- 1Department of Thoracic Surgery, The First People’s Hospital of Jiande, Jiande, China
- 2Department of Thoracic Surgery, Affiliated Zhongshan Hospital of Dalian University, Dalian, China
- 3Department of Radiology, The First People’s Hospital of Jiande, Jiande, China
- 4Department of Radiology, Affiliated Zhongshan Hospital of Dalian University, Dalian, China
Background: Visceral pleural changes (VPC) is increasingly detected in early-stage lung adenocarcinoma, but its clinical and prognostic significance is unclear. This retrospective multicenter study aims to evaluate the influence of VPC on OS and DFS in patients with stage IA lung adenocarcinoma.
Methods: Overall, 494 patients with stage IA lung adenocarcinoma from two centers were enrolled, including 202 VPC-positive (VPC+) and 292 VPC-negative (VPC-) patients. After 1:1 propensity score matching (PSM), 284 patients (142 per group) were analyzed. The Kaplan–Meier method was used to compare survival between groups, and Cox regression analysis identified independent prognostic factors for OS and DFS.
Results: Kaplan–Meier analysis showed no significant OS difference between VPC+ and VPC- group (HR 0.67, 95% CI 0.31–1.47, P = 0.320). However, DFS was significantly better in VPC+ patients compared to VPC- patients (HR 0.51, 95% CI 0.27–0.94, P = 0.028). Specifically, 5-year OS was 96.5% in VPC+ vs. 95.8% in VPC- (P = 0.845), and 5-year DFS was 95.8% in VPC+ vs. 92.3% in VPC-(P = 0.259), with no significant differences. Median OS was 76.0 months before PSM and 76.0 months after PSM. For DFS, median time was 76.0 months before PSM and 76.0 months after PSM. Cox regression identified operative time as an independent OS prognostic factor (HR 1.01, 95% CI 1.00–1.11, P = 0.039), while VPC- (HR 0.40, 95% CI 0.19–0.83, P = 0.015) and pathological stage IA3 (HR 3.12, 95% CI 1.08–9.00, P = 0.035) were independent DFS prognostic factors.
Conclusion: In patients with stage IA lung adenocarcinoma, VPC- is associated with worse DFS compared to VPC+, while no significant difference in OS was observed. Pathological stage were significant prognostic factors for DFS.
Introduction
Lung adenocarcinoma, the most common histological subtype of non-small cell lung cancer (NSCLC), constitutes a significant proportion of early-stage lung cancers (1). Patients with stage IA lung adenocarcinoma generally have a favorable prognosis, with a 5-year overall survival (OS) rate exceeding 80% (2, 3). However, the prognostic factors for stage IA lung adenocarcinoma specifically remain to be fully elucidated.
Visceral pleural invasion (VPI) is a known adverse prognostic factor in NSCLC, particularly in tumors ≤3 cm in diameter, where it predicts lymph node metastasis and postoperative recurrence (4–6). Consequently, the 8th edition of the TNM classification recommends upstaging tumors with VPI from IA to IB (7). On chest computed tomography (CT), signs such as pleural retraction, pleural traction lines, and pleural indentation are often associated with VPI (8–12). However, visceral pleural changes (VPC) do not always progress to VPI.
Recent advancements in imaging techniques have enabled the detection of subtle pleural changes. However, the clinical significance of VPC in patients with early-stage lung adenocarcinoma is still not fully understood. VPC may represent an early interaction between the tumor and the pleura, potentially influencing tumor biology and progression (9–11). The specific impact of VPC on stage IA lung adenocarcinoma remains to be further investigated.
This retrospective analysis, including 494 patients, aims to explore the prognostic significance of VPC in stage IA lung adenocarcinoma and determine if it can serve as an independent prognostic factor for OS and disease-free survival (DFS).
Patients and methods
Study population and eligibility criteria
This retrospective study was based on the data of patients who underwent surgical resection for lung cancer at The First People’s Hospital of Jiande (Jiande, China) and Affiliated Zhongshan Hospital of Dalian University (Dalian, China) from January 2012 to December 2018. All patients underwent preoperative high-resolution chest CT (1–1.25 mm). CT images were acquired in the supine position during inspiratory breath-hold. The inclusion criteria were (1) patients who underwent lung resection and were diagnosed with lung adenocarcinoma and (2) patients with a tumor size of ≤3 cm. The exclusion criteria were (1) prior lung resection surgery; (2) non-primary pulmonary malignancies; (3) transfer to other hospitals; (4) non-adenocarcinoma histopathology; (5) pathologically confirmed adenocarcinoma in situ or minimally invasive adenocarcinoma; (6) non-stage IA lung adenocarcinoma; (7) loss to follow-up; (8) palliative surgery; (9) centrally located lung cancer; (10) Neoadjuvant therapy with preoperative radiotherapy or chemotherapy; and (11) conversion to thoracotomy during surgery.
All pathological diagnoses were based on hospital pathology reports. All patients were restaged according to the 8th edition of the TNM classification established by the International Association for the Study of Lung Cancer (7).
The study adhered to the principles outlined in the Declaration of Helsinki and was approved by the Ethics Committee of The First People’s Hospital of Jiande (Ethics Committee Approval Number: 20250523-KY-002-01). The requirement for written informed consent was waived due to the retrospective nature of the study. To protect patient privacy, all personal identifiers were removed from the dataset before analysis, and only de-identified data were used. The original data were accessible only to the authors of the study. Furthermore, data access was restricted to the research team, and all data were stored securely.
Data collection
The following data were retrospectively collected: demographic characteristics (sex, age, body mass index, and smoking history), comorbidities (hypertension, diabetes mellitus, coronary heart disease, and chronic obstructive pulmonary disease), VPC, density classification, nodule depth, TNM stage, surgical approaches, resection site, type of lung resection, total number of lymph nodes retrieved, number of mediastinal lymph nodes retrieved, total lymph node stations explored, mediastinal lymph node stations explored, surgical duration, intraoperative blood loss volume, drainage time and volume, length of postoperative hospital stay, OS, and DFS.
Pulmonary nodule imaging characteristics and VPC
Pure ground-glass nodules (pGGN) were characterized by a mild, uniform increase in lung tissue density on chest CT, presenting with a translucent, frosted-glass appearance. The internal vascular and bronchial structures remained clearly visible, and there were no solid components. Mixed ground-glass nodules (mGGN), or part-solid nodules, were those that contained both ground-glass density and solid components, with the solid portion having a higher density (0 < consolidation-to-tumor ratio [CTR] < 1), which may obscure vascular and bronchial structures. Solid nodules (SNs) were those composed entirely of solid components of uniform density, lacking ground-glass or cystic elements, and with a CT value close to that of soft tissue (CTR = 1) (13).
Nodule depth was defined as the distance from the pulmonary nodule to the pleura, relative to the distance from the pleura to the hilum, categorized into the outer one-third and the inner two-thirds, and it did not include centrally located lung masses (Figure 1).
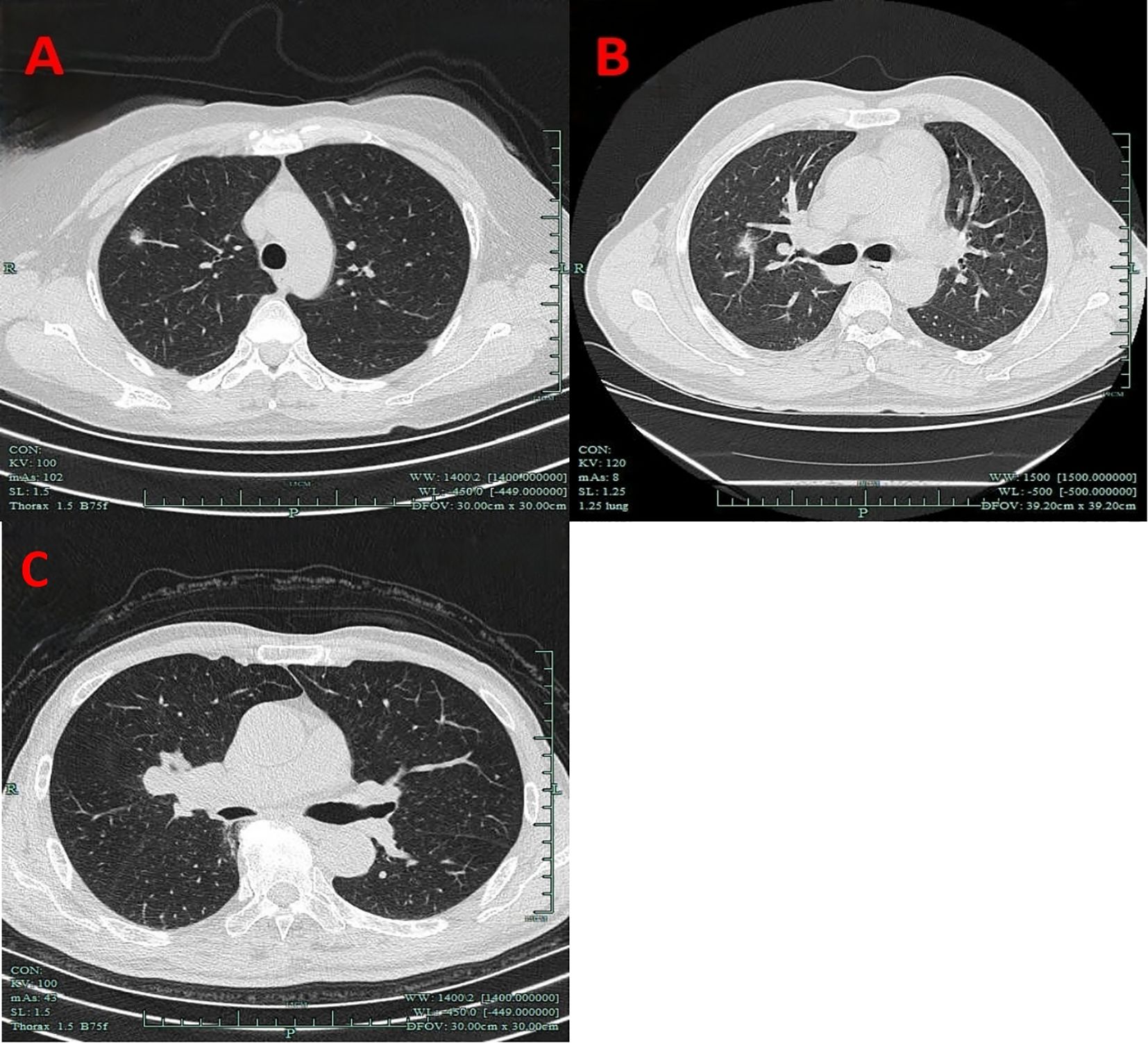
Figure 1. Imaging manifestations of nodule depth. (A) The outer one-third. (B) The inner two-thirds. (C) Centrally located lung masses.
