- 1H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, United States
- 2Morsani College of Medicine, University of South Florida, Tampa, FL, United States
Introduction: BCMA-directed CAR T-cell therapies have improved outcomes in relapsed and refractory multiple myeloma (MM); however, the majority of patients relapse within 1 to 3 years following treatment. Managing disease progression after CAR T-cell therapy remains a major challenge, particularly in aggressive subtypes including extramedullary disease (EMD) and paramedullary disease (PMD). Real-world data on progression patterns post-CAR T cell therapy and the impact of EMD or PMD on outcomes of patients who relapsed post-CAR T-cell therapy remain scarce.
Methods: In this single-center, retrospective study, we evaluated progression patterns and survival outcomes in 106 MM patients who progressed after commercial CAR T-cell therapy (ide-cel or cilta-cel) between May 2021 and December 2023. Overall survival (OS) was defined from the time of post-CAR T-cell therapy progression to death or last follow-up, and progression-free survival (PFS) from post-CAR T-cell therapy progression to progression on the next line of therapy.
Results: Biochemical relapse occurred in 82% of patients, with EMD or PMD present in 51% at progression. Baseline EMD at the time of CAR T-cell infusion was detected in 33% of patients and was associated with significantly inferior PFS (3.6 vs. 7.0 months, p=0.0076) and OS (4.8 vs. 21.0 months, p=0.00086) compared to those without EMD. Similarly, the presence of EMD at progression was associated with shorter PFS (4.7 vs. 8.5 months, p=0.022) and OS (7.4 vs. 21.1 months, p=0.035). Patients who were EMD positive at both baseline and progression had the poorest outcomes. PMD at baseline or progression was not significantly associated with worse survival.
Discussion: Our findings highlight that post-CAR T-cell progression in MM is heterogeneous and that EMD confers an adverse prognosis, emphasizing the critical need for imaging surveillance. Strategies such as bridging therapies aimed at reducing tumor burden prior to CAR T-cell infusion or maintenance therapies post-CAR T-cell therapy warrant further investigation to optimize responses and improve long-term survival in this high-risk population.
Introduction
Multiple myeloma (MM) makes up 1.8% of all new cancer cases in the United States, and it is characterized by a remitting relapsing course ultimately leading to refractoriness to treatment (1). Patients with MM typically experience disease progression either biochemically or symptomatically with end-organ damage and extramedullary diseases (EMD) or paramedullary disease (PMD) (2). EMD is characterized by soft tissue plasmacytomas resulting from hematogenous spread of malignant cells outside the bone marrow, while PMD occurs due to direct tumor extension from skeletal lesions following cortical bone disruption (3). EMD and PMD are uncommon at initial diagnosis but occurs more frequently at the time relapse or disease progression (3–5). Patients with EMD or PMD at time of diagnosis or progression tend to have worse outcomes than those without (6–9).
Idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) are B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T-cell (CAR T-cell) therapies that have demonstrated significant efficacy in patients with heavily pretreated MM patients, as shown in the pivotal KarMMa-1 and CARTITUDE-1 trials, respectively (10, 11). Subsequently, the phase 3 KarMMa-3 and CARTITUDE-4 trials evaluated the use of ide-cel and cilta-cel in earlier lines of therapy, demonstrating superior progression-free survival (PFS) compared to standard regimens in patients with relapsed and refractory MM (12, 13). Despite these advances, relapse after CAR-T-cell therapies remains a major challenge, often presenting in aggressive forms such as EMD or PMD, which are associated with particularly poor outcomes.
The detailed knowledge of patterns of progression after BCMA directed CAR T-cell therapy is lacking in the literature. Understanding how patients progress and how this affects prognosis is important to developing optimal disease monitoring strategies and interventions. Our study aims to assess the patterns of progression after commercial BCMA directed CAR T-cell therapy and to assess the impact of the presence of EMD and PMD at baseline prior to CAR T-cell therapy and at time of progression on the survival outcomes.
Methods
Study design and settings
This is a single-center retrospective study conducted at the H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida, with prior approval from the institutional review board.
Patients
All adult patients (≥18 years) with MM who received commercial BCMA-directed CAR T-cell therapy (ide-cel or cilta-cel) between May 1, 2021, and December 31, 2023, and subsequently experienced disease progression were included in the study. At our institution, baseline laboratory tests, imaging, and bone marrow assessments are performed on all MM patients prior to CAR T-cell infusion. On day 90 post-CAR T-cell therapy, repeat laboratory tests, imaging (positron emission tomography, computed tomography, or whole-body magnetic resonance imaging), and bone marrow assessments are conducted to evaluate treatment response. Subsequently patients are monitored per standard of care with the treating physicians with monitoring of serum and urine paraprotein as well as needed imaging tests. In the event of suspected disease progression, a restaging work up with imaging and bone marrow biopsy is performed.
Data collection and clinical assessment
Laboratory, imaging, and bone marrow data were collected at three time points: baseline prior or at the time of CAR T-cell infusion, at day 90 post-infusion, and at disease progression. Response and progression were evaluated using the International Myeloma Working Group (IMWG) criteria (14). Biochemical relapse is defined as a 25% or greater increase from the lowest response level in any of the following markers: serum M-protein (with an absolute increase ≥ 0.5 g/dL), urine M-protein (with an absolute increase ≥ 200 mg/24h), or the difference between involved and uninvolved free light chain (FLC) levels in patients without measurable serum or urine M-protein (with an absolute increase in FLC ≥ 10 mg/dL) with or without evidence of end-organ damage. Biopsy confirmation was not universally required to confirm EMD or PMD, and they were identified through imaging with EMD showing myeloma involvement in soft tissues and all soft tissue lesions were reviewed and non-contiguous with bone, disease and PMD characterized by direct tumor extension from skeletal lesions. Longitudinal assessment was used retrospectively to increase confidence in the diagnosis of EMD and PMD. At the time of progression post-CAR T-cell therapy, bone marrow involvement was defined by the presence of clonal plasma cells at or above 5%. High-risk cytogenetics were defined as the presence of del(17p)/monosomy 17, t(4,14), or t(14,16) on fluorescence in situ hybridization testing. Overall survival (OS) was defined as the time from progression after CAR T-cell therapy to death from any cause or last follow-up. PFS was defined as the time from progression after CAR T-cell therapy to progression on the earliest subsequent therapy.
Statistical analysis
The distribution of progression patterns was analyzed overall, as well as based on the presence of any EMD or PMD and the type of CAR T-cell therapy using the chi-square test for categorical variables and independent t-test for continuous variables. Kaplan-Meier survival curves were used to estimate PFS and OS, with log-rank tests applied to compare survival outcomes across selected variables, including baseline and progression EMD or PMD status and the type of CAR T-cell therapy. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using R program version 3.5.1.
