- 1Department of Oncology, Cancer Prevention and Treatment Institute of Chengdu, Chengdu Fifth People’s Hospital (The Second Clinical Medical College, Affiliated Fifth People’s Hospital of Chengdu University of Traditional Chinese Medicine), Chengdu, China
- 2Department of Pathology, Chengdu Fifth People’s Hospital (The Second Clinical Medical College, Affiliated Fifth People’s Hospital of Chengdu University of Traditional Chinese Medicine), Chengdu, China
- 3Department of Imaging, Chengdu Fifth People’s Hospital (The Second Clinical Medical College, Affiliated Fifth People’s Hospital of Chengdu University of Traditional Chinese Medicine), Chengdu, China
Objective: This study aims to investigate the prognostic factors and treatment outcomes of primary breast leiomyosarcoma (PBL). We present a contemporary case of postoperative recurrence and metastasis, and conduct a systematic review to comprehensively analyze all reported cases over the past 54 years.
Method: We describe a 48-year-old female with primary breast leiomyosarcoma managed with multimodal therapy, including surgery, chemotherapy, radiotherapy, and immunotherapy. Additionally, a systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. We searched multiple electronic databases for studies on PBL published between 1969 and 2023. Patient demographics, clinical characteristics, and treatment strategies were extracted from the eligible studies. Kaplan-Meier survival analysis was employed to assess overall survival (OS), and Cox proportional hazards regression models were used to evaluate prognostic factors, including age, tumor size, and treatment approach.
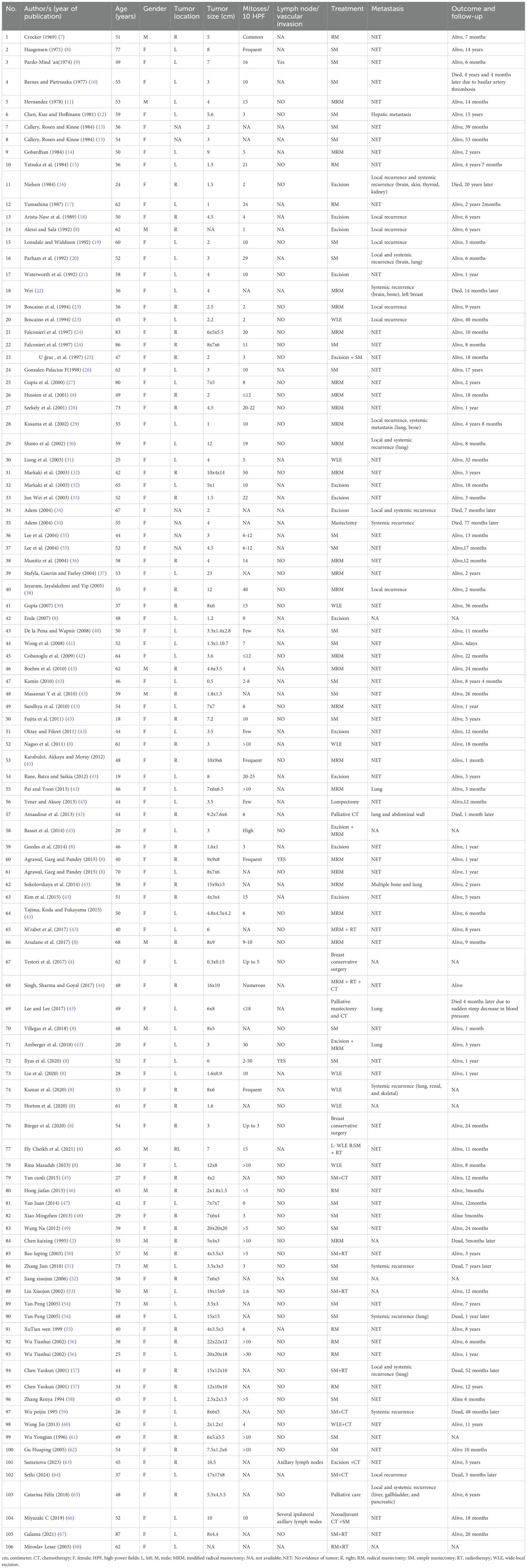
Result: The systematic search identified 98 eligible studies, which collectively reported on 106 patients with PBL. The PRISMA 2020 flow diagram illustrated the study selection process. Among the patients, 86.8% were female, and 50.9% of tumors originated in the left breast. The mean pretreatment tumor diameter was 6.38 ± 4.98 cm. Surgical intervention was performed in 88.1% of cases, predominantly mastectomy. Survival analysis revealed a median OS of 18 months. Subgroup analysis demonstrated significantly shorter OS in patients aged ≤37 years at diagnosis or with tumors >7 cm (P<0.05). Multivariate Cox regression identified younger age at diagnosis as an independent predictor of poor prognosis (HR: 4.514, 95% CI: 1.146-17.784, P = 0.031).
Conclusion: Surgical resection remains the cornerstone of treatment for PBL. Our findings, derived from a PRISMA-guided systematic review, highlight younger age at diagnosis as a significant adverse prognostic factor, underscoring the need for tailored therapeutic strategies for this high-risk subgroup.
Introduction
Primary breast leiomyosarcoma, first described by Schmidt in 1887, is an exceedingly rare malignant mesenchymal tumor of the breast (1, 2). Breast sarcomas collectively account for approximately 1% of all breast malignancies, encompassing various histological subtypes including leiomyosarcoma, fibrosarcoma, angiosarcoma, and lymphosarcoma (3, 4). As a distinct entity, primary breast leiomyosarcoma predominantly affects postmenopausal women and demonstrates an intermediate prognosis - generally more favorable than other breast sarcomas but less favorable than epithelial breast carcinomas (5, 6). Despite numerous case reports in the literature, no standardized treatment protocol has been established for breast leiomyosarcoma.
Notably, while multiple case reports and limited series analyses have been published until 2024, a comprehensive systematic review incorporating survival analysis and prognostic factor evaluation of all reported cases over the past 54 years remains lacking. Therefore, we shared the diagnosis and treatment process of a 48-year-old female case of postoperative recurrence and metastasis of primary breast leiomyosarcoma, and conducted a literature review and secondary analysis of all case reports on primary breast leiomyosarcoma in the past 54 years.
Materials and methods
Study design
Given the absence of standardized treatment protocols for primary breast leiomyosarcoma, current management strategies are largely extrapolated from sarcoma therapies in other anatomical sites. To systematically characterize primary breast leiomyosarcoma, features and treatment outcomes, we conducted a comprehensive literature review of studies published between 1969 and 2023. Eligible publications in English and Chinese were identified through database searches, rigorously screened using predefined inclusion/exclusion criteria, and analyzed to evaluate associations between age, tumor size, and overall survival (OS).
Eligibility criteria and research question
This systematic review was conducted to address the following question: “What are the prognostic factors and treatment outcomes for patients with primary breast leiomyosarcoma?” The eligibility criteria were structured using the PICOS framework: (1) Population: Patients of any age or gender with a histologically confirmed primary breast leiomyosarcoma. (2) Exposure: The diagnosis of primary breast leiomyosarcoma. (3) Comparators: Not applicable. (4) Outcomes: The primary outcome was overall survival (OS), defined as the time from diagnosis to death from any cause. Secondary outcomes included patient demographics, tumor characteristics (size, location), treatment details (surgery, chemotherapy, radiotherapy), and recurrence. (5) Study Designs: All published case reports, case series, and observational studies reporting on primary breast leiomyosarcoma were eligible for inclusion.
Search strategy
Two independent reviewers (R.G. and W.Z.) performed a systematic search of PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), and Wanfang Database for studies published from inception until December 31, 2023. The search strategy utilized the key terms “breast” AND “leiomyosarcoma” across all fields. The overall search strategy was (1) breast (all fields) and (2) leiomyosarcoma (all fields). Searches in electronic databases combined the terms 1 and 2. The complete, database-specific search strategies, including all keywords and Boolean operators, are provided in Supplementary File 1.
Study selection criteria
The study selection process was conducted independently by the same two reviewers (R.G. and W.Z.). The reviewers independently screened the titles and abstracts of all retrieved records against the eligibility criteria. Studies that clearly did not meet the criteria were excluded. The full texts of all records that appeared relevant or whose eligibility was uncertain based on the title/abstract were retrieved. The same two reviewers then independently assessed these full-text articles for final inclusion. The following exclusion criteria were applied: (i) cases without a pathologically confirmed diagnosis of primary breast leiomyosarcoma; (ii) cases with missing critical information on age, tumor size, or survival outcomes; (iii) literature for which the full text was unavailable.
At both screening stages, any disagreement between the two reviewers regarding the inclusion or exclusion of a study was first addressed through discussion. If a consensus could not be reached, the final decision was made by a third senior reviewer (L.H.).