VPC represent a broad concept that includes any form of pleural involvement, such as thickening or adhesion, without penetrating the elastic layer or invading the visceral pleura (14). The key imaging manifestations of VPC include pleural traction, pleural tail sign, pleural attachment, and pleural indentation (Figure 2). In this study, VPC referred exclusively to visceral pleural involvement without visceral pleural invasion (VPI).
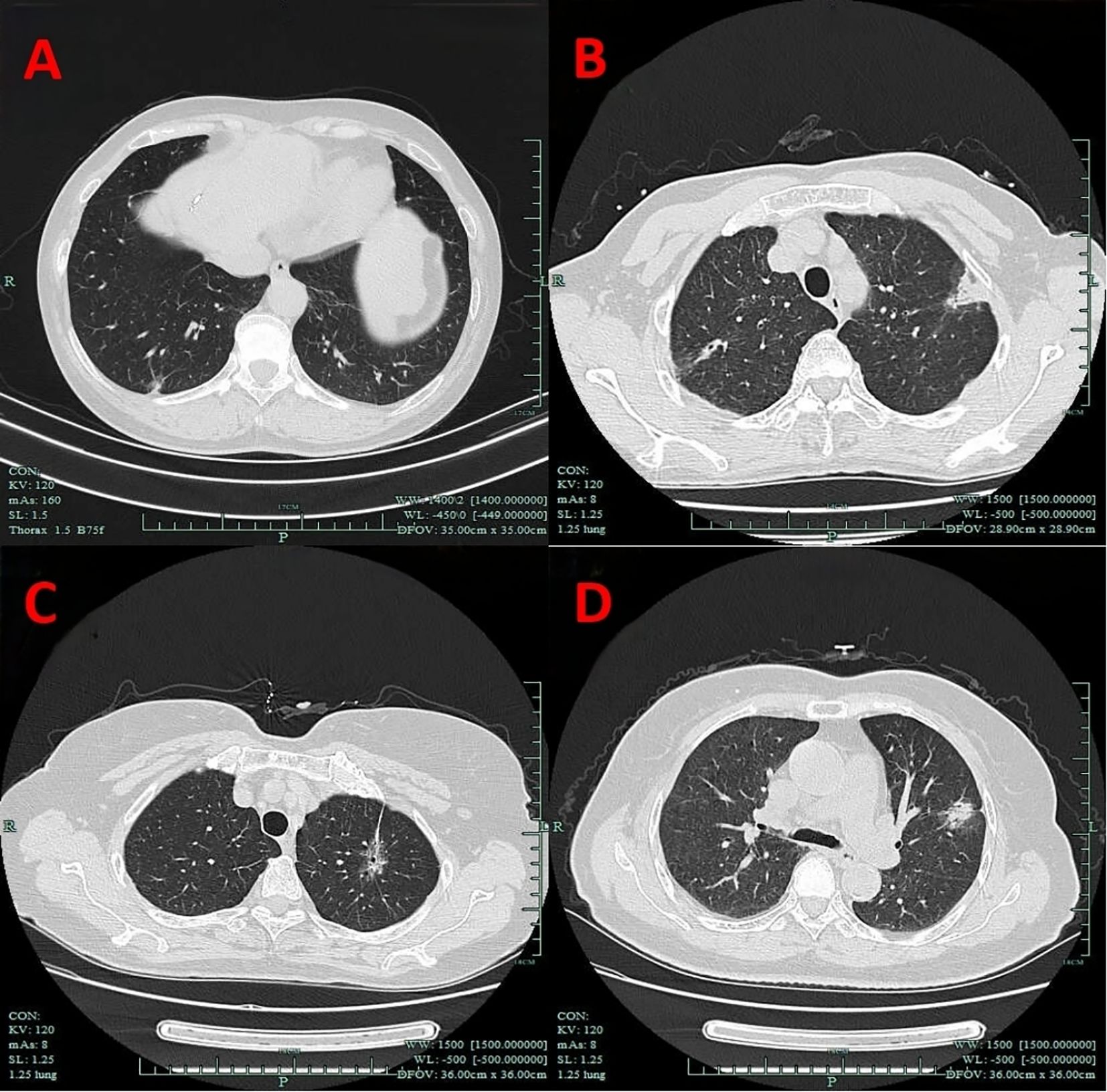
Figure 2. Imaging manifestations of VPC. (A) Pleural indentation. (B) Pleural attachment. (C) Pleural traction. (D) Pleural tail sign. VPC, visceral pleural changes.
Radiological assessments for both nodule characteristics and VPC were independently conducted by a thoracic surgeon and a thoracic radiologist from each institution. For patients with uncertain findings, a consensus was reached through joint discussion.
Follow-up
Follow-up data were collected through retrospective analysis of patients’ imaging records from their respective hospitals. A designated thoracic surgeon and a thoracic radiologist systematically reviewed postoperative imaging to detect tumor recurrence, encompassing both locoregional recurrence and distant metastasis, and documented the timing of each follow-up. For patients who were lost to follow-up or chose to undergo postoperative imaging at other hospitals, a standardized telephone follow-up system was used to collect the survival data.
OS was defined as the time from the date of diagnosis until death from any cause or until March 2025, whichever came first. DFS was defined as the time from the date of diagnosis to the first event of disease progression, whether locoregional or distant; death from any cause; or until March 2025, whichever came first.
Statistical analysis
We conducted PSM at a 1:1 ratio to enhance the intergroup comparability and reduce potential bias. Propensity scores were calculated using a logistic regression model that included age, density classification, nodule depth, TNM stage, total number of lymph nodes retrieved, and total lymph node stations explored. We used the nearest-neighbor matching method with a caliper value of 0.2 and without replacement to perform the PSM. This approach helped to balance the distribution of potential confounders between the groups (P > 0.05).
To evaluate the balance in the covariates between the groups after PSM, we used the standardized mean difference (SMD). Generally, an SMD of <0.10 indicates good balance, 0.10–0.34 suggests minor imbalance, 0.35–0.64 indicates moderate imbalance, 0.65–1.19 indicates substantial imbalance, and ≥1.20 reflects very large imbalance. Our results showed that all covariates were within the acceptable range for balance.
For normally distributed continuous data, the Student’s t-test was used for comparisons between groups, and the data are presented as the mean ± standard deviation. For non-normally distributed continuous data, the Wilcoxon rank-sum test was used for comparisons, and the data are presented as the median with interquartile range (25th–75th percentiles). Categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate, and are presented as percentages.
Kaplan–Meier plots were used for the univariable analysis of OS and DFS, and the log-rank test was used for survival comparisons between the groups. Variables significant at P < 0.05 in the univariable analysis were included in the multivariable Cox proportional-hazards regression analysis to determine independent prognostic factors.
All analyses were performed using GraphPad Prism 8 and R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics and perioperative results
From January 2012 to December 2018, 684 patients underwent VATS for lung cancer with a tumor size of ≤3 cm at The First People’s Hospital of Jiande (n = 309) or the Affiliated Zhongshan Hospital of Dalian University (n = 375). After excluding 190 patients, 494 were included in the final analysis (Figure 3). The cohort included 202 patients with VPC and 292 without VPC. The nodule types were classified as pGGN in 191 patients (38.7%), mGGN in 187 (37.9%), and SN in 116 (23.4%). The surgical approaches were uniportal VATS (U-VATS) in 286 patients (57.9%) and multiportal VATS (M-VATS) in 208 patients (42.1%). The tumor location was peripheral (outer one-third lung zone) in 294 patients (59.5%) and central (inner two-thirds perihilar region) in 200 patients (40.5%). The median OS time was 76.0 months (interquartile range (IQR): 73.0 - 83.75 months) and 76.0 months (IQR: 73.0 - 83.0 months) for DFS.
