- Department of Pathology, Affiliated Hospital of Jining Medical University, Jining, Shandong, China
Gastric SMARCA4-deficient carcinosarcoma and sarcomatoid carcinoma are rare with poor prognosis. In the present study, two male patients were hospitalized due to abdominal manifestations, specifically abdominal pain and a sense of fullness and discomfort. Gastroscopic examination revealed huge raised lesions in the stomach of both individuals, subsequently leading to laparoscopic radical total gastrectomy. Histopathological analysis of surgical specimens obtained from both patients demonstrated the presence of carcinomatous and sarcomatous components. In Case 1, the sarcoma region was negative for the SMARCA4, epithelial markers cytokeratin (CK) and epithelial membrane antigen (EMA), CK, EMA, and SMARCA4 were positively expressed in the adenocarcinoma area, thereby warranting carcinosarcoma diagnosis. The sarcomatous component was identified as a tumor characterized by SMARCA4 deficiency. In Case 2, the tumor cells exhibited positive staining for CK and Vimentin, negative staining for SMARCA4, and the patient was positive for SMARCA4-deficient sarcomatoid carcinoma.
Introduction
SMARCA4 mutations occur in 7% of human tumors (1) and affect multiple organ systems. The mutation frequency of SMARCA4 varies significantly across different tumor types. It has been reported to be mutated in 15% of Burkitt’s lymphoma (2), 10-35% of non-small-cell lung carcinoma (3, 4), 5-10% of medulloblastoma and melanoma (5–7). The mutation rate of SMARCA4 in gastric cancer is relatively low, approximately 2% (8).SMARCA4-deficient tumors are typically poorly differentiated or undifferentiated, exhibit highly malignant and invasive characteristics, and show resistance to chemotherapy (4). Histologically, adenocarcinoma is the most frequently observed cancer (8), while sarcomatoid and carcinosarcomas variants are uncommon.
This study presents two cases of SMARCA4-deficient gastric tumors: one case of carcinosarcoma and one of sarcomatoid carcinoma. It elaborates on their histological similarities and differences and further explores potential therapeutic approaches for SMARCA4-deficient gastric tumors. The main clinicopathologic characteristics of these two patients are summarized in Table 1.
