- Department of Radiology, Ningbo Yinzhou No. 2 Hospital, Ningbo, Zhejiang, China
Introduction: This study was conducted to evaluate any association between extraprostatic extension (EPE) grade with the risk of pelvic lymph node metastasis (PLNM) of prostate cancer (PCa).
Methods: Magnetic resonance imaging data, as well as clinical and pathological data were collected for 317 patients undergoing radical prostatectomy (RP) along with pelvic lymph node dissection (PLND) at Ningbo Yinzhou No. 2 Hospital from January 2019 to January 2024. The collected magnetic resonance images were scored employing the EPE grade. The factors associated with PLNM were analyzed through Chi-square test and independent sample T-test. Independent risk factors associated with PLNM were identified through Multivariate analyses. The area under the curve (AUC) was calculated and the diagnostic performance of the model was assessed by analyzing the receiver operating characteristic (ROC) curve. The clinical net benefit of EPE grade, biopsy positive rate, and the combined model were examined using clinical decision curves.
Results: Among 317 patients, 33had PLNM. Multifactor analysis demonstrated EPE grade and biopsy positive rate as independent risk factors for PLNM of PCa. The AUC of EPE grade and biopsy positive rate was, respectively, 0.879 and 0.877, and the diagnostic efficiency of PLNM between the two was not statistically significant (P > 0.05). However, when the two approaches were combined, the diagnostic efficiency improved significantly, and the AUC increased to 0.921 (P < 0.05). The analysis of the clinical decision curve revealed a significantly higher clinical net benefit of the combined model than that of the EPE grade and biopsy positive rate.
Conclusions: The EPE grade and biopsy positive rate exhibit an independent correlation with PLNM of PCa. In addition, the combination of the two can significantly enhance the accuracy of predicting PLNM of PCa.
1 Introduction
Prostate cancer (PCa) is the most common cancer in men (1), and pelvic lymph node metastasis (PLNM) is an important factor associated with poor prognosis (2). Pelvic lymph node dissection (PLND) is the “gold standard” for PLNM diagnosis in PCa; however, the indication, scope, and patient benefit of PLND remain controversial (3). The European Association of Urology (EAU) guidelines recommend PLND when the risk of lymph node metastasis is predicted to be ≥5% using the Briganti nomogram (4), while the National Comprehensive Cancer Network (NCCN) recommend PLND when the risk of lymph node metastasis is predicted to be ≥2% using the MSKCC nomogram (5). Although sentinel lymph node biopsy has a diagnostic role, standard pelvic lymph node (SPLND) dissection can only remove 38% of suspicious lymph nodes (6). Multiple studies have shown that the number of lymph nodes obtained and the positive rate are positively correlated with the extent of PLND (7, 8). Heidenreich reported that the lymph node detection rate of extended pelvic lymph node dissection (EPLND) is approximately twice that of SPLND (9). Notably, EPLND significantly increases postoperative complications compared to SPLND (10).
Preoperative diagnosis of PLNM of PCa is crucial for accurate staging and determining optimal treatment. PLNM in PCa usually appear small on magnetic resonance imaging (MRI) (11), and if a threshold of 0.8-1.0cm in short diameter is employed as a criterion, the clinical stage may be underestimated due to low detection sensitivity (12). Ultra-small iron oxide particle MRI or choline positron emission tomography-computed tomography (PET-CT) has demonstrated high accuracy in detecting PLNM (13, 14), nonetheless, the executability of these techniques is low. Therefore, accurately predicting PLNM before radical prostatectomy (RP) of PCa remains the key and challenging aspect of clinical work.
Recent studies analyzing magnetic resonance images to quantitatively grade the likelihood of extraprostatic invasion in PCa, have shown that higher extraprostatic extension (EPE) grade is associated with higher tumor stage, Gleason score, or biochemical recurrence (15). According to these results, a higher EPE grade is associated with more aggressive PCa and a poorer prognosis. Consequently, EPE grade may also be associated with PLNM in PCa.
2 Materials and methods
2.1 Study population
Clinical data of 338 patients who underwent radical prostatectomy (RP) and PLND at Ningbo Yinzhou No. 2 Hospital from January 2019 to January 2024 were collected. Inclusion criteria were: (1) clear magnetic resonance images, (2) less than 3 months of the interval between MRI examination and operation, and (3) complete clinicopathological data. Exclusion criteria: (1) neoadjuvant hormone therapy prior to surgery, (2) preoperative MRI images showed that the tumor had invaded the seminal vesicle or other organs, or imaging findings revealed distant metastasis. Finally, a total of 317 patients were included in the study (Figure 1).
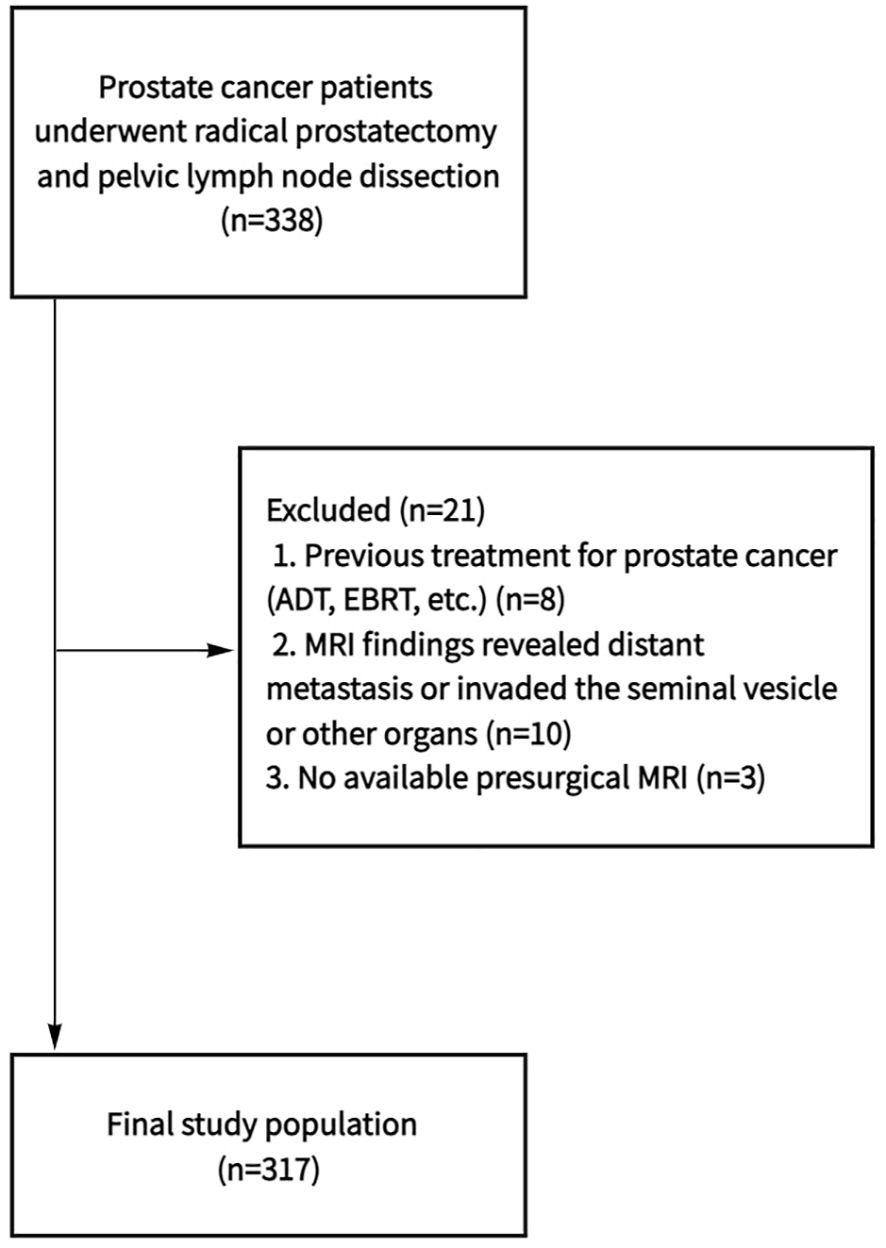
Figure 1. Flowchart shows determination of final study population. ADT, androgen deprivation therapy; EBRT, external beam radiation therapy; MRI, magnetic resonance imaging.