Results
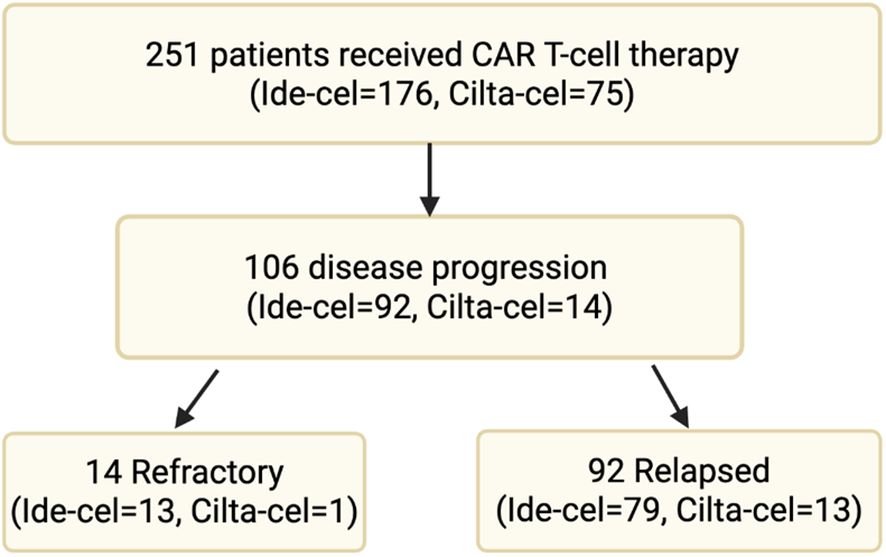
A total of 251 patients with MM received commercial anti-BCMA CAR T-cell therapy: either ide-cel (n=176) or cilta-cel (n=75) between May 2021 and December 2023. Of them, 106 patients (ide-cel n=92, cilta-cel n=14) had disease progression at the time of data cut off and were included in the study. Among them, 14 patients (ide-cel n=13 and cilta-cel n=1) had refractory disease, defined as progression within 60 days of CAR T-cell infusion. The remaining 92 patients (ide-cel n=79, cilta-cel n=13) had relapsed disease, with progression occurring beyond 60 days post-infusion (Figure 1).
Baseline characteristics
The median age of the included patients was 66 years (Interquartile Range (IQR): 58-73), with the majority being male (n=59, 56%) and white (n=70, 66%). IgG was the most common heavy chain involved, seen in 60 patients (57%), while kappa was the predominant light chain in 71 patients (67%). Oligo- or non-secretory disease was observed in 4 patients (4%). Anemia (hemoglobin < 10 g/dL) prior to CAR T-cell infusion was present in 58 patients (55%), which was more frequent than neutropenia (absolute neutrophil count < 1000/mL) in 11 patients (10%) and thrombocytopenia (platelets count < 100 x 109/L) in 37 patients (35%). The median number of prior lines of treatment was 6 (IQR: 5-7), and most patients (n=84, 79%) had undergone a prior autologous stem cell transplant. High-risk cytogenetics were found in 38% of patients (n=40), with del(17p) present in 30% (n=31), t(4,14) in 13% (n=13) and t(14,16) in 6% (n=6). By day 90 post-CAR T-cell therapy, 67% of patients had achieved an objective-response rate based on IMWG response criteria with statistically lower response in patients with EMD compared with those without EMD (47% vs 72%, p=0.01). Otherwise, baseline characteristics were well balanced, with no statistically significant differences between patients with and without EMD (Table 1).
Patterns of progression
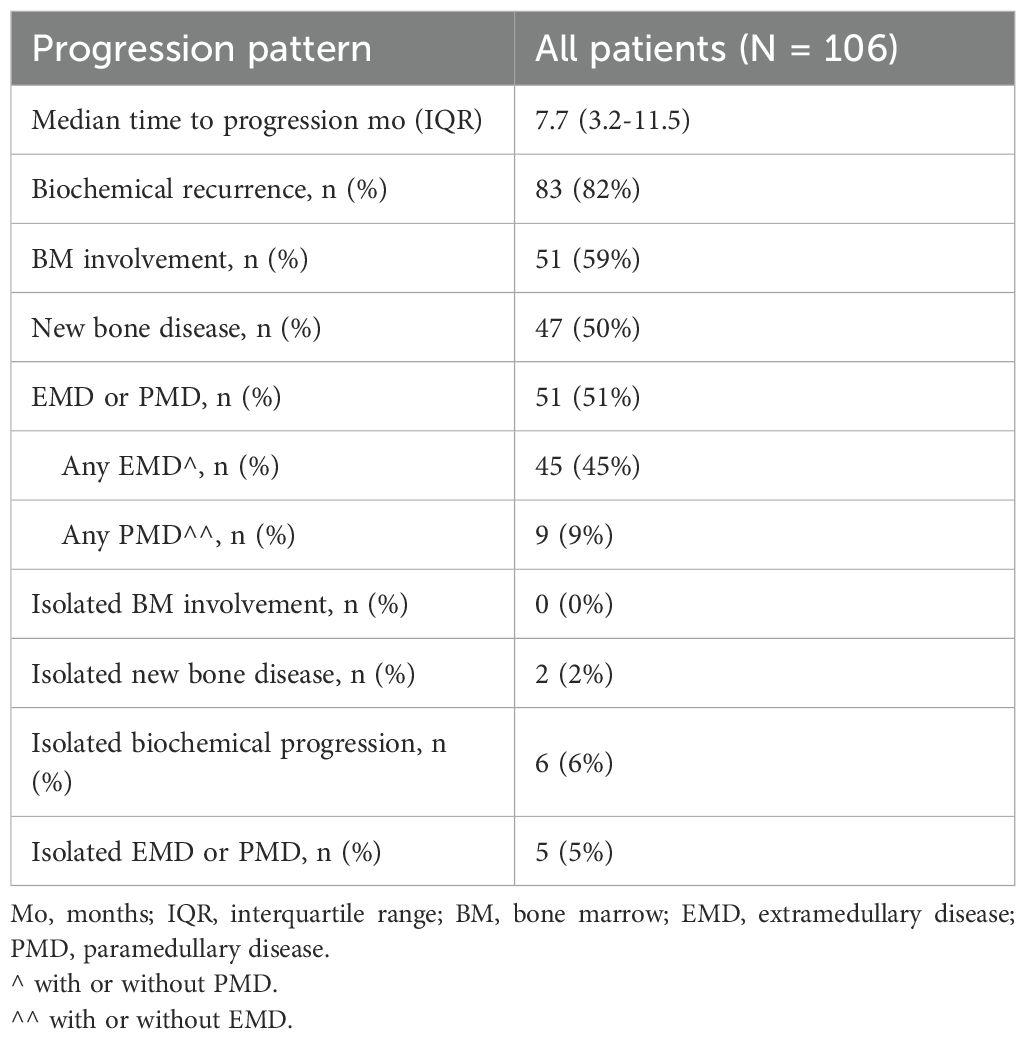
The median time to progression in this cohort of patients was 7.7 months (IQR: 3.2–11.5 months). Biochemical recurrence with or without symptomatic disease was the most frequent pattern of relapse, occurring in 82% of patients (n=83), followed by bone marrow involvement in 59% (n=51), EMD or PMD in 51% (n=51), and new bone lesions in 50% (n=47). Isolated sites of relapse were relatively uncommon, with isolated biochemical progression seen in 6% (n=6), isolated EMD or PMD in 5% (n=5), isolated bone marrow involvement in 0% (n=0), and isolated new bone disease in 2% (n=2) (Table 2). There were no significant differences in progression patterns between patients treated with ide-cel and those treated with cilta-cel.
