- 1Department of General Surgery, Zigong Fourth People’s Hospital, Zigong, Sichuan, China
- 2Department of Gastrointestinal Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Objective: The potential benefits of low pneumoperitoneum pressure (LPP) in laparoscopic gastrointestinal surgery, particularly gastric procedures, remain insufficiently investigated. This meta-analysis aims to systematically evaluate the advantages of LPP in laparoscopic gastrointestinal surgery compared to standard pneumoperitoneum pressure (SPP).
Methods: A comprehensive literature search was conducted in Embase, Web of Science, PubMed, and Cochrane Library databases from inception to April 10, 2025. Studies comparing LPP with SPP in laparoscopic gastrointestinal surgery, including both randomized controlled trials (RCTs) and observational studies, were systematically reviewed. Data were analyzed using RevMan 5.3 software, with primary outcomes including postoperative pain at rest, pain in post-anesthesia care unit (PACU), and activity-related pain.
Results: Twelve studies were included in the meta-analysis. Compared with SPP, LPP significantly reduced postoperative pain at rest (SMD = -0.40, 95% CI: -0.68 to -0.12, P = 0.005) and pain in PACU (SMD = -1.06, 95% CI: -1.65 to -0.47, P = 0.0004). Additionally, LPP was associated with faster recovery of gastrointestinal function (SMD = -0.27, 95% CI: -0.50 to -0.05, P = 0.02). However, no significant differences were observed between the two groups in terms of activity-related pain, operative time, intraoperative blood loss, surgical field visibility, length of hospital stay, anastomotic leakage, or postoperative complications. Notably, LPP was more frequently associated with intraoperative adjustments to pneumoperitoneum pressure (OR = 4.01, 95% CI: 2.48 to 6.50, P < 0.00001).
Conclusions: In laparoscopic gastrointestinal surgery, LPP provides clinically relevant benefits by reducing postoperative pain at rest and in PACU, as well as accelerating gastrointestinal recovery. However, surgeons should be aware of the potential need for more frequent intraoperative adjustments to pneumoperitoneum pressure when using LPP.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/search, identifier CRD420251037390.
Background
Laparoscopic surgery has been widely adopted in the management of gastrointestinal disease and is currently regarded as the gold standard for treating gastrointestinal malignancies. A critical prerequisite for laparoscopic procedures is carbon dioxide insufflation to establish pneumoperitoneum, which maintains an optimal surgical field. For laparoscopic gastrointestinal surgery, the SPP typically ranges between 12–15 mmHg. However, European consensus guidelines recommend maintaining the lowest feasible pneumoperitoneum pressure while ensuring safety (1), as elevated intra-abdominal pressure during pneumoperitoneum may adversely impact patient outcomes (2, 3). Increased intra-abdominal pressure elevates the diaphragm, potentially compromising hemodynamic stability and triggering elevated systemic biomarkers of physiological stress (4). Emerging evidence suggests that LPP mitigates pneumoperitoneum-related adverse effects, including reduced postoperative pain and enhanced recovery (5, 6). A recent prospective cohort study demonstrated that LPP significantly decreases postoperative analgesic requirements and accelerates time to first flatus (7). Furthermore, a contemporary RCT confirmed comparable operative duration and intraoperative blood loss between LPP and SPP in laparoscopic colorectal surgery (8). For gastric procedures, preliminary investigations by Yu Z et al. (9) and Zhang YW et al. (10) have endorsed the safety and efficacy of LPP, though evidence remains scarce. While interest in LPP for laparoscopic gastrointestinal surgery is growing, synthesized evidence remains limited. Existing meta-analyses by Hamid et al. (11) and Dourado et al. (12) focused exclusively on colorectal surgeries and were constrained by small sample sizes. To address these limitations, this study updates the literature pool and incorporates emerging data on laparoscopic gastric surgeries. By expanding the scope and sample size, we aim to provide a robust evaluation of the clinical benefits and safety profile of LPP across the spectrum of laparoscopic gastrointestinal procedures.
Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13) and prospectively registered in the PROSPERO international registry (Registration No. CRD420251037390).
Literature search strategy
Two researchers (FXP/KEL) independently searched the Embase, Web of Science, PubMed, and Cochrane Library databases from their inception to April 10, 2025. The search strategy incorporated a combination of Medical Subject Headings (MeSH) and free-text terms. MeSH terms included Colorectal Neoplasm, Laparoscopy, and Stomach Neoplasm. Free-text terms comprised Colorectal Tumor, Colorectal Cancer, Colorectal Carcinoma, Gastric Neoplasm, Gastric Cancer, Stomach Cancer, Peritoneoscopy, Celioscopy, Laparoscopic Assisted Surgery, Laparoscopic Surgical Procedure, Pneumoperitoneum, abdominal pressure, and intraabdominal pressure. Boolean operators (AND/OR) were utilized to link MeSH terms with free-text terms, ensuring comprehensive coverage of relevant studies.
Inclusion and exclusion criteria
Inclusion Criteria: i) Patients aged >18 years, diagnosed with gastrointestinal diseases and undergoing laparoscopic surgery. ii) Intervention: Low pneumoperitoneum pressure; control group: standard or high pneumoperitoneum pressure. iii) Complete outcome data reported. Exclusion Criteria: i) Animal studies. ii) Meta-analyses. iii) Single-arm studies. iv) Letters or commentaries. v) Case reports.
Quality assessment of literature
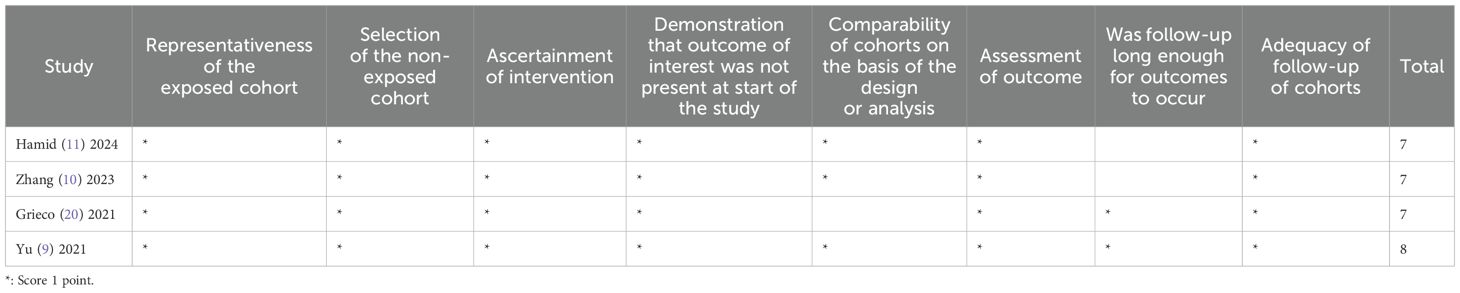
The quality assessment was independently conducted by two researchers (FXP/KEL). For the included RCTs, the Cochrane Risk of Bias tool (14) was used to evaluate risks of bias, including selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. For non-randomized studies, the Newcastle-Ottawa Scale (NOS) (15) was applied to assess bias, covering the following criteria: representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of intervention, demonstration that the outcome of interest was absent at the study’s initiation, comparability of cohorts based on design or analysis, outcome assessment, adequacy of follow-up duration for outcomes to occur, and completeness of cohort follow-up. Any discrepancies between the two researchers were resolved through discussion or adjudication by a third researcher.
Data extraction
Zotero software was utilized for managing retrieved literature. Two researchers independently screened the literature by reviewing titles, abstracts, and full texts. Data from the ultimately included studies were extracted, encompassing the following information: first author, publication year, study type, sample size, patient age, BMI, ASA classification, and additional data including primary and secondary outcomes.
Observed outcomes
Primary outcomes: Postoperative resting pain; pain in PACU; activity-related pain. Secondary outcomes: Operative time; intraoperative blood loss; surgical field visibility; time to first postoperative flatus; length of hospital stay; anastomotic leakage; postoperative complications; Intra−operative pressure changes. Surgical field visibility refers to the clarity of the operative area under endoscopic visualization and the ease of surgical manipulation. Evaluation Criteria: The Leiden Surgical Rating Scale (L-SRS) was employed for assessment, which evaluates three key parameters: (i) clarity of the surgical field, (ii) instrument maneuverability, and (iii) degree of intraoperative interference. The L-SRS utilizes a 5-point scoring system, where 1 indicates extremely poor visibility, 2 denotes poor, 3 represents acceptable, 4 signifies good, and 5 corresponds to optimal surgical field conditions.