2.2 Magnetic resonance imaging technology
Prostate MRI examination of all patients was performed 3 months prior to surgery using 1.5TMRI (GE SIGNA Voyager) scanner. The scan sequences included T1-weighted imaging (T1WI), dynamic enhanced T1WI, high-resolution T2-weighted imaging (T2WI), and diffusion-weighted imaging (DWI). The imaging scan area comprised the internal iliac artery bifurcation from the external iliac artery to the inguinal area.
2.3 Image analysis
EPE grade were assessed by two experienced radiologists who were blinded to the PLND and pathology results during MRI review, as: grade 0: no suspicion of pathological extrinsic invasion, grade 1: envelope swelling or irregular envelope or tumor envelope contact length ≥ 15mm, grade 2: ≥15 mm contact length of the envelope, with irregular and bulging envelope, grade 3: tumor extension into periprostatic space or invasion of adjacent anatomic structure in magnetic resonance images (Figure 2). Drew a vertical line through the urethra as the center line to divide the prostate into left and right halves, and located the dominant tumor on the left and right sides respectively.
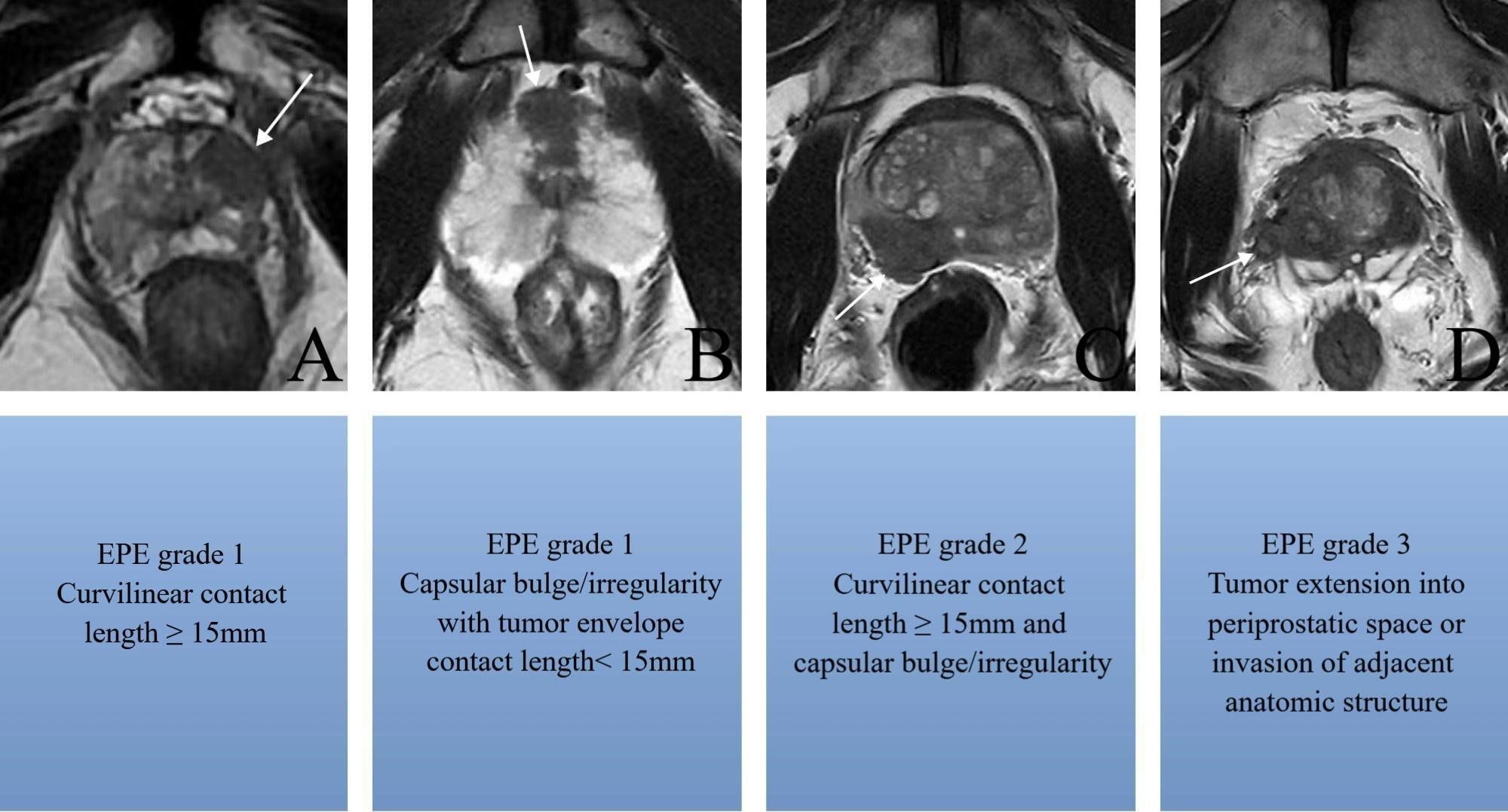
Figure 2. Axial T2-weighted MR images show (A) tumor envelope contact length ≥ 15mm without irregular envelope (arrow), (B) envelope swelling and irregular envelope with tumor envelope contact length< 15mm (arrow), (C) ≥15 mm contact length of the envelope with irregular and bulging envelope(arrow), (D) tumor extension into periprostatic space or invasion of adjacent anatomic structure in magnetic resonance images (arrow).
