- 1Department of Gastrointestinal Gland Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Clinical Research Lab, Guangxi Key Laboratory of Enhanced Recovery After Surgery for Gastrointestinal Cancer, Nanning, China
- 3Department of Gastrointestinal, Hernia and Enterofistula Surgery, The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
A Correction on
MiR-29b-3p inhibits migration and invasion of papillary thyroid carcinoma by down-regulating COL1A1 and COL5A1
By Wang C, Wang Y, Fu Z, Huang W, Yu Z, Wang J, Zheng K, Zhang S, Li S and Chen J (2022). Front. Oncol. 12:837581. doi: 10.3389/fonc.2022.837581
In the published article, there was an error in Figure 2 as published. In Figure 2E, the EDU experimental result images for the B-CPAP cell line NC inhibitor treatment group were misplaced. The corrected Figure 2 and its caption appear below.
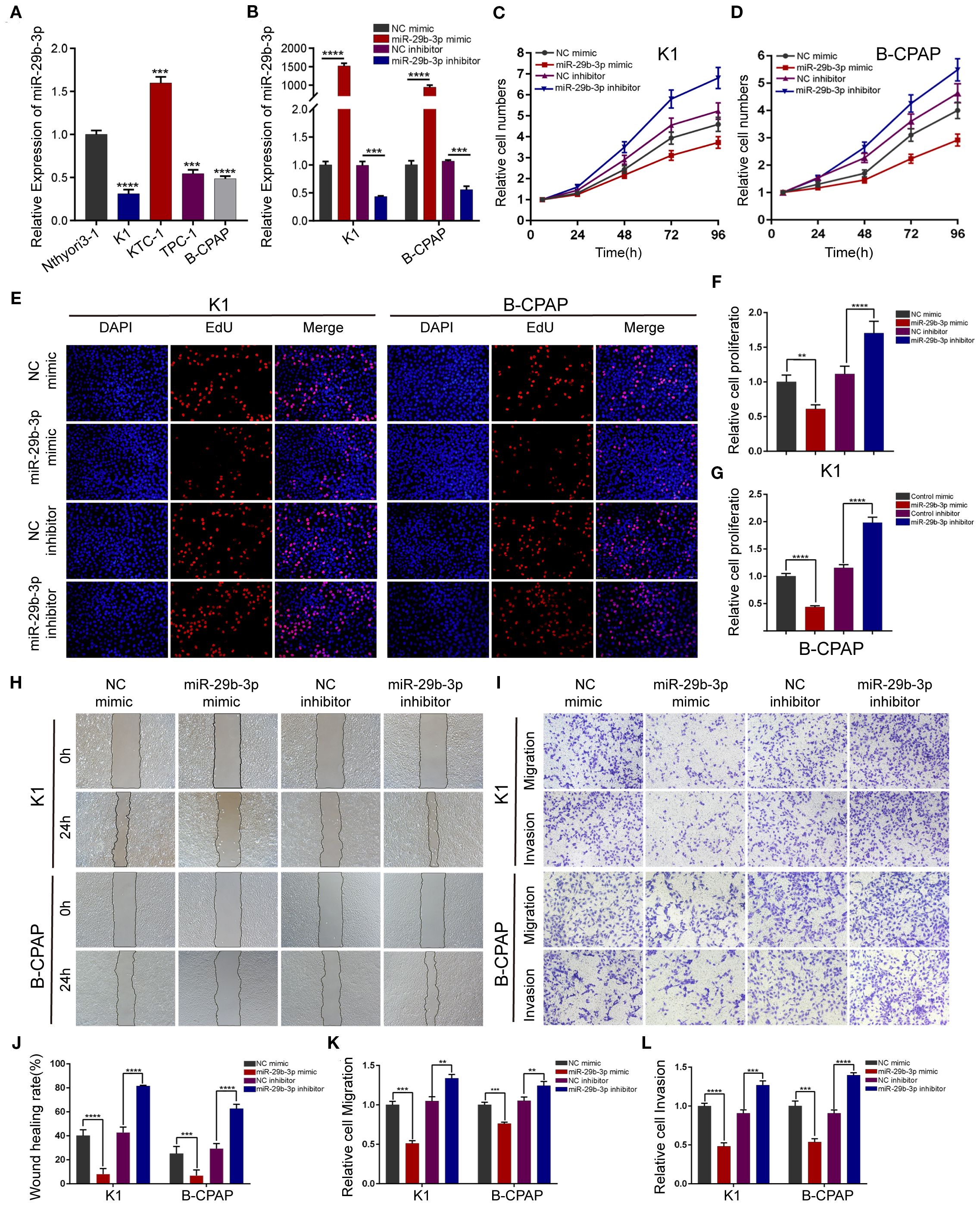
Figure 2. miR-29b-3p inhibits PTC cell migration and invasion in vitro. (A) Expression of miR-29b-3p was verified by qRT-PCR in PTC cell lines (K1, KTC-1, TPC-1, and B-CPAP) and normal thyroid cell lines (Nthyori3–1); two lowly expressing (K1 and B-CPAP) cell lines were selected for further study. (B) The knockdown and overexpression efficiencies of miR-29b-3p was verified by qRT-PCR after transfection with miR-29b-3p mimic/inhibitor or NC mimic/inhibitor. Cell