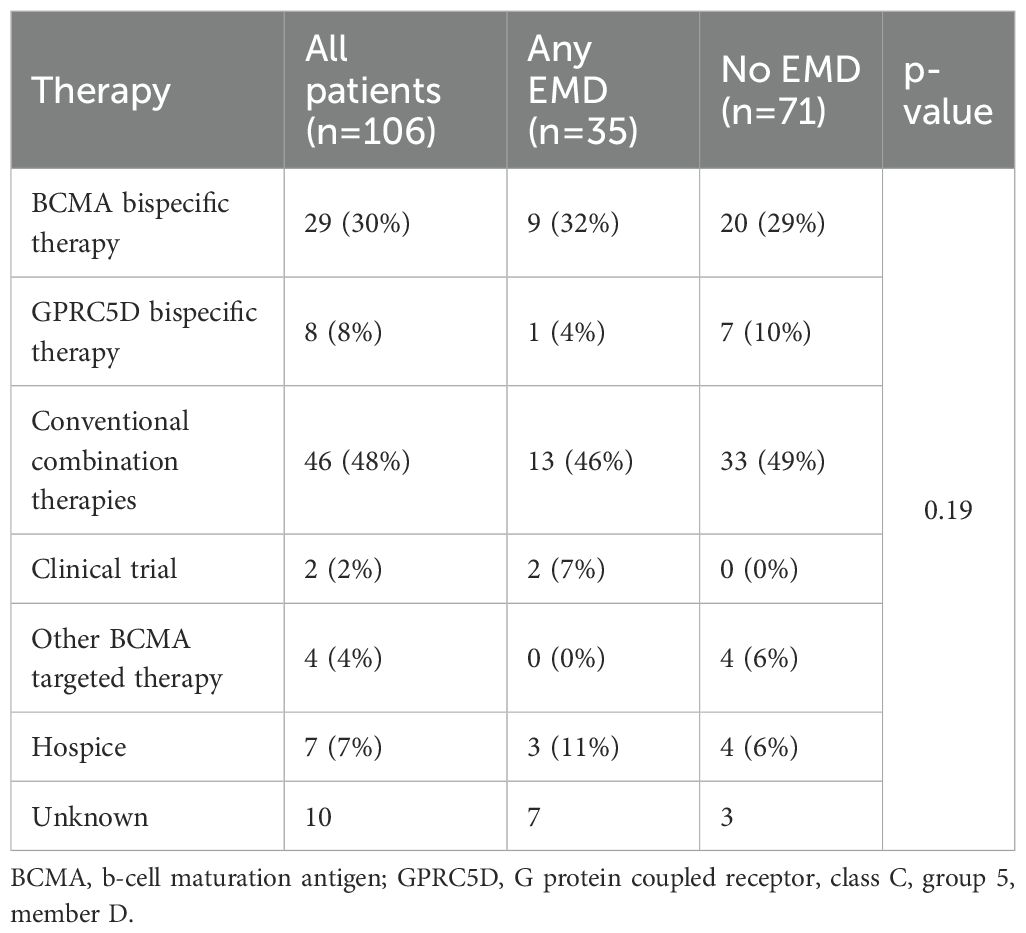
After progression on CAR T-cell therapy, conventional combination regimens were most frequently used (48%) line of therapy, followed by BCMA-directed (30%) and GPRC5D-directed bispecifics (8%). Treatment patterns were comparable between patients with and without EMD, with no statistically significant differences observed (Table 3).
EMD and PMD
Baseline EMD or PMD at the time of CAR T-cell infusion was detected in 46 patients (43%), including 35 patients (33%) with any EMD (with or without PMD) and 18 patients (17%) with any PMD (with or without EMD). The most common sites of EMD/PMD were skin and soft tissue in 13 patients (13%), followed by lymph nodes and paraspinal masses in 9 patients (9%) each. At progression, similar patterns were observed: skin and soft tissue in 16 patients (17%), lymph nodes in 13 (17%), and lung/pleura in 14 (15%). Patients with baseline EMD were more likely to have EMD at progression (p=0.035); similarly, those with baseline PMD were more likely to have PMD at progression (p=0.049). Figure 2 shows the transition of EMD and PMD status from baseline at the time of CAR T-cell infusion to time of progression. Among 60 patients (57%) who were EMD- and PMD-negative at baseline, 35 (58%) remained negative, 18 (30%) developed EMD only, 3 (5%) developed PMD only, 2 (3%) developed both EMD and PMD, and 2 (3%) had unknown status at progression. Of the 7 patients (7%) with both EMD and PMD at baseline, 4 (57%) transitioned to EMD only, and 3 (43%) became EMD- and PMD-negative. Among 28 patients (26%) with EMD only at baseline, 17 (61%) remained unchanged, 1 (4%) progressed to both EMD and PMD, 7 (25%) became negative, and 3 (11%) had unknown progression status. Of the 11 patients (10%) with PMD only, 4 (36%) remained in the same category, 3 (27%) transitioned to EMD only, and 3 (27%) became negative for both EMD and PMD.
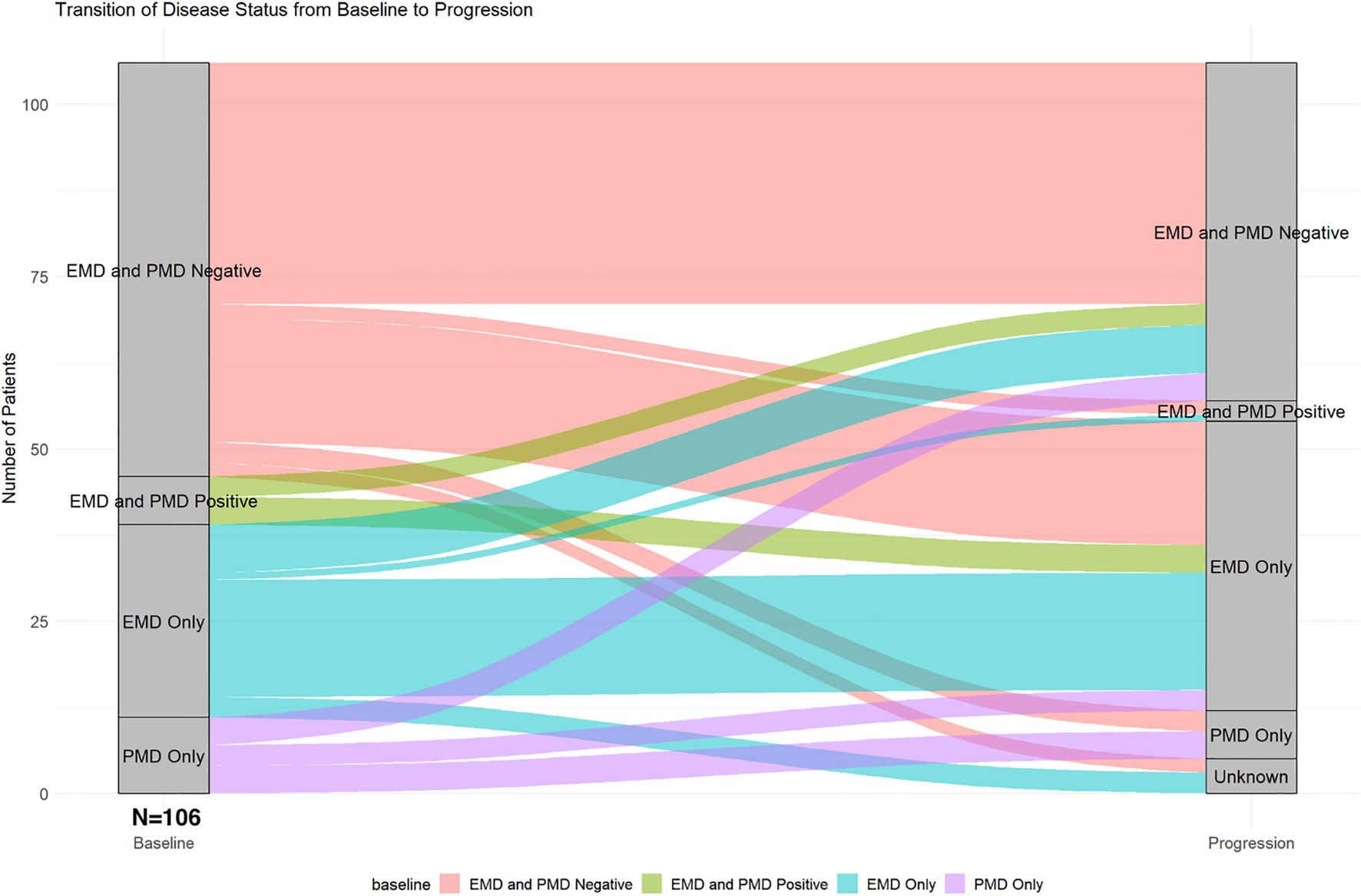
Figure 2. Transition of extramedullary and paramedullary disease status from baseline to progression.
