- Department of Radiotherapy, The Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, Nanjing, China
Malignant melanoma (MM) is a highly aggressive tumor, with a median overall survival (mOS) of only 8 to 12 months for its metastatic form. However, studies focusing on the efficacy of different radiotherapy (RT) fractionation regimens for MM are limited. Here, we report the case of a 60-year-old male who presented with a one-month history of intermittent abdominal pain and was subsequently diagnosed with MM. Following disease progression on systemic therapy, the patient was treated with different fractionation regimens, including 5 Gy per fraction and 3 Gy per fraction. After the failure of immunotherapy, RT effectively controlled the tumor burden. Notably, the patient received different doses of RT and achieved different outcomes. This case report demonstrates that RT could serve as a viable option for patients who have developed resistance to immunotherapy and low-dose RT may enhance tumor immune response when combined with immunotherapy.
1 Introduction
Malignant melanoma (MM) is s one of the most metastatic human cancers that can arise in the skin, mucous membranes, uvea, and leptomeninges (1). Melanoma of unknown primary (MUP) is defined as metastatic melanoma without a detectable primary lesion, typically found in lymph nodes, subcutaneous tissues, or other distant sites. MUP has a relatively low incidence, accounting for 3-4% of all melanoma cases (2–4). According to the American Joint Committee on Cancer (AJCC) 8th edition staging manual, MUP presenting in lymph nodes or subcutaneous tissue is classified as stage III disease, in contrast, stage IV disease is characterized by distant metastases, including visceral metastases (5). Surgical resection remains the primary treatment for melanoma but is only effective for pre-stage IV disease with minimal regional metastasis (6, 7). For unresectable metastatic melanoma, systemic therapies, particularly immunotherapy and targeted therapy, have become the mainstay of treatment (8, 9). Although melanoma is often radioresistant, radiotherapy remains useful for unresectable or recurrent cases (7).