2.4 Clinical and pathological assessment
In the European Association of Urology Risk Group, patients of PCa categorized as moderate or high risk were treated with EPLND. This procedure includes the dissection of external and internal iliac lymph nodes, obturator lymph nodes, as well as common iliac lymph nodes, following the assessment by the surgeon on treatment effectiveness and possible complications. Prior to surgery, all patients underwent a standard transperineal prostate biopsy (12 needles); for suspected lesions on MRI images, an additional 1–3 needle biopsy was performed. Postoperative specimens were evaluated by two pathologists having more than 10 years of experience.
2.5 Variables
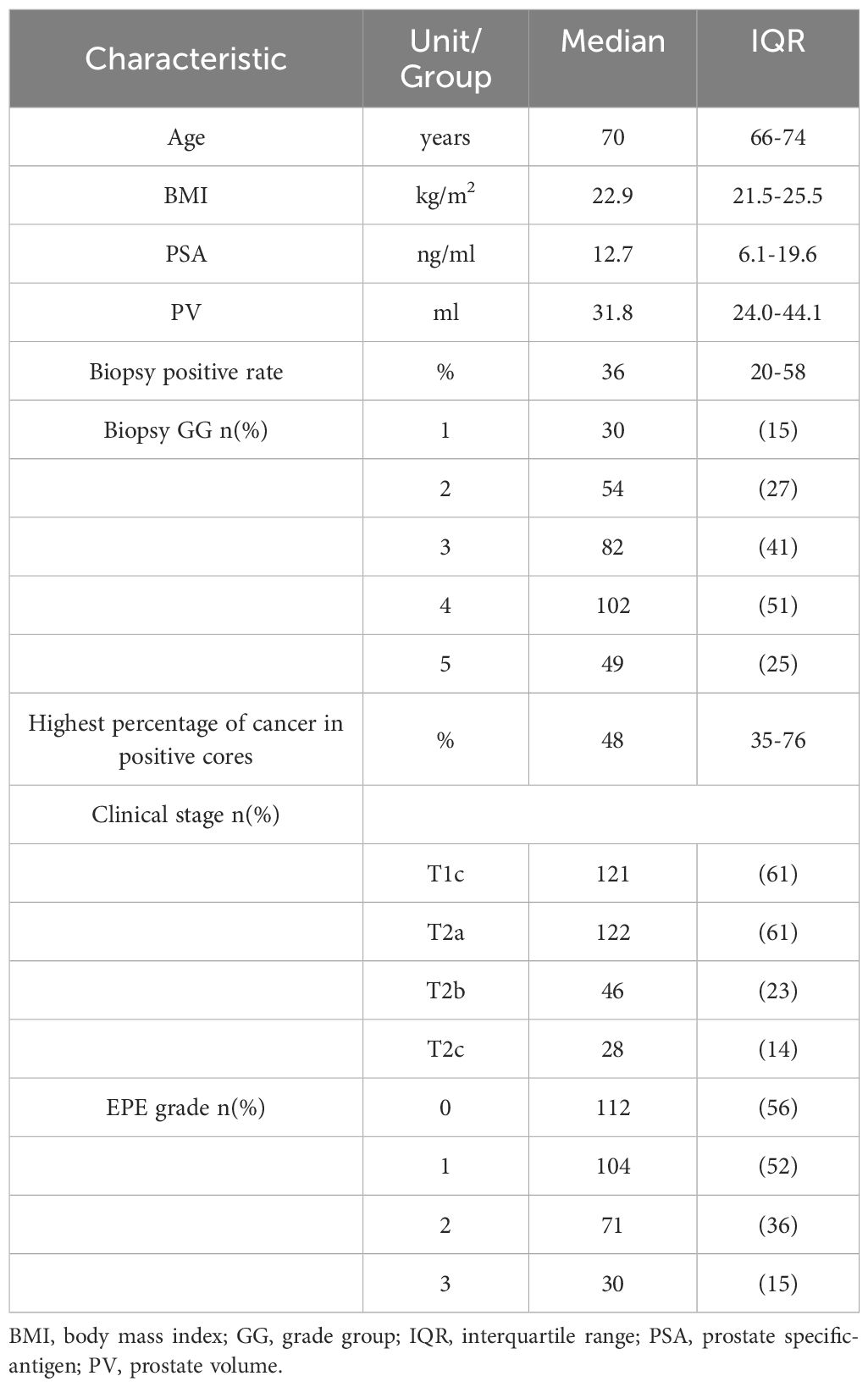
Clinical data included age, body mass index (BMI), prostate-specific antigen (PSA) level, prostate volume (PV), biopsy positive rate, biopsy grade group (GG) (Gleason score ≤6, 3 + 4, 4 + 3, 8, 9–10 corresponding to groups 1-5, respectively), highest percentage of cancer in positive cores, Clinical stage, and the EPE grade of PCa.
2.6 Statistical analysis
Statistical analysis was performed using SPSS(V17.0), Stata (V17.0), and MedCalc(V20.0). The Kappa test was used to evaluate the consistency between the two radiologists. Inter-group comparisons were carried out using the independent sample t-test or Chi-square test. Unless otherwise indicated, data were expressed as the median of the quartile range (IQRS). The independent risk factors for PLNM of PCa were determined through multivariate logistic regression analysis. The receiver operating characteristic (ROC) curves of the subjects corresponding to each independent risk factor and the combined model of independent risk factors were prepared. The area under the curves (AUC) was calculated, and the differences were compared employing the Delong test, the differences being statistically significant at P < 0.05. The decision analysis curve for each independent risk factor and the combined independent risk factor was drawn when predicting PLNM. Next, the clinical benefit of each independent risk factor as well as the combined independent risk factor in predicting PLNM were evaluated by comparing the relative position of each curve and the net profit rate corresponding to different risk thresholds.
3 Results
3.1 Descriptive statistics
This study included 317patients with PCa, 33of whom had PLNM, 8 (mean) Lymph Nodes were resected in PLNM+ group, 19 (58%). 8(24%) and 6 (18%) cases had 1, 2 and > 2 positive Lymph Node, while 16, 13and 4 cases had Lymph Node metastasis on the left (L), right (R), and bilaterally. The locations of dominant tumor with PLNM were as follows: 19 cases on the left side and 14 cases on the right side. Among the 19 cases with dominant tumor on the left side, 13 cases had left PLNM, 3 cases had right PLNM, and 3 case hade bilateral PLNM. Among the 14 cases with dominant tumor on the right side, 10 cases had right PLNM, 3 cases had left PLNM, and 1 case hade bilateral PLNM.