Survival data
The median PFS after progression from CAR T-cell therapy to progression on the earliest subsequent therapy for all patients was 5.8 months (95% Confidence Interval [CI]: 4.7-8.5) (Figure 3A). Patients with baseline EMD or PMD had significantly shorter median PFS after progression from CAR-T cell therapy compared to those without (4.1 months (95%CI: 3.5-5.6) vs. 7.4 months (95%CI: 6.4-12.8), p=0.012) (Figure 3B). Similarly, patients with any baseline EMD had a shorter median PFS 3.6 months (95%CI: 3.0-5.6) versus 7.0 months (95%CI: 6.3-12.5) for those without any baseline EMD (p=0.0061) (Figure 3C). Baseline any PMD was not associated with a significant difference in PFS (Figure 3D). Patients with EMD or PMD at progression post-CAR T-cell therapy had a median PFS of 4.8 months (95% CI: 4.1-6.4), significantly shorter than 8.6 months (95% CI: 6.6-NA) in those without (p=0.04) (Figure 3E). The presence of any EMD at progression was also associated with inferior PFS (4.7 months (95% CI: 3.5-7.4) vs. 8.5 months (95% CI: 5.8-NA), p=0.022) (Figure 3F). Any PMD at progression did not significantly impact PFS (Figure 3G).
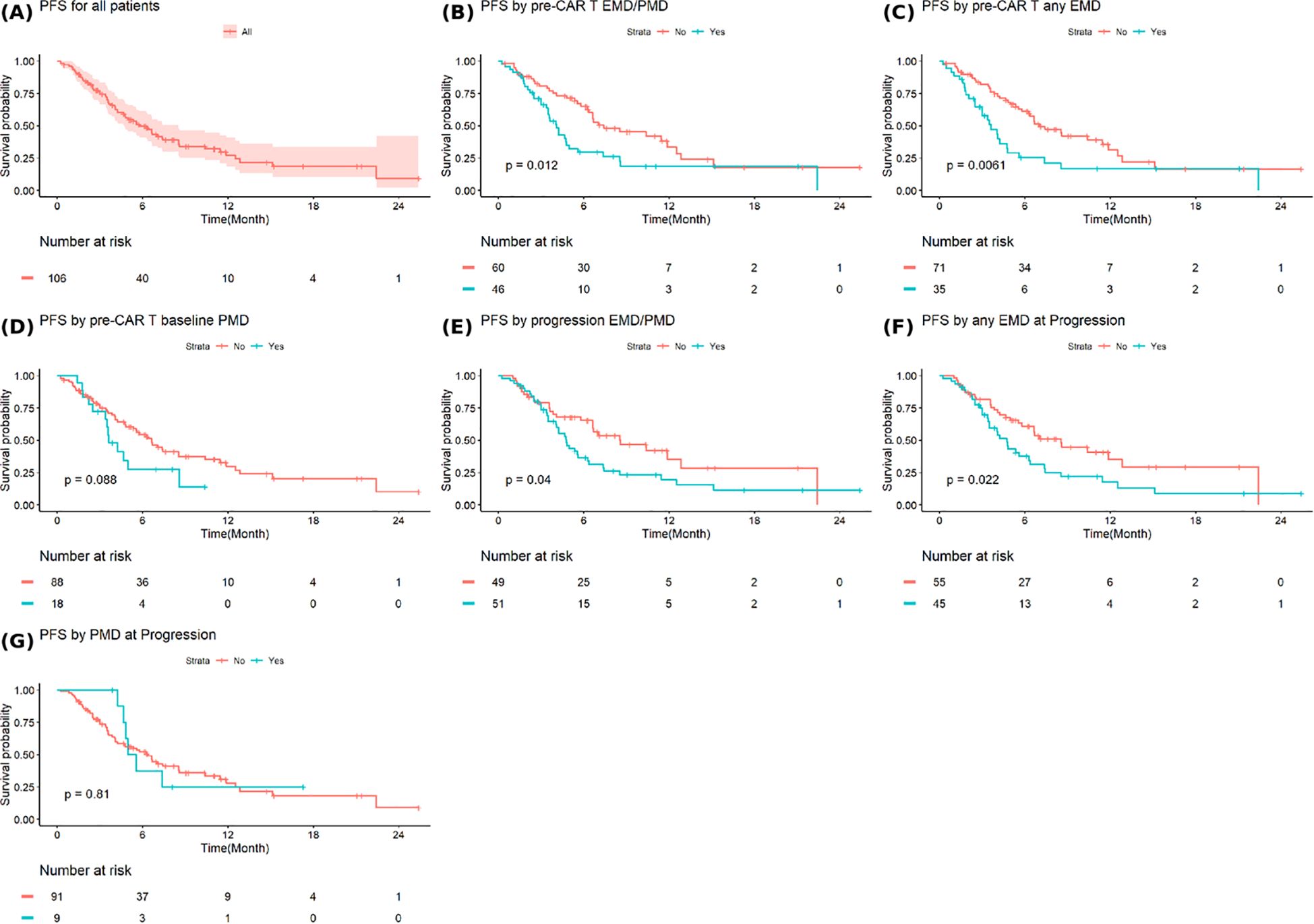
Figure 3. Progression free survival in (A) all patients and based on (B) extramedullary or paramedullary disease (EMD or PMD) at baseline (C) any EMD at baseline (D) any PMD at baseline (E) EMD or PMD at progression (F) any EMD at progression (G) any PMD at progression. * Progression free survival (PFS2) is defined as the time from progression after CAR T-cell therapy to progression on the earliest subsequent therapy.