In this report, we describe a patient with MUP who received multiple courses of radiotherapy (RT). We observed that the irradiated lesions remained stable, with some demonstrating a partial response (PR).
2 Case presentation
2.1 Patient
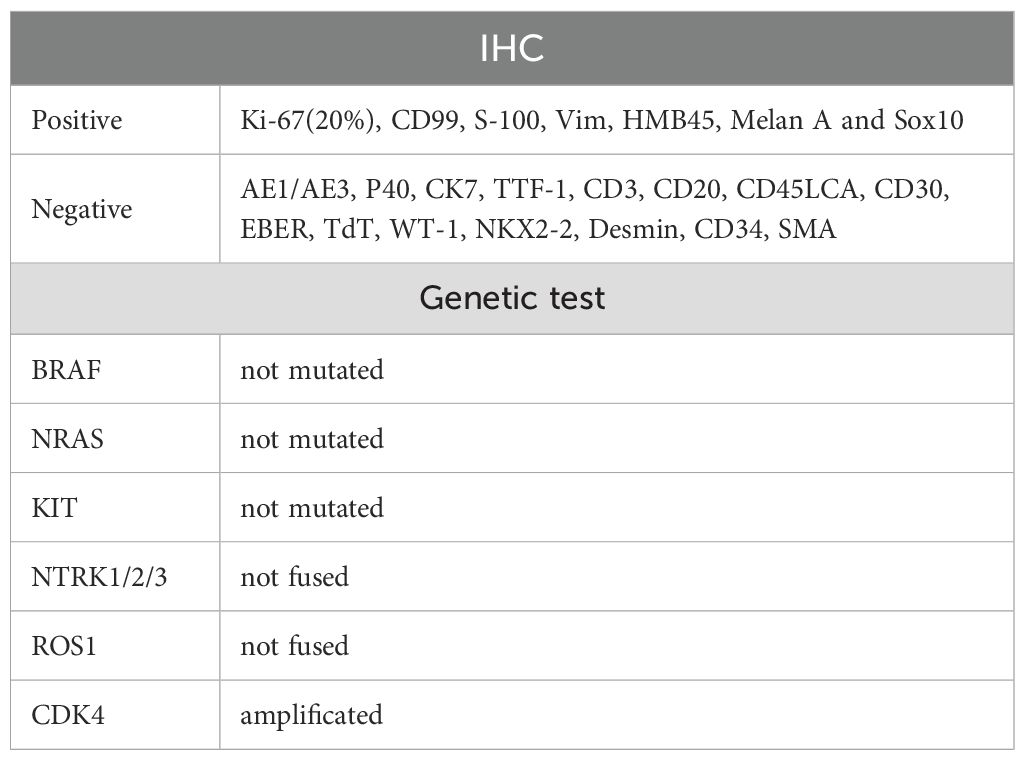
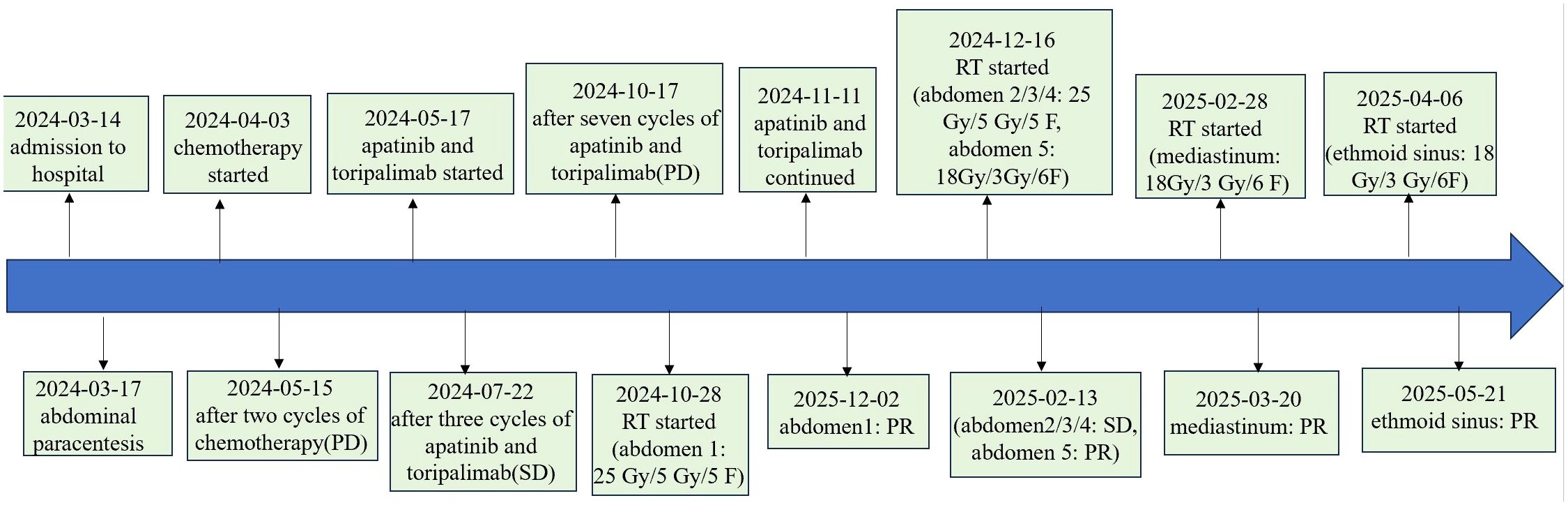
On March 14, 2024, a 60-year-old male presented with abdominal pain. A computed tomography (CT) scan revealed multiple soft-tissue nodules in the abdominopelvic cavity, thoracic cavity, and retroperitoneal space (With a total of six lesions measuring greater than 1 cm, and the largest measuring 9.51 × 4.30 cm), along with enlarged lymph nodes in the anterior mediastinum, bilateral phrenic-diaphragmatic angles, lower esophagus, hepatic hilum, perigastric space, and retroperitoneum. Additionally, inflammatory changes were noted in the left ethmoid sinus. Three days later, the patient underwent abdominal paracentesis. The pathological results showed, microscopically, that round and oval cells were densely arranged in sheets, constituting a tumor lesion. Immunohistochemical (IHC) staining was positive for Ki-67 (20%), CD99, S-100, Vimentin, HMB-45, Melan-A, and SOX10, while being negative for SMA. Genetic testing revealed CDK4 amplification but no mutation in BRAF, NRAS, KIT, and no fusion in NTRK1/2/3 or ROS1(Table 1).
The patient was diagnosed with stage IV MUP according to the 8th edition of the American Joint Committee on Cancer (AJCC) cutaneous melanoma staging system. He initially received two cycles of chemotherapy with albumin-bound paclitaxel (300 mg) and carboplatin (600 mg). However, disease progression was observed. Subsequently, he was treated with toripalimab (240 mg) and apatinib (250 mg). After three cycles, he achieved stable disease (SD). However, after completion of seven cycles, a repeat CT revealed further disease progression, with the largest lesion increasing in diameter from 10.2 cm to 14 cm.
