- 1Senior Department of Cardiology, the Sixth Medical Center of PLA General Hospital, Beijing, China
- 2National Clinical Research Center for Otolaryngologic Diseases, Beijing, China
- 3Beijing Key Laboratory of Hearing Impairment Prevention and Treatment, Beijing, China
- 4Senior Department of Otolaryngology Head and Neck Surgery, People's Liberation Army General Hospital, Beijing, China
Background: To derive and validate a prognostic nomogram for predicting postoperative recurrence in patients with glomus jugulare tumor(GJT) to assist clinical decision-making.
Methods: A retrospective analysis was conducted on the clinical data of a total of 318 patients diagnosed with GJT at a single tertiary medical center. The study collected information on patient demographics, clinical symptoms and signs, examination results, and the extent of tumor growth. Patients were categorized into two groups based on DFS (Disease - free survival): those who experienced recurrence and those who did not. A nomogram model was developed using logistic regression to analyze the risk of postoperative recurrence.
Results: Multivariate logistic regression analysis identified age, immunohistochemical expression levels of Ki-67 and S-100 and tumor invasion extent were significantly associated as independent predictors. These independent predictors were incorporated into a nomogram. The logistic regression-based nomogram showed excellent predictive accuracy of the nomogram model in the training set, validation set, and test set, with corresponding areas under the curve (AUC) of 0.863, 0.711, and 0.784, respectively.
Conclusions: The nomogram effectively predicts GJT recurrence, validated internally and externally, aiding clinical risk stratification.
Introduction
Surgical resection is the primary treatment for tympanojugular paragangliomas(TJPGLs), which account for nearly 40% of head and neck paragangliomas (HNPGLs). These tumors may severely affect various cranial nerves and cause significant clinical manifestations. However, the majority of patients achieve long-term disease-free survival following surgery. Studies indicate a recurrence rate of approximately 6.5% within 5 years following definitive treatment, with nearly one-third of patients experiencing recurrence more than 10 years after initial surgery (1, 2).
Currently, neither the NCCN (National Comprehensive Cancer Network) nor CSCO (Chinese Society of Clinical Oncology) guidelines provide dedicated protocols for TJPGLs management. Relevant recommendations are dispersed within broader guidelines for head and neck cancers or neuroendocrine tumors. These guidelines lack clarity on whether adjuvant radiotherapy (RT) reduces recurrence rates in the overall HNPGL population (a point of ongoing debate) and fail to define strategies for identifying high-risk patients or implementing appropriate postoperative interventions. Existing evidence suggests adjuvant RT may prolong DFS in patients with near-total resection or those at high risk of recurrence (3–5).Nevertheless, the potential risks of local discomfort, pharyngeal edema, and neurological injury associated with RT must be considered. Consequently, the precise identification of high-risk recurrence patients for adjuvant RT holds promise for reducing overall recurrence rates while minimizing overtreatment, thereby advancing precision medicine objectives.
Therefore, there is a critical need for effective recurrence prediction models. Existing histopathological scoring systems (e.g., PASS, GAPP, M-GAPP) exhibit suboptimal sensitivity and specificity (6, 7). Notably, the latest classification system groups pheochromocytomas (PHEOs) and sympathetic paragangliomas (PGLs) under a unified staging scheme. Recent analyses comparing historical data for PHEOs and PGLs (1, 2, 8–11) have revealed distinct recurrence risk profiles between these entities. This indicates that while prognostic factors for HNPGLs may overlap with those of systemic PGLs, they likely possess unique characteristics, underscoring the necessity for dedicated research to elucidate HNPGL-specific prognostic determinants.
TJPGLs is often carried out according to the Fisch or Glasscock–Jackson (12) classification system. To avoid the bias of Tympanic ball paragangliomas (TBPs) in Fisch A and B grades, all patients in this study were classified as glomus jugulare tumor in Fisch C and D grades, which are of glomus jugulare origin. This study analyzed clinical data from 318 patients, identifying independent predictors of recurrence and integrating them into a visual nomogram model. The model demonstrated robust predictive performance across training, validation, and test datasets. It provides clinicians with an intuitive and practical tool for individualized recurrence risk assessment. This facilitates the optimization of postoperative management strategies, including the identification of candidates for adjuvant RT and the design of tailored surveillance protocols, ultimately promoting precision medicine in the postoperative care of GJT patients.
Materials and methods
This study retrospectively analyzed patients with GJT diagnosed in a tertiary medical centers between January 2003 and December 2022, confirmed by imaging and pathology. The inclusion criteria were: (1) Surgical treatment; (2) 2 years of follow-up data; (3) Complete records; (4) Glomus jugulare region; (5) Gross total resection (GTR: no visible tumor residue) or subtotal resection (STR: <5% residual tumor). The exclusion criteria were: (1) Preoperative radio/chemotherapy; (2) No surgery; (3) Incomplete data; (4) Partial resection; (5) Postoperative adjuvant therapy.
The 277 patients treated from January 2003 to December 2020 were randomly divided into a training set (70%, 194 patients) and a validation set (30%, 83 patients). The remaining 41 patients treated from January 2021 to December 2022 were assigned to the test set. All patients had a 2-year postoperative follow-up to monitor tumor recurrence. Ethical approval was granted by the Ethical Committee of a tertiary referral center.
For each patient, the following data were extracted from the database: (1) Patient characteristics, namely sex, age, clinical symptoms, disease duration, perioperative patient status, and intraoperative data. (2) Radiological data: computed tomography (CT) scans, magnetic resonance imaging (MRI) and digital subtraction angiography (DSA). (3) Intraoperative data: specifically operative time, blood loss, tumor growth patterns, and relationships with surrounding bone and nerves were assessed using imaging, surgical videos, and operative records. (4) Postoperative data: length of hospitalization, and DFS at ≥2 years from the date of surgery, were collected. All surgeries were performed by a single surgical team.
Categorical variables are expressed as frequencies and percentages, and quantitative variables as means ± standard deviations. Associations between variables and recurrence were analyzed using Chi-square or Mann-Whitney U tests (significance threshold: p ≤ 0.05) according to the data type. The identified factors were incorporated into a binary logistic regression model. Factors with a significance level less than 0.05 were included in the nomogram model construction. For variables that were not significant (i.e., with a significance level greater than 0.05), stepwise regression (bidirectional) was used to select modeling variables. For the binary logistic regression, the p-values, odds ratios(OR), and 95% confidence intervals (CIs) of independent risk factors were provided. The model fit was assessed using the Hosmer-Lemeshow goodness-of-fit test, and the model fit situation was observed.
Statistical analyses were performed using SPSS 25.0 (IBM SPSS Statistics) and R software (version 4.4.2). The t test was used to compare continuous variables, while the χ2 test was used for categorical variables. Univariable and multivariable logistic regression analyses were conducted to identify variables potentially associated with postoperative recurrence. Binary logistic regression was used for all predictive modeling of recurrence outcomes. Statistical significance was defined as P < 0.05. The predictive performance across the training set, test set, and validation set was evaluated using the area under the receiver operating characteristic curve (AUC-ROC). The statistical significance threshold for this study was set at p ≤ 0.05 (two-tailed).