The median OS after progression from CAR T-cell therapy for all patients was 12.5 months (95%CI: 8.6-NA) (Figure 4A). Patients with baseline EMD or PMD had a significantly shorter median OS of 5.7 months (95% CI: 3.9-NA) compared to 21.1 months (95% CI: 10.6-NA) in those without baseline involvement (p=0.012) (Figure 4B). Similarly, patients with baseline any EMD experienced inferior median OS of 4.8 months (95% CI: 3.4–NA) versus 21.1 months (95% CI: 10.6–NA) among those without EMD (p=0.00086) (Figure 4C). Baseline any PMD was not associated with a significant difference in OS (Figure 4D). Patient with EMD or PMD at progression post-CAR T-cell therapy continued to show worse outcomes, with a median OS of 7.4 months (95% CI: 5.6-NA) compared to 21.1 months (95% CI: 10.6-NA) in those without, although this did not reach statistical significance (p=0.061) (Figure 4E). However, the presence of any EMD at progression was significantly associated with shorter OS (7.4 months (95% CI: 5.4-NA) vs. 21.1 months (95% CI: 10.6-NA), p=0.035) (Figure 4F). Any PMD at progression did not significantly affect OS (Figure 4G).
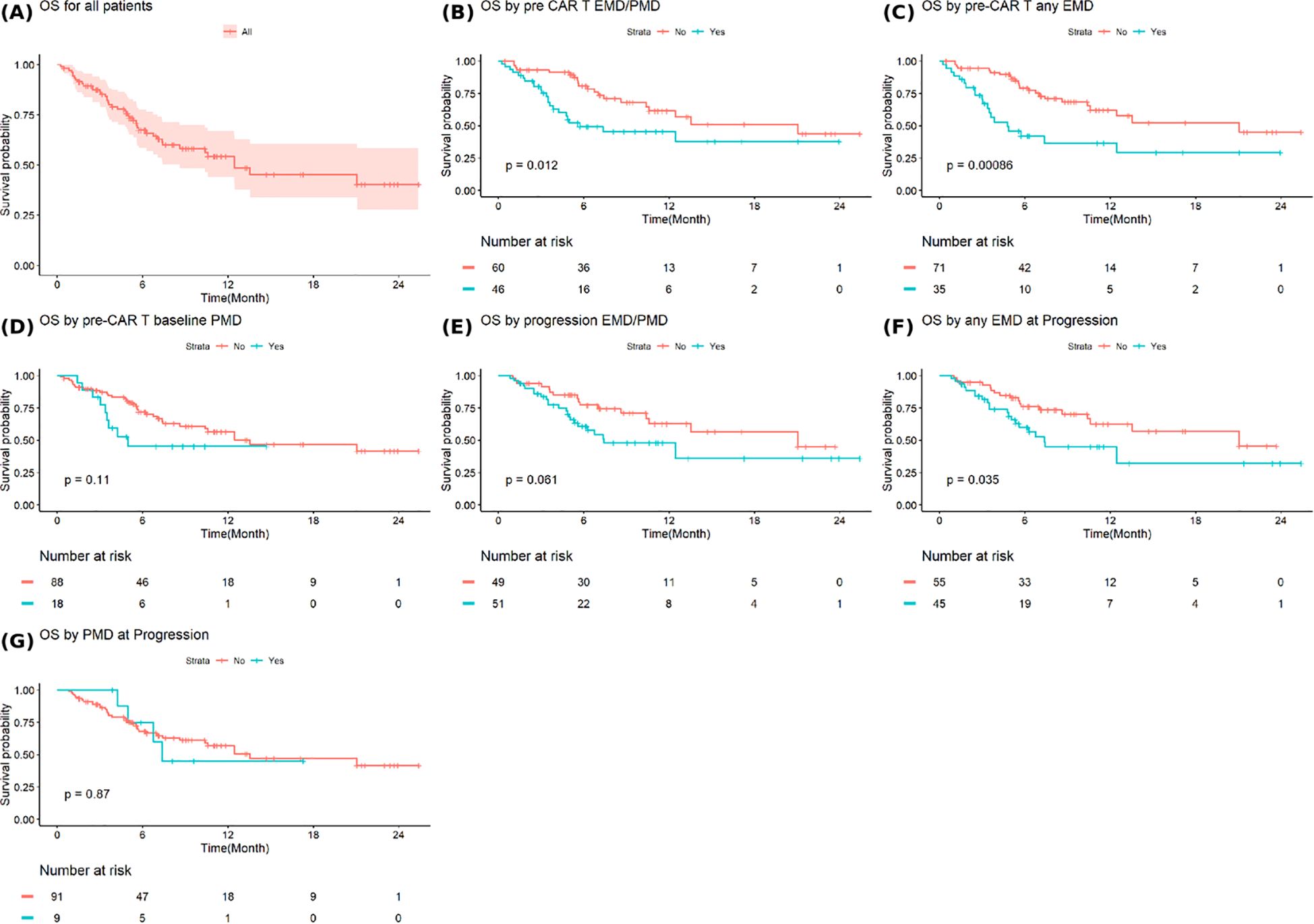
Figure 4. Overall survival in (A) all patients and based on (B) extramedullary or paramedullary disease (EMD or PMD) at baseline (C) any EMD at baseline (D) any PMD at baseline (E) EMD or PMD at progression (F) any EMD at progression (G) any PMD at progression. * Overall survival is defined as the time from progression after CAR T-cell therapy to death from any cause or last follow-up.
Figure 5 shows PFS and OS stratified by both baseline and progression any EMD status. Patients with EMD present at both baseline and progression had the worst outcomes, post-CAR T-cell therapy progression, with a median PFS of 4 months (95%CI: 3-7.4; p=0.016) and a median OS of 4.9 months (95%CI: 3.0-NA, p=0.006) compared to other groups. In contrast, patients who were negative for EMD at baseline and progression experienced the most favorable outcomes post-CAR T-cell therapy progression with a median PFS of 8.6 months (95%CI: 6.6-NA) and a median OS of 21.0 months (95%CI: 13.6-NA).
