- 1OncoRay – National Center for Radiation Research in Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, TUD Dresden University of Technology, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany
- 2Helmholtz-Zentrum Dresden-Rossendorf, Institute of Radiooncology – OncoRay, Dresden, Germany
- 3Department of Radiotherapy and Radiation Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, TUD Dresden University of Technology, Dresden, Germany
- 4German Cancer Consortium (DKTK), Partner Site Dresden, and German Cancer Research Center (DKFZ), Heidelberg, Germany
Proton therapy (PT) has the potential to deliver conformal doses to the tumor while sparing normal tissue, but is highly susceptible to treatment uncertainties. The occurrence of anatomical changes during PT treatments has a major impact on the delivered dose, often necessitating plan adaptations that are typically performed offline and require a few days before the adapted plan is ready. In order to react promptly to detected anatomical changes, online adaptive proton therapy (OAPT) has been proposed with the goal of adapting the plan while the patient is on the treatment couch. First OAPT workflows for daily plan adaptation that are effective against interfractional anatomical variations have reached clinical application. However, even faster OAPT workflows are needed to cope with faster anatomical changes. Near real-time adaptive PT (NAPT) relying on online in vivo treatment verification can be a potential solution for many tumor entities (e.g., thoraco-abdominal tumors), which would greatly benefit from the conformality of PT, but are presently challenging to treat with proton beams due to the influence of intrafractional variations. In addition, NAPT offers the opportunity to achieve the long-awaited closed PT feedback loop. In this paper we review the required tasks and necessary components in an OAPT workflow for the application of near real-time adaptation, proceeding sequentially from volumetric imaging for online plan adaptation up to online verification during delivery. Available technology and upcoming developments are discussed. Several aspects regarding regulatory approval, cost-benefit related issues and additional beyond-the-loop tasks are also addressed.
1 Introduction
Due to the characteristic behavior of proton beams and their energy release in tissues, proton therapy (PT) is particularly sensitive to anatomy variations occurring during the course of the treatment (1). A modification of the patient anatomy may result in underdosage to the tumor, unnecessary high doses to the normal tissue or even overdosage to the organs-at-risk (OAR), potentially leading to severe side effects. PT remains constrained for certain entities (2), particularly in the abdominal region. In parallel with the advent of latest technological developments, new radiotherapy (RT) treatment modalities have emerged that introduce an adaptive approach. The concept of adaptive radiotherapy (ART) based on a systematic feedback loop is well-known (3) and can be classified into three main time regimes: offline (adaptation performed between fractions), online (adaptation performed directly prior to delivery, while the patient is on the treatment couch) and real-time (adaptation performed within fraction delivery) (4). Online ART has become a reality in photon-based radiotherapy (XRT) with the availability of hybrid systems of imaging plus linear accelerator (LINAC), like magnetic resonance (MR)-LINACs (5), tomotherapy (6) and systems based on cone-beam computed tomography (CBCT) (7). Within ART, positron emission tomography (PET)-integrated systems have also been recently proposed (8) that target biological changes in the tumor rather than anatomical variations, thereby propelling the concept of biology-driven adaptation (9).
Robust optimization in PT, as implemented in commercial treatment planning systems (TPS) today, accounts only for positional shifts within an otherwise unchanged anatomy. However, anatomical changes, such as variations in cavity filling or tumor size, can lead to clinically significant differences. Given the large impact of anatomical variations on the conformity of the proton dose distribution, the spread of online ART has sparked interest for potential applications in PT (10, 11), with many PT centers actively working towards clinical implementation of this novel approach (12–14). The concept of online adaptive PT (OAPT) is not yet as established as its photon counterpart and refers generally to the opportunity of performing a plan adaptation (and related tasks) while the patient is on the treatment couch during the daily treatment session. This review focuses on online adaptations due to anatomical changes. There are different flavors of OAPT (Figure 1). They depend on the one hand on the timing of the addressed anatomical changes (and the necessity of potential plan adaptations per treatment and fraction) and on the other hand on the time needed to perform the adaptation itself (15).

Figure 1. Different workflows representing the multifaceted OAPT landscape, including daily and post-delivery tasks. Standard PT tasks (in white) are differentiated from major OAPT components (in blue). Workflow-specific OAPT components are highlighted in orange. Note that, while integrated CBCT systems are now widely established for patient setup in PT, in the context of OAPT an in-room 3D imaging system capable of providing a high-quality volumetric image for planning is essential and is crucial for near real-time applications. In standard DAPT (A) the online adaptation follows volumetric imaging and online contouring tasks. To reduce the daily burden, daily adaptation can be performed on demand (B) only after evaluation of the actual adaptation need. Finally, NAPT (C) relies on treatment verification in parallel with delivery to account for unexpected intrafractional anatomical variations and trigger the image acquisition and eventual adaptation only after a treatment deviation is detected. This differs from standard (A) or evaluation-triggered (B) DAPT, where the adaptation happens before delivery. If no treatment deviation is identified, the clinical workflow can proceed from setup directly to delivery as in standard clinical practice. Real-time adjustments involving motion management techniques are not represented. Treatment verification without triggering capability may be used to independently monitor the delivery also in workflows (A, B).
Interfractional changes like weight changes, tumor shrinkages or growth, relevant changes in cavity filling, and substantially different fill levels of the digestive organs with related shifts of nearby soft tissue happen on a timescale of hours or days (16). These variations can be dealt with daily adaptive PT (DAPT), with the goal of adapting the plan to the daily anatomy. In a standard DAPT workflow (Figure 1A), the online plan adaptation follows the conventional setup and 3D imaging. This way the treatment plan is adapted to the current daily anatomy based on an image acquired prior the delivery. Attempts at implementing such a workflow have been recently proposed (12) and first clinical realizations have been reported (17, 18).
Generating the online adapted plan at each fraction places an undeniable burden on patient comfort and daily workload. To reduce the daily burden, an evaluation-based DAPT “on demand” is envisaged (Figure 1B), where the online adaptation is possibly performed after an assessment of its need based on the daily acquired 3D image and/or projected dose distribution or even on the information from pre-delivery range assessment like range probing (19). If there is no need for adaptation, the workflow can continue with the delivery of the initial treatment plan.
However, in order to address faster changes in organ position and deformation, an even faster action is required that is capable of detecting and correcting for intrafractional variations. Such anatomical changes can happen on timescales of minutes (like urinary activity, organ drift), or seconds (like peristalsis, respiratory movements, cardiac motion) (20–22). In particular, respiratory movements are usually handled prospectively via techniques of motion management, like suppression of motion, 4D imaging and optimization, continuous motion surrogate monitoring, and appropriate changes in the beam delivery sequence (gating, repainting), etc. (15, 16). Motion management alone, however, is not able to compensate for the entire intrafractional range of movements, especially when dealing with irregular and/or unexpected variations, highlighting the need for a near real-time adaptive approach that includes online generation of an adapted plan (2, 23). For example, entities like thoraco-abdominal (e.g., lung, esophageal, liver, pancreas), gynecological and genitourinary tumors are greatly influenced by unexpected intrafractional variations and located in close position to gastrointestinal, cardiac, pulmonary and/or urinary OAR (2, 16), thus requiring steep dose distributions. For such entities a shift towards near real-time adaptation has already been observed in XRT after the advent of MR-guided radiotherapy (24–26). This emphasizes the necessity of adopting similar approaches for PT, which can offer superior conformality but is presently limited. With near real-time adaptation in PT, analogous to XRT, it would be possible to continuously monitor treatment delivery and promptly react to irregular intrafraction anatomical changes as they occur. This differs from standard or evaluation-triggered DAPT, in which the adaptation is only performed before the treatment delivery. Near real-time adaptive PT (NAPT) (Figure 1C) can address this issue by adapting the treatment plan using information on treatment deviations from online treatment verification systems that run concurrently with delivery. Upon identification of a deviation between the delivered and the expected proton dose, an online adaptive workflow is initiated with the acquisition of a 3D image and the assessment of the adaptation need. On the one hand, this approach would allow a rapid response to unforeseen anatomical changes and, on the other hand, it would provide a safety net to ensure that the planned dose is delivered as indicated and that further plan adaptation is only applied when necessary. This NAPT workflow, which involves imaging, contour generation, plan adaptation, and approval under stricter time constraints, is not yet available in a truly real-time setting due to the time currently required to complete these critical operations before resuming delivery.
In this sense, at the time of this review an actual “real-time” adaptive PT can only be represented by operations of motion management happening during the daily treatment session that do not require a beam delivery interruption or a treatment plan modification, leading instead to adjustments of the beam geometry or delivery sequence (15, 27, 28). These real-time operations have also been previously referred to as an in-line adaptive workflow (11).
This review outlines step-by-step the necessary components within a daily treatment session to implement a NAPT closed feedback loop after standard patient setup, describing the current research status and highlighting areas that require further investigation. Each major step in the workflow (volumetric imaging for online treatment planning, online contour generation, decision on online adaptation need, online plan adaptation – optimization strategy and dose calculation, pre-delivery quality assurance of the online adapted plan, online treatment verification) will be discussed in dedicated sections. Additional aspects regarding integration, regulatory approval and tasks that are performed outside the loop will also be addressed.
2 Volumetric imaging for online treatment planning
The availability of 3D imaging in the treatment room, preferably keeping the patient in treatment position, is essential for identifying anatomical changes in the timeframe for OAPT. Given the stricter timeframe and potential for adaptation during paused/suspended delivery, 3D imaging in treatment position is especially relevant for in NAPT. The quality of the acquired images must be adequate for the online plan adaptation of proton-based treatments, i.e., it must ensure the correct retrieval of the stopping power ratio (SPR) for accurate range and dose calculation. Image-guided XRT has propelled the development of volumetric image guidance tools (29), many of which are now being investigated for OAPT. Here pre-delivery imaging techniques based on in-room (fan-beam) CT, CBCT, MR imaging (MRI) and proton CT are discussed in terms of technological advancement, the ability to retrieve the current 3D patient’s anatomy and related SPR map for proton dose calculation, and the suitability for near real-time adaptation (Table 1). In-room imaging tools providing additional information for real-time motion management are briefly mentioned.

Table 1. Overview of the main in-room pre-delivery volumetric imaging systems depicting their properties and suitability for online (near real-time) plan adaptation in PT.
In-room (fan-beam) CT is a well-known pre-treatment imaging solution for checking the anatomy in treatment position and for setup and positioning. Developments of mobile CT scanners (Figure 2A) to perform in-room CT for OAPT have been observed (37, 38) and proposed for clinical use (39), although the integration in a fast and efficient workflow is challenging. CT scanners on rails have a fixed position in the room (Figure 2B). Despite the associated costs and impact on the overall available space in the treatment room, they are at present gaining traction for use in DAPT workflows (12, 17). In-room CT offers several advantages regarding image quality, the most accurate prediction of the SPR for PT planning including dual-energy CT (DECT) based SPR prediction (39, 40) and the option to perform 4D-imaging to monitor interfractional changes in the respiratory pattern (16), however, it is currently not optimal for near real-time adaptation. In-room CT scans are acquired distant from the actual delivery position and usually incapable of returning images matched to the isocenter. Before proceeding further with the adaptation, the in-room CT images must be first registered to the reference image (e.g., planning CT), in order to share the same reference frame. Moreover, the reference frame of the in-room CT and its position within the treatment room must be also correctly related with the room coordinate system. In order to correctly locate at room isocenter a specific point of the treatment volume identified in the CT scan, a precise and reproducible position of the robot for patient positioning (or patient positioning system) is required. Securing sufficient accuracy of the patient table position over the large distance from the PT gantry to the CT gantry is paramount. The integration of in-room CT systems with the delivery system still represents a challenge and is heavily facility-dependent (29). As a result, the insertion of such systems into a closed feedback loop for near real-time adaptation in PT is a complex undertaking. Still, in-room CTs are first choice for OAPT workflows as the fan-beam CT imaging is the gold-standard for accurate SPR prediction and dose calculation (39).

Figure 2. Overview of discussed volumetric imaging solution for treatment planning: (A) mobile CT scanner (reproduced from https://www.itnonline.com/content/orlando-health-and-mevion-offer-advanced-diagnostic-image-guidance-proton-therapy with permission), (B) Siemens in-room CT on rails at OncoRay (reproduced from https://www.hzdr.de/db/Cms?pOid=43592&pNid=99 with permission), (C) Varian gantry-mounted CBCT at Paul Scherrer Institute, (D) CNAO C-arm mounted CBCT (reproduced from (36) under CC-BY 4.0 license), (E) IBA nozzle-mounted CBCT (reproduced from https://www.itnonline.com/content/iba-and-philips-step-commercial-collaboration-brazilian-proton-therapy-market with permission), (F) Medphoton (Brainlab) couch-mounted CBCT at MedAustron, (G) Medphoton (Brainlab) ceiling-mounted CBCT at Maastro Proton Therapy (courtesy of Mevion Medical Systems and Maastro Proton Therapy), (H) full-body, rotatable MR scanner integrated at horizontal beamline at OncoRay, (reproduced from https://www.hzdr.de/db/Cms?pOid=71010&pNid=0 with permission) (I) experimental proton CT prototype (reproduced from (35) under a CC-BY 4.0 license).
Over the past decade, CBCT has become available in newly built PT centers, with commercially available options from respective PT vendors seamlessly integrated into the treatment room. In detail, there are different configurations, like gantry-mounted (Figure 2C), mounted on robotic C-arms (Figure 2D), nozzle-mounted (Figure 2E), couch-mounted (Figure 2F), or ceiling-mounted (Figure 2G) (29). CBCT allows imaging at isocenter position and has thus received great interest for OAPT (18, 41). Its use in online adaptive XRT workflows is already established, with commercial systems such as Varian’s Ethos or Elekta’s C-arm LINACs (42–44), including novel developments of Elekta Evo and ONE (45), and the emerging implementation of 4D-CBCT enabling time-resolved imaging (46). However, the inherently low image quality of CBCT presents a significant challenge for its use in OAPT. Due to its technical design, CBCT is more susceptible to X-ray scattering within the patient or detector and beam hardening compared to fan-beam CT—especially in larger patients and at wide cone angles, such as in the upper abdomen or pelvis. Additional factors, including signal lag, limited detector efficiency, and field-of-view truncation, further degrade image quality. Since volumetric CBCT data is reconstructed from 2D projections acquired at different beam angles during a single gantry rotation (typically lasting 60 to 120 s) without patient couch movement, any patient motion can introduce additional artifacts. As a result, uncorrected CBCT images generally lack the image quality required to meet the strict CT number stability demands in PT for accurate dose calculation (47, 48). Hence, the adoption and clinically reliable validation of correction techniques is of utmost importance to allow for accurate proton dose calculations. Various correction methods to utilize CBCTs for dose computation in PT have been already investigated in several studies (49–52), and include deforming the planning (fan-beam) CT to the CBCT using deformable image registration (DIR), scatter correction methods, image correction utilizing deep convolution neural networks, histogram matching, and corrections via look-up tables. While CBCT offers an appealing option for near real-time adaptation at the isocenter (minimizing both the time and uncertainties associated with patient repositioning required for in-room CT), its routine clinical implementation for dose optimization remains unrealized despite extensive research efforts. As an initial step toward clinical translation, corrected CBCT datasets could be used exclusively for dose recalculations and as a trigger for acquiring a new fan-beam CT scan if clinically significant dosimetric changes are detected. With sufficient experience across a broad range of patient cases, direct plan adaptation based on CBCT with unified settings could then be introduced as a subsequent step. Moreover, CBCT integration into many commercial delivery and planning systems simplifies the integration into the OAPT closed feedback loop and gives the opportunity to perform prompt checks of the current anatomy, for example in case a treatment deviation is identified during delivery (e.g., by a complimentary treatment verification system). CBCT, however, like in-room CT, cannot be used to continuously monitor the patient anatomy and tumor location during beam delivery.
The CT-based solutions discussed so far assume that the patient is imaged and treated in recumbent position. Recently, interest in upright treatment and imaging has increased significantly (53). The idea of treating patients in a seated position is not new (54), but has gained renewed attention due to the development of commercial systems for vertical CT imaging together with reported clinical applications (55–58). The main driving factor towards the investigation of upright CT imaging and treatment is the opportunity of reducing the costs of the expensive PT facilities. In fact, employment of upright positioning would allow for a gantry-less treatment room, where the patient is rotated relative to the fixed beam instead of moving the beam around the patient with a gantry (59). An additional aspect to consider is that upright CT imaging offers in principle high flexibility and the opportunity of imaging at the isocenter. This is relevant for OAPT treatments, especially when aiming at faster time regimes. For certain tumor entities, treatment in an upright patient position may offer potential benefits and, in some cases, could even be more comfortable for patients. However, given the limited experience with this emerging technology – including specialized treatment chairs and volumetric imaging systems (59, 60) – several challenges remain. These must be addressed before definitive conclusions can be drawn regarding the treatment quality and clinical outcomes of upright PT. The positioning accuracy and reproducibility need to be quantified for different treatment sites. Upright PT will very much rely on fast online adaptation capabilities in case the positioning accuracy is inferior to supine treatments and most importantly clinical evidence of safe and efficient treatment needs to be generated (61, 62).
