- 1Federal Institute for Population Research Das Bundesinstitut für Bevölkerungsforschung (BiB), Wiesbaden, Germany
- 2Department of Pharmaceutics, Xiangya School of Pharmaceutical Sciences, Central South University, Changsha, Hunan, China
- 3Department of Immunology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia
- 4Department of Biology, Faculty of Education, Omdurman Islamic University, Omdurman, Sudan
Editorial on the Research Topic
Oxidative stress in breast cancer
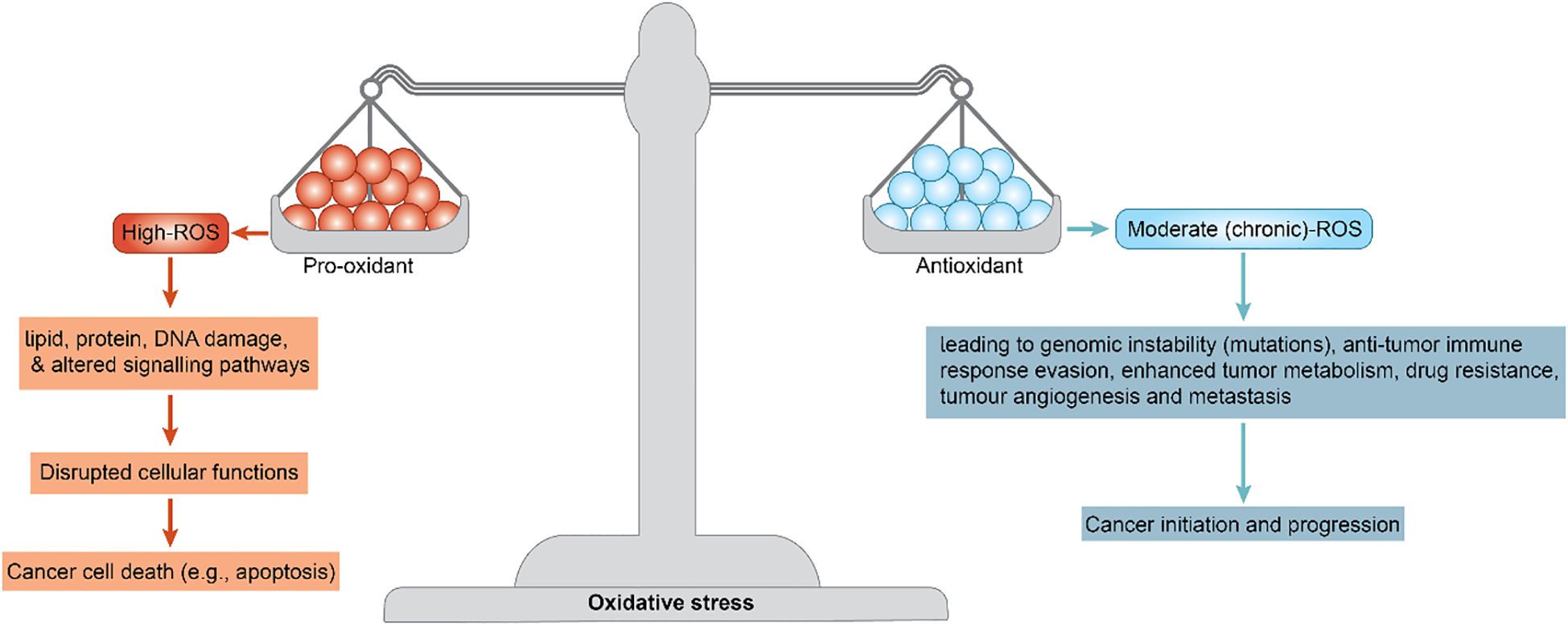
Breast cancer is one of the most prevalent and challenging malignancies worldwide. In addition to well-characterized genetic and hormonal drivers, there is increasing recognition that oxidative stress plays a pivotal role in breast cancer biology (1). Excess reactive oxygen species (ROS) can cause lipid, protein, and DNA damage. It can also alter signaling pathways and disrupt cellular functions, leading to cancer cell death (e.g., apoptosis) via oxidative stress imbalance. Moderate (chronic) ROS levels can contribute to cancer initiation, leading to genomic instability (mutations), an anti-tumor immune response, enhanced tumor metabolism, drug resistance, tumor angiogenesis, and metastasis (Figure 1) (2, 3).