Statistical analysis
Statistical analyses were performed using RevMan 5.3.1 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration) (16). Dichotomous variables were expressed as odds ratios (OR) with 95% confidence intervals (CI), while continuous outcomes were reported as standardized mean differences (SMD) with 95% CI. Results were visualized using forest plots. Heterogeneity across studies was assessed using Higgins I² statistics. I² ≤ 50%: Indicates low heterogeneity, and a fixed-effects model was applied. I² > 50%: Indicates substantial heterogeneity, and a random-effects model was used. If significant heterogeneity was detected, subgroup or sensitivity analyses were conducted to explore potential sources of heterogeneity. The GRADE quality assessment for the primary outcomes and some of the secondary outcomes was conducted using the GRADEpro GDT software (version 3.6).
Results
Study selection
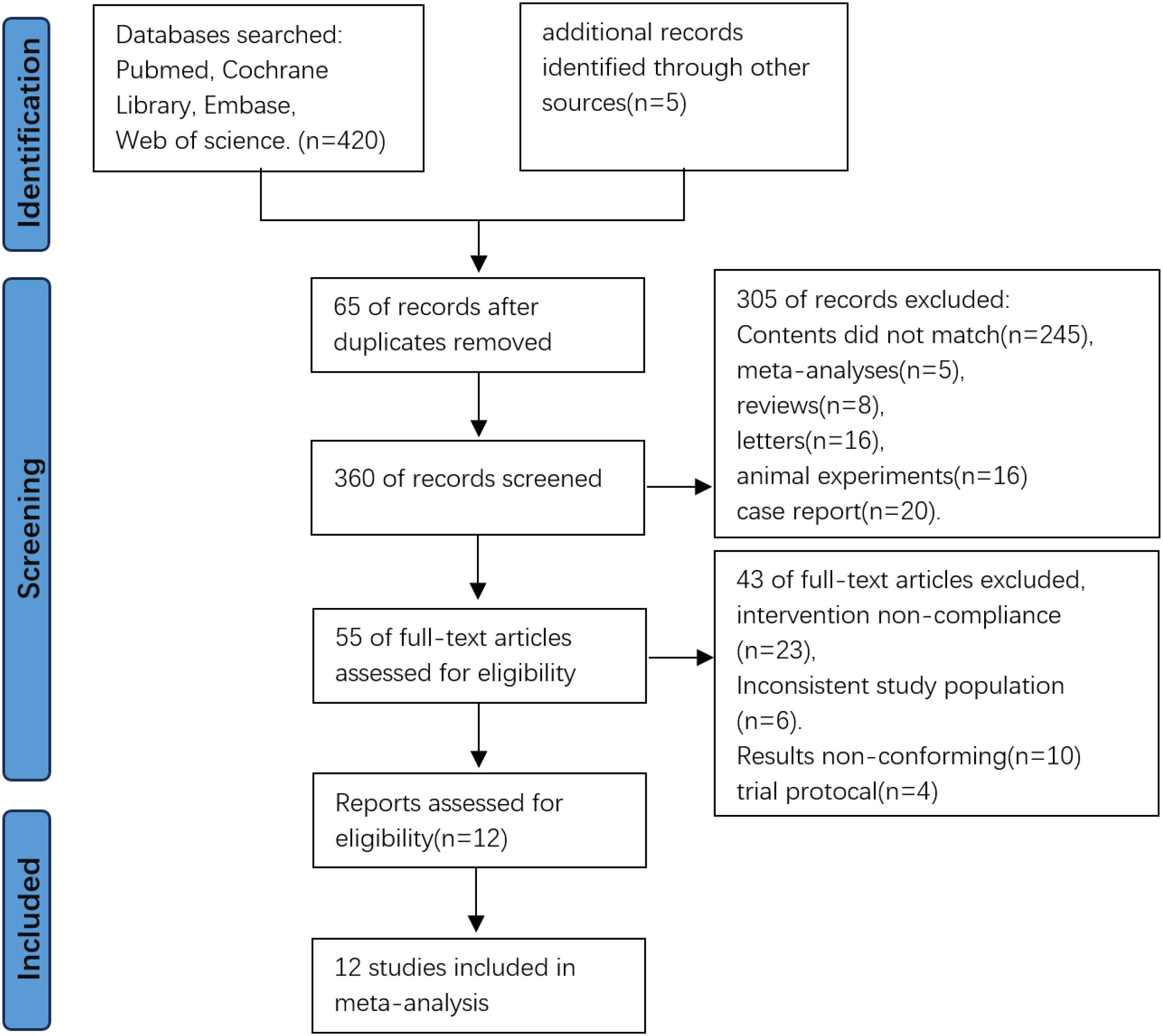
A total of 425 records were identified through database searches. After removing 65 duplicates, 360 records remained for screening. Following title and abstract screening, 305 records were excluded, resulting in 55 full-text articles assessed for eligibility, upon full-text review, 43 articles were excluded due to irrelevance or unmet criteria. Finally, 12 studies were included in the meta-analysis (Figure 1).
Study characteristics
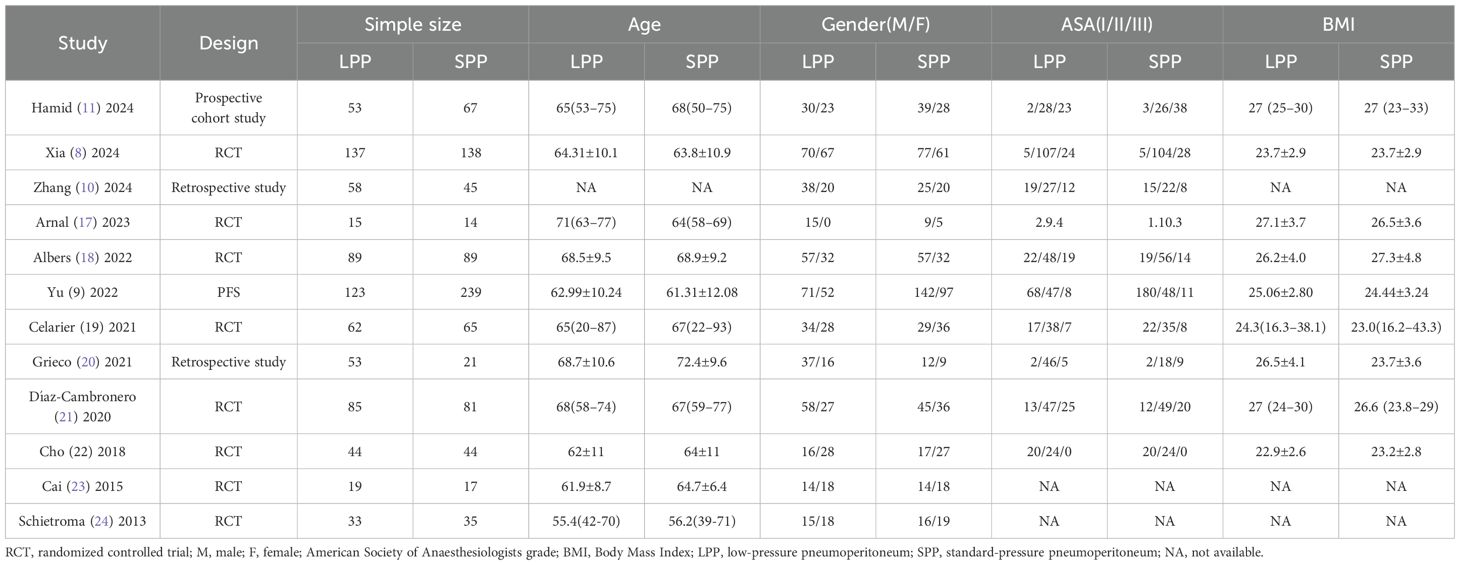
A total of 12 studies (7–10, 17–24) were included, comprising 8 RCTs (8, 17–19, 21–24), 2 retrospective studies (10, 20), 1 prospective cohort study (7), and 1 propensity score-matched analysis (9). The pooled cohort consisted of 1,626 patients, with 771 cases (47.42%) allocated to the LPP group (8–10 mmHg) and 855 cases (52.58%) to the SPP group (12–15 mmHg). While minor protocol variations existed across studies regarding pneumoperitoneum pressure thresholds, the majority adhered to predefined pressure ranges. Three studies specifically evaluated laparoscopic gastric procedures (9, 10, 24), whereas the remaining nine focused on colorectal surgeries. Notably, 75% of the included studies (9 out of 12) were published post-2020, reflecting heightened research interest in optimizing pneumoperitoneum pressures. Geographically, contributions were equally distributed, with 6 studies originating from Asian countries and 6 from European nations (Table 1).