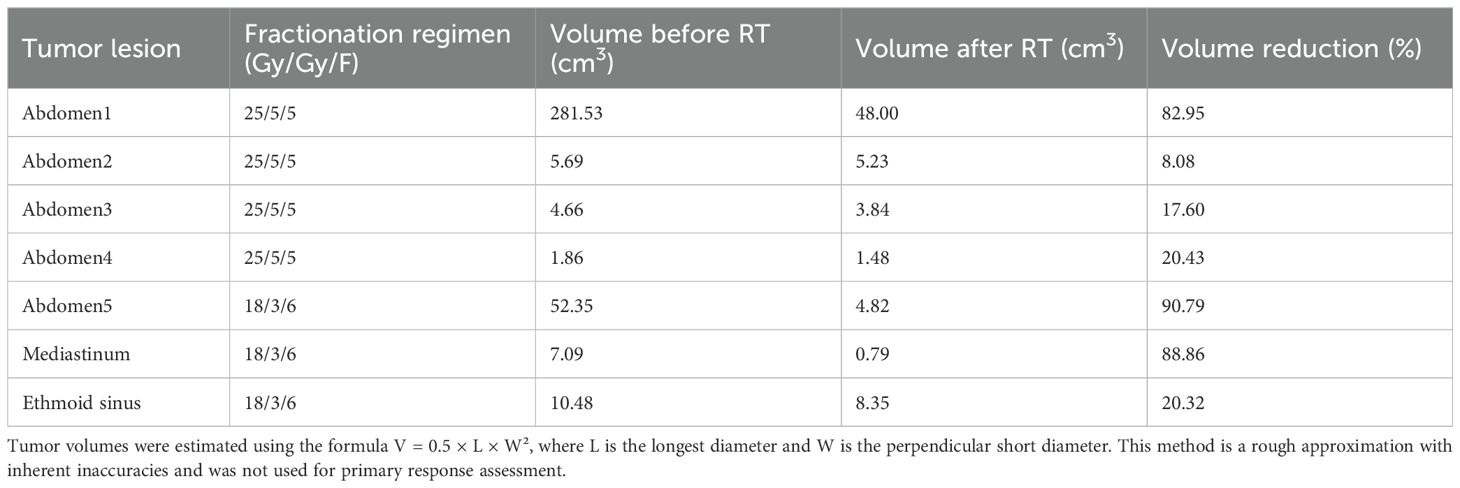
On October 28, 2024, the patient commenced stereotactic body radiation therapy (SBRT), with the largest abdominal lesion receiving a total dose of 25 Gy in 5 fractions (Abdomen1: 25 Gy in 5 fractions). Subsequently, the patient commenced a three-week cycle of combination therapy with toripalimab and apatinib on November 11, 2024. A follow-up CT scan one month later demonstrated PR in the irradiated lesion. (Figures 1A, B). Two weeks later, he received further RT for the remaining larger abdominal lesions (Abdomen2/3/4: 25 Gy in 5 fractions). To minimize gastrointestinal toxicity, a lesion near the stomach was treated with a lower-dose regimen (Abdomen5: 18 Gy in 6 fractions).
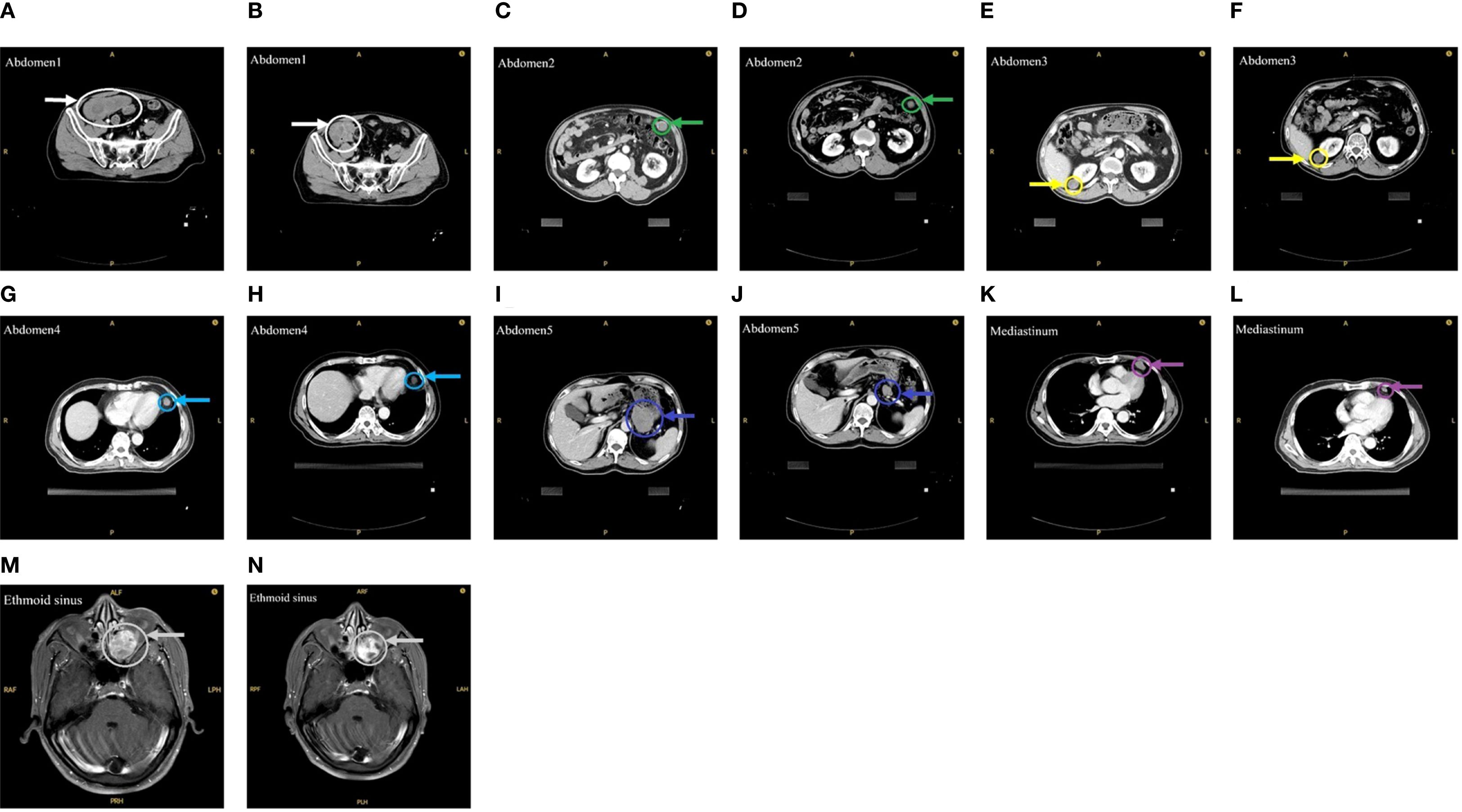
Figure 1. Imaging before and after RT. (A) Before the first RT. (B) After the first RT. (C, E, G, I) Before the second RT. (D, F, H, J) After the second RT. (K) Before the third RT. (L) After the third RT. (M) Before the fourth RT. (N) After the fourth RT.
One month after this course of RT, evaluation showed PR in the low-dose field and SD in the high-dose fields (Figures 1C-J). Subsequently, the mediastinal lesions were irradiated (Mediastinum: 18 Gy in 6 fractions). Three weeks later, imaging showed regression of the mediastinal lesions (Figures 1K, L). During this period, the patient developed nasal bleeding, and magnetic resonance imaging (MRI) revealed a metastasis in the ethmoid sinus. The same radiation dose was administered to this site (Ethmoid sinus: 18 Gy in 6 fractions). A follow-up MRI one month later showed regression of the ethmoid sinus lesion in the (Figures 1M, N). After RT, overall tumor burden markedly decreased (Table 2). Among the 7 lesions, 3 achieved PR and 4 showed SD. Notably, the 4 lesions treated with high-dose irradiation exhibited an average reduction of 32.27%, while the 3 lesions receiving low-dose irradiation demonstrated a more pronounced average shrinkage of 66.66%.