Results
This study enrolled a total of 318 patients meeting the inclusion criteria. All patients underwent GTR or STR during surgery and were followed up for over 2 years postoperatively. The recurrence criteria for PGLs were further refined as follows: local recurrence (disease in non-chromaffin-derived tissues within the primary tumor resection area), distant metastasis (disease in non-chromaffin-derived tissues outside the primary tumor region, such as bone metastasis), or new primary/uncertain (disease in chromaffin-derived tissues outside the primary tumor area, such as CBT or VGPL). Local recurrence is defined as pathologically confirmed tumor regrowth at the original site during post-operative follow-up, meeting growth rate criteria (volume increase ≥20% or annual diameter increase ≥2-3 mm) (5, 13). Based on these criteria, 68 patients (21.4%) experienced postoperative recurrence, with 3 patients undergoing surgical treatment more than three times. Among the entire cohort, the mean follow-up period was 8.7 years with a range of 2 to 22 years.
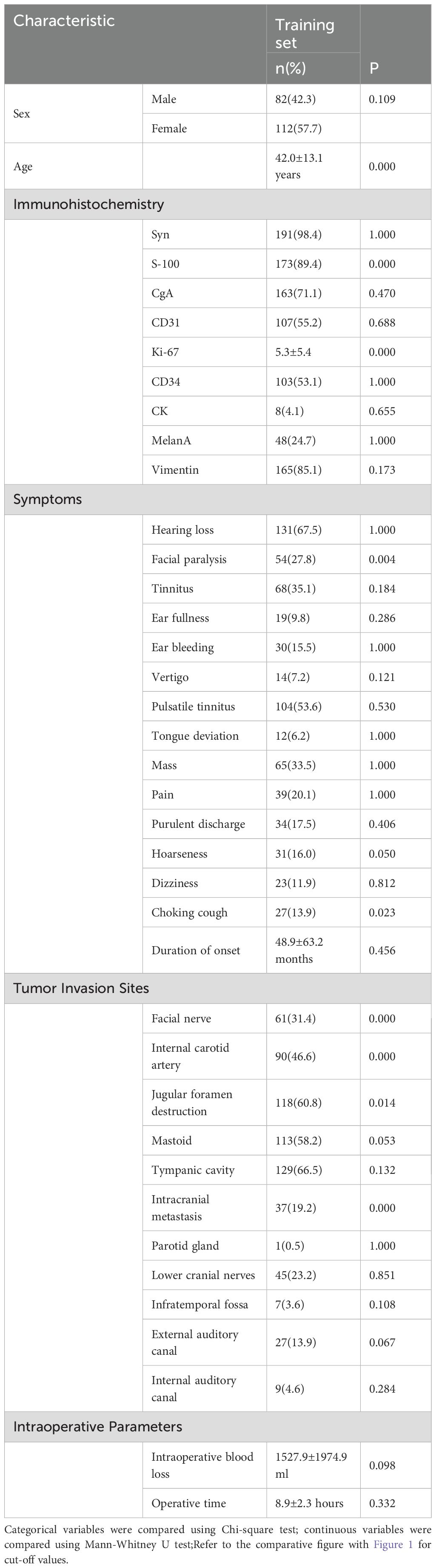
Drawing on previous literature and considering the characteristics and anatomical specificity of GJT, potential factors influencing postoperative recurrence of systemic PGLs were screened and included in this study. Among the 194 patients in the training set, chi-square tests were performed for categorical variables and Mann-Whitney U tests for continuous variables. Variables with p ≤ 0.05 were seen as linked to recurrence. The clinical characteristics of patients are as follows. (Table 1) (Figure 1).
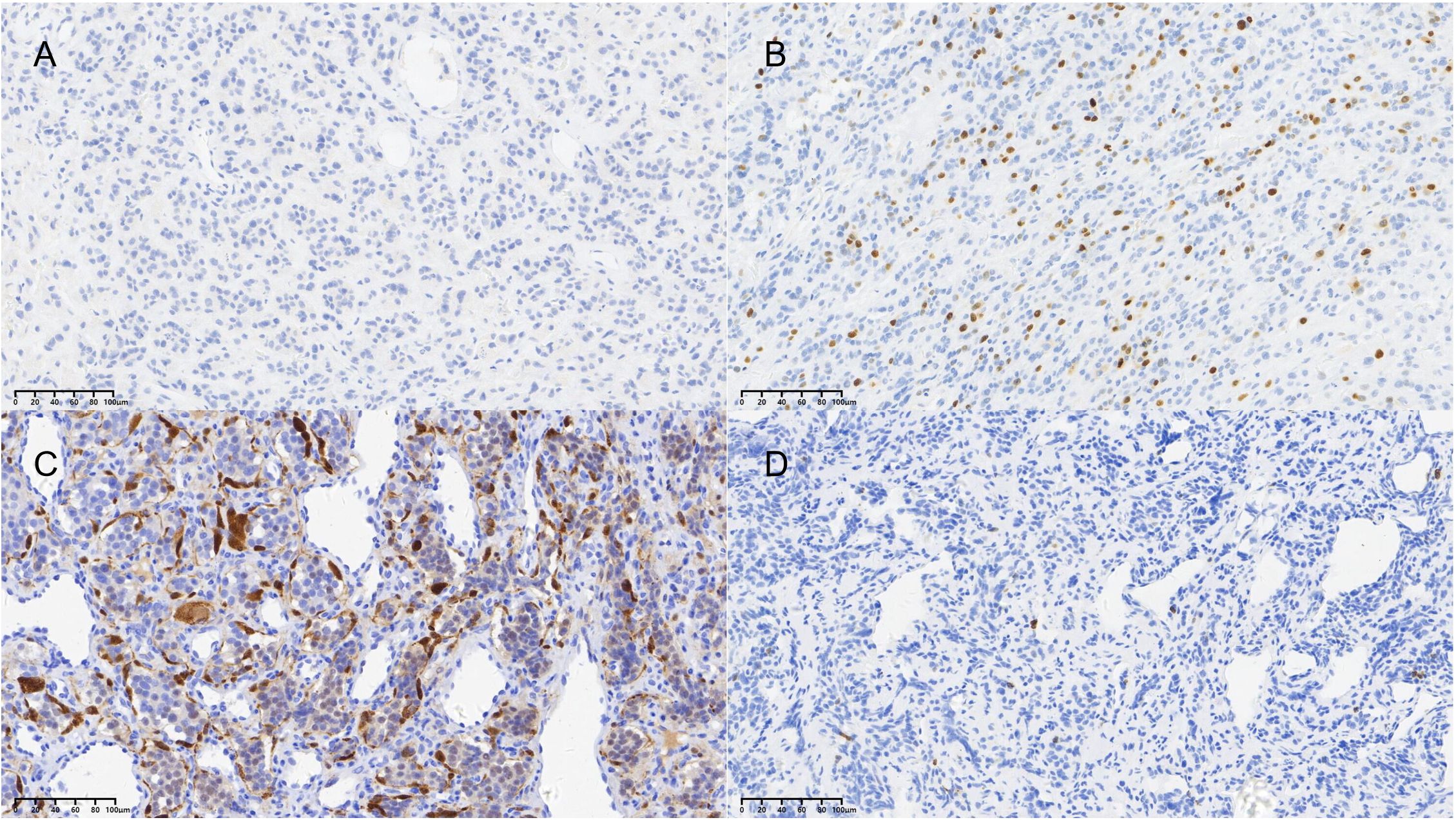
Figure 1. Representative immunohistochemical staining of key prognostic markers. (A) Positive nuclear staining for Ki-67 (brown chromogen, hematoxylin counterstain; original magnification ×20). (B) Positive staining for S-100 protein highlighting sustentacular cells (brown chromogen, hematoxylin counterstain; original magnification ×20). The graduated mark represents the actual magnification scale for the pathological tissue section.