Risk of bias analysis
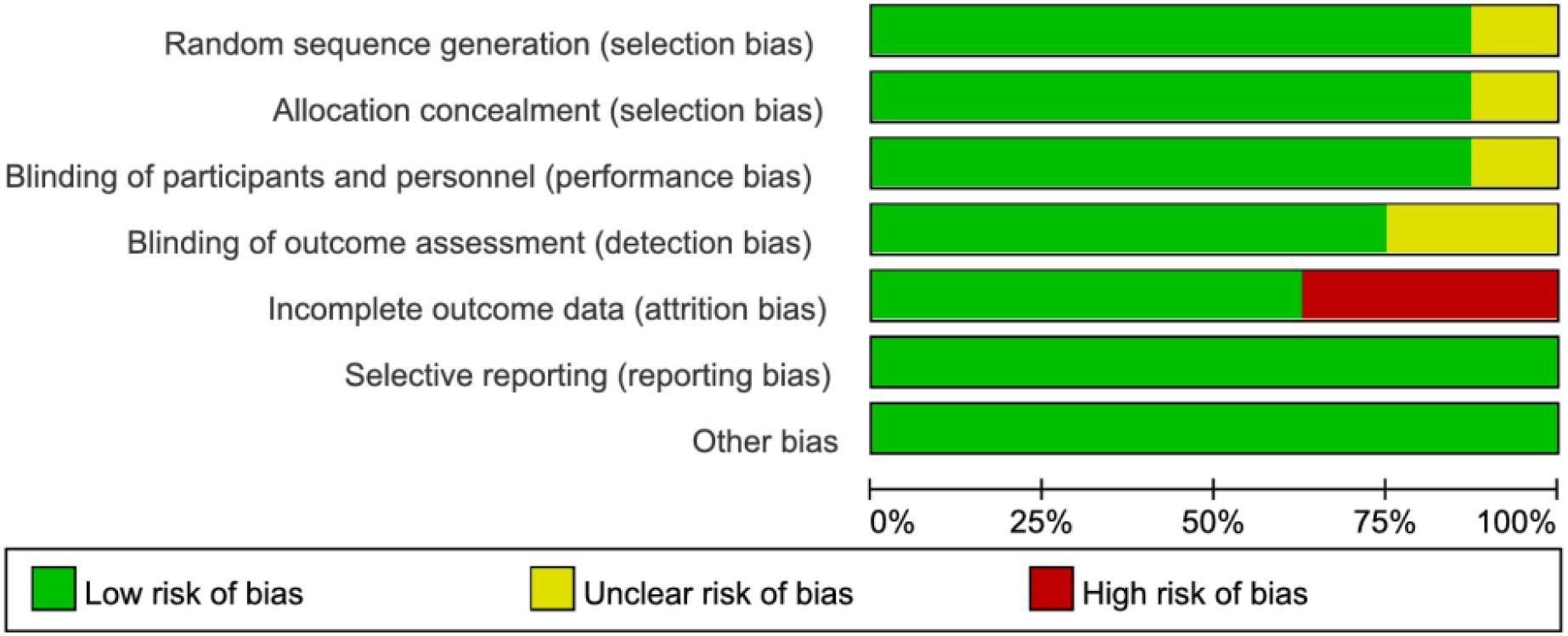
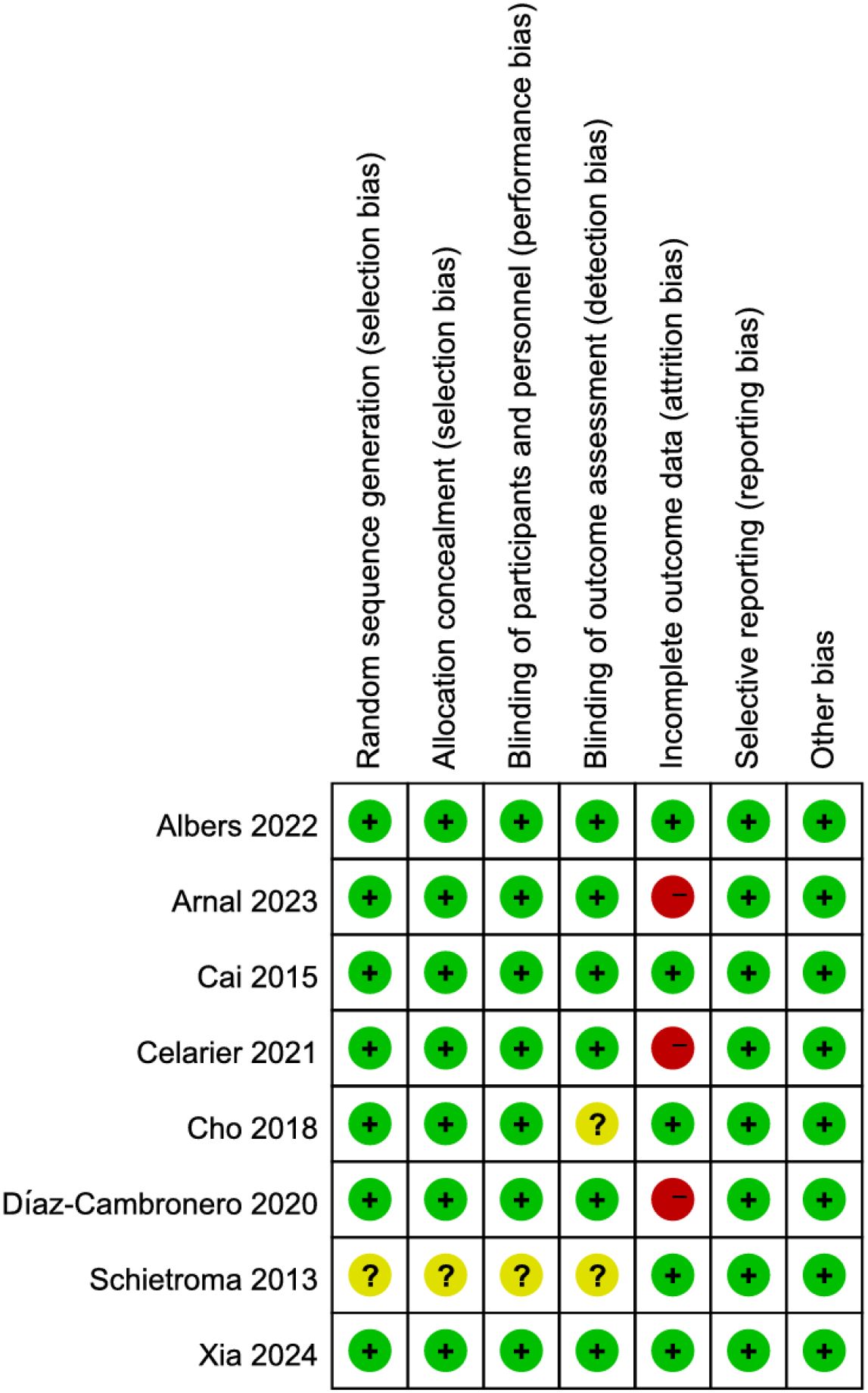
Among the 8 RCTs evaluated, three studies (17, 19, 21) exhibited a high risk of attrition bias due to incomplete outcome reporting. The trial by Schietroma M et al. (24) demonstrated methodological limitations, including insufficient documentation of random sequence generation, allocation concealment, and blinding procedures for investigators, participants, or outcome assessors. All other RCTs maintained adequate methodological quality without additional high-risk biases (Figures 2, 3). For the four observational studies included, quality assessment scores ranged between 7–8 stars (maximum 9-star scale), indicating moderate-to-high methodological reliability (Table 2).
Results of evidence quality assessment
The evidence grading of the primary and some secondary outcomes indicated that the quality of evidence ranged from moderate to low. The downgrading was primarily due to inconsistencies in study design, small sample sizes, imprecision of results, substantial heterogeneity across studies, and the number of studies included is small (Supplementary 1).
Postoperative resting pain, pain in PACU, and activity-related pain
Five studies analyzed postoperative resting pain in patients, while only two studies evaluated pain in PACU and activity-related pain. The meta-analysis results demonstrated LPP laparoscopic surgery significantly reduced postoperative resting pain (SMD = -0.40, 95% CI: -0.68 to -0.12, P = 0.005, I² = 66%, Figure 4A) and pain in PACU (SMD = -1.06, 95% CI: -1.65 to -0.47, P = 0.0004, I² = 0%, Figure 4B). However, no significant difference was observed in postoperative activity-related pain between the two groups (SMD = -0.58, 95% CI: -1.44 to 0.28, P = 0.18, I² = 92%, Figure 4C). A subgroup analysis of postoperative resting pain, stratified by geographic region (European vs. Asian countries), revealed reduced heterogeneity within subgroups. LPP significantly improved postoperative resting pain in European patients (SMD = -0.92, 95% CI: -1.22 to -0.63, P < 0.00001, I² = 45%) but showed no significant benefit for Asian patients (SMD = -0.18, 95% CI: -0.58 to 0.22, P = 0.38, I² = 0%). However, the limited number of studies in each subgroup may compromise the reliability of these findings.