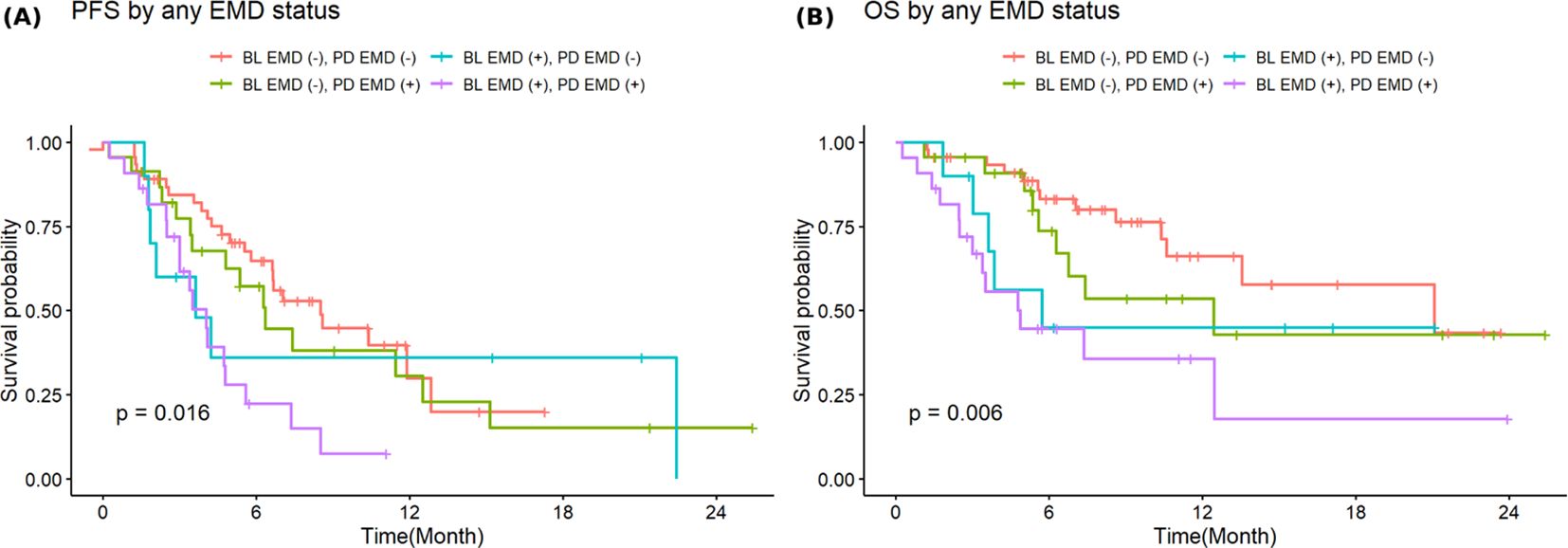
Figure 5. Stratified progression-free (A) and overall survival (B) by combined baseline and progression extramedullary disease.
Discussion
This single-center, retrospective real-world analysis characterizes the patterns of disease progression following standard-of-care CAR T-cell therapy in patients with MM, with a specific focus on the impact of EMD and PMD on survival outcomes. Our findings demonstrate that post-CAR T-cell therapy progression is heterogeneous, with the majority of patients experiencing biochemical relapse, but is associated with a high rate of EMD and PMD. While about 5% of patient had EMD or PMD as the sole evidence of progression and an additional 2% progressed solely with new bone disease, these findings highlight the importance of imaging in the follow up and monitoring of patients with multiple myeloma post-BCMA directed CAR T-cell therapy. Notably, none of the patients in our cohort exhibited isolated bone marrow involvement at the time of progression, suggesting that routine bone marrow monitoring following BCMA-directed CAR T-cell therapy, in the absence of other signs of progression, have limited clinical utility to detect isolated marrow relapse.
Our study highlights that the presence of EMD, at baseline or at the time of progression, is associated with inferior PFS and OS after progression post-CAR T-cell therapy, reinforcing the prognostic significance of this disease feature. While the survival outcomes of patients with PMD alone is numerically inferior to those without, the difference did not reach statistical significance likely reflecting the limited sample size and this warrants further investigation. Although patients with baseline EMD or PMD are more likely to exhibit EMD or PMD at progression, the absence of these features at baseline does not eliminate their emergence at progression, underscoring the importance of imaging monitoring after CAR T-cell therapy and at the time of disease progression.
With advancements in novel therapeutic agents and improved survival in MM, the incidence of EMD has become increasingly common, particularly at the time of relapse. The pathophysiology underlying EMD is multifactorial and may involve heightened systemic inflammation and a more immunosuppressive tumor microenvironment. These features are often associated with high tumor burden and increased metabolic activity, as visualized on positron emission tomography imaging. Additionally, EMD is frequently characterized by bulky, metabolically active lesions that impair T-cell functionality, both in the collected autologous T cells used for CAR T-cell manufacturing and in the infused CAR T-cells. This contributes to a mismatch between effector cells and tumor burden, ultimately requiring robust CAR T-cell expansion to achieve meaningful and sustained responses. Notably, high metabolic tumor volume has been linked to both increased toxicity and reduced efficacy of BCMA-directed CAR T-cell therapy in MM (15–20).
In the KarMMa-1 trial in a heavily pretreated cohort, EMD, defined as soft-tissue lesions not contiguous with bone, was observed in 39% of patients, with ide-cel maintaining the objective response rate in this high-risk subgroup (10). In KarMMa-3, in patients with 2–4 prior lines, EMD was more broadly defined to include both true extramedullary soft-tissue disease and bone-related soft-tissue plasmacytomas, with an incidence of 24%, and its presence correlated with significantly shorter PFS (21). Similarly, the CARTITUDE-1 trial reported EMD, including both bone-based and extramedullary plasmacytomas, in 20% of patients, where it was associated with reduced PFS (22). In the CARTITUDE-4 trial, 21.2% of patients had EMD, which was likewise linked to inferior PFS and OS outcomes (13). Real-world experiences have reported higher incidence of EMD compared to clinical trials. For example, in a study of 159 patients treated with ide-cel, 48% had either a history of or active EMD at the time of infusion (23). Another multicenter analysis involving 152 patients who received commercial CAR T-cell therapy found that 31% had active EMD at infusion (24). Both studies demonstrated that the presence of EMD was associated with inferior response rates and survival outcomes.
Overall, our findings align with existing literature demonstrating inferior outcomes in patients with EMD. The high incidence of EMD in our study is likely due to the heavily pretreated nature of our cohort as the risk of developing EMD increases with cumulative treatment exposure and disease refractoriness (25). Some studies define EMD strictly as hematogenous spread without adjacent bone involvement, while others include both EMD and PMD. These inconsistencies in classification highlight the need for caution when interpreting and comparing results across studies.
Our study demonstrated that the presence of EMD at the time of progression after CAR T-cell therapy was associated with inferior survival outcomes. While most published studies have focused on the prognostic impact of EMD prior to CAR T-cell infusion, our findings highlight the significance of disease characteristics at progression. Notably, patients with baseline EMD were more likely to have EMD at progression, and this ultra-high-risk group experienced significantly worse survival outcomes, underscoring the aggressive and persistent nature of this disease phenotype, even after CAR T-cell therapy.
To improve outcomes in patients with EMD undergoing standard-of-care CAR T-cell therapy, there is a critical need to establish standardized treatment protocols tailored to this high-risk population. Bridging therapies designed to reduce tumor burden prior to CAR T-cell infusion may be beneficial in enhancing treatment efficacy. Among these, radiation therapy has shown promise as a localized bridging strategy, effectively reducing bulky disease without significantly increasing toxicity, and potentially improving CAR T-cell expansion and response (24, 26). However, data on the optimal type, timing, and integration of bridging therapies remain limited. Maintenance therapy following CAR T-cell therapy, including the use of lenalidomide or novel bispecific antibodies, is currently under investigation and may improve the durability of response, particularly in high-risk patient populations (27–29). Prospective studies are warranted to better define the role of these interventions and optimize outcomes in this challenging subgroup.