Finally, MRI is noteworthy for its ability to provide high-quality soft-tissue imaging without exposing patients to additional ionizing radiation from imaging. In XRT, MRI has already been integrated into daily adaptive workflows using MR-LINACs, where it enables continuous anatomical monitoring during treatment. Although MRI offers superior soft-tissue contrast compared to CT, it is inferior in terms of geometrical accuracy (distortions) and dose calculation applicability. The enhanced soft-tissue contrast may be relevant for NAPT and for challenging cases affected by significant intra-fractional variations (30, 63). However, MR-only dose calculation remains more complex in PT than in XRT, as accurate material characterization of bones and air from MR scans is crucial. As with CBCT, generating synthetic CT datasets from MR scans is being explored as a method for enabling SPR prediction, though it remains challenging. Various approaches, including bulk density overrides, anatomical atlases, voxel-based intensity assignments, DIR with CT datasets, and deep learning techniques, have been proposed to address this issue (31, 64). There are also significant challenges in integrating MRI into a PT treatment room: minimizing proton beam deflection in MR scanners (65) and ensuring the compatibility of dosimetry equipment with MRI (66, 67). Due to the impact of gantry rotation, which involves moving large amounts of steel, on the magnetic field of an MR scanner, integrating MRI into a gantry room seems practically impossible. As a result, translational research is currently focused on integrating MR scanners at horizontal beam lines. Since this could potentially be combined with an upright positioning, it may offer fewer limitations regarding the degrees of freedom for beam directions. Despite the challenges, research in MR-integrated PT has advanced to the point of initial clinical studies (32), with ongoing developments (Figure 2H) (33, 68–76). Several solutions are currently under pre-clinical exploration, including in-room/integrated or off-room/shuttle-based MR scanners. However, it is clear that further research and improvements are needed to define robust and reliable clinical MR-only workflows in PT. Depending on the clinical use case – such as initial treatment planning or dose re-calculation to assess adaptation needs – MR-based synthetic CT approaches do not necessarily need to match the accuracy of state-of-the-art fan-beam CT. A significant step forward would be the ability to trigger treatment adaptation through improved in-room imaging, allowing for re-planning and tailoring treatment in response to anatomical changes. At this stage, it remains uncertain whether MR-only PT treatment planning can achieve the same level of accuracy and precision as current CT-based methods, such as DECT-based direct SPR prediction. For the future potential of MR guidance in adaptive PT, further research is needed to assess its clinical applicability.
CT imaging with proton beams instead of X-rays is a promising technology to overcome the uncertainty in the conversion from X-ray attenuation to SPR (77–80). Despite ongoing research activities over the last decades, this technique is still in an early stage of development, i.e., first prototype proton CT scanners (Figure 2I) are constructed and experimentally validated, but a clinical system is currently not available (34, 35, 81). Current applications are mostly limited to head sizes, since today’s PT facilities equipped with cyclotrons are not capable to deliver proton beams with sufficient energy to cross body regions like abdomen and pelvis. Some dedicated synchrotron-based systems used for PT can overcome this limitation by reaching proton energies of 330 MeV corresponding to a range in water of 60 cm (82, 83). As demonstrated in a recent study (84), further improvements in image reconstruction are crucial to reduce the partly severe ring and streak artifacts in proton CT images. Furthermore, it was shown that DECT-based direct SPR prediction can achieve a comparable SPR accuracy as proton CT (84). Hence, it will not be trivial to demonstrate a benefit of proton CT compared to X-ray CT in terms of clinical outcome or cost efficiency.
Further non-volumetric imaging techniques may be combined with the above mentioned, in order to reconstruct the full range of intrafractional movements in the patient. Exemplary solutions are the visualization of external markers, the detection of internal markers or electronic transponders, online fluoroscopy for correlation of internal motion and external surrogate signal, ultrasound imaging and surface tracking (85). These motion management solutions are already quite established in image-guided routines and do not constitute per se an issue for their integration in a near real-time adaptive workflow, although stricter timing criteria and the accurate combination of multimodal information must be considered. A full review of motion management systems suitable in PT has been reported (16) and is beyond the scope of this review as these techniques do not provide 3D images suitable for dose calculation and (adaptive) treatment planning.
3 Online contour generation
One of the challenges associated with imaging for online plan adaptation is the rapid generation of contours on the 3D images, which provide the essential anatomy labels for the adapted plan and are crucial for performing dose-volume histogram (DVH)-based assessments. Despite being a long-known issue (86), this remains a major bottleneck for the development of an OAPT workflow (and online ART in general) (10) and is in general a time-consuming task also for the initial planning. Several strategies have been investigated to speed up the process and numerous commercial solutions for automatic segmentation of medical images and contouring (i.e., segmentation specifically aimed at generating contours for radiation therapy) are available (87–90). However, auto-contouring operations usually require manual checks, which often leads to further manual adjustments (91, 92). While this is acceptable for daily adaptive workflows, it places an unfeasible burden on the total adaptation time in NAPT. A fully integrated NAPT loop requires minimal user intervention, with ideally no involvement of a radiation oncologist in the treatment session. This is in contrast to what generally happens now for online photon-based ART, where the presence of the radiation oncologist is requested at least for some fractions (93). Moreover, not only the timing aspect, but also the accuracy of the auto-contouring process must be carefully addressed (94, 95).
Contour propagation based on (rigid or deformable) registration has already been investigated for clinical use in OAPT (12, 17, 96–98). This approach is well-established and already available in many commercial software products for treatment planning. However, it may be more suitable for tumor sites with minimal motion (e.g., brain, nasal cavities). Its application in more mobile anatomical areas or low-contrast regions, such as thoraco-abdominal tumors, could be more challenging. Additionally, many contouring tools incorporate prior knowledge, such as atlas-based or model-based segmentation. However, their performance depends heavily on factors like the contrast of the specific structure and the representativeness of the selected atlases or models (86), meaning these methods are not truly patient-specific. Several studies have explored the suitability of propagated contours in relation with intra-observer variability and need for manual adjustments with promising results (99–104), although patient-specific factors still play a significant role.
Auto-segmentation based on artificial intelligence (AI) is gaining increasing popularity and several approaches for auto-contouring are available or rapidly evolving. The development of deep learning (DL)-based segmentation in particular has driven a great number of studies lately (105). DL auto-contouring may in the future have the potential to outperform previous solutions, due to its capability to identify features in the data thanks to prior training and it may then be applicable to a broader range of tumor sites. However, current limitations stem primarily from the size and representativeness of the datasets used for training. Despite these limitations, many commercial software solutions offering DL-based auto-contouring are already available and are being extensively researched (106–108) and evaluated in clinical settings (109). Studies evaluating the impact of DL auto-contouring on contouring time have also been reported (110, 111), including comparisons with other auto-contouring techniques, such as atlas-based or DIR-based methods (112). Time savings compared to manual contouring have been shown, although no clear advantage for a specific method has been observed, and corrections are still required. Recently, strategies to account for the uncertainties related with the auto-contouring within the optimization process have also been proposed (113, 114). These methods could effectively mitigate the impact of contour uncertainties thus facilitating the adoption of automated contouring tools. However, further research is required at this stage.
Additional contouring best practices should also be considered. First and foremost, it is crucial to emphasize that contouring protocols must remain consistent between the planning CT and the additional images required for OAPT. Best practices include using pre-determined contouring strategies for specific structures, maintaining consistent naming conventions, and clearly differentiating between targets, OAR, and normal tissues. Further research is expected on the topic, with recent studies suggesting that manual corrections might be required only for the target contours and discarded for the OAR (115). A consensus has not been reached yet.
Moreover, the development of auto-contouring techniques for OAPT requires the definition of rigorous safety checks on the resulting anatomical structures. In fact, evaluating the acceptability of the contours and establishing suitable assessment metrics is another essential part of the process (95, 104, 116), and automatic safety protocols are of utmost importance for the application of auto-segmentation in OAPT. Final checks on the quality of the current structures can be considered as a part of the online quality assurance (QA) process and will be discussed in more details in the dedicated section.
4 Decision on online adaptation need
In conventional scheduled DAPT (Figure 1A), adaptation is performed at every treatment session. Alternatively, adaptation can be triggered (Figure 1B, C) while the patient is on the treatment couch, based on new 3D imaging, a re-calculated dose from the scheduled plan, or external signals such as treatment verification data. In this context, triggered NAPT (Figure 1C) can help reduce overall workload, since the adaptation would not be necessarily performed at every fraction. Online adaptation may be more efficient when performed only when clinically necessary or when a clear benefit is anticipated. This means, it is crucial to rapidly assess whether adaptation is deemed advantageous on the basis of the collected data (e.g., 3D image in treatment position, updated contours, re-calculated dose distribution and DVH parameters), before proceeding with the adaptive workflow. For photon-based ART, several workflows have been proposed depending on the timing of the adaptation, frequency of imaging and tumor characteristics (117). The approach of directly performing a plan adaptation, as exemplified in certain MR-LINAC workflows (24–26) and in conventional scheduled DAPT (17), poses a challenge as the online adaptation and related safety checks are time-consuming. Essentially, a timely decision-making with appropriate assessment tools that determine the adaptation demand has to be much faster than the plan adaptation and its QA itself.
A straightforward approach to assess the need for adaptation is the use of pre-determined thresholds and checklists that include anatomical and dosimetric information, such as variations in structure volume, center of mass, and DVH properties for the scheduled plan on the updated anatomy (12). In this context, dose calculation plays a key role, requiring a fast and efficient process.
Moreover, the development of automated decision support platforms for assessing the need for adaptation is expected to be greatly advanced thanks to AI. Several AI-based decision support systems have already been proposed in photon-based ART for different purposes, such as plan auditing (118), individualized treatment decisions for head and neck cancer (119, 120) and esophageal cancer (121), with the first commercial ART software platforms available for deciding when a plan adaptation is needed (122).
It’s important to note that the decision to adapt could be made at different levels depending on the availability of collected data, by extracting and processing relevant features. This could involve using only the anatomical information from 3D images, incorporating contour data, considering information from the dose distributions only, or a combination of these factors. Various AI techniques could be used to support this process. The capacity to extract pertinent information about the adaptation need from the images alone, without the necessity for initial plan recalculations or even updated contours, would facilitate a significantly more expedient decision-making process.
5 Online plan adaptation: optimization strategy and dose calculation
The online plan adaptation process involves two key aspects: the optimization algorithm used for the adapted plan generation and the dose engine used for the dose calculation. Relevant research in both areas is ongoing in the PT community. Several solutions to fasten the plan adaptation process have been proposed and comprehensively discussed (11). An overview of the most prominent OAPT plan adaptation algorithms and dose engines will be presented, with focus on near real-time implications.
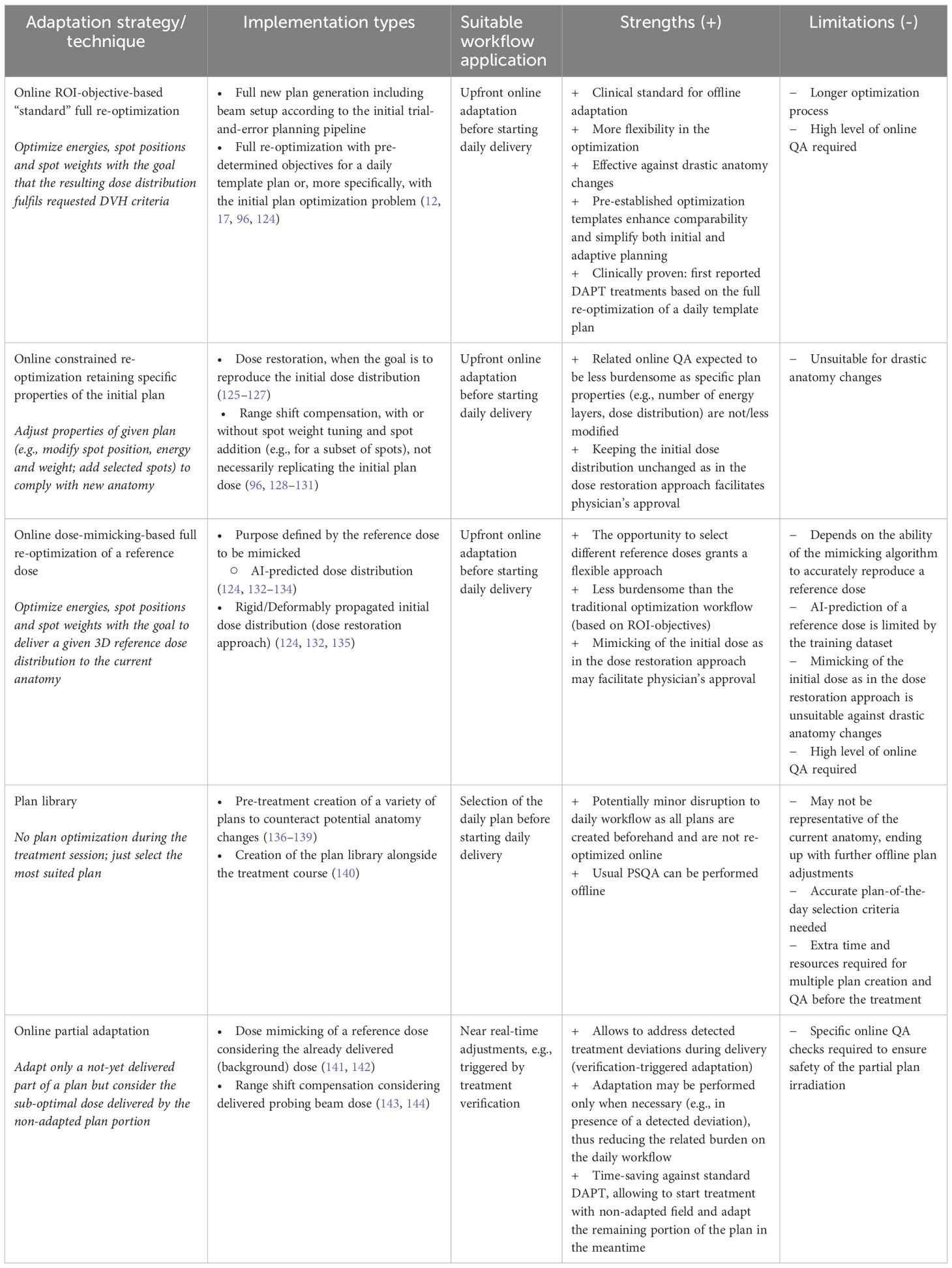
In PT, the time required for standard plan optimization is largely determined by the number of robustness scenarios considered. In contrast, XRT can account for setup errors through an appropriate planning target volume (PTV) concept, and range uncertainties are negligible. Therefore, reducing optimization times is particularly critical for enabling fast online adaptations in PT compared with XRT. The main strategies for generating online adapted plans that align with the current anatomy can be broadly classified based on the underlying optimization technique and/or on the final goal of the adaptation (123). The principal plan adaptation strategies are: “standard” region-of-interest (ROI)-objective-based full re-optimization, constrained re-optimization retaining specific properties of the initial plan with or without the goal of reproducing the initial dose (dose restoration), and dose-mimicking-based full re-optimization (which can be tuned to mimic different dose distributions depending on the application). Furthermore, the use of plan libraries has been put forth as a potential strategy for OAPT (123). Additional adaptive strategies that could be applied after a partial delivery of the plan are particularly relevant for NAPT and will also be addressed down below. A detailed outline of the discussed strategies is presented in Table 2.
The generation of a fully new plan (which entails new energies, spot positions and weights, and potentially also beam angles and/or air gaps) following the initial (ROI-objective-based) pipeline and the conventional trial-and-error approach is still the gold standard for offline adaptation (4, 15). However, it is a time-consuming process and may require several iterations for tuning the plan parameters by the user, before achieving an optimal quality. The goal of an online adaptation is not to achieve the best plan but rather a plan that reliably meets clinical constraints. Hence, methodologies to speed up full re-optimization are under research. For example, full re-optimization may be based on a pre-established template plan, without the need for online adjustment of optimization parameters, like objectives, beam angles, etc. To improve efficiency and ensure consistency, (non-)patient-specific ROI-objective-based templates can be applied to both initial and adapted plans, reducing manual input. Patient-specific templates can either apply the final objectives from the initial plan (124) or be created de novo during the pre-treatment planning phase (12, 17, 96). In the latter case, compared to standard clinical practice, the “daily” plan template allows for potentially more aggressive beam arrangements and robustness settings, though it increases pre-treatment workload.
Differently from full re-optimization, online constrained re-optimization techniques enforce direct adjustments of plan parameters (96, 123, 128–131), such as the spot energy/range, isocenter position and/or tuning spot weights for a subset of spots, rather than ROI objectives, offering a faster and less time-intensive online plan adaptation. In fact, in these adaptive methods, the spot positions and energy layers shall remain unchanged compared to the initial plans. This strategy is subject to some limitations, in that it may prove inadequate for compensating for significant anatomical variations and deformations. In such cases, the addition of energy layers or other more substantial modifications to the original plan may be required. These techniques have been applied with or without the goal of restoring the initial planned dose and have been previously referred to as simply “re-optimization” (11). In this context, “dose restoration” gained attention as an online constrained re-optimization technique specifically aimed at reproducing the initial dose distribution in adapted plans, for example in rigid dose restoration approaches, such as the one described by Jagt et al. (125) for prostate cancer. Since then, it has been investigated for other tumor sites (124, 126, 127, 132, 135) in more broadly defined approaches aimed at reproducing the initial dose distribution or the initial plan quality in adapted plans. The key motivation for dose restoration is its potential to save time by reducing the impact of contouring, as the initial structures could be used on the current anatomy. Sometimes, it is claimed that constrained online re-optimization techniques simplify online QA by preserving certain properties of the initial plan, but this cannot be the main argument, as a high level of QA is still required to ensure a safe adapted treatment. Rigid dose restoration is achieved by propagating the initial structures and restoring the initial dose distribution against density changes, assuming no differences in position or shape of the targets and OAR. This replicates the “static dose cloud approximation” from conventional radiotherapy, where patient shifts and density variations have minimal impact on isodose shape and position. However, applying this concept to PT has limitations. Despite efforts to enhance robustness (126), the assumption that the initial dose distribution suits the current anatomy fails in many cases. Complex anatomical changes, such as deformations, position variations, or poor registrations, can undermine the technique’s effectiveness (127). It is thus not ideal for abdominal or thoracic sites, which may require NAPT interventions. Additionally, current time performances do not meet daily OAPT or real-time requirements, suggesting a need for faster hardware or alternative solutions for clinical viability (127).
Dose mimicking has been proposed (124, 132–134) as an alternative adaptive plan optimization strategy, where the optimizer aims at generating a completely new treatment plan (energies, spot positions and spot weights) that delivers a certain given reference dose distribution, similar as to what has been initially investigated in the context of XRT (145). The dose mimicking operation can have different goals, depending on the reference dose to be replicated. For example, the reference dose may be an AI-predicted dose on the new anatomy, in a “dose prediction plus mimicking” approach (132, 133, 146, 147). However, the reference dose may also be the dose distribution of the initial plan copied to the new image (132). This last approach aims at restoring the initial dose cloud and can therefore be regarded as another technical realization of dose restoration. Deformed dose restoration based on dose mimicking has been investigated as a potential solution to address some of the limitations of the rigid approach. The aim is to restore a dose cloud that has been adapted to the new anatomy through the use of guided dose deformation (135), where specific control structures serve as guiding reference for the deformation.