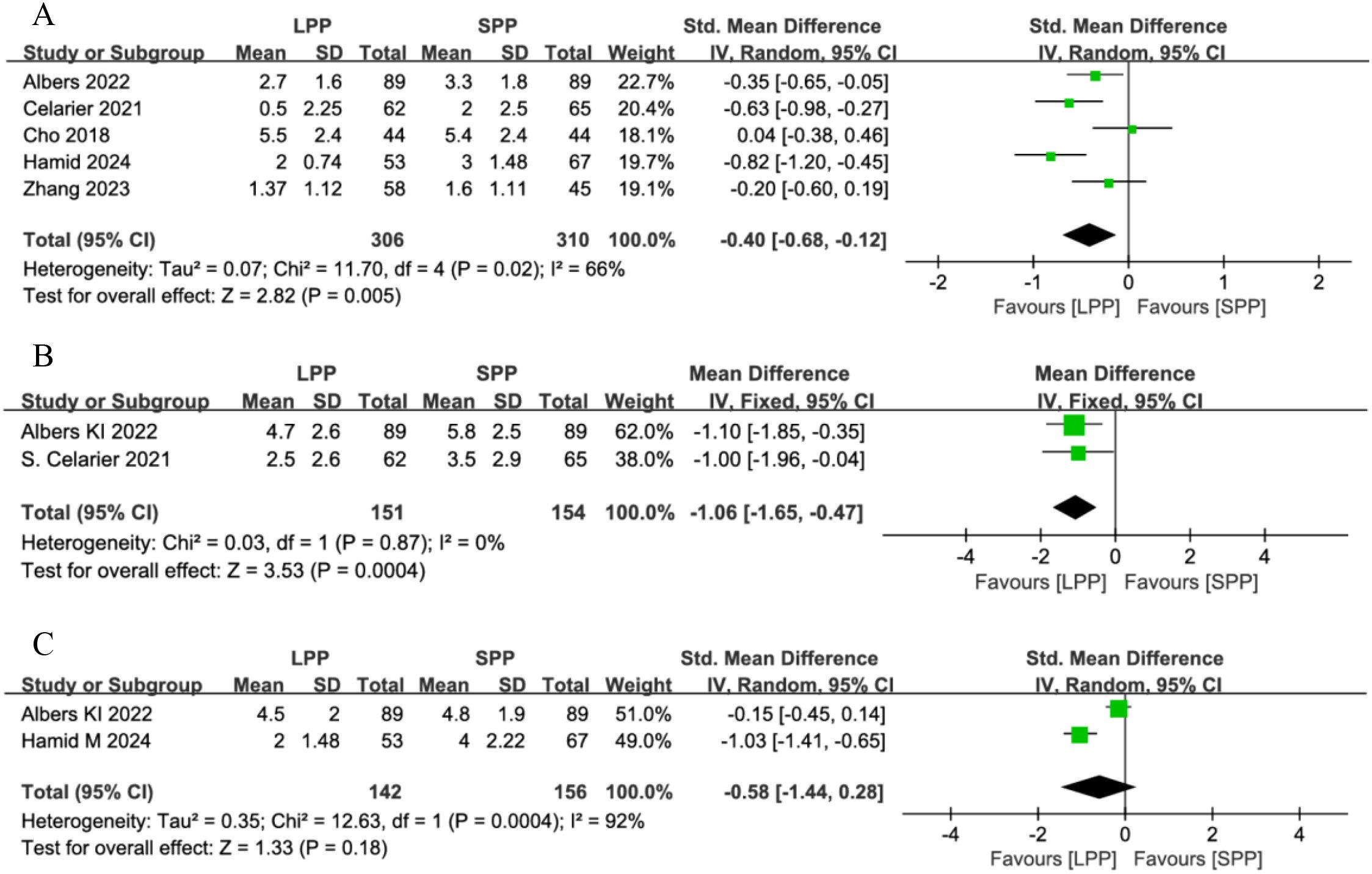
Figure 4. (A) Forest plot of postoperative resting pain comparing LPP and SPP. (B) Funnel plot of pain in PACU Comparing LPP and SPP. (C) Forest Plot of activity-related pain Comparing LPP and SPP. LPP, low pneumoperitoneum pressure; SPP, Standard Pneumoperitoneum Pressure; PACU, post-anesthesia care unit.
Operative time, intraoperative blood loss, and surgical field visibility
Ten studies analyzed operative time, five studies evaluated intraoperative blood loss, and four studies assessed the surgical field. One study was excluded from the surgical field analysis due to dichotomous data that could not be pooled. Meta-analysis revealed no significant differences between LPP and SPP laparoscopy in operative time (SMD = -0.09, 95% CI: -0.20 to 0.02, P = 0.12, I² = 0%, Figure 5A), intraoperative blood loss (SMD = -0.00, 95%CI: -0.13 to 0.13, P = 0.95, I² = 0%, Figure 5B), or surgeon’s visual field (SMD = -0.03, 95% CI: -0.32 to 0.26, P = 0.84, I² = 64%, Figure 5C). For the surgical field outcomes, significant heterogeneity was observed among studies. Sensitivity analysis excluding the study by Xia L (8) reduced heterogeneity (I² = 0%), but the limited number of remaining studies compromises the reliability of this conclusion.
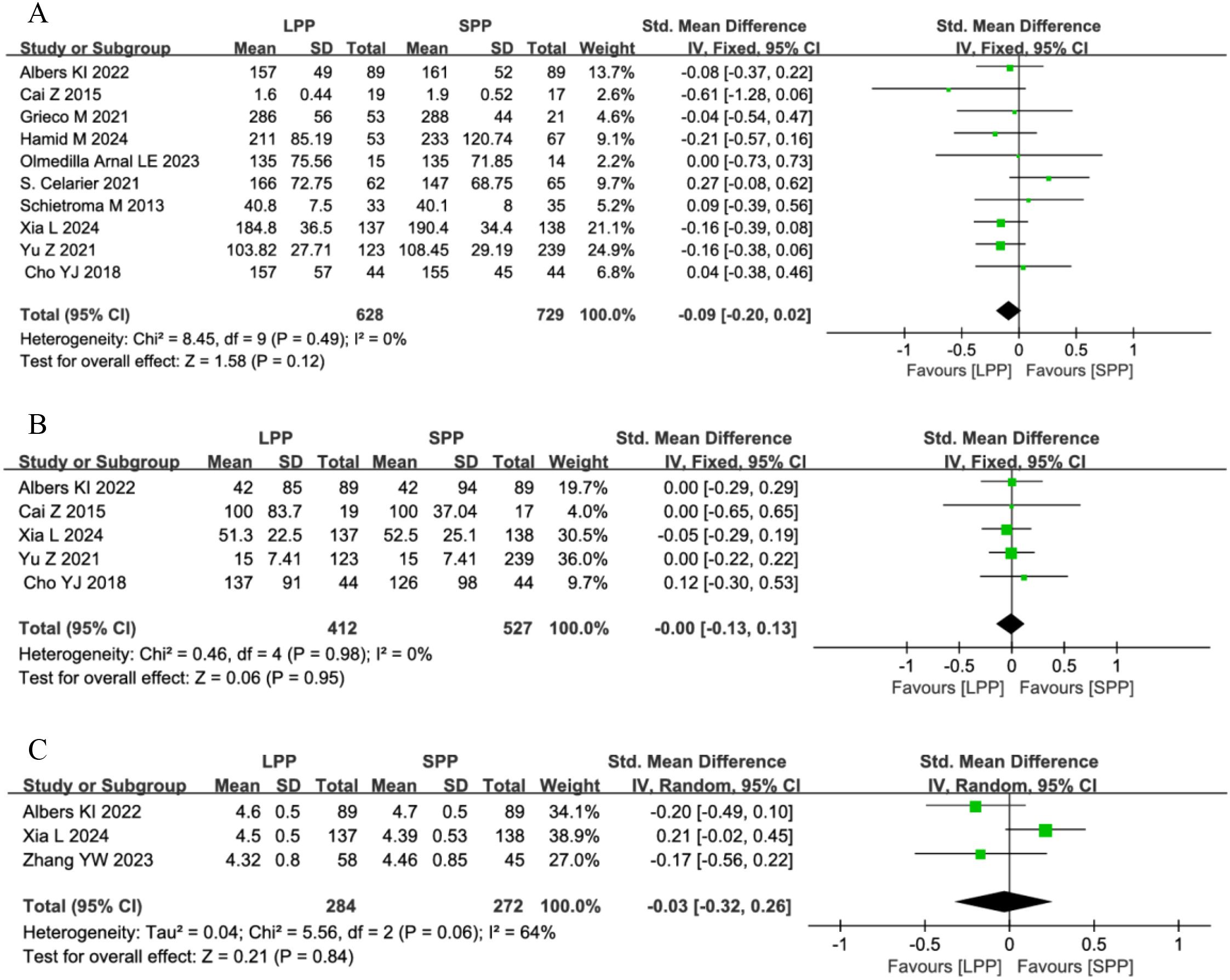
Figure 5. (A) Forest plot of operative time comparing LPP and SPP. (B) Forest Plot of intraoperative blood loss Comparing LPP and SPP. (C) Forest Plot of surgical field visibility Comparing LPP and SPP.
