- Department of Hematology, Shaoxing People’s Hospital, Shaoxing, China
Systemic light chain amyloidosis (AL) is an uncommon disorder of clonal plasma cells, marked by the creation of amyloid-forming immunoglobulin light chains that result in amyloid fibril formation and deposition, causing dysfunction in multiple organs. There is no established treatment protocol for patients with relapsed or refractory AL. Teclistamab is a bispecific antibody targeting B-cell maturation antigen (BCMA) and is approved for treating relapsed or refractory multiple myeloma (MM). The objective of our study was to report real-world data on the safety and efficacy of teclistamab in patients with relapsed/refractory AL.
Introduction
Systemic light chain amyloidosis (AL) is a condition involving plasma cells that leads to the misfolding and extracellular accumulation of immunoglobulin light chains, leading to harm in various organ systems such as the heart (60%), kidneys (50% - 80%), liver (25% - 60%), soft tissue (40%), peripheral/autonomic nervous system (15%), and gastrointestinal tract (5%) (1). AL can lead to serious dysfunction in multiple organ systems, and approximately 30% of patients may die within six months of being diagnosed (2).
Therapeutic strategies for AL aim at the clonal plasma cells and have historically used anti-plasma cell agents effective in multiple myeloma (MM) treatment. The sole Food and Drug Administration (FDA)-approved treatment plan for AL, which is also the standard initial therapy, consists of daratumumab, cyclophosphamide, bortezomib, and dexamethasone. Despite enhancements in therapies that include bortezomib, the rates of achieving a complete hematologic response are still below expectations. There is a high rate of early mortality, with outcomes differing according to the degree and seriousness of organ involvement, and treatment-related toxicity is common (2). And Patients with relapsed AL who are refractory to daratumumab lack a standard treatment protocol and usually receive combination therapies based on MM treatment models (3, 4).
As of now, the FDA has granted accelerated approval to B-cell maturation antigen (BCMA)-targeting bispecific antibodies (bsAbs), specifically teclistamab, for use in relapsed/refractory myeloma. As a bispecific antibody, Teclistamab redirects T-cells by targeting CD3 on T-cells and the BCMA on plasma cells. Teclistamab produced profound and sustained responses in individuals with relapsed or refractory (R/R) MM during the MajesTEC-1 trial (5). BCMA-targeting bsAbs are an enticing treatment option for AL due to several reasons: (a) rapid attainment of profound hematologic responses in myeloma is crucial for organ response in AL; (b) there is a reduced occurrence of severe cytokine release syndrome (CRS) compared to other T-cell redirecting treatments like chimeric antigen receptor T-cell therapy. No clinical trials have yet provided data on the safety and efficacy of teclistamab in AL. This report discusses a case involving a patient with R/R AL who received successful treatment using the bispecific antibody teclistamab.
Case report
A 64-year-old man with a history of hypertension was admitted to the hospital on June 23, 2023, due to persistent lower limb swelling that had lasted over six months. The examination conducted during hospitalization indicated Hemoglobin (119g/L, 130-175g/L) and Serum creatinine (115.2μmol/L, 59-104μmol/L), serum protein electrophoresis and immunofixation electrophoresis were notable for a monoclonal IgA λ immunoglobulin, serum kappa FLC was 31.2 mg/L, lambda FLC was 219 mg/L with a kappa to lambda ratio of 0.14. 24-hour proteinuria: 4.1g/d (0-0.15g/d). Tspot, thyroid function, glycosylated hemoglobin, ANA, ANCA, anti-glomerular basement membrane antibody, NTproBNP, troponin and CMV-DNA were normal. Pathological examination following a renal biopsy showed AL-type amyloidosis in the kidneys (Figure 1). The cardiac MRI revealed heart involvement and an enlarged left atrium, with diffuse thickening of the left ventricular septal wall. According to the modified European criteria, by the Mayo 2012 criteria, he was at stage II, with renal stage II AL.