An explored alternative to online plan adaptation is the use of pre-determined plan libraries, where multiple treatment plans are created in advance to account for potential anatomical variations (e.g., organ filling changes). These plans undergo the elaborate routine QA before they can be selected for delivery based on the patient’s anatomy at each fraction, minimizing workflow disruption. This approach, which hence does not represent a truly online plan adaptation, is not expected to become standard clinical practice in PT (148, 149). Several retrospective planning studies across different tumor sites exist (136–139), and a first clinical application for an intra-thoracic tumor has been reported (140). In silico studies have explored various methods for generating plan libraries, such as motion modeling, marker tracking, and adjusting setup margins using retrospective control CTs. In the reported clinical case, the plan library was built progressively during treatment based on acquired control CTs. Inherent limitations of the strategy prevent a broader adoption of plan libraries. The plans available in the library may not include the full range of geometry variations and may end up being not suitable for the daily delivery; this is particularly relevant for NAPT aiming to tackle unexpected intrafractional changes.
Adaptive strategies as the ones described so far focus on the adaptation of the entire plan before starting any beam delivery in the daily treatment session. To streamline daily adaptive workflows, more flexible approaches are desirable – such as selectively adapting only portions of the plan, while a part of the fraction dose is already delivered. This can enable parallel operations, thereby improving overall efficiency (141). Dealing with a sudden change detected during delivery as in the foreseen near real-time adaptive setting is an additional challenge. The employment of online in vivo treatment verification will not only offer a tool to control the compliance of the delivered (adapted) treatment, but will also enable a transformation in the way the online adaptation is applied. In fact, the detection of a treatment deviation above an established threshold may serve as a signal to trigger a subsequent image acquisition in treatment position and, ultimately, an adaptation of the plan in the fully closed near real-time adaptive loop. This so-called adaptation triggered by treatment verification might in principle happen at any time during the delivery, propelling the need for plan adaptation strategies to be employed mid-delivery or even multiple times during delivery. In this context, the concept of a “partial adaptation”, whereby only a portion of the plan is adapted, is also of paramount importance. Without the ability to adapt only the undelivered portion of the scheduled plan in response to a detected treatment deviation and to consider the delivered dose up to the trigger, NAPT triggered by verification would be unfeasible. Planning strategies that introduce a partial adaptation for PT have recently been proposed (141, 143) and further investigated in combination with the potential trigger information from a treatment verification system (142, 144).
A fast dose calculation is essential within the iterative adaptation algorithms and beyond. Dose calculation plays also a key role in multiple steps of the online adaptive workflow, including initial plan recalculation on the current anatomy for adaptation decisions and independent dose verification for QA (11). Unlike photon dose calculation, proton dose calculation presents unique challenges. Early clinical approaches in PT relied on analytical algorithms such as pencil beam and ray casting, which provided fast and efficient performance (150–152). These methods have been explored for the use in OAPT, with strategies proposed to improve their accuracy (153–155). However, their limitations have been well-documented, particularly when compared to Monte Carlo (MC)-based dose calculation, which offers greater precision (150, 156). MC-based dose calculation represents nowadays the gold standard regarding accuracy, is broadly clinically applied and MC-based dose engines have been incorporated into all relevant commercial TPS (150, 151). Significant efforts have been made to develop powerful hardware (157, 158) capable of faster performance, in combination with novel MC-simulation strategies (157, 159). While MC-based dose calculation still poses challenges for near real-time adaptation due to its time requirements, it is increasingly being used in OAPT workflows. This is evident in its application for independent pre-delivery checks of online adapted plans and log-file-based post-delivery QA, as will be discussed in the next section. Additionally, its superior accuracy and anticipated improvements in time performance make it the preferred method for proton dose calculations.
Finally, several studies propose novel AI-based approaches for proton dose computation, such as knowledge-based planning (160), long short-term memory networks (161) or transformers (162). Many of these approaches aim at achieving the same level of MC accuracy at shorter calculation time (157, 163–165). There are still limitations in applicability to different sites or different field arrangements than the ones included for the training of the AI algorithms. Hence recent works focus on generalizable algorithms that could return MC accuracy in sub-second speed, which could be potentially relevant for near real-time applications (166), if not during optimization then at least for fast secondary dose calculation in the online QA (see next section), including AI-based dose calculation models specifically designed for OAPT (167–170).
6 Pre-delivery QA of the online adapted plan
QA of the treatment plan, so-called patient-specific QA (PSQA), is an essential step to ensure the treatment safety. PSQA aims at checking 1) that the calculated dose distribution for the plan is correct, 2) the correct data transfer between the TPS and the delivery system, and 3) the deliverability of the plan (171). In most PT centers, PSQA still involves dosimetric measurements for plan deliveries to water and water-equivalent phantoms, which are time-consuming and limit the availability of the treatment room. Recently, the combination of log-file-based verification with an independent dose calculation became a valid alternative for the above-described three aims, that already found its way in clinical application in several centers (14, 17, 172). It is clear that, within the context of OAPT, phantom-based PSQA procedures are unfeasible due to the strict time constraints (10, 11), requiring new strategies for measurement-less PSQA that can be performed during the treatment session and before delivery. Therefore, the trend towards phantomless PSQA is synergistic with the implementation of OAPT. Still, an offline (phantomless) PSQA and a PSQA of a treatment plan that was just generated a few seconds or minutes before application represent different conditions and requirements. A dummy-irradiation to generate a log file is not possible for OAPT and therefore the log file results will only be available after patient treatment. Therefore, QA checks that can be performed before delivery are crucial. One key component is a secondary dose calculation using an independent dose engine. Another essential element is the implementation of ‘sanity checks’, which are conducted at various stages of the workflow before the online adapted plan is approved and delivered. They confirm the plausibility of adapted plan changes and verify data integrity before treatment. Unlike conventional pre-treatment PSQA, online adaptive workflows have access to additional information, particularly the initial plan as reference. This enables two types of verification: 1) Hard checks, which correspond to standard pre-treatment QA procedures and require an exact match with the reference plan (such as patient name, etc. 2) Soft checks, which assess deviations from the reference plan within defined tolerances. For soft checks, defining appropriate thresholds is complex and requires careful consideration to ensure clinical safety and treatment accuracy.
For example, soft checks can be applied to evaluate the obtained structures on the actual anatomy, ensuring that the online contouring process has been performed correctly. This is particularly important when contouring is automated using dedicated software, which is desirable given the time constraints of OAPT. Exemplary structure parameters can be selected to be part of this structure pre-delivery QA to assess intensity and geometry, like the center of mass, the volume, the average CT numbers of specific structures, etc. (12, 173). For the online adapted plan, different inputs can be used and assessed in secondary dose calculations, depending on the evaluation’s specific goal. Machine steering files have been proposed as inputs to evaluate the correct data transfer and deliverability, as they contain treatment plan information converted into delivery system instructions (174). However, access to these files may not always be feasible. In such cases, an alternative approach is to run the evaluation in the TPS, use external software, or work with exported DICOM files. Selected plan parameters, such as energy layers, spot numbers, cumulative monitor units, etc. can then be evaluated with hard or soft checks, depending on the accepted tolerance relative to the initial reference plan, for which standard PSQA was performed. Automation, safety and speed are central to the online PSQA process, requiring seamless integration of multiple QA levels and parallel execution of tasks. For example, the online PSQA in standard DAPT can be performed alongside patient setup and setup verification. The aforementioned QA procedures and introduced sanity checks do not require actual plan delivery and are well-suited for an online (near real-time) timeframe.
Proton range assessment using range probing is an additional pre-delivery QA method, also applicable to OAPT workflows, and is currently under translational research. Its purpose is to verify the accuracy of the SPR prediction used in treatment planning (175, 176). Range probing utilizes high-energy, low-dose proton beams (177) to measure the remaining energy of protons after traversing the patient. This is achieved using either multi-layer ionizazion chambers (MLIC) (178) or flat panel detectors (179), allowing for a direct comparison between the measured energy loss and the simulated values from the planning CT or a pre-treatment image of the current fraction, when available. In the context of OAPT, range probing is primarily intended to validate CBCT-based SPR prediction (179–181). Following initial proof-of-principle and phantom-based validations, the first proof-of-concept clinical implementation has been performed (19). For widespread clinical adoption, integration into PT systems would be necessary, potentially achievable through flat panel detectors. However, no medical product is currently available for routine clinical use.
7 Online treatment verification and detection of deviations during delivery
In vivo treatment verification during delivery has specifically two main goals: 1) to provide a safety net, checking that the adaptation worked out as intended; 2) to detect deviations due to inter-/intrafractional changes in the anatomy that may trigger NAPT. It is also highly desirable when aiming at reduced target margins. The first-to-clinic daily adaptive workflows do not include treatment verification, and are based solely on the detection of anatomical changes on the daily image taken before the delivery is started (12, 17, 18). As previously introduced, the detection of a relevant treatment deviation during delivery and the ability to act on it promptly is essential for near real-time adaptation, making online treatment verification a crucial step in the workflow – effectively closing the feedback loop. A number of techniques have been proposed for the verification of range in vivo (Table 3). The investigated in vivo range verification techniques have been differentiated depending on the measured information (e.g. positrons or prompt gamma rays), timing (online or offline), dimension (1D, 2D, 3D) (1) and applicability (general or specific to a tumor location) (177). The most relevant ones are discussed below in relation with potential applications within a near real-time adaptive workflow.

Table 3. Overview of the close-to-clinic in vivo treatment verification approaches for PT in terms of their suitability for near real-time adaptation.
One of the first in-vivo verification techniques studied was PET imaging during particle therapy delivery (previously also referred to as PT-PET) (182–184). Positron emitters (10C, 11C, 15O among the most commonly used) are produced via nuclear interactions between the incident protons and atoms of the patient’s tissue and PET-based treatment verification relies on the coincident detection of 511-keV photons produced by the annihilation of the emitted positrons with electrons. Unlike particle therapy with heavier ions, when using proton beams positron emitters can only originate from the target atoms, rather than from projectile fragmentation. Because the nuclear interactions and thereby the positron emission are closely related to the dose deposition, the positron activity can be used to approximate the proton beam range, usually by comparing the measured activity distribution with an expected one derived from the treatment plan (185) and deriving a range deviation. Given that PET imaging is a well-established diagnostic technique, there has been considerable interest in adapting it for the use in in vivo treatment verification (186). Several solutions have been proposed, each with their specific advantages and disadvantages: online in-beam PT-PET involves PET imaging directly after each field delivery, and sometimes during irradiation; online in-room PT-PET entails PET imaging with an in-room PET scanner after the patient is moved to the scanner position post-treatment; offline PT-PET requires transferring the patient to a nearby room, where PET imaging is performed using a standard diagnostic PET scanner (187). While offline PT-PET can rely on standard diagnostic systems, online PT-PET systems are usually dedicated in-house developed systems that can be used in the room while the patient is on the couch, hence they must respect certain geometrical constraints (188).
While many research groups have investigated PET-based range verification in clinical studies (184, 188, 189), and several hundred patients have been monitored at a few pioneering institutions, the method has not found its way into broad clinical application. This is probably due to the inherent limitations of the approach: due to the half-life time of the positron emitters in the range of minutes for the most prominent positron emitters (20 min for 11C, 2 min for 15O and 19 s for 10C) a spot-wise evaluation is not possible, as the signals from different pencil beam spots and even from subsequent treatment fields overlap each other. Additionally, the biological wash-out effect (190), which describes the movement of positron emitters between their generation and decay, leads to a blurring of the original activity distribution. Intrafractional movement needs to be monitored and considered correctly to correlate the locations of positron emitter generation and dose deposition with the location of positron emission (191–193). The positron activity produced is relatively low, around 6.6 kBq per Gy and cm³ (194), compared to 10–100 kBq/cm³ in diagnostic nuclear medicine. As a result, the derived signal is heavily influenced by statistical uncertainty, and interpreting clinically relevant changes at the dose distribution level remains challenging, particularly due to the differences in activity and dose distribution, especially when using proton beams.
Despite its limitations and restricted applicability, research in the field of PT-PET continues. An experimental intrafractional adaptive PT workflow based on PET verification has recently been proposed and investigated pre-clinically (144). Additionally, the use of short-lived isotopes, like 20N with a half-live of 11 ms (195, 196), has been suggested, however their activity concentration is about two orders of magnitude below that of conventional emitters mentioned above. Further developments of in-beam PET detectors (197), application of AI to the obtained PET images (198), time-of-flight PET (199, 200) and combined techniques (201) have also been reported.
Currently, the most promising in vivo treatment verification technique is the detection of prompt gamma (PG) rays, i.e., photons that are almost instantaneously (<ns) produced after nuclear interactions of the beam with the nuclei of the traversed tissues (1, 177, 202–204). These emitted PG rays are correlated with the depth-dose profile of protons. In general, the timeframe of PG ray’s emission makes PG ray-based treatment verification (PGTV) particularly well-suited for NAPT. Differently from PET-based treatment verification, PGTV is not affected by activity build-up or decay times, hence it could provide an instantaneous information about treatment deviations, and the PG rays have a low probability to undergo secondary processes before reaching the detectors. Moreover, PGTV may be employed to continuously monitor the delivery.
The use of PG rays for proton range verification was first proposed in 2003 (202). A range of different PGTV techniques is now actively studied, the main ones comprising PG imaging (PGI) (205), PG timing (PGT) (206) and PG spectroscopy (PGS) (207). PGI aims at recording the spatial distribution of the emitted PG rays, PGT is based on their temporal distribution, and PGS relies on the local energy spectrum of the emitted PG rays, relating the PG spectrum to the proton-energy-dependent cross section of the respective nuclear reactions. With improvements in detectors, different domains of information could also be combined, enabling so-called multi-feature treatment verification.
PGI is currently the most advanced of the available PGTV techniques. It is in clinical application since 2015 (208, 209) and has since been applied in more than 470 field deliveries within a retrospective clinical study (210), proving the feasibility under realistic conditions. PGS is in clinical application since 2020 (211). Recently, a multi-institutional comparison of PGI and PGS has been performed under controlled conditions, providing standard conditions and methodology that allow fair comparisons and performance characterization of different PGTV systems, which are hopefully used in future studies proposing novel PGTV techniques (212). Accuracy of PGTV systems on spot level is currently around 2–3 mm for range verification, given a high spot dose (205). While all approaches measure primarily the PG information per pencil beam spot, there are quite different approaches of evaluating this raw data to conclude on clinically relevant treatment deviations. Usually, first a spot-wise range shift is determined by comparing the measured PG signal with an expected PG signal (213, 214). However, in real-world application that spot-wise information has limited accuracy, especially for low weighted spots, but more importantly a conclusion on clinically relevant treatment deviation is difficult from range information at the spot-level. One proposal is to reconstruct the 3D dose distribution from PG information, while another approach is to condense the PG data into a classification that identifies relevant treatment changes (either binary, such as yes/no, or multi-class, distinguishing the source of error). The classification method has been successfully tested and validated on clinically acquired PGI data for prostate treatments (215) achieving very promising results. Furthermore, the classification approach has been explored on different complexity levels for head and neck treatments (216, 217). Yet another approach, called spot boosting, proposes to manipulate the clinical optimized treatment plan by boosting the dose for a limited number of pencil beam spots and to irradiate those spots first, to selectively use them for PG range verification at those selected spot locations (218).
Despite its proven clinical applicability, no medical products for PGTV are currently available. A significant technological challenge is the integration of the PGTV system near the isocenter (e.g., direct attachment to the nozzle or gantry). However, with several clinical studies (219) underway, focusing not just on technical proof-of-concept but also on clinical impact and treatment intervention – particularly in the context of OAPT – PGTV is well-positioned to play a key role in future OAPT-ready treatment systems. For example, an upcoming clinical interventional trial (DEPICT) planned at OncoRay, Dresden, aims to evaluate the capability of PGI to trigger an online intervention. In this case, the acquisition of a CT scan will be triggered if PGI detects a clinically relevant treatment deviation, based on a model (215) trained on paired PGI and ground-truth dose distribution data from a retrospective patient cohort (Figure 3). While this study may not involve online adaptation, it represents an important first step toward establishing a closed feedback loop reliant on treatment verification (in this case, PGI). This progress is also driving the investigation of novel PGTV techniques (220).

Figure 3. Schematic representation of the envisioned workflow for the DEPICT clinical trial. Treatment delivery is regularly monitored using PGI. If relevant treatment deviations are detected, a cCT scan acquisition is triggered to assess the need for plan adaptation immediately after the fraction dose delivery. If adaptation is required, an updated treatment plan is created offline for the next fraction. Otherwise, treatment proceeds with the next fraction as usual. (Courtesy of Jonathan Berthold).
Recently, treatment verification based on ionoacoustic imaging, which utilizes the generation of ultrasound waves from the periodic temperature increase in tissue caused by the pulsed proton beam, has been studied in simplified, non-clinical experimental settings (221–228). The localized energy deposition from a proton pulse around the Bragg peak causes a local increase in temperature and pressure. This generates an acoustic wave that propagates isotropically and that can be detected when it reaches the patient’s skin. By analyzing the time of flight of the ionoacoustic signal, the position of the Bragg peak can be determined. However, treatment verification using ultrasound detection is still in its early stages and faces several challenges, including the propagation of ultrasound through heterogeneous tissues with varying sound speeds, reflection and attenuation, transducer characteristics, and background noise. These factors significantly degrade the signal-to-noise ratio under realistic conditions, raising questions about its clinical applicability.
In addition to using dedicated systems during the delivery of treatment to monitor its compliance, log-file-based dose calculation offers the opportunity to check the actual delivered dose after the delivery is completed (229, 230), or even mid-treatment in recently reported investigations (231). Since log files are mostly not employed at the time of treatment and usual applications aim at reliable post-delivery quality controls and dose accumulation, they will be discussed in the next section dedicated to tasks “beyond” the closed OAPT feedback loop.