The median (IQR) age was 70 (66-74)years, the BMI was 22.9 (21.5-25.5) kg/m2, the PSA was 12.7 (6.1-19.6) ng/mL, the PV was31.8 (24.0-44.1) mL, the biopsy positive rate was 36 (20-58) %, and Highest percentage of cancer in positive cores was 48 (35-76)%. Biopsy GG 1, 2, 3, 4, and 5 corresponded to, respectively,30, 54, 82, 102, 49 cases. Clinical stage T1c, T2a, T2b, and T2c, respectively, corresponded to 121, 122, 46, and 28 cases. The EPE grades 0, 1, 2, and 3, respectively, corresponded to112, 104, 71, and 30cases (Table 1). The Kappa coefficient for observer consistency in EPE grade was 0.820, indicating a strong consistency.
3.2 Factors associated with pelvic lymph node metastasis
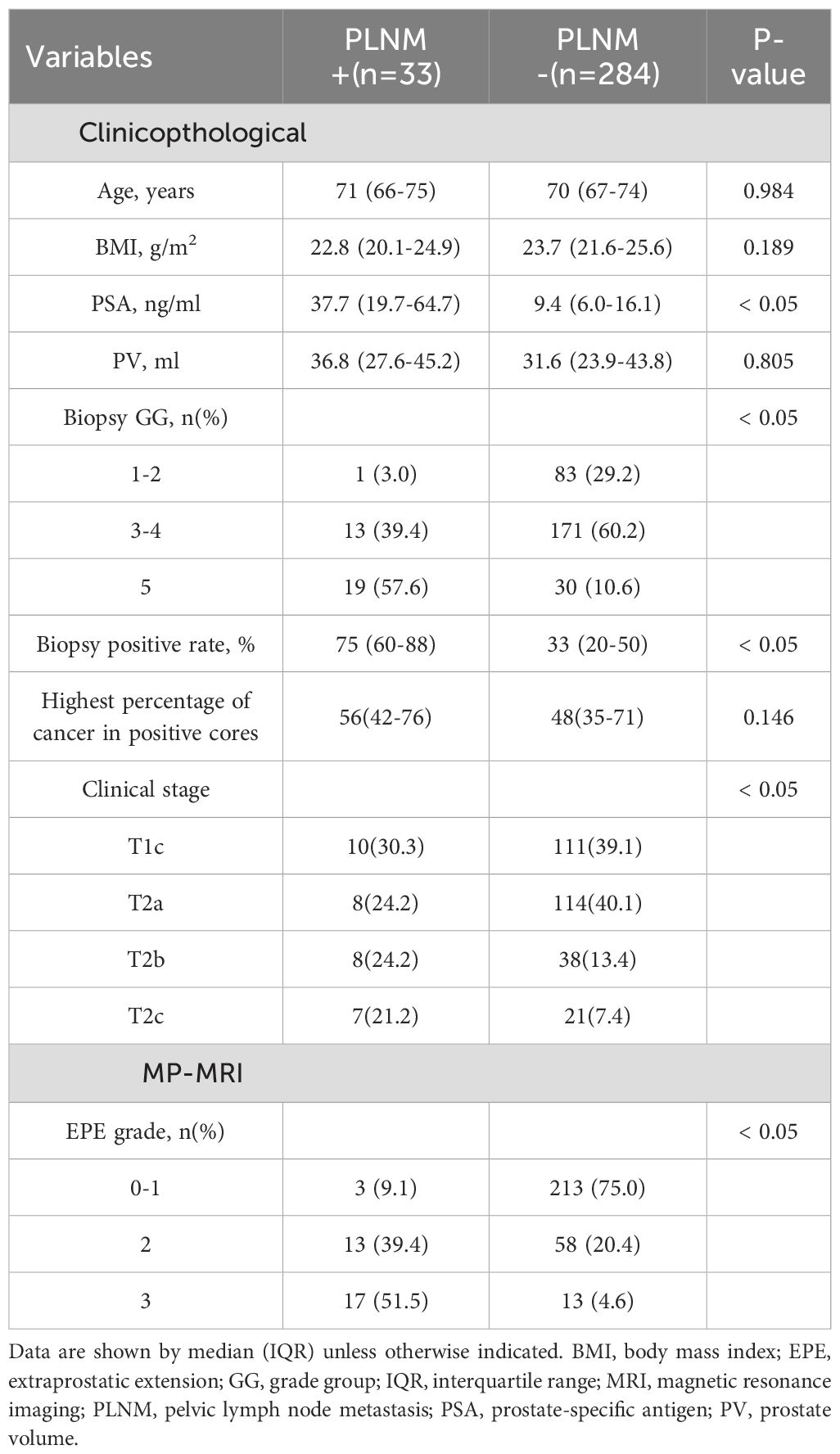
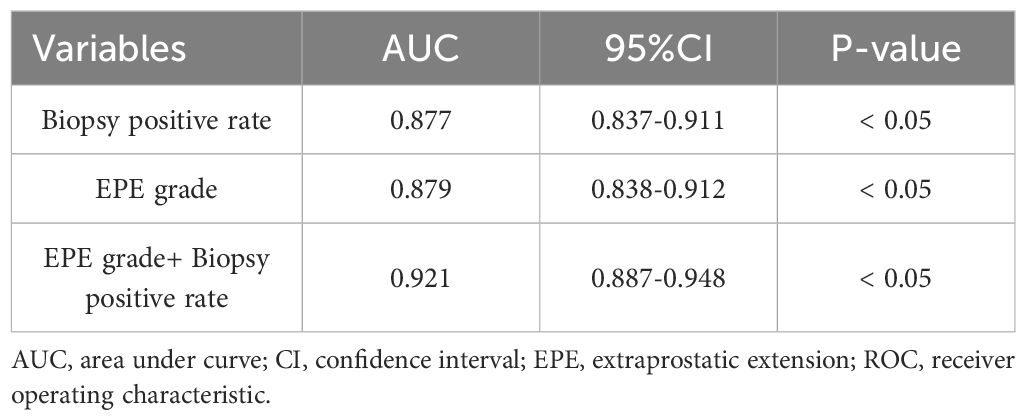
PSA levels were significantly higher in patients with positive PLNM than those in negative patients (37.7[19.7-64.7]vs.9.4 [6.0-16.1] ng/ml, p< 0.05). Patients with positive PLNM also had a higher biopsy-positive rate than that in patients with negative PLNM (75[60-88]vs.33[20-50]%, p< 0.05). Clinical stage, Biopsy GG and EPE grades also differed significantly between positive and negative patients with PLNM (p< 0.05) (Table 2). Multivariate analysis revealed that for PLNM of PCa, biopsy positive rate (p< 0.05) and EPE grade (p< 0.05) were independent risk factors (Table 3). When the EPE grade was greater than or equal to level 2, the Youden index was the largest. The sensitivity, specificity, negative predictive value, and positive predictive value for predicting PLNM were 90.9%, 75.7%, 98.6%, and 29.7%, respectively. The AUC of EPE grade for predicting lymph node metastasis was 0.879.