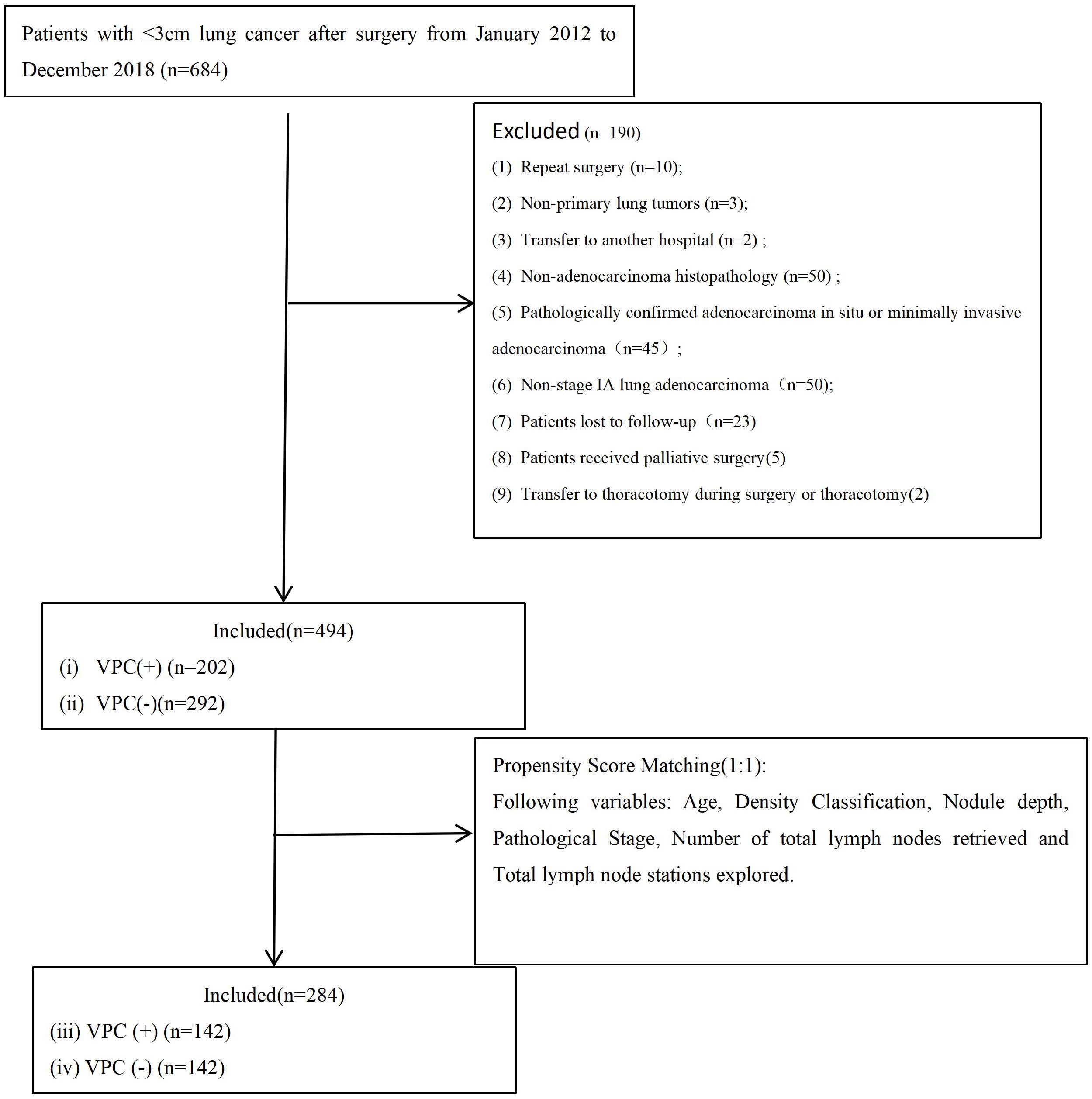
Figure 3. Flowchart of patient selection. VPC+, visceral pleural changes-positive; VPC−, visceral pleural changes-negative.
After 1:1 PSM, the data of 284 patients (142 with and 142 without VPC) were analyzed (Figure 3). The matched subgroups comprised 71 patients with pGGN (25.0%), 136 with mGGN (47.9%), and 77 with SN (27.1%). The surgical approaches were U-VATS in 159 patients (56.0%) and M-VATS in 125 patients (44.0%). The tumor location remained peripheral in 171 patients (60.2%) and central in 113 patients (39.8%). The post-PSM The median OS time was 76.0 months (IQR: 73.0 - 84.25 months) and 76.0 months (IQR: 73.0 - 84.0 months) for DFS. All preoperative variables were balanced between the groups (P > 0.05). The baseline characteristics of the patients are summarized in Table 1.
Prognostic factor analysis
The univariable analysis revealed associations of OS with age (P = 0.036), pathological stage (IA3) (P = 0.047) and number of total lymph nodes retrieved (P = 0.038). DFS was significantly associated with pleural indentation status (P = 0.031), smoking (P = 0.040), and pathological stage (IA3) (P = 0.001) (Table 2).
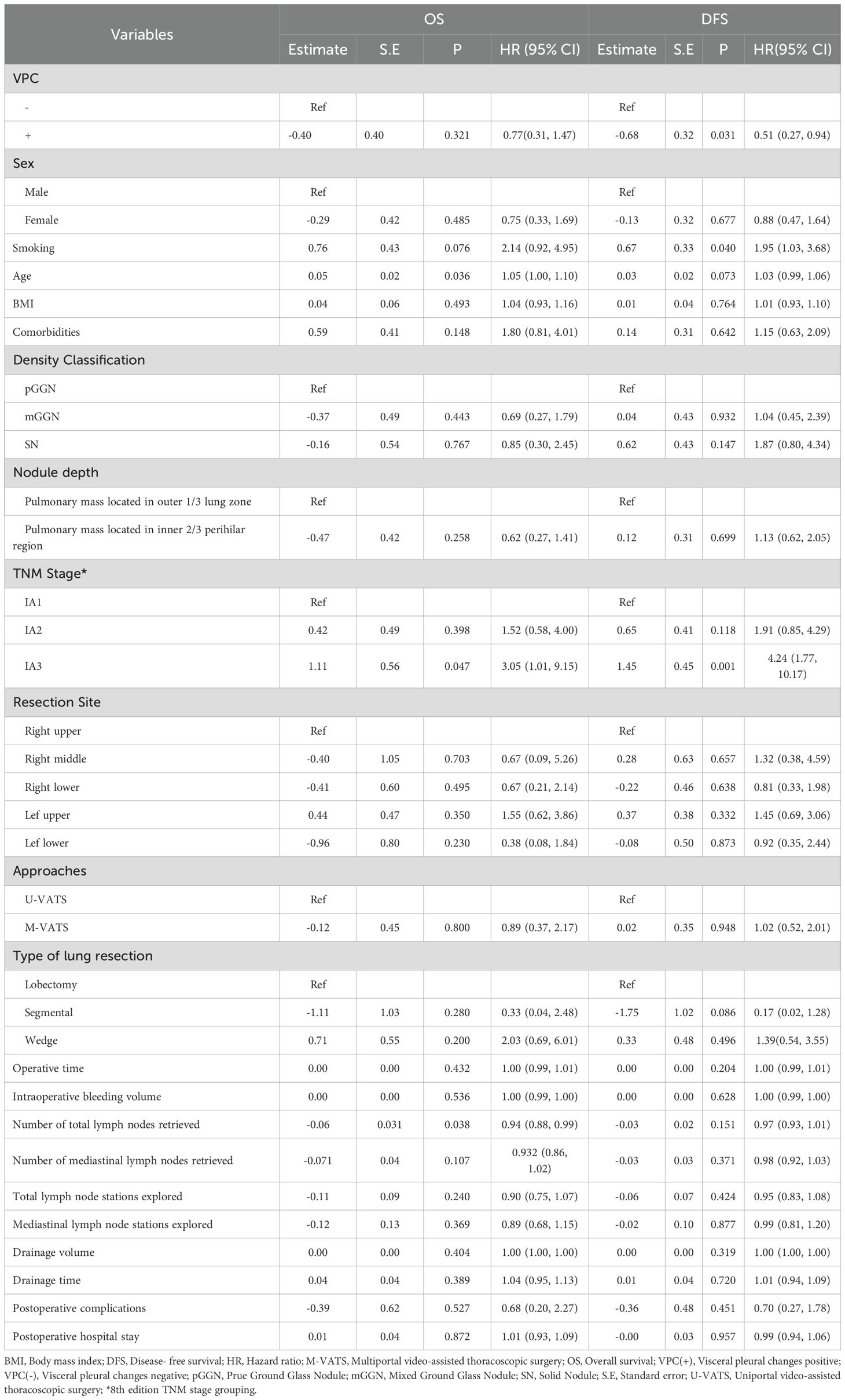
Table 2. Univariate analysis of the factors influencing OS and DFS in patients with lung adenocarcinoma.
Multivariable Cox proportional-hazards regression identified number of total lymph nodes retrieved (hazard [HR] 0.92, 95% confidence interval [CI] 0.87–0.99, P = 0.027) as an independent predictor of OS. VPC+ status (HR 0.49, 95% CI 0.27–0.91, P = 0.025) and IA3 (HR 4.06, 95% CI 1.67–9.87, P = 0.002) were independent predictors of DFS (Table 3).