8 Beyond the loop
The advent of NAPT offers the potential to finally close the long-awaited feedback loop during the treatment session (3). This entails a number of essential tasks and requirements that extend beyond the daily treatment workflow and that need to be carefully addressed.
First, the technological realization of a NAPT solution with a closed feedback loop requires changes in soft- and hardware. While introducing new medical products is a standard procedure within the medical technology industry, this process is not typically applied to in-house developments in translational research. In these cases, institutions face significant challenges to be compliant with the Medical Device Regulation (MDR) in Europe or the regulations by the Food and Drug Administration (FDA) in the United States. Especially the MDR requirements not only demand substantial resources, but they can also delay the clinical application of new technologies. At this stage, it is highly advisable for pioneering institutions to seek structured guidance from MDR experts as early as possible to streamline the development process and ensure compliance. Additionally, adopting a pragmatic approach is essential in determining the depth of legal requirements to be met (e.g., risk analysis, documentation). Medical physicists are well-positioned to make these pragmatic decisions, as they have long been responsible for risk assessment and patient safety, well before the introduction of the MDR. For in-house developments, the goal should be to integrate this expertise and methodology into the MDR process as seamlessly as possible, while minimizing the burden of additional bureaucracy. In this context, it is important to recognize the priorities of the European Society for Radiotherapy and Oncology (ESTRO) within the framework of the European Alliance for Medical Radiation Protection Research (EURAMED) (232), which emphasize interdisciplinary collaboration to improve clinical outcomes and minimize side effects. ESTRO advocates for the clinical implementation of new radiotherapy technologies, as a means of advancing precision medicine. This focus aligns with ongoing research and regulatory efforts aimed at supporting clinical trials and ensuring that emerging technologies like OAPT are thoroughly evaluated for their clinical efficacy, time efficiency, and cost-effectiveness.
Secondly, as anticipated, the introduction of log-file-based dose recalculation allows to perform additional checks on the treatment compliance. They usually lie outside of the “online” loop, since they are mostly performed only after the clinical delivery is concluded. This “log-file-based QA” relies on treatment delivery log files from machine’s internal beam monitors to reconstruct the delivered dose under actual treatment conditions (gantry angle and patient-specific anatomy). This approach eliminates the necessity for phantom measurements at a fixed gantry angle of 0° and as such has already found its successful way in routine clinical application with available medical products (233). It has been investigated in numerous workflow scenarios in PT, including fully automated PSQA solutions (14), and pioneering OAPT workflows (12, 17). Log-file-based 4D dose reconstruction for the irradiation of moving targets has also been investigated (234), and novel methods that can be applied within the delivery (231) offer a powerful tool for monitoring the delivered dose during treatment. The real-time availability of the delivered dose would allow to monitor the impact of movement and verify the efficacy of employed motion mitigation techniques. Moreover, it is an important step towards the introduction of near real-time adaptations relying on information on the actual delivered dose. In addition, the availability of log files in OAPT can help improve the dose accumulation quality (10, 11). Although this would be a powerful resource to evaluate treatment outcome and make predictions regarding adaptation needs, dose accumulation is currently not used routinely in clinical practice, due to the related uncertainties in image and dose registration having a great impact on the final dose distribution (10, 11, 235, 236). Despite its scarce clinical application (148), the interest towards dose accumulation in research is increasing, in relation with the development of OAPT. NAPT may benefit greatly from the availability of such a tool. Many recent studies have explored strategies to account for registration uncertainties in dose accumulation (237–240), even including DL, which may be a great asset for future applications of the accumulated dose (241).
Lastly, an efficient interconnection between the involved systems in the workflow is crucial for the implementation of the proposed near real-time adaptive closed feedback loop. Research to date has focused on the single tasks (10, 11) and the clinical landscape is actually quite fragmented (149), with only very first clinical DAPT applications reported in non-complex settings (17). The establishment of OAPT workflows is an open problem demanding multidisciplinary and multivendor collaborations. The process requires the overall development of novel components of hard- and software or the integration of novel modules within established systems (e.g., oncology information system (OIS), TPS). These systems must perform key functions for online adaptive workflows, such as real-time imaging integration, automatic adaptation of treatment plans based on changes in the patient or tumor, dynamic re-contouring of targets and OAR, online QA operations, incorporation of data from real-time treatment verification, etc. Additionally, they must seamlessly combine data from various sources (e.g., imaging, treatment logs, and contouring software) and ensure that different platforms work together effectively to enable timely, safe, and efficient treatment delivery. Recently, a few academic-industrial consortia have emerged to work on potential solutions addressing the different tasks in OAPT workflows, highlighting the great interest in the topic and the push towards its clinical implementation (242, 243). Among these, the ProtOnART consortium (243) stands out for the direct collaboration between academic institutions (OncoRay, Dresden and PARTICLE, Leuven) and industries (IBA, Louvain-la-Neuve and RaySearch AB, Stockholm), with the goal of providing an OAPT solution that could be accessible to multiple centers, rather than being limited to a specific in-house application. Unlike other initiatives focused primarily on research, the ProtOnART consortium has a strong translational foundation, with the goal of treating its first patients in 2026, focusing on lung and esophagus cancer in the first step. Specifically, the consortium’s short-term objective is to establish an efficient online daily adaptive workflow for PT demonstrated in clinical practice by its clinical partners. The ultimate goal of ProtOnART, however, is to enable NAPT, where plan adaptations are made during or between proton field deliveries, bringing this technology into clinical reality and thus making it highly relevant for the purpose of this review.
It is crucial to emphasize that the implementation of efficient data streams and the timely performance of tasks will be essential to achieve the overall objective of NAPT. For example, the possibility of performing parallel tasks is essential, such as initiating the assessment of adaptation needs while the contouring process is still ongoing, commencing the online plan adaptation process before the final contours have been approved, or sending the online adapted plan to the delivery machine before the final results of the online QA process have been obtained. Together with this, a thorough documentation of the online adaptive session is demanded which goes beyond the current standards for reporting and documenting PT treatments (244), with new guidelines currently under development. This documentation might take the form of extended plan reports, dedicated to specific tasks. Image-related documentation could include imaging protocols, the employed image registration (if images are not taken at the isocenter), contouring protocols documenting how different regions of interest were obtained and if manual adjustments were necessary. The reason for the online plan adaptation should also be reported, for example, by noting the DVH criteria that have triggered an automated adaptation due to exceeding predefined thresholds or other clinical motivations. The initial plan used for dose recalculation and assessment, details of the online plan adaptation (e.g., robustness settings, optimization settings) and the results of the DVH-metrics comparison could all be part of the online adapted plan documentation. Moreover, reports of the online QA containing the results of the performed sanity checks should be automatically provided. Finally, the timings for each step in the workflow, which would provide a basis for performance evaluations, should also be recorded. Whenever applicable, documentation should be appropriately stored in the OIS.
It is also important to recognize that while triggered NAPT has the potential to streamline certain aspects of the workflow, it may simultaneously introduce new complexities both inside and outside the treatment room. These include increased demands on staff for monitoring, decision-making, and QA, as well as the need for additional training to effectively operate new clinical tools and automation systems. All professional groups involved in the workflow would be affected, similar to current practices in online adaptive XRT: physicians would face additional workload for reviewing online-generated contours; medical physicists would take on responsibilities for online QA verification and offline review of the adapted plan; and radiotherapy therapists (RTTs) would need to manage a modified in-room workflow, potentially including increased responsibilities like in RTT-only workflows (245, 246). In this context, it is essential to establish clear intervention protocols that define decision rules, intervention thresholds, and corresponding actions. Such structured approaches enable efficient RTT-led workflows, ensuring that physicians and medical physicists are only required to intervene when unexpected or out-of-tolerance situations arise. Despite these initial challenges and the associated learning curve, it is expected that with growing clinical experience and ongoing technological advancements, the workflow will become more streamlined, thereby reducing the additional training and workload over time. In this regard, valuable insights can be drawn from the experience gained in implementing online adaptive XRT (247).
9 Summary and outlook
OAPT is on the path to becoming a clinical reality, but it still remains greatly limited at present. The driving force behind this progress lies in unmet clinical needs, such as the growing demand for more precise and timely treatments and the inclusion of previously undertreated entities. These needs are prompting ongoing research and technological advancements aimed at enabling faster and more efficient adaptations. This review provided a comprehensive overview of the key components and tasks essential for the successful implementation of an OAPT workflow, ranging from daily adaptive to near real-time, with main focus on the latter. First in-house clinical implementations of daily adaptive workflows have already been successfully carried out (17), indicating that the necessary tools and frameworks for standard DAPT workflows are already in place. The first clinical application of DAPT has confirmed the feasibility of performing OAPT within a reasonable time frame during a treatment session (Figure 4). Moreover, interest is increasing in developing solutions that extend beyond in-house applications (243). Additionally, the establishment of a closed feedback loop for OAPT in clinical settings is already within reach, thanks to advancements in treatment verification techniques, as outlined. These developments create a solid foundation for accelerating treatment timelines, eventually moving towards near real-time applications.
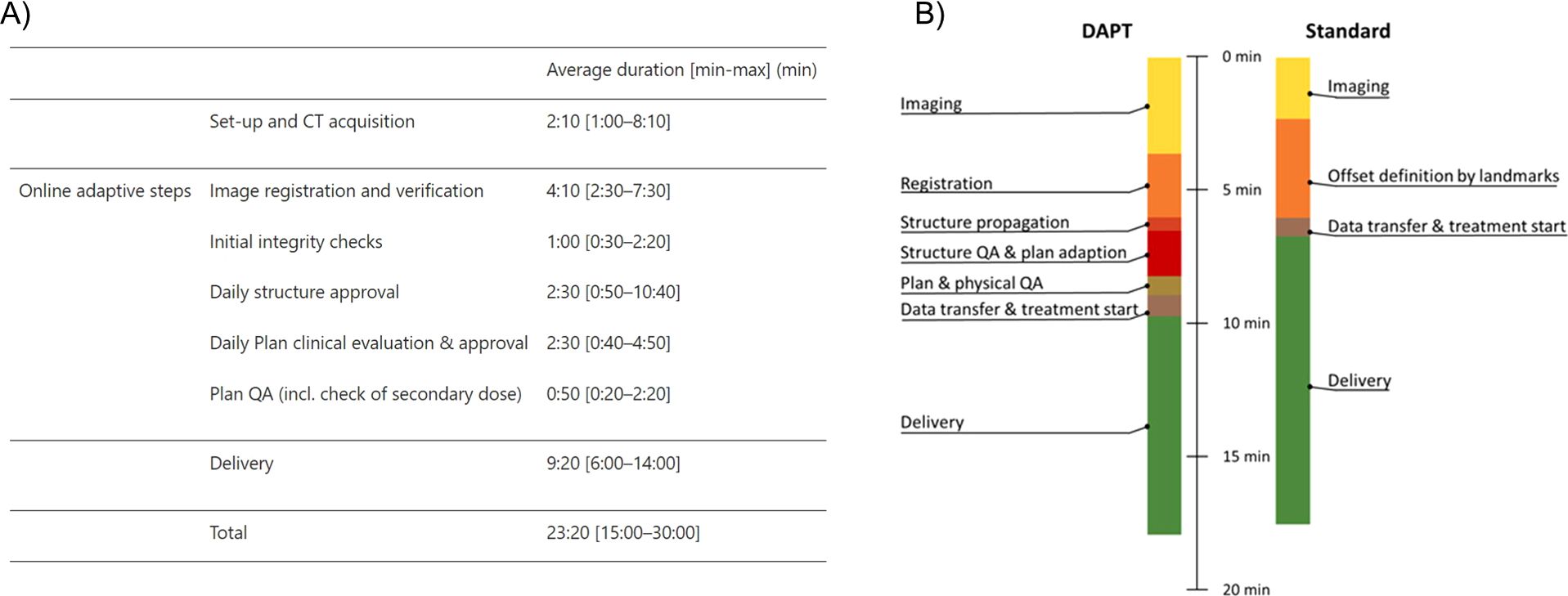
Figure 4. Timing reports for the first clinical applications of DAPT (A) and previous experimental tests of the same workflow (B), reproduced from (17) and (12) under a CC-BY 4.0 license, respectively. Panel (A) details the timings for the first reported clinical fractions, while panel (B) shows the average timings from the experimentally delivered fractions. The first clinical results align closely with the experimental tests, demonstrating the feasibility of the workflow within a typical daily appointment timeframe.
At the same time, the requisite effort demands for the definition of clear guidelines and clinical approaches to be carefully redesigned. For a widespread use, several aspects of OAPT must be carefully assessed. This involves a multidimensional evaluation, considering factors such as clinical benefits, time efficiency, cost-effectiveness, and more.
The lack of evidence and the scarcity of clinical outcome data on PT represent in general a significant challenge to the establishment of solid indications for this modality, that needs to be overcome. The issue is thoroughly discussed in the PT community (248–251) and recently results of several completed clinical studies (including randomized clinical trials) have emerged with positive results showing improved clinical outcomes, reduction in toxicity, mitigation of acute and late effects, and consequential reduction in total cost burden (252–254). In this context, it is important that OAPT be introduced within clinical studies from the outset to assess its benefits across all dimensions. This includes evaluating its ability to enhance clinical outcomes, its competitiveness with XRT, its potential to enable hypofractionation, and its role in treating new tumor entities.
Economic factors are another critical consideration in the development of efficient OAPT and represent a significant limitation within the broader context of PT, especially when compared to XRT (250). While evaluations of opportunity costs for online adaptive treatments have already been presented for XRT (255), similar analyses are still lacking for PT. However, as the availability of PT centers continues to increase (252) and the number of patients grows, more clinical data will emerge, creating valuable opportunities for the development and effective use of OAPT.
The clinical benefit and cost must be related with other aspects to get a full evaluation, like the potentially prolonged time of treatment and overall available time in the treatment room. However, OAPT could also enable hypofractionation thereby leading to an overall reduction of treatment time per patient. Novel studies exploring possible scenarios for the introduction of OAPT in PT centers with focus on clinical outcome and time expenditures have been recently published (256, 257) and further similar investigations are expected. Borderías et al. (256) specifically highlighted that when online adaptation is time-consuming and results in some patients being treated with XRT instead, there is a trade-off between clinical benefit (expressed as reduced normal tissue complication probability, NTCP, for the whole cohort) and the time available for adaptation (Figure 5). While the study results are highly illustrative, one should keep in mind that the general transferability to other institutions might be limited due to the made assumptions on facility utilization. According to the study, the benefit of online adaptation is contingent on reducing the setup margin, and a maximum allowable adaptation time can be defined, beyond which the modelled clinical advantage is no longer maintained. Furthermore, the availability of online adaptive strategies for PT with reduced setup margins would favor the employment of hypofractionation or dose escalation, which are currently limited by the treatment-induced toxicities. This has already been observed in XRT (258–261). Likewise, NTCP-based adaptation would also be facilitated (130), as well as emerging approaches like simulation-free workflows, which are gaining significant interest in XRT (262–264). In this sense NAPT bringing forward innovative adaptive strategies would thus be an invaluable tool for the introduction and exploration of novel therapies, enabling the treatment of previously inaccessible tumor entities.
Figure 5. Variation in NTCP for 2-year mortality with three online adaptive proton therapy (OAPT) strategies using different setup margins for the lung cancer patient cohort investigated in (256) with institution-specific assumptions on facility occupancy and utilization. The dashed red line represents the NTCP gain for transitioning from conventional RT to non-adapted intensity-modulated PT. Points above this line indicate situations where OAPT strategies provide an NTCP gain across all patients. Seven different scenarios (Sideal, …,S6) are investigated, ranging from instantaneous OAPT for all patients (Sideal) to more realistic scenarios where an increasing patient proportion is treated with RT. For 2-year mortality, a maximum of 6 minutes of additional time per fraction per patient is allowed to maintain an overall NTCP gain without setup error reduction (blue line). With setup error reduction, 13.7 and 19 minutes of extra time per fraction can still provide an NTCP gain (orange and green lines). For details refer to (256). Reproduced from (256) with permission from Elsevier.
In order to ensure a careful and nuanced balance between the benefits of OAPT treatments and the present limitations, it is important to consider each patient indication carefully. Factors such as the potential for hypofractionation or dose escalation enabled by OAPT, treatment time, margin reduction, clinical outcomes, available clinical resources, and the comparison between DAPT, NAPT, and offline adaptive workflows must all be weighed. It is crucial to recognize that there is no one-size-fits-all approach, and each patient’s specific circumstances should guide the choice of the most appropriate adaptive treatment strategy.
Thus, in light of the growing range of available workflows and adaptive strategies in PT and the interest in the topic of OAPT from both vendors and clinical users, further clinical implementations towards faster time regimes are soon to be expected, leading to a multifaceted technological landscape and approach, with decisions dependent on the specifics of each patient case and available workflow.
