- 1Department of Radiotherapy, The Second Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of Anus and Intestine Surgery, The Second Hospital of Hebei Medical University, Shijiazhuang, China
Primary tracheal carcinoma is a rare malignant tumor; its optimal treatment strategy is not yet formally proven. Surgery, especially complete surgical excision, is often considered as the first-line treatment, and chemotherapy has been reported ineffective. There is a growing body of evidence suggesting that radiotherapy may offer better local control and improve survival outcomes for cases of incomplete excision, questionable surgical margins, or unresectable lesions. However, the effect, total dose, and fraction dose of radiotherapy remain controversial. We report two cases of primary tracheal squamous cell carcinoma at the tracheal carina: a 74-year-old man with an unresectable tracheal carcinoma at the carina and a 55-year-old woman with a tracheal tumor. Both of them were treated with radical radiotherapy, demonstrating satisfactory local control. Finally, we review the current progress of radiotherapy in primary tracheal carcinoma.
Introduction
Primary tracheal carcinoma (PTC) is a rare malignant tumor, accounting for <0.5% of all malignant tumors. Data from MD Anderson Cancer Center showed that only 74 patients have been diagnosed with primary cancers of the trachea from 1945 to June 2004, while over this 60-year period, approximately 600,000 new patients have been registered (1). Squamous cell carcinoma (SCC) and adenoid cystic carcinoma (ACC) are the most frequent histologic types (2). SCC is the most frequent histologic type in western countries (56.2% vs. 21.3%), while ACC is most common in China (50.7% vs. 30.4%) and United States (48% vs. 28%) (3–6). SCC patients appear to have poorer survival, with a 25% 5-year overall survival (OS) rate compared to those diagnosed with ACC (3, 7).
The most common symptom is dyspnea, hemoptysis, and cough. To date, surgery, especially complete surgical excision, is considered as the first-line treatment in localized PTC and shows benefit for long-term survival (8, 9). Chemotherapy was reported ineffective, but for patients with recurrent or metastatic disease, targeted and immune treatment seem to have good perspectives (10). Radiotherapy (RT) was