- 1Guangdong Medical University, Zhanjiang, Guangdong, China
- 2Department of Hepatobiliary Surgery, Zhongshan City People’s Hospital, Zhongshan, Guangdong, China
Background: Laparoscopic hepatopancreatoduodenectomy (LHPD) is a while very complex procedure for biliary malignancies combined with intrahepatic bile duct invasion, but there are few reports of related surgeries due to high postoperative complications and mortality. In this study, we report a case of tubular adenoma with high-grade intraepithelial neoplasia of the homologous left hepatic bile duct combined with the common bile duct.
Case presentation: A 53-year-old female was admitted to the hospital with jaundice for one week. Imaging studies showed space-occupying lesions in both the left intrahepatic and common bile duct. We performed LHPD, and the patient was discharged on postoperative day 13 without bile and pancreatic fistula. Pathology confirmed tubular adenoma with high-grade intraepithelial neoplasia in both sites.
Conclusion: LHPD can be an option for radical surgery in carefully screened patients with biliary malignancies with intrahepatic invasion.
Background
Hepaticopancreaticoduodenectomy (HPD) is a high-risk surgical procedure performed to treat complex malignant tumors in the hepatobiliary and pancreatic regions. The extent of resection includes the liver, extrahepatic biliary system, and pancreaticoduodenum (1, 2). Although Takasaki et al. reported in 1980 that HPD could be a potential radical surgery for gallbladder cancer with invasion of the liver parenchyma or extrahepatic bile duct, research on its effectiveness for treating biliary tumors is still ongoing (2, 3). However, HPD is not widely accepted in Western countries because of its high postoperative morbidity and mortality rates (4). A North American cohort study revealed that the overall morbidity and mortality rates were significantly higher with HPD than with major hepatectomy (MH) and pancreaticoduodenectomy (PD) (87% vs. 51% vs. 52% for morbidity; 26% vs. 7.6% vs. 1.4% for mortality, respectively) (5). Owing to advancements in surgical procedures and accumulated experience, R0 resection after HPD for biliary tumors can significantly reduce mortality rates in patients with a sufficient residual liver volume and a sufficient functional liver remnant (FLR) greater than 40%. However, strict screening of suitable candidates is essential (5–7). Laparoscopic pancreaticoduodenectomy (LPHD) offers advantages over traditional approaches, including reduced trauma, less blood loss, a clearer surgical field, and fewer postoperative complications. It has been attempted in some larger surgical centers (8–11). A case in which LPHD was performed to treat synchronous tumors of the lower common bile duct and intrahepatic bile duct is reported here. Postoperative pathology revealed tubular adenoma with high-grade intraepithelial neoplasia.