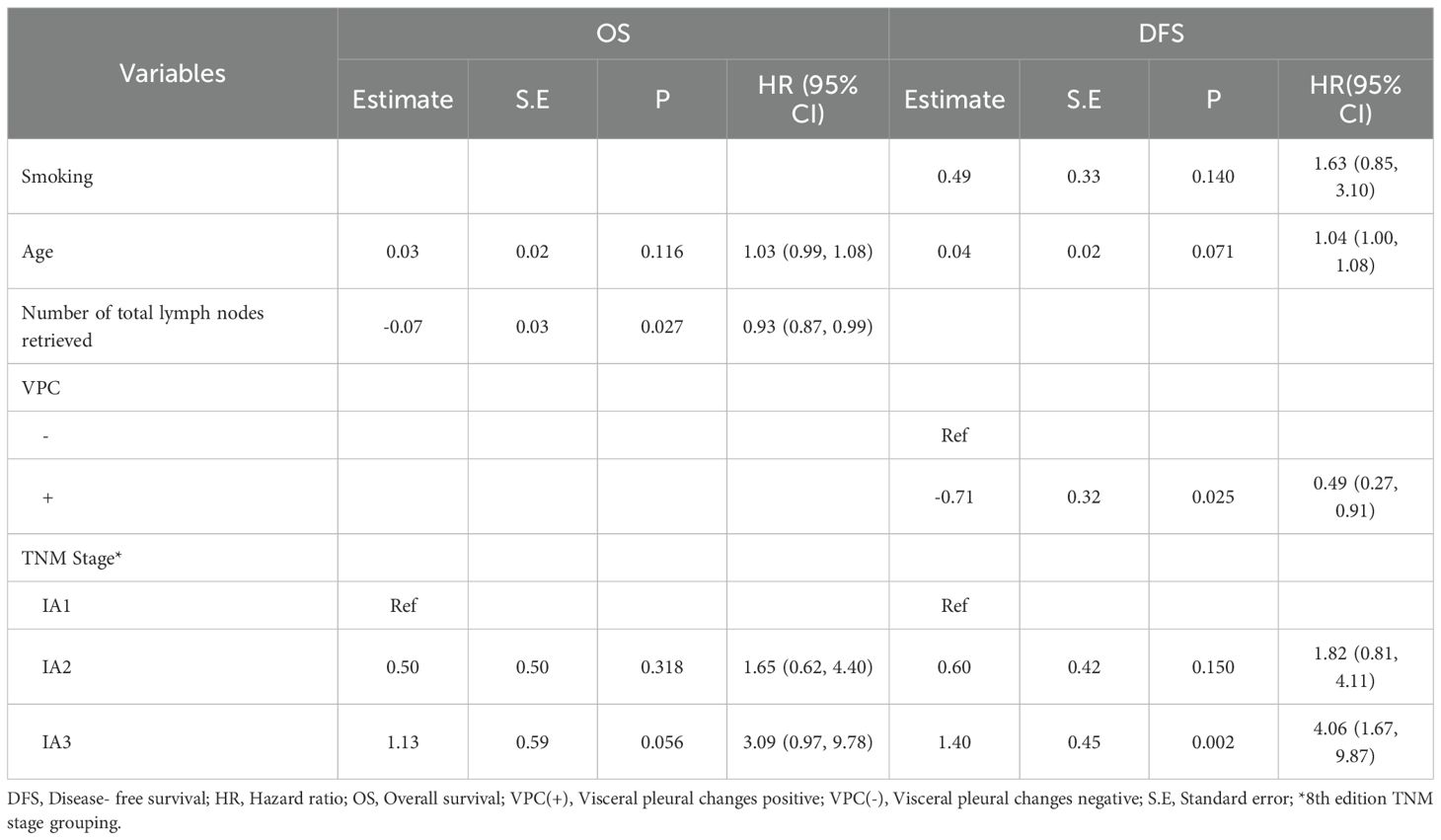
Table 3. Multivariable analysis of the factors influencing OS and DFS in patients with lung adenocarcinoma.
Survival analysis
Following the prognostic factor analysis, survival analysis was performed. After PSM, VPC-positive (VPC+) and VPC-negative (VPC−) patients had median OS durations of 77.0 months (IQR: 74.0 - 90.0 months) and 75.0 months (IQR: 73.0 - 78.75 months), respectively (HR 0.67, 95% CI 0.31-1.47, P = 0.320), and median DFS durations of 77.0 months (IQR: 74.0 - 89.0 months) months and 75.0 months (IQR: 73.0 - 78.0 months), respectively (HR 0.51, 95% CI 0.27-0.94, P = 0.028) (Figure 4, Figure 5). No significant differences were found in the rates of 5-year OS (97.2% vs. 97.9%, respectively, P > 0.05) or 5-year DFS (97.2% vs. 95.1%, respectively, P = 0.541) between the VPC+ and VPC− groups.
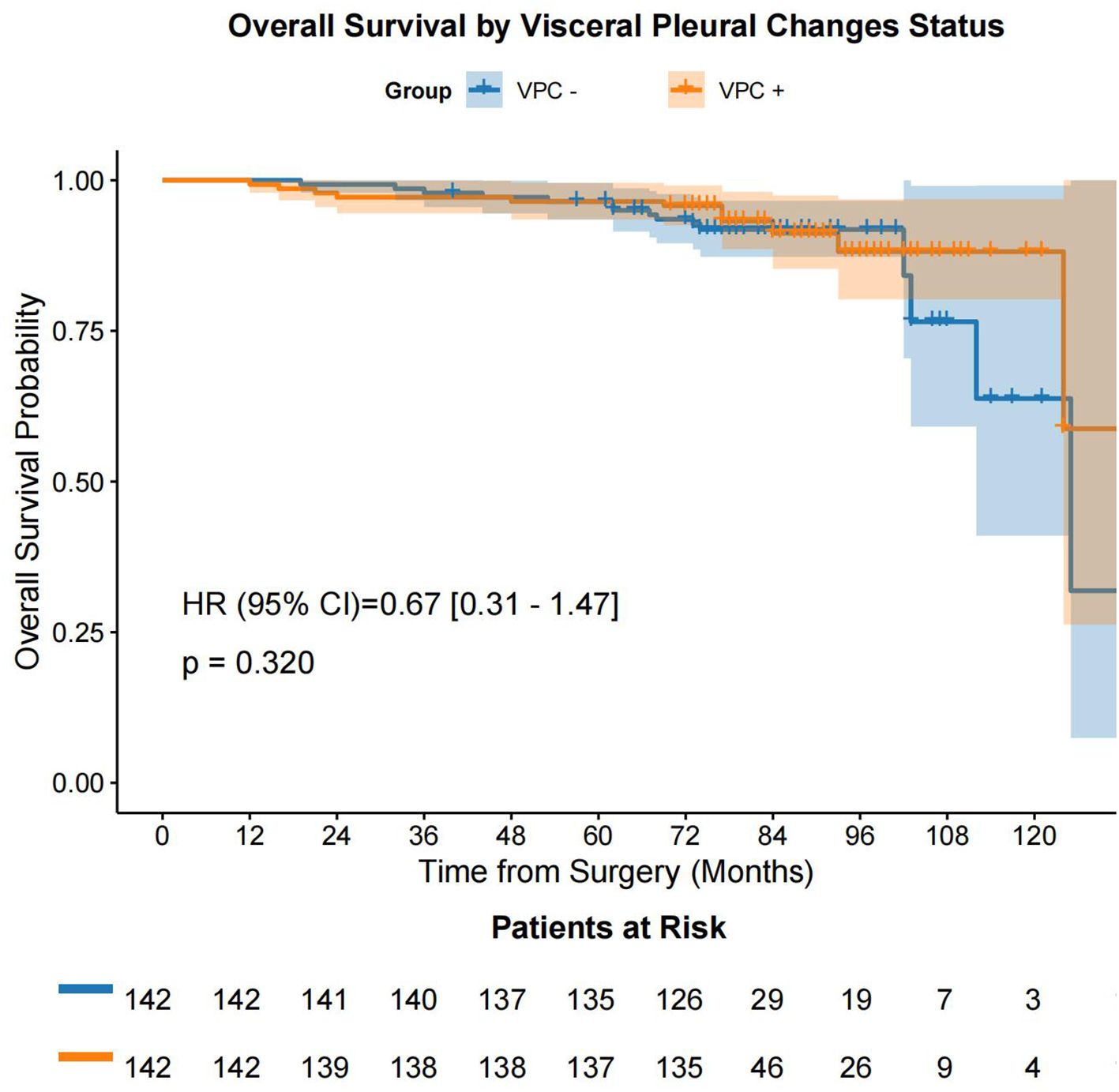
Figure 4. OS in the VPC+ and VPC− groups. OS, overall survival; VPC+, visceral pleural changes-positive; VPC−, visceral pleural changes-negative.