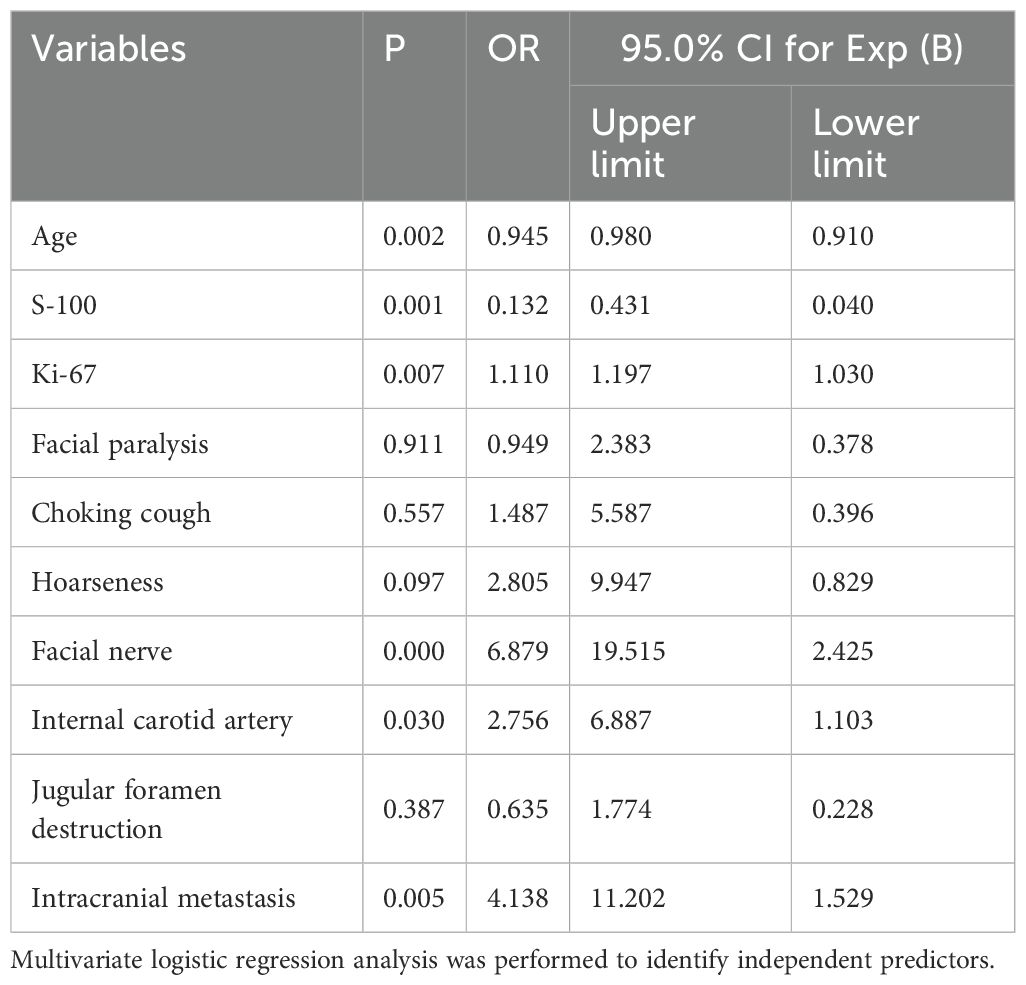
Univariate and binary logistic regression analyses were performed. Univariate analysis of 194 patients identified variables with P < 0.05, which were subsequently included in the logistic regression analysis. The results demonstrated that immunohistochemistry findings, clinical symptoms, and tumor invasion patterns were significantly associated with postoperative recurrence. (Table 2).
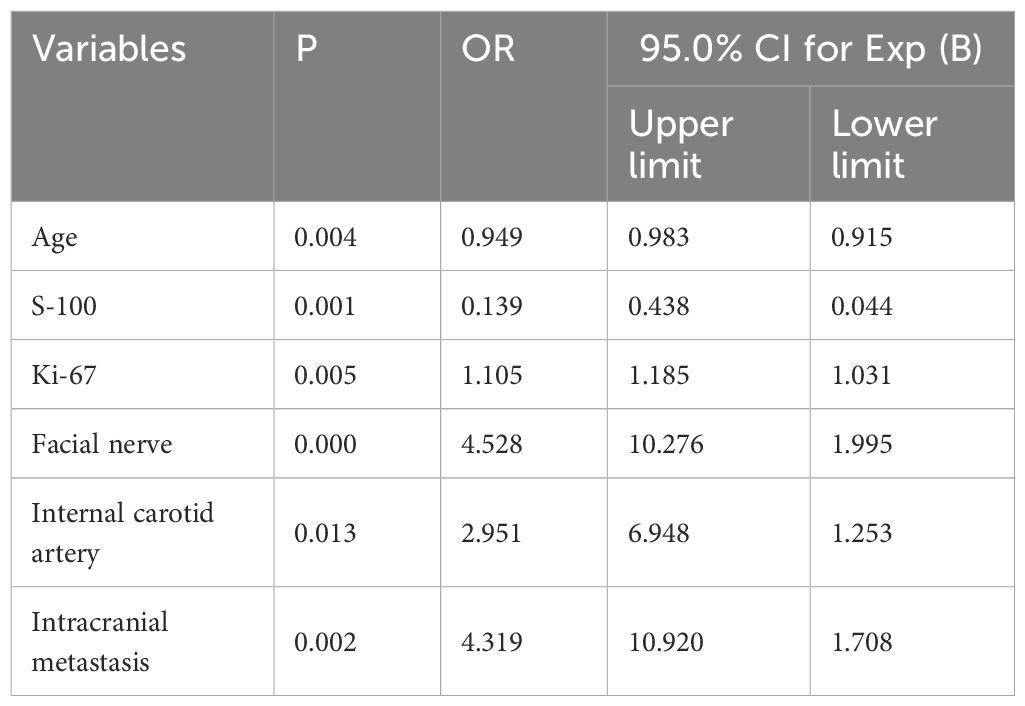
Stepwise regression (forward and backward) further optimized variable selection for modeling, identifying the final independent variables included in the model. (Table 3).