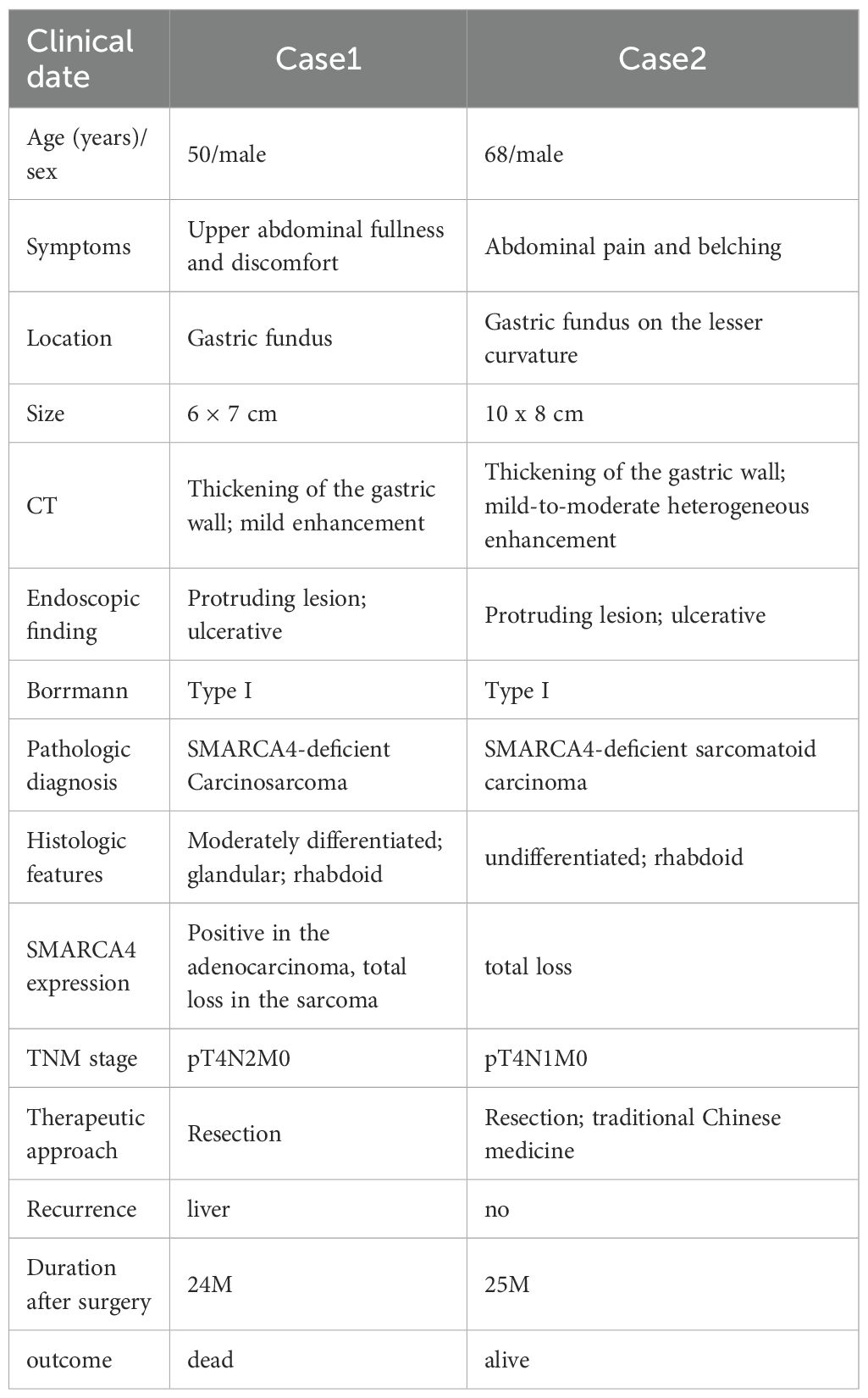
Table 1. The clinicopathologic features of gastric SMARCA4-deficient carcinosarcoma and sarcomatoid carcinoma.
Case presentation
Case1
A 50-year-old male patient was admitted to the hospital for upper abdominal fullness and discomfort lasting 6 months and difficulty in swallowing for more than 20 days. The patient had no specific medical or family history. Physical examination revealed deep tenderness in the upper abdomen. The serum tumor-related antigen was 99.47 U/mL (normal, 0–95 U/mL); all other laboratory results were normal. Enhanced computed tomography (CT) showed thickening of the gastric cardia wall in the lower esophageal segment with mild enhancement (Figure 1A); multiple hepatic cysts. Gastroscopy revealed a large protruding lesion on the gastric fundus mucosa with a ruptured surface, Borrmann type I (Figure 1C). Four biopsy specimens were taken from the edge of the ulceration. The biopsy pathology was diagnosed as tumor with SMARCA4 deficiency. Immunohistochemistry result: HMB-45(-), S-100(-), EMA(-), CAM5.2(-), ERG(-), INI-1(+), CK局灶(+), CK7(-), CK5/6(-), p40(-), Vimentin(+), SMARCA4(-), Ki-67(+,70-80%).
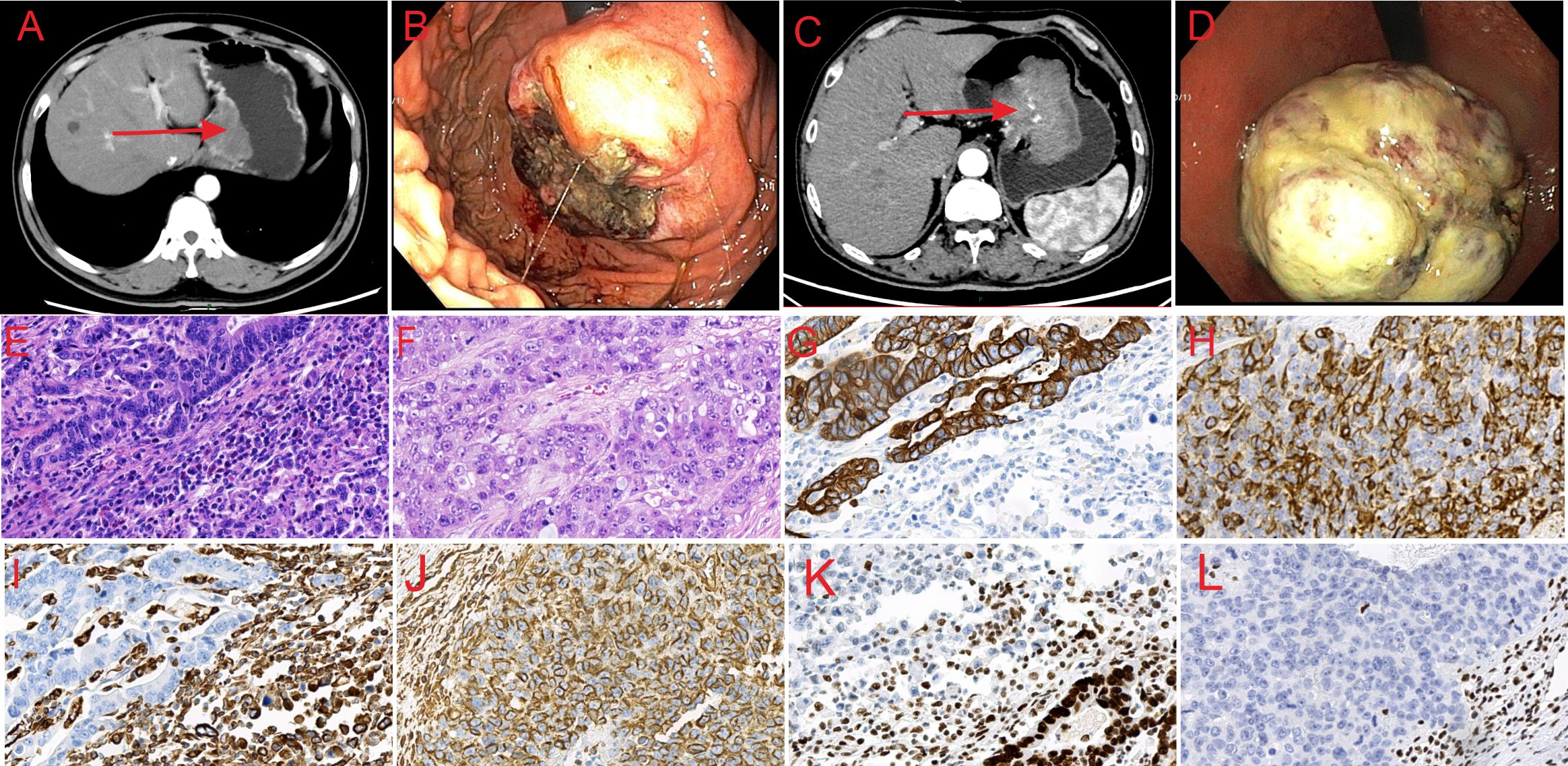
Figure 1. Abdominal CT, endoscopic and histological features of the lesions. (A) CT image of SMARCA4-deficient carcinosarcoma showing thickening of the lower esophageal segment and gastric cardia wall. (B) CT image of SMARCA4-deficient sarcomatoid carcinoma showing thickening of the gastric lesser curvature and side-cardia wall, with an irregular mass shadow. (C) Endoscopic image of SMARCA4-deficient carcinosarcoma showing a large, protruding lesion on the gastric fundus mucosa, with a ruptured surface. (D) Endoscopic image of SMARCA4-deficient sarcomatoid carcinoma showing a large, protruding lesion near the gastric fundus on the lesser curvature. (E) Microscopic image of SMARCA4-deficient carcinosarcoma (HE, ×40). (F) Microscopic image of SMARCA4-deficient sarcomatoid carcinoma (HE, ×40). (G) CK staining shows positive expression in the adenocarcinoma area and negative