Time to first postoperative flatus and hospital stay: Four studies analyzed the time to first postoperative flatus, and seven studies evaluated postoperative hospital stay. Meta-analysis demonstrated that patients undergoing LPP laparoscopy for gastrointestinal surgery had a significantly shorter time to first flatus compared to the SPP group (SMD = -0.27, 95% CI: -0.50 to -0.05, P = 0.02, I² = 29%, Figure 6A). However, no significant difference was observed in postoperative hospital stay between the groups (SMD = 0.03, 95% CI: -0.1 to 0.15, P = 0.69, I² = 0%, Figure 6B).
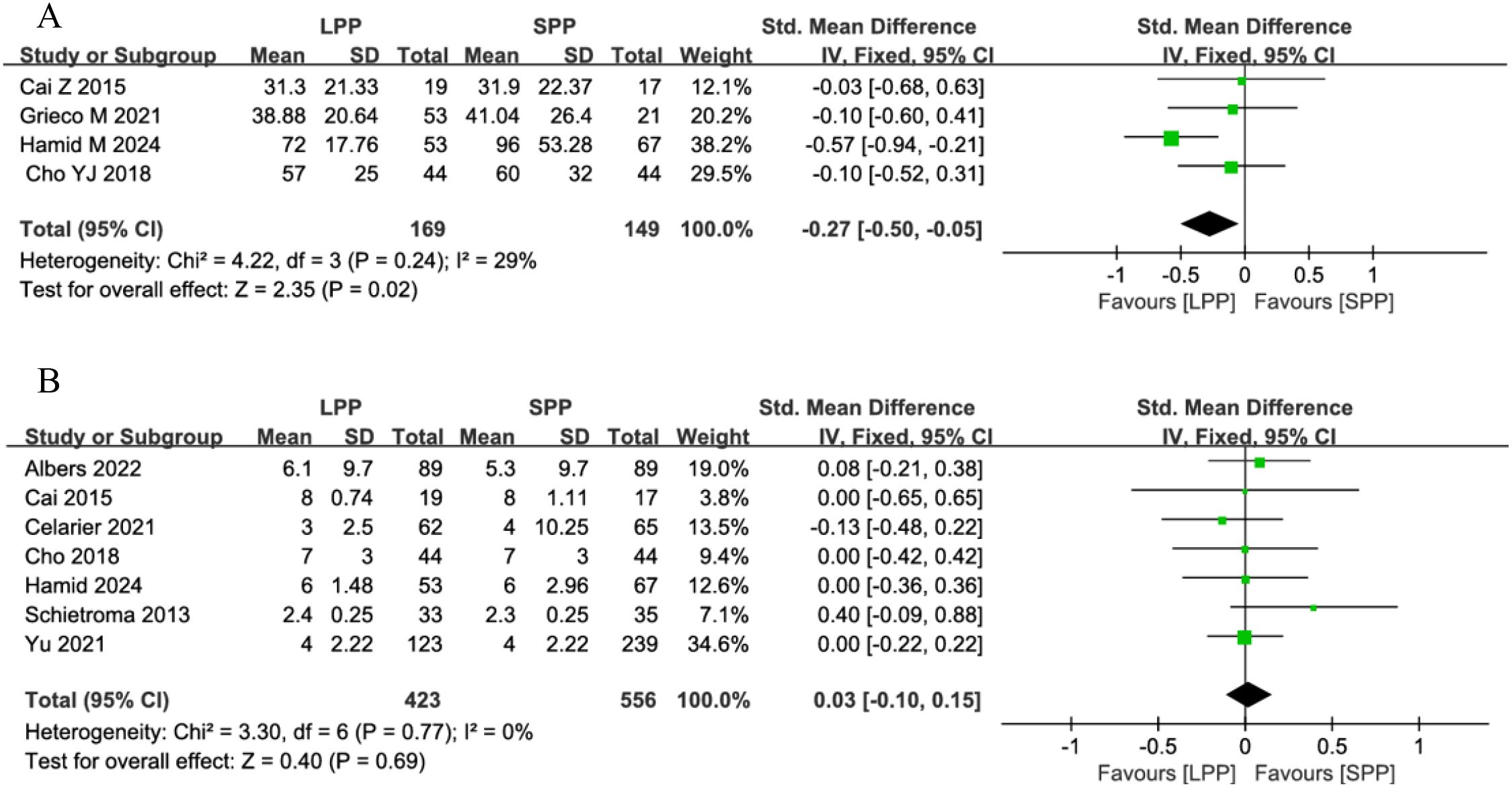
Figure 6. (A) Forest plot of time to first postoperative flatus comparing LPP and SPP. (B) Forest plot of hospital stay comparing LPP and SPP.
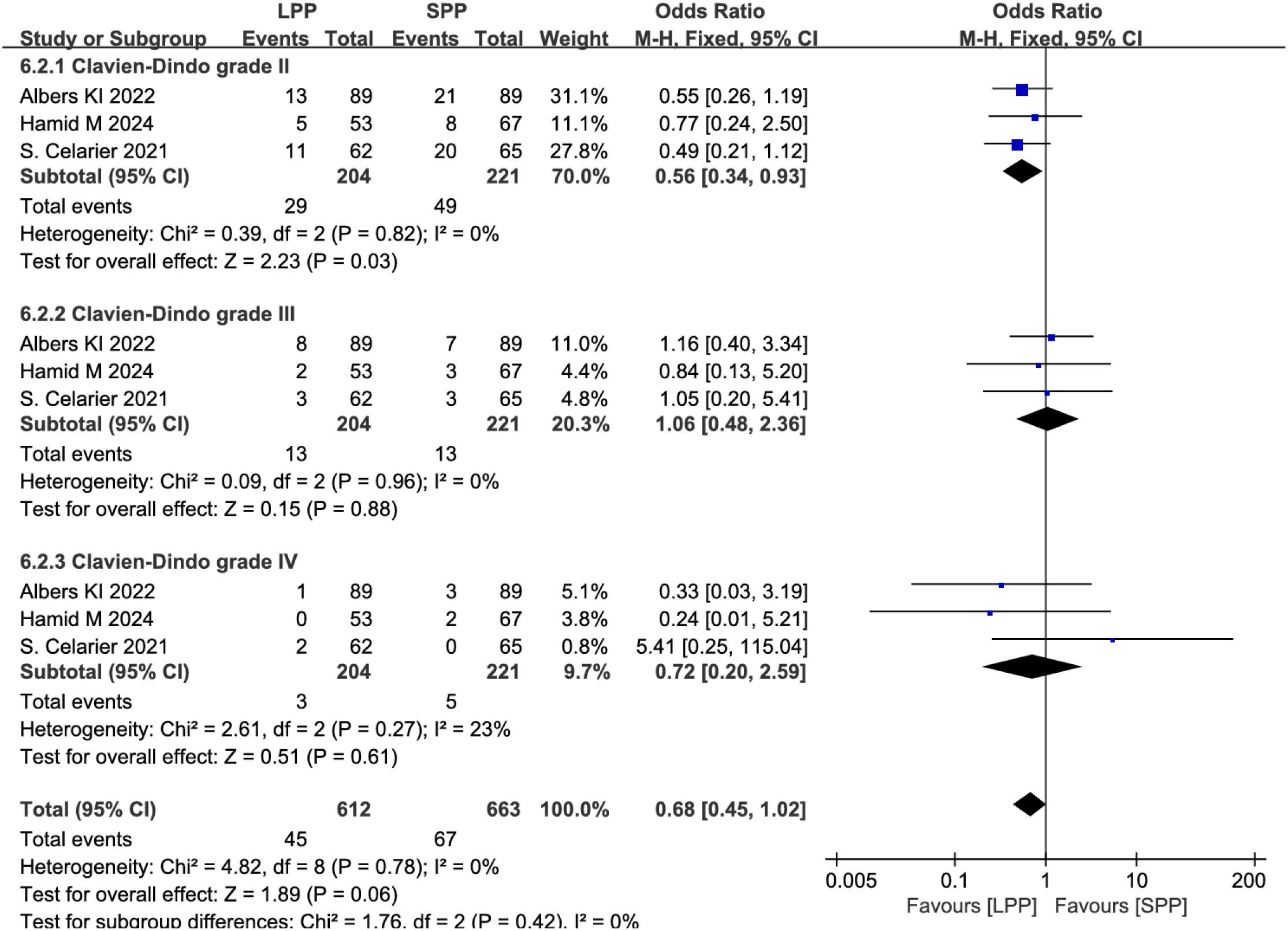
Postoperative complications and anastomotic leakage: Eight studies analyzed postoperative complications, and six studies evaluated anastomotic leakage. Meta-analysis revealed no significant differences between LPP and SPP laparoscopy in postoperative complications (OR = 0.78, 95% CI: 0.55 to 1.09, P = 0.14, I² = 7%, Figure 7A) and anastomotic leakage (OR = 1.17, 95% CI: 0.50 to 2.69, P = 0.72, I² = 23%, Figure 7B). Further subgroup analysis of postoperative complication grades between the two groups showed that LPP laparoscopy was associated with a reduced incidence of Clavien-Dindo grade II complications (OR = 0.56, 95% CI: 0.34 to 0.93, P = 0.03, I² = 0%, Figure 8), while no differences were observed between the two groups in Clavien-Dindo grade III and IV complications.