A nomogram was established based on logistic regression analysis, showing the weight of each included variable as a prognostic factor for recurrence in GJT patients after surgery. (Figure 2) In the nomogram, age and Ki-67 are included as continuous variables, while tumor invasion sites are incorporated as categorical variables. Patients with intracranial metastasis and negative S-100 expression had significantly higher odds of recurrence. The calibration plots demonstrated satisfactory consistency between the nomogram-predicted and actual observed values (Figure 3). The model showed good performance in both low-probability and high-probability predictions. Moderate deviations between observed and predicted probabilities were noted in the intermediate probability range (approximately 0.3-0.7).
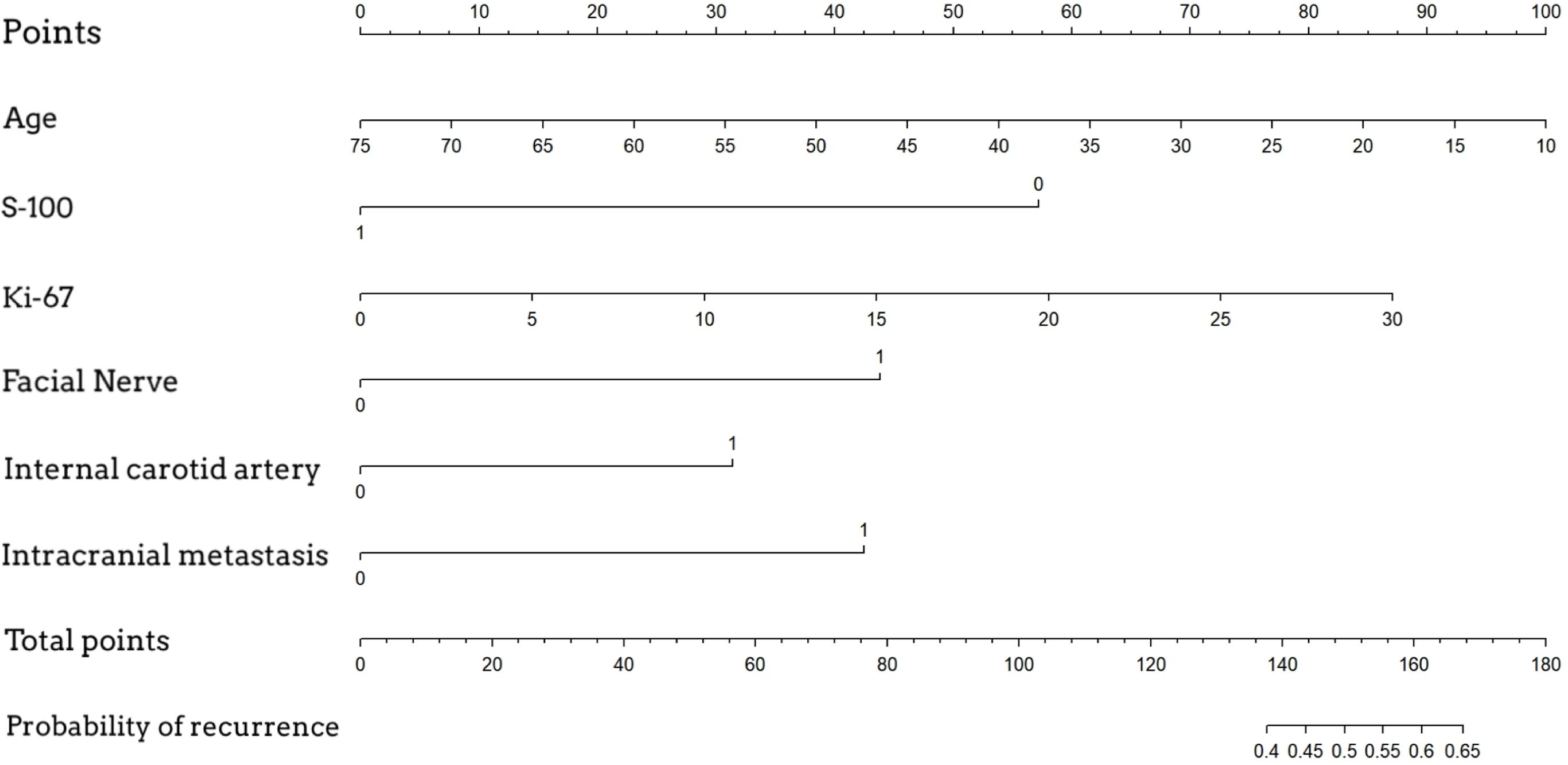
Figure 2. Nomogram for predicting postoperative recurrence of glomus jugulare tumors. To use the nomogram, locate the patient's value for each variable, draw a line upward to the 'Points' axis to determine the score for each variable, sum all the points, and then locate the total points on the 'Total Points' axis. A line drawn downward to the 'Risk of Recurrence' axis will indicate the individual's estimated probability of recurrence.