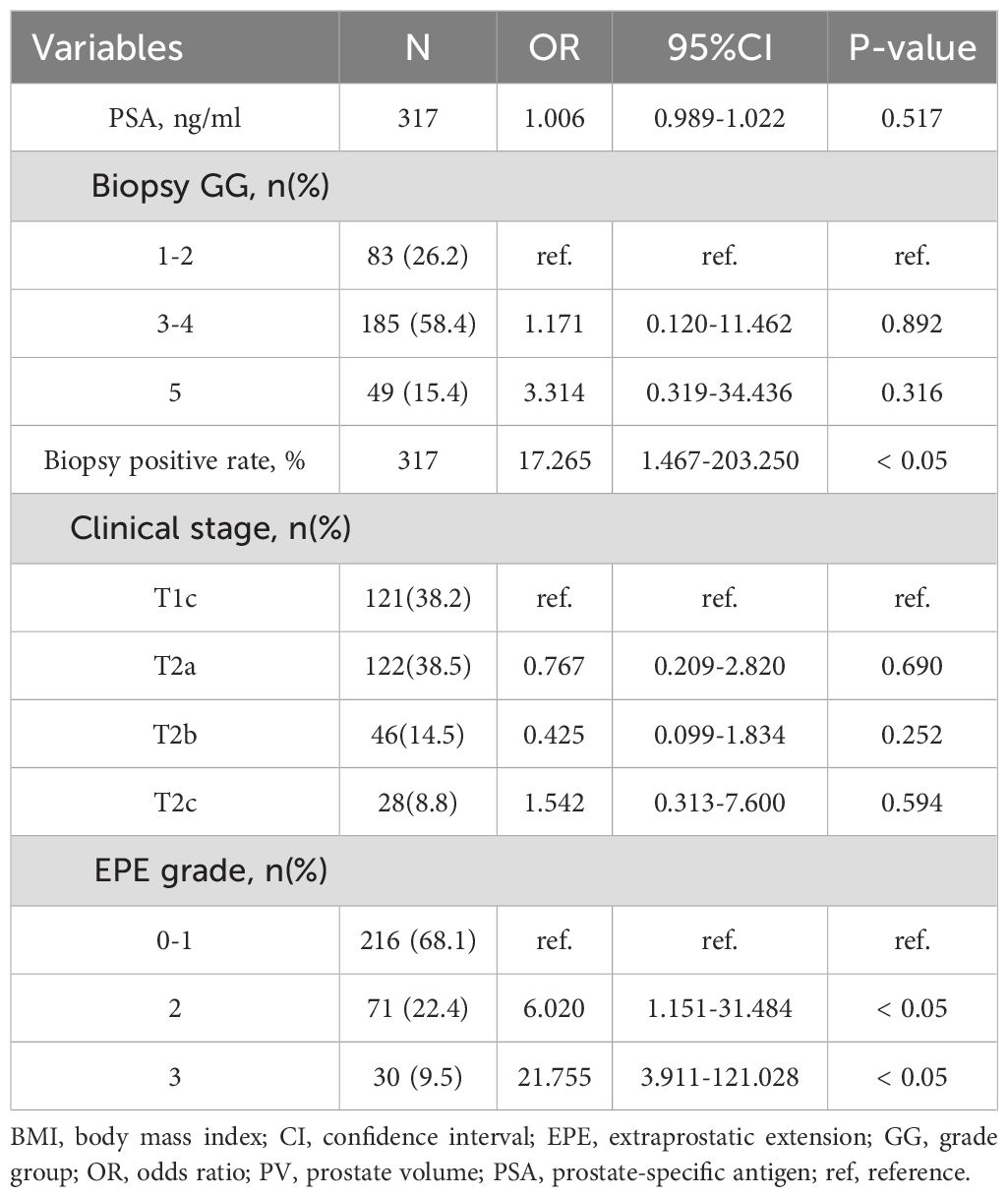
Table 3. Multivariate analysis for predicting pelvic lymph node metastasis using clinical and MRI parameters (n=317).
3.3 Analysis of receiver operating characteristic curves of subjects with pelvic lymph node metastasis
The ROC curve analysis of subjects with PLNM revealed that the respective AUC of EPE grade and the biopsy positive rate were 0.879 (95%CI 0.838-0.912, p< 0.05) and 0.877 (95%CI 0.837-0.911) (p< 0.05) (Table 4) (Figure 3). The prediction value of EPE grade and biopsy positive rate for PLNM did not differ significantly (p > 0.05). Considering both EPE grade and biopsy positive rate (AUC = 0.921), the prediction performance of PLNM was improved significantly compared with any single index considered alone and the difference was statistically significant (p< 0.005).