Data extraction and management
To ensure the accuracy and consistency of data collection, a standardized data extraction form was developed a priori. The following data were extracted from each included study: first author, publication year, patient age and sex, tumor size and location, treatment modalities (surgery, chemotherapy, radiotherapy), and survival outcomes (overall survival (OS) time, status).
The data extraction was performed independently by two reviewers (R.G. and W.Z.) using this standardized form. To minimize errors and confirm data accuracy, the two reviewers cross-checked each other’s completed extraction forms. Any discrepancies or uncertainties in the extracted data were identified and then resolved by jointly reviewing the original source document. In cases where critical data (e.g., specific treatment details or exact survival times) were ambiguous or missing from the published report, we attempted to contact the corresponding authors via email to obtain clarification. No automation tools were used in the data collection process.
Outcomes
The primary outcome of this systematic review was OS, defined as the time interval from the date of pathological diagnosis to death from any cause or the date of last follow-up for surviving patients. Secondary outcomes included key tumor characteristics (size, location, lymph node or vascular invasion status) and primary treatment modalities (surgery, chemotherapy, radiotherapy).
Regarding data completeness for each outcome, we sought the most definitive result available in each study. For OS, this meant extracting the final survival status at the longest reported follow-up time for each case, rather than survival rates at multiple pre-specified time points, due to inconsistent reporting across the included literature.
Risk of bias assessment
The methodological quality of the included case reports and case series was assessed using the respective critical appraisal tools from the Joanna Briggs Institute (JBI). Two reviewers (X.J. and X.L.) independently conducted the assessments. If a consensus could not be achieved, a third senior reviewer (L.H.) was consulted to make the final decision. Given the retrospective and descriptive nature of the majority of included studies, which often lacked detailed reporting on specific criteria (e.g., unambiguous description of diagnostic criteria or follow-up schedules), the overall quality was variable. The primary aim of this assessment was to transparently characterize the strengths and limitations of the available evidence base rather than to exclude studies.
Statistical analysis
Demographic and clinical characteristics of the study cohort, including mean age and tumor diameter, were analyzed using descriptive statistics. Continuous variables are presented as mean ± standard deviation. To evaluate survival outcomes, Kaplan-Meier (KM) analysis was performed to estimate median OS in all eligible patients. Survival distributions between subgroups were compared using log-rank tests. Univariate and multivariate Cox proportional hazards regression models were employed to assess the prognostic significance of key variables, including tumor characteristics (size and location), demographic factors (age and gender) and treatment modalities. These analyses were conducted to identify independent predictors of survival outcomes in breast leiomyosarcoma patients. All statistical tests were two-sided, with P < 0.05 considered statistically significant.
Results
Case presentation
A 48-year-old woman presented with a 2×3 cm right breast mass discovered during routine physical examination. Following simple lumpectomy at a local hospital, pathological examination confirmed primary breast leiomyosarcoma (Figure 1), which was subsequently verified by our institutional review. The patient received no adjuvant therapy postoperatively. Two years later, she developed progressive lower back pain refractory to non-steroidal anti-inflammatory drugs (NSAIDs), accompanied by lumbar stiffness and ambulatory difficulty. Initial lumbar magnetic resonance imaging (MRI) demonstrated compression fractures without definitive intervention, and her symptoms progressively worsened to complete mobility impairment.
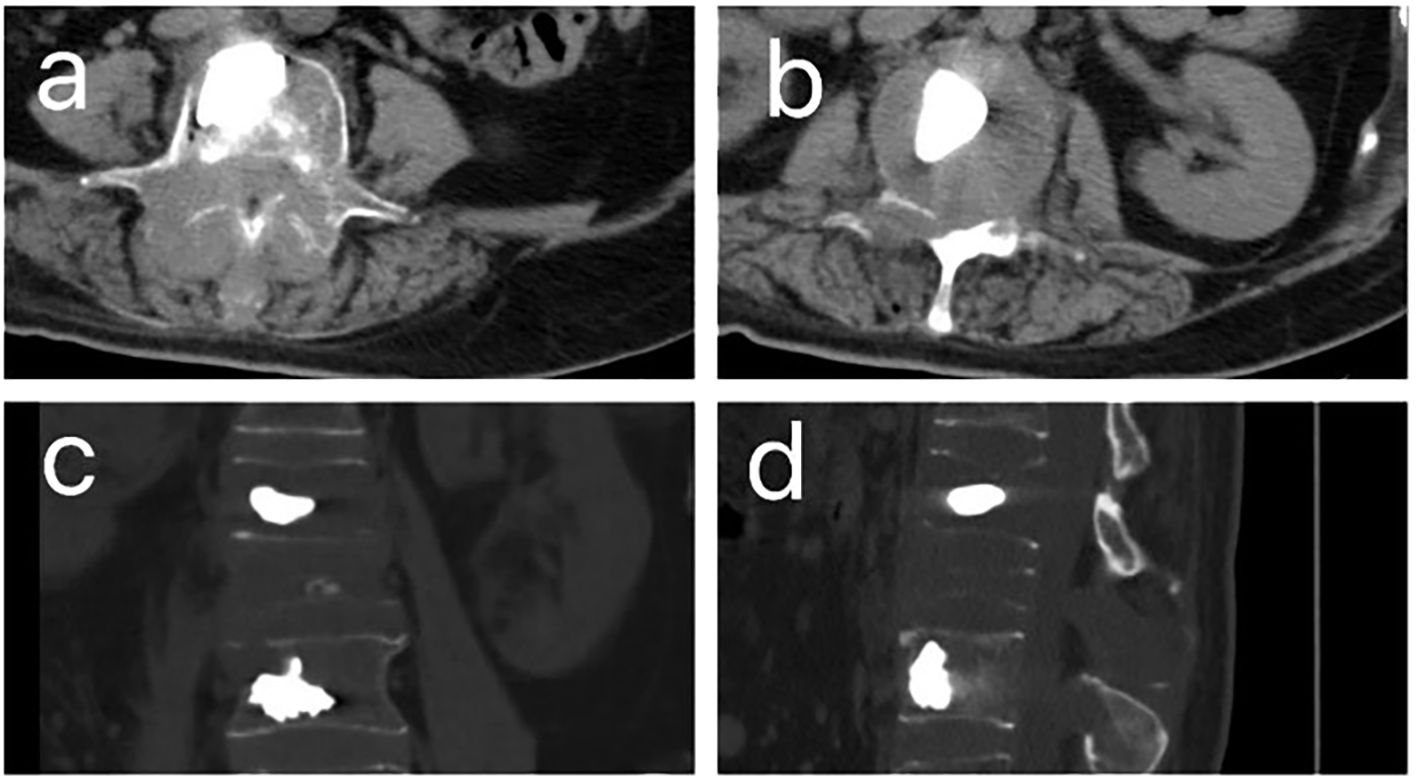
Figure 1. CT images after percutaneous kyphoplasty (PKP) of patient. (A, B). The horizontal plane CT image of patient after PKP. (C). The coronal CT images of patient after PKP. (D) The sagittal CT images of patient after PKP.
Ten months following symptom onset, repeat MRI revealed multiple thoracolumbar pathological fractures with posterior element destruction. The patient was subsequently referred to our orthopedic service with significant functional impairment (Eastern Cooperative Oncology Group Performance Status (ECOG) score is 3, bedbound status). She underwent C-arm guided percutaneous kyphoplasty (PKP) with biopsy of lumbar vertebrae 2 and 4 under local anesthesia, which demonstrated extensive osteolytic destruction involving the laminae, pedicles, and vertebral bodies. Notably, lumbar vertebrae 3 showed near-complete bony obliteration (Figure 2). Histopathological analysis confirmed metastatic breast leiomyosarcoma, supported by characteristic immunohistochemical profile: positive for smooth muscle actin (SMA), cluster of differentiation (CD) 34, and CD10, negative for Desmin and CD117, and with a low proliferative index (Ki-67 5-10%).