Case report
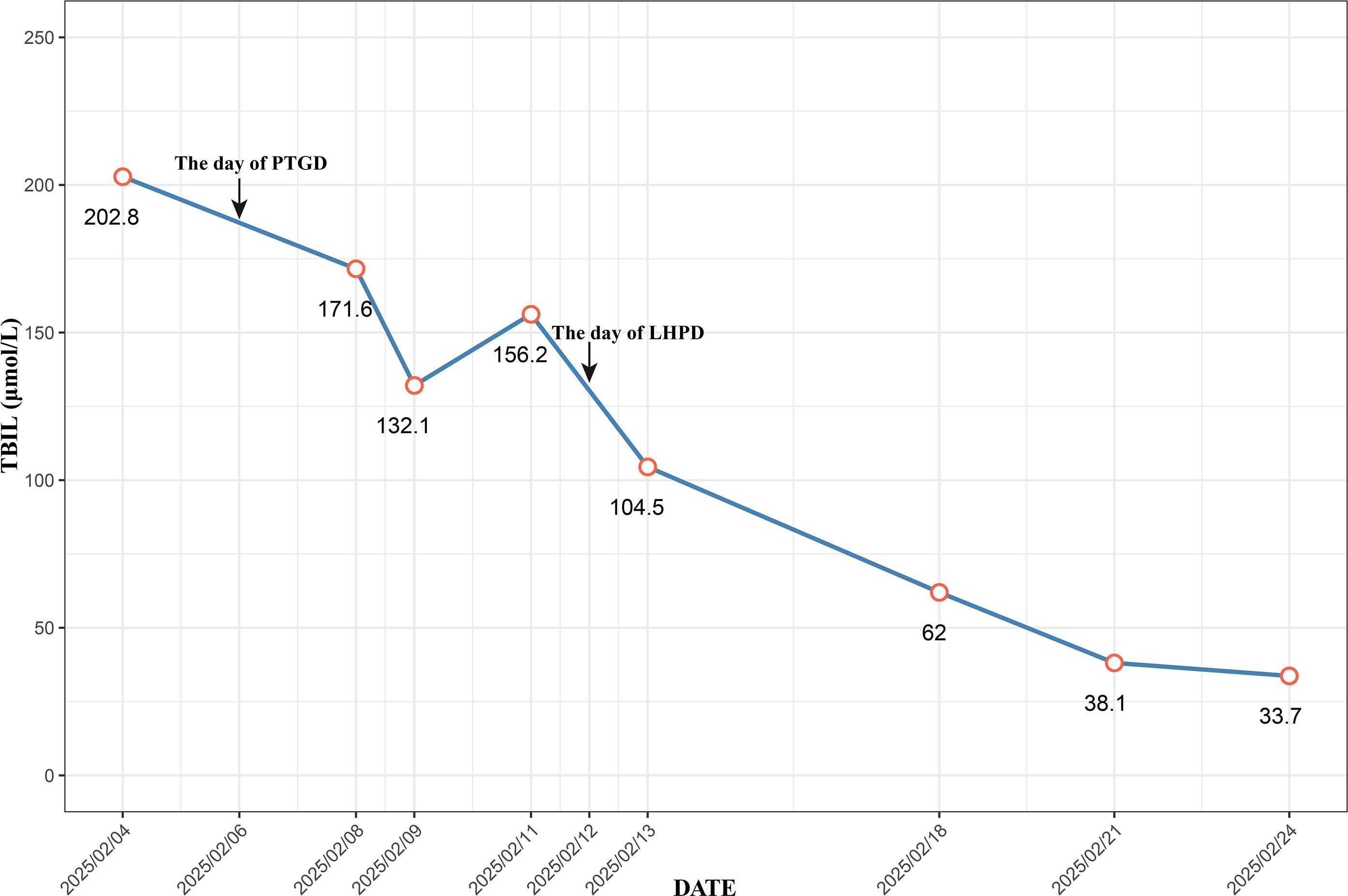
A 53-year-old female was admitted to Zhongshan People’s Hospital on February 3, 2025, due to jaundice that had persisted for more than one week. The patient had previously undergone laparoscopic total hysterectomy and adnexectomy. Physical examination revealed yellowing of the skin and sclera and tenderness in the right upper abdomen and subxiphoid region, with all other findings being negative. Preoperative laboratory tests revealed hepatitis B and significantly elevated levels of bilirubin, transaminases, and tumor markers (Table 1). Contrast-enhanced computed tomography (CE-CT) and magnetic resonance cholangiopancreatography (MRCP) revealed stenosis of the lower common bile duct lumen, local thickening of the bile duct wall and multiple enhancing space-occupying lesions, significant dilation of the left hepatic duct and intrahepatic bile duct lumen, and multiple irregular filling defects (Figure 1). On the basis of these findings, the preoperative diagnosis was intrahepatic cholangiocarcinoma combined with common bile duct carcinoma. Considering the patient’s significantly elevated total bilirubin, impaired preoperative liver function, and high risk of surgical bleeding, we performed ultrasound-guided percutaneous transhepatic gallbladder drainage (PTGD) to rapidly reduce biliary pressure and prevent the progression of acute liver failure. Postoperatively, the daily bile drainage volume was approximately 390 mL. After six days of drainage, the patient’s total bilirubin decreased to 156.2 μmol/L, representing a 77% reduction (Figure 2). On February 12, 2025, we performed LHPD.
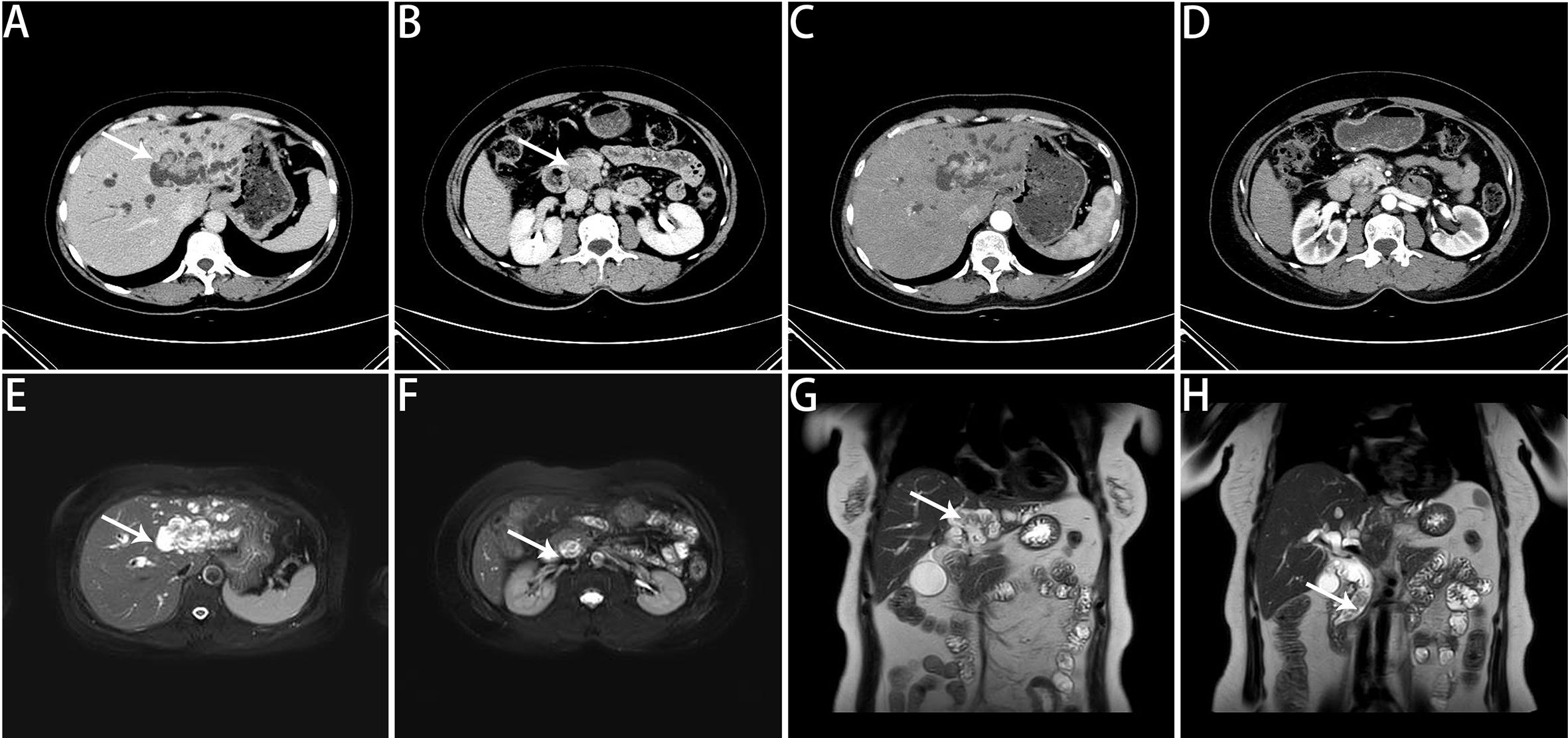
Figure 1. Preoperative imaging findings. (A) Noncontrast CT scan revealing multiple isodense nodules within the left intrahepatic bile ducts. (B) Noncontrast CT scan showing multiple isodense nodules within the distal common bile duct. (C) CE-CT in the arterial phase revealing space-occupying lesions with marked enhancement and irregular borders in the left intrahepatic bile ducts. (D) CE-CT in the arterial phase revealing markedly enhanced space-occupying lesions in the distal common bile duct. (E) MRCP image revealing multiple irregular space-occupying lesions in the left intrahepatic bile ducts. (F) MRCP image showing multiple irregular space-occupying lesions in the distal common bile duct. (G) MRCP coronal view showing multiple irregular space-occupying lesions in the left intrahepatic bile ducts. (H) MRCP coronal view showing multiple irregular space-occupying lesions in the distal common bile duct. The white arrows indicate lesions.