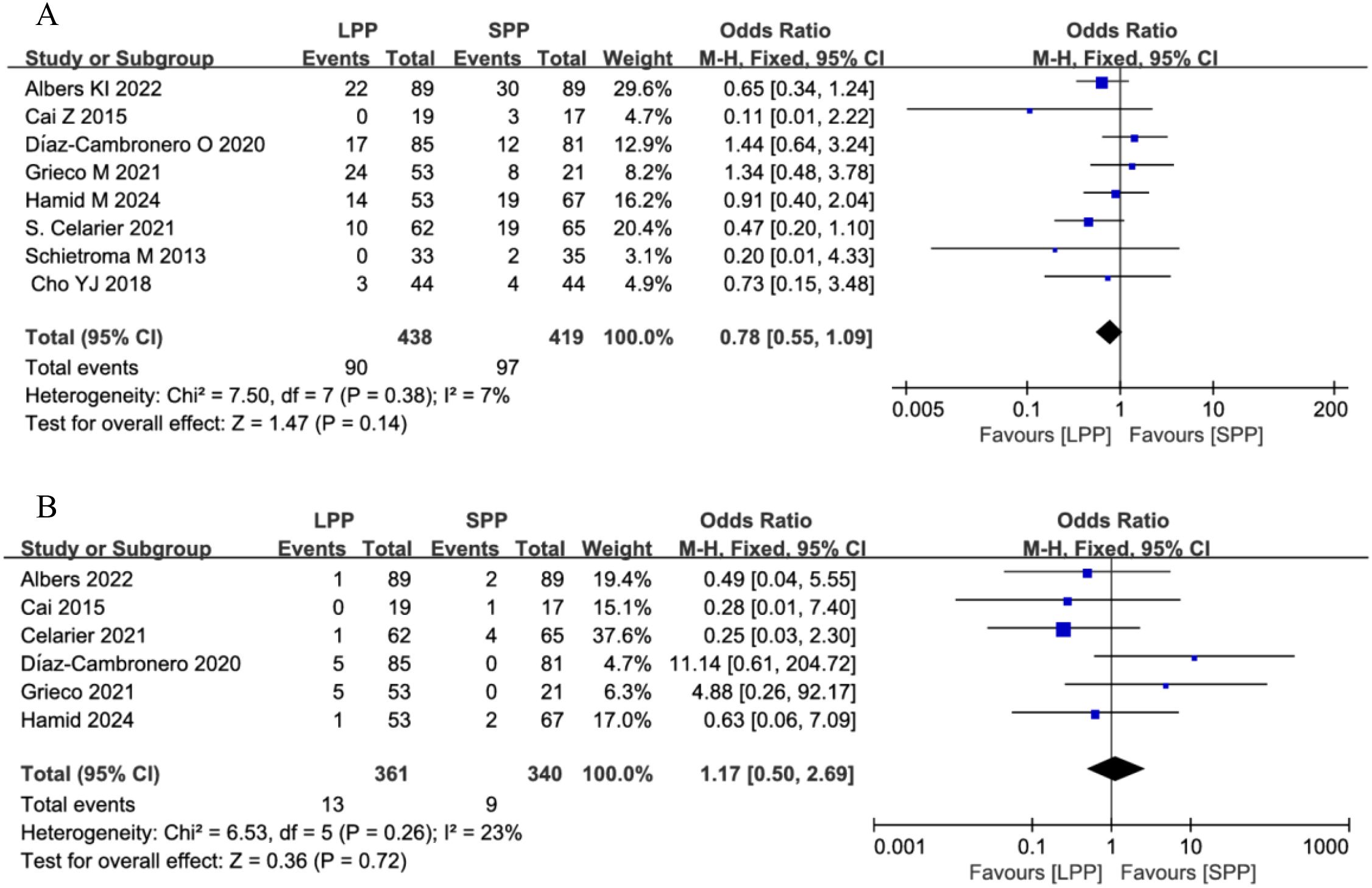
Figure 7. (A) Forest plot of postoperative complications comparing LPP and SPP. (B) Forest plot of anastomotic leakage comparing LPP and SPP.
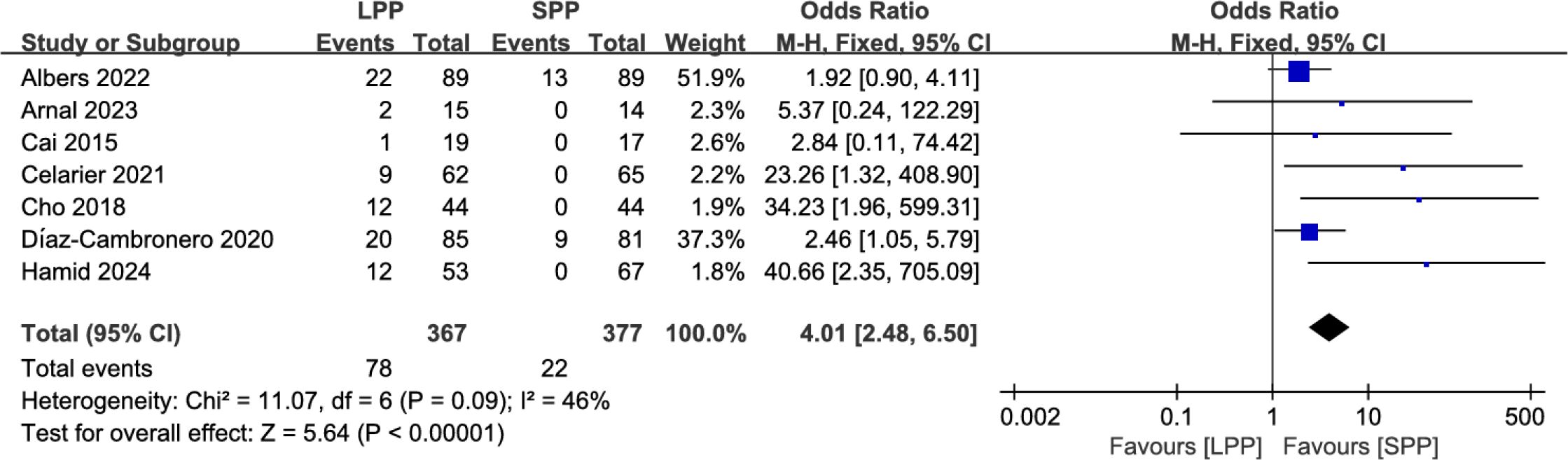
Intra−operative pressure changes: Seven studies analyzed the number of patients requiring intraoperative pressure changes. Meta-analysis indicated that significantly more patients in the LPP laparoscopy group underwent intraoperative pneumoperitoneum pressure adjustments compared to the SPP group (OR = 4.01, 95% CI: 2.48 to 6.50, P < 0.00001, I² = 46%, Figure 9).