proliferation ability of PTC cells was determined by CCK-8 assays (C, D) and EdU assays (E–G) after miR-29b-3p overexpression or knockdown. The effects of miR-29b-3p overexpression and knockdown in PTC cells on cell migration and invasion were analyzed by wound healing assays (H) and Transwell assays (I). (J, L) Results of the Transwell assays and wound healing assays. In the figure, **p < 0.01, ***p < 0.001,****p < 0.0001.
In the published article, there was an error in Figure 4 as published. In Figure 4N, image arrangement errors occurred for the K1 cell line miR-29b-3p Mimic treatment group and the B-CPAP cell line NC Mimic, miR-29b-3p Mimic, and miR-29b-3p Mimic + OE COL5A1 treatment groups. The corrected Figure 4 and its caption appear below.
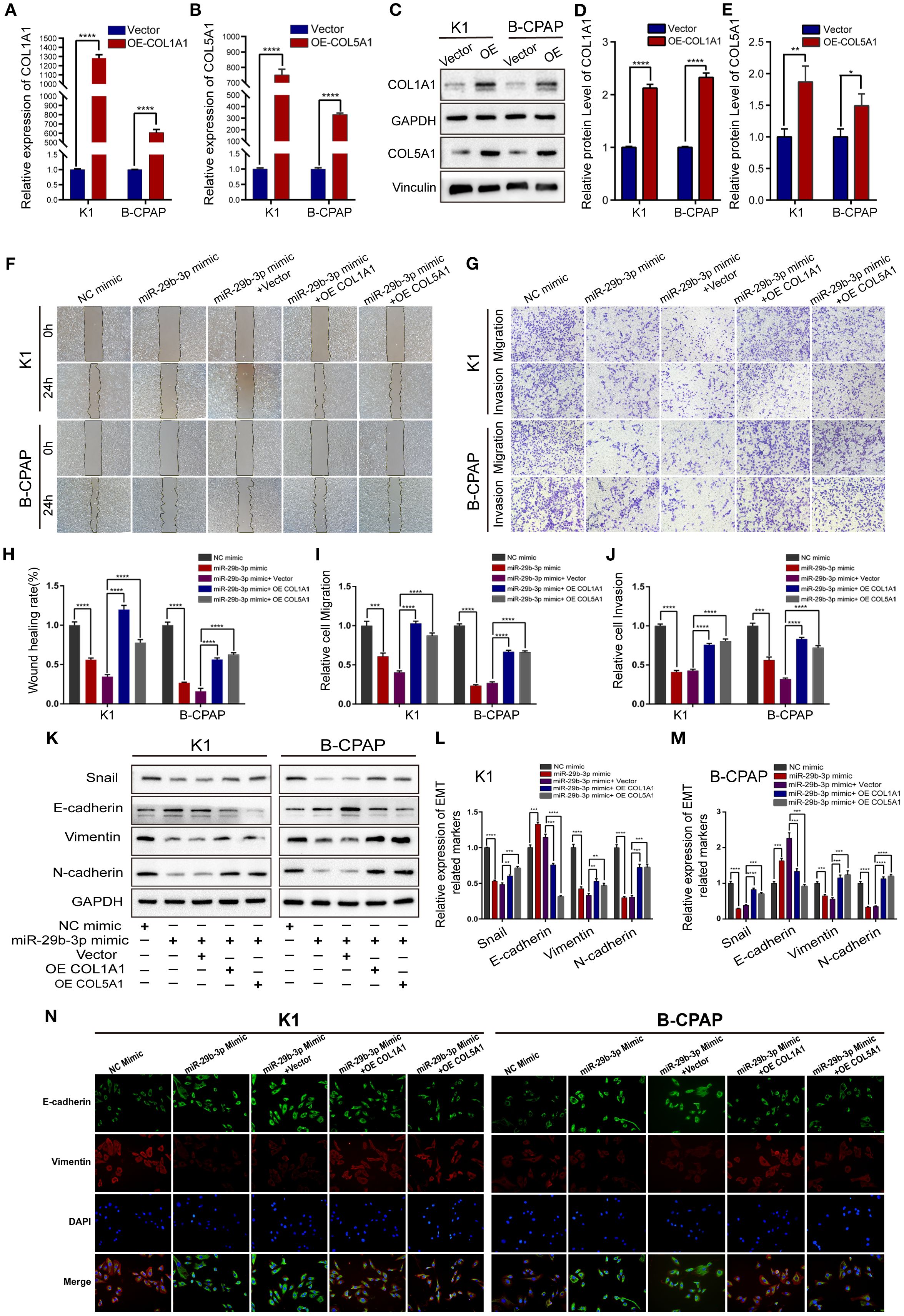
Figure 4. Overexpression of COL1A1 or COL5A1 partially blocks the suppressive effects of miR-29b-3p on invasion in PTC cells. (A, B) qRT-PCR and (C) Western blot were performed to assess the transfection efficiency of COL1A1 and COL5A1 overexpression plasmids. (D, E) Quantification of COL1A1 and COL5A1 levels in Western blot normalized over GAPDH and Vinculin. The wound healing assays (F) and Transwell assays (G) demonstrated that the overexpression of COL1A1 and COL5A1 could efficiently alleviate the miR-29b-3p mimic-induced inhibition of the