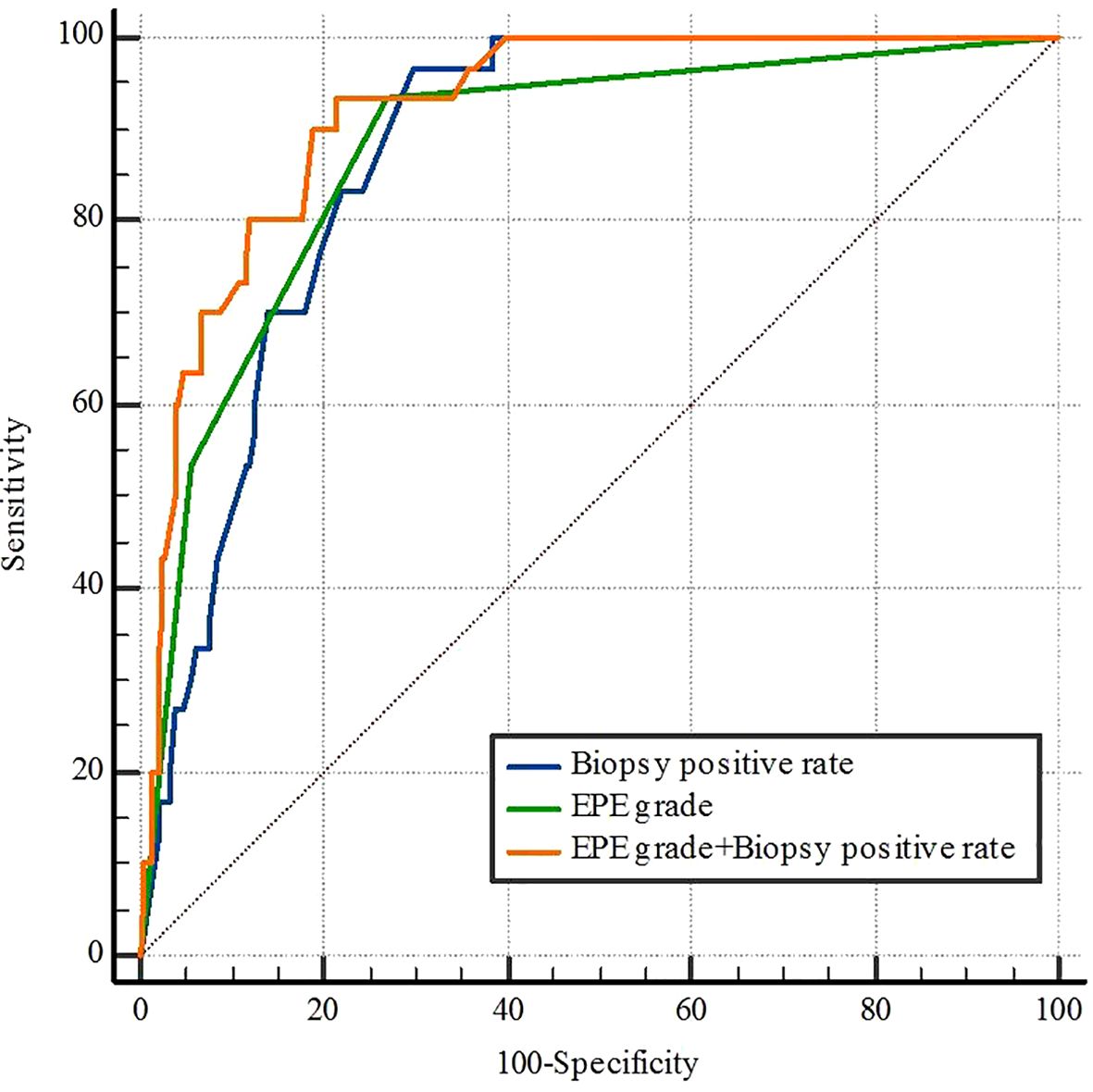
Figure 3. Receiver operating characteristic curves for biopsy positive rate, EPE grade, and EPE grade+ Biopsy positive rate of pelvic lymph node metastasis. EPE, extraprostatic extension.
3.4 The clinical decision curve of biopsy positive rate, extraprostatic extension grade, and combined model
Analysis of the clinical decision curve, revealed that the biopsy positive rate, the EPE grade, and the combined model predicting PLNM were positioned at the upper right of the two extreme curves at different risk thresholds, indicating a higher net benefit to all participants. The combined model, at most risk thresholds, had a significantly higher net benefit than the biopsy positive rate and the EPE grade (Figure 4).
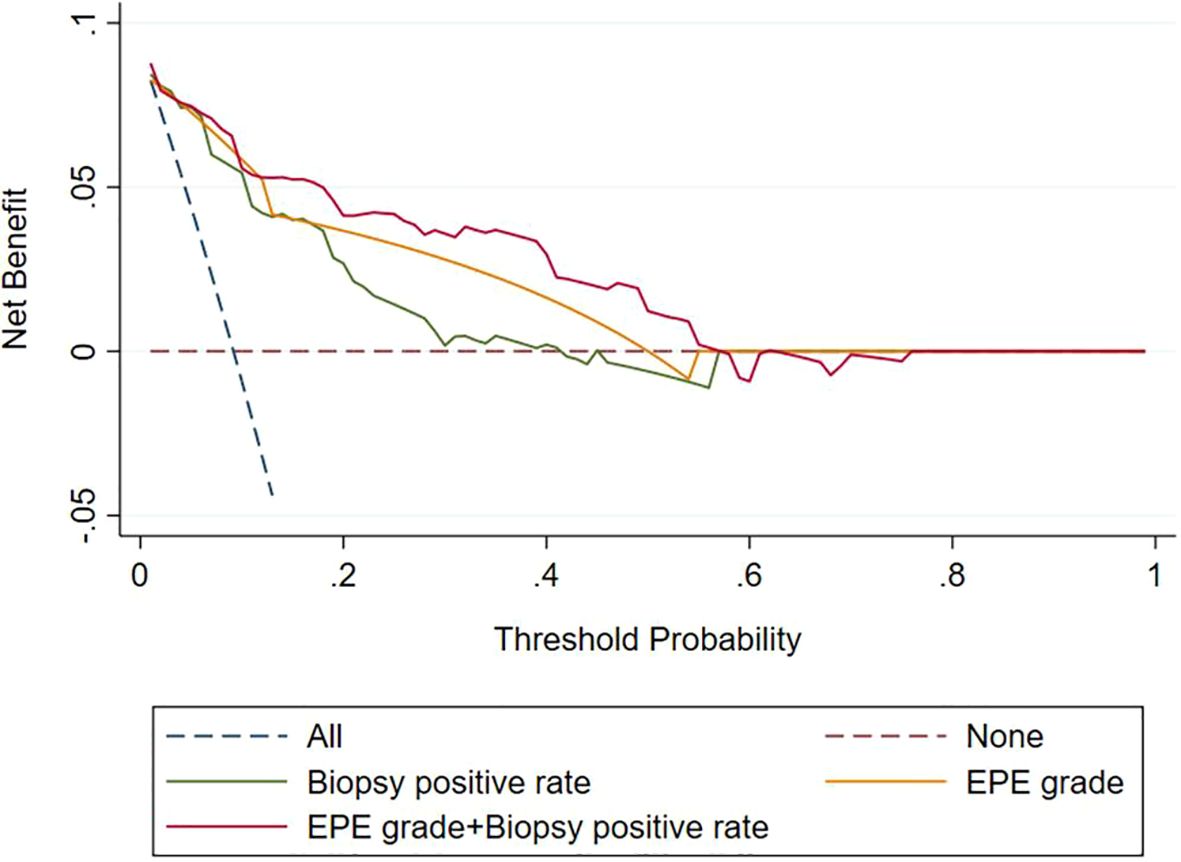
Figure 4. Decision curves of the biopsy positive rate, EPE grade, and EPE grade+ Biopsy positive rate for diagnosing pelvic lymph node metastasis. EPE, extraprostatic extension.
4 Discussion
We used preoperative MRI and clinicopathological data to assess the risk of PLNM in 317 patients with PCa undergoing RP and PLND and found EPE grade and biopsy positive rate as independent risk factors. A combination of these independent risk factors improved the accuracy of predicting PLNM in PCa.
