous activities and a long walk in EORTC-QLQ-C30), functional well-being (all items in this domain in FACT-G, e.g. able to work and enjoy life) and social/family well-being (6 of the 7 items in this domain in FACT-G, e.g. getting support from family and friends). Symptom items related pain (e.g. bone and back pain in EORTC-QLQ-MY20) and fatigue/energy level (e.g. items in FACIT-fatigue), as well as items regarding infections (e.g. worry about getting infections in FACT-N), memory (e.g. able to remember things in FACT-BMT) and overall health (e.g. rating own health in EQ5D) were described as ‘relevant’ or above. Interestingly, several symptom items such as nausea/vomiting, poor appetite, hair loss, sore eyes/mouth, headaches, and tremors were described as ‘not relevant’ by this patient group.
When looking at responses from patient subgroups with renal impairment (n=43), bone disease (n=86) and spinal disease (n=66), the QOL items that these subgroups found relevant largely mirrored the items highlighted in the whole MM patient group. When comparing responses between newly diagnosed and relapsed patients, QOL items related to risk of infections, worries about future health and thoughts regarding their illness had higher weighted mean scores in the newly diagnosed patient group. In the relapsed disease group, items related to the disease’s impact on usual activities (e.g., being able to work), concerns around disease progression, bone pain and tiredness had higher weighted mean scores.
In contrast to the MM patients, MM HCPs graded all 172 items as ‘relevant’ or over. Forty-five items (26%) were ‘very relevant’, 115 (67%) were ‘fairly relevant’, and 12 (7%) items were graded as ‘relevant’. Items considered as ‘very relevant’ included those related to physical and role functioning (e.g. all items in these domains in EORTC-QLQ-C30), all items in the EQ5D, ability to work (e.g. able to work in FACT-G, and concerns about keeping one’s job in FACT-BMT), as well as symptom items on pain and fatigue.
Discussion
As myeloma patients survive longer with improved therapies, maintaining QOL is a significant treatment endpoint. Increasingly, researchers are incorporating appropriate QOL measures as primary endpoints now that we have a plethora of therapeutics options. This real-world survey was one of few studies that interrogated which items MM patients found relevant from commonly used QOLQs. With its simple design, uptake of this study was good and it included patients with a wide age range, time from diagnosis, different stages of disease, different symptom burden at presentation or relapse and variable therapy lines exposure. Despite it being a long survey of over 172 questions, it was feasible to conduct, with only 16% of items in the survey skipped by patients.
Apart from doctors, various HCPs within the multidisciplinary care team also participated in the survey, allowing us to understand how their views differ from those of the patients. It was surprising to find out that MM patients found the majority (74%) of QOL items as ‘irrelevant’ or ‘slightly relevant’. Only 26% of items were identified and reported as ‘relevant’ or above. Most of these questionnaires were designed and validated when traditional chemotherapeutic regimens were main treatment options for MM. For example, EORTC QLQ-C30 and -MY20 were developed in 1993 and 1996 respectively, long before proteasome inhibitors, anti-CD38, IMiDs, bispecific T cell engagers and CAR T Cell therapies became new standard of care for treating MM. Patients are also now more likely to be on continuous treatment until disease progression without any treatment breaks, leading to cumulative toxicities. Multiple newer targeted agents are being increasingly prescribed in combination. These have unique side effect profiles, which may not be accurately assessed by current QOLQs. Despite this, researchers continue to draw conclusions from these existing QOLQs in clinical trials that test newer agents (4, 5). There have been attempts to develop new PRO tools such as MyPOS (6) and HM-PRO (7), which have included important additional items related to sex life, information needs and confidence in the clinical team. However, despite the rigorous process involved in developing these newer tools, they remain underused compared to older ones. This survey highlighted the need to update and validate new representative modules for more reliable, harmonised QOL reporting.