often recommended for cases with incomplete excision, with the surgical margin being questionable, or with unresectable lesions, which could enable better local control to be achieved, but the effect, total dose, and fractionation schedules remain controversial (11, 12). Here we reported two cases of primary tracheal squamous cell carcinoma at the tracheal carina, who received radical RT that demonstrated satisfactory local control, and we reviewed the current progress of radiotherapy in primary tracheal carcinoma.
Case presentation
Case 1
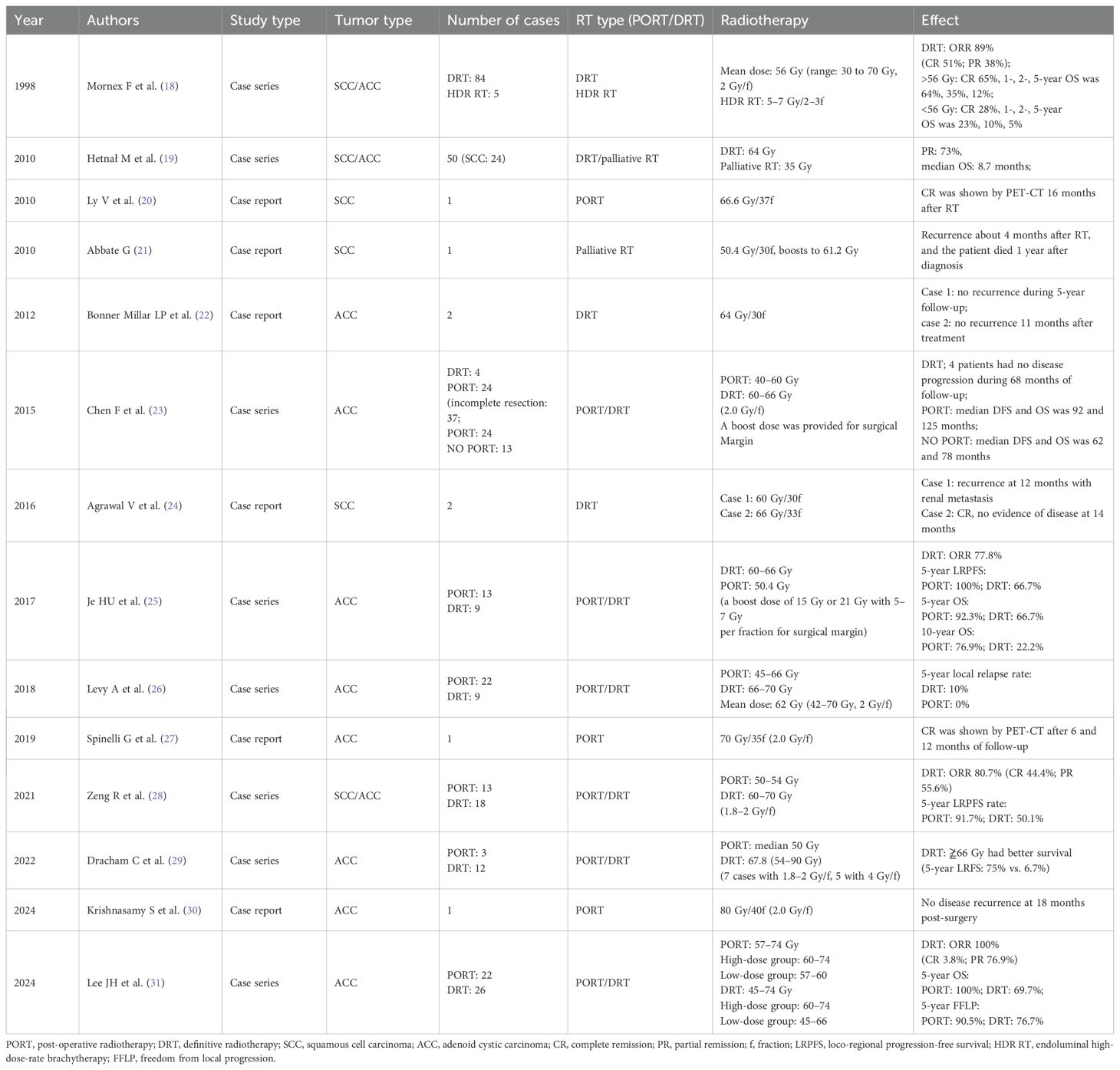
A 74-year-old man presented with a 2-month history of intermittent coughing, expectoration, and blood-tinged sputum, without fever, dyspnea, and thoracalgia. His past medical history was characterized by a kidney stone treated with extracorporeal lithotripsy and arrhythmia (sinus arrhythmia has persisted for over 50 years without oral medication treatment). An enhanced chest computed tomography (CT) showed an opacity at the tracheal carina (Figures 1A, B). To verify the nature of the lesion, fiberoptic bronchoscope examination and histopathologic biopsy were performed at a local hospital. The fiberoptic bronchoscope was used to verify the lesion, and the pathological diagnosis of the biopsy was squamous cell carcinoma with focal neuroendocrine (NE) marker expression (Figures 1H, I). Immunohistochemistry showed the following results: CD56 (+), CgA (-), CK (+), CK5/6 (+), CK7 (+), ki-67 (+, 50%), P40 (+), Syn (–), TTF-1 (–), and NapsinA (–). Then, the patient visited our hospital, and a positron emission tomography (PET)/CT scan revealed the thickness of the tracheal carina, with a maximum diameter of 11 mm and showing a strong 18F-fluorodeoxyglucose (18-FDG) uptake (SUVmax 10.6) without distant metastasis (Figures 1E–G). In terms of laboratory examination, the results of routine blood and urine examinations were normal, as were those of the biochemistry examination. Based on the results above, the patient was diagnosed with tracheal SCC. The American Joint Committee on Cancer (AJCC) does not have TNM stage definitions for tracheal tumors; however, the stage was cT4N0M0, stage IIIA according to AJCC for lung cancer staging or cT1N0M0, stage I according to the classification proposed by Bhattacharyya (13).