Discussion
Laparoscopic minimally invasive surgery has been widely adopted for gastrointestinal diseases, including benign and malignant conditions. Maintaining stable pneumoperitoneum pressure during laparoscopy is critical. Studies suggest that LPP may reduce postoperative risks such as gas embolism and pain (6, 25), while high-pressure pneumoperitoneum (HPP) may adversely affect heart rate, respiration, and circulation (26, 27). Elevated pneumoperitoneum pressure can lead to reduced cardiac output, restricted venous return, decreased renal blood flow, and elevated biochemical markers (4). In elderly patients with reduced skin elasticity, HPP increases the risk of acidosis (28). Given these detrimental effects, LPP has been increasingly implemented across multiple surgical disciplines, including hepatobiliary surgery (25, 29, 30), gynecology (31), and urology (32–34), with demonstrated safety and efficacy. However, research on LPP in gastrointestinal surgery, particularly laparoscopic gastric procedures, remains limited. Schietroma M et al. (24) pioneered the application of LPP in gastric surgery in 2013, reporting its potential to attenuate postoperative inflammatory responses. More recently, a 2024 RCT confirmed comparable operative duration and intraoperative blood loss between LPP and SPP in laparoscopic colorectal surgery, further validating its safety and feasibility (8). Despite these advancements, synthesized evidence remains scarce. Only two meta-analyses have explored this topic: Hami M et al. (11) concluded that LPP is safe and feasible for colorectal surgery but could not establish definitive clinical benefits, while Dourado J et al. (12) demonstrated that LPP reduces postoperative pain with safety comparable to SPP. Both analyses were constrained by limited sample sizes and narrow scopes. To address these gaps, our study expanded the literature search to incorporate updated RCTs, non-RCTs, and gastric surgery data, ultimately including 12 studies. Our findings suggest that LPP in laparoscopic gastrointestinal surgery significantly reduces postoperative pain at rest (SMD = -0.40, 95% CI: -0.68 to -0.12, P = 0.005) and in PACU (SMD = -1.06, 95% CI: -1.65 to -0.47, P = 0.0004), though no advantage was observed for activity-related pain. Additionally, patients undergoing LPP experienced accelerated recovery of gastrointestinal function, as evidenced by a shorter time to first flatus (SMD = -0.27, 95% CI: -0.50 to -0.05, P = 0.02). However, these benefits were counterbalanced by a higher likelihood of intraoperative pressure adjustments (OR = 4.01, 95% CI: 2.48 to 6.50, P < 0.00001, P < 0.00001), reflecting technical challenges in maintaining optimal surgical exposure under LPP. Regarding postoperative complications between the two groups, our results showed that LPP was associated with a reduced incidence of Clavien-Dindo grade II complications, while no significant differences were observed in the rates of grade III and IV complications. However, in obese patients undergoing laparoscopic surgery, the incidence of grade III and IV complications was higher in the LPP group compared to the SPP group. Moreover, the intraoperative bleeding rate was significantly higher in the LPP group (35). In terms of anesthetic implications, the study by Albers KI et al. (18) found no significant difference between the two groups in the intraoperative consumption of anesthetic agents such as propofol, remifentanil, and esketamine. Notably, LPP was associated with a reduction in postoperative nausea symptoms. Deep neuromuscular blockade (NMB) has been identified as a critical adjunct in achieving optimal conditions for LPP laparoscopic surgery. Studies have shown that procedures performed under LPP with only moderate NMB are associated with a higher incidence of intraoperative adverse events (36). Nemes et al. (37) attributed this to inadequate intraoperative neuromuscular monitoring. Furthermore, research by Kim et al. (38) indicated that combining deep NMB with LPP laparoscopic surgery alleviates postoperative pain and promotes faster recovery of bowel function. These findings were corroborated by our meta-analysis. Additionally, our results showed that LPP did not impair the surgeon’s visual field during surgery (SMD = -0.03, 95% CI: -0.32 to 0.26, P = 0.84), further supporting the feasibility of LPP in clinical practice. Collectively, these results indicate that LPP offers selective advantages but does not universally outperform SPP in laparoscopic gastrointestinal surgery. The observed heterogeneity in outcomes—such as the pronounced reduction in resting pain among European cohorts compared to Asian populations—suggests that patient-specific factors or regional surgical practices may influence efficacy. In addition, LPP should be applied with greater caution in obese patients. Future research should prioritize large-scale RCTs to evaluate LPP’s impact on hemodynamic stability, respiratory function, and acid-base balance, while standardizing protocols for pressure thresholds and intraoperative techniques to minimize confounding. Such efforts will be critical to refining clinical guidelines and optimizing patient outcomes in this evolving field.
Study limitations
Several limitations should be acknowledged. First, the analysis of certain outcomes—including pain in PACU, surgical field visibility, and activity-related pain—was based on a limited number of studies, with substantial heterogeneity observed (I² > 50%), which may compromise the reliability of pooled results. Second, variations in pneumoperitoneum pressure settings between intervention (LPP) and control (SPP) groups across studies introduced potential confounding, leading to instability in meta-analytical estimates. Finally, inconsistent application of nerve-sparing techniques between groups in some included studies may have influenced postoperative pain and functional recovery outcomes.
Conclusion
LPP in laparoscopic gastrointestinal surgery can reduce postoperative resting pain and pain in PACU, while also improving gastrointestinal functional recovery. However, LPP does not demonstrate comprehensive superiority, and the reduction in postoperative resting pain may vary among populations. Caution is required when adopting LPP protocols during surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
KLu: Methodology, Writing – original draft, Writing – review & editing. XP: Data curation, Formal Analysis, Software, Writing – review & editing. KLan: Data curation, Formal Analysis, Software, Writing – review & editing. FZ: Conceptualization, Writing – review & editing. YC: Conceptualization, Writing – review & editing. HY: Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The meta-analysis is funded by the The Health Commission of Zigong City (Project Number: 23yb018), but the funder has no authority over the study design, collection, management, analysis, and interpretation of data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1665112/full#supplementary-material
References
1. Neudecker J, Sauerland S, Neugebauer E, Bergamaschi R, Bonjer HJ, Cuschieri A, et al. The European Association for Endoscopic Surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surg Endosc. (2002) 16:1121–43. doi: 10.1007/s00464-001-9166-7
2. Galizia G, Prizio G, Lieto E, Castellano P, Pelosio L, and Imperatore V. Hemodynamic and pulmonary changes during open, carbon dioxide pneumoperitoneum, and abdominal wall-lifting cholecystectomy: a prospective, randomized study. Surg Endosc. (2001) 15:477–83. doi: 10.1007/s004640000343
3. Wallace DH, Serpell MG, Baxter JN, and O’Dwyer PJ. Randomized trial of different insufflation pressures for laparoscopic cholecystectomy. Br J Surg. (1997) 84:455–8. doi: 10.1002/bjs.1800840408
4. Andrei VE, Schein M, Margolis M, Rucinski JC, and Wise L. Liver enzymes arecommonly elevated following laparoscopic cholecystectomy: iselevated intra-abdominal pressure the cause? Dig Surg. (1998) 15:256–9. doi: 10.1159/000018624
5. Sun C, Yu J, Xu P, Liu L, Zhang Z, Guo C, et al. Low pneumoperitoneum pressure reduces gas embolism during laparoscopic liver resection: A randomized controlled trial. Ann Surg. (2024) 279:588–97. doi: 10.1097/SLA.0000000000006130.4
6. Ortenzi M, Montori G, Sartori A, Balla A, Botteri E, Piatto G, et al. Low-pressure versus standard-pressure pneumoperitoneum in laparoscopic cholecystectomy: a systematic review and meta-analysis of randomized controlled trials. Surg endoscopy. (2022) 36:7092–113. doi: 10.1007/s00464-022-09201-1
7. Hamid M, Zaman S, Mostafa OES, Deutsch A, Bird J, Kawesha A, et al. Low vs. conventional intra-abdominal pressure in laparoscopic colorectal surgery: a prospective cohort study. Langenbecks Arch Surg. (2024) 410:12. doi: 10.1007/s00423-024-03579-3
8. Xia L, Xu X, Zhang C, Cao G, Chen L, Chen E, et al. Low pneumoperitoneum pressure on venous thromboembolism in laparoscopic colorectal cancer surgery: A randomized controlled study. Eur J Surg Oncol. (2024) 50:108672. doi: 10.1016/j.ejso.2024.108672
9. Yu Z, Yu L, Wu JX, Yu T, Yang XG, Zhang BX, et al. Low-pressure pneumoperitoneum with abdominal wall lifting versus standard pressure pneumoperitoneum in laparoscopic fundoplication for gastroesophageal reflux disease: A propensity score-matched analysis. Surg Laparosc Endosc Percutan Tech. (2021) 32:46–53. doi: 10.1097/SLE.0000000000000990
10. Zhang YW, Li Y, Huang WB, Wang J, Qian XE, Yang Y, et al. Utilization of deep neuromuscular blockade combined with reduced abdominal pressure in laparoscopic radical gastrectomy for gastric cancer: An academic perspective. World J Gastrointest Surg. (2023) 15:1405–15. doi: 10.4240/wjgs.v15.i7.1405
11. Hamid M, Mostafa OES, Mohamedahmed AYY, Zaman S, Kumar P, Waterland P, et al. Comparison of low versus high (standard) intra abdominal pressure during laparoscopic colorectal surgery: systematic review and meta-analysis. Int J Colorectal Dis. (2024) 39:104. doi: 10.1007/s00384-024-04679-8
12. Dourado J, Rogers P, Horesh N, Emile SH, Aeschbacher P, and Wexner SD. Low-pressure versus standard-pressure pneumoperitoneum in minimally invasive colorectal surgery: a systematic review, meta-analysis, and meta-regression analysis. Gastroenterol Rep (Oxf). (2024) 12:goae052. doi: 10.1093/gastro/goae052
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
14. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.5. Cochrane. (2024). Available online at: www.cochrane.org/handbook.
15. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2015). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed May 2024).
16. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li TJ, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Bristol, UK: John wiley & Sons. (2019). p. 20. doi: 10.1002/9781119536604
17. Olmedilla Arnal LE, Cambronero OD, Mazzinari G, Pérez Peña JM, Zorrilla Ortúzar J, Rodríguez Martín M, et al. An individualized low-pneumoperitoneum-pressure strategy may prevent a reduction in liver perfusion during colorectal laparoscopic surgery. Biomedicines. (2023) 11:891. doi: 10.3390/biomedicines11030891
18. Albers KI, Polat F, Helder L, Panhuizen IF, Snoeck MMJ, Polle SBW, et al. Quality of recovery and innate immune homeostasis in patients undergoing low-pressure versus standard-pressure pneumoperitoneum during laparoscopic colorectal surgery (RECOVER): A randomized controlled trial. Ann Surg. (2022) 276:e664–73. doi: 10.1097/SLA.0000000000005491
19. Celarier S, Monziols S, Célérier B, Assenat V, Carles P, Napolitano G, et al. Low-pressure versus standard pressure laparoscopic colorectal surgery (PAROS trial): a phase III randomized controlled trial. Br J Surg. (2021) 108:998–1005. doi: 10.1093/bjs/znab069
20. Grieco M, Tirelli F, Agnes A, Santocchi P, Biondi A, and Persiani R. High-pressure CO2 insufflation is a risk factor for postoperative ileus in patients undergoing TaTME. Updates Surg. (2021) 73:2181–7. doi: 10.1007/s13304-021-01043-1
21. Díaz-Cambronero O, Mazzinari G, Flor Lorente B, García Gregorio N, Robles-Hernandez D, Olmedilla Arnal LE, et al. Effect of an individualized versus standard pneumoperitoneum pressure strategy on postoperative recovery: a randomized clinical trial in laparoscopic colorectal surgery. Br J Surg. (2020) 107:1605–14. doi: 10.1002/bjs.11736
22. Cho YJ, Paik H, Jeong SY, Park JW, Jo WY, Jeon Y, et al. Lower intra-abdominal pressure has no cardiopulmonary benefits during laparoscopic colorectal surgery: a double-blind, randomized controlled trial. Surg Endosc. (2018) 32:4533–42. doi: 10.1007/s00464-018-6204-2
23. Cai Z, Malbrain ML, Sun J, Pan R, Ma J, Feng B, et al. Does elevated intra-abdominal pressure during laparoscopic colorectal surgery cause acute gastrointestinal injury? Wideochir Inne Tech Maloinwazyjne. (2015) 10:161–9. doi: 10.5114/wiitm.2015.52210
24. Schietroma M, Carlei F, Cecilia EM, Piccione F, Sista F, De Vita F, et al. A prospective randomized study of systemic inflammation and immune response after laparoscopic nissen fundoplication performed with standard and low-pressure pneumoperitoneum. Surg Laparosc Endosc Percutan Tech. (2013) 23:189–96. doi: 10.1097/SLE.0b013e3182827e51
25. Luo W, Jin D, Huang J, Zhang J, Xu Y, Gu J, et al. Low pneumoperitoneum pressure reduces gas embolism during laparoscopic liver resection: A randomized controlled trial. Ann Surg. (2024) 279:588–97. doi: 10.1097/SLA.0000000000006130
26. Darmawan KF, Sinclair T, and Dunn JCY. Comparison of laparoscopic and open pediatric inguinal hernia repairs at two institutions. Pediatr Surg Int. (2018) 34:1293–8. doi: 10.1007/s00383-018-4360-z
27. Yashwashi T, Kaman L, Kajal K, Dahiya D, Gupta A, Meena SC, et al. Effects of low- and high-pressure carbon dioxide pneumoperitoneum on intracranial pressure during laparoscopic cholecystectomy. Surg Endosc. (2020) 34:4369–73. doi: 10.1007/s00464-019-07207-w
28. Park JS, Ahn EJ, Ko DD, Kang H, Shin HY, Baek CH, et al. Effects of pneumoperitoneal pressure and position changes on respiratory mechanics during laparoscopic colectomy. Korean J Anesthesiol. (2012) 63:419–24. doi: 10.4097/kjae.2012.63.5.419
29. Tian F, Sun X, Yu Y, Zhang N, Hong T, Liang L, et al. Comparison of low-pressure and standard-pressure pneumoperitoneum laparoscopic cholecystectomy in patients with cardiopulmonary comorbidities: a double blinded randomized clinical trial. BMC Surg. (2024) 24:348. doi: 10.1186/s12893-024-02606-w
30. Srikanth MVVS, Arumugaswamy PR, Rathore YS, Chumber S, Yadav R, Maitra S, et al. Comparison of inflammatory markers in low-pressure pneumoperitoneum with deep neuromuscular block versus standard pressure pneumoperitoneum among patients undergoing laparoscopic cholecystectomy for gallstone disease: a randomized control trial. Surg Endosc. (2024) 38:4648–56. doi: 10.1007/s00464-024-11026-z
31. Esa U, Hardy Mohamad Zaini R, Mazlan MZ, Omar AA, Che Omar S, and Rosedi A. Evaluation of surgical condition during laparoscopic gynaecological surgery in patients with moderate vs. deep neuromuscular block in low-pressure pneumoperitoneum. Anaesthesiol Intensive Ther. (2024) 56:121–8. doi: 10.5114/ait.2024.141209
32. Peng Y, Zhu M, and Chen C. Application of different CO2 pneumoperitoneum pressure in laparoscopic pyeloplasty for infants with ureteropelvic junction obstruction. Front Pediatr. (2024) 12:1380985. doi: 10.3389/fped.2024.1380985
33. Keating K, Holdren C, Eames R, Pulford Cerror, Peifer D, et al. The impact of low-pressure pneumoperitoneum in robotic assisted radical prostatectomy II: a prospective, randomized, double blinded trial. World J Urol. (2024) 42:347. doi: 10.1007/s00345-024-05038-6
34. Reijnders-Boerboom GTJA, Jacobs LMC, Helder LS, Panhuizen IF, Brouwer MPJ, Albers KI, et al. Recovery and immune function after low pressure pneumoperitoneum during robot-assisted radical prostatectomy: a randomised controlled trial. BJU Int. (2024) 134:416–25. doi: 10.1111/bju.16397
35. Özgen G, Toydemir T, and Yerdel MA. Low-pressure pneumoperitoneum during laparoscopic sleeve gastrectomy: a safety and feasibility analysis. Obes Surg. (2023) 33:1984–8. doi: 10.1007/s11695-023-06625-z
36. Özdemir-van Brunschot DMD, Braat AE, van der Jagt MFP, Scheffer GJ, Martini CH, Langenhuijsen JF, et al. Deep neuromuscular blockade improves surgical conditions during low-pressure pneumoperitoneum laparoscopic donor nephrectomy. Surg Endosc. (2018) 32:245–51. doi: 10.1007/s00464-017-5670-2
37. Nemes R and Renew JR. Clinical practice guideline for the management of neuromuscular blockade: what are the recommendations in the USA and other countries? Curr Anesthesiol Rep. (2020) 10:90–8. doi: 10.1007/s40140-020-00372-y
38. Kim MH, Lee KY, Lee KY, Min BS, and Yoo YC. Maintaining optimal surgical conditions with low insufflation pressures is possible with deep neuromuscular blockade during laparoscopic colorectal surgery: A prospective, randomized, double-blind, parallel-group clinical trial. Med (Baltimore). (2016) 95:e2920. doi: 10.1097/MD.0000000000002920
Keywords: pneumoperitoneum pressure, postoperative pain, laparoscopic gastrointestinal surgery, gastrointestinal disease, LPP
Citation: Lu K, Peng X, Lan K, Zhang F, Cheng Y and Yang H (2025) Low pneumoperitoneum pressure facilitates postoperative pain relief and gastrointestinal function recovery in laparoscopic gastrointestinal surgery: a systematic review and meta-analysis. Front. Oncol. 15:1665112. doi: 10.3389/fonc.2025.1665112
Received: 13 July 2025; Accepted: 04 August 2025;
Published: 21 August 2025.
Edited by:
Rocco Ricciardi, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Luis Capitán-Morales, University of Seville, SpainGörkem Özgen, Altınbaş University, Türkiye
Copyright © 2025 Lu, Peng, Lan, Zhang, Cheng and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Yang, MzkxMDkyMDY1NkBxcS5jb20=
 Kai Lu
Kai Lu Xuefeng Peng1
Xuefeng Peng1