As of May 2025, with over one month elapsed since the final RT session, all irradiated lesions in this patient have maintained PR or SD status. The patient’s Eastern Cooperative Oncology Group (ECOG) performance status was 1, with minimal symptom burden including only mild fatigue. Treatment-related toxicities were limited to grade 1 radiation dermatitis, which showed improvement with symptomatic management. A timeline of the treatment course is provided in Figure 2.
2.2 Radiotherapy
All treatments were delivered using a Varian TrueBeam linear accelerator with 6 MV photon energy, where the dose rate was 1.2 Gy/min for high-dose regimens and 0.6 Gy/min for low-dose regimens. SBRT was used for high-dose irradiation (25 Gy in 5 fractions), while conventional fractionation was employed for low-dose irradiation (18Gy in 6 fractions). RT was administered once daily, five consecutive days per week (Monday to Friday). Response to RT was monitored via serial CT and MRI, with lesion dimensions measured according to RECIST 1.1 criteria. Treatment details, including target volume delineations, and plan evaluation, are provided in Supplementary Materials.
3 Discussion
Melanoma is a highly aggressive malignancy with a rapid progression and poor prognosis, causing approximately 55,000 deaths worldwide annually (10). The diagnosis can be aided by IHC and genetic testing. Common positive IHC markers include S-100, SOX-10 and Melan-A (11), while frequent genetic alterations include BRAF and NRAS mutations (11–15). In this case, the patient’s non-specific clinical presentation and inconclusive imaging findings complicated the initial diagnosis. However, the diagnosis of MM was confirmed through IHC (positive for S-100, SOX10, HMB45, and Melan-A) and histopathological examination. With no prior history of melanoma and no detectable primary lesion upon comprehensive assessment, the patient was diagnosed with melanoma of unknown primary MUP. Notably, inflammatory changes in the left ethmoid sinus were noted at the patient’s initial admission. Following treatment, a lesion developed in the ethmoid sinus. Given the spontaneous regression potential of MM, whether the lesion represented a primary or metastatic focus remained unclear. The patient’s initial treatment with chemotherapy was ineffective.