At present, preoperative lymph node staging of PCa relies mainly on CT and MRI, but these imaging methods cannot identify PLNM. Hovels (16) found 39% and 42% sensitivity, respectively, of MRI and CT in predicting lymph node metastasis. PSMA PET-CT demonstrates low sensitivity and high specificity in predicting PLNM in PCa (17). The radiomics nomogram currently has good predictive efficacy for PLNM of PCa, but its reliability needs to be verified further (18, 19).
In this group, the metastasis rate of PLNM of PCa was 10.4%, and that of PLNM of EPE grades 0-I, 2, and 3 were, respectively, 1.4%, 18.3%, and 56.6%, with statistically significant differences between groups. The purpose of EPE grading is to stratify the risk of PCa extrinsic invasion. Studies on the efficacy of EPE grade have found the correlation of EPE grade with pathological Gleason score and clinical stage (20). The probability of lymph node metastasis also correlates highly with tumor stage and pathological Gleason score (21). Outside the prostate capsule, there is a dense lymphatic drainage network. As the tumor grows in size and breaks through the prostate capsule, the density of lymphatic vessels around the tumor increases, hastening the occurrence of pelvic lymph node metastasis of PCa (22). This may explain the study findings that the majority of patients with PLNM had high EPE grade on magnetic resonance images. Therefore, theoretically, the risk of PLNM increases significantly in patients with EPE grade 2 and 3. For such patients, surgical indications should be strictly adhered to, and the risk of PLNM must be fully explained to the patients and their families before surgery.
Clinicopathological factors PLNM of PCa with high accuracy, and PSA and Gleason scores are independent predictors of PLNM of PCa (23). Porcaro found a positive correlation between PSA level and PLNM (P = 0.012) (24), and Yiakoumos also reported PSA density as an important predictor of PLNM of PCa (25). One study based on the SEER database and the American Cancer Database demonstrated a very low risk of PLNM for PCa with a Gleason score of 6 or less and a significantly higher risk, with a Gleason score of 8-10 (26). The risk factors for PLNM of PCa include the number of positive needles and biopsy positive rate, and biopsy positive rate serves as an independent predictor (27). According to recent studies, peripheral monocyte count is also one of the best predictors of PLNM of PCa (28). In this study, the differences between positive and negative PLNM in PSA, biopsy positive rate, and biopsy GG were statistically significant, among which biopsy positive rate was an independent risk factor for PLNM of PCa.
A combination of MRI with clinicopathological indicators approach can enhance the accuracy of predicting PLNM of PCa. Multivariate models incorporating MRI tumor volume, tumor T stage, PSA, and biopsy GG demonstrated a high predictive value for PLNM in PCa (29). In this study, the AUC for predicting PLNM through EPE grade was 0.879, and the AUC for predicting PLNM increased to 0.921 upon employing the combination of EPE grade and the biopsy positive rate, with statistical significance. This study indicates that the combined biopsy positive rate and EPE grade has significant clinical value in predicting PLNM of PCa. To a certain extent, it can provide important reference for clinical treatment decisions regarding PCa. When the EPE grade is greater than or equal to level 2, it suggests that urologists should consider performing PLND for patients of PCa.
In this study, among the 33 patients of PCa in this group who had PLNM, 23 patients had PLNM on the same side as the main lesion of the tumor, 6 patients had PLNM on the contralateral side as the main lesion of the tumor, and 4 cases had bilateral PLNM simultaneously. This was quite similar to that reported by Weckermann (30). It indicates that unilateral lymph node dissection on the side where the main tumor is located alone has a relatively high risk of missing pelvic lymph node metastasis on the contralateral side.
The study also had some limitations. First, the sample size of PLNM+ cases (n=33) is small for multivariable regression, risking model over fitting, Small PLNM+ cohort inflates confidence intervals. Second, as a retrospective study, there are fixed limitations in data collection and a risk of selection bias. Thirdly, All patients came from the same center, resulting in a relatively small overall sample size, the evidence level of the research results is not high, and large-scale, multi-center, and prospective studies are needed for further verification. Lastly, MRI protocol (1.5T) may limit the assessment of EPE grade of PCa.
5 Conclusion
To conclude, there is an independent correlation between EPE grade and the biopsy positive rate with PLNM of PCa. A comprehensive evaluation of these factors is helpful in predicting the probability of PLNM of PCa before surgery, which will be helpful for urologists in deciding whether to perform PLND during RP.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ningbo Yinzhou No 2 Hospital Medical Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
J-GW: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Resources, Validation, Visualization, Writing – original draft. L-LY: Data curation, Supervision, Writing – review & editing. P-PH: Investigation, Software, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wong MC, Goggins WB, Wang HH, Fung FD, Leung C, Wong SY, et al. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. (2016) 70:862–74. doi: 10.1016/j.eururo.2016.05.043
2. Hatano K, Tanaka J, Nakai Y, Nakayama M, Kakimoto KI, Nakanishi K, et al. Utility of index lesion volume assessed by multiparametric MRI combined with Gleason grade for assessment of lymph node involvement in patients with high-risk prostate cancer. Jpn J Clin Oncol. (2020) 50:333–7. doi: 10.1093/jjco/hyz170
3. Lestingi JFP, Guglielmetti GB, Trinh QD, Coelho RF, Pontes J Jr, Bastos DA, et al. Extended versus limited pelvic lymph node dissection during radical prostatectomy for intermediate- and high-risk prostate cancer: early oncological outcomes from a randomized phase 3 trial. Eur Urol. (2021) 79:595–604. doi: 10.1016/j.eururo.2020.11.040
4. Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. (2011) 59:61–71. doi: 10.1016/j.eururo.2010.10.039
5. Mohler J, Bahnson RR, Boston B, Busby JE, D’Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. (2010) 8:162–200. doi: 10.6004/jnccn.2010.0012