In contrast to MM patients, MM HCPs considered all QOL items relevant for assessing patients’ QOL. The discrepancy reflects the difference in the perceptions of QOL priorities between MM patients and HCPs. The results from this survey could help and alert HCPs to focus their attention, resources and treatment decision plans on relevant items highlighted by patients. For example, MM patients graded fatigue, infection and pain as relevant QOL issues. Pain from bone disease is a very common feature in myeloma. Optimal pain control is challenging and likely involves multiple strategies combining targeted chemotherapy, radiotherapy, and pain medications in a personalised and multidisciplinary approach (8). Spinal bracing, kyphoplasty and vertebroplasty can be considered for those with painful vertebral fractures but more studies are needed as data regarding clinical outcomes and QOL on patients with spinal interventions is scarce. Fatigue is another common, distressing symptom graded as very relevant by MM patients. One way to tackle this problem would be to promote physical activity, which has been shown to improve not only fatigue but also physical functioning and HRQOL in cancer patients (9). Tailored exercise programmes have been shown to be safe and effective in MM and could be incorporated into the pre- and rehabilitation pathways (10). Infections remain a major concern for both patients and HCPs, particularly for those receiving bispecific T cell engagers and CAR T cell therapy. Clinicians should follow up-to-date guidance on antimicrobial prophylaxis and infection management in these patients (11). They should also explore ways to improve treatment tolerability, such as better infection risk assessment and using frailty-adjusted dose modification (12). Overall, having harmonised criteria for QOL will support MM patients and empower their HCPs in treatment management targeting superior QOL outcomes.
The limitations of this study include its single-centred design and the fact that it was not powered for subgroup analysis. Although it included a broad MM population, a small percentage of patients received new therapies such as CAR-T therapy and bispecific T cell engagers. Nonetheless, the feedback from this real-world survey highlighted the need for more relevant QOLQs to access QOL in modern-day MM patients. The lack of relevant questionnaires may explain their limited use in routine clinical environments. Further qualitative studies are needed to ensure new concepts and treatment side effects are included in future QOL tools.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for this study involving humans because this was an optional, anonymised survey. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements because participation of the survey was optional and participants were anonymised.
Author contributions
CL: Formal analysis, Writing – original draft, Data curation, Writing – review & editing, Investigation. SB: Writing – original draft, Writing – review & editing, Formal analysis, Data curation. DM: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. YL: Investigation, Writing – review & editing, Methodology, Conceptualization, Project administration. JL: Writing – review & editing, Investigation. OM: Investigation, Writing – review & editing. ED: Writing – review & editing, Investigation. NC: Writing – review & editing, Investigation. NR: Investigation, Writing – review & editing. KX: Investigation, Writing – review & editing. JS: Writing – review & editing, Investigation. XP: Investigation, Writing – review & editing. RP: Writing – review & editing, Investigation. LL: Investigation, Writing – review & editing. AM: Investigation, Writing – review & editing. EB: Writing – review & editing. KY: Conceptualization, Writing – review & editing. CK: Writing – original draft, Formal analysis, Methodology, Conceptualization, Supervision, Investigation, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We thank all patients and healthcare professionals who completed the survey for this study.
Conflict of interest
CK had a nonrestricted educational grant from Celgene/BMS for research.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1656912/full#supplementary-material
References
1. Kent EE, Ambs A, Mitchell SA, Clauser SB, Smith AW, and Hays RD. Health-related quality of life in older adult survivors of selected cancers: Data from the SEER - MHOS linkage. Cancer. (2015) 121:758–65. doi: 10.1002/cncr.29119
2. Seitzler S, Finley-Oliver E, Simonelli C, and Baz R. Quality of life in multiple myeloma: considerations and recommendations. Expert Rev Hematol. (2019) 12:419–24. doi: 10.1080/17474086.2019.1613886