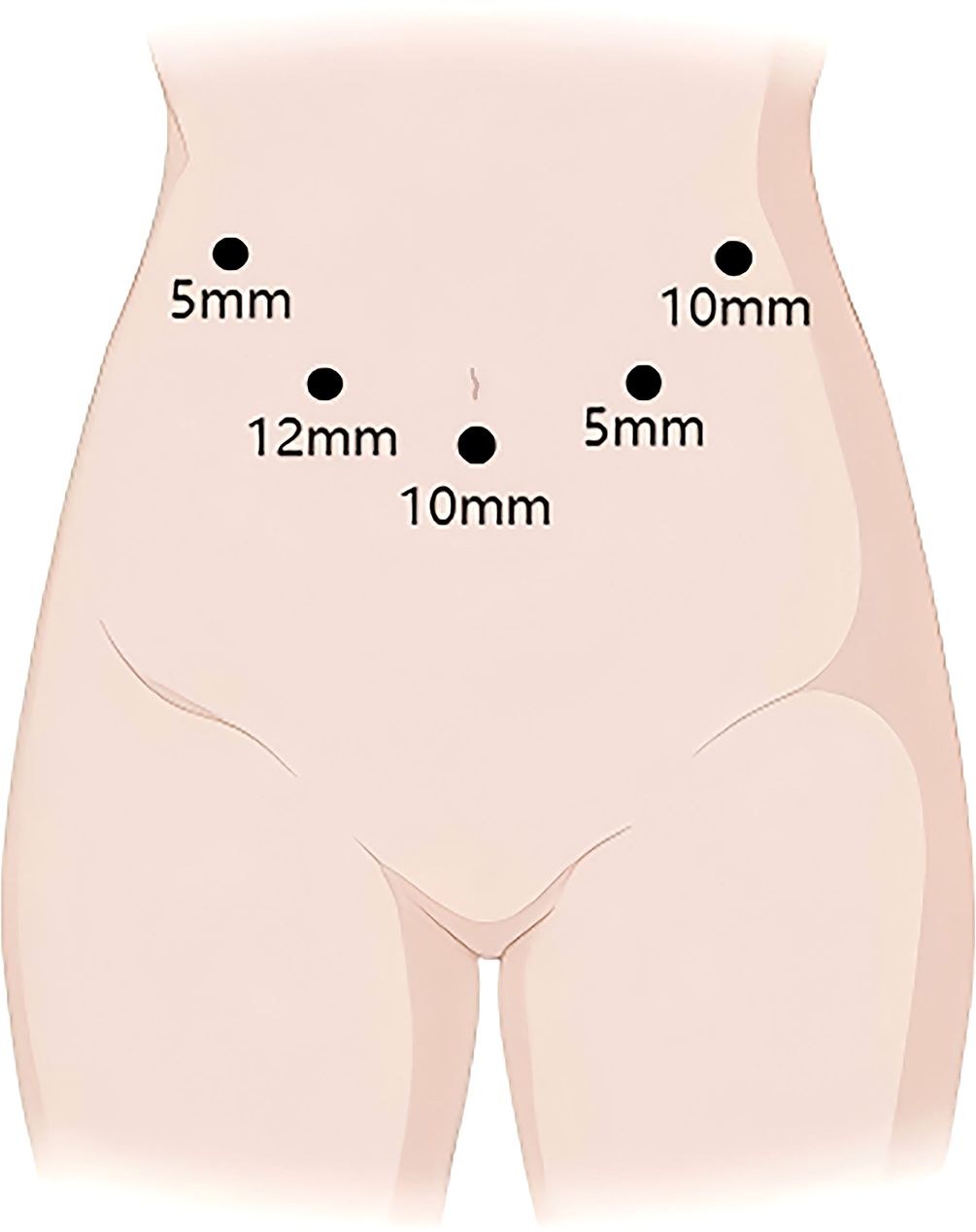
The patient is placed in the supine split-leg position. The surgery was performed using the V-shaped 5-port technique (Figure 3). First, First, the gallbladder triangle was dissected and the gallbladder was removed. An ultrasonic scalpel was used to separate the falciform ligament, left coronary ligament, and hepatogastric ligament so that the left side of the liver could be elevated. The lymph nodes along the upper border of the pancreas and the hepatic hilar lymph nodes were dissected. The left hepatic artery, gastroduodenal artery, and left portal vein branch underwent ligation and transection. The ischemic segment of the left hemiliver was demarcated, and the liver parenchyma was incised, consequently exposing the segment VIb branch of the middle hepatic vein for subsequently dissection. The incision was continued cephalad to transect the hepatic parenchyma, thereby exposing the left hepatic duct. The origin of the left hepatic duct was clamped with double bulldog clamps and then transected, and no tumor was observed at the resection margin. The liver parenchyma was further divided up to the root of the left hepatic vein, and finally, the left hepatic vein was transected using an Endo GIA Stapler, indicating the completion of left hemiliver resection (Figure 4B). PD was performed using a venous approach. First, the gastrocolic ligament and adhesions of the posterior gastric wall were divided, the pancreas and duodenum were mobilized, and the abdominal aorta, inferior vena cava, left renal vein, and superior mesenteric artery root were surgically exposed. The right gastroepiploic artery and right gastric artery were divided, and then the gastric antrum and pancreatic neck were transected (Figure 4C). Finally, the jejunum was transected approximately 15 cm distal to the ligament of Treitz, the common hepatic duct was divided 1 cm below the confluence of the left and right hepatic ducts, and PD was completed.