Author contributions
VG: Methodology, Conceptualization, Writing – original draft, Investigation, Visualization, Writing – review & editing. KS: Visualization, Methodology, Writing – review & editing, Conceptualization, Supervision. CR: Resources, Methodology, Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank Dr. Lena Nenoff and Lukas Wolter for the fruitful discussions and feedback about specific aspects of the reviewed topic, Dr. Jonathan Berthold for discussing the upcoming clinical trial DEPICT and related figures and Dr. Fabian Hennings for discussing online adaptive workflows related time expenditures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Knopf A-C and Lomax A. In vivo proton range verification: a review. Phys Med Biol. (2013) 58:R131–60. doi: 10.1088/0031-9155/58/15/r131
2. Mohan R. A review of proton therapy – Current status and future directions. Precis Radiat Oncol. (2022) 6:164–76. doi: 10.1002/pro6.1149
3. Yan D, Vicini F, Wong J, and Martinez A. Adaptive radiation therapy. Phys Med Biol. (1997) 42:123–32. doi: 10.1088/0031-9155/42/1/008
4. Glide-Hurst CK, Lee P, Yock AD, Olsen JR, Cao M, Siddiqui F, et al. Adaptive radiation therapy (ART) strategies and technical considerations: A state of the ART review from NRG oncology. Int J Radiat Oncol Biol Phys. (2021) 109:1054–75. doi: 10.1016/j.ijrobp.2020.10.021
5. Ng J, Gregucci F, Pennell RT, Nagar H, Golden EB, Knisely JPS, et al. MRI-LINAC: A transformative technology in radiation oncology. Front Oncol. (2023) 13:1117874. doi: 10.3389/fonc.2023.1117874
6. Tu K-Y, Huang Y-S, Lau J, and Lee H-H. Adaptive Tomotherapy for locally advanced unresectable pancreatic neuroendocrine tumor: Case report and literature review. Front Oncol. (2022) 12:1045752. doi: 10.3389/fonc.2022.1045752
7. Liu H, Schaal D, Curry H, Clark R, Magliari A, Kupelian P, et al. Review of cone beam computed tomography based online adaptive radiotherapy: current trend and future direction. Radiat Oncol. (2023) 18. doi: 10.1186/s13014-023-02340-2
8. Oderinde OM, Shirvani SM, Olcott PD, Kuduvalli G, Mazin S, and Larkin D. The technical design and concept of a PET/CT linac for biology-guided radiotherapy. Clin Transl Radiat Oncol. (2021) 29:106–12. doi: 10.1016/j.ctro.2021.04.003
9. Thorwarth D. Biologically adapted radiation therapy. Z Med Phys. (2018) 28:177–83. doi: 10.1016/j.zemedi.2017.08.001
10. Albertini F, Matter M, Nenoff L, Zhang Y, and Lomax A. Online daily adaptive proton therapy. Br J Radiol. (2019) 93: 20190594. doi: 10.1259/bjr.20190594
11. Paganetti H, Botas P, Sharp GC, and Winey B. Adaptive proton therapy. Phys Med Biol. (2021) 66:22TR01. doi: 10.1088/1361-6560/ac344f
12. Nenoff L, Matter M, Charmillot M, Krier S, Uher K, Weber DC, et al. Experimental validation of daily adaptive proton therapy. Phys Med Biol. (2021) 66:205010. doi: 10.1088/1361-6560/ac2b84
13. Stock M, Georg D, Ableitinger A, Zechner A, Utz A, Mumot M, et al. The technological basis for adaptive ion beam therapy at MedAustron: Status and outlook. Z Med Phys. (2018) 28:196–210. doi: 10.1016/j.zemedi.2017.09.007
14. Guterres Marmitt G, Pin A, Ng Wei Siang K, Janssens G, Souris K, Cohilis M, et al. Platform for automatic patient quality assurance via Monte Carlo simulations in proton therapy. Phys Med. (2020) 70:49–57. doi: 10.1016/j.ejmp.2019.12.018
15. Green OL, Henke LE, and Hugo GD. Practical clinical workflows for online and offline adaptive radiation therapy. Semin Radiat Oncol. (2019) 29:219–27. doi: 10.1016/j.semradonc.2019.02.004
16. Pakela JM, Knopf A, Dong L, Rucinski A, and Zou W. Management of motion and anatomical variations in charged particle therapy: past, present, and into the future. Front Oncol. (2022) 12:806153. doi: 10.3389/fonc.2022.806153
17. Albertini F, Czerska K, Vazquez M, Andaca I, Bachtiary B, Besson R, et al. First clinical implementation of a highly efficient daily online adapted proton therapy (DAPT) workflow. Phys Med Biol. (2024) 69:215030. doi: 10.1088/1361-6560/ad7cbd
18. National Library of Medicine (U.S.). Online proton adaptive radiotherapy (PARTy) (2024). Available online at: https://clinicaltrials.gov/ct2/show/NCT06310655 (Accessed December 5, 2025). Identifier NCT06310655.
19. Meijers A, Seller Oria C, Free J, Langendijk JA, Knopf AC, and Both S. Technical Note: First report on an in vivo range probing quality control procedure for scanned proton beam therapy in head and neck cancer patients. Med Phys. (2021) 48:1372–80. doi: 10.1002/mp.14713
20. Langen KM and Jones DTL. Organ motion and its management. Int J Radiat Oncol Biol Phys. (2001) 50:265–78. doi: 10.1016/s0360-3016(01)01453-5
21. Díez S, García J, and Sendra F. Analysis and evaluation of periodic physiological organ motion in radiotherapy treatments. Radiother Oncol. (2004) 73:325–9. doi: 10.1016/j.radonc.2004.08.023
22. Mostafaei F, Tai A, Omari E, Song Y, Christian J, Paulson E, et al. Variations of MRI-assessed peristaltic motions during radiation therapy. PloS One. (2018) 13:e0205917. doi: 10.1371/journal.pone.0205917
23. von Siebenthal M, Székely G, Lomax AJ, and Cattin PC. Systematic errors in respiratory gating due to intrafraction deformations of the liver. Med Phys. (2007) 34:3620–9. doi: 10.1118/1.2767053
24. Daamen LA, de Mol van Otterloo SR, van Goor IWJM, Eijkelenkamp H, Erickson BA, Hall WA, et al. Online adaptive MR-guided stereotactic radiotherapy for unresectable Malignancies in the upper abdomen using a 1. 5T MR-linac. Acta Oncol. (2021) 61:111–5. doi: 10.1080/0284186x.2021.2012593
25. Lee SL, Bassetti M, Meijer GJ, and Mook S. Review of MR-guided radiotherapy for esophageal cancer. Front Oncol. (2021) 11:628009. doi: 10.3389/fonc.2021.628009
26. Portelance L, Corradini S, Erickson B, Lalondrelle S, Padgett K, van der Leij F, et al. Online magnetic resonance-guided radiotherapy (oMRgRT) for gynecological cancers. Front Oncol. (2021) 11:628131. doi: 10.3389/fonc.2021.628131
27. Bertholet J, Knopf A, Eiben B, McClelland J, Grimwood A, Harris E, et al. Real-time intrafraction motion monitoring in external beam radiotherapy. Phys Med Biol. (2019) 64:15TR01. doi: 10.1088/1361-6560/ab2ba8
28. Riboldi M, Orecchia R, and Baroni G. Real-time tumour tracking in particle therapy: technological developments and future perspectives. Lancet Oncol. (2012) 13:e383–91. doi: 10.1016/s1470-2045(12)70243-7
29. Landry G and Hua C. Current state and future applications of radiological image guidance for particle therapy. Med Phys. (2018) 45: e1086-95. doi: 10.1002/mp.12744
30. Hoffmann A, Oborn B, Moteabbed M, Yan S, Bortfeld T, Knopf A, et al. MR-guided proton therapy: a review and a preview. Radiat Oncol. (2020) 15. doi: 10.1186/s13014-020-01571-x
31. Oborn BM, Dowdell S, Metcalfe PE, Crozier S, Mohan R, and Keall PJ. Future of medical physics: Real-time MRI-guided proton therapy. Med Phys. (2017) 44: e77-90. doi: 10.1002/mp.12371
32. Bauer J, Beyer C, Dorsch S, Ellerbrock M, Hansmann T, Klüter S, et al. TOWARDS DAILY ONLINE MR-IMAGE-GUIDED PARTICLE THERAPY: FIRST CLINICAL APPLICATION OF A SHUTTLE-BASED WORKFLOW AT THE HEIDELBERG ION-BEAM THERAPY CENTER. Int J Part Ther. (2024) 12:100243. doi: 10.1016/j.ijpt.2024.100243
33. Pestana R, Seidensaal K, Beyer C, Herfarth K, Debus J, Klüter S, et al. TOWARDS MRI-GUIDED ONLINE ADAPTIVE PARTICLE THERAPY IN THE PELVIC REGION. Int J Part Ther. (2024) 12:100242. doi: 10.1016/j.ijpt.2024.100242
34. Johnson RP, Bashkirov V, DeWitt L, Giacometti V, Hurley RF, Piersimoni P, et al. A fast experimental scanner for proton CT: technical performance and first experience with phantom scans. IEEE Trans Nucl Sci. (2016) 63:52–60. doi: 10.1109/tns.2015.2491918
35. Johnson RP. Meeting the detector challenges for pre-clinical proton and ion computed tomography. Phys Med Biol. (2024) 69:11TR02. doi: 10.1088/1361-6560/ad42fc
36. Rossi M, Belotti G, Paganelli C, Pella A, Barcellini A, Cerveri P, et al. Image-based shading correction for narrow-FOV truncated pelvic CBCT with deep convolutional neural networks and transfer learning. Med Phys. (2021) 48:7112–26. doi: 10.1002/mp.15282
37. Oliver JA, Zeidan OA, Meeks SL, Shah AP, Pukala J, Kelly P, et al. The Mobius AIRO mobile CT for image-guided proton therapy: Characterization & commissioning. J Appl Clin Med Phys. (2017) 18:130–6. doi: 10.1002/acm2.12084
38. Sun B, Yang D, Lam D, Zhang T, Dvergsten T, Bradley J, et al. Toward adaptive proton therapy guided with a mobile helical CT scanner. Radiother Oncol. (2018) 129:479–85. doi: 10.1016/j.radonc.2018.08.021
39. Peters N, Wolfahrt P, and Richter C. X-ray computed tomography for treatment planning: current status and innovations. In: Paganelli C, Gianoli C, and Knopf A, editors. Imaging in particle therapy: current practice and future trends. Bristol, UK: IOP Publishing (2024). p. 4–(1-18). doi: 10.1088/978-0-7503-5117-1
40. Richter C, Wolfahrt P, and Richter C. Dual-energy CT in radiation oncology. In: Alkadhi H, Euler A, Maintz D, and Sahani D, editors. Spectral imaging: dual-energy, multi-energy and photon-counting CT. Cham, Switzerland: Springer Cham (2022). p. 333–46. doi: 10.1007/978-3-030-96285-2
41. Nesteruk KP, Bobić M, Lalonde A, Winey BA, Lomax AJ, and Paganetti H. CT-on-rails versus in-room CBCT for online daily adaptive proton therapy of head-and-neck cancers. Cancers. (2021) 13:5991. doi: 10.3390/cancers13235991
42. Archambault Y, Boylan C, Bullock D, Morgas T, Peltola J, Ruokokoski E, et al. Making on-line adaptive radiotherapy possible using artificial intelligence and machine learning for efficient daily re-planning. Med Phys Int. (2020) 8:77–86. Available online at http://mpijournal.org/pdf/2020-02/MPI-2020-02-p077.pdf (Accessed November 25, 2025)
43. Stanley DN, Harms J, Pogue JA, Belliveau J, Marcrom SR, McDonald AM, et al. A roadmap for implementation of kV-CBCT online adaptive radiation therapy and initial first year experiences. J Appl Clin Med Phys. (2023) 24. doi: 10.1002/acm2.13961
44. Groot Koerkamp ML, Bol GH, Kroon PS, Krikke LL, Harderwijk T, Zoetelief AJ, et al. Bringing online adaptive radiotherapy to a standard C-arm linac. Phys Imaging Radiat Oncol. (2024) 31:100597. doi: 10.1016/j.phro.2024.100597
45. Elekta AB. Elekta launches AI-powered adaptive CT-Linac for next-level cancer care (2024). Available online at: https://ir.elekta.com/investors/press-releases/2024/elekta-launches-ai-powered-adaptive-ct-linac-for-next-level-cancer-care/ (Accessed Apr 10, 2025).
46. O’Brien RT, Dillon O, Lau B, George A, Smith S, Wallis A, et al. The first-in-human implementation of adaptive 4D cone beam CT for lung cancer radiotherapy: 4DCBCT in less time with less dose. Radiother Oncol. (2021) 161:29–34. doi: 10.1016/j.radonc.2021.05.021
47. MacKay RI. Image guidance for proton therapy. Clin Oncol. (2018) 30:293–8. doi: 10.1016/j.clon.2018.02.004
48. Schulze R, Heil U, Groβ D, Bruellmann D, Dranischnikow E, Schwanecke U, et al. Artefacts in CBCT: a review. Dentomaxillofac Radiol. (2011) 40:265–73. doi: 10.1259/dmfr/30642039
49. Herrick M, Penfold S, Santos A, and Hickson K. A systematic review of volumetric image guidance in proton therapy. Phys Eng Sci Med. (2023) 46:963–75. doi: 10.1007/s13246-023-01294-9
50. Giacometti V, Hounsell AR, and McGarry CK. A review of dose calculation approaches with cone beam CT in photon and proton therapy. Phys Med. (2020) 76:243–76. doi: 10.1016/j.ejmp.2020.06.017
51. Reiners K, Dagan R, Holtzman A, Bryant C, Andersson S, Nilsson R, et al. CBCT-based dose monitoring and adaptive planning triggers in head and neck PBS proton therapy. Cancers. (2023) 15:3881. doi: 10.3390/cancers15153881
52. Peng H, Wu C, Nguyen D, Schuemann J, Mairani A, Pu Y, et al. Recent advancements of artificial intelligence in particle therapy. IEEE Trans Radiat Plasma Med Sci. (2023) 7:213–24. doi: 10.1109/trpms.2023.3241102
53. Upright Radiotherapy Research Consortium. New research and training project launched — Upright Radiotherapy Research Consortium (2024). Available online at: https://www.uprightresearchconsortium.com/news/new-research-and-training-project-launched (Accessed December 9, 2025).