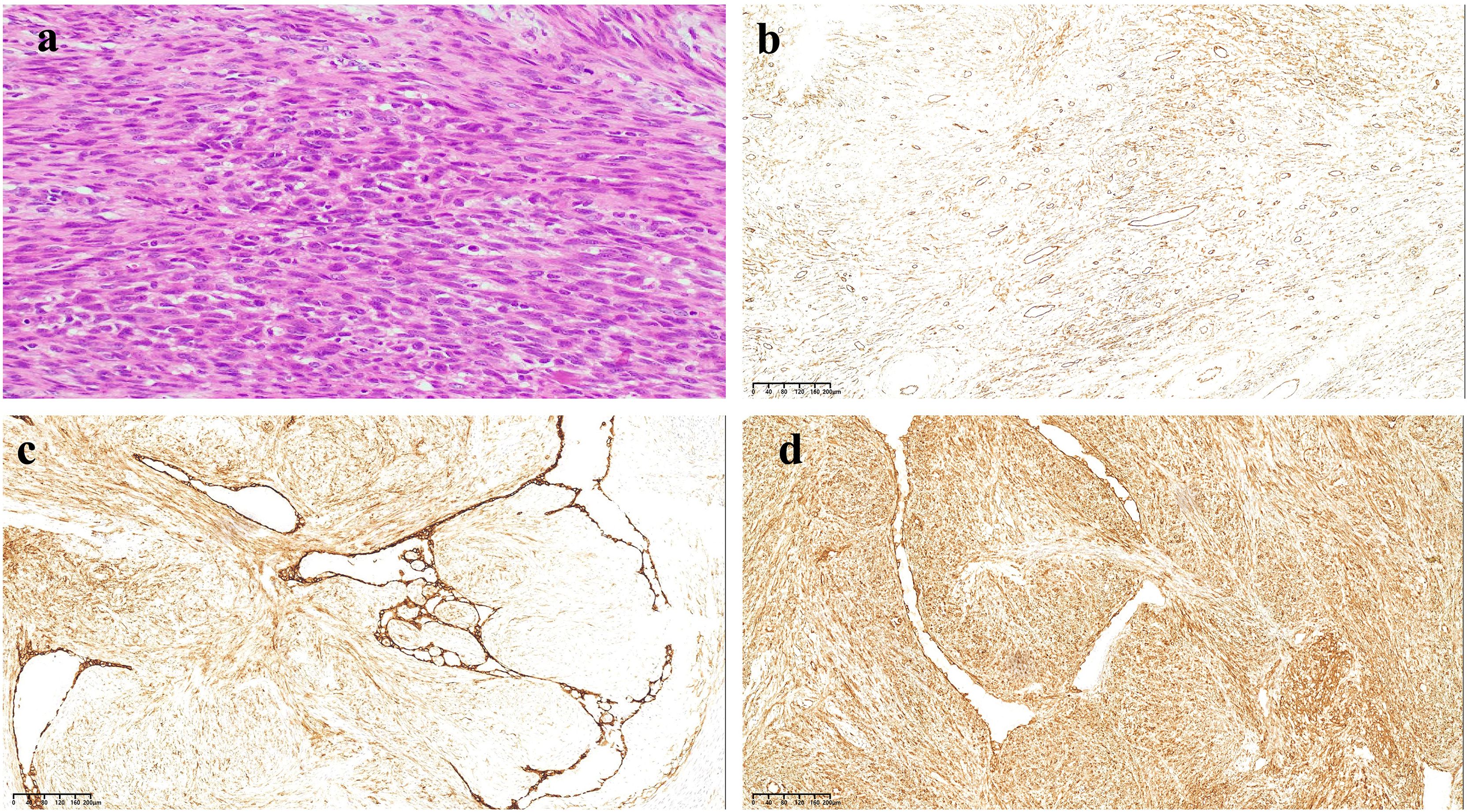
Figure 2. HE and immunohistochemical staining results of tumor tissue. (a) HE staining results show the tumor cells were spindle-shaped, infiltrative, and interlaced, with cigar-like nuclei, eosinophilic cytoplasm, perinuclear vacuoles, and has moderately anisotropic, nuclear schizophrenic and atypical nuclear schizophrenic images. (b) The tumor cells were positive for CD34 (×200). (c) The tumor cells were positive for CD10 (×200). (d) The tumor cells were positive for SMA (×200).
The patient came to our department for further treatment after the surgery. CT scan indicates that the patient only has bone metastasis and no recurrence or metastasis in other areas. Considering the extremely high risk of paraplegia, after sufficient communication with the patient and their family, IMRT technology was used to complete lumbar palliative radiation therapy. The radiation dose prescription was 95% DT P-GTVm1 = 6000cGy/30F/200cGy, P-GTVm2 = 4000cGy/20F/200cGy, P-CTV=3600cGy/20F/180cGy. After radiotherapy, 6 cycles of pembrolizumab combined with albumin bound paclitaxel and carboplatin were performed. The specific usage was pembrolizumab 200mg, albumin paclitaxel 260mg/m2, carboplatin AUC = 5 on day 1 every three weeks. After 6 cycles of treatment, the patient chose pembrolizumab immune maintenance therapy, administered every 3 weeks at a dose of 200mg each time, while also receiving bisphosphate to prevent bone related events. After treatment, the patient did not experience any further lumbar pain and returned to normal daily activities. The ECOG score was 1 point.
One year later, the patient began to experience back pain, which gradually worsened. Complete CT and MRI examinations revealed metastatic tumors in multiple vertebral bodies and some ribs of the thoracic and lumbar vertebrae, of which the thoracic 10 vertebral bodies were metastatic and invaded the spinal cord. Considering the progression of the tumor, palliative radiotherapy for the thoracic 10 vertebrae was performed at a dose of 95% DT P-GTVm=4500cGy/15F/300cGy, P-CTV=3000cGy/15F/200cGy. At the same time, 6 cycles of pembrolizumab combined with systemic intravenous chemotherapy were performed again. The chemotherapy regimen was pembrolizumab (200 mg on day 1), epirubicin hydrochloride (70 mg/m2 on day 1), ifosfamide (2000 mg/m2/day on day 1 to day 5), and mesna europrotection (400 mg/m2/day on day 1 to day 5). At present, the patient’s living condition is good, and as of the last follow-up, the total survival time of the patient is as long as 4 years.
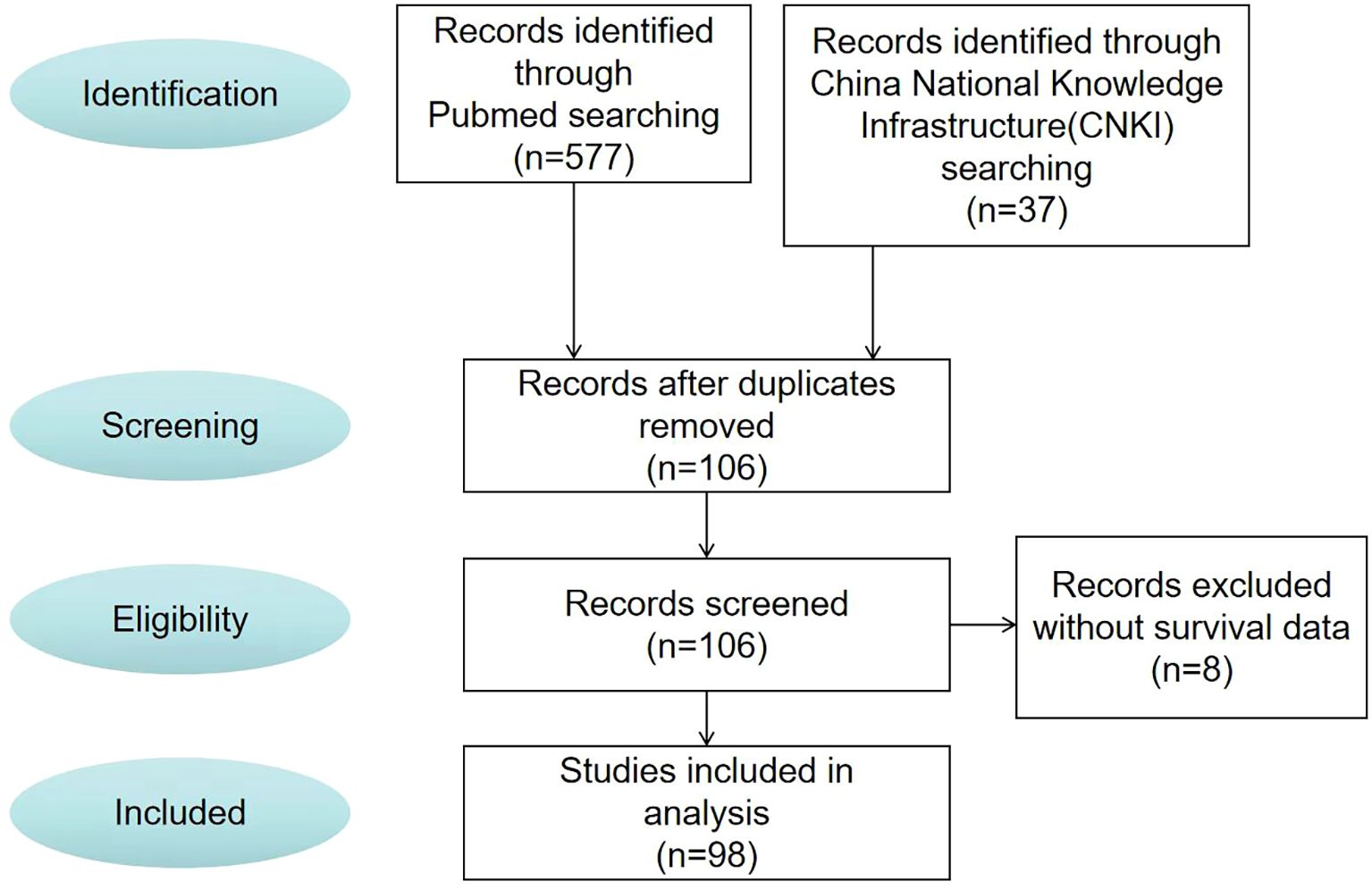
Study selection flowchart
After the screening process (Figure 3), 98 papers were deemed to meet our system evaluation criteria. Table 1 lists the data of 106 cases in 98 articles. However, through further information extraction and screening of the literature, a total of 98 patients were included in subsequent survival analysis and Cox multivariate risk analysis.