expression in the sarcoma area of SMARCA4-deficient carcinosarcoma (×40). (H) CK staining showing positive expression in SMARCA4-deficient sarcomatoid carcinoma (×40). (I) Vimentin staining showed negative expression in the adenocarcinoma area and positive expression in the sarcoma area of SMARCA4-deficient carcinosarcoma (×40). (J) Vimentin staining showing positive expression in SMARCA4-deficient sarcomatoid carcinoma (×40). (K) SMARCA4 staining showing positive expression in the adenocarcinoma area and negative expression in the sarcoma area of SMARCA4-deficient carcinosarcoma (×40). (L) SMARCA4 staining showing negative expression in SMARCA4-deficient sarcomatoid carcinoma (×40).
The patient underwent radical total gastrectomy with esophagojejunostomy, but declined postoperative adjuvant treatment. Lymph nodes no. 1, 2, 3, 5, 6, 7, 8, 9 and 11 were removed during the operation. The tumor measured approximately 7 cm × 6 cm. Histopathological analysis revealed a mixed tumor composed of moderately differentiated adenocarcinoma and sarcoma. In the adenocarcinoma region, cancer cells formed glandular tubular structures with fibrinoid necrosis. The boundary between the adenocarcinoma and sarcomatous areas was unclear. Sarcomatous cells were loosely arranged, spindle-shaped, uniform in size, and displayed bizarre nuclei, numerous mitotic figures, rich cytoplasm, eccentric nuclei, large nuclei, obvious nucleoli, and many inflammatory cell infiltrations (Figure 1E). The tumor invaded the entire thickness of the gastric wall and the nerve. Cancer emboli were observed in the vessels. There were three lymph node metastases: one was an adenocarcinoma, and two were sarcoma components. Immunohistochemistry revealed that CK (Figure 1G), EMA, and SMARCA4 (Figure 1K) were positively expressed in the adenocarcinoma area, whereas Vimentin (Figure 1I) was negative; CK (Figure 1G), EMA, SMARCA4 (Figure 1K), ERG, Desmin, CD34, HMB-45, S100, CK7, CK5/6, and p40 were negatively expressed in the sarcoma area, while Ki-67(+50–60%), Vimentin (Figure 1I), and INI-1 were positively expressed. The final diagnosis was carcinosarcoma with a SMARCA4 deficiency in the sarcoma area; pT4N2M0.