Oxidative stress may be considered to play a “double-edged sword role”; while chronic ROS exposure can promote malignancy in cancers, acute or high ROS bursts can be cytotoxic to cancer cells and are exploited by many therapies.
This Research Topic’s five papers illustrate diverse facets of oxidative stress in breast cancer ―from immune modulation and drug resistance to therapy side effects and biomarkers―and are summarized below.
A review, entitled From Immunogenic Cell Death to Immunogenic Modulation: Select Chemotherapy Regimens Induce a Spectrum of Immune-enhancing Activities in the Tumor Microenvironment <investigated the mechanisms by which specific chemotherapeutic agents promote anti-tumor immunity through the induction of immunogenic cell death (ICD) or immunogenic modulation in cancer cells. Fabian et al. propose the novel concept of “immunogenic cell stress” as a pivotal paradigm within this field of enquiry. The authors posit that this oxidative immunostimulation can be leveraged by combining standard chemotherapies (e.g., anthracyclines) with immunotherapy to capitalize on immunogenic cell stress. The objective of this approach is to convert “cold” tumors into “hot” ones from an immunological perspective by leveraging therapy-induced oxidative stress.
The study Integrative Dissection of 5-hydroxytryptamine Receptors (5-HTRs; HTR1A, HTR1B, HTR2A, HTR2B, HTR2C, HTR4, and HTR7)-related Signature in the Prognosis and Immune Microenvironment of Breast Cancer examined how chronic psychological stress (depression) might influence breast cancer via 5-hydroxytryptamine (5-HT) receptors. Among the receptors, the expression of HTR2A/2B exhibited a positive correlation with the infiltration of immune cells, including CD8+ T cells and macrophages. Moreover, the inhibition of HTR2A expression may reduce CD8+ T cell proliferation while promoting the invasion and metastasis of breast cancer cells in zebrafish and mouse models. The findings indicate that HTR2A/2B may significantly modulate the tumor microenvironment, thereby influencing cancer progression. These findings also support the hypothesis that depression is associated with worse breast cancer outcomes, potentially through stress-related inflammation and oxidative stress.
Zhan et al. identified a prognostic tumor gene signature centered on HTR2A and HTR2B (serotonin receptor genes). HTR2A/B expression was reduced in more aggressive cancers, and higher levels of these receptors correlated with better recurrence-free survival and greater T-cell infiltration in tumors. The loss of HTR2A/B signaling resulted in weaker immunity and more metastasis in experimental models. While the study did not measure oxidative stress directly, the results suggest that robust serotonin signaling may counteract the harmful effects of chronic stress, which often elevates oxidative stress and suppresses immunity.
Clinically, this implies that treating depression or modulating 5-HT pathways could benefit breast cancer patients by preserving an effective anti-tumor immune environment.
The mini review Targeting Ferroptosis: A Promising Strategy to Overcome Drug Resistance in Breast Cancer emphasized the pathway of ferroptosis as a strategy to overcome breast cancer drug resistance. Ferroptosis is a form of cell death driven by iron-dependent lipid peroxidation and toxic ROS accumulation. Many drug-resistant cancer cells evade apoptosis but remain susceptible to this oxidative form of death. Peng et al. note that drug-resistant breast tumor cells often upregulate antioxidant systems (glutathione, GPX4, etc.) to avoid ferroptosis. Disabling these defenses can re-sensitize tumors: for example, blocking cystine uptake or inhibiting GPX4 unleashes lethal lipid peroxidation in chemo-resistant cells. Inducing ferroptosis in models can eliminate therapy-resistant cells and reduce tumors. Therapies that trigger ferroptosis (e.g. small-molecule inhibitors or nanoparticle-delivered agents) could be combined with standard treatments to eradicate residual disease. Selective induction of ferroptosis in tumors is challenging, but this strategy exploits oxidative stress to circumvent drug resistance.