6. Mattei A, Fuechsel FG, Bhatta Dhar N, Warncke SH, Thalmann GN, Krause T, et al. The template of the primary lymphatic landing sites of the prostate should be revisited: results of a multimodality mapping study. Eur Urol. (2008) 53:118–25. doi: 10.1016/j.eururo.2007.07.035
7. Bader P, Burkhard FC, Markwalder R, and Studer UE. Is a limited lymph node dissection an adequate staging procedure for prostate cancer? J Urol. (2002) 168:514–8. doi: 10.1016/s0022-5347(05)64670-8
8. Heidenreich A, Ohlmann CH, and Polyakov S. Anatomical extent of pelvic lymphadenectomy in patients undergoing radical prostatectomy. Eur Urol. (2007) 52:29–37. doi: 10.1016/j.eururo.2007.04.020
9. Heidenreich A, Varga Z, and Von Knobloch R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol. (2002) 167:1681–6. doi: 10.1016/S0022-5347(05)65177-4
10. Bluestein DL, Bostwick DG, Bergstralh EJ, and Oesterling JE. Eliminating the need for bilateral pelvic lymphadenectomy in select patients with prostate cancer. J Urol. (1994) 151:1315–20. doi: 10.1016/s0022-5347(17)35239-4
11. McMahon CJ, Rofsky NM, and Pedrosa I. Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology. (2010) 254:31–46. doi: 10.1148/radiol.2541090361
12. Briganti A, Abdollah F, Nini A, Suardi N, Gallina A, Capitanio U, et al. Performance characteristics of computed tomography in detecting lymph node metastases in contemporary patients with prostate cancer treated with extended pelvic lymph node dissection. Eur Urol. (2012) 61:1132–8. doi: 10.1016/j.eururo.2011.11.008
13. Birkhäuser FD, Studer UE, Froehlich JM, Triantafyllou M, Bains LJ, Petralia G, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur Urol. (2013) 64:953–60. doi: 10.1016/j.eururo.2013.07.032
14. Vag T, Heck MM, Beer AJ, Souvatzoglou M, Weirich G, Holzapfel K, et al. Preoperative lymph node staging in patients with primary prostate cancer: comparison and correlation of quantitative imaging parameters in diffusion-weighted imaging and 11C-choline PET/CT. Eur Radiol. (2014) 24:1821–6. doi: 10.1007/s00330-014-3240-8
15. Mehralivand S, Shih JH, Harmon S, Smith C, Bloom J, Czarniecki M, et al. A grading system for the assessment of risk of extraprostatic extension of prostate cancer at multiparametric MRI. Radiology. (2019) 290:709–19. doi: 10.1148/radiol.2018181278
16. Hövels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. (2008) 63:387–95. doi: 10.1016/j.crad.2007.05.022
17. Klingenberg S, Jochumsen MR, Ulhøi BP, Fredsøe J, Sørensen KD, Borre M, et al. (68)Ga-PSMA PET/CT for primary lymph node and distant metastasis NM staging of high-risk prostate cancer. J Nucl Med. (2021) 62:214–20. doi: 10.2967/jnumed.120.245605
18. Liu X, Zhang Y, Sun Z, Wang X, Zhang X, Wang X, et al. Prediction of pelvic lymph node metastasis in prostate cancer using radiomics based on T(2)-weighted imaging. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2022) 47:1025–36. doi: 10.11817/j.issn.1672-7347.2022.210692
19. Liu X, Wang X, Zhang Y, Sun Z, Zhang X, and Wang X. Preoperative prediction of pelvic lymph nodes metastasis in prostate cancer using an ADC-based radiomics model: comparison with clinical nomograms and PI-RADS assessment. Abdom Radiol (NY). (2022) 47:3327–37. doi: 10.1007/s00261-022-03583-5
20. Kim SH, Cho SH, Kim WH, Kim HJ, Park JM, Kim GC, et al. Predictors of extraprostatic extension in patients with prostate cancer. J Clin Med. (2023) 12:2077–0383. doi: 10.3390/jcm12165321
21. Xu N, Ke ZB, Chen YH, Wu YP, Chen SH, Wei Y, et al. Risk factors for pathologically confirmed lymph nodes metastasis in patients with clinical T2N0M0 stage prostate cancer. Front Oncol. (2020) 10:1547. doi: 10.3389/fonc.2020.01547
22. Kostis G, Ioannis L, Helen K, and Helen P. The expression of vascular endothelial growth factor-C correlates with lymphatic microvessel density and lymph node metastasis in prostate carcinoma: An immunohistochemical study. Urol Ann. (2014) 6:224–30. doi: 10.4103/0974-7796.134275
23. Wang X, Zhang X, Li H, Zhang M, Liu Y, and Li X. Application of machine learning algorithm in prediction of lymph node metastasis in patients with intermediate and high-risk prostate cancer. J Cancer Res Clin Oncol. (2023) 149:8759–68. doi: 10.1007/s00432-023-04816-w
24. Porcaro AB, Tafuri A, Sebben M, Processali T, Pirozzi M, Amigoni N, et al. High body mass index predicts multiple prostate cancer lymph node metastases after radical prostatectomy and extended pelvic lymph node dissection. Asian J Androl. (2020) 22:323–9. doi: 10.4103/aja.aja_70_19
25. Yiakoumos T, Kälble T, and Rausch S. Prostate-specific antigen density as a parameter for the prediction of positive lymph nodes at radical prostatectomy. Urol Ann. (2015) 7:433–7. doi: 10.4103/0974-7796.152118
26. Daugherty M, Sedaghatpour D, Bratslavsky G, and Shapiro O. Is pelvic lymph node dissection necessary in patients with biopsy proven Gleason 6 prostate cancer? - analysis of the SEER database. Can J Urol. (2018) 25:9414–20.
27. Porcaro AB, de Luyk N, Corsi P, Sebben M, Tafuri A, Mattevi D, et al. Clinical factors predicting and stratifying the risk of lymph node invasion in localized prostate cancer. Urol Int. (2017) 99:207–14. doi: 10.1159/000458763
28. Zhou JW, Mao YH, Liu Y, Liang HT, Samtani CC, Fu YW, et al. A novel robust nomogram based on peripheral monocyte counts for predicting lymph node metastasis of prostate cancer. Asian J Androl. (2021) 23:409–14. doi: 10.4103/aja.aja_89_20
29. Brembilla G, Dell’Oglio P, Stabile A, Ambrosi A, Cristel G, Brunetti L, et al. Preoperative multiparametric MRI of the prostate for the prediction of lymph node metastases in prostate cancer patients treated with extended pelvic lymph node dissection. Eur Radiol. (2018) 28:1969–76. doi: 10.1007/s00330-017-5229-6
Keywords: prostate cancer, magnetic resonance imaging, lymph node metastasis, extraprostatic extension, biopsy positive rate
Citation: Wang J-g, Ying L-l and He P-p (2025) The influence of extraprostatic extension grade on the detection of pelvic lymph node metastasis in prostate cancer. Front. Oncol. 15:1663809. doi: 10.3389/fonc.2025.1663809
Received: 11 July 2025; Accepted: 10 October 2025;
Published: 22 October 2025.
Edited by:
Xiaobo Wu, The Chinese University of Hong Kong, ChinaReviewed by:
Yi Xiao Liu, The Chinese University of Hong Kong, ChinaZhumeilin Zhu, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, China
Copyright © 2025 Wang, Ying and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-guang Wang, dHNqdW5ndWFuZ0AxNjMuY29t
 Jun-guang Wang
Jun-guang Wang Ling-ling Ying
Ling-ling Ying