The patient underwent a re-examination 12 months after surgery. CEA, CA199, and CA125 were all within the normal ranges. Color ultrasound showed that the hypoechoic area in the left lobe of the liver was inclined to be a benign lesion, which required dynamic observation, and multiple cysts in the liver. Eighteen months after surgery, the patient was readmitted to hospital for liver metastasis detected by a CT scan at an out-of-town hospital, but refused further examinations and treatment. The patient ultimately survived for 24 months. The patient’s timeline of consultations is shown in Figure 2.
Case2
A 68-year-old male patient was admitted to the hospital for abdominal pain and belching lasting over 1 month. The patient had no specific medical or family history. Laboratory tests showed normal levels of CA19-9, carcinoembryonic antigen, and alpha-fetoprotein. Enhanced CT revealed thickening of the gastric lesser curvature at the cardia wall, with an irregular mass, and mild-to-moderate heterogeneous enhancement (Figure 1B). Gastroscopy showed a large protruding lesion near the gastric fundus on the lesser curvature, with ulceration on the oral side, Borrmann type I (Figure 1D). Six biopsy specimens were taken from the oral side. The biopsy pathology was diagnosed as adenocarcinoma of the gastric body with necrosis. The patient underwent radical total gastrectomy with esophagojejunostomy. Perigastric lymph nodes were removed during the operation.
The tumor measured approximately 10 cm × 8 cm. Microscopically, the tumor cells were arranged disorderly, without the structural hierarchy of normal tissues. They contained cancerous and sarcomatoid components. The cancerous component contained moderately differentiated epithelial-like nest cells, while the sarcomatoid component featured diffuse spindle growth cells invading the entire thickness of the gastric wall (Figure 1F). Cancer emboli were detected in the vessels. CK (Figure 1H), Vimentin (Figure 1J) and Ki-67 (>75%) were positive in the tumor cells, whereas SMARCA4 (Figure 1L), CD20, LCA, CD3, HMB-45, Melan-A, Epstein-Barr virus (EBV) were negative. Mismatch repair proteins were intact. The final diagnosis was SMARCA4-deficient sarcomatoid carcinoma; pT4N1M0.
After surgery, the patient refused chemotherapy and received traditional Chinese medicine (TCM) treatment at another hospital, with no recurrence or metastasis, during the 25-month telephone follow-up. The patient’s timeline of consultations is shown in Figure 3.
Discussion
The SWitch/sucrose non-fermentable (SWI/SNF) chromatin remodeling complex is a multi-protein complex consisting of 10–15 subunits, regulates gene activity through chromatin remodeling processes, maintains genomic stability, and controls cell growth, differentiation, and other biological behaviors. SMARCA4 is the primary SWI/SNF complex subunit that generates energy through the catalysis of ATP hydrolysis, which is essential for ensuring the normal function of the complex, thereby maintaining the normal cell physiological function and gene expression patterns (9).
SMARCA4 mutations occur in almost all small cell carcinoma of the ovary hypercalcemic type (10) and 8% of non-small cell lung cancer cases (3). Gastric SMARCA4-deficient carcinosarcoma and sarcomatoid carcinoma are particularly rare. In the literature review, we identified only 4 previously reported cases of SMARCA4-deficient gastrointestinal tumors with sarcomatoid components that had a definitive pathological diagnosis. These cases were designated as carcinosarcomas or sarcomatoid carcinomas (11–13). A summary of these cases is provided in Table 2. The patients ranged in age from 46 to 73 years (mean, 66.3 years) with a male-to-female ratio of 3:1. The tumors were located at the esophagogastric junction (1 case), rectum (1 case), and colon (2 cases), with sizes ranging from 3.5 to 17 cm. Including the 2 cases reported in the present study, 5 out of 6 patients underwent surgical resection, among whom 2 received other postoperative adjuvant therapies and achieved favorable outcomes. Detailed information is presented in Table 2. SMARCA4-deficient tumors with sarcomatoid components in the chest and rectum (12, 14) are often associated with claudin-4 and SMARCA2 deficiencies; they had larger primary tumor volumes and shorter survival times than other types of SMARCA4-deficient tumors.