Baseline characteristics of breast leiomyosarcoma patients
The clinical characteristics of 106 cases are listed in Table 2. The average age of the patients was 50.9 ± 14.4 years old, ranging from 18–87 years. There were 92 female cases, accounting for 86.8% of patients and the male cases accounted for 13.2%. 50.9% of patients had their primary tumor located on the left side. The average size of the primary tumor was 6.38 ± 4.98 cm. At the initial diagnosis, most patients (55.7%) have no lymph node or vascular invasion. Surgical treatment is the main treatment method for patients with primary fibrosarcoma of the breast, accounting for a high proportion 98.1%, including wide local excision (WLE), radical mastectomy (RM), simple mastectomy (SM) and modified radical mastectomy (MRM). The mean overall survival was about 34.53 months.
Survival outcomes
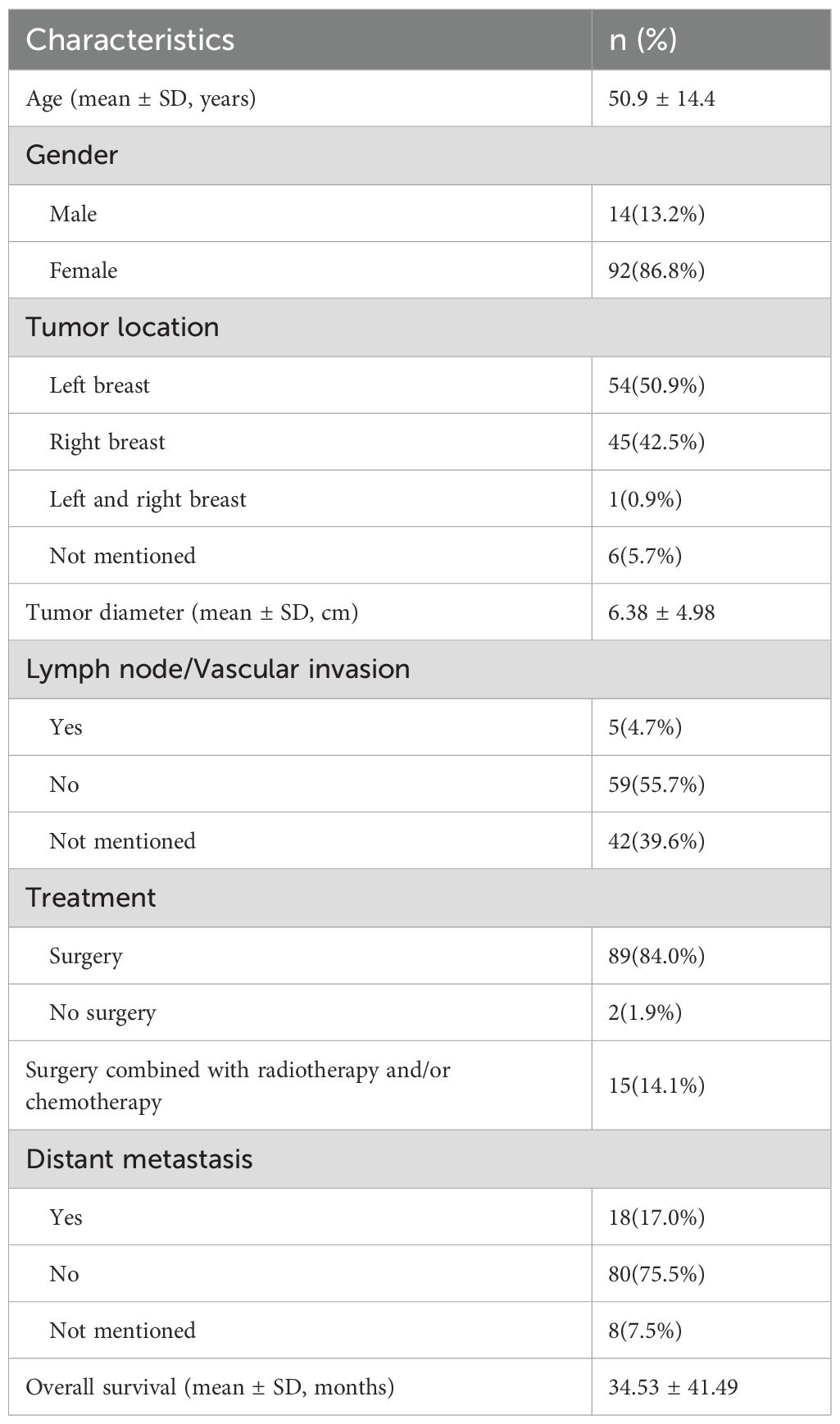
A total of 98 patients were included in the OS analysis (Figure 3). The clinical characteristics of 98 cases are listed in Table 3. There were 84 female cases, accounting for 85.7% of patients and the male cases accounted for 14.3%. 51.0% of patients had their primary tumor located on the left side. Similarly, Surgical treatment is the main treatment method for primary breast fibrosarcoma patients.
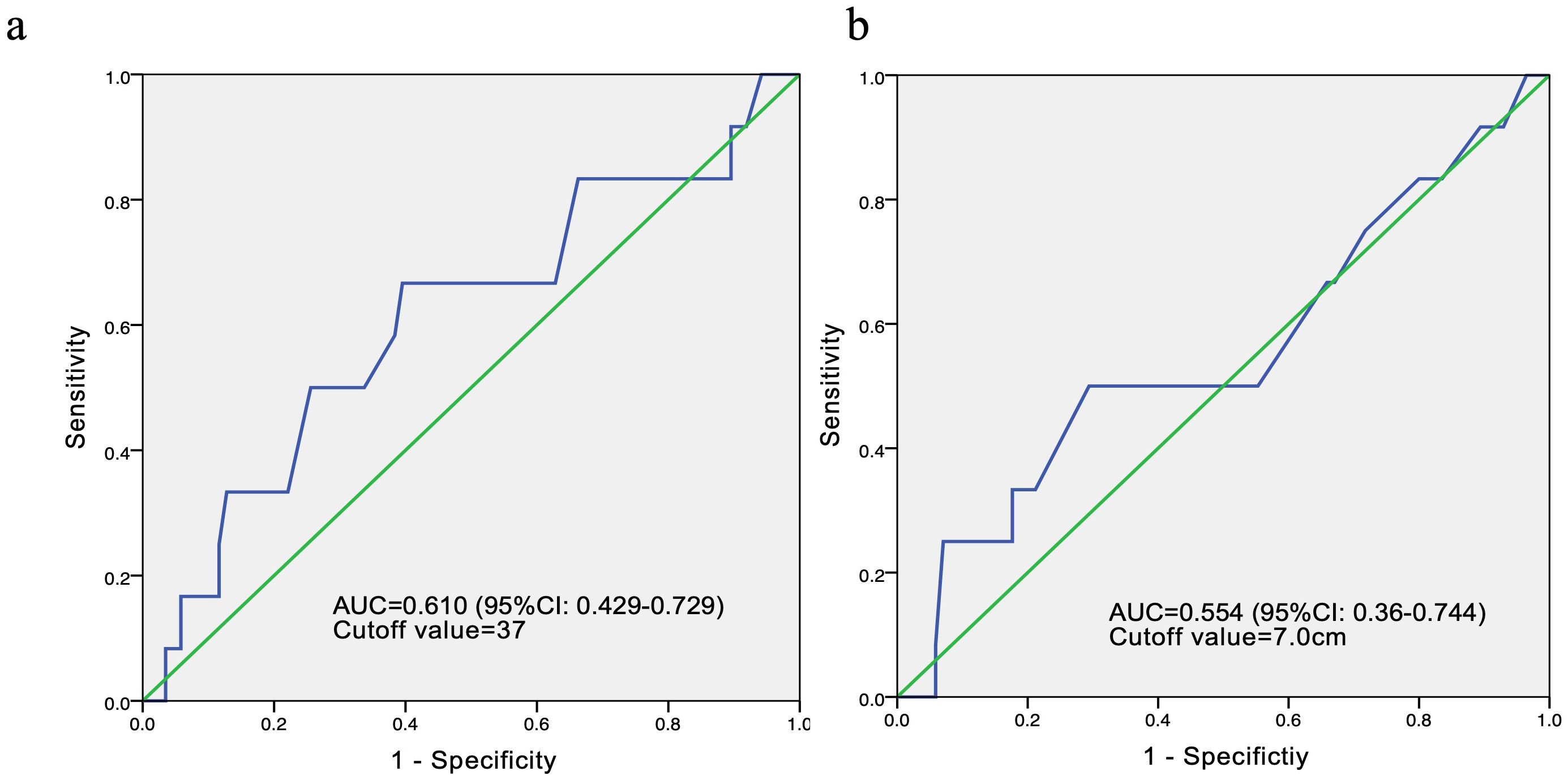
In order to conduct survival and Cox multivariate analysis, we use the ROC curve to calculate the cutoff values for age and the maximum diameter of the tumor. The results showed that the cutoff value for age was 37 years, with an area under the curve (AUC) of 0.610 and a 95% confidence interval of 0.429-0.729. The sensitivity and specificity are 0.667 and 0.86, respectively. The cutoff value for the maximum diameter of the tumor was 7cm, with an AUC of 0.554 and a 95% confidence interval of 0.360-0.744 (Figure 4). The sensitivity and specificity are 0.5 and 0.318, respectively.