migration and invasion activities of K1 and B-CPAP cells. (H–J) Quantified results of the wound-healing assays and Transwell assays. Inhibition of miR-29b-3p mimic-induced EMT by overexpressed COL1A1 or COL5A1; the EMT-related proteins were investigated by (K) Western blot and (N) immunofluorescence. (L, M) The EMT-related proteins expression for each group was analyzed by Image J software. In the figure, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In the published article, there was an error in Figure 5 as published. In Figure 5L, the immunofluorescence experimental result images for the B-CPAP cell line si-COL1A1–2 treatment group were misplaced during assembly. The corrected Figure 5 and its caption appear below.
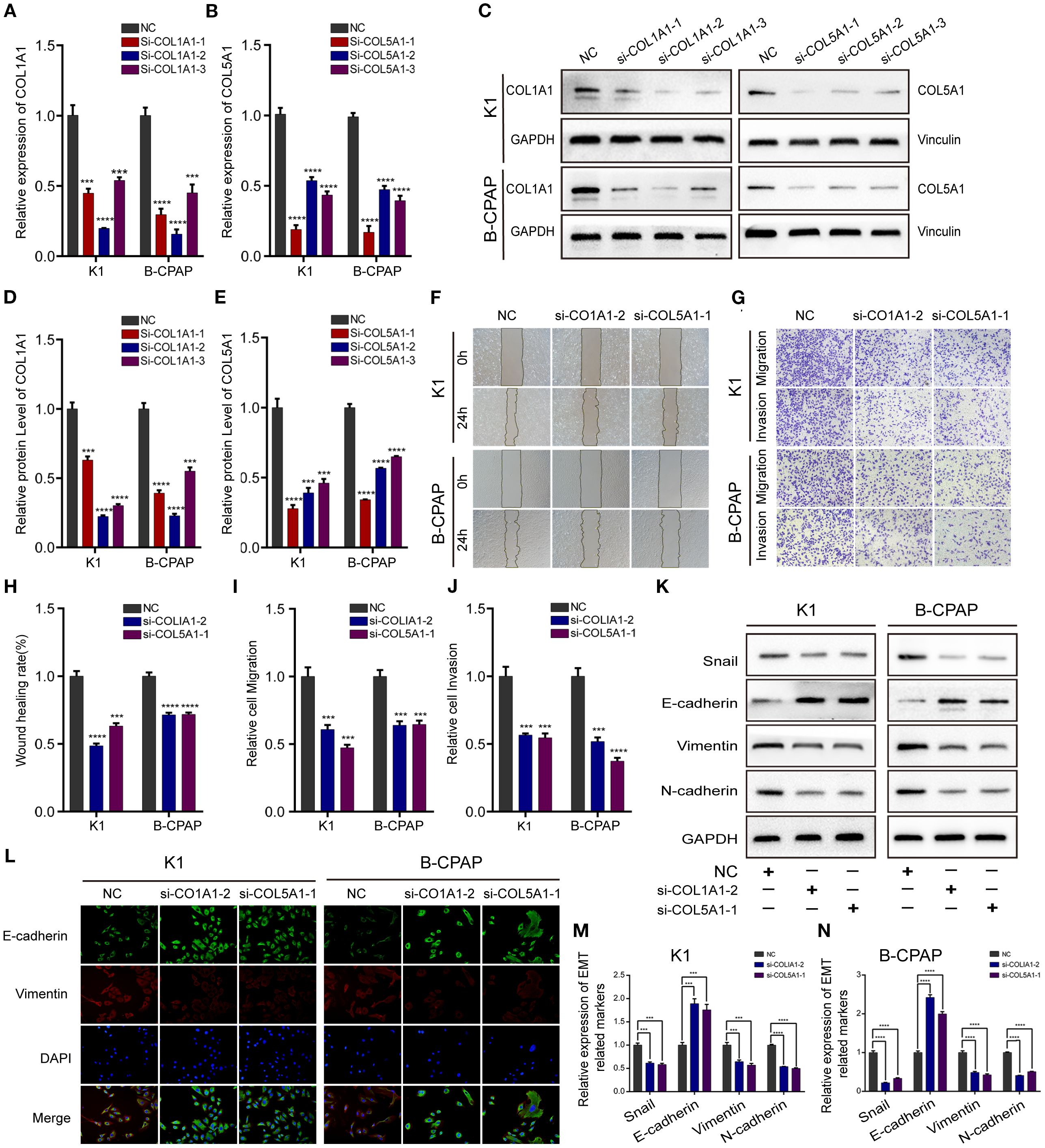
Figure 5. Down-regulating COL1A1 or COL5A1 expression can inhibit migration, invasion, and EMT in PTC cells. The knockdown efficiency of COL1A1 and COL5A1 were analyzed by (A, B) qRT-PCR and (C–E) and Western blotting. Additionally, (F) wound healing and (G) Transwell assays were conducted to evaluate PTC cells migration and invasion, which proved that both si-COL1A1–2 and si-COL5A1–1 significantly impaired migration and invasion ability in K1 and B-CPAP cells. (H–J) The statistical results of wound healing assays and Transwell assays. (K) Western blot and (L) immunofluorescence