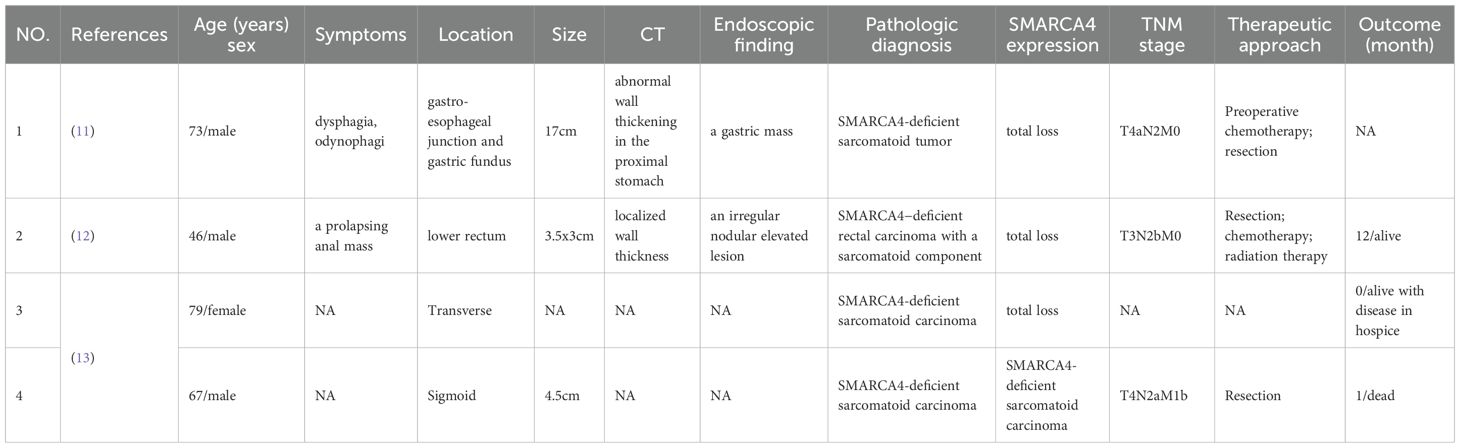
Table 2. Reported cases: clinicopathologic features of SMARCA4-deficient gastrointestinal tumors with sarcomatoid components.
Carcinosarcomas and sarcomatoid carcinomas share some morphological overlap; however, they are distinct tumor entities (15). The SMARCA4-deficient carcinosarcoma reported in this study is an unclassified pleomorphic sarcoma, which is immunoreactive to the stromal marker Vimentin but non-immunoreactive to the epithelial markers CK, EMA. The cancerous component is a moderately differentiated adenocarcinoma. There is no transitional zone between the two components. The SMARCA4-deficient sarcomatoid carcinoma is essentially a special type of epithelial-derived carcinoma, in which tumor cells undergo mesenchymal-like morphological transformation. Tumor cells express CK and Vimentin. The cancerous component manifests as nests of epithelial-like cells, and there is a distinct transitional zone between the two components.
The cases described in this study require differentiation from malignant rhabdoid tumors, lymphomas, epithelioid gastrointestinal stromal tumors, melanomas, undifferentiated gastric carcinomas, NUT midline carcinomas, and other neoplastic entities. Most of these differential diagnoses can be accomplished using specific immunohistochemical markers or molecular detection approaches. For example, malignant rhabdoid tumors predominantly affect infants and young children, primarily arising in the central nervous system or soft tissues, with occasional involvement of the gastrointestinal tract. Immunohistochemically, the tumor cells exhibit expression of Vimentin, CKpan, and EMA. Lymphoma cells characteristically express lymphoid markers such as LCA. Epithelioid gastrointestinal stromal tumor cells are arranged in a nest-like or sheet-like pattern with epithelioid morphology, and their identification can be confirmed by positive immunohistochemical staining for CD117 and DOG1. Melanomas show positivity for immunohistochemical markers including HMB45, MelanA, and S100. As an epithelial-derived neoplasm, undifferentiated gastric carcinoma typically expresses epithelial markers and generally lacks or rarely exhibits expression of mesenchymal markers (e.g., Vimentin). In NUT midline carcinoma, abrupt foci of squamous differentiation are frequently observed, and the tumor cells harbor detectable BRD-NUT fusion and positive NUT expression.