Limitations of our study include the relatively small sample size and the predominance of patients treated with ide-cel, with a minority having progressed after receiving cilta-cel. Response assessments were conducted at the discretion of the treating physician, without independent review, which may introduce variability. Additionally, EMD and PMD was identified solely through radiographic imaging without histopathologic confirmation. Although imaging is essential for detecting EMD and PMD, relying exclusively on radiologic findings may overestimate its prevalence, as false positives are possible in the absence of biopsy-proven disease. Despite these limitations, our study offers meaningful insights into real-world patterns of progression following commercial CAR T-cell therapy and demonstrates that the presence of EMD is associated with poor outcomes, underscoring the need for targeted strategies to improve prognosis in this high-risk population.
Conclusion
In this real-world retrospective analysis, we characterized patterns of disease progression following commercial anti-BCMA CAR T-cell therapy and demonstrated that the presence of EMD, both at baseline and at the time of progression, is associated with significantly inferior survival outcomes after progression post-CAR T-cell therapy. Our findings reinforce the aggressive and refractory nature of EMD and highlight that patients with these high-risk features are more likely to progress early and die sooner than those without such involvement. This underscores the clinical relevance of assessing and monitoring for EMD and PMD throughout the treatment course of patients undergoing CAR T-cell therapy.
Given the poor prognosis associated with EMD, there is a critical need for therapeutic strategies to improve outcomes in this high-risk subgroup. Bridging therapies aimed at reducing tumor burden prior to CAR T-cell infusion may enhance CAR T-cell expansion, persistence, and antitumor activity. Additionally, maintenance therapies post-infusion may prolong response durability. However, the optimal approach to bridging and maintenance remains undefined and warrants prospective investigation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Moffitt Cancer Center Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZA: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review & editing. FA: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review & editing. WF: Formal Analysis, Methodology, Software, Writing – review & editing. GD: Data curation, Methodology, Resources, Software, Writing – review & editing. DH: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. AG-C: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – review & editing. BB: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. OC: Conceptualization, Methodology, Supervision, Writing – review & editing. HL: Conceptualization, Methodology, Supervision, Writing – review & editing. CF: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – review & editing. TN: Conceptualization, Methodology, Validation, Visualization, Writing – review & editing. FL: Methodology, Supervision, Validation, Visualization, Writing – review & editing. KS: Conceptualization, Methodology, Validation, Visualization, Writing – review & editing. RB: Conceptualization, Formal Analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MA: Conceptualization, Formal Analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
DH reports research support from Bristol-Myers Squibb, Janssen, Kite Pharma, Karyopharm, and Adaptive Biotech; consulting or advisory role for Bristol-Myers Squibb, Janssen, Legend Biotech, Pfizer, Kite Pharma, AstraZeneca, and Karyopharm. AG-C reports research support from Janssen; honoraria from Janssen, Bristol-Myer Squibb, Sanofi, Cellectar, Pfizer, Amgen, and Kite Pharma.
BB reports consulting or advisory role for Bristol-Meyers Squibb, Kite Pharma, Pfizer, Takeda, and Johnson and Johnson. OC reports honoraria for consultancy with Legend Biotech Inc, Bristol-Myers Squibb, and Janssen Biotech Inc. CF reports research funding from Bristol-Myers Squibb, Janssen and Roche/Genetech; honoraria and consulting for Bristol-Myers Squibb, Seattle genetics, Celgene, Abbvie, Sanofi, Incyte, Amgen, Onk therapeutics & Janssen. TN reports clinical trial support by Novartis to the institution and clinical trial support drug only supply by Karyopharm to the institution. FL reports Scientific Advisory Role/Consulting Fees for A2, Allogene, Amgen, Bluebird Bio, Bristol-Myers Squibb, Calibr, Caribou, Cowen, EcoR1, Gerson Lehrman Group GLG, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Sana, Umoja, Pfizer. Data Safety Monitoring Board: Data and Safety Monitoring Board for the NCI Safety Oversight CAR T-cell Therapies Committee. Research Contracts or Grants to my Institution for Service: Kite Pharma Institutional, Allogene Institutional, CERo Therapeutics Institutional, Novartis Institutional, BlueBird Bio Institutional, 2SeventyBio Institutional, Bristol-Myers Squibb Institutional, National Cancer Institute R01CA244328 MPI: Locke; P30CA076292 PI: Cleveland, Leukemia and Lymphoma Society Scholar in Clinical Research PI: Locke. Patents, Royalties, Other Intellectual Property: Several patents held by the institution in my name unlicensed in the field of cellular immunotherapy. Education or Editorial Activity: Aptitude Health, ASH, BioPharma Communications CARE Education, Clinical Care Options Oncology, Imedex, Society for Immunotherapy of Cancer. KS reports grants from Abbvie, Karyopharm; honoraria for lectures from Karyopharm, Janssen, Adaptive, GlaxoSmith Kline, Bristol-Myers Squibb, Sanofi, Regeneron; advisory board for Janssen, GlaxoSmith Kline. RB reports research support from Abbvie, Regeneron, Karyopharm, Bristol-Myers Squibb, Janssen; consulting or advisory role for Pfizer, Janssen, and Sanofi. MA reports research support form Janssen, Bristol-Myers Squibb, Pfizer, and AstraZeneca; consulting or advisory role for Sanofi, Janssen and Bristol-Myers Squibb.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Surveillance epidemiology and end results program. In: Cancer Stat Facts: Myeloma. USA: SEER database. Available online at: https://seer.cancer.gov/statfacts/html/mulmy.html (Accessed January 2025).