The study Effect of Fangxia-Dihuang Decoction on Doxorubicin-induced Cognitive Impairment in Breast Cancer Animal Model addressed the issue of chemo brain in a breast cancer mouse model. Doxorubicin (DOX) caused cognitive deficits in mice, with elevated oxidative stress markers and inflammatory cytokines in the brain. Wang et al. tested Fangxia-Dihuang Decoction (FXDH), a traditional herbal medicine, as a neuroprotective treatment during DOX treatment. Mice receiving FXDH performed better on memory tests and had lower levels of lipid peroxidation and inflammation compared to DOX-only controls.
FXDH boosted antioxidant defenses (e.g. GSH, SOD) and reduced neuroinflammation in the hippocampus, preventing DOX-induced neuronal damage. Oxidative stress and inflammation are key drivers of chemo brain and counteracting them can preserve cognitive function. Antioxidant and anti-inflammatory supplements (like components of FXDH) could mitigate cognitive side effects of chemotherapy. Such interventions might improve patients’ quality of life and treatment tolerance by reducing oxidative damage in the nervous system.
The study Association between Neutrophil-lymphocyte Ratio and Female Breast Cancer: An observational study from the NHANES 2001–2018 investigated the neutrophil-to-lymphocyte ratio (NLR), a systemic inflammation marker, in relation to breast cancer. Xiong et al. found that higher NLRs were associated with greater odds of breast cancer. Women in the highest NLR quartile had approximately 67% higher odds of breast cancer than those in the lowest quartile (OR ~1.67). This trend held across subgroups and was confirmed in an external cohort, indicating a robust link between NLR and breast cancer. An elevated NLR suggests an immune system biased toward a neutrophil-driven inflammatory response. Neutrophils release ROS and other mediators, creating a pro-oxidative, tumor-promoting environment. This supports the idea that chronic inflammation and oxidative stress can facilitate cancer development. NLR is an easy, cost-effective biomarker that could help identify individuals with high inflammatory status for monitoring or preventive measures.
This comprehensive body of research demonstrates that oxidative stress plays a dual role in the development of breast cancer. While excess ROS and inflammation can drive tumor growth and spread and treatment resistance, these pathways can also be targeted for therapy. Strategies to mitigate oxidative stress and inflammation, such as stress relief and tissue preservation, can improve outcomes and quality of life.
Strategies to augment oxidative stress within tumors, such as inducing ferroptosis or leveraging immunogenic cell stress, have the potential to enhance cell killing. The potential for reducing oxidative damage while exploiting tumor-specific vulnerabilities is significant.
Future therapeutic interventions will integrate antioxidant and pro-oxidant strategies to boost treatment efficacy while minimizing damage to normal cells.
Author contributions
RW: Writing – review & editing, Writing – original draft. ZL: Writing – review & editing. RM: Writing – review & editing. AM: Conceptualization, Writing – review & editing, Writing – original draft, Validation, Visualization.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mussa A, Hamid M, Hajissa K, Murtadha AH, Al-Hatamleh MAI, Mokhtar NF, et al. Pharmacological Vitamin C-induced high H2O2 generation mediates apoptotic cell death by caspase 3/7 activation in breast cancer tumor spheroids. J Trans Med. (2025) 23:31. doi: 10.1186/s12967-024-06016-7
2. Mussa A, Mohd Idris RA, Ahmed N, Ahmad S, Murtadha AH, Tengku Din TADAA, et al. High-dose vitamin C for cancer therapy. Pharm (Basel). (2022) 15:711. doi: 10.3390/ph15060711
Keywords: oxidative stress, ROS - reactive oxygen species, breast cancer, vitamin C, antioxidant, pro-oxidant
Citation: Westerman R, Liu Z, Mohamud R and Mussa A (2025) Editorial: Oxidative stress in breast cancer. Front. Oncol. 15:1661039. doi: 10.3389/fonc.2025.1661039
Received: 07 July 2025; Accepted: 09 September 2025;
Published: 16 September 2025.
Edited and reviewed by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesCopyright © 2025 Westerman, Liu, Mohamud and Mussa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronny Westerman, cm9ubnkud2VzdGVybWFuQGJpYi5idW5kLmRl
 Ronny Westerman
Ronny Westerman Zhenbao Liu
Zhenbao Liu Rohimah Mohamud
Rohimah Mohamud Ali Mussa
Ali Mussa