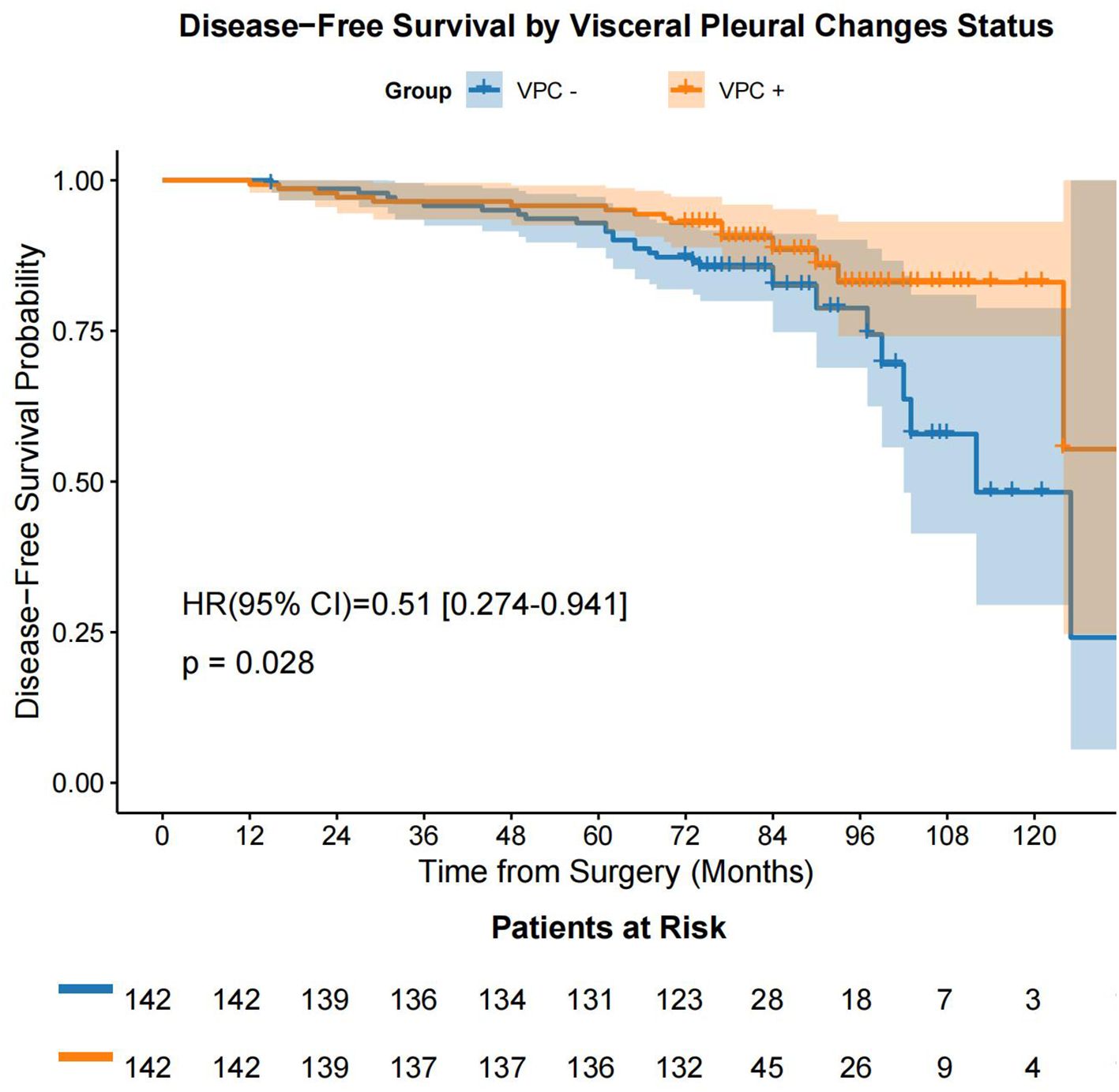
Figure 5. DFS in the VPC+ and VPC− groups. DFS, disease-free survival; VPC+, visceral pleural changes-positive; VPC−, visceral pleural changes-negative.
Subgroup survival analysis
Using the data after PSM, we performed a subgroup survival analysis by categorizing patients into three groups: pGGN (n = 71), mGGN (n = 136), and SN (n = 77). The analysis revealed no significant differences in OS (P = 0.730) and DFS (P = 0.150) among the three groups (Figure 6, Figure 7).
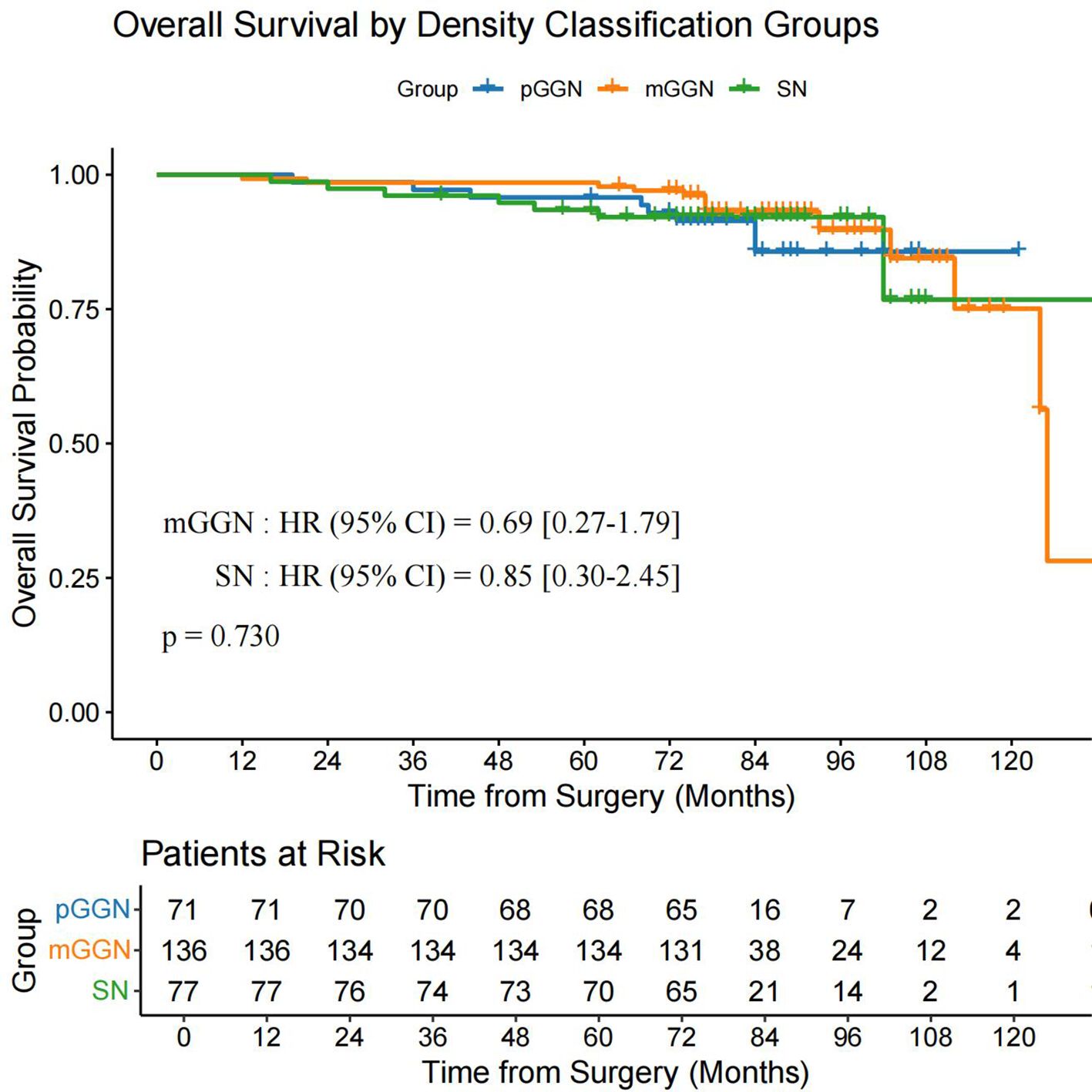
Figure 6. OS in the pGGN, mGGN, and SN groups. mGGN, mixed ground-glass nodules; OS, overall survival; pGGN, pure ground-glass nodules; SN, solid nodules.