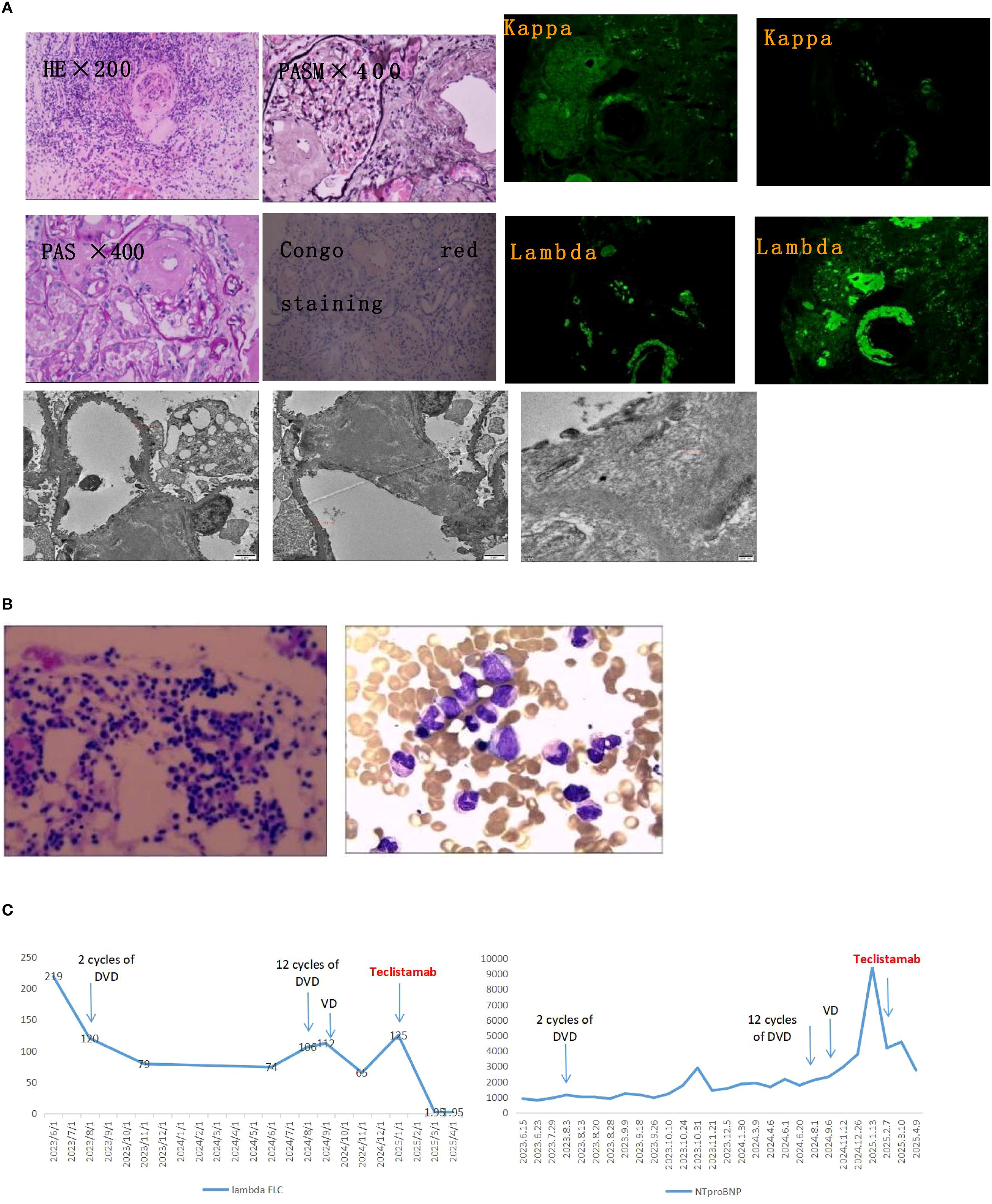
Figure 1. Diagnostic pathology and longitudinal treatment response. (A) Renal pathological assessment. Upper left: Congo red staining under polarized light shows characteristic apple-green birefringence, confirming amyloid deposits. Upper right: Immunofluorescence demonstrates strong lambda light chain restriction (+++) with kappa light chain negativity, diagnostic for AL-lambda amyloidosis. Bottom: Transmission electron microscopy reveals randomly oriented, non-branching amyloid fibrils measuring 8–10 nm in diameter. (B) Bone marrow evaluation. Wright-Giemsa staining shows no evidence of myeloma cell infiltration or clonal plasma cell expansion. (C) Serial biomarker trends. The vertical dashed line denotes initiation of teclistamab therapy. Lambda free light chain (λ FLC) levels (mg/L, left axis) rapidly declined to undetectable ranges post-treatment. Concurrently, NT-proBNP levels (pg/mL, right axis) showed substantial reduction, reflecting cardiac functional improvement. DVD, daratumumab-bortezomib-dexamethasone; VD, bortezomib-dexamethasone; NT-proBNP, N-terminal pro-brain natriuretic peptide.
He began treatment with 2 cycles of DVD (daratumumab, bortezomib, and dexamethasone), the lambda FLC decreased to 120 mg/L, yet there was no improvement in renal function (Serum creatinine rose to 162 μmol/L) and NTproBNP (1132 pg/ml, 0-125pg/ml). 9 cycles of treatment with DVD were completed, the lambda FLC decreased to 74 mg/L, yet there was no improvement in renal function (Serum creatinine rose to 272 μmol/L) and NTproBNP (2154 pg/ml). And the patient’s immunofixation electrophoresis remained positive. During the 12th cycle of the DVD regimen, he relapsed with worsening renal function (Serum creatinine rose to 333 μmol/L) and NTproBNP (2307 pg/ml), and rising lambda FLC (106 mg/L). The medication also caused the patient to develop peripheral neuropathy as an adverse reaction. The patient felt numbness and discomfort in their limbs, along with occasional nighttime pain. Following this, the treatment was altered to a VD regimen (bortezomib and dexamethasone) for 5 rounds. However, the treatment goal was not met. The patient’s immunofixation electrophoresis continued to be positive, and there was a decline in renal function (Serum creatinine rose to 365 μmol/L), an increase in NTproBNP (9421 pg/ml), and elevated lambda FLC (125 mg/L). We once more examined the bone marrow and did not find a restricted plasma cell clone (Figure 1).