KM survival indicated that the median OS of these patients was 18.0 months (Figure 5). In subgroup analysis, patients with age ≤ 37 years at initial diagnosis or tumor diameter >7 cm before treatment had a shorter OS, and the differences were statistically significant.
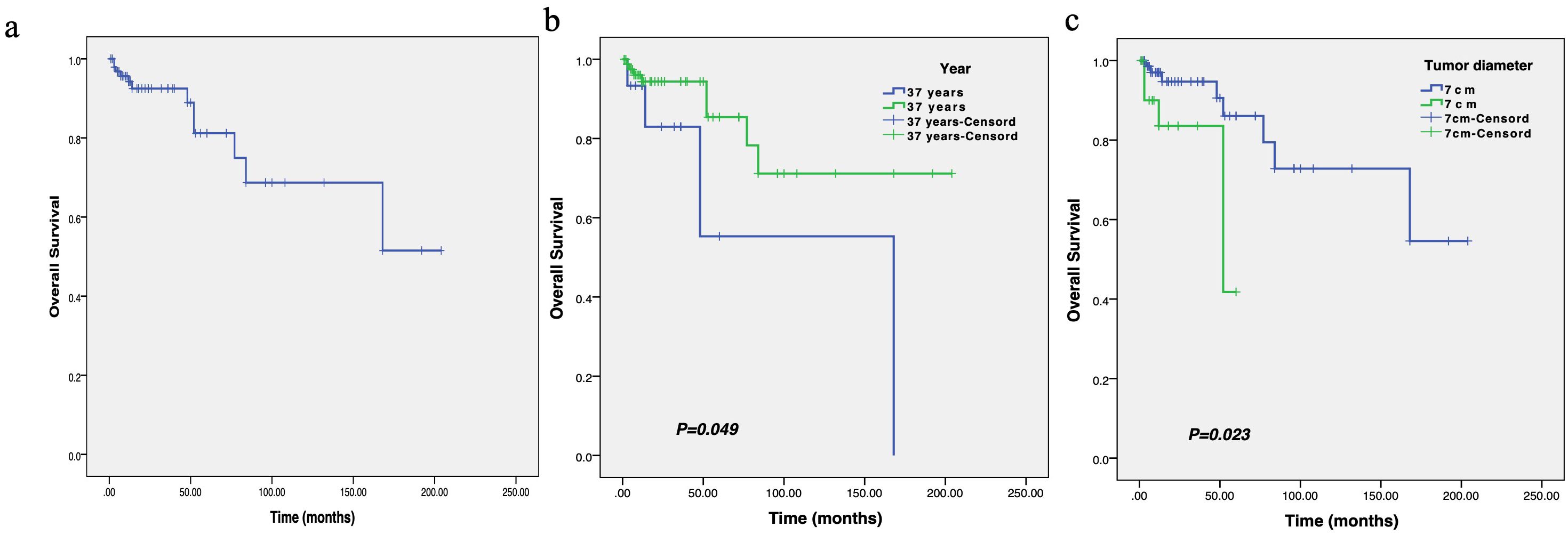
Figure 5. The survival curve of overall survival in patients with breast leiomyosarcoma (a), as well as the comparison of overall survival between different ages (b) and tumor diameters (c).
Prognostic factors from Cox regression
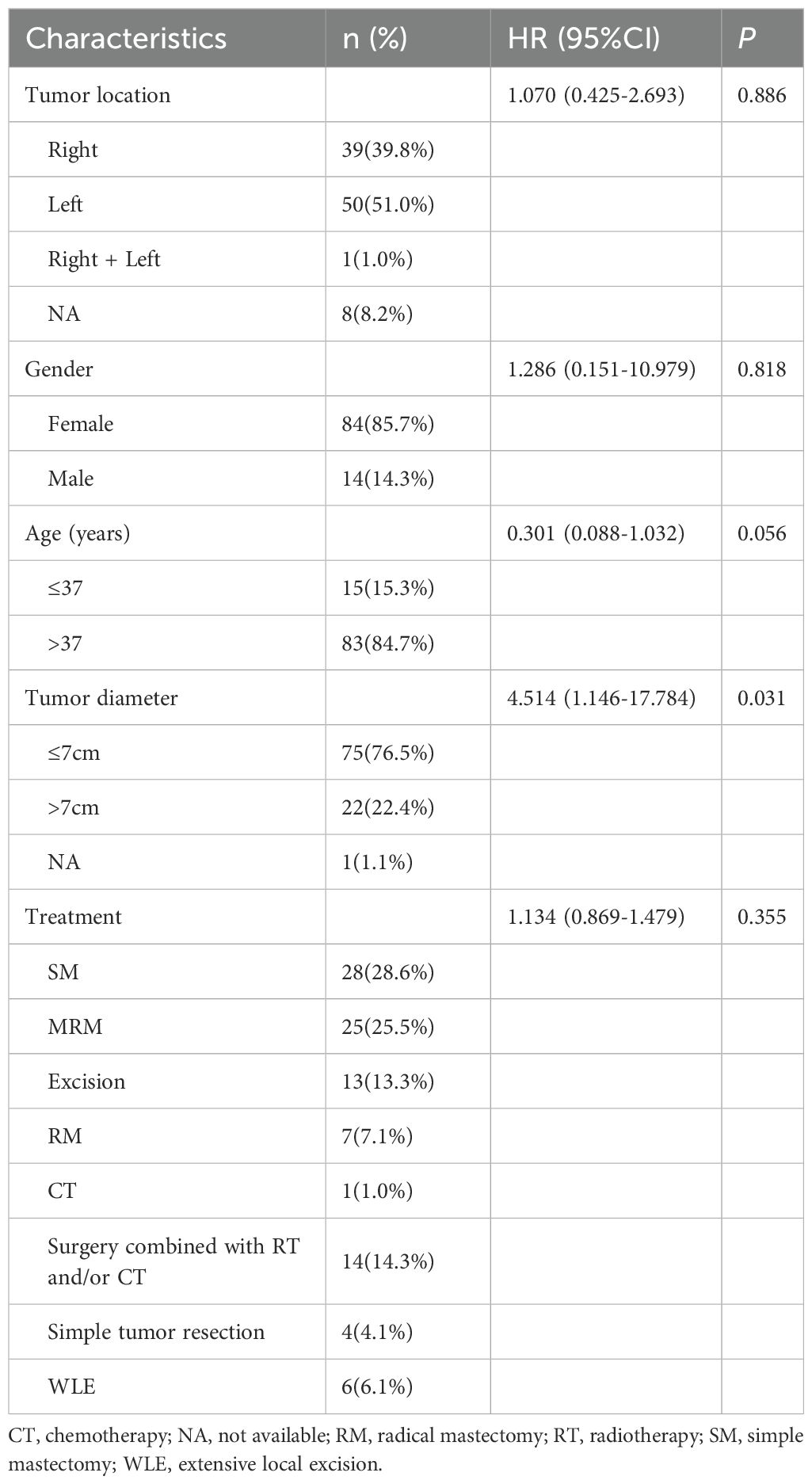
Cox proportional hazards univariate regression analysis consisted of data on tumor diameter, tumor location, patient age, gender and treatment method in 98 patients (Table 3). The results showed that the tumor diameter ≤ 7 cm could significantly improve the prognosis of patients with breast leiomyosarcoma (HR 4.514, 95% CI 1.146-17.784, P = 0.031).