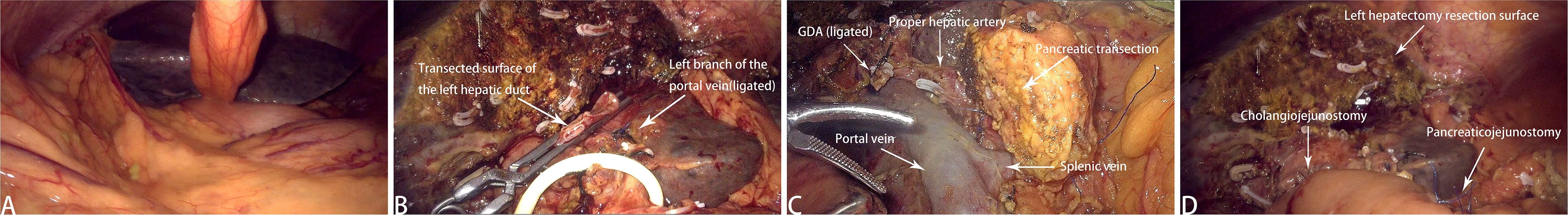
Figure 4. Intraoperative images. (A) Intraoperative laparoscopic overview. (B) Resected left hemihepatectomy sample. (C) Surgical field, including the Heidelberg triangle and the pancreatic transection surface. (D) Postoperative wound following LHPD.
The digestive tract was reconstructed using the Child’s method. The modified Blumgart technique was used for pancreaticojejunostomy—a stent was placed in the main pancreatic duct and the pancreatic duct was continuously sutured to the jejunum using 5–0 polydioxanone sutures. For choledochojejunostomy, an end-to-side anastomosis was created using 4–0 barbed sutures. The jejunum was anastomosed to the posterior gastric wall incision approximately 50 cm from the stoma using an Endo GIA Stapler, marking the completion of gastrojejunostomy (Figure 4D). The surgical field was inspected to ascertain the presence or absence of bleeding, and the specimen was removed for pathological analysis. The abdominal cavity was irrigated with warm distilled water, and an abdominal drainage tube was placed. The operation lasted 575 minutes, with an estimated blood loss of approximately 200 ml.
After the operation, we conducted nursing monitoring for the patient, actively enhancing anti-infection treatment, nutritional support and correcting hypoproteinemia. We focused on monitoring changes in the volume and characteristics of the drainage fluid from the abdominal drainage tube and regularly repeated blood tests and abdominal CT scans to detect any fluid accumulation or infection in the surgical area. If fluid accumulation was detected, we promptly performed ultrasound-guided catheter drainage to prevent pancreatic fistula from eroding the blood vessels. The patient recovered smoothly postoperatively, with no signs of pancreatic leakage, biliary leakage, or liver failure. The patient was discharged on postoperative day 13. Immunohistochemistry revealed that the tubular adenoma with high-grade intraepithelial neoplasia in the lower common bile duct and left intrahepatic bile duct, CK19 (–). All the lymph nodes were not involved, and no tumor cells were found in the specimen resection margin (Figure 5).
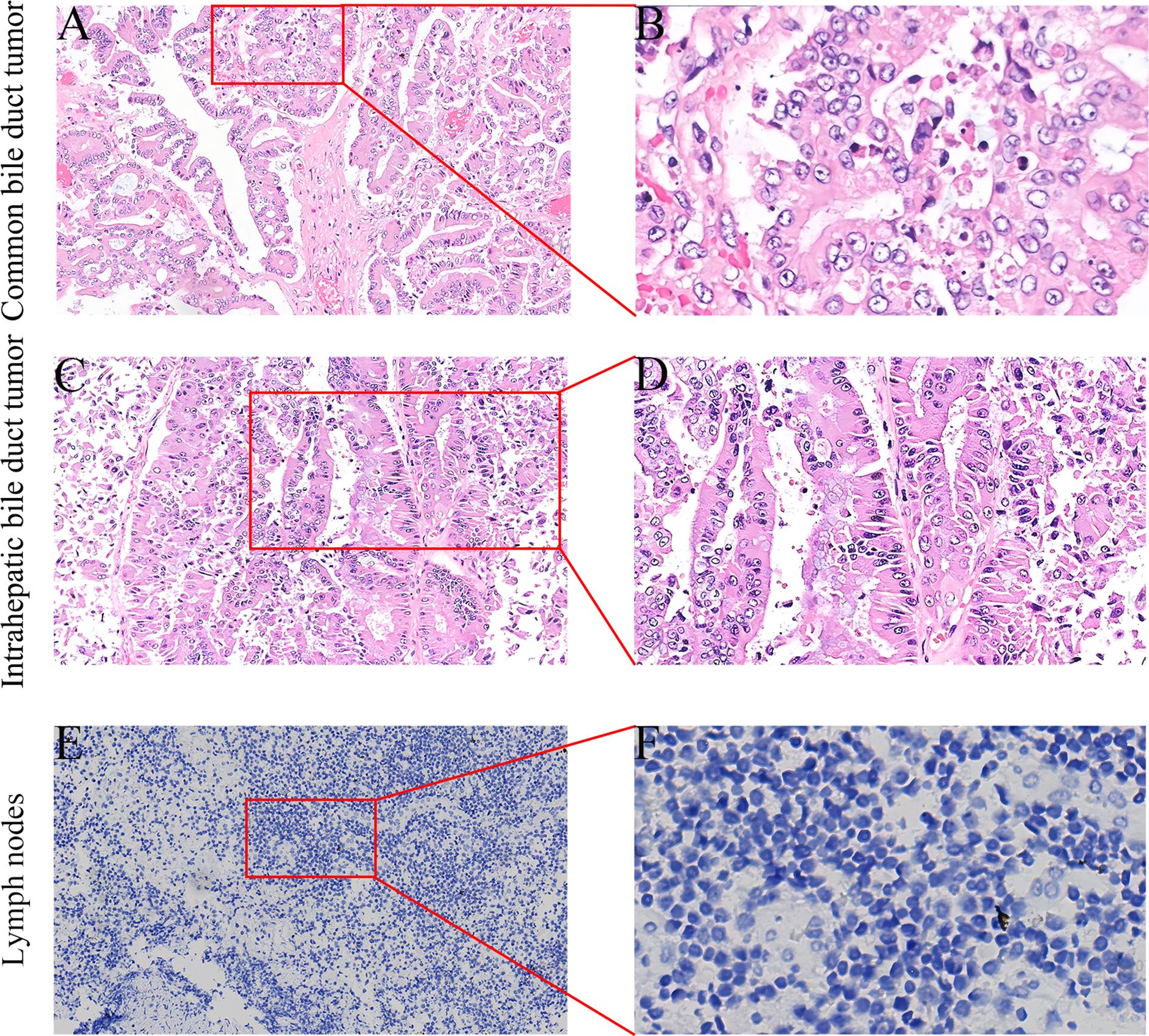
Figure 5. Postoperative specimen immunohistochemistry (IHC) results (A-F). (A) HE staining of the common bile duct mass (×100). (B) Histologically, hyperplastic glands with a crowded arrangement and focal cribriform pattern are observed (×400). (C) HE staining of the left intrahepatic bile duct mass (×100). (D) Microscopically, the cells exhibit a columnar morphology with enlarged hyperchromatic nuclei, increased cytologic atypia, disorganized arrangement, and loss of polarity (×200). (E) Negative CK19 IHC staining (×100). (F) Microscopically, only blue-stained nuclei and clear cytoplasm are observed, with no high-grade intraepithelial neoplastic cells (×400).
Discussion
HPD is a complex surgical intervention for malignant biliary tumors. Although its indications remain unclear, the most suitable cases are those involving advanced biliary malignancies with local hepatic invasion. The procedure is suitable for patients in whom R0 resection is feasible and who have no distant metastases (12, 13). However, owing to the high postoperative mortality rate, severe complications, and unclear survival benefits, many surgeons do not recommend this procedure (4, 5, 14). Although most literature reports a high postoperative mortality rate and poor survival prognosis, in