In recent years, immune checkpoint inhibitors (ICIs) and targeted therapies have significantly improved survival outcomes for patients with advanced melanoma (16). High tumor mutational burden (TMB) is a biomarker for better response to ICIs (17), making ICI-based therapy a cornerstone for metastatic MM (18). Combining anti-angiogenic agents with PD-1 inhibitors can enhance anti-tumor activity and mitigate resistance (19). For instance, toripalimab plus axitinib showed a 48.3% objective response rate (ORR) in advanced mucosal melanoma (20), and lenvatinib plus pembrolizumab provided durable responses in patients with advanced MM who had progressed on prior anti-PD-1 therapy (21). In our case, the patient achieved SD with a PD-1 inhibitor plus an anti-angiogenic drug, suggesting initial efficacy, but eventually developed resistance after seven cycles (22).
Compared to cutaneous melanoma, other melanoma subtypes have fewer BRAF mutations and more frequent KIT mutations (11, 23). This patient had neither, making him ineligible for BRAF or KIT inhibitors. CDK4 gene amplification, an important genetic feature in MM (24), can be targeted (25), but clinical trials of the CDK4 inhibitor abemaciclib have shown low ORRs (0-3.8%) in advanced MM patients (26, 27), and no CDK4 inhibitor has been approved for melanoma treatment to date. Therefore, CDK4 inhibitors were not administered, but clinical trials are needed to clarify their role.
To date, the patient has received four courses of RT. MM is traditionally considered radioresistant, partly due to a low α/β ratio and a high capacity for sublethal damage repair under conventional fractionation (7, 28). Under conventional fractionation, MM has a strong ability to repair sublethal damage, and the cytotoxic effect of conventional fractionation may be offset by efficient sublethal damage repair in melanoma cells (7). Early studies on melanoma showed a complete response rate of 82% (range 67-92%) for patients receiving >4 Gy/F and only 36% (range 21-46%) for <4 Gy/F (25–29). These findings have led to the widespread adoption of hypofractionated radiotherapy for melanoma treatment. The most commonly used regimen delivers 30 Gy in 5 fractions of 6 Gy each, administered twice weekly, with comparable efficacy observed across both cutaneous and mucosal subtypes (30). However, RTOG8305 was a prospective clinical study that included 137 patients with MM, with one group of patients treated with high-dose RT (32 Gy in 4 fractions) and one group treated with low-dose RT (50Gy in 20 fractions) (31). There was no significant difference in tumor regression or local failure rates between the two groups, with an increase in grade 4 toxicity in the high-dose group (31). TROG96-06, a randomized prospective clinical study, reached the same conclusions using the same dose (32). Currently, there is no consensus on the mode and dose of segmentation for MM.
More recently, the combination of ICI and RT has shown promise, even in patients who have failed prior anti-PD-1 therapy (33–35). Preclinical evidence indicates that RT enhances antitumor immunity through multiple mechanisms, such as promoting dendritic cell-mediated antigen presentation, increasing the release of immune-stimulatory mediators, and fostering a pro-inflammatory tumor microenvironment (TME) (36). Funck-Brentano et al. analyzed 26 consecutive patients with advanced melanoma who progressed on ICI and reported that 10 patients (38%) achieved a complete response (CR) or partial response (PR) following combined ICI and hypofractionated RT (37). However, the immunostimulatory effects of RT are influenced by dose and fractionation. High-dose irradiation can induce immunogenic tumor cell death and release tumor-specific antigens (38), while low-dose irradiation may enhance the activation and stimulation of immune cells as well as modulate the stromal microenvironment, thereby potentiating the efficacy of immunotherapy (39, 40). A phase I trial of ipilimumab and SBRT suggested that lower radiation doses (e.g., 24 Gy in 3 fractions) might be more synergistic with immunotherapy, as higher doses could have an antagonistic effect on the immune response (41–43). One case report described a patient with metastatic vaginal mucosal melanoma who was treated with combined immunotherapy and RT. The patient received varying RT doses: high-dose (30 Gy in 5 fractions) to two liver metastases, low-dose (5 Gy in 5 fractions) to another liver lesion, and low-dose (6 Gy in 6 fractions) to a right inguinal lesion, followed by continued immunotherapy. At 24-month follow-up, all irradiated lesions achieved complete response (CR) (44). Another recent case reported local improvement with low-dose scatter radiation (0.9-1.8 Gy) in a patient with stage IV MUP (45). In the present case, we hypothesize that low-dose irradiation may more effectively induce immunogenic cell death and facilitate tumor antigen release. Nevertheless, no significant abscopal effect was observed throughout the treatment course.