Although there were differences in OS between subgroups aged over 37 and under 37 years, there was no statistically significant difference between age and OS in multivariate analysis (HR 0.301, 95% CI 0.088-1.032, P = 0.056). Whereas, the patient gender (HR 1.286, 95% CI 0.151-10.979, P = 0.818), tumor location (HR 1.070, 95% CI 0.425-2.693, P = 0.886) and treatment method (HR 1.134, 95% CI 0.869-1.479, P = 0.355) did not have a significant effect on the OS of breast leiomyosarcoma.
Discussion
Breast sarcoma is a rare non-epithelial malignant tumor originating from the mesenchymal tissue of the breast, with approximately 4.6 new cases per million women per year, accounting for less than 1% of all breast malignancies (69, 70). Same with other soft tissue sarcomas, primary breast sarcomas are associated with genetic disorders such as familial adenomatous polyposis and neurofibromatosis type 1 (71). Risk factors include a history of radiotherapy, chronic lymphoedema, vinyl chloride exposure and Epstein-Barr virus (EBV) infection (72). Leiomyosarcomas are rare one of the subtypes to which it belongs, and its exact origin is unclear. It may develop from mesenchymal cells or smooth muscle cells within blood vessels and is most likely to occur in the vascular and muscular tissues of this anatomical region near the areola (1).
The clinical presentation of breast leiomyosarcoma is often a slow-growing large palpable mass, painless, firm, and lobulated, typically found in postmenopausal women (3). There is a tendency for skin and muscle invasion, but areola changes and nipple discharge are relatively rare (6). It is difficult to distinguish from other breast tumors in clinical practice because physical examination and imaging results are often similar to other malignant tumors (43), and are often mistaken for benign causes (lobular tumors and fibroadenomas) (8), and the diagnosis can only be finally confirmed through histological examination and immunohistochemical analysis after biopsy. Histopathology showed marked cellular heterogeneity, atypical mitoses, vascular invasion and necrosis (1). Immunohistochemistry demonstrated that leiomyosarcoma staining positive for desmin, smooth muscle actin and vimentin, whereas it was negative for epithelial markers, cytokeratin and S-100 (43, 73, 74).
Currently, there are insufficient guidelines for the treatment of breast leiomyosarcoma, probably due to the rarity of the disease in this location. As a result, the diagnostic and therapeutic approaches to this type of tumor are highly heterogeneous and require more specific treatment strategies and guidelines (43, 75). Because of the high rate of local recurrence, surgery with adequate resection margins is the only potential treatment for patients with sarcomas. A previous study showed that for optimal efficacy, a minimum negative margin of 3 cm should be achieved; however, a 2 cm margin can be used for breast protection (76). Several studies have reported metastatic spread to lungs, liver and bone, with lymph node involvement being extremely rare (5, 43, 66).
Our study also confirms that there are very few patients with primary breast leiomyosarcoma who are initially diagnosed with lymph node metastasis or vascular invasion. Routine lymph node dissection and sentinel lymph node biopsy is not recommended as it has no impact on patient survival (77). However, biopsy should be performed if lymph node metastasis is suspected on imaging. After surgical resection, radiotherapy is recommended for local control. Adjuvant radiotherapy after breast-conserving mastectomy has been shown to improve disease-free survival and local control of recurrence, especially if resection margins are inadequate (72). Chemotherapy may be indicated for tumors larger than 5 cm, high-grade tumors or advanced cancers (8).
Research suggests that while some patients with leiomyosarcoma (LMS) may benefit from immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors like nivolumab and pembrolizumab) (78), histological subtype analyses reveal that LMS has the lowest response rates compared to subtypes such as alveolar soft part sarcoma and undifferentiated pleomorphic sarcoma, which show the highest (78). Studies on combining these agents with chemotherapy (e.g., doxorubicin, dacarbazine) or radiotherapy in advanced LMS have demonstrated limited but promising clinical activity, with efficacy potentially dependent on the tumor’s immune microenvironment characteristics (79). It is hypothesized that by modulating this microenvironment, immunotherapy may convert immunologically “cold” tumors into “hot” ones, thereby promoting immune cell infiltration. Recent findings highlight that interactions between small venous smooth muscle cells and endothelial cells in breast tumors are critical for the infiltration of immune cells (e.g., T cells, B cells), suggesting a potential target for enhancing immunotherapy efficacy (80).
Current research directions primarily focus on combining immunotherapy with chemotherapy, targeted therapy, and radiotherapy. For instance, chemotherapy may create a favorable context for immunotherapy by inducing immunogenic cell death. Novel strategies, including those using genetically engineered tumor cells, are also under investigation. Adjunctive approaches such as certain biological therapies (e.g., interferon, interleukin-2) and adoptive cell therapies aim to modulate the body’s immune response against tumors. However, specific application data for these therapies in breast leiomyosarcoma remain limited.
However, our research found that patients with tumors larger than 7cm have a worse prognosis. Does this mean that patients with tumors larger than 7 cm may need to receive additional treatment besides surgery, in addition to other high-risk factors. Although, it is unclear whether treatment is beneficial or has any impact on morbidity and mortality. The combination of anthracyclines with the addition of ifosfamide has been described as first-line chemotherapy (43). There is also emerging evidence to support the use of neoadjuvant chemotherapy for the treatment of metastatic disease, but the results remain uncertain (66).
Hematogenous spread is the most common mode of metastasis in leiomyosarcoma (43). Distant hematogenous metastases to bone, liver, lungs, central nervous system and spine reported in about 25% of cases, and usually detected after a latent period of 15–20 years (5, 74). In patients with metastatic disease, palliative chemotherapy or palliative surgery may be offered to slow disease progression and control local complications (43, 81). Patients with this malignancy have a relatively poor prognosis and a high risk of recurrence compared to other types of breast cancer, with 5-year disease-free survival rates ranging from 33%-52% (82), making frequent follow-up and monitoring for post-excision recurrence necessary.
Our study provides the first systematic evidence that surgical approach, gender, age, and tumor location are not significantly associated with the prognosis of primary breast leiomyosarcoma. In contrast, tumor size was identified as an independent predictor of survival. Consequently, initial tumor size may be considered a key factor in guiding treatment decisions and identifying patients at high risk of recurrence.
But, there a key limitation of this review stems from the inherent methodological constraints of the available primary literature, which predominantly comprises single case reports and small, retrospective case series. Our systematic quality assessment using the JBI tools confirmed these limitations, highlighting frequent deficiencies in standardized follow-up and comprehensive outcome reporting. Consequently, the generalizability and robustness of our pooled findings should be interpreted with caution.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
RG: Data curation, Formal Analysis, Investigation, Writing – original draft. WZ: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. XJ: Investigation, Writing – original draft. XL: Data curation, Investigation, Writing – original draft. LH: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported in part by the Chengdu University of Traditional Chinese Medicine College Cooperation Innovation Fund (LH202402003); Research Project of Chengdu Municipal Health Commission (WXLH202403256); the Science and technology project of the Health planning committee of Sichuan and Chengdu (2024276 & 2020035 & 2021115); Xinglin Scholars Program of Chengdu University of Traditional Chinese Medicine (YYZX2021039), Chengdu Fifth People’s Hospital Scientific Research Project (KYJJ2021-05) and Chengdu Fifth People’s Hospital Teaching Reform Research Project (JGZX202214).
Acknowledgments
We appreciate the support of the participating patients and the assistance of the participating doctors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1662132/full#supplementary-material
References
1. Osório C, Rodrigues EF, Santos M, Santos T, and Nora M. Fungating breast wound: A rare manifestation of primary breast leiomyosarcoma. Cureus. (2023) 15:e33398. doi: 10.7759/cureus.33398