54. Munzenrider JE, Austin-Seymour M, Blitzer PJ, Gentry R, Goitein M, Gragoudas ES, et al. Proton therapy at Harvard. Strahlentherapie. (1985) 161:756–63. Available online at https://pubmed.ncbi.nlm.nih.gov/3001976/ (Accessed November 25, 2025)
55. Boisbouvier S, Boucaud A, Tanguy R, and Grégoire V. Upright patient positioning for pelvic radiotherapy treatments. Tech Innov Patient Support Radiat Oncol. (2022) 24:124–30. doi: 10.1016/j.tipsro.2022.11.003
56. Pryanichnikov AA, Sokunov VV, and Shemyakov AE. Some results of the clinical use of the proton therapy complex “Prometheus. Phys Part Nuclei Lett. (2018) 15:981–5. doi: 10.1134/s1547477118070592
57. Gordon K, Gulidov I, Smyk D, Semenov A, Golubev K, Lemaeva A, et al. Upright proton therapy for esthesioneuroblastoma: a single-institution experience. Front Oncol. (2024) 14:1348291. doi: 10.3389/fonc.2024.1348291
58. Lemaeva A, Gulidov I, Smyk D, Agapova Y, Koryakin S, Eremina I, et al. A single-center experience of the upright proton therapy for skull-base chordomas and chondrosarcomas: Updated results. Clin Transl Radiat Oncol. (2024) 48:100814. doi: 10.1016/j.ctro.2024.100814
59. Boisbouvier S, Underwood T, McNamara J, and Probst H. Upright patient positioning for gantry-free breast radiotherapy: feasibility tests using a robotic chair and specialised bras. Front Oncol. (2023) 13:1250678. doi: 10.3389/fonc.2023.1250678
60. Feldman J, Pryanichnikov A, Shwartz D, Hillman Y, Wygoda M, Blumenfeld P, et al. Study of upright patient positioning reproducibility in image-guided proton therapy for head and neck cancers. Radiother Oncol. (2024) 201:110572. doi: 10.1016/j.radonc.2024.110572
61. Volz L, Sheng Y, Durante M, and Graeff C. Considerations for upright particle therapy patient positioning and associated image guidance. Front Oncol. (2022) 12:930850. doi: 10.3389/fonc.2022.930850
62. Volz L, Korte J, Martire MC, Zhang Y, Hardcastle N, Durante M, et al. Opportunities and challenges of upright patient positioning in radiotherapy. Phys Med Biol. (2024) 69:18TR02. doi: 10.1088/1361-6560/ad70ee
63. Moteabbed M, Smeets J, Hong TS, Janssens G, Labarbe R, Wolfgang JA, et al. Toward MR-integrated proton therapy: modeling the potential benefits for liver tumors. Phys Med Biol. (2021) 66:195004. doi: 10.1088/1361-6560/ac1ef2
64. Yawson AK, Paul KM, Beyer C, Dorsch S, Klüter S, Welzel T, et al. Pseudo-SPR map generation from MRI using U-net architecture for ion beam therapy application. Lect Notes Comput Sci. (2023) 14122: 257–67. doi: 10.1007/978-3-031-48593-0_19
65. Gantz S, Hietschold V, and Hoffmann AL. Characterization of magnetic interference and image artefacts during simultaneous in-beam MR imaging and proton pencil beam scanning. Phys Med Biol. (2020) 65:215014. doi: 10.1088/1361-6560/abb16f
66. Fuchs H, Padilla-Cabal F, Zimmermann L, Palmans H, and Georg D. MR-guided proton therapy: Impact of magnetic fields on the detector response. Med Phys. (2021) 48:2572–9. doi: 10.1002/mp.14660
67. Fuchs H, Palmans H, Heilemann G, Zuschlag D, Georg D, and Kuess P. Dosimetry in MRgPT: Impact of magnetic fields on TLD dose response during proton irradiation. Med Phys. (2024) 52:633–9. doi: 10.1002/mp.17454
68. Cobanaj M, Lebbink F, Horst F, Traneus E, and Hoffmann A. VERIFICATION OF PROTON DOSE CALCULATION IN THE MAGNETIC FIELD OF AN IN-BEAM MR SCANNER. Int J Part Ther. (2024) 12:100157. doi: 10.1016/j.ijpt.2024.100157
69. Oborn BM, Semioshkina E, van der Kraaij E, and Hoffmann AL. Simulation and experimental benchmarking of a proton pencil beam scanning nozzle model for development of MR-integrated proton therapy. Med Phys. (2024) 51:6196–205. doi: 10.1002/mp.17279
70. Schieferecke J, Gantz S, Hoffmann A, and Pawelke J. Investigation of contrast mechanisms for MRI phase signal-based proton beam visualization in water phantoms. Magn Reson Med. (2023) 90:1776–88. doi: 10.1002/mrm.29752
71. Gebauer B, Pawelke J, Hoffmann A, and Lühr A. Technical note: Experimental dosimetric characterization of proton pencil beam distortion in a perpendicular magnetic field of an in-beam MR scanner. Med Phys. (2023) 50:7294–303. doi: 10.1002/mp.16448
72. Gantz S, Karsch L, Pawelke J, Schieferecke J, Schellhammer S, Smeets J, et al. Direct visualization of proton beam irradiation effects in liquids by MRI. Proc Natl Acad Sci USA. (2023) 120(23):e2301160120. doi: 10.1073/pnas.2301160120
73. Marot M, Surla S, Burke E, Brons S, Runz A, Greilich S, et al. Proton beam dosimetry in the presence of magnetic fields using Farmer-type ionization chambers of different radii. Med Phys. (2023) 50:4590–9. doi: 10.1002/mp.16368
74. Dietrich KA, Klüter S, Dinkel F, Echner G, Brons S, Orzada S, et al. An essentially radiation-transparent body coil integrated with a patient rotation system for MR-guided particle therapy. Med Phys. (2024) 51:4028–43. doi: 10.1002/mp.17065
75. Dorsch S, Paul K, Beyer C, Karger CP, Jäkel O, Debus J, et al. Quality assurance and temporal stability of a 1.5 T MRI scanner for MR-guided Photon and Particle Therapy. Z Med Phys. (2023). 35:204–217. doi: 10.1016/j.zemedi.2023.04.004
76. Fuchs H, Padilla-Cabal F, Oborn BM, and Georg D. Commissioning a beam line for MR-guided particle therapy assisted by in silico methods. Med Phys. (2022) 50:1019–28. doi: 10.1002/mp.16143
77. Schulte R, Bashkirov V, Li T, Liang Z, Mueller K, Heimann J, et al. Conceptual design of a proton computed tomography system for applications in proton radiation therapy. IEEE Trans Nucl Sci. (2004) 51:866–72. doi: 10.1109/tns.2004.829392
78. Penfold SN, Rosenfeld AB, Schulte RW, and Schubert KE. A more accurate reconstruction system matrix for quantitative proton computed tomography. Med Phys. (2009) 36:4511–8. doi: 10.1118/1.3218759
79. Poludniowski G, Allinson NM, and Evans PM. Proton radiography and tomography with application to proton therapy. Br J Radiol. (2015) 88:20150134. doi: 10.1259/bjr.20150134
80. Prall M, Durante M, Berger T, Przybyla B, Graeff C, Lang PM, et al. High-energy proton imaging for biomedical applications. Sci Rep. (2016) 6: 27651. doi: 10.1038/srep27651
81. Johnson RP. Review of medical radiography and tomography with proton beams. Rep Prog Phys. (2017) 81:016701. doi: 10.1088/1361-6633/aa8b1d
82. Pryanichnikov AA, Shwartz D, Feldmand J, Seco J, and Spadea MF. Dual-energy extraction for proton therapy and imaging: validation on a clinical synchrotron-based facility. arXiv [Preprint]. (2025). doi: 10.48550/ARXIV.2506.19736
83. Feldman J, Pryanichnikov A, Achkienasi A, Polyansky I, Hillman Y, Raskin S, et al. Commissioning of a novel gantry-less proton therapy system. Front Oncol. (2024) 14:1417393. doi: 10.3389/fonc.2024.1417393
84. Dedes G, Dickmann J, Niepel K, Wesp P, Johnson RP, Pankuch M, et al. Experimental comparison of proton CT and dual energy x-ray CT for relative stopping power estimation in proton therapy. Phys Med Biol. (2019) 64:165002. doi: 10.1088/1361-6560/ab2b72
85. Lane SA, Slater JM, and Yang GY. Image-guided proton therapy: A comprehensive review. Cancers. (2023) 15:2555. doi: 10.3390/cancers15092555
86. Sharp G, Fritscher KD, Pekar V, Peroni M, Shusharina N, Veeraraghavan H, et al. Vision 20/20: Perspectives on automated image segmentation for radiotherapy. Med Phys. (2014) 41:050902. doi: 10.1118/1.4871620
87. Hizam DA, Tan LK, Saad M, Muaadz A, and Ung NM. Comparison of commercial atlas-based automatic segmentation software for prostate radiotherapy treatment planning. Phys Eng Sci Med. (2024) 47:881–94. doi: 10.1007/s13246-024-01411-2
88. Gibbons E, Hoffmann M, Westhuyzen J, Hodgson A, Chick B, and Last A. Clinical evaluation of deep learning and atlas-based auto-segmentation for critical organs at risk in radiation therapy. J Med Radiat Sci. (2022) 70:15–25. doi: 10.1002/jmrs.618
89. Radici L, Ferrario S, Borca VC, Cante D, Paolini M, Piva C, et al. Implementation of a commercial deep learning-based auto segmentation software in radiotherapy: evaluation of effectiveness and impact on workflow. Life. (2022) 12:2088. doi: 10.3390/life12122088
90. La Macchia M, Fellin F, Amichetti M, Cianchetti M, Gianolini S, Paola V, et al. Systematic evaluation of three different commercial software solutions for automatic segmentation for adaptive therapy in head-and-neck, prostate and pleural cancer. Radiat Oncol. (2012) 7. doi: 10.1186/1748-717x-7-160
91. Vaassen F, Boukerroui D, Looney P, Canters R, Verhoeven K, Peeters S, et al. Real-world analysis of manual editing of deep learning contouring in the thorax region. Phys Imaging Radiat Oncol. (2022) 22:104–10. doi: 10.1016/j.phro.2022.04.008
92. Brouwer CL, Boukerroui D, Oliveira J, Looney P, Steenbakkers RJHM, Langendijk JA, et al. Assessment of manual adjustment performed in clinical practice following deep learning contouring for head and neck organs at risk in radiotherapy. Phys Imaging Radiat Oncol. (2020) 16:54–60. doi: 10.1016/j.phro.2020.10.001
93. Ricci JC, Rineer J, Shah AP, Meeks SL, and Kelly P. Proposal and Evaluation of a Physician-Free, Real-Time On-Table Adaptive Radiotherapy (PF-ROAR) Workflow for the MRIdian MR-Guided LINAC. J Clin Med. (2022) 11:1189. doi: 10.3390/jcm11051189
94. Heilemann G, Buschmann M, Lechner W, Dick V, Eckert F, Heilmann M, et al. Clinical implementation and evaluation of auto-segmentation tools for multi-site contouring in radiotherapy. Phys Imaging Radiat Oncol. (2023) 28:100515. doi: 10.1016/j.phro.2023.100515
95. Mackay K, Bernstein D, Glocker B, Kamnitsas K, and Taylor A. A review of the metrics used to assess auto-contouring systems in radiotherapy. Clin Oncol. (2023) 35:354–69. doi: 10.1016/j.clon.2023.01.016
96. Bobić M, Choulilitsa E, Lee H, Czerska K, Christensen JB, Mayor A, et al. Multi-institutional experimental validation of online adaptive proton therapy workflows. Phys Med Biol. (2024) 69:165021. doi: 10.1088/1361-6560/ad6527
97. Qiao Y, Jagt T, Hoogeman M, Lelieveldt BPF, and Staring M. Evaluation of an open source registration package for automatic contour propagation in online adaptive intensity-modulated proton therapy of prostate cancer. Front Oncol. (2019) 9:1297. doi: 10.3389/fonc.2019.01297
98. Nenoff L, Matter M, Amaya EJ, Josipovic M, Knopf A-C, Lomax AJ, et al. Dosimetric influence of deformable image registration uncertainties on propagated structures for online daily adaptive proton therapy of lung cancer patients. Radiother Oncol. (2021) 159:136–43. doi: 10.1016/j.radonc.2021.03.021
99. Riegel AC, Antone JG, Zhang H, Jain P, Raince J, Rea A, et al. Deformable image registration and interobserver variation in contour propagation for radiation therapy planning. J Appl Clin Med Phys. (2016) 17:347–57. doi: 10.1120/jacmp.v17i3.6110
100. Woerner AJ, Choi M, Harkenrider MM, Roeske JC, and Surucu M. Evaluation of deformable image registration-based contour propagation from planning CT to cone-beam CT. Technol Cancer Res Treat. (2017) 16:801–10. doi: 10.1177/1533034617697242
101. Rigaud B, Simon A, Castelli J, Lafond C, Acosta O, Haigron P, et al. Deformable image registration for radiation therapy: principle, methods, applications and evaluation. Acta Oncol. (2019) 58:1225–37. doi: 10.1080/0284186x.2019.1620331
102. Nash D, Palmer AL, van Herk M, McWilliam A, and Vasquez Osorio E. Suitability of propagated contours for adaptive replanning for head and neck radiotherapy. Phys Med. (2022) 102:66–72. doi: 10.1016/j.ejmp.2022.09.002
103. Pukala J, Johnson PB, Shah AP, Langen KM, Bova FJ, Staton RJ, et al. Benchmarking of five commercial deformable image registration algorithms for head and neck patients. J Appl Clin Med Phys. (2016) 17:25–40. doi: 10.1120/jacmp.v17i3.5735
104. Hvid CA, Elstrøm UV, Jensen K, Alber M, and Grau C. Accuracy of software-assisted contour propagation from planning CT to cone beam CT in head and neck radiotherapy. Acta Oncol. (2016) 55:1324–30. doi: 10.1080/0284186x.2016.1185149
105. Feng M, Valdes G, Dixit N, and Solberg TD. Machine learning in radiation oncology: opportunities, requirements, and needs. Front Oncol. (2018) 8:110. doi: 10.3389/fonc.2018.00110
106. Finnegan RN, Quinn A, Horsley P, Chan J, Stewart M, Bromley R, et al. Geometric and dosimetric evaluation of a commercial AI auto-contouring tool on multiple anatomical sites in CT scans. J Appl Clin Med Phys. (2025). 26: e70067. doi: 10.1002/acm2.70067
107. Fan M, Wang T, Lei Y, Patel PR, Dresser S, Ghavidel BB, et al. Evaluation and failure analysis of four commercial deep learning-based autosegmentation software for abdominal organs at risk. J Appl Clin Med Phys. (2025) 26: e70010. doi: 10.1002/acm2.70010
108. Ng CKC. Performance of commercial deep learning-based auto-segmentation software for prostate cancer radiation therapy planning: A systematic review. Information. (2025) 16:215. doi: 10.3390/info16030215
109. Langmack KA, Alexander GG, Gardiner J, McKenna A, and Shawcroft E. An audit of the impact of the introduction of a commercial artificial intelligence-driven auto-contouring tool into a radiotherapy department. Br J Radiol. (2024) 98:375–82. doi: 10.1093/bjr/tqae255
110. Doolan PJ, Charalambous S, Roussakis Y, Leczynski A, Peratikou M, Benjamin M, et al. A clinical evaluation of the performance of five commercial artificial intelligence contouring systems for radiotherapy. Front Oncol. (2023) 13:1213068. doi: 10.3389/fonc.2023.1213068
111. Goddard L, Velten C, Tang J, Skalina KA, Boyd R, Martin W, et al. Evaluation of multiple-vendor AI autocontouring solutions. Radiat Oncol. (2024) 19. doi: 10.1186/s13014-024-02451-4
112. Ng CKC, Leung VWS, and Hung RHM. Clinical evaluation of deep learning and atlas-based auto-contouring for head and neck radiation therapy. Appl Sci. (2022) 12:11681. doi: 10.3390/app122211681
113. Smolders A, Bengtsson I, Forsgren A, Lomax A, Weber DC, Fredriksson A, et al. Robust optimization strategies for contour uncertainties in online adaptive radiation therapy. Phys Med Biol. (2024) 69:165001. doi: 10.1088/1361-6560/ad6526
114. Nenoff L, Buti G, Bobić M, Lalonde A, Nesteruk KP, Winey B, et al. Integrating structure propagation uncertainties in the optimization of online adaptive proton therapy plans. Cancers. (2022) 14:3926. doi: 10.3390/cancers14163926
115. Smolders A, Choulilitsa E, Czerska K, Bizzocchi N, Krcek R, Lomax A, et al. Dosimetric comparison of autocontouring techniques for online adaptive proton therapy. Phys Med Biol. (2023) 68:175006. doi: 10.1088/1361-6560/ace307
116. Baroudi H, Brock KK, Cao W, Chen X, Chung C, Court LE, et al. Automated contouring and planning in radiation therapy: what is ‘Clinically acceptable’? Diagnostics. (2023) 13:667. doi: 10.3390/diagnostics13040667
117. Heukelom J and Fuller CD. Head and neck cancer adaptive radiation therapy (ART): conceptual considerations for the informed clinician. Semin Radiat Oncol. (2019) 29:258–73. doi: 10.1016/j.semradonc.2019.02.008
118. Liu S, Chapman KL, Berry SL, Bertini J, Ma R, Fu Y, et al. Implementation of a knowledge-based decision support system for treatment plan auditing through automation. Med Phys. (2023) 50:6978–89. doi: 10.1002/mp.16472
119. Benedick T, Li M, Wang E, Pawooskar-Almeida S, Le A, and Liu B. Utilizing data-mining and deep learning methods on retrospective head and neck radiation therapy cases for decision support of individualized treatment planning. In: Medical imaging 2023: imaging informatics for healthcare, research, and applications. ellingham, Washington, USA: SPIE. (2023). 4. doi: 10.1117/12.2656180
120. Rachi T, Ariji T, and Takahashi S. Development of machine-learning prediction programs for delivering adaptive radiation therapy with tumor geometry and body shape changes in head and neck volumetric modulated arc therapy. Adv Radiat Oncol. (2023) 8:101172. doi: 10.1016/j.adro.2023.101172
121. Draguet C, Barragán-Montero AM, Vera MC, Thomas M, Populaire P, Defraene G, et al. Automated clinical decision support system with deep learning dose prediction and NTCP models to evaluate treatment complications in patients with esophageal cancer. Radiother Oncol. (2022) 176:101–7. doi: 10.1016/j.radonc.2022.08.031
122. Gros SAA, Santhanam AP, Block AM, Emami B, Lee BH, and Joyce C. Retrospective clinical evaluation of a decision-support software for adaptive radiotherapy of head and neck cancer patients. Front Oncol. (2022) 12:7777933. doi: 10.3389/fonc.2022.7777933
123. Oud M, Breedveld S, Rojo-Santiago J, Giżyńska MK, Kroesen M, Habraken S, et al. A fast and robust constraint-based online re-optimization approach for automated online adaptive intensity modulated proton therapy in head and neck cancer. Phys Med Biol. (2024) 69:075007. doi: 10.1088/1361-6560/ad2a98
124. Draguet C, Populaire P, Vera MC, Fredriksson A, Haustermans K, Lee JA, et al. A comparative study on automatic treatment planning for online adaptive proton therapy of esophageal cancer: which combination of deformable registration and deep learning planning tools performs the best? Phys Med Biol. (2024) 69:205013. doi: 10.1088/1361-6560/ad80f6
125. Jagt T, Breedveld S, Van De Water S, Heijmen B, and Hoogeman M. Near real-time automated dose restoration in IMPT to compensate for daily tissue density variations in prostate cancer. Phys Med Biol. (2017) 62:4254–72. doi: 10.1088/1361-6560/aa5c12
126. Bernatowicz K, Geets X, Barragan A, Janssens G, Souris K, and Sterpin E. Feasibility of online IMPT adaptation using fast, automatic and robust dose restoration. Phys Med Biol. (2018) 63:085018. doi: 10.1088/1361-6560/aaba8c
127. Borderías Villarroel E, Geets X, and Sterpin E. Online adaptive dose restoration in intensity modulated proton therapy of lung cancer to account for inter-fractional density changes. Phys Imaging Radiat Oncol. (2020) 15:30–7. doi: 10.1016/j.phro.2020.06.004