3. Pimentel JL. A note on the usage of Likert Scaling for research data analysis. USM R&D J. (2010) 18:109–12.
4. Martin T, Lin Y, Agha M, Cohen AD, Htut M, Stewart AK, et al. Health-related quality of life in patients given ciltacabtagene autoleucel for relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b–2, open-label study. Lancet Haematol. (2022) 9:e897–905. doi: 10.1016/S2352-3026(22)00284-8
5. Mohty M, Bahlis NJ, Nooka AK, DiBonaventura M, Ren J, and Conte U. Impact of elranatamab on quality of life: Patient-reported outcomes from MagnetisMM -3. Br J Haematol. (2024) 204:1801–10. doi: 10.1111/bjh.19346
6. Osborne TR, Ramsenthaler C, Schey SA, Siegert RJ, Edmonds PM, and Higginson IJ. Improving the assessment of quality of life in the clinical care of myeloma patients: the development and validation of the Myeloma Patient Outcome Scale (MyPOS). BMC Cancer. (2015) 15:280. doi: 10.1186/s12885-015-1261-6
7. Goswami P, Oliva EN, Ionova T, Else R, Kell J, Fielding AK, et al. Hematological Malignancy specific patient-reported outcome measure (HM-PRO): construct validity study. Front Pharmacol. (2020) 11. doi: 10.3389/fphar.2020.01308
8. Rana S, Maharjan S, Sookdeo SD, and Schmidt P. Pain Management in Multiple Myeloma Patients: A Literature Review. Cureus. (2024) 16(3):e55975. doi: 10.7759/cureus.55975
9. Lecat CSY, McCourt O, Land J, Yong K, and Fisher A. Multiple myeloma and physical activity. BMC Res Notes. (2021) 14:171. doi: 10.1186/s13104-021-05591-y
10. Koutoukidis DA, Land J, Hackshaw A, Heinrich M, McCourt O, Beeken RJ, et al. Fatigue, quality of life and physical fitness following an exercise intervention in multiple myeloma survivors (MASCOT): an exploratory randomised Phase 2 trial utilising a modified Zelen design. Br J Cancer. (2020) 123:187–95. doi: 10.1038/s41416-020-0866-y
11. Raje N, Anderson K, Einsele H, Efebera Y, Gay F, Hammond SP, et al. Monitoring, prophylaxis, and treatment of infections in patients with MM receiving bispecific antibody therapy: consensus recommendations from an expert panel. Blood Cancer J. (2023) 13:116. doi: 10.1038/s41408-023-00879-7
12. Coulson AB, Royle KL, Pawlyn C, Cairns DA, Hockaday A, Bird J, et al. F railty-adjusted therapy i n T ransplant N on- E ligible patient s with newly diagno s ed Multiple Myeloma (FiTNEss (UK-MRA Myeloma XIV Trial)): a study protocol for a randomised phase III trial. BMJ Open. (2022) 12:e056147. doi: 10.1136/bmjopen-2021-056147
Keywords: myeloma, quality of life, PROM (patient reported outcome measurement), healthcare professional (HCP), mutliple myeloma, health related qualitiy of life
Citation: Lecat CSY, Bristogiannis S, Mehta D, Lwin Y, Land J, McCourt O, Dowling E, Correia N, Rabin NK, Xu K, Sive J, Papanikolaou X, Popat R, Lee L, McMillan A, Boyle EM, Yong K and Kyriakou C (2025) Current quality of life questionnaires are not relevant for assessing QOL issues in multiple myeloma patients in the era of modern therapies: results from a survey with myeloma patients and myeloma healthcare professionals. Front. Oncol. 15:1656912. doi: 10.3389/fonc.2025.1656912
Received: 30 June 2025; Accepted: 09 September 2025;
Published: 01 October 2025.
Edited by:
Nicola Sgherza, AOU Policlinico Consorziale di Bari, ItalyReviewed by:
Srinivas Devarakonda, The Ohio State University, United StatesNatalie Callander, University of Wisconsin-Madison, United States
Copyright © 2025 Lecat, Bristogiannis, Mehta, Lwin, Land, McCourt, Dowling, Correia, Rabin, Xu, Sive, Papanikolaou, Popat, Lee, McMillan, Boyle, Yong and Kyriakou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charalampia Kyriakou, Y2hhcmFsYW1waWEua3lyaWFrb3UxQG5ocy5uZXQ=
 Catherine S. Y. Lecat
Catherine S. Y. Lecat Sotirios Bristogiannis2
Sotirios Bristogiannis2 Ke Xu
Ke Xu Jonathan Sive
Jonathan Sive Annabel McMillan
Annabel McMillan Kwee Yong
Kwee Yong