2. Kaixin C and Fengming L. A case of smooth muscle sarcoma of the male breast. Pract J Cancer. (1995), 11.
3. Ilyas MIM, Nazir S, and Xiao PQ. Breast leiomyosarcoma: A systematic review and recommendations for management. Int Surg. (2019) 104:196–202. doi: 10.9738/INTSURG-D-15-00183.1
4. Testori A, Meroni S, Voulaz E, Alloisio M, De Sanctis R, Bossi P, et al. Primary breast leiomyosarcoma and synchronous homolateral lung cancer: a case report. J Thorac Dis. (2017) 9:E1054–9. doi: 10.21037/jtd.2017.10.98
5. Karabulut Z, Akkaya H, and Moray G. Primary leiomyosarcoma of the breast: A case report. J Breast Cancer. (2012) 15:124–7. doi: 10.4048/jbc.2012.15.1.124
6. Duncan MA and Lautner MA. Sarcomas of the breast. Surg Clinics North America. (2018) 98:869–76. doi: 10.1016/j.suc.2018.03.013
7. Crocker DJ and Murad TM. Ultrastructure of fibrosarcoma in a male breast. Cancer. (1969) 23:891–9. doi: 10.1002/1097-0142(196904)23:4<891::AID-CNCR2820230426>3.0.CO;2-Z
8. Masadah R, Anwar F, Nelwan BJ, and Faruk M. Primary leiomyosarcoma of the breast: A case report and literature review. Int J Surg Case Rep. (2023) 106:108290. doi: 10.1016/j.ijscr.2023.108290
9. Pardo-Mindán J, Garcia-Julian G, and Eizaguirre Altuna M. Leiomyosarcoma of the breast. Report of a case. Am J Clin Pathol. (1974) 62:477–80. doi: 10.1093/ajcp/62.4.477
10. Barnes L and Pietruszka M. Sarcomas of the breast: a clinicopathologic analysis of ten cases. Cancer. (1977) 40:1577–85. doi: 10.1002/1097-0142(197710)40:4<1577::AID-CNCR2820400430>3.0.CO;2-D
11. Hernandez FJ. Leiomyosarcoma of male breast originating in the nipple. Am J Surg Pathol. (1978) 2:299–304. doi: 10.1097/00000478-197809000-00006
12. Chen KT, Kuo TT, and Hoffmann KD. Leiomyosarcoma of the breast: a case of long survival and late hepatic metastasis. Cancer. (1981) 47:1883–6. doi: 10.1002/1097-0142(19810401)47:7<1883::AID-CNCR2820470728>3.0.CO;2-T
13. Callery CD, Rosen PP, and Kinne DW. Sarcoma of the breast. A study of 32 patients with reappraisal of classification and therapy. Ann Surg. (1985) 201:527–32. doi: 10.1097/00000658-198504000-00020
15. Yatsuka K, Mihara S, Isobe M, Edakuni S, Takeoka A, Kakegawa T, et al. Leiomyosarcoma of the breast–a case report and an electron microscopic study. Japanese J Surg. (1984) 14:494–8. doi: 10.1007/BF02469792
16. Nielsen BB. Leiomyosarcoma of the breast with late dissemination. Virchows Archiv A Pathol Anat Histopathol. (1984) 403:241–5. doi: 10.1007/BF00694900
18. Arista-Nasr J, Gonzalez-Gomez I, Angeles-Angeles A, Illanes-Baz E, Brandt-Brandt H, and Larriva-Sahd J. Primary recurrent leiomyosarcoma of the breast. Case report with ultrastructural and immunohistochemical study and review of the literature. Am J Clin Pathol. (1989) 92:500–5. doi: 10.1093/ajcp/92.4.500
19. Lonsdale RN and Widdison A. Leiomyosarcoma of the nipple. Histopathology. (1992) 20:537–9. doi: 10.1111/j.1365-2559.1992.tb01042.x
20. Parham DM, Robertson AJ, Hussein KA, and Davidson AI. Leiomyosarcoma of the breast; cytological and histological features, with a review of the literature. Cytopathology. (1992) 3:245–52. doi: 10.1111/j.1365-2303.1992.tb00514.x
21. Waterworth PD, Gompertz RH, Hennessy C, Henry JA, and Lennard TW. Primary leiomyosarcoma of the breast. Br J Surg. (1992) 79:169–70. doi: 10.1002/bjs.1800790225
22. Wei CH, Wan CY, Chen A, and Tseng HH. Epithelioid leiomyosarcoma of the breast: report of a case. J Formos Med Assoc. (1993) 92:379–81.
23. Boscaino A, Ferrara G, Orabona P, Donofrio V, Staibano S, and De Rosa G. Smooth muscle tumors of the breast: clinicopathologic features of two cases. Tumori. (1994) 80:241–5. doi: 10.1177/030089169408000316
24. Falconieri G, Della Libera D, Zanconati F, and Bittesini L. Leiomyosarcoma of the female breast: report of two new cases and a review of the literature. Am J Clin Pathol. (1997) 108:19–25. doi: 10.1093/ajcp/108.1.19
25. Uğraş S, Dilek ON, Karaayvaz M, Dilek H, Peker O, and Barut I. Primary leiomyosarcoma of the breast. Surg Today. (1997) 27:1082–5. doi: 10.1007/BF02385794
26. González-Palacios F. Leiomyosarcoma of the female breast. Am J Clin Pathol. (1998) 109:650–1. doi: 10.1093/ajcp/109.5.650
27. Gupta RK, Kenwright D, Naran S, Lallu S, and Fauck R. Fine needle aspiration cytodiagnosis of leiomyosarcoma of the breast. A case report. Acta Cytol. (2000) 44:1101–5. doi: 10.1159/000328606
28. Székely E, Madaras L, Kulka J, Járay B, and Nagy L. Leiomyosarcoma of the female breast. Pathol Oncol Res: POR. (2001) 7:151–3. doi: 10.1007/BF03032583
29. Kusama R, Fujimori M, Hama Y, Shingu K, Ito K, Mochizuki Y, et al. Stromal sarcoma of the breast with leiomyosarcomatous pattern. Pathol Int. (2002) 52:534–9. doi: 10.1046/j.1440-1827.2002.01386.x
30. Shinto O, Yashiro M, Yamada N, Matsuoka T, Ohira M, Ishikawa T, et al. Primary leiomyosarcoma of the breast: report of a case. Surg Today. (2002) 32:716–9. doi: 10.1007/s005950200133
31. Liang WC, Sickle-Santanello BJ, Nims TA, and Accetta PA. Primary leiomyosarcoma of the breast: a case report with review of the literature. Breast J. (2003) 9:494–6. doi: 10.1046/j.1524-4741.2003.09613.x
32. Markaki S, Sotiropoulou M, Hanioti C, and Lazaris D. Leiomyosarcoma of the breast. A clinicopathologic and immunohistochemical study. Eur J Obstet Gynecol Reprod Biol. (2003) 106:233–6. doi: 10.1016/S0301-2115(02)00226-9
33. Jun Wei X, Hiotis K, Garcia R, and Hummel Levine P. Leiomyosarcoma of the breast: a difficult diagnosis on fine-needle aspiration biopsy. Diagn Cytopathol. (2003) 29:172–8. doi: 10.1002/dc.10359
34. Adem C, Reynolds C, Ingle JN, and Nascimento AG. Primary breast sarcoma: clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer. (2004) 91:237–41. doi: 10.1038/sj.bjc.6601920
35. Lee J, Li S, Torbenson M, Liu QZ, Lind S, Mulvihill JJ, et al. Leiomyosarcoma of the breast: a pathologic and comparative genomic hybridization study of two cases. Cancer Genet Cytogenet. (2004) 149:53–7. doi: 10.1016/S0165-4608(03)00286-3
36. Munitiz V, Rios A, Canovas J, Ferri B, Sola J, Canovas P, et al. Primitive leiomyosarcoma of the breast: case report and review of the literature. Breast (Edinburgh Scotland). (2004) 13:72–6. doi: 10.1016/j.breast.2003.09.004
37. Stafyla VK, Gauvin JM, and Farley DR. A 53-year-old woman with a leiomyosarcoma of the breast. Curr Surg. (2004) 61:572–5. doi: 10.1016/j.cursur.2004.05.008
38. Jayaram G, Jayalakshmi P, and Yip CH. Leiomyosarcoma of the breast: report of a case with fine needle aspiration cytologic, histologic and immunohistochemical features. Acta Cytol. (2005) 49:656–60. doi: 10.1159/000326256
39. Gupta RK. Needle aspiration cytology and immunohistologic findings in a case of leiomyosarcoma of the breast. Diagn Cytopathol. (2007) 35:254–6. doi: 10.1002/dc.20618
40. De la Pena J and Wapnir I. Leiomyosarcoma of the breast in a patient with a 10-year-history of cyclophosphamide exposure: a case report. cases J. (2008) 1:301. doi: 10.1186/1757-1626-1-301
41. Wong LC, Huang PC, Luh SP, and Huang CS. Primary leiomyosarcoma of the nipple-areola complex: report of a case and review of literature. J Zhejiang Univ Sci B. (2008) 9:109–13. doi: 10.1631/jzus.B0720246
42. Cobanoglu B, Sezer M, Karabulut P, Ozer S, and Murat A. Primary leiomyosarcoma of the breast. Breast J. (2009) 15:423–5. doi: 10.1111/j.1524-4741.2009.00752.x
43. Amberger M, Park T, Petersen B, and Baltazar GA. Primary breast leiomyosarcoma with metastases to the lung in a young adult: Case report and literature review. Int J Surg Case Rep. (2018) 47:34–7. doi: 10.1016/j.ijscr.2018.04.010
44. Singh G, Sharma D, and Goyal S. Primary leiomyosarcoma of breast presenting with metastasis: an atypical presentation with dismal prognosis. Indian J Med Paediatric Oncol. (2017) 38:535–7. doi: 10.4103/ijmpo.ijmpo_139_16
45. Cunli Y, Lindu H, and Xiulin X. Report of a case of breast leiomyosarcoma. Med Forum. (2015) 19:2838 + 2881.
46. Jiafan H, Ningxia W, Rongzheng L, Zhao P, Weili H, and Xin H. A case of primary breast smooth muscle sarcoma in a man. Chin J Breast Disease(Electronic Edition). (2013) 7:66–8.