In our report, we observed that the lesion treated with a lower dose (18Gy in 6 fractions) demonstrated superior tumor burden reduction compared to those treated with the higher-dose fractions. This finding appears inconsistent with some literature but highlights a critical point: the radiosensitivity of lesions can be heterogeneous, even within the same patient. Studies have revealed significant heterogeneity in RT responses, which arises from complex interactions between radiation dose and TME (46, 47). Using B78 melanoma and MyC-CaP prostate cancer mouse models, Jagodinsky et al. demonstrated that varying radiation doses can induce distinct biological and treatment outcomes even within a single tumor (48).
Several limitations inherent to this case report should be acknowledged. First, the absence of correlative data at the molecular level and immunological parameters precludes validation of the proposed mechanistic hypotheses. Second, the short follow-up period limits the assessment of long-term local control and overall survival outcome. Furthermore, conclusions are constrained by the nature of a single-case report. Finally, the primary site of origin remains undetermined throughout the treatment course, and it is unclear whether the ethmoid sinus lesion represents a primary tumor or a metastatic deposit.
4 Conclusion
In summary, we present a case of MUP with multiple metastases where the diagnosis was confirmed by pathological and immunohistochemical analysis. RT provided effective local control after the patient developed resistance to systemic immunotherapy. This case suggests that RT is a viable option following the development of immune resistance and that lower-dose fractionation may, in some instances, elicit a superior anti-tumor response. However, determining the optimal timing, dose, and fractionation schedule for RT, especially in combination with immunotherapy, remains a significant challenge. Further research is imperative to develop individualized and optimized treatment strategies for patients with MM.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study of human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients or patients next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JS: Writing – original draft, Writing – review & editing, Data curation, Investigation. YG: Investigation, Data curation, Writing – review & editing. PZ: Supervision, Project administration, Writing – review & editing. HG: Writing – review & editing, Resources, Data curation. LW: Supervision, Investigation, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the Wu Jieping Medical Foundation Clinical Research Special Funding (320.6750.2024-13-50); the Xinrui Cancer Research Support Program (cphcf-2023-255).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1662686/full#supplementary-material.
References
1. Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P, Basset-Seguin N, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur J Cancer. (2022) 170:236–55. doi: 10.1016/j.ejca.2022.03.008
2. Persa O-D, Hassel JC, Steeb T, Erdmann M, Karimi B, Stege H, et al. Brief communication: treatment outcomes for advanced melanoma of unknown primary compared with melanoma with known primary. J Immunother. (2024) 47:384–7. doi: 10.1097/CJI.0000000000000537
3. Scott JF, Conic RZ, Thompson CL, Gerstenblith MR, and Bordeaux JS. Stage IV melanoma of unknown primary: A population-based study in the United States from 1973 to 2014. J Am Acad Dermatol. (2018) 79:258–265.e4. doi: 10.1016/j.jaad.2018.03.021
4. Verver D, van der Veldt A, van Akkooi A, Verhoef C, Grünhagen D, and Louwman W. Treatment of melanoma of unknown primary in the era of immunotherapy and targeted therapy: A Dutch population-based study. Int J Cancer. (2020) 146:26–34. doi: 10.1002/ijc.32229
5. Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: evidence-based changes in the american joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. (2017) 67:472–92. doi: 10.3322/caac.21409
6. Levine SM and Shapiro RL. Surgical treatment of malignant melanoma: practical guidelines. Dermatol Clin. (2012) 30(3):487–501. doi: 10.1016/j.det.2012.04.009
7. Testori A, Rutkowski P, Marsden J, Bastholt L, Chiarion-Sileni V, Hauschild A, et al. Surgery and radiotherapy in the treatment of cutaneous melanoma. Ann Oncol. (2009) 20 Suppl 6:vi22–29. doi: 10.1093/annonc/mdp257