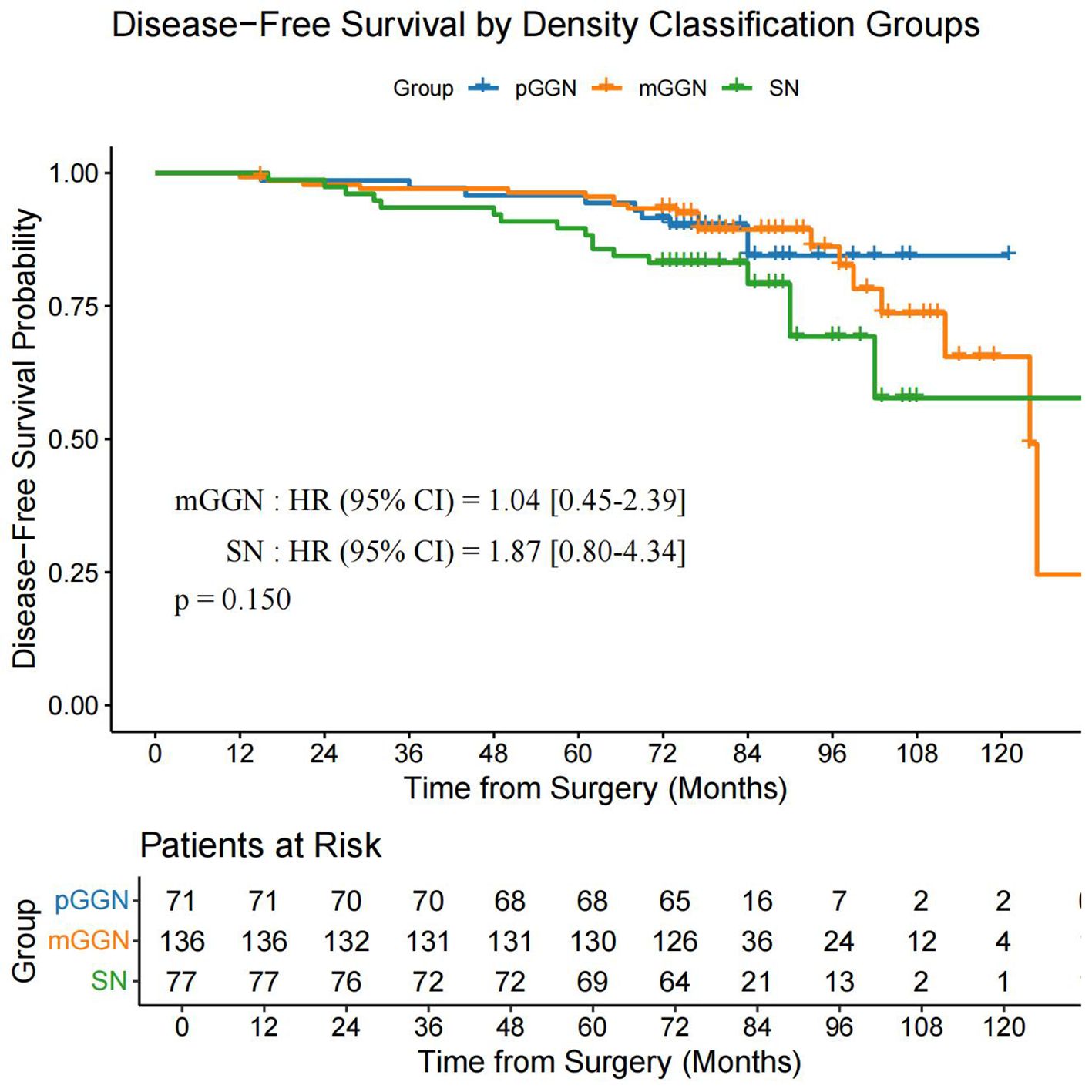
Figure 7. DFS in the pGGN, mGGN, and SN groups. DFS, disease-free survival; pGGN, pure ground-glass nodules; mGGN, mixed ground-glass nodules; SN, solid nodules.
Discussion
This study investigated the prognostic significance of VPC on DFS and OS in patients with stage IA lung adenocarcinoma. After PSM, the median follow-up duration for both groups of patients exceeded 6 years. The results suggested that VPC status may influence DFS but not OS. Specifically, patients with VPC+ status had a longer mean DFS than those without VPC (VPC−), although no significant difference in OS was observed between the two groups. Age emerged as an independent prognostic factor for OS, while VPC status and pathological stage (IA3) were identified as independent prognostic factors for DFS. Furthermore, no significant differences in 5-year OS and DFS were found between the VPC+ and VPC− groups.
In previous studies, VPI has been considered as a poor prognostic factor in patients with lung cancer (15–20). However, two studies from China indicated that in stage I NSCLC, VPI is not a prognostic factor (21, 22). These findings highlight the significant heterogeneity in the involvement of the visceral pleura in lung cancer prognosis. In contrast, few studies have evaluated the prognostic role of VPC in early-stage lung adenocarcinoma. Unlike VPI, which promotes tumor progression and lymph node metastasis, VPC may indicate an early, non-invasive interaction between the tumor and the pleura. Histopathologically, VPC may be associated with reactive fibrosis, inflammatory changes, or thickened septal edema, rather than true invasion (23–25). This distinction is crucial, as it suggests that VPC might represent a distinct biological interaction between the tumor and pleura that does not necessarily progress to invasion.
A previous study (26) showed that in T1-stage patients, those with pleural contact had significantly worse 3-year cause-specific mortality and OS rates than those without pleural contact (17.6% [95% CI 10.7%–25.9%] vs. 6.6% [95% CI 3.5%–11.1%], P < 0.01, and 58.2% [95% CI 47.6%–67.5%] vs. 77.6% [95% CI 70.5%–83.2%], P < 0.01, respectively). Multivariate analysis indicated that pleural contact was associated with cause-specific mortality (HR 1.96, 95% CI 1.09–3.52, P = 0.03) and OS (HR 1.59, 95% CI 1.08–2.34, P = 0.02), suggesting that pleural contact is linked to significantly worse survival in patients with clinical T1N0M0 lung cancer. Unlike the extensively studied VPI, our study suggested that VPC also has clinical relevance. A key distinction is that VPC do not always progress to VPI. In the present study, we included patients with VPC, but we excluded patients with VPI. The results showed that VPC and pathological stage were independent prognostic factors for DFS (VPC: HR 0.49, 95% CI 0.27–0.91, P = 0.025; IA3: HR 4.06, 95% CI 1.67–9.87, P = 0.002), but not for OS. The Kaplan–Meier analysis revealed no significant difference in OS between the VPC+ and VPC− groups (P = 0.320), but there was a significant difference in DFS between the groups (P = 0.028). This supports VPC as an early indicator of tumor-pleura interaction impacting DFS.
The observed DFS advantage (P = 0.025) without a corresponding OS benefit (P = 0.320) in VPC+ patients warrants consideration. A key consideration is the potential impact of effective salvage therapies following recurrence. In contemporary oncology practice, patients with recurrent lung adenocarcinoma, particularly those with targetable mutations or PD-L1-positive disease, have access to increasingly effective post-recurrence treatments, including targeted agents and immunotherapy, which demonstrably improve survival outcomes in advanced settings (27, 28). Patients in the VPC− group experienced recurrence earlier and thus had greater opportunity to receive and benefit from these potent salvage options. This may have substantially extended their survival, thereby mitigating the OS advantage potentially conferred by the prolonged DFS in the VPC+ group. The later recurrence timing in the VPC+ cohort could also dilute the measurable OS impact of salvage therapy over the study period. Therefore, the efficacy of modern salvage treatments represents a plausible primary explanation for the DFS-OS dissociation observed here. This underscores that while VPC status identifies patients with a lower risk of early recurrence, achieving a survival advantage in the era of effective salvage therapy may require strategies that prevent recurrence more durably.
In contrast to some previous studies that demonstrated worse prognosis in patients with VPC+ (26, 29, 30), our study found that VPC- was associated with worse DFS. This discrepancy may be attributed to differences in study design, patient selection, and the specific criteria used to define VPC. Our study excluded patients with VPI, focusing solely on early-stage lung adenocarcinoma, which may have different biological behavior compared to more advanced stages. Additionally, our use of propensity score matching (PSM) to balance confounding factors may have influenced the observed outcomes.
Notably, we did not account for adjuvant therapies in our analysis, which might have restricted the comprehensiveness of our findings, particularly when assessing the relationship between VPC and prognosis. Pleural indentation has been linked to adverse outcomes in NSCLC, as it may heighten the risk of invasion into the lymph-rich visceral pleura, thereby facilitating tumor dissemination (31, 32). Additionally, pleural attachment has been identified as a risk factor for local recurrence after radiotherapy, as well as a risk factor for lower survival rates in lung adenocarcinoma (11, 32–34). However, our study did not differentiate between the various features of VPC; thus, it did not emphasize the significance of pleural indentation. Pre-treatment CT showing pleural attachment has predictive value.
The correlation between radiographic and pathological findings further highlights this critical distinction. Kim et al. (35) reported that CT-defined pleural contact demonstrated low positive predictive value (44%–56%) for pathological VPI and lacked independent prognostic significance for DFS (P > 0.05). Consistent with these observations, Hsu et al. (9) identified that only specific patterns of pleural retraction, notably type 2 pleural retraction characterized by linear traction with soft tissue components at the pleural terminus, could predict VPI with 71% accuracy. In our multicenter cohort, VPC encompassed a spectrum of features, including pleural retraction, pleural tail sign, pleural attachment, and pleural indentation. These radiographic manifestations likely represent localized stromal reactions rather than invasive tumor fronts, explaining the DFS advantage in VPC+ subgroups.
The marginal significance of DFS (P = 0.028) might be due to the limited sample size and the relatively small number of events. Unlike VPI, which is well - studied and recognized as a poor prognostic factor, VPC remains largely unexplored. Our findings indicate that VPC might represent a distinct biological interaction between the tumor and pleura that does not necessarily progress to invasion.
This interpretation aligns with Yang et al.’s (36) detailed radiographic–pathological correlation, which described pleural retraction signs as thick linear tractions with soft tissue components at the pleural margin, often accompanied by tumor-induced pleural buckling, findings strongly associated with VPI on final pathology. However, current controversies persist regarding the CT-based morphological criteria for T-staging. No consensus has been established on whether pleural contact warrants T-stage upstaging (37). This uncertainty stems from the persistent challenges in establishing a definitive radiographic–pathological correlation, as CT evidence of pleural contact or retraction cannot reliably determine the pathological T-stage (10). Notably, while radiographic VPC may indicate the tumor–pleural interaction, our study corroborates the existing literature, suggesting that these findings frequently represent reactive fibroelastotic changes rather than true pleural penetration (9, 35, 36). These observations have critical therapeutic implications.