47. Juan Y and Gang L. Report of a case of breast epithelioid smooth muscle sarcoma diagnosed by ultrasound. Jilin Med J. (2014) 35:7551.
48. Mingzhen X. Clinicopathological observations on primary smooth muscle sarcoma of the breast. Natl Med Front China. (2013) 8:93–4.
49. Na W and Hongzhang C. A case of primary smooth muscle sarcoma of the breast. J Modern Oncol. (2012) 20:621–2.
50. Luping B, Futing J, and Ming H. Report of a case of smooth muscle sarcoma of the male breast. J Chin Phys. (2003) 5:276.
51. Jian Z. A case of primary breast smooth muscle sarcoma in a male. J Dalian Med Univ. (2010) 32:496.
53. Xiaojun L, Guangxian Z, Chuansheng X, and Junying L. A case of giant breast smooth muscle sarcoma in a male. J Air Force Med Univ. (2002), 1721.
54. Peng Y, Hongjiang W, and Zhongyu W. 2 cases of breast smooth muscle sarcoma. China J Gen Surg. (2006), 84.
55. Xu T, Chunlin W, and Qixiang G. Report of a case of smooth muscle sarcoma of the breast. Pract Oncol J. (1999) 279.
56. Tianhui W. Two cases of primary giant smooth muscle sarcoma of the breast. J Cancer Control Treat. (2001) 143.
57. Yaokun C and Qiming S. Primary smooth muscle sarcoma of the breast (with report of 2 cases). J Pract Oncol. (2001) 16.
58. Renya Z. A case of primary angio-smooth muscle sarcoma in the breast. Cancer Res Prev Treat. (1994) 127.
60. Jin W, Yu F, Junying A, Nannan L, Qiang G, and Li F. A case of primary smooth muscle sarcoma of the breast. Chin J Pathol. (2013) 42:766–7.
61. Yongjun W and Cuiling L. A case of primary smooth muscle sarcoma of the breast. Chin J Oncol. (1997) 42.
62. Huaping G and Xuyou L. A case of primary smooth muscle sarcoma of the breast. Cancer Res Prev Treat. (2005), 222–57.
63. Samenova D, Midlenko A, Khamzina Y, Kaldybayev M, and Khamzina S. Primary leiomyosarcoma of the breast: A successful surgical treatment in a 45-year-old woman. Am J Case Rep. (2023) 24:e939437. doi: 10.12659/AJCR.939437
64. Sethi E, Misra S, and Ahuja A. Primary leiomyosarcoma with osteosarcomatous differentiation of the breast. Autopsy Case Rep. (2024) 14:e2024476. doi: 10.4322/acr.2024.476
65. Félix C, Bispo M, and Chagas C. Pancreatic metastasis from primary leiomyosarcoma of the breast. GE Portuguese J Gastroenterol. (2019) 26:223–5. doi: 10.1159/000489866
66. Miyazaki C, Shiozawa M, Koike R, Ogihara K, Sasaki Y, Shiba S, et al. Neoadjuvant chemotherapy for primary sarcoma of the breast: a case report. J Med Case Rep. (2019) 13:289. doi: 10.1186/s13256-019-2197-2
67. Galama R, Matoso J, Capela G, Bôto C, Duarte C, and Mendes AR. A rare etiology of a large tumoral mass of the breast - Case report primary leiomyosarcoma and osteosarcoma of the breast. Int J Surg Case Rep. (2021) 78:201–3. doi: 10.1016/j.ijscr.2020.12.050
68. Lesar M, Juzbasić S, Stanec M, Vrdoljak DV, Milas I, and Bublić J. Leiomyosarcoma of the breast. Acta Med Croatica: Casopis Hravatske Akademije Medicinskih Znanosti. (2003) 57:315–7.
69. Surov A, Holzhausen H-J, Ruschke K, and Spielmann RP. Primary breast sarcoma: prevalence, clinical signs, and radiological features. Acta Radiol. (2011) 52:597–601. doi: 10.1258/ar.2011.100468
70. McGowan TS, Cummings BJ, O'Sullivan B, Catton CN, Miller N, and Panzarella T. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys. (2000) 46:383–90. doi: 10.1016/S0360-3016(99)00444-7
71. Lee JS, Yoon K, Onyshchenko M, and Verhoef C. Sarcoma of the breast: clinical characteristics and outcomes of 991 patients from the national cancer database. Sarcoma. (2021) 2021:1–7. doi: 10.1155/2021/8828158
72. Cheikh TE, Hamza K, Hicham B, Fatiha EM, Hajar EO, Mustapha B, et al. Leiomyosarcoma of the male breast: Case report. Ann Med Surg. (2021) 67:102495. doi: 10.1016/j.amsu.2021.102495
73. Yener O and Aksoy F. Leiomyosarcoma of the female breast: report of a case. Indian J Surg. (2011) 75:90–2. doi: 10.1007/s12262-011-0353-z
74. Singh G, Sharma D, and Goyal S. Primary leiomyosarcoma of breast presenting with metastasis: an atypical presentation with dismal prognosis. Indian J Med Paediatric Oncol. (2021) 38:535–7. doi: 10.4103/ijmpo.ijmpo_139_16
75. Rupert M.P.A.C.K.L. and Fehl CAAJ. A patient-centered approach for the treatment of fungating breast wounds. J Adv Practitioner Oncol. (2020) 11:503–10. doi: 10.6004/jadpro.2020.11.5.6
76. Anastasiou E, Lorentz KO, Stein GJ, and Mitchell PD. Prehistoric schistosomiasis parasite found in the Middle East. Lancet Infect Dis. (2014) 14:553–4. doi: 10.1016/S1473-3099(14)70794-7
77. Lamyman MJ, Giele HP, Critchley P, Whitwell D, Gibbons M, and Athanasou NA. Local recurrence and assessment of sentinel lymph node biopsy in deep soft tissue leiomyosarcoma of the extremities. Clin Sarcoma Res. (2011) 1:7. doi: 10.1186/2045-3329-1-7
78. Wagner MJ, Zhang Y, Cranmer LD, Loggers ET, Black G, McDonnell S, et al. A phase 1/2 trial combining avelumab and trabectedin for advanced liposarcoma and leiomyosarcoma. Clin Cancer Res. (2022) 28:2306–12. doi: 10.1158/1078-0432.CCR-22-0240
79. Italiano A, Bellera C, and D'Angelo S. PD1/PD-L1 targeting in advanced soft-tissue sarcomas: a pooled analysis of phase II trials. J Hematol Oncol. (2020) 13:55. doi: 10.1186/s13045-020-00891-5
80. Xi C, Wencheng Z, Dong Q, Yong G, Cihui Y, Yuwen W, et al. Tumor regression after combination of radiation and PD-1 antibody nivolumab treatment in a patient with metastatic mediastinal leiomyosarcoma: a case report. Cancer Biol Ther. (2019) 20:408–12. doi: 10.1080/15384047.2018.1537577
81. Amaadour L, Benbrahim Z, Moumna K, Boudahna L, Amarti A, Arifi S, et al. Primary breast leiomyosarcoma. Case Rep Oncol Med. (2013) 2013:1–4. doi: 10.1155/2013/732730
Keywords: breast leiomyosarcoma, breast sarcoma, prognosis, overall survival, tumor size
Citation: Guo R, Zhang W, Ji X, Liu X and He L (2025) Primary breast leiomyosarcoma: prognostic factors and treatment outcomes – a systematic review and case report (1969–2023). Front. Oncol. 15:1662132. doi: 10.3389/fonc.2025.1662132
Received: 10 July 2025; Accepted: 14 October 2025;
Published: 30 October 2025.
Edited by:
Mustafa Cem Algin, TC Saglik Bakanligi Eskisehir Sehir Hastanesi, TürkiyeReviewed by:
Narges Bazgir, Shahid Beheshti University, IranElif Berna Koksoy, Ankara University, Türkiye
Copyright © 2025 Guo, Zhang, Ji, Liu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Zhang, end3ODlAMTI2LmNvbQ==; Lang He, aGVsYW5nMTI2QG91dGxvb2suY29t
†These authors have contributed equally to this work
 Rui Guo1†
Rui Guo1† Weiwei Zhang
Weiwei Zhang Lang He
Lang He