8. Domingues B, Lopes JM, Soares P, and Pópulo H. Melanoma treatment in review. ImmunoTargets Ther. (2018) 7:35. doi: 10.2147/ITT.S134842
9. Sood S, Jayachandiran R, and Pandey S. Current advancements and novel strategies in the treatment of metastatic melanoma. Integr Cancer Ther. (2021) 20:1534735421990078. doi: 10.1177/1534735421990078
10. SChadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet. (2018) 392:971–84. doi: 10.1016/S0140-6736(18)31559-9
11. Tímár J and Ladányi A. Molecular pathology of skin melanoma: epidemiology, differential diagnostics, prognosis and therapy prediction. Int J Mol Sci. (2022) 23:5384. doi: 10.3390/ijms23105384
12. Kropp LM, De Los Santos JF, McKee SB, and Conry RM. Radiotherapy to control limited melanoma progression following ipilimumab. J Immunother. (2016) 39:373–8. doi: 10.1097/CJI.0000000000000142
13. Liang Y, Maeda O, Nishida K, Chretien B, and Ando Y. Genomic profiles of patients with skin melanoma in the era of immune checkpoint inhibitors. Cancer Sci. (2025) 116:1107–14. doi: 10.1111/cas.16338
14. Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. (2015) 386:444–51. doi: 10.1016/S0140-6736(15)60898-4
15. Teixido C, Castillo P, Martinez-Vila C, Arance A, and Alos L. Molecular markers and targets in melanoma. Cells. (2021) 10:2320. doi: 10.3390/cells10092320
16. Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. (2019) 381:626–36. doi: 10.1056/NEJMoa1904059
17. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. (2013) 500:415–21. doi: 10.1038/nature12477
18. Robert C, Ribas A, Schachter J, Arance A, Grob J-J, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. (2019) 20:1239–51. doi: 10.1016/S1470-2045(19)30388-2
19. Malekan M, Haass NK, Rokni GR, Gholizadeh N, Ebrahimzadeh MA, and Kazeminejad A. VEGF/VEGFR axis and its signaling in melanoma: Current knowledge toward therapeutic targeting agents and future perspectives. Life Sci. (2024) 345:122563. doi: 10.1016/j.lfs.2024.122563
20. Lian B, Li Z, Wu N, Li M, Chen X, Zheng H, et al. Phase II clinical trial of neoadjuvant anti-PD-1 (toripalimab) combined with axitinib in resectable mucosal melanoma. Ann Oncol. (2024) 35:211–20. doi: 10.1016/j.annonc.2023.10.793
21. Arance A, de la Cruz-Merino L, Petrella TM, Jamal R, Ny L, Carneiro A, et al. Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on a programmed cell death protein-1 or programmed death ligand 1 inhibitor given as monotherapy or in combination. J Clin Oncol. (2023) 41:75–85. doi: 10.1200/JCO.22.00221
22. Lim SY, Shklovskaya E, Lee JH, Pedersen B, Stewart A, Ming Z, et al. The molecular and functional landscape of resistance to immune checkpoint blockade in melanoma. Nat Commun. (2023) 14:1516. doi: 10.1038/s41467-023-36979-y
23. Du Y, Bai X, and Si L. Immunotherapy for mucosal melanoma. Oncol Trans Med. (2023) 9:254. doi: 10.1097/ot9.0000000000000019
24. Sheppard KE and McArthur GA. The cell-cycle regulator CDK4: an emerging therapeutic target in melanoma. Clin Cancer Res. (2013) 19:5320–8. doi: 10.1158/1078-0432.CCR-13-0259
25. Garutti M, Targato G, Buriolla S, Palmero L, Minisini AM, and Puglisi F. CDK4/6 inhibitors in melanoma: A comprehensive review. Cells. (2021) 10:1334. doi: 10.3390/cells10061334
26. Tolaney SM, Sahebjam S, Le Rhun E, Bachelot T, Kabos P, Awada A, et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin Cancer Res. (2020) 26:5310–9. doi: 10.1158/1078-0432.CCR-20-1764
27. Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. (2016) 6:740–53. doi: 10.1158/2159-8290.CD-16-0095
28. Doss LL and Memula N. The radioresponsiveness of melanoma. Int J Radiat Oncol Biol Phys. (1982) 8:1131–4. doi: 10.1016/0360-3016(82)90060-8
29. Harwood AR and Lawson VG. Radiation therapy for melanomas of the head and neck. Head Neck Surg. (1982) 4:468–74. doi: 10.1002/hed.2890040605
30. Ballo MT and Ang KK. Radiation therapy for Malignant melanoma. Surg Clin North Am. (2003) 83:323–42. doi: 10.1016/S0039-6109(02)00096-8
31. Sause WT, Cooper JS, Rush S, Ago CT, Cosmatos D, Coughlin CT, et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys. (1991) 20:429–32. doi: 10.1016/0360-3016(91)90053-7