Surgical resection remains the main treatment for SMARCA4-deficient tumors. However, it is still necessary to combine other adjuvant therapies to delay the progression of the disease. SMARCA4/2 deficiency reportedly restricts the flow of calcium ions mediated by IP3R3 from cancer cells to the mitochondria, leading to resistance to chemotherapeutic drugs (16). So most patients show poor responsiveness to chemotherapy (17). For example, a case report of a 73-year-old male with gastric SMARCA4-deficient high-grade sarcomatoid carcinoma showed that the patient was unresponsive to chemotherapy (11); however, a case report of a 59-year-old male with gastric SMARCA4-deficient sarcoma demonstrated that the patient had a good response to combined chemotherapy with doxorubicin and ifosfamide after surgery and remained alive during 49 months of follow-up (18). Both patients in this article refused postoperative chemotherapy, making it impossible to evaluate the efficacy of chemotherapy.
As a homologous subunit of SMARCA4 in the SWI/SNF complex, SMARCA2 has emerged as an attractive therapeutic target for SMARCA4-deficient tumors through a synthetic lethal effect (19). However, SMARCA2 is also mutated or inactivated in a variety of solid tumors. Previous studies have documented SMARCA2 mutations or deletion in 5-10% of non-small-cell lung carcinoma (20, 21), 10% of esophageal adenocarcinoma (22), 5-9% of gastric cancer (23, 24), 1.3% of colorectal carcinoma (25), 3% of clear cell renal cell carcinoma (26), and 5% adenoid cystic carcinomas (27). Notably, the loss of SMARCA2 has been observed in some tumors with SMARCA4 deficiency, such as 90-100% of small cell carcinoma of the ovary (hypercalcemic type) (28, 29), 67-80% of thoracic SMARCA4-deficient undifferentiated tumors (14, 30), 15-30% of SMARCA4-deficient non-small cell lung cancer (20, 21), 11% of SMARCA4-deficient gastric adenocarcinoma (23), 15% of SMARCA4-deficient colorectal cancer (25), 70% of undifferentiated endometrial carcinoma (31). This phenomenon limits the application of SMARCA2-targeted synthetic lethal therapies. SMARCA2-induced synthetic lethality also requires the complete loss of SMARCA4 function (1) and is ineffective against tumors with reduced or heterogeneous SMARCA4 expression. Second-generation histone deacetylase inhibitors (HDACis) can reactivate the expression of SMARCA2-dependent IP3R3, reduce the limitation of IP3R3-mediated calcium ion flow to mitochondria caused by SMARCA4/2 deficiency, enhancing their response to chemotherapy (16). However, they are unsuitable for SMARCA4-deficient tumors without SMARCA2 deletion. SMARCA4-deficient lung and ovarian cancer cells show increased sensitivity to the KDM6 inhibitor GSK-J4, which can be an effective treatment option after cisplatin resistance (32). However, its effect on gastric cancer cells is unknown. Programmed death-ligand 1 (PDL1) immunotherapy remains the most effective therapeutic option and is not restricted by PDL1 expression levels (33). In our study, TCM treatment after surgery has led to no recurrence or metastasis during the 25-month follow-up period. It indicates that TCM treatment can become another widely applicable postoperative adjuvant treatment method.
This study provides a comprehensive description of two rare cases of primary gastric SMARCA4-deficient carcinosarcoma and sarcomatoid carcinoma. Histologically, the difference was the distinct responses of the sarcomatous components to epithelial markers. TCM treatment may serve as a post-surgical treatment option. However, due to the small sample size in this study, further studies are needed to better characterize these two tumor subtypes and their response to chemotherapy.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by the Ethical Committee of the Affiliated Hospital of Jining Medical University (No.2022-11-C017). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RZ: Writing – review & editing, Data curation, Writing – original draft. JZ: Data curation, Writing – original draft. XZ: Resources, Writing – review & editing. RYZ: Writing – review & editing, Supervision. LL: Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Jining Key Research and Development Program, Project No. 2024YXNS110.