128. Botas P, Kim J, Winey B, and Paganetti H. Online adaption approaches for intensity modulated proton therapy for head and neck patients based on cone beam CTs and Monte Carlo simulations. Phys Med Biol. (2018) 64:015004. doi: 10.1088/1361-6560/aaf30b
129. Lalonde A, Bobić M, Winey B, Verburg J, Sharp GC, and Paganetti H. Anatomic changes in head and neck intensity-modulated proton therapy: Comparison between robust optimization and online adaptation. Radiother Oncol. (2021) 159:39–47. doi: 10.1016/j.radonc.2021.03.008
130. Lalonde A, Bobić M, Sharp GC, Chamseddine I, Winey B, and Paganetti H. Evaluating the effect of setup uncertainty reduction and adaptation to geometric changes on normal tissue complication probability using online adaptive head and neck intensity modulated proton therapy. Phys Med Biol. (2023) 68:115018. doi: 10.1088/1361-6560/acd433
131. Bobić M, Lalonde A, Nesteruk KP, Lee H, Nenoff L, Gorissen BL, et al. Large anatomical changes in head-and-neck cancers – A dosimetric comparison of online and offline adaptive proton therapy. Clin Transl Radiat Oncol. (2023) 40:100625. doi: 10.1016/j.ctro.2023.100625
132. Borderias-Villarroel E, Fredriksson A, Cvilic S, Di Perri D, Longton E, Pierrard J, et al. Dose mimicking based strategies for online adaptive proton therapy of head and neck cancer. Phys Med Biol. (2023) 68:105002. doi: 10.1088/1361-6560/accb38
133. Maes D, Holmstrom M, Helander R, Saini J, Fang C, and Bowen SR. Automated treatment planning for proton pencil beam scanning using deep learning dose prediction and dose-mimicking optimization. J Appl Clin Med Phys. (2023) 24:e14065. doi: 10.1002/acm2.14065
134. Kaushik S, Stützer K, Ödén J, Fredriksson A, and Toma-Dasu I. Adaptive intensity modulated proton therapy using 4D robust planning: a proof-of-concept for the application of dose mimicking approach. Phys Med Biol. (2024) 69:185010. doi: 10.1088/1361-6560/ad75e0
135. Miyazaki K, Fujii Y, Yamada T, Kanehira T, Miyamoto N, Matsuura T, et al. Deformed dose restoration to account for tumor deformation and position changes for adaptive proton therapy. Med Phys. (2022) 50:675–87. doi: 10.1002/mp.16149
136. Jagt TZ, Breedveld S, van Haveren R, Nout RA, Astreinidou E, Heijmen BJM, et al. Plan-library supported automated replanning for online-adaptive intensity-modulated proton therapy of cervical cancer. Acta Oncol. (2019) 58:1440–5. doi: 10.1080/0284186x.2019.1627414
137. Pilskog S, Abal B, Øvrelid KS, Engeseth GM, Ytre-Hauge KS, and Hysing LB. Plan selection in proton therapy of locally advanced prostate cancer with simultaneous treatment of multiple targets. Int J Radiat Oncol Biol Phys. (2020) 106:630–8. doi: 10.1016/j.ijrobp.2019.11.007
138. Oud M, Breedveld S, Giżyńska M, Kroesen M, Hutschemaekers S, Habraken S, et al. An online adaptive plan library approach for intensity modulated proton therapy for head and neck cancer. Radiother Oncol. (2022) 176:68–75. doi: 10.1016/j.radonc.2022.09.011
139. Hamaide V, Souris K, Dasnoy D, Glineur F, and Macq B. Real-time image-guided treatment of mobile tumors in proton therapy by a library of treatment plans: a simulation study. Med Phys. (2023) 50:465–79. doi: 10.1002/mp.16084
140. Troost EGC, Menkel S, Tschiche M, Thiele J, Jaster M, Haak D, et al. Towards online adaptive proton therapy: first report of plan-library-based plan-of-the-day approach. Acta Oncol. (2021) 61:231–4. doi: 10.1080/0284186x.2021.1994154
141. Gambetta V, Fredriksson A, Menkel S, Richter C, and Stützer K. The partial adaptation strategy for online-adaptive proton therapy: A proof of concept study in head and neck cancer patients. Med Phys. (2024) 51:5572–81. doi: 10.1002/mp.17178
142. Gambetta V, Pieta V, Berthold J, Hölscher T, Fredriksson A, Richter C, et al. Partial adaptation for online-adaptive proton therapy triggered by during-delivery treatment verification: Feasibility study on prostate cancer treatments. Phys Imaging Radiat Oncol. (2025) 34:100755. doi: 10.1016/j.phro.2025.100755
143. Chen M, Zhong Y, Shao Y, Jiang S, and Lu W. Mid-range probing—towards range-guided particle therapy. Phys Med Biol. (2018) 63:13NT01. doi: 10.1088/1361-6560/aaca1b
144. Chen M, Yang D, Zhu XR, Ma L, Grosshans DR, Shao Y, et al. Investigation of intra-fractionated range guided adaptive proton therapy (RGAPT): II. Range-shift compensated on-line treatment adaptation and verification. Phys Med Biol. (2024) 69:155006. doi: 10.1088/1361-6560/ad56f2
145. McIntosh C, Welch M, McNiven A, Jaffray DA, and Purdie TG. Fully automated treatment planning for head and neck radiotherapy using a voxel-based dose prediction and dose mimicking method. Phys Med Biol. (2017) 62:5926–44. doi: 10.1088/1361-6560/aa71f8
146. Zhang L, Holmes JM, Liu Z, Vora SA, Sio TT, Vargas CE, et al. Beam mask and sliding window-facilitated deep learning-based accurate and efficient dose prediction for pencil beam scanning proton therapy. Med Phys. (2023) 51:1484–98. doi: 10.1002/mp.16758
147. Vazquez I, Liang D, Salazar RM, Gronberg MP, Sjogreen C, Williamson TD, et al. Deep learning techniques for proton dose prediction across multiple anatomical sites and variable beam configurations. Phys Med Biol. (2025) 70:075016. doi: 10.1088/1361-6560/adc236
148. Trnkova P, Zhang Y, Toshito T, Heijmen B, Richter C, Aznar MC, et al. A survey of practice patterns for adaptive particle therapy for interfractional changes. Phys Imaging Radiat Oncol. (2023) 26:100442. doi: 10.1016/j.phro.2023.100442
149. Zhang Y, Trnkova P, Toshito T, Heijmen B, Richter C, Aznar M, et al. A survey of practice patterns for real-time intrafractional motion-management in particle therapy. Phys Imaging Radiat Oncol. (2023) 26:100439. doi: 10.1016/j.phro.2023.100439
150. Saini J, Traneus E, Maes D, Regmi R, Bowen SR, Bloch C, et al. Advanced Proton Beam Dosimetry Part I: review and performance evaluation of dose calculation algorithms. Transl Lung Cancer Res. (2018) 7:171–9. doi: 10.21037/tlcr.2018.04.05
151. De Martino F, Clemente S, Graeff C, Palma G, and Cella L. Dose calculation algorithms for external radiation therapy: an overview for practitioners. Appl Sci. (2021) 11:6806. doi: 10.3390/app11156806
152. Schaffner B, Pedroni E, and Lomax A. Dose calculation models for proton treatment planning using a dynamic beam delivery system: an attempt to include density heterogeneity effects in the analytical dose calculation. Phys Med Biol. (1999) 44:27–41. doi: 10.1088/0031-9155/44/1/004
153. Nenoff L, Matter M, Jarhall AG, Winterhalter C, Gorgisyan J, Josipovic M, et al. Daily adaptive proton therapy: is it appropriate to use analytical dose calculations for plan adaption? Int J Radiat Oncol Biol Phys. (2020) 107:747–55. doi: 10.1016/j.ijrobp.2020.03.036
154. Burlacu T, Lathouwers D, and Perkó Z. A deterministic adjoint-based semi-analytical algorithm for fast response change computations in proton therapy. J Comput Theor Transp. (2023) 52:1–41. doi: 10.1080/23324309.2023.2166077
155. Burlacu T, Lathouwers D, and Perkó Z. Yet anOther Dose Algorithm (YODA) for independent computations of dose and dose changes due to anatomical changes. Phys Med Biol. (2024) 69:165003. doi: 10.1088/1361-6560/ad6373
156. Taylor PA, Kry SF, and Followill DS. Pencil beam algorithms are unsuitable for proton dose calculations in lung. Int J Radiat Oncol Biol Phys. (2017) 99:750–6. doi: 10.1016/j.ijrobp.2017.06.003
157. Holmes J, Feng H, Zhang L, Fix MK, Jiang SB, and Liu W. Fast Monte Carlo dose calculation in proton therapy. Phys Med Biol. (2024) 69:17TR01. doi: 10.1088/1361-6560/ad67a7
158. Feng H, Patel SH, Wong WW, Younkin JE, Penoncello GP, Morales DH, et al. GPU-accelerated Monte Carlo-based online adaptive proton therapy: A feasibility study. Med Phys. (2022) 49:3550–63. doi: 10.1002/mp.15678
159. Shan J, Feng H, Morales DH, Patel SH, Wong WW, Fatyga M, et al. Virtual particle Monte Carlo: A new concept to avoid simulating secondary particles in proton therapy dose calculation. Med Phys. (2022) 49:6666–83. doi: 10.1002/mp.15913
160. Xu Y, Cyriac J, De Ornelas M, Bossart E, Padgett K, Butkus M, et al. Knowledge-based planning for robustly optimized intensity-modulated proton therapy of head and neck cancer patients. Front Oncol. (2021) 11:737901. doi: 10.3389/fonc.2021.737901
161. Neishabouri A, Wahl N, Mairani A, Köthe U, and Bangert M. Long short-term memory networks for proton dose calculation in highly heterogeneous tissues. Med Phys. (2021) 48:1893–908. doi: 10.1002/mp.14658
162. Pastor-Serrano O, Dong P, Huang C, Xing L, and Perkó Z. Sub-second photon dose prediction via transformer neural networks. Med Phys. (2023) 50:3159–71. doi: 10.1002/mp.16231
163. Wu C, Nguyen D, Xing Y, Montero AB, Schuemann J, Shang H, et al. Improving proton dose calculation accuracy by using deep learning. Mach Learn Sci Technol. (2021) 2:015017. doi: 10.1088/2632-2153/abb6d5
164. Spezialetti M, Lapenna F, Caianiello P, Fracchiolla F, Muciaccia F, Placidi G, et al. (2021). Using deep learning for fast dose refinement in proton therapy, in: 2021 IEEE International Conference on Systems, Man, and Cybernetics (SMC). doi: 10.1109/smc52423.2021.9658879
165. Wang W, Chang Y, Liu Y, Liang Z, Liao Y, Qin B, et al. Feasibility study of fast intensity-modulated proton therapy dose prediction method using deep neural networks for prostate cancer. Med Phys. (2022) 49:5451–63. doi: 10.1002/mp.15702
166. Pastor-Serrano O and Perkó Z. Millisecond speed deep learning based proton dose calculation with Monte Carlo accuracy. Phys Med Biol. (2022) 67:105006. doi: 10.1088/1361-6560/ac692e
167. Neishabouri A, Bauer J, Abdollahi A, Debus J, and Mairani A. Real-time adaptive proton therapy: An AI-based spatio-temporal mono-energetic dose calculation model (CC-LSTM). Comput Biol Med. (2025) 188:109777. doi: 10.1016/j.compbiomed.2025.109777
168. Pang B, Chen S, Zeng Y, Liu M, Zhang Q, Quan H, et al. Lightweight and universal deep learning model for fast proton spot dose calculation at arbitrary energies. Phys Med Biol. (2025) 70:105014. doi: 10.1088/1361-6560/add3b9
169. Radonic D, Xiao F, Wahl N, Voss L, Neishabouri A, Delopoulos N, et al. Proton dose calculation with LSTM networks in presence of a magnetic field. Phys Med Biol. (2024) 69:215019. doi: 10.1088/1361-6560/ad7f1e
170. Liu Y, Shang X, Li N, Wang Z, Fang C, Zou Y, et al. An AI dose-influence matrix engine for robust pencil beam scanning protons therapy. Med Phys. (2024) 52:1903–13. doi: 10.1002/mp.17602
171. Matter M, Nenoff L, Meier G, Weber DC, Lomax AJ, and Albertini F. Alternatives to patient specific verification measurements in proton therapy: a comparative experimental study with intentional errors. Phys Med Biol. (2018) 63:205014. doi: 10.1088/1361-6560/aae2f4
172. Ates O, Pirlepesov F, Zhao L, Hua C, and Merchant TE. Development of a log file analysis tool for proton patient QA, system performance tracking, and delivered dose reconstruction. J Appl Clin Med Phys. (2023) 24:e13972. doi: 10.1002/acm2.13972
173. Bookbinder A, Bobić M, Sharp GC, and Nenoff L. An operator-independent quality assurance system for automatically generated structure sets. Phys Med Biol. (2024) 69:175003. doi: 10.1088/1361-6560/ad6742
174. Meier G, Besson R, Nanz A, Safai S, and Lomax AJ. Independent dose calculations for commissioning, quality assurance and dose reconstruction of PBS proton therapy. Phys Med Biol. (2015) 60:2819–36. doi: 10.1088/0031-9155/60/7/2819
175. Schneider U, Pedroni E, and Lomax A. The calibration of CT Hounsfield units for radiotherapy treatment planning. Phys Med Biol. (1996) 41:111–24. doi: 10.1088/0031-9155/41/1/009
176. Farace P, Righetto R, and Meijers A. Pencil beam proton radiography using a multilayer ionization chamber. Phys Med Biol. (2016) 61:4078–87. doi: 10.1088/0031-9155/61/11/4078
177. Parodi K and Polf JC. In vivo range verification in particle therapy. Med Phys. (2018) 45:e1036-50. doi: 10.1002/mp.12960
178. Mumot M, Algranati C, Hartmann M, Schippers JM, Hug E, and Lomax AJ. Proton range verification using a range probe: definition of concept and initial analysis. Phys Med Biol. (2010) 55:4771–82. doi: 10.1088/0031-9155/55/16/010
179. Seller Oria C, Free J, Marmitt GG, Knäusl B, Brandenburg S, Knopf AC, et al. Technical note: Flat panel proton radiography with a patient specific imaging field for accurate WEPL assessment. Med Phys. (2023) 50:1756–65. doi: 10.1002/mp.16208
180. Oria CS, Marmitt GG, Both S, Langendijk JA, Knopf AC, and Meijers A. Classification of various sources of error in range assessment using proton radiography and neural networks in head and neck cancer patients. Phys Med Biol. (2020) 65:235009. doi: 10.1088/1361-6560/abc09c
181. Seller Oria C, Thummerer A, Free J, Langendijk JA, Both S, Knopf AC, et al. Range probing as a quality control tool for CBCT-based synthetic CTs: In vivo application for head and neck cancer patients. Med Phys. (2021) 48:4498–505. doi: 10.1002/mp.15020
182. Pawelke J, Enghardt W, Haberer T, Hasch BG, Hinz R, Kramer M, et al. In-beam PET imaging for the control of heavy-ion tumour therapy. IEEE Trans Nucl Sci. (1997) 44:1492–8. doi: 10.1109/23.632694
183. Parodi K, Enghardt W, and Haberer T. In-beam PET measurements of β+radioactivity induced by proton beams. Phys Med Biol. (2001) 47:21–36. doi: 10.1088/0031-9155/47/1/302
184. Parodi K, Bortfeld T, Enghardt W, Fiedler F, Knopf A, Paganetti H, et al. PET imaging for treatment verification of ion therapy: Implementation and experience at GSI Darmstadt and MGH Boston. Nucl Instrum Methods Phys Res A. (2008) 591:282–6. doi: 10.1016/j.nima.2008.03.075
185. Parodi K, Paganetti H, Cascio E, Flanz JB, Bonab AA, Alpert NM, et al. PET/CT imaging for treatment verification after proton therapy: A study with plastic phantoms and metallic implants. Med Phys. (2007) 34:419–35. doi: 10.1118/1.2401042
186. Parodi K, Yamaya T, and Moskal P. Experience and new prospects of PET imaging for ion beam therapy monitoring. Z Med Phys. (2023) 33:22–34. doi: 10.1016/j.zemedi.2022.11.001
187. Shakirin G, Braess H, Fiedler F, Kunath D, Laube K, Parodi K, et al. Implementation and workflow for PET monitoring of therapeutic ion irradiation: a comparison of in-beam, in-room, and off-line techniques. Phys Med Biol. (2011) 56:1281–98. doi: 10.1088/0031-9155/56/5/004
188. Fiorina E, Ferrero V, Baroni G, Battistoni G, Belcari N, Camarlinghi N, et al. Detection of interfractional morphological changes in proton therapy: A simulation and in vivo study with the INSIDE in-beam PET. Front Phys. (2021) 8:578388. doi: 10.3389/fphy.2020.578388
189. Moglioni M, Kraan AC, Baroni G, Battistoni G, Belcari N, Berti A, et al. In-vivo range verification analysis with in-beam PET data for patients treated with proton therapy at CNAO. Front Oncol. (2022) 12:929949. doi: 10.3389/fonc.2022.929949
190. Toramatsu C, Yoshida E, Wakizaka H, Mohammadi A, Ikoma Y, Tashima H, et al. Washout effect in rabbit brain: in-beam PET measurements using 10 C, 11 C and 15 O ion beams. BioMed Phys Eng Express. (2018) 4:035001. doi: 10.1088/2057-1976/aaade7
191. Gianoli C, De Bernardi E, Ricotti R, Kurz C, Bauer J, Riboldi M, et al. First clinical investigation of a 4D maximum likelihood reconstruction for 4D PET-based treatment verification in ion beam therapy. Radiotherapy Oncol. (2017) 123:339–45. doi: 10.1016/j.radonc.2017.04.018
192. Laube K, Menkel S, Bert C, Enghardt W, Helmbrecht S, Saito N, et al. 4D particle therapy PET simulation for moving targets irradiated with scanned ion beams. Phys Med Biol. (2013) 58:513–33. doi: 10.1088/0031-9155/58/3/513
193. Stützer K, Bert C, Enghardt W, Helmbrecht S, Parodi K, Priegnitz M, et al. Experimental verification of a 4D MLEM reconstruction algorithm used for in-beam PET measurements in particle therapy. Phys Med Biol. (2013) 58:5085–111. doi: 10.1088/0031-9155/58/15/5085
194. Priegnitz M, Möckel D, Parodi K, Sommerer F, Fiedler F, and Enghardt W. In-beam PET measurement of 7Li3+ irradiation induced β+-activity. Phys Med Biol. (2008) 53:4443–53. doi: 10.1088/0031-9155/53/16/015
195. Dendooven P, Buitenhuis HJT, Diblen F, Heeres PN, Biegun AK, Fiedler F, et al. Short-lived positron emitters in beam-on PET imaging during proton therapy. Phys Med Biol. (2015) 60:8923–47. doi: 10.1088/0031-9155/60/23/8923
196. Cuccagna C, Battistoni G, Bisogni MG, Cerello P, Del Guerra A, Ferrero V, et al. Few-seconds range verification with short-lived positron emitters in carbon ion therapy. Phys Med. (2024) 118:103209. doi: 10.1016/j.ejmp.2024.103209
197. Brzeziński K, Baran J, Borys D, Gajewski J, Chug N, Coussat A, et al. Detection of range shifts in proton beam therapy using the J-PET scanner: a patient simulation study. Phys Med Biol. (2023) 68:145016. doi: 10.1088/1361-6560/acdd4c
198. Zhang R, Mu D, Ma Q, Wan L, Xiao P, Qi P, et al. Proton spot dose estimation based on positron activity distributions with neural network. Med Phys. (2024) 51:7226–39. doi: 10.1002/mp.17297
199. Vandenberghe S, Mikhaylova E, D’Hoe E, Mollet P, and Karp JS. Recent developments in time-of-flight PET. EJNMMI Phys. (2016) 3. doi: 10.1186/s40658-016-0138-3
200. Conti M and Bendriem B. The new opportunities for high time resolution clinical TOF PET. Clin Transl Imaging. (2019) 7:139–47. doi: 10.1007/s40336-019-00316-5
201. Yoshida E, Tashima H, Nagatsu K, Tsuji AB, Kamada K, Parodi K, et al. Whole gamma imaging: a new concept of PET combined with Compton imaging. Phys Med Biol. (2020) 65:125013. doi: 10.1088/1361-6560/ab8e89
202. Stichelbaut F and Jongen Y. (2003). Verification of the proton beam position in the patient by the detection of prompt gamma-rays emission, in: 39th meeting of the Particle Therapy Co-Operative Group (PTCOG).