experienced surgical centers, the postoperative mortality rate can be controlled below 10%. Moreover, the 5-year overall survival rate of patients with R0 resection is comparable to that of patients who have undergone extensive liver resection (5-year survival: 41% vs 40%, P = 0.328) (15). It is worth noting that all patients experienced varying degrees of postoperative complications. Because HPD involves the resection of the liver, gallbladder, pancreas, duodenum, and part of the stomach, it disrupts the continuity of the digestive tract. The reconstruction steps are intricate, increasing the risks of abdominal adhesions and ischemic anastomotic tissues. Most HPD studies have reported complications such as wound infection (4% - 41.7%), intra-abdominal infection (12% - 69.5%), bile leakage (5% - 50%), and pancreatic fistula (19% - 77%) (15–23). Liver failure caused by HPD is the most common cause of perioperative death. An American retrospective study of 480 HPD patients revealed that extended hepatic resection was significantly associated with an increased risk of postoperative mortality, with postoperative complication rates and in-hospital mortality rates as high as 87% and 18.2% (P < 0.001) (24). A study by Welch et al. also found that the incidence of liver failure after HPD surgery was as high as 56%, compared with only 14% after extensive hepatectomy. In this context, preoperative portal vein embolization (PVE) is recommended to induce compensatory hyperplasia of the non-resected liver lobe to increase the future liver remnant (FLR), improve surgical safety and feasibility, and reduce postoperative liver failure-related mortality. In addition, associating liver partition and portal vein ligation for staged hepatectomy (ALLPS) has been used to rapidly increase the volume of the remnant liver. However, its role in HPD remains unclear due to its high mortality and morbidity (5, 13).
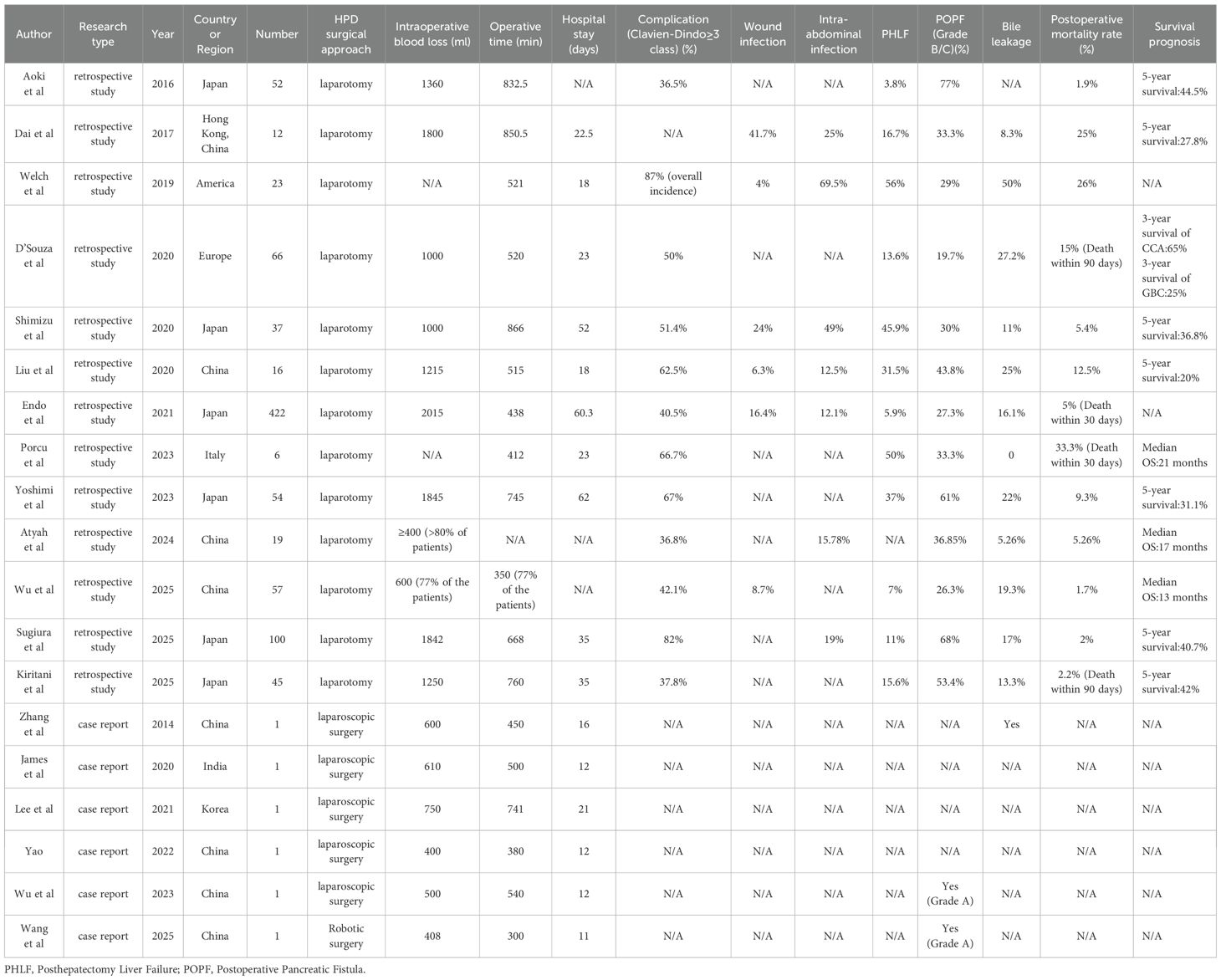
Against this background, LHPD has gradually attracted people’s attention due to its minimally invasive characteristics and technical potential. Compared with open surgery, the application of minimally invasive surgery in major abdominal surgeries has significantly reduced the incidence of postoperative complications and mortality. Multiple studies revealed that compared with traditional open procedures, Laparoscopic pancreaticoduodenectomy(LPD) and laparoscopic liver resection (LLR) not only significantly reduce the average length of hospital stay and shortens the postoperative recovery time but also do not significantly affect long-term survival rates (3-year OS: LPD 59.1% vs. open pancreaticoduodenectomy [OPD] 54.3%, p = 0.33; 5-year OS: LLR 78.6% vs. open liver resection [OLR] 75.7%) (25–28). Existing LHPD literature reports indicate that surgical blood loss was controlled within 400–600 ml. No severe postoperative complications occurred. Only 2 cases of mild pancreatic fistula and 1 case of biliary fistula were reported after surgery, which were considered acceptable. Yao’s report emphasizes that the proficiency of surgeons in laparoscopic techniques is a key factor in reducing postoperative complications, particularly proficiency in LPD and LLR, and emphasizes the need for standardized surgical procedures (Table 2) (8).