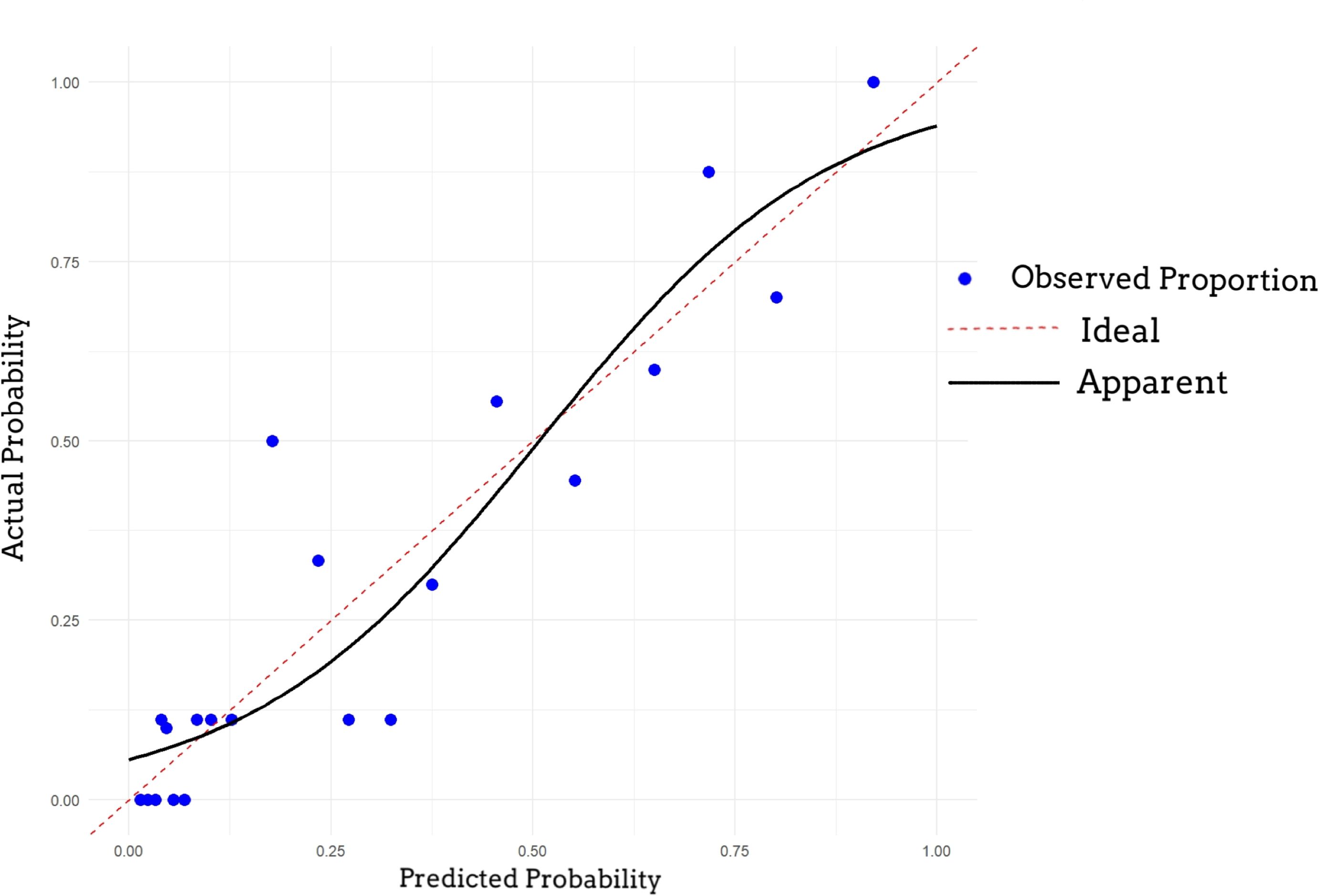
Figure 3. Calibration curve of the nomogram. The 45-degree dotted line represents the ideal prediction. The solid line represents the performance of the nomogram, where a closer fit to the diagonal indicates better calibration between predicted probability and observed outcome.
The data of various influencing factors from the validation and test sets (Table 4) are incorporated into the model for validation.
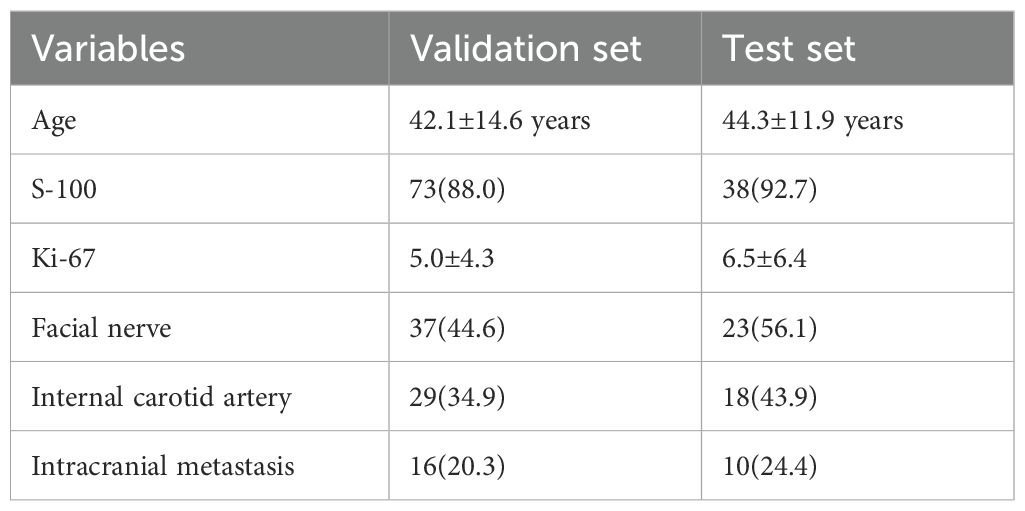
Table 4. Number of Positive Cases or Mean Values of Predictive Factors in the Validation and Test Sets.
The ROC curves demonstrated acceptable predictive accuracy for postoperative recurrence in the training set (AUC: 0.863; Figure 4A), validation set (AUC: 0.711; Figure 4B), and test set (AUC: 0.784; Figure 4C).
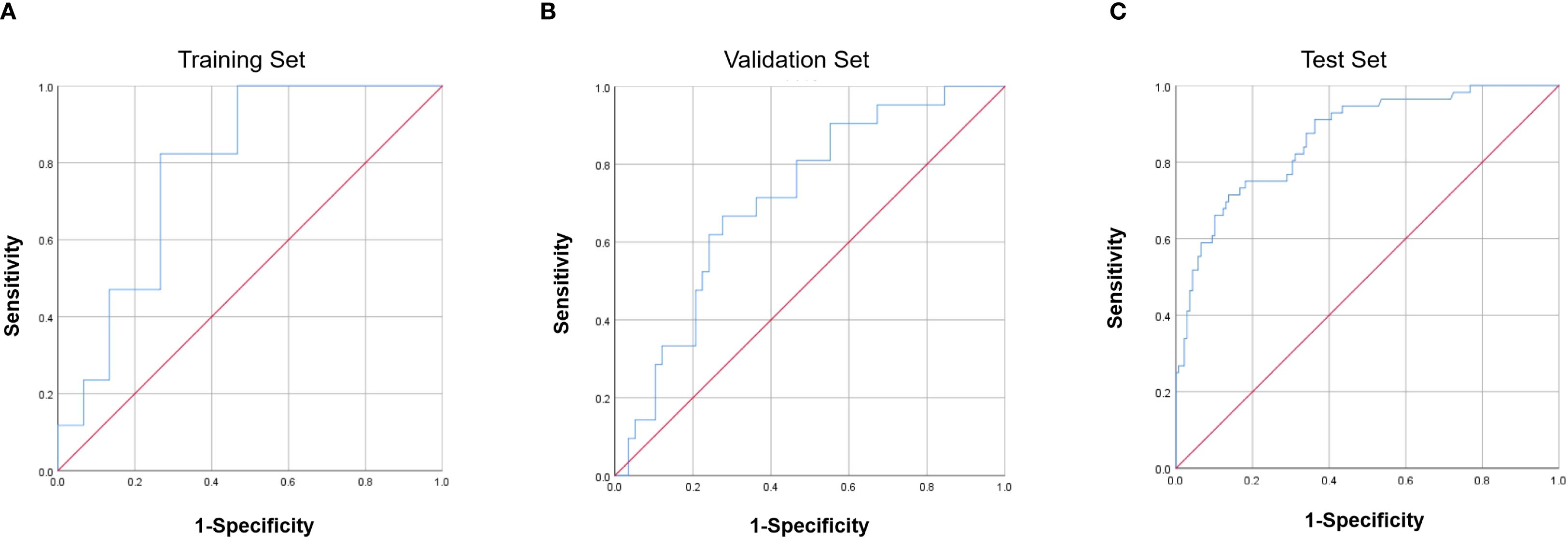
Figure 4. Receiver operating characteristic (ROC) curves of the nomogram. (A) Training set (AUC = 0.863). (B) Validation set (AUC = 0.711). (C) Test set (AUC = 0.784). AUC, area under the curve.