analyses were performed to determine the expression of EMT-related proteins detection in PTC cells transfected with si-COL1A1–2 and si-COL5A1–1. (M, N) Western blotting assay showing the protein expression levels of EMT-related in PTC cells. In the figure, ***p < 0.001, ****p < 0.0001.
The original version of this article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: microRNAs, miR-29b-3p, papillary thyroid carcinoma, downregulation, COL1A1, COL5A1, gene expression
Citation: Wang C, Wang Y, Fu Z, Huang W, Yu Z, Wang J, Zheng K, Zhang S, Li S and Chen J (2025) Correction: MiR-29b-3p inhibits migration and invasion of papillary thyroid carcinoma by down-regulating COL1A1 and COL5A1. Front. Oncol. 15:1657780. doi: 10.3389/fonc.2025.1657780
Received: 01 July 2025; Accepted: 19 September 2025;
Published: 03 October 2025.
Edited and reviewed by:
Carlos Pérez-Plasencia, National Autonomous University of Mexico, MexicoCopyright © 2025 Wang, Wang, Fu, Huang, Yu, Wang, Zheng, Zhang, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junqiang Chen, Y2hlbmp1bnFpYW5nQGd4bXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Congjun Wang
Congjun Wang Ye Wang
Ye Wang Zhao Fu
Zhao Fu Weijia Huang
Weijia Huang Zhu Yu
Zhu Yu Jiancheng Wang
Jiancheng Wang Kaitian Zheng
Kaitian Zheng Siwen Zhang
Siwen Zhang Shen Li3
Shen Li3 Junqiang Chen
Junqiang Chen