Our successful implementation of HPD is based on three key factors. The first is patient selection criteria: patients with relatively localized left intrahepatic bile duct tumors, postoperative FLR > 40%, a low incidence of postoperative liver failure, and normal preoperative ICG and liver function tests, indicating the patient’s ability to tolerate extensive liver surgery. The second is surgical operation: Pancreatic fistula is the most frequent complication following HPD and primarily results from pancreaticojejunostomy during the PD procedure. The high incidence may be attributed to risk factors specific to biliary tract cancer patients, soft pancreatic texture, prolonged operative time, and postoperative hepatic dysfunction (29). In this case, the patient underwent pancreaticojejunostomy using the modified Blumgart technique. This method involves the U-shaped placement of sutures, which reduces the shear force of the suture while completely wrapping the jejunum around the pancreatic stump, preventing pancreatic fluid leakage and reducing the incidence of pancreatic fistula (30). To prevent anastomotic stenosis, we placed a stent in the pancreatic duct to drain pancreatic fluid and further reduce the risk of postoperative pancreatic fistula. Continuous sutures were used on the anterior and posterior walls of the bile duct-jejunal anastomosis to reduce the risk of postoperative bile fistula. However, the mucosa of the left hepatic duct and common hepatic duct resection margins were normal. To reduce the risk of postoperative biliary stenosis, we retained part of the common hepatic duct and did not choose the right hepatic duct for anastomosis. In addition, we carefully ligated the blood vessels that are prone to bleeding due to pancreatic fistula (such as the gastrocolic trunk, dorsal pancreatic artery, gastroduodenal artery, and inferior pancreaticoduodenal artery) to prevent bleeding caused by the erosion of blood vessels by pancreatic fistula.
The limitation of this case lies in the fact that intraoperative frozen pathological examination was not performed on the resection margins of the left hepatic duct and common hepatic duct. Instead, based on the intraoperative observation that the mucosal morphology of the resection margins of the left hepatic duct and common hepatic duct was normal, and combined with preoperative imaging findings, it was judged that the left intrahepatic bile duct tumor and the lower segment tumor of the common bile duct did not invade the bile duct resection margins. This empirical speculation carried a certain degree of risk. However, ultimately, the patient achieved an R0 resection. Postoperatively, the patient did not develop serious complications and recovered well.
Overall, integrating the literature and the experience of this case, the following key aspects should be emphasized when performing LHPD: Firstly, strict patient selection criteria are required. This includes evaluating the FLR, conducting ICG tests, assessing the preoperative liver function, and determining whether R0 resection can be achieved, etc., in order to reduce the surgical risk (31). For patients with severe preoperative jaundice, percutaneous biliary drainage can be used to reduce bilirubin. For patients with insufficient FLR, PVE can be considered. Secondly, due to the complexity of the surgery, the operating surgeon should have extensive experience in LPD and LLR. Precise handling of areas such as choledochojejunostomy or pancreaticojejunostomy is necessary to reduce the risk of complications such as pancreatic fistula and ensure the safety of the surgery. In conclusion, LHPD is feasible and safe in experienced surgical centers and among strictly selected patients.