Discussion
In 2017, the World Health Organization (WHO) renamed the “malignant” nomenclature as “metastatic”, and the terms “benign” and “malignant” were abandoned. The most recent 2022 WHO classification suggests treating all tumors as malignant lesions with variable metastatic potential (14), highlighting their aggressiveness and high risk of recurrence.
This study is the first to conduct large-scale data collection and systematic analysis of patients treated with gross total resection or near-total resection alone. We comprehensively summarized disease-related therapeutic factors, developed a recurrence-predicting nomogram model based on prognostic predictors, and validated its stability and accuracy using an independent validation set and an test dataset. These findings provide actionable insights to guide clinical decision-making in the management of GJT.
With a mean follow-up of 8.7 years post-surgery, this study observed a recurrence rate of 21.4% demonstrated the highest recurrence risk among HNPGL, aligning with established findings. Among recurrent cases, 3 patients underwent surgery alone more than three times, with 4 developing local or distant metastases detected during subsequent treatments.
Our analysis identified age, immunohistochemical markers (S-100 and Ki-67), tumor invasion of the facial nerve or internal carotid artery, and intracranial metastasis as independent predictors of postoperative recurrence. Notably, the tumor invasion site showed significant associations in both univariate and multivariate analyses and contributed high weight in the nomogram model.
Studies on systemic PGLs (15) and GJT have shown a significant correlation between younger age and increased risks of both local recurrence and distant metastasis. In the study by Richter et al. (16) age was dichotomized at 42 years as a predictive factor, revealing elevated recurrence probabilities in patients younger than 42, though youth itself was not an independent predictor of recurrence. Consistent with these findings, our study confirms that younger patients are more susceptible to postoperative recurrence and incorporates age as a continuous variable in the nomogram.
Previous studies predominantly incorporated tumor volume as a predictor of recurrence. However, the complexity of anatomical locations and inherent inaccuracies in manual measurements may confound research outcomes and model development. To address these limitations, our study focused on surgically relevant factors by integrating tumor invasion sites into the predictive framework, thereby minimizing measurement bias and establishing a more direct correlation with intraoperative decision-making. The nomogram revealed that patients with intracranial invasion, carotid artery adhesion, or facial nerve invasion remained at high recurrence risk even after near-total resection. Facial nerve involvement emerged as secondary predictors. We hypothesize that preoperative irreversible neural deficits (House-Brackmann grade VI facial paralysis) may prompt surgeons to resect or reconstruct severely adherent nerves, whereas preserved preoperative function (House-Brackmann grade II-III facial weakness) often leads to nerve preservation despite intraoperative adhesions. This conservative approach risks residual superficial tumor tissue (“near-total resection”), potentially elevating recurrence likelihood.
Carotid artery adhesion is one of the factors affecting recurrence and is directly related to intraoperative bleeding. If not properly managed, it may lead to severe intracranial ischemic lesions in patients with poor contralateral blood supply compensation after surgery, resulting in serious consequences such as hemiplegia. Therefore, it is essential for patients to undergo preoperative angiography. According to the study by Andrea Bacciu et al (17), preoperative endovascular intervention, in selected cases, facilitates gross total tumor removal and significantly reduces the risk of intraoperative ICA injury. For these highly vascularized lesions, preoperative carotid artery stenting or permanent preoperative balloon occlusion is a key step (18). However, massive bleeding from the carotid artery and its branches during tumor resection remains a key factor in severe surgical complications and mortality.
In this study, Ki-67 emerged as a significant predictor of recurrence in GJT. Existing research on PGLs presents conflicting perspectives. For instance, Thompson et al. (6) argued that the Ki-67 index is not an isolated or independent predictive factor but interacts with clinical and histopathological features to collectively influence patient prognosisIn contrast, Yunying Cui et al. identified Ki-67 ≥3% as an independent predictor of pheochromocytoma and paraganglioma (PPGL) recurrence,Yiming Ding (19), in a smaller cohort, reported that Ki-67 exhibited excessive variability and lacked prognostic significance. In our study, among 52 patients with Ki-67 >10%, 22 experienced postoperative recurrence, and Ki-67 was significantly associated with recurrence risk (P = 0.008). These findings align with the widely adopted Pheochromocytoma of the Adrenal Gland Scaled Score (PASS) system—originally developed for systemic PGLs—which posits that Ki-67 reflects tumor proliferative activity and aggressiveness. Elevated Ki-67 indices may indicate heightened cellular proliferation, thereby increasing risks of recurrence or disease progression.
The S-100 protein family plays critical roles in cellular processes such as proliferation, apoptosis, differentiation, calcium ion homeostasis, and inflammation, and is implicated in various cancers (20). Deficiency of S-100 proteins can lead to tumor recurrence through multiple mechanisms, including upregulation of the fibroblast growth factor (FGF) pathway in tumor cells (21), alterations in cellular morphology and mechanical properties to enhance invasiveness, suppression of p53 function to promote proliferation and survival, and modulation of calcium ion balance, which may disrupt physiological functions such as cell adhesion, migration, and proliferation (22). Previous studies emphasize (23), that sustentacular cells in PGLs typically express S-100 and constitute a structural component of these tumors. Notably, as tumor malignancy increases, S-100 protein expression in sustentacular cells often becomes negative, indicating enhanced invasive potential. Researchers such as Charlie (24), Ling-Ling Wang (25), Caipu (23) have incorporated S-100 expression into scoring systems for evaluating PGLs recurrence risk. The findings of this study align with prior research, suggesting that the mechanism by which S-100 loss drives tumor recurrence in GJT may parallel that observed in PGLs at other anatomical sites. However, further experimental validation is required to confirm these molecular pathways.
Based on the analysis of the aforementioned predictive factors, the independent variables were incorporated into a nomogram model to develop a clinically applicable predictive model, and its performance was rigorously evaluated. As shown in Figure 3, the model demonstrated good fit with clinical data from the past 18 years and exhibited reasonable accuracy. However, the predictive accuracy for the recent 2-year testing dataset was slightly lower compared to the validation set, which may be attributed to the relatively short postoperative follow-up period. Specifically, the 41 patients in the testing set were newly diagnosed cases with a postoperative follow-up duration limited to 2 years. According to previous studies (26), the median time to postoperative recurrence is typically over 10 years, suggesting that the shorter follow-up period in this cohort may have influenced the model’s performance evaluation.