32. Burmeister BH, Mark Smithers B, Burmeister E, Baumann K, Davis S, Krawitz H, et al. A prospective phase II study of adjuvant postoperative radiation therapy following nodal surgery in Malignant melanoma-Trans Tasman Radiation Oncology Group (TROG) Study 96.06. Radiother Oncol. (2006) 81:136–42. doi: 10.1016/j.radonc.2006.10.001
33. Anderson ES, Postow MA, Wolchok JD, Young RJ, Ballangrud Å, Chan TA, et al. Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer. (2017) 5:76. doi: 10.1186/s40425-017-0282-x
34. Nardin C, Mateus C, Texier M, Lanoy E, Hibat-Allah S, Ammari S, et al. Tolerance and outcomes of stereotactic radiosurgery combined with anti-programmed cell death-1 (pembrolizumab) for melanoma brain metastases. Melanoma Res. (2018) 28:111–9. doi: 10.1097/CMR.0000000000000413
35. Trommer-Nestler M, Marnitz S, Kocher M, Rueß D, Schlaak M, Theurich S, et al. Robotic stereotactic radiosurgery in melanoma patients with brain metastases under simultaneous anti-PD-1 treatment. Int J Mol Sci. (2018) 19:2653. doi: 10.3390/ijms19092653
36. Procureur A, Simonaggio A, Bibault J-E, Oudard S, and Vano Y-A. Enhance the immune checkpoint inhibitors efficacy with radiotherapy induced immunogenic cell death: A comprehensive review and latest developments. Cancers (Basel). (2021) 13:678. doi: 10.3390/cancers13040678
37. Funck-Brentano E, Baghad B, Fort M, Aouidad I, Roger A, Beauchet A, et al. Efficacy of late concurrent hypofractionated radiotherapy in advanced melanoma patients failing anti-PD-1 monotherapy. Int J Cancer. (2020) 147:1707–14. doi: 10.1002/ijc.32934
38. Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, and Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. (2014) 3:e28518. doi: 10.4161/onci.28518
39. Rodriguez-Ruiz ME, Garasa S, Rodriguez I, Solorzano JL, Barbes B, Yanguas A, et al. Intercellular adhesion molecule-1 and vascular cell adhesion molecule are induced by ionizing radiation on lymphatic endothelium. Int J Radiat Oncol Biol Phys. (2017) 97:389–400. doi: 10.1016/j.ijrobp.2016.10.043
40. Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. (2013) 24:589–602. doi: 10.1016/j.ccr.2013.09.014
41. Sundahl N, De Wolf K, Kruse V, Meireson A, Reynders D, Goetghebeur E, et al. Phase 1 dose escalation trial of ipilimumab and stereotactic body radiation therapy in metastatic melanoma. Int J Radiat Oncol Biol Phys. (2018) 100:906–15. doi: 10.1016/j.ijrobp.2017.11.029
42. Schaue D, Ratikan JA, Iwamoto KS, and McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat OncologyBiologyPhysics. (2012) 83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049
43. Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. (2017) 8:15618. doi: 10.1038/ncomms15618
44. Sezen D, Patel RR, Tang C, Onstad M, Nagarajan P, Patel SP, et al. Immunotherapy combined with high- and low-dose radiation to all sites leads to complete clearance of disease in a patient with metastatic vaginal melanoma. Gynecol Oncol. (2021) 161:645–52. doi: 10.1016/j.ygyno.2021.03.017
45. Deng X, Xiang K, He X, Chen S, Guo Q, Wu H, et al. Good response of stage IV melanoma to high−dose radiation therapy combined with immunotherapy: A case report. Oncol Lett. (2024) 28:598. doi: 10.3892/ol.2024.14731
46. Dagogo-Jack I and Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. (2018) 15:81–94. doi: 10.1038/nrclinonc.2017.166
47. Burrell RA, McGranahan N, Bartek J, and Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. (2013) 501:338–45. doi: 10.1038/nature12625
Keywords: malignant melanoma, radiotherapy, melanoma of unknown primary, immunotherapy, targeted therapy, case report
Citation: Shen J, Ge Y, Zhang P, Gao H and Wang L (2025) Diverse radiotherapy fractionation in malignant melanoma: a case report. Front. Oncol. 15:1662686. doi: 10.3389/fonc.2025.1662686
Received: 18 July 2025; Accepted: 10 September 2025;
Published: 26 September 2025.
Edited by:
Tao Song, Zhejiang Provincial People’s Hospital, ChinaReviewed by:
Matthew Scarpelli, Purdue University, United StatesNeda Milosavljevic, University of Kragujevac, Serbia
Treshita Dey, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGI), India
Copyright © 2025 Shen, Ge, Zhang, Gao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijun Wang, ZHJfd2FuZ2xqQG5qbXUuZWR1LmNu
 Jiayi Shen
Jiayi Shen Yizhi Ge
Yizhi Ge Puchang Zhang
Puchang Zhang Lijun Wang
Lijun Wang