Conclusion
For carefully selected patients with biliary tract malignancies and intrahepatic invasion, experienced surgeons can perform LHPD as a radical surgical treatment for such patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study involving human participants was reviewed and approved by the Institutional Review Board and Ethics Committee of Zhongshan city People’s Hospital. Informed consent was obtained from all participants. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YF: Writing – original draft. DH: Investigation, Writing – review & editing. YC: Investigation, Methodology, Writing – review & editing. QL: Investigation, Software, Writing – review & editing. RH: Conceptualization, Data curation, Writing – review & editing. KH: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Science and Technology Plan Project of Zhongshan City, Guangdong Province (Project No.: 2024B1039), but the funding was had no involvement in the study design; data collection/analysis; interpretation of results; writing of the manuscript.
Acknowledgments
The authors thank Dr. Kun He (Department of Hepatobiliary Surgery, Zhongshan City People’s Hospital) for their guidance and assistance in this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HPD, Hepaticopancreaticoduodenectomy; LHPD, Laparoscopic Hepaticopancreaticoduodenectomy; MH, major hepatectomyand; PD, pancreaticoduodenectomy; FLR, functional liver remnant; CE-CT, Contrast-enhanced computed tomography; MRCP, resonance cholangiopancreatography; PVE, portal vein embolization; POPF, postoperative pancreatic fistula; LPD, Laparoscopic pancreaticoduodenectomy; LLR, laparoscopic liver resection; IHC, immunohistochemistry.
References
1. Torres OJM, Alikhanov R, Li J, Serrablo A, Chan AC, and de Souza MFE. Extended liver surgery for gallbladder cancer revisited: Is there a role for hepatopancreatoduodenectomy? Int J Surg. (2020) 82s:82–6. doi: 10.1016/j.ijsu.2020.05.085
2. Endo I, Hirahara N, Miyata H, Yamamoto H, Matsuyama R, Kumamoto T, et al. Mortality, morbidity, and failure to rescue in hepatopancreatoduodenectomy: An analysis of patients registered in the National Clinical Database in Japan. J Hepatobiliary Pancreat Sci. (2021) 28:305–16. doi: 10.1002/jhbp.918
3. Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, and Nagino M. Review of hepatopancreatoduodenectomy for biliary cancer: an extended radical approach of Japanese origin. J Hepatobiliary Pancreat Sci. (2014) 21:550–5. doi: 10.1002/jhbp.80
4. D’Souza MA, Valdimarsson VT, Campagnaro T, Cauchy F, Chatzizacharias NA, D’Hondt M, et al. Hepatopancreatoduodenectomy -a controversial treatment for bile duct and gallbladder cancer from a European perspective. HPB (Oxford). (2020) 22:1339–48. doi: 10.1016/j.hpb.2019.12.008
5. Welch JC, Gleeson EM, Karachristos A, and Pitt HA. Hepatopancreatoduodenectomy in North America: are the outcomes acceptable? HPB (Oxford). (2020) 22:360–7. doi: 10.1016/j.hpb.2019.08.010
6. Shimizu A and Soejima Y. ASO author reflections: is major hepatopancreatoduodenectomy beneficial for patients with advanced and widespread extrahepatic cholangiocarcinoma? Ann Surg Oncol. (2021) 28:2026–7. doi: 10.1245/s10434-020-09261-4
7. Sun J, Xie TG, Ma ZY, Wu X, and Li BL. Current status and progress in laparoscopic surgery for gallbladder carcinoma. World J Gastroenterol. (2023) 29:2369–79. doi: 10.3748/wjg.v29.i16.2369
8. Yao GL. Laparoscopic hepatopancreaticoduodenectomy for synchronous gallbladder cancer and extrahepatic cholangiocarcinoma: a case report. World J Surg Oncol. (2022) 20:190. doi: 10.1186/s12957-022-02628-9
9. Wu B, Bai Y, and Yu S. Laparoscopic hepatopancreatoduodenectomy for synchronous intrahepatic and extrahepatic cholangiocarcinoma: A case report. Oncol Lett. (2023) 26:449. doi: 10.3892/ol.2023.14036
10. Zhang MZ, Xu XW, Mou YP, Yan JF, Zhu YP, Zhang RC, et al. Resection of a cholangiocarcinoma via laparoscopic hepatopancreato- duodenectomy: a case report. World J Gastroenterol. (2014) 20:17260–4. doi: 10.3748/wjg.v20.i45.17260
11. James M, Kalayarasan R, Gnanasekaran S, and Pottakkat B. Laparoscopic hepatopancreatoduodenectomy for locally advanced gall bladder cancer. J Minim Access Surg. (2021) 17:369–72. doi: 10.4103/jmas.JMAS_179_20
12. Wu X, Li M, Wu W, Wang X, Li H, Bao R, et al. Hepatopancreatoduodenectomy for advanced biliary Malignancies. Chin Med J (Engl). (2022) 135:2851–8. doi: 10.1097/cm9.0000000000002067
13. Fancellu A, Sanna V, Deiana G, Ninniri C, Turilli D, Perra T, et al. Current role of hepatopancreatoduodenectomy for the management of gallbladder cancer and extrahepatic cholangiocarcinoma: A systematic review. World J Gastrointest Oncol. (2021) 13:625–37. doi: 10.4251/wjgo.v13.i6.625
14. Zhou Y, Zhang Z, Wu L, and Li B. A systematic review of safety and efficacy of hepatopancreatoduodenectomy for biliary and gallbladder cancers. HPB (Oxford). (2016) 18:1–6. doi: 10.1016/j.hpb.2015.07.008