Compared to patients who received postoperative radiotherapy, who exhibit recurrence rates ranging from 0% to 18% (27–29), those treated with surgery alone show higher recurrence rates in previous literature (30). A systematic review encompassing >2,740 patients (10) reported 89.1% efficacy with stereotactic radiotherapy. Therefore, it is recommended that patients identified by this model as having a recurrence risk undergo adjuvant radiotherapy to reduce postoperative recurrence. Additionally, the necessity of postoperative surveillance should be emphasized for high-risk patients, who must be informed that recurrence risks may persist for over 10 years. While some studies suggest (31) that frequent short-term follow-up has limited utility in detecting GJT recurrence, we recommend that high-risk populations adhere to the National Comprehensive Cancer Network (NCCN) and Endocrine Society guidelines: follow-up every 3–6 months for the first 2 years postoperatively, followed by annual evaluations to monitor for recurrence.
Limitations
Our study has several limitations that should be acknowledged. First, as a retrospective single-center study, the data were derived from historical medical records and examinations, which are subject to recall bias and data incompleteness. Second, the follow-up duration for the testing cohort was significantly shorter than that of other comparative groups, necessitating further validation through multicenter prospective studies with extended follow-up periods. Finally, due to limited documentation in the collected patient records, our study could not incorporate genetic factors such as Succinate Dehydrogenase Complex Iron Sulfur Subunit B(SDHB) gene mutations into the model. Prior research highlights the critical role of SDHB mutations in evaluating local recurrence and distant metastasis (29, 32), and most systemic paraganglioma risk assessment models include SDHB status as a key factor in their scoring systems (33). In our dataset, only a small subset of patients underwent genetic testing, precluding multivariate analysis of SDHB mutations. As a result, this variable was excluded from the nomogram construction. It should be noted that integrating SDHB gene status as a predictor would likely alter the risk contributions of the other factors within the model.
Conclusion
Our study identified Ki-67 expression, S-100 protein status, tumor location, and age as significant predictors of postoperative recurrence in GJT patients. We developed and validated a nomogram model to predict recurrence risk in GJT patients, demonstrating its clinical utility. For patients classified as high-risk by the model, adjuvant radiotherapy is recommended to reduce recurrence, and the necessity of rigorous postoperative surveillance should be emphasized. This model provides a practical tool to guide individualized clinical management and improve outcomes in GJT patients.
The COVID-19 pandemic may have impacted follow-up compliance and timing for some patients, particularly those in the test set (2020–2022), potentially affecting recurrence detection.
The clinical presentation and management of GJT can exhibit geographical variations influenced by genetic predisposition and environmental factors. As highlighted in a recent review by Palade et al. (34), the incidence and predominant location of these tumors may differ across populations, which is a crucial consideration for the generalizability of prognostic models. Our study, derived from a single tertiary center’s experience, identifies a set of robust predictors. However, their relative weight and the model’s overall performance should be validated in international, multi-ethnic cohorts to ensure broad clinical applicability.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Chinese PLA General Hosptial. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the reason for waiving written informed consent is that the study utilized existing patient cases from the hospital, and verbal informed consent was approved. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. QL: Conceptualization, Investigation, Software, Writing – review & editing. XG: Data curation, Methodology, Writing – review & editing. TK: Data curation, Methodology, Writing – review & editing. JC: Supervision, Writing – review & editing. SY: Project administration, Resources, Supervision, Writing – review & editing. WS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Clinical Research Center for Otolaryngologic Diseases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
AUC: Area Under the Curve
CBT: Carotid Body Tumor
CI: Confidence Interval
CgA: Chromogranin A
CK: Cytokeratin
CT: Computed Tomography
DFS: Disease-Free Survival
DSA: Digital Subtraction Angiography
GAPP: Grading of Adrenal Pheochromocytoma and Paraganglioma
GJT: Glomus Jugulare Tumor
GTR: Gross Total Resection
HNPGL: Head and Neck Paraganglioma
ICA: Internal Carotid Artery
M-GAPP: Modified Grading of Adrenal Pheochromocytoma and Paraganglioma
MRI: Magnetic Resonance Imaging
NCCN: National Comprehensive Cancer Network
OR: Odds Ratio
PASS: Pheochromocytoma of the Adrenal Gland Scaled Score
PHEO: Pheochromocytoma
PGL: Paraganglioma
PPGL: Pheochromocytoma and Paraganglioma
ROC: Receiver Operating Characteristic
RT: Radiotherapy
SDHB: Succinate Dehydrogenase Complex Iron Sulfur Subunit B
STR: Subtotal Resorption
TBPs: Tympanic ball paragangliomas
TJPGL: Tympanojugular Paraganglioma
VGPL: Vagal Paraganglioma
WHO: World Health Organization
References
1. Parisien-La Salle S, Chbat J, Lacroix A, Perrotte P, Karakiewicz P, Saliba I, et al. Postoperative recurrences in patients operated for pheochromocytomas and paragangliomas: new data supporting lifelong surveillance. Cancers (Basel). (2022) 14:ed2022. doi: 10.3390/cancers14122942
2. Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, and Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. (2005) 90:2110–6. doi: 10.1210/jc.2004-1398
3. Wanna GB, Sweeney AD, Carlson ML, Latuska RF, Rivas A, Bennett ML, et al. Subtotal resection for management of large jugular paragangliomas with functional lower cranial nerves. Otolaryngol Head Neck Surg. (2014) 151:991–5. doi: 10.1177/0194599814552060
4. van Hulsteijn LT, Corssmit EP, Coremans IE, Smit JW, Jansen JC, Dekkers OM, et al. Regression and local control rates after radiotherapy for jugulotympanic paragangliomas: systematic review and meta-analysis. Radiother Oncol. (2013) 106:161–8. doi: 10.1016/j.radonc.2012.11.002
5. Rougier G, Rochand A, Bourdais R, Gal J, Bénézery K, Bozec A, et al. Long-term outcomes in head and neck paragangliomas managed with intensity-modulated radiotherapy. Laryngoscope. (2022) 133:607–14. doi: 10.1002/lary.30232