Due to his resistant disease, the patient received teclistamab, which the FDA (5) has approved for use in MM after four lines of treatment. At the beginning, the serum creatinine level rose to 260μmol/L, NTproBNP was measured at 4177 pg/ml, and lambda FLC was 125 mg/L. He managed the gradual increase (teclistamab 0.06mg/kg d1, 0.3mg/kg d2, 60mg d8,15,22) in dosing without developing CRS or immune effector cell-associated neurotoxicity syndrome (ICANS). The encouraging news is that after completing one cycle, both serum immunofixation and lambda FLC were negative. The patient reached a state of complete remission. Subsequently, he switched to receiving subcutaneous injection every two weeks starting from cycle 3 (teclistamab 60mg q2w). Throughout the treatment, no further adverse reactions were observed, and the patient’s cardiac issues (NTproBNP was measured at 2738 pg/ml) improved considerably (Figure 1). Regrettably, the patient’s kidney function did not enhance as expected and continued to decline, the serum creatinine level rose to 362μmol/L. It might be that the kidneys’ structure is impaired, leading to difficulties in regaining their functions. The medical examination during the hospitalization on April 9th, 2025, indicated Hemoglobin (106g/L) and Serum creatinine (362μmol/L), serum immunofixation and lambda FLC were negative, 24-hour proteinuria: 3.2g/d, NTproBNP (2738 pg/ml).
This patient was generally hard to manage because of relapse, refractory, and declining cardiac and renal function. The use of teclistamab led to a swift hematologic and clinical response, with the patient maintaining a lasting response to the treatment. Trials generally exclude patients who have severe kidney failure along with MM or AL. Patients with severe kidney dysfunction frequently need hemodialysis or peritoneal dialysis, which may also impact the effectiveness of the medication. Perhaps it is also related to the fact that the drugs are difficult to improve the patient’s kidney function. This research is based on a single case report, which is limited by its small sample size, and its findings may not apply to all patients with R/R AL. There is still debate over the follow-up consolidation therapy for R/R AL patients who have achieved complete remission. Additional research involving more patients and extended follow-up periods is necessary to verify the effectiveness and safety of anti-BCMA bispecific antibodies in R/R AL.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Shaoxing People’s Hospital Academic Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZC: Writing – original draft. ZZ: Writing – review & editing. JY: Writing – original draft. LF: Writing – review & editing. JF: Writing – review & editing. WF: Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Medical Science and Technology Project of Zhejiang Provincial Health Commission Grand (2023KY353), Medical and Health Science and Technology Program of Zhejiang Province (2023KY1255), This work was supported by the Jiebang Guashuai Project of Shaoxing People’s Hospital (2025JBPY01), the Cultivation of High-level Innovative Health Talents Program of Zhejiang Province.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gertz MA and Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: A systematic review. Jama. (2020) 324:79–89. doi: 10.1001/jama.2020.5493
2. Muchtar E, Gertz MA, Kumar SK, Lacy MQ, Dingli D, Buadi FK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. (2017) 129:2111–9. doi: 10.1182/blood-2016-11-751628
3. Khwaja J, Bomsztyk J, Mahmood S, Wisniowski B, Sha R, Tailor A, et al. High response rates with single-agent belantamab mafodotin in relapsed systemic AL amyloidosis. Blood Cancer J. (2022) 12:128. doi: 10.1038/s41408-022-00717-2
4. Forgeard N, Elessa D, Carpinteiro A, Belhadj K, Minnema M, Roussel M, et al. Teclistamab in relapsed or refractory AL amyloidosis: a multinational retrospective case series. Blood. (2024) 143:734–7. doi: 10.1182/blood.2023022937
Keywords: amyloidosis, teclistamab, relapsed/refractory, bispecific antibody, safety and efficacy
Citation: Chen Z, Zhang Z, Yu J, Fu L, Fu J and Feng W (2025) Case Report: Safety and efficacy assessment of teclistamab in relapsed/refractory amyloidosis: a case study. Front. Oncol. 15:1664171. doi: 10.3389/fonc.2025.1664171
Received: 11 July 2025; Accepted: 02 September 2025;
Published: 18 September 2025.
Edited by:
Yangmin Zhu, Guangdong Second Provincial General Hospital, ChinaReviewed by:
Wenqing Zhang, Shanghai Jiao Tong University, ChinaCihan Heybeli, Dokuz Eylul University, Türkiye
Copyright © 2025 Chen, Zhang, Yu, Fu, Fu and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiying Feng, ZmVuZ3dlaXlpbmcxOTk3QDEyNi5jb20=
 Zhe Chen
Zhe Chen Zhijian Zhang
Zhijian Zhang Jieni Yu
Jieni Yu Leihua Fu
Leihua Fu Weiying Feng
Weiying Feng