The influence of nodule characteristics on VPC and VPI is significant. Prior to PSM, statistically significant differences in VPC were observed across different nodule types, including ground-glass, mixed, and solid nodules (P < 0.001). For pGGN, existing studies have demonstrated that pathological VPI does not occur without solid components or pleural changes (9, 23, 38). This indicates that pleural changes in lesions with pGGN are mostly benign interactions.
The situation becomes more complex with mGGN and SN. Previous studies have highlighted pleural retraction as a predictor of VPI in part-solid lung cancers, but the relatively low consolidation ratio in these lesions often results in subtle pleural retraction signs (38, 39). In the present study, 75% of VPC+ nodules were part-solid or solid, which are frequently associated with invasive adenocarcinoma components. However, accompanying pleural fibrosis may modulate the behavior of the tumor. A previous study suggested that stromal fibrosis promotes extracellular matrix remodeling, creating a physical barrier that impedes tumor cell migration (37). This is consistent with the absence of VPI in pGGN with pleural changes (9, 40), as their fibrotic reactions may suppress invasive progression. Conversely, VPI+ tumors can bypass these barriers by breaching the elastic layer and entering subpleural lymphatics, thereby enabling systemic dissemination (41, 42). It should be noted that these mechanisms are based on assumptions about the physical movement of malignant cells rather than being based on validated metastatic processes, which are considered far more complex (23, 43).
Yang et al. (13) demonstrated that VPI significantly impacts both OS and recurrence-free survival in patients with tumors >1 cm in size or a CTR of >50%, whereas VPI shows no prognostic significance in tumors ≤1 cm or with a CTR of ≤50%. These findings highlight the critical clinical relevance of VPI to the proportion of solid components and solid sizes. However, our study used a simplified categorization of pGGN, mGGN, and SN without detailed CTR stratification. The subgroup analysis revealed no significant differences in OS (P = 0.730) or DFS (P = 0.150) among these groups. This suggests that VPC may have distinct prognostic implications compared to VPI, which warrants further investigation in larger cohorts with detailed CTR stratification.
Nodule features exert differential impacts on VPC and VPI. VPC in ground-glass nodules predominantly correlates with benign pathological changes, while VPC in mixed/solid nodules may involve complex interactions requiring further study. These distinctions have prognostic implications for DFS and OS.
This study has several strengths. First, PSM balanced potential confounders, enhancing the comparability of the VPC+ and VPC− groups. Second, the multicenter design increased the external validity by incorporating data from two centers. Third, robust statistical methods, including Cox proportional-hazards regression and Kaplan–Meier analyses, were used to evaluate the influence of VPC on DFS and OS. Finally, our results highlight VPC as a potential prognostic indicator for DFS in early-stage lung adenocarcinoma, offering a valuable direction for future research.
However, this study also has several limitations. The retrospective design may have introduced selection and information biases, potentially affecting the generalizability of the findings. Data collection from medical records could have led to incomplete or inaccurate data, particularly for VPC assessments that rely on imaging and pathological reports, thus introducing subjectivity. The broad definition of VPC, encompassing multiple imaging features, was not stratified by subtype and lacked formal inter-rater reliability assessment, potentially introducing variability in progress and prognostic conclusions. While PSM mitigates some confounding, it cannot eliminate all biases. The small sample size, especially the limited number of VPC+ cases, may have reduced statistical power. Additionally, information on adjuvant therapies was not collected, so their potential impact on DFS and OS could not be evaluated. These limitations underscore the need for larger prospective studies to validate our findings.
In conclusion, our findings suggest that VPC status may relate to DFS in patients with stage IA lung adenocarcinoma, but not to OS. This implies that VPC might be a valuable factor for risk assessment and postoperative follow-up planning in patients with early-stage lung adenocarcinoma. However, owing to the retrospective nature of the study, our results require cautious interpretation. More research, especially prospective studies with larger cohorts, is needed to verify the role of VPC as a prognostic marker and define how VPC assessment could help to manage stage IA lung adenocarcinoma.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The First People’s Hospital of Jiande. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin. The requirement for written informed consent was waived due to the retrospective nature of the study.
Author contributions
YR: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. YY: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. JH: Data curation, Funding acquisition, Investigation, Methodology, Writing – original draft. HX: Data curation, Funding acquisition, Investigation, Methodology, Writing – original draft. WC: Data curation, Investigation, Methodology, Writing – original draft. CL: Data curation, Investigation, Methodology, Writing – original draft. PS: Data curation, Investigation, Methodology, Writing – original draft. YH: Data curation, Investigation, Methodology, Writing – original draft. ZZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research were supported by Hangzhou Science and Technology Bureau (Grant No. B20240282), Jiande Municipal Science and Technology Bureau (Grant No. 2023JZX07), and the Interdisciplinary Project of Dalian University (Grant No. DLUXK-2023-QN-012).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, body mass index; CI, confidence interval; CT, computed tomography; CTR, consolidation-to-tumor ratio; DFS, disease-free survival; HR, hazard ratio; mGGN, mixed ground-glass nodules; M-VATS, multiportal video-assisted thoracoscopic surgery; NSCLC, non-small cell lung cancer; OS, overall survival; pGGN, pure ground-glass nodules; PSM, propensity score matching; SBRT, stereotactic body radiation therapy; SD, standard deviation; SE, standard error; SMD, standardized mean difference; SN, solid nodules; TNM, tumor–node–metastasis; U-VATS, uniportal video-assisted thoracoscopic surgery; VPC(+), visceral pleural changes-positive; VPC(−), visceral pleural changes-negative; VPI, visceral pleural invasion.
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
2. Asamura H, Nishimura KK, Giroux DJ, Chansky K, Hoering A, Rusch V, et al. IASLC lung cancer staging project: the new database to inform revisions in the ninth edition of the TNM classification of lung cancer. J Thorac Oncol. (2023) 18:564–75. doi: 10.1016/j.jtho.2023.01.088
3. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:497–530. doi: 10.6004/jnccn.2022.0025
4. Yoshida J, Nagai K, Asamura H, Goya T, Koshiishi Y, Sohara Y, et al. Visceral pleura invasion impact on non-small cell lung cancer patient survival: its implications for the forthcoming TNM staging based on a large-scale nation-wide database. J Thorac Oncol. (2009) 4:959–63. doi: 10.1097/JTO.0b013e3181a85d5e
5. Mathey-Andrews C, Abruzzo AR, Venkateswaran S, Potter AL, Senthil P, Beqari J, et al. Segmentectomy vs lobectomy for early non-small cell lung cancer with visceral pleural invasion. Ann Thorac Surg. (2024) 117:1007–14. doi: 10.1016/j.athoracsur.2023.06.020
6. Dai ZY, Shen C, Wang X, Wang FQ, and Wang Y. Could less be enough: sublobar resection vs lobectomy for clinical stage IA non-small cell lung cancer patients with visceral pleural invasion or spread through air spaces. Int J Surg. (2025) 111:2675–85. doi: 10.1097/JS9.0000000000002249
7. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage grouVPCngs in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
8. Jiang L, Liang W, Shen J, Chen X, Shi X, He J, et al. The impact of visceral pleural invasion in node-negative non-small cell lung cancer: a systematic review and meta-analysis. Chest. (2015) 148:903–11. doi: 10.1378/chest.14-2765
9. Hsu JS, Han IT, Tsai TH, Lin SF, Jaw TS, Liu GC, et al. Pleural tags on CT scans to predict visceral pleural invasion of non-small cell lung cancer that does not abut the pleura. Radiology. (2016) 279:590–6. doi: 10.1148/radiol.2015151120
10. Onoda H, Higashi M, Murakami T, Tao H, Yokoyama S, Kunihiro Y, et al. Correlation between pleural tags on CT and visceral pleural invasion of peripheral lung cancer that does not appear touching the pleural surface. Eur Radiol. (2021) 31:9022–9. doi: 10.1007/s00330-021-07869-y