6. Wachtel H, Hutchens T, Baraban E, Schwartz LE, Montone K, LiVolsi V, et al. Predicting metastatic potential in pheochromocytoma and paraganglioma: A comparison of PASS and GAPP scoring systems. J Clin Endocrinol Metab. (2020) 105:e4661–70. doi: 10.1210/clinem/dgaa608
7. Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. (2011) 48:1739–49. doi: 10.1016/j.ejca.2011.07.016
8. Iacobone M, Schiavi F, Bottussi M, Favaro A, Porzionato A, Fassina A, et al. Is genetic screening indicated in apparently sporadic pheochromocytomas and paragangliomas? Surgery. (2011) 150:1194–201. doi: 10.1016/j.surg.2011.09.024
9. Mete O, Asa SL, Gill AJ, Kimura N, de Krijger RR, and Tischler A. Overview of the 2022 WHO classification of paragangliomas and pheochromocytomas. Endocr Pathol. (2022) 33:90–114. doi: 10.1007/s12022-022-09704-6
10. Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from Malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. (2002) 26:551–66. doi: 10.1097/00000478-200205000-00002
11. Al Subhi AR, Boyle V, and Elston MS. Systematic review: incidence of pheochromocytoma and paraganglioma over 70 years. J Endocr Soc. (2022) 6:bvac105. doi: 10.1210/jendso/bvac105
12. Jackson CG, Welling DB, Chironis P, Glasscock ME 3rd, and Woods CI. Glomus tympanicum tumors: contemporary concepts in conservation surgery. Laryngoscope. (1989) 99:875–84. doi: 10.1288/00005537-198909000-00001
13. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. (2000) 92:205–16. doi: 10.1093/jnci/92.3.205
14. Torresan F, Beber A, Schiavone D, Cavedon E, Iacobone M, Mian C, et al. Long-term outcomes after surgery for pheochromocytoma and sympathetic paraganglioma. Cancers (Basel). (2023) 15:ed2023. doi: 10.3390/cancers15112890
15. Parasiliti-Caprino M, Lucatello B, Lopez C, Giraudo G, Maletta F, Perotti P, et al. Predictors of recurrence of pheochromocytoma and paraganglioma: a multicenter study in Piedmont, Italy. Hypertens Res. (2019) 43:500–10. doi: 10.1038/s41440-019-0338-x
16. Richter S, Pacak K, Kunst HPM, Tischler AS, De Krijger RR, Van Nederveen FH, et al. Management and follow-up strategies for patients with head and neck paraganglioma. Eur J Endocrinol. (2024) 190:389–98. doi: 10.1093/ejendo/lvae113
17. Bacciu A, Prasad SC, Sist N, Rossi G, Piras G, Presutti L, et al. Management of the cervico-petrous internal carotid artery in class C tympanojugular paragangliomas. Head Neck. (2015) 38:899–905. doi: 10.1002/hed.24063
18. Sanna M, De Donato G, Piazza P, and Falcioni M. Revision glomus tumor surgery. Otolaryngol Clin North Am. (2006) 39:763–82, vii. doi: 10.1016/j.otc.2006.04.004
19. Ding Y, Li L, Han D, Chen X, Wang Z, Mao C, et al. Head and neck Malignant paragangliomas: experience from a single institution. Ear Nose Throat J. (2021) 103:298–304. doi: 10.1177/01455613211046615
20. Allgöwer C, Kretz AL, von Karstedt S, Wittau M, Henne-Bruns D, Lemke J, et al. Friend or foe: S100 proteins in cancer. Cancers (Basel). (2020) 12:ed2020. doi: 10.3390/cancers12082037
21. Perego M, Tyurin VA, Tyurina YY, Yellets J, Nacarelli T, Lin C, et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci Transl Med. (2020) 12:eabb5817. doi: 10.1126/scitranslmed.abb5817
22. Fei F, Qu J, Zhang M, Li Y, and Zhang S. S100A4 in cancer progression and metastasis: A systematic review. Oncotarget. (2017) 8:73219–39. doi: 10.18632/oncotarget.18016
23. Chun C, Song L, Xu G, Liu C, Wang Z, Zhang Y, et al. Analysis of clinical and pathological characteristics of retroperitoneal paraganglioma and associated prognostic factors. J Surg Oncol. (2024), 47–55. doi: 10.1002/jso.v130.1
24. Pierre C, Agopiantz M, Brunaud L, Bihain F, Nomine C, Renaud F, et al. COPPS, a composite score integrating pathological features, PS100 and SDHB losses, predicts the risk of metastasis and progression-free survival in pheochromocytomas/paragangliomas. Virchows Arch. (2019) 475:721–34. doi: 10.1007/s00428-019-02553-5
25. Wang LL, Wei XJ, Zhang QC, Zhao J, Zhang CD, Li Y, et al. Morphological and immunohistochemical characteristics associated with metastatic and recurrent progression in pheochromocytoma/paraganglioma: A cohort study. Ann Diagn Pathol. (2022) 59:151981. doi: 10.1016/j.anndiagpath.2022.151981
26. Elshaikh MA, Mahmoud-Ahmed AS, Kinney SE, Wood BP, Lee JH, Barnett GH, et al. Recurrent head-and-neck chemodectomas: a comparison of surgical and radiotherapeutic results. Int J Radiat Oncol Biol Phys. (2002) 52:953–6. doi: 10.1016/S0360-3016(01)02751-1
27. Papaspyrou K, Mann WJ, and Amedee RG. Management of head and neck paragangliomas: review of 120 patients. Head Neck. (2009) 31:381–7. doi: 10.1002/hed.20967
28. Scheick SM, Morris CG, Amdur RJ, Kirwan JM, and Mendenhall WM. Long-term outcomes after radiosurgery for temporal bone paragangliomas. Am J Clin Oncol. (2018) 41:223–6. doi: 10.1097/COC.0000000000000255
29. Mediouni A, Ammari S, Wassef M, Laccourreye O, and Bonfils P. Malignant head/neck paragangliomas. Comp study Eur Ann Otorhinolaryngol Head Neck Dis. (2013) 131:159–66. doi: 10.1016/j.anorl.2012.09.011
30. Gottfried ON, Liu JK, and Couldwell WT. Comparison of radiosurgery and conventional surgery for the treatment of glomus jugulare tumors. Neurosurg Focus. (2004) 17:E4. doi: 10.3171/foc.2004.17.2.4
31. Contrera KJ, Yong V, Reddy CA, Greskovich JF, Lamarre ED, Scharpf J, et al. Recurrence and progression of head and neck paragangliomas after treatment. Otolaryngol Head Neck Surg. (2020) 162:504–11. doi: 10.1177/0194599820902702
32. Fliedner SM, Lehnert H, and Pacak K. Metastatic paraganglioma. Semin Oncol. (2010) 37:627–37. doi: 10.1053/j.seminoncol.2010.10.017
33. Bima C, Bioletto F, Lopez C, Bollati M, Procopio M, Ponzetto F, et al. Clinical and pathological tools for predicting recurrence and/or metastasis in patients with pheochromocytoma and paraganglioma. Biomedicines. (2022) 10:ed2022. doi: 10.3390/biomedicines10081813
Keywords: GJT, nomogram, postoperative recurrence, prognostic factors, AUC
Citation: Li K, Lu Q, Guo X, Kou T, Chen J, Yang S and Shen W (2025) Multifactorial analysis and construction of a nomogram model for postoperative recurrence of glomus jugulare tumor. Front. Oncol. 15:1662079. doi: 10.3389/fonc.2025.1662079
Received: 08 July 2025; Accepted: 12 September 2025;
Published: 06 October 2025.
Edited by:
Daniela Vrinceanu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Stefan Janik, Medical University of Vienna, AustriaAdrian Costache, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2025 Li, Lu, Guo, Kou, Chen, Yang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weidong Shen, c2hlbndlaWRvbmcxOTcwQDE2My5jb20=
 Kun Li
Kun Li Qi Lu4
Qi Lu4 Shiming Yang
Shiming Yang