15. Shimizu A, Motoyama H, Kubota K, Notake T, Fukushima K, Ikehara T, et al. Safety and oncological benefit of hepatopancreatoduodenectomy for advanced extrahepatic cholangiocarcinoma with horizontal tumor spread: shinshu university experience. Ann Surg Oncol. (2021) 28:2012–25. doi: 10.1245/s10434-020-09209-8
16. Aoki T, Sakamoto Y, Kohno Y, Akamatsu N, Kaneko J, Sugawara Y, et al. Hepatopancreaticoduodenectomy for biliary cancer: strategies for near-zero operative mortality and acceptable long-term outcome. Ann Surg. (2018) 267:332–7. doi: 10.1097/sla.0000000000002059
17. Dai WC, Chok KS, Cheung TT, Chan AC, Chan SC, and Lo CM. Hepatopancreatoduodenectomy for advanced hepatobiliary Malignancies: a single-center experience. Hepatobiliary Pancreat Dis Int. (2017) 16:382–6. doi: 10.1016/s1499-3872(17)60039-0
18. Liu F, Hu HJ, Ma WJ, Wang JK, Ran CD, Regmi P, et al. Is radical resection of hilar cholangiocarcinoma plus partial resection of pancreatic head justified for advanced hilar cholangiocarcinoma? ANZ J Surg. (2020) 90:1666–70. doi: 10.1111/ans.15955
19. Porcu A, Deiana G, Feo CF, Ninniri C, Turilli D, Tanda L, et al. Hepatopancreatoduodenectomy for the treatment of extrahepatic cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. (2023) 22:430–3. doi: 10.1016/j.hbpd.2022.08.011
20. Yoshimi Y, Noji T, Okamura K, Tanaka K, Matsui A, Nakanishi Y, et al. The short- and long-term surgical results of consecutive hepatopancreaticoduodenectomy for wide-spread biliary Malignancy. Ann Surg Oncol. (2024) 31:90–6. doi: 10.1245/s10434-023-14406-2
21. Atyah MM, Luo Y, Liu R, Yang Z, and Xu L. The application of hepatopancreatoduodenectomy in advanced gallbladder carcinoma: Patients’ selection, surgical outcome and influence on survival compared to radical cholecystectomy. Asian J Surg. (2024) 22:S1015–9584. doi: 10.1016/j.asjsur.2024.10.139
22. Sugiura T, Ohgi K, Ashida R, Yamada M, Kato Y, Otsuka S, et al. Hepatopancreatoduodenectomy for extrahepatic cholangiocarcinoma: A series of 100 consecutive cases from an expert center in Japan. Ann Surg Oncol. (2025) 32:6531–40. doi: 10.1245/s10434-025-17515-2
23. Kiritani S, Kawaguchi Y, Nishioka Y, Mihara Y, Ichida A, Takamoto T, et al. Long-term outcomes of hepatopancreatoduodenectomy for perihilar cholangiocarcinoma: A comparative study with conventional hepatectomy. Eur J Surg Oncol. (2025) 51:109633. doi: 10.1016/j.ejso.2025.109633
24. Tran TB, Dua MM, Spain DA, Visser BC, Norton JA, and Poultsides GA. Hepato-pancreatectomy: how morbid? Results from the national surgical quality improvement project. HPB (Oxford). (2015) 17:763–9. doi: 10.1111/hpb.12426
25. Wang Q, Li HJ, Dai XM, Xiang ZQ, and Zhu Z. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: Systematic review and meta-analysis of propensity-score matched studies. Int J Surg. (2022) 105:106821. doi: 10.1016/j.ijsu.2022.106821
26. Yin T, Qin T, Wei K, Shen M, Zhang Z, Wen J, et al. Comparison of safety and effectiveness between laparoscopic and open pancreatoduodenectomy: A systematic review and meta-analysis. Int J Surg. (2022) 105:106799. doi: 10.1016/j.ijsu.2022.106799
27. Zhu P, Liao W, Zhang WG, Chen L, Shu C, Zhang ZW, et al. A prospective study using propensity score matching to compare long-term survival outcomes after robotic-assisted, laparoscopic, or open liver resection for patients with BCLC stage 0-A hepatocellular carcinoma. Ann Surg. (2023) 277:e103–11. doi: 10.1097/sla.0000000000005380
28. Qin T, Zhang H, Pan S, Liu J, Li D, Chen R, et al. Effect of laparoscopic and open pancreaticoduodenectomy for pancreatic or periampullary tumors: three-year follow-up of a randomized clinical trial. Ann Surg. (2024) 279:605–12. doi: 10.1097/sla.0000000000006149
29. Mizuno T, Ebata T, Yokoyama Y, Igami T, Yamaguchi J, Onoe S, et al. Major hepatectomy with or without pancreatoduodenectomy for advanced gallbladder cancer. Br J Surg. (2019) 106:626–35. doi: 10.1002/bjs.11088
30. Cao F, Tong X, Li A, Li J, and Li F. Meta-analysis of modified Blumgart anastomosis and interrupted transpancreatic suture in pancreaticojejunostomy after pancreaticoduodenectomy. Asian J Surg. (2020) 43:1056–61. doi: 10.1016/j.asjsur.2020.01.011
Keywords: laparoscopic hepaticopancreaticoduodenectomy, surgery, case report, intrahepatic cholangiocarcinoma, bile duct tumor
Citation: Fang Y, Huang D, Chang Y, Luo Q, Huang R and He K (2025) Laparoscopic hepaticopancreaticoduodenectomy for synchronous intrahepatic cholangiocarcinoma and distal common bile duct tumors: a case report. Front. Oncol. 15:1665399. doi: 10.3389/fonc.2025.1665399
Received: 14 July 2025; Accepted: 24 October 2025;
Published: 10 November 2025.
Edited by:
Wenbo Zou, No.924 Hospital of PLA Joint Logistic Support Force, ChinaReviewed by:
Nikolaos Benetatos, University of Patras, GreeceZhaohui Tang, Shanghai Jiao Tong University, China
Copyright © 2025 Fang, Huang, Chang, Luo, Huang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun He, aGVrdW44MEAxMjYuY29t
†ORCID: Kun He, orcid.org/0000-0002-0818-9026
 Yuhui Fang1
Yuhui Fang1 Kun He
Kun He