Acknowledgments
The authors thanked the patients’ family members for their cooperation and regular follow-up. We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fernando TM, Piskol R, Bainer R, Sokol ES, Trabucco SE, Zhang Q, et al. Functional characterization of SMARCA4 variants identified by targeted exome-sequencing of 131,668 cancer patients. Nat Commun. (2020) 11:5551. doi: 10.1038/s41467-020-19402-8
2. Love C, Sun Z, Jima D, Li G, Zhang J, Miles R, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. (2012) 44:1321–5. doi: 10.1038/ng.2468
3. Schoenfeld AJ, Bandlamudi C, Lavery JA, Montecalvo J, Namakydoust A, Rizvi H, et al. The genomic landscape of SMARCA4 alterations and associations with outcomes in patients with lung cancer. Clin Cancer Res. (2020) 26:5701–8. doi: 10.1158/1078-0432.CCR-20-1825
4. Liang X, Gao X, Wang F, Li S, Zhou Y, Guo P, et al. Clinical characteristics and prognostic analysis of SMARCA4-deficient non-small cell lung cancer. Cancer Med. (2023) 12:14171–82. doi: 10.1002/cam4.6083
5. Jones DTW, Jäger N, Kool M, Zichner T, Hutter B, Sultan M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. (2012) 488:100–5. doi: 10.1038/nature11284
6. Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. (2012) 488:106–10. doi: 10.1038/nature11329
7. Becker TM, Haferkamp S, Dijkstra MK, Scurr LL, Frausto M, Diefenbach E, et al. The chromatin remodelling factor BRG1 is a novel binding partner of the tumor suppressor p16INK4a. Mol Cancer. (2009) 8:4. doi: 10.1186/1476-4598-8-4
8. Huang SC, Ng KF, Yeh TS, Cheng CT, Chen MC, Chao YC, et al. The clinicopathological and molecular analysis of gastric cancer with altered SMARCA4 expression. Histopathology. (2020) 77:250–61. doi: 10.1111/his.14117
9. Wang Y, Hoang L, Ji JX, and Huntsman DG. The SWI/SNF complex mutations in gynecologic cancers: molecular mechanisms and models. Annu Rev Pathol. (2020) 15:467–92. doi: 10.1146/annurev-pathmechdis-012418-012917
10. Witkowski L, Carrot-Zhang J, Albrecht S, Fahiminiya S, Hamel N, Tomiak E, et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet. (2014) 46:438–43. doi: 10.1038/ng.2931
11. Bhat V, Koneru M, Knapp K, Joneja U, Morrison J, and Hong YK. Identification and treatment of SMARCA4 deficient poorly differentiated gastric carcinoma. Am Surgeon. (2022) 0:1–3. doi: 10.1177/00031348221146972
12. Meda Y, Miyake H, Nagai H, Yoshioka Y, Yuasa N, Kiriyama A, et al. SMARCA4-deficient rectal carcinoma with a sarcomatoid component: a case report. Clin J Gastroenterol. (2022) 15:419–26. doi: 10.1007/s12328-022-01602-y
13. Golconda U, McHugh KE, Allende SD, Collins K, Henn P, Lacambra M, et al. Colorectal carcinoma with sarcomatoid components report of 15 cases and literature review of an exceedingly rare carcinoma subtype. Am J Surg Pathol. (2023) 00:1–10. doi: 10.1097/PAS.0000000000002172
14. Rekhtman N, Montecalvo J, Chang JC, Alex D, Ptashkin RN, Ai N, et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol. (2020) 15:231–47. doi: 10.1016/j.jtho.2019.10.023
15. Li Y, Cui L, Chen Y, and Wang F. Carcinosarcoma and sarcomatoid carcinoma of the stomach: Two case reports. Med (Baltimore). (2021) 100:e24697. doi: 10.1097/MD.0000000000024697
16. Xue Y, Morris JL, Yang K, Fu Z, Zhu X, Johnson F, et al. SMARCA4/2 loss inhibits chemotherapy-induced apoptosis by restricting IP3R3-mediated Ca(2+) flux to mitochondria. Nat Commun. (2021) 12:5404. doi: 10.1038/s41467-021-25260-9
17. Cui M, Lemmon K, Jin Z, and Uboha NV. Esophageal carcinoma with SMARCA4 mutation: Unique diagnostic challenges. Pathol - Res Pract. (2023) 248:154692. doi: 10.1016/j.prp.2023.154692
18. Ota T, Ishikawa T, Yasuda R, Yasuda T, Okayama T, Inoue K, et al. The first case of SMARCA4−deficient sarcoma of stomach. Clin J Gastroenterol. (2022) 15:531–6. doi: 10.1007/s12328-022-01606-8
19. Cantley J, Ye X, Rousseau E, Januario T, Hamman BD, Rose CM, et al. Selective PROTAC-mediated degradation of SMARCA2 is efficacious in SMARCA4 mutant cancers. Nat Commun. (2022) 13:6814. doi: 10.1038/s41467-022-34562-5