203. Pinto M. Prompt-gamma imaging in particle therapy. Eur Phys J Plus. (2024) 139. doi: 10.1140/epjp/s13360-024-05664-4
204. Krimmer J, Dauvergne D, Létang JM, and Testa É. Prompt-gamma monitoring in hadrontherapy: A review. Nucl Instrum Methods Phys Res A. (2018) 878:58–73. doi: 10.1016/j.nima.2017.07.063
205. Cheon B-W and Min CH. Prompt gamma imaging system in particle therapy: a mini-review. Front Phys. (2024) 12:1356572. doi: 10.3389/fphy.2024.1356572
206. Werner T, Berthold J, Enghardt W, Hueso-Gonzalez F, Kogler T, Petzoldt J, et al. (2017). Range verification in proton therapy by prompt gamma-ray timing (PGT): steps towards clinical implementation, in: 2017 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC). pp. 1–5. doi: 10.1109/nssmic.2017.8532807
207. Verburg JM and Seco J. Proton range verification through prompt gamma-ray spectroscopy. Phys Med Biol. (2014) 59:7089–106. doi: 10.1088/0031-9155/59/23/7089
208. Richter C, Pausch G, Barczyk S, Priegnitz M, Keitz I, Thiele J, et al. First clinical application of a prompt gamma based in vivo proton range verification system. Radiother Oncol. (2016) 118:232–7. doi: 10.1016/j.radonc.2016.01.004
209. Xie Y, Bentefour EH, Janssens G, Smeets J, Vander Stappen F, Hotoiu L, et al. Prompt gamma imaging for in vivo range verification of pencil beam scanning proton therapy. Int J Radiat Oncol Biol Phys. (2017) 99:210–8. doi: 10.1016/j.ijrobp.2017.04.027
210. Evaluation of prompt-gamma imaging for range verification for the proton irradiation of tumor patients (PRIMA. In: German clinical trial register. DRKS00009224.
211. Verburg JM, Hueso-Gonzalez F, Tattenberg S, Wohlfahrt P, Ruggieri T, and Bortfeld T. First-in-human use of prompt gamma-ray spectroscopy for proton range verification. In: 2020 Joint AAPM | COMP virtual meeting (2020). Hoboken, New Jersey, USA: Wiley. Available online at: https://aapm.onlinelibrary.wiley.com/doi/10.1002/mp.14316.(Accessed November 27, 2025)
212. Hueso-González F, Berthold J, Wohlfahrt P, Bortfeld T, Khamfongkhruea C, Tattenberg S, et al. Inter-center comparison of proton range verification prototypes with an anthropomorphic head phantom. Phys Med Biol. (2024) 69:225010. doi: 10.1088/1361-6560/ad8856
213. Sterpin E, Janssens G, Smeets J, Stappen FV, Prieels D, Priegnitz M, et al. Analytical computation of prompt gamma ray emission and detection for proton range verification. Phys Med Biol. (2015) 60:4915–46. doi: 10.1088/0031-9155/60/12/4915
214. Huang Z, Tian L, Janssens G, Smeets J, Xie Y, Kevin Teo B-K, et al. An experimental validation of a filtering approach for prompt gamma prediction in a research proton treatment planning system. Phys Med Biol. (2024) 69:155025. doi: 10.1088/1361-6560/ad6116
215. Berthold J, Pietsch J, Piplack N, Khamfongkhruea C, Thiele J, Hölscher T, et al. Detectability of anatomical changes with prompt-gamma imaging: first systematic evaluation of clinical application during prostate-cancer proton therapy. Int J Radiat Oncol Biol Phys. (2023) 117:718–29. doi: 10.1016/j.ijrobp.2023.05.002
216. Pietsch J, Khamfongkhruea C, Berthold J, Janssens G, Stützer K, Löck S, et al. Automatic detection and classification of treatment deviations in proton therapy from realistically simulated prompt gamma imaging data. Med Phys. (2022) 50:506–17. doi: 10.1002/mp.15975
217. Khamfongkhruea C, Berthold J, Janssens G, Petzoldt J, Smeets J, Pausch G, et al. Classification of the source of treatment deviation in proton therapy using prompt-gamma imaging information. Med Phys. (2020) 47:5102–11. doi: 10.1002/mp.14393
218. Tian L, Landry G, Dedes G, Pinto M, Kamp F, Belka C, et al. A new treatment planning approach accounting for prompt gamma range verification and interfractional anatomical changes. Phys Med Biol. (2020) 65:095005. doi: 10.1088/1361-6560/ab7d15
219. Berthold J, Khamfongkhruea C, Petzoldt J, Thiele J, Hölscher T, Wohlfahrt P, et al. First-in-human validation of CT-based proton range prediction using prompt gamma imaging in prostate cancer treatments. Int J Radiat Oncol Biol Phys. (2021) 111:1033–43. doi: 10.1016/j.ijrobp.2021.06.036
220. Everaere P, Dauvergne D, Gallin-Martel M-L, Hérault J, Koudia A, Koumeir C, et al. Prompt gamma energy integration: a new method for online-range verification in proton therapy with pulsed-beams. Front Phys. (2024) 12:1371015. doi: 10.3389/fphy.2024.1371015
221. Parodi K and Assmann W. Ionoacoustics: A new direct method for range verification. Mod Phys Lett A. (2015) 30:1540025. doi: 10.1142/s0217732315400258
222. Kirsch L, Assmann W, Gerlach S, Schmidt A-K, Bender M, Parodi K, et al. Ionoacoustic monitoring of relativistic heavy ion beams. Nucl Instrum Methods Phys Res A. (2023) 1057:168755. doi: 10.1016/j.nima.2023.168755
223. Schauer J, Wieser HP, Huang Y, Ruser H, Lascaud J, Würl M, et al. Proton beam range verification by means of ionoacoustic measurements at clinically relevant doses using a correlation-based evaluation. Front Oncol. (2022) 12:925542. doi: 10.3389/fonc.2022.925542
224. Schauer J, Wieser HP, Lascaud J, Huang Y, Vidal M, Herault J, et al. Range verification of a clinical proton beam in an abdominal phantom by co-registration of ionoacoustics and ultrasound. Phys Med Biol. (2023) 68:125009. doi: 10.1088/1361-6560/acd834
225. Freijo C, Herraiz JL, Sanchez-Parcerisa D, and Udias JM. Dictionary-based protoacoustic dose map imaging for proton range verification. Photoacoustics. (2021) 21:100240. doi: 10.1016/j.pacs.2021.100240
226. Caron J, Gonzalez G, Pandey PK, Wang S, Prather K, Ahmad S, et al. Single pulse protoacoustic range verification using a clinical synchrocyclotron. Phys Med Biol. (2023) 68:045011. doi: 10.1088/1361-6560/acb2ae
227. Jiang Z, Sun L, Yao W, Wu QJ, Xiang L, and Ren L. 3D in vivo dose verification in prostate proton therapy with deep learning-based proton-acoustic imaging. Phys Med Biol. (2022) 67:215012. doi: 10.1088/1361-6560/ac9881
228. Kellnberger S, Assmann W, Lehrack S, Reinhardt S, Thirolf P, Queirós D, et al. Ionoacoustic tomography of the proton Bragg peak in combination with ultrasound and optoacoustic imaging. Sci Rep. (2016) 6. doi: 10.1038/srep29305
229. Li H, Sahoo N, Poenisch F, Suzuki K, Li Y, Li X, et al. Use of treatment log files in spot scanning proton therapy as part of patient-specific quality assurance. Med Phys. (2013) 40:021703. doi: 10.1118/1.4773312
230. Scandurra D, Albertini F, van der Meer R, Meier G, Weber DC, Bolsi A, et al. Assessing the quality of proton PBS treatment delivery using machine log files: comprehensive analysis of clinical treatments delivered at PSI Gantry 2. Phys Med Biol. (2016) 61:1171–81. doi: 10.1088/0031-9155/61/3/1171
231. Galeone C, Steinsberger T, Donetti M, Martire MC, Milian FM, Sacchi R, et al. Real-time delivered dose assessment in carbon ion therapy of moving targets. Phys Med Biol. (2024) 69:205001. doi: 10.1088/1361-6560/ad7d59
232. ESTRO. Priorities of ESTRO for engagement within the EURAMED framework (2025). Available online at: https://www.estro.org/About/Newsroom/Newsletter/Research-Projects/Priorities-of-ESTRO-for-engagement-within-the-EURA (Accessed April 09, 2025).
233. Su Z, Hsi WC, O’neil M, and Sabouri P. A comprehensive evaluation of an independent quality assurance system for intensity modulated proton therapy treatment plans and deliveries. Int J Radiat Oncol Biol Phys. (2024) 120:e589. doi: 10.1016/j.ijrobp.2024.07.1298
234. Meijers A, Jakobi A, Stützer K, Guterres Marmitt G, Both S, Langendijk JA, et al. Log file-based dose reconstruction and accumulation for 4D adaptive pencil beam scanned proton therapy in a clinical treatment planning system: Implementation and proof-of-concept. Med Phys. (2019) 46:1140–9. doi: 10.1002/mp.13371
235. Murr M, Brock KK, Fusella M, Hardcastle N, Hussein M, Jameson MG, et al. Applicability and usage of dose mapping/accumulation in radiotherapy. Radiother Oncol. (2023) 182:109527. doi: 10.1016/j.radonc.2023.109527
236. Nenoff L, Amstutz F, Murr M, Archibald-Heeren B, Fusella M, Hussein M, et al. Review and recommendations on deformable image registration uncertainties for radiotherapy applications. Phys Med Biol. (2023) 68:24TR01. doi: 10.1088/1361-6560/ad0d8a
237. Nenoff L, Ribeiro CO, Matter M, Hafner L, Josipovic M, Langendijk JA, et al. Deformable image registration uncertainty for inter-fractional dose accumulation of lung cancer proton therapy. Radiother Oncol. (2020) 147:178–85. doi: 10.1016/j.radonc.2020.04.046
238. Amstutz F, Nenoff L, Albertini F, Ribeiro CO, Knopf AC, Unkelbach J, et al. An approach for estimating dosimetric uncertainties in deformable dose accumulation in pencil beam scanning proton therapy for lung cancer. Phys Med Biol. (2021) 66:105007. doi: 10.1088/1361-6560/abf8f5
239. Wu X, Amstutz F, Weber DC, Unkelbach J, Lomax AJ, and Zhang Y. Patient-specific quality assurance for deformable IMRT/IMPT dose accumulation: Proposition and validation of energy conservation based validation criterion. Med Phys. (2023) 50:7130–8. doi: 10.1002/mp.16564
240. Ng Wei Siang K, Both S, Oldehinkel E, Langendijk JA, and Wagenaar D. Assessment of residual geometrical errors of clinical target volumes and their impact on dose accumulation for head and neck radiotherapy. Radiother Oncol. (2023) 188:109856. doi: 10.1016/j.radonc.2023.109856
241. Smolders A, Lomax A, Weber DC, and Albertini F. Deep learning based uncertainty prediction of deformable image registration for contour propagation and dose accumulation in online adaptive radiotherapy. Phys Med Biol. (2023) 68:245027. doi: 10.1088/1361-6560/ad0282
242. The RAPTOR consortium. Towards interventional particle therapy for advanced cancer care (2021). Available online at: https://raptor-consortium.com/raptor-white-paper/ (Accessed December 9, 2025).
243. ProtOnART – a new consortium for proton online adaptive radiation therapy (2022). Available online at: https://www.oncoray.de/news/article/protonart-a-new-consortium-for-proton-online-adaptive-radiation-therapy (Accessed December 9, 2025).
244. International Commission on Radiation Units and Measurements. ICRU report 78: Prescribing, recording, and reporting proton beam therapy (2007). Available online at: https://www.icru.org/report/prescribing-recording-and-reporting-proton-beam-therapy-icru-report-78/ (Accessed April 09, 2025).
245. Goudschaal K, Azzarouali S, Visser J, Admiraal M, Wiersma J, van Wieringen N, et al. Clinical implementation of RTT-only CBCT-guided online adaptive focal radiotherapy for bladder cancer. Clin Trans Radiat Oncol. (2025) 50:100884. doi: 10.1016/j.ctro.2024.100884
246. Beckert R, Schiff JP, Morris E, Samson P, Kim H, and Laugeman E. The impact of an Advanced Practice Radiation Therapist contouring for a CBCT-based adaptive radiotherapy program. Tech Innov Patient Support Radiat Oncol. (2024) 30:100242. doi: 10.1016/j.tipsro.2024.100242
247. Chetty IJ, Cai B, Chuong MD, Dawes SL, Hall WA, Helms AR, et al. Quality and safety considerations for adaptive radiation therapy: an ASTRO white paper. Int J Radiat OncologyBiologyPhysics. (2025) 122:838–64. doi: 10.1016/j.ijrobp.2024.10.011
248. Olsen DR, Bruland ØS, Frykholm G, and Norderhaug IN. Proton therapy – A systematic review of clinical effectiveness. Radiother Oncol. (2007) 83:123–32. doi: 10.1016/j.radonc.2007.03.001
249. Li M, Li X, Yao L, Han X, Yan W, Liu Y, et al. Clinical efficacy and safety of proton and carbon ion radiotherapy for prostate cancer: A systematic review and meta-analysis. Front Oncol. (2021) 11:709530. doi: 10.3389/fonc.2021.709530
250. Chen Z, Dominello MM, Joiner MC, and Burmeister JW. Proton versus photon radiation therapy: A clinical review. Front Oncol. (2023) 13:1133909. doi: 10.3389/fonc.2023.1133909
251. Zhou P, Du Y, Zhang Y, Zhu M, Li T, Tian W, et al. Efficacy and safety in proton therapy and photon therapy for patients with esophageal cancer. JAMA Netw Open. (2023) 6:e2328136. doi: 10.1001/jamanetworkopen.2023.28136
252. Press RH and Mehta MP. Proton therapy: current status and controversies. JCO Oncol Pract. (2024) 20:747–9. doi: 10.1200/op.24.00132
253. Efstathiou JA, Yeap BY, Michalski JM, Horick N, Zietman AL, Christodouleas JP, et al. Prostate advanced radiation technologies investigating quality of life (PARTIQoL): phase III randomized clinical trial of proton therapy vs. IMRT for localized prostate cancer. Int J Radiat Oncol Biol Phys. (2024) 120:S1. doi: 10.1016/j.ijrobp.2024.08.012
254. Frank SJ, Busse P, Rosenthal DI, Hernandez M, Swanson DM, Garden AS, et al. Phase III randomized trial of intensity-modulated proton therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for the treatment of head and neck oropharyngeal carcinoma (OPC). JCO. (2024) 42:6006–6. doi: 10.1200/jco.2024.42.16_suppl.6006
255. McComas KN, Yock A, Darrow K, and Shinohara ET. Online adaptive radiation therapy and opportunity cost. Adv Radiat Oncol. (2023) 8:101034. doi: 10.1016/j.adro.2022.101034
256. Borderías-Villarroel E, Barragán-Montero A, and Sterpin E. Time is NTCP: Should we maximize patient throughput or perform online adaptation on proton therapy systems? Radiother Oncol. (2024) 198:110389. doi: 10.1016/j.radonc.2024.110389
257. Oud M, Breedveld S, Giżyńska M, Chen YH, Habraken S, Perkó Z, et al. Dosimetric advantages of adaptive IMPT vs. Enhanced workload and treatment time – A need for automation. Radiother Oncol. (2024) 201:110548. doi: 10.1016/j.radonc.2024.110548
258. Thomsen SN, Møller DS, Knap MM, Khalil AA, Shcytte T, and Hoffmann L. Daily CBCT-based dose calculations for enhancing the safety of dose-escalation in lung cancer radiotherapy. Radiother Oncol. (2024) 200:110506. doi: 10.1016/j.radonc.2024.110506
259. Rhee DJ, Beddar S, Jaoude JA, Sawakuchi G, Martin R, Perles L, et al. Dose escalation for pancreas SBRT: potential and limitations of using daily online adaptive radiation therapy and an iterative isotoxicity automated planning approach. Adv Radiat Oncol. (2023) 8:101164. doi: 10.1016/j.adro.2022.101164
260. Ermongkonchai T, Khor R, Muralidharan V, Tebbutt N, Lim K, Kutaiba N, et al. Stereotactic radiotherapy and the potential role of magnetic resonance-guided adaptive techniques for pancreatic cancer. World J Gastroenterol. (2022) 28:745–54. doi: 10.3748/wjg.v28.i7.745
261. Bainbridge HE, Menten MJ, Fast MF, Nill S, Oelfke U, and McDonald F. Treating locally advanced lung cancer with a 1.5 T MR-Linac – Effects of the magnetic field and irradiation geometry on conventionally fractionated and isotoxic dose-escalated radiotherapy. Radiother Oncol. (2017) 125:280–5. doi: 10.1016/j.radonc.2017.09.009
262. MacDonald RL, Fallone C, Chytyk-Praznik K, Robar J, and Cherpak A. The feasibility of CT simulation-free adaptive radiation therapy. J Appl Clin Med Phys. (2024) 25: e14438. doi: 10.1002/acm2.14438
263. de Leon J, Jelen U, Carr M, Crawford D, Picton M, Tran C, et al. Adapting outside the box: Simulation-free MR-guided stereotactic ablative radiotherapy for prostate cancer. Radiother Oncol. (2024) 200:110527. doi: 10.1016/j.radonc.2024.110527
Keywords: cancer, proton therapy, online adaptive proton therapy, near real-time adaptation, volumetric imaging, online plan optimization, online QA, treatment verification
Citation: Gambetta V, Stützer K and Richter C (2025) Current status and upcoming developments for online adaptive proton therapy enabling a closed feedback loop for near real-time adaptation. Front. Oncol. 15:1660605. doi: 10.3389/fonc.2025.1660605
Received: 06 July 2025; Accepted: 17 November 2025; Revised: 10 November 2025;
Published: 17 December 2025.
Edited by:
Konrad P. Nesteruk, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Mirek Fatyga, Mayo Clinic Arizona, United StatesPeilin Liu, William Beaumont Hospital, United States
Mislav Bobić, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2025 Gambetta, Stützer and Richter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Richter, Y2hyaXN0aWFuLnJpY2h0ZXJAb25jb3JheS5kZQ==
 Virginia Gambetta
Virginia Gambetta Kristin Stützer
Kristin Stützer Christian Richter
Christian Richter