2. Chakraborty R, Liu HD, Rybicki L, Tomer J, Khouri J, Dean RM, et al. Progression with clinical features is associated with worse subsequent survival in multiple myeloma. Am J Hematol. (2019) 94:439–45. doi: 10.1002/ajh.25415
3. Bladé J, Beksac M, Caers J, Jurczyszyn A, von Lilienfeld-Toal M, Moreau P, et al. Extramedullary disease in multiple myeloma: a systematic literature review. Blood Cancer J. (2022) 12:45. doi: 10.1038/s41408-022-00643-3
4. Jiménez-Segura R, Rosiñol L, Cibeira MT, Fernández de Larrea C, Tovar N, Rodríguez-Lobato LG, et al. Paraskeletal and extramedullary plasmacytomas in multiple myeloma at diagnosis and at first relapse: 50-years of experience from an academic institution. Blood Cancer J. (2022) 12:135. doi: 10.1038/s41408-022-00730-5
5. Short KD, Rajkumar SV, Larson D, Buadi F, Hayman S, Dispenzieri A, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. (2011) 25:906–8. doi: 10.1038/leu.2011.29
6. He J, Yue X, He D, Zhao Y, Yang Y, Zheng G, et al. Multiple extramedullary-bone related and/or extramedullary extraosseous are independent poor prognostic factors in patients with newly diagnosed multiple myeloma. Front Oncol. (2021) 11:668099. doi: 10.3389/fonc.2021.668099
7. Beksac M, Seval GC, Kanellias N, Coriu D, Rosiñol L, Ozet G, et al. A real world multicenter retrospective study on extramedullary disease from Balkan Myeloma Study Group and Barcelona University: analysis of parameters that improve outcome. Haematologica. (2020) 105:201–8. doi: 10.3324/haematol.2019.219295
8. Gao S, Li Q, Dong F, Yang P, Chen Y, Wang J, et al. Clinical characteristics and survival outcomes of newly diagnosed multiple myeloma patients presenting with extramedullary disease: A retrospective study. Leuk Res. (2022) 115:106793. doi: 10.1016/j.leukres.2022.106793
9. Wang J, Shen N, Shen X, Zhang R, Jin Y, Li J, et al. Survival trends and prognostic factors of patients with newly diagnosed multiple myeloma accompanied with extramedullary disease. Ann Med. (2023) 55:2281657. doi: 10.1080/07853890.2023.2281657
10. Munshi NC, Anderson LD Jr., Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. (2021) 384:705–16. doi: 10.1056/NEJMoa2024850
11. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. (2021) 398:314–24. doi: 10.1016/S0140-6736(21)00933-8
12. Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. New Engl J Med. (2023) 388:1002–14. doi: 10.1056/NEJMoa2213614
13. San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos M-V, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. New Engl J Med. (2023) 389:335–47. doi: 10.1056/NEJMoa2303379
14. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. (2016) 17:e328–e46. doi: 10.1016/S1470-2045(16)30206-6
15. Qi Y, Li H, Qi K, Zhu F, Cheng H, Chen W, et al. Clinical outcomes and microenvironment profiling in relapsed/refractory multiple myeloma patients with extramedullary disease receiving anti-BCMA CAR T-cell-based therapy. Am J Hematol. (2024) 99:2286–95. doi: 10.1002/ajh.27469
16. Freeman CL, Noble J, Menges M, Villanueva R, Nakashima JY, Figura NB, et al. Tumor burden quantified by Soluble B-Cell Maturation Antigen and Metabolic Tumor Volume determine myeloma CAR-T outcomes. Blood. (2025) 145(15):1645–57. doi: 10.1182/blood.2024024965
17. Tan JY, Low MH, Chen Y, and Lim FLWI. CAR T cell therapy in hematological Malignancies: implications of the tumor microenvironment and biomarkers on efficacy and toxicity. Int J Mol Sci. (2022) 23(13):6931. doi: 10.3390/ijms23136931
18. Que Y, Xu M, Xu Y, Almeida VDF, Zhu L, Wang Z, et al. Anti-BCMA CAR-T cell therapy in relapsed/refractory multiple myeloma patients with extramedullary disease: A single center analysis of two clinical trials. Front Immunol. (2021) 12:755866. doi: 10.3389/fimmu.2021.755866
19. Nakamoto-Matsubara R, Nardi V, Horick N, Fukushima T, Han RS, Shome R, et al. Integration of clinical outcomes and molecular features in extramedullary disease in multiple myeloma. Blood Cancer J. (2024) 14:224. doi: 10.1038/s41408-024-01190-9
20. Freeman CL, Noble J, Menges M, Villanueva R, Nakashima JY, Figura NB, et al. Tumor burden quantified by soluble B-cell maturation antigen and metabolic tumor volume determines myeloma CAR-T outcomes. Blood. (2025) 145:1645–57. doi: 10.1182/blood.2024024965
21. Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, Manier S, et al. Ide-cel vs standard regimens in triple-class-exposed relapsed and refractory multiple myeloma: updated KarMMa-3 analyses. Blood. (2024) 144:2389–401. doi: 10.1182/blood.2024024582
22. Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Ciltacabtagene autoleucel, an anti–B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. (2023) 41:1265–74. doi: 10.1200/JCO.22.00842
23. Hansen DK, Sidana S, Peres LC, Leitzinger CC, Shune L, Shrewsbury A, et al. Idecabtagene vicleucel for relapsed/refractory multiple myeloma: real-world experience from the myeloma CAR T consortium. J Clin Oncol. (2023) 41:2087–97. doi: 10.1200/JCO.22.01365
24. Dima D, Abdallah AO, Davis JA, Awada H, Goel U, Rashid A, et al. Impact of extraosseous extramedullary disease on outcomes of patients with relapsed-refractory multiple myeloma receiving standard-of-care chimeric antigen receptor T-cell therapy. Blood Cancer J. (2024) 14:90. doi: 10.1038/s41408-024-01068-w
25. Sevcikova S, Minarik J, Stork M, Jelinek T, Pour L, and Hajek R. Extramedullary disease in multiple myeloma - controversies and future directions. Blood Rev. (2019) 36:32–9. doi: 10.1016/j.blre.2019.04.002
26. Manjunath SH, Cohen AD, Lacey SF, Davis MM, Garfall AL, Melenhorst JJ, et al. The safety of bridging radiation with anti-BCMA CAR T-cell therapy for multiple myeloma. Clin Cancer Res. (2021) 27:6580–90. doi: 10.1158/1078-0432.CCR-21-0308
27. Shi X, Yan L, Shang J, Kang L, Yan Z, Jin S, et al. Anti-CD19 and anti-BCMA CAR T cell therapy followed by lenalidomide maintenance after autologous stem-cell transplantation for high-risk newly diagnosed multiple myeloma. Am J Hematol. (2022) 97:537–47. doi: 10.1002/ajh.26486
28. Holstein SA, Grant SJ, and Wildes TM. Chimeric antigen receptor T-cell and bispecific antibody therapy in multiple myeloma: moving into the future. J Clin Oncol. (2023) 41:4416–29. doi: 10.1200/JCO.23.00512
Keywords: multiple myeloma, CAR T-cell therapy, progression, extramedullary disease, survival outcomes
Citation: Abuhelwa Z, Amisha F, Fan W, De Avila G, Hansen DK, Grajales-Cruz AF, Blue B, Castaneda O, Liu H, Freeman CL, Nishihori T, Locke FL, Shain KH, Baz R and Alsina M (2025) Post-progression outcomes following BCMA-directed CAR T-cell therapy in myeloma: impact of extramedullary and paramedullary disease. Front. Oncol. 15:1663814. doi: 10.3389/fonc.2025.1663814
Received: 11 July 2025; Accepted: 08 October 2025;
Published: 27 October 2025.
Edited by:
Hongtao Liu, University of Wisconsin System, United StatesReviewed by:
Lorenzo Iovino, Fred Hutchinson Cancer Center, United StatesYangmin Zhu, Guangdong Second Provincial General Hospital, China
Copyright © 2025 Abuhelwa, Amisha, Fan, De Avila, Hansen, Grajales-Cruz, Blue, Castaneda, Liu, Freeman, Nishihori, Locke, Shain, Baz and Alsina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melissa Alsina, bWVsaXNzYS5hbHNpbmFAbW9mZml0dC5vcmc=
 Ziad Abuhelwa
Ziad Abuhelwa Fnu Amisha1,2
Fnu Amisha1,2 Ciara L. Freeman
Ciara L. Freeman Taiga Nishihori
Taiga Nishihori Melissa Alsina
Melissa Alsina