11. Zhang C, Wang L, Cai X, Li M, Sun D, and Wang P. Tumour-pleura relationship on CT is a risk factor for occult lymph node metastasis in peripheral clinical stage IA solid adenocarcinoma. Eur Radiol. (2023) 33:3083–91. doi: 10.1007/s00330-023-09476-5
12. Sun Q, Li P, Zhang J, Yip R, Zhu Y, Yankelevitz DF, et al. CT predictors of visceral pleural invasion in patients with non-small cell lung cancers 30 mm or smaller. Radiology. (2024) 310:e231611. doi: 10.1148/radiol.231611
13. Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. (1999) 354:99–105. doi: 10.1016/S0140-6736(99)06093-6
14. Travis WD, Brambilla E, Rami-Porta R, Vallières E, Tsuboi M, Rusch V, et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol. (2008) 3:1384–90. doi: 10.1097/JTO.0b013e31818e0d9f
15. Yang Z, Li Y, Guo C, Xing Y, Liu C, Zhang J, et al. The prognostic value of visceral pleural infiltration in ≤3 cm nonsmall cell lung cancer presenting with ground glass opacity: an inverse probability of treatment weighting study. Int J Surg. (2024) 110:7871–9. doi: 10.1097/JS9.0000000000001803
16. Van Schil PE, Asamura H, Nishimura KK, Rami-Porta R, Kim YT, Bertoglio P, et al. The international association for the study of lung cancer lung cancer staging project: proposals for the revisions of the T-descriptors in the forthcoming ninth edition of the TNM classification for lung cancer. J Thorac Oncol. (2024) 19:749–65. doi: 10.1016/j.jtho.2023.12.006
17. Ruan Z, Zhuo X, and Xu C. Diagnosis, treatment, and prognosis of stage IB non-small cell lung cancer with visceral pleural invasion. Front Oncol. (2024) 13:1310471. doi: 10.3389/fonc.2023.1310471
18. Wu LL, Liu X, Jiang WM, Huang W, Lin P, Long H, et al. Stratification of patients with stage IB NSCLC based on the 8th edition of the american joint committee on cancer (AJCC) staging manual. Front Oncol. (2020) 10:571. doi: 10.3389/fonc.2020.00571
19. Zhai WY, Wong WS, Duan FF, Liang DC, Gong L, Dai SQ, et al. Distinct prognostic factors of ground glass opacity and pure-solid lesion in pathological stage I invasive lung adenocarcinoma. World J Oncol. (2022) 13:259–71. doi: 10.14740/wjon1499
20. Seok Y and Lee E. Visceral pleural invasion is a significant prognostic factor in patients with partly solid lung adenocarcinoma sized 30 mm or smaller. Thorac Cardiovasc Surg. (2018) 66:150–5. doi: 10.1055/s-0036-1586757
21. Fu F, Zhang Y, Wen Z, Zheng D, Gao Z, Han H, et al. Distinct prognostic factors in patients with stage I non-small cell lung cancer with radiologic part-solid or solid lesions. J Thorac Oncol. (2019) 14:2133–42. doi: 10.1016/j.jtho.2019.08.002
22. Chan EY, Amirkhosravi F, Nguyen DT, Chihara RK, Graviss EA, and Kim MP. Lobectomy provides the best survival for stage I lung cancer patients despite advanced age. Ann Thorac Surg. (2022) 114:1824–32. doi: 10.1016/j.athoracsur.2022.03.031
23. Zhao Q, Wang JW, Yang L, Xue LY, and Lu WW. CT diagnosis of pleural and stromal invasion in Malignant subpleural pure ground-glass nodules: an exploratory study. Eur Radiol. (2019) 29:279–86. doi: 10.1007/s00330-018-5558-0
24. Wang Y, Lyu D, Yu D, Hu S, Ma Y, Huang W, et al. Intratumoral and peritumoral radiomics combined with computed tomography features for predicting the invasiveness of lung adenocarcinoma presenting as a subpleural ground-glass nodule with a consolidation-to-tumor ratio ≤50. J Thorac Dis. (2024) 16:5122–37. doi: 10.21037/jtd-24-243
25. Mutsaers SE and Krymskaya VP. Pleural fibrosis: now that’s what mTORC(ing) about. Am J Respir Cell Mol Biol. (2024) 70:8–10. doi: 10.1165/rcmb.2023-0327ED
26. Eriguchi T, Takeda A, Tsurugai Y, Sanuki N, Kibe Y, Hara Y, et al. Pleural contact decreases survival in clinical T1N0M0 lung cancer patients undergoing SBRT. Radiother Oncol. (2019) 134:191–8. doi: 10.1016/j.radonc.2019.02.005
27. Mok T, Nakagawa K, Park K, Ohe Y, Girard N, Kim HR, et al. Nivolumab plus chemotherapy in epidermal growth factor receptor-mutated metastatic non-small-cell lung cancer after disease progression on epidermal growth factor receptor tyrosine kinase inhibitors: final results of checkMate 722. J Clin Oncol. (2024) 42:1252–64. doi: 10.1200/JCO.23.01017
28. Yang JC, Lee DH, Lee JS, Fan Y, de Marinis F, Iwama E, et al. Phase III KEYNOTE-789 study of pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor–Resistant, EGFR-mutant, metastatic nonsquamous non-small cell lung cancer. J Clin Oncol. (2024) 42:4029–39. doi: 10.1200/JCO.23.02747
29. Seok Y, Jeong JY, and Lee E. Extent of visceral pleural invasion and the prognosis of surgically resected node-negative non-small cell lung cancer. Thorac Cancer. (2017) 8:197–202. doi: 10.1111/1759-7714.12424
30. Lin X, Liu K, Li K, Chen X, Chen B, Li S, et al. A CT-based deep learning model: visceral pleural invasion and survival prediction in clinical stage IA lung adenocarcinoma. iScience. (2023) 27:108712. doi: 10.1016/j.isci.2023.108712
31. Chen Y, Huang Q, Zhong H, Li A, Lin Z, and Guo X. Correlations between iodine uptake, invasive CT features and pleural invasion in adenocarcinomas with pleural contact. Sci Rep. (2023) 13:16191. doi: 10.1038/s41598-023-43504-0
32. Li L, Yang Q, Luo D, Wang X, Liu Z, and Huang R. Baseline computed tomography imaging findings could assist in early diagnosis of visceral pleural invasion for newly discovered early subpleural non-small cell lung cancer: T1 or T2. J Thorac Dis. (2024) 16:5779–91. doi: 10.21037/jtd-24-294
33. Yamamoto T, Kadoya N, Shirata Y, Koto M, Sato K, Matsushita H, et al. Impact of tumor attachment to the pleura measured by a pretreatment CT image on outcome of stage I NSCLC treated with stereotactic body radiotherapy. Radiat Oncol. (2015) 10:35. doi: 10.1186/s13014-015-0343-6
34. Wang H, Schabath MB, Liu Y, Berglund AE, Bloom GC, Kim J, et al. Semiquantitative computed tomography characteristics for lung adenocarcinoma and their association with lung cancer survival. Clin Lung Cancer. (2015) 16:e141–63. doi: 10.1016/j.cllc.2015.05.007
35. Kim H, Goo JM, Kim YT, and Park CM. CT-defined visceral pleural invasion in T1 lung adenocarcinoma: lack of relationship to disease-free survival. Radiology. (2019) 292:741–9. doi: 10.1148/radiol.2019190297
36. Yang S, Yang L, Teng L, Zhang S, Cui Y, Cao Y, et al. Visceral pleural invasion by pulmonary adenocarcinoma ≤3 cm: the pathological correlation with pleural signs on computed tomography. J Thorac Dis. (2018) 10:3992–9. doi: 10.21037/jtd.2018.06.125
37. Snoeckx A. Visceral pleural invasion: predictable on CT? Quant Imaging Med Surg. (2019) 9:2019–22. doi: 10.21037/qims.2019.11.03
38. Heidinger BH, Schwarz-Nemec U, Anderson KR, de Margerie-Mellon C, Monteiro Filho AC, Chen Y, et al. Visceral pleural invasion in pulmonary adenocarcinoma: differences in CT patterns between solid and subsolid cancers. Radiol Cardiothorac Imaging. (2019) 1:e190071. doi: 10.1148/ryct.2019190071
39. Wang F, Pan X, Zhang T, Zhong Y, Wang C, Li H, et al. Predicting visceral pleural invasion in lung adenocarcinoma presenting as part-solid density utilizing a nomogram model combined with radiomics and clinical features. Thorac Cancer. (2024) 15:23–34. doi: 10.1111/1759-7714.15151
40. Kim HJ, Cho JY, Lee YJ, Park JS, Cho YJ, Yoon HI, et al. Clinical significance of pleural attachment and indentation of subsolid nodule lung cancer. Cancer Res Treat. (2019) 51:1540–8. doi: 10.4143/crt.2019.057
41. Zhao LL, Xie HK, Zhang LP, Zha JY, Zhou FY, Jiang GN, et al. Visceral pleural invasion in lung adenocarcinoma ≤3 cm with ground-glass opacity: a clinical, pathological and radiological study. J Thorac Dis. (2016) 8:1788–97. doi: 10.21037/jtd.2016.05.90
42. Kao CC, Wang HC, Lin MW, Tsai TM, Hsu HH, Liao HC, et al. Predicting visceral pleural invasion in resected lung adenocarcinoma via computed tomography. Cancers (Basel). (2025) 17:1414. doi: 10.3390/cancers17091414
Keywords: disease-free survival, lung adenocarcinoma, overall survival, propensity score matching, visceral pleural changes
Citation: Ruan Y, You Y, Han J, Xue H, Cao W, Long C, Sun P, Hu Y and Zhao Z (2025) Prognostic significance of visceral pleural changes in stage IA lung adenocarcinoma: a retrospective study. Front. Oncol. 15:1658916. doi: 10.3389/fonc.2025.1658916
Received: 03 July 2025; Accepted: 12 September 2025;
Published: 25 September 2025.
Edited by:
Nanna Maria Sijtsema, University Medical Center Groningen, Groningen, NetherlandsReviewed by:
Yoshiki Murakumo, Kitasato University, JapanMarcello Migliore, King Faisal Specialist Hospital and Research Centre, Saudi Arabia
Jingke She, Hunan University, China
Copyright © 2025 Ruan, You, Han, Xue, Cao, Long, Sun, Hu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhilong Zhao, emhpbG9uZzUwOUAxMjYuY29t
†These authors have contributed equally to this work
 Yingding Ruan
Yingding Ruan Yuhe You2†
Yuhe You2† Hongsheng Xue
Hongsheng Xue