20. Herpel E, Rieker RJ, Dienemann H, Muley T, Meister M, Hartmann A, et al. SMARCA4 and SMARCA2 deficiency in non–small cell lung cancer: immunohistochemical survey of 316 consecutive specimens. Ann Diagn Pathol. (2017) 26:47–51. doi: 10.1016/j.anndiagpath.2016.10.006
21. Sun S, Li Q, Zhang Z, Xiong S, Zhang Y, Liu Q, et al. SMARCA2 deficiency in NSCLC: a clinicopathologic and immunohistochemical analysis of a large series from a single institution. Environ Health Prev Med. (2022) 27:3–3. doi: 10.1265/ehpm.21-00254
22. Schallenberg S, Bork J, Essakly A, Alakus H, Buettner R, Hillmer AM, et al. Loss of the SWI/SNF-ATPase subunit members SMARCF1 (ARID1A), SMARCA2 (BRM), SMARCA4 (BRG1) and SMARCB1 (INI1) in oesophageal adenocarcinoma. BMC Cancer. (2020) 20(1):12. doi: 10.1186/s12885-019-6425-3
23. Zhang Z, Li Q, Sun S, Li Z, Cui Z, Liu Q, et al. Expression of SMARCA2 and SMARCA4 in gastric adenocarcinoma and construction of a nomogram prognostic model. Int J Clin Oncol. (2023) 28:1487–500. doi: 10.1007/s10147-023-02403-0
24. Suzuki H, Huang S-C, Ng K-F, Chang IY-F, Chang C-J, Chao Y-C, et al. The clinicopathological significance of SWI/SNF alterations in gastric cancer is associated with the molecular subtypes. PloS One. (2021) 16(1):e0245356. doi: 10.1371/journal.pone.0245356
25. Ahadi MS, Fuchs TL, Clarkson A, Sheen A, Sioson L, Chou A, et al. Switch/sucrose-non-fermentable (SWI/SNF) complex (SMARCA4, SMARCA2, INI1/SMARCB1)-deficient colorectal carcinomas are strongly associated with microsatellite instability: an incidence study in 4508 colorectal carcinomas. Histopathology. (2022) 80:906–21. doi: 10.1111/his.14612
26. Xia Qy, Rao Q, Cheng L, Shen Q, Shi Ss, Li L, et al. Loss of BRM expression is a frequently observed event in poorly differentiated clear cell renal cell carcinoma. Histopathology. (2014) 64:847–62. doi: 10.1111/his.12334
27. Ho AS, Kannan K, Roy DM, Morris LGT, Ganly I, Katabi N, et al. The mutational landscape of adenoid cystic carcinoma. Nat Genet. (2013) 45:791–8. doi: 10.1038/ng.2643
28. Jelinic P, Schlappe BA, Conlon N, Tseng J, Olvera N, Dao F, et al. Concomitant loss of SMARCA2 and SMARCA4 expression in small cell carcinoma of the ovary, hypercalcemic type. Modern Pathol. (2016) 29:60–6. doi: 10.1038/modpathol.2015.129
29. Karnezis AN, Wang Y, Ramos P, Hendricks WPD, Oliva E, D’Angelo E, et al. Dual loss of the SWI/SNF complex ATPases SMARCA4/BRG1 and SMARCA2/BRM is highly sensitive and specific for small cell carcinoma of the ovary, hypercalcaemic type. J Pathol. (2015) 238:389–400. doi: 10.1002/path.4633
30. Sauter JL, Graham RP, Larsen BT, Jenkins SM, Roden AC, and Boland JM. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Modern Pathol. (2017) 30:1422–32. doi: 10.1038/modpathol.2017.61
31. Ramalingam P, Croce S, and McCluggage WG. Loss of expression of SMARCA4 (BRG1), SMARCA2 (BRM) and SMARCB1 (INI1) in undifferentiated carcinoma of the endometrium is not uncommon and is not always associated with rhabdoid morphology. Histopathology. (2017) 70:359–66. doi: 10.1111/his.13091
32. Romero OA, Vilarrubi A, Alburquerque-Bejar JJ, Gomez A, Andrades A, Trastulli D, et al. SMARCA4 deficient tumours are vulnerable to KDM6A/UTX and KDM6B/JMJD3 blockade. Nat Commun. (2021) 12:4319. doi: 10.1038/s41467-021-24618-3
Keywords: gastric tumor, SMARCA4-deficient, carcinosarcoma, sarcomatoid carcinoma, case report
Citation: Zhao R, Zhang Jj, Zhi X, Zhang Ry and Li L (2025) Primary gastric SMARCA4-deficient carcinosarcoma and sarcomatoid carcinoma: Two case reports. Front. Oncol. 15:1663811. doi: 10.3389/fonc.2025.1663811
Received: 11 July 2025; Accepted: 30 September 2025;
Published: 15 October 2025.
Edited by:
Xiaojun Liu, Gansu Provincial Hospital, ChinaReviewed by:
Narendra Bharathy, Children’s Cancer Therapy Development Institute, United StatesGenlin Lu, Longyou People’s Hospital, China
Teodor Florin Georgescu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2025 Zhao, Zhang, Zhi, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Li, bGlsZWlAbWFpbC5qbm1jLmVkdS5jbg==
 Ran Zhao
Ran Zhao Lei Li
Lei Li