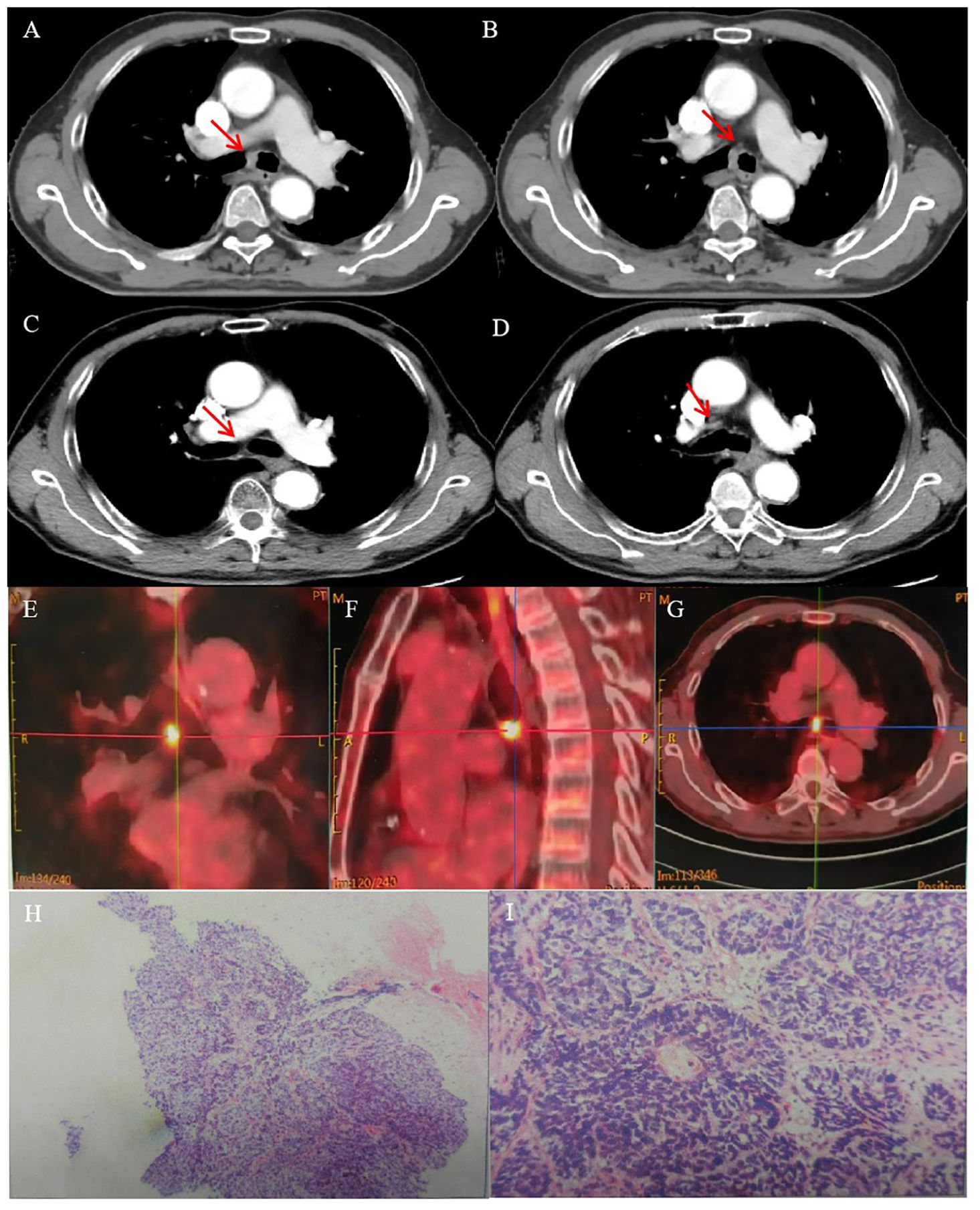
Figure 1. CT images and RT target of case 1 patient. (A, B) Computed tomography (CT) revealed a tracheal lesion at the carina. (C, D) The lesion disappeared 2 months after RT. (E–G) PET/CT revealed the thickness of the trachea of carina with a maximum diameter of 11 mm and showing strong 18F-fluorodeoxyglucose (18-FDG) uptake (SUVmax 10.6). (H, I) Photomicrograph of case 1 patient with primary tracheal carcinoma. The neoplasm showed the characteristic features of PTC. (A) ×100. (B) ×400.
The patient refused an operation due to his advanced age. He received intensity-modulated conformal radiation therapy (IMRT) of TOMOtherapy (USA), the target volume of which was described as follows: according to contrast-enhanced CT and PET/CT images to delineate the target, the gross tumor volume (GTV) comprised a gross tumor, the clinical target volume (CTV) had an area of 2 cm above and below the tumor, and CTV was enlarged 5 mm circumferentially to form the planning target volume (PTV). GTV was also enlarged 5 mm circumferentially to form the PGTV. The dose of PGTV was 66 Gy in 33 fractions of 2 Gy, while that of PTV was 54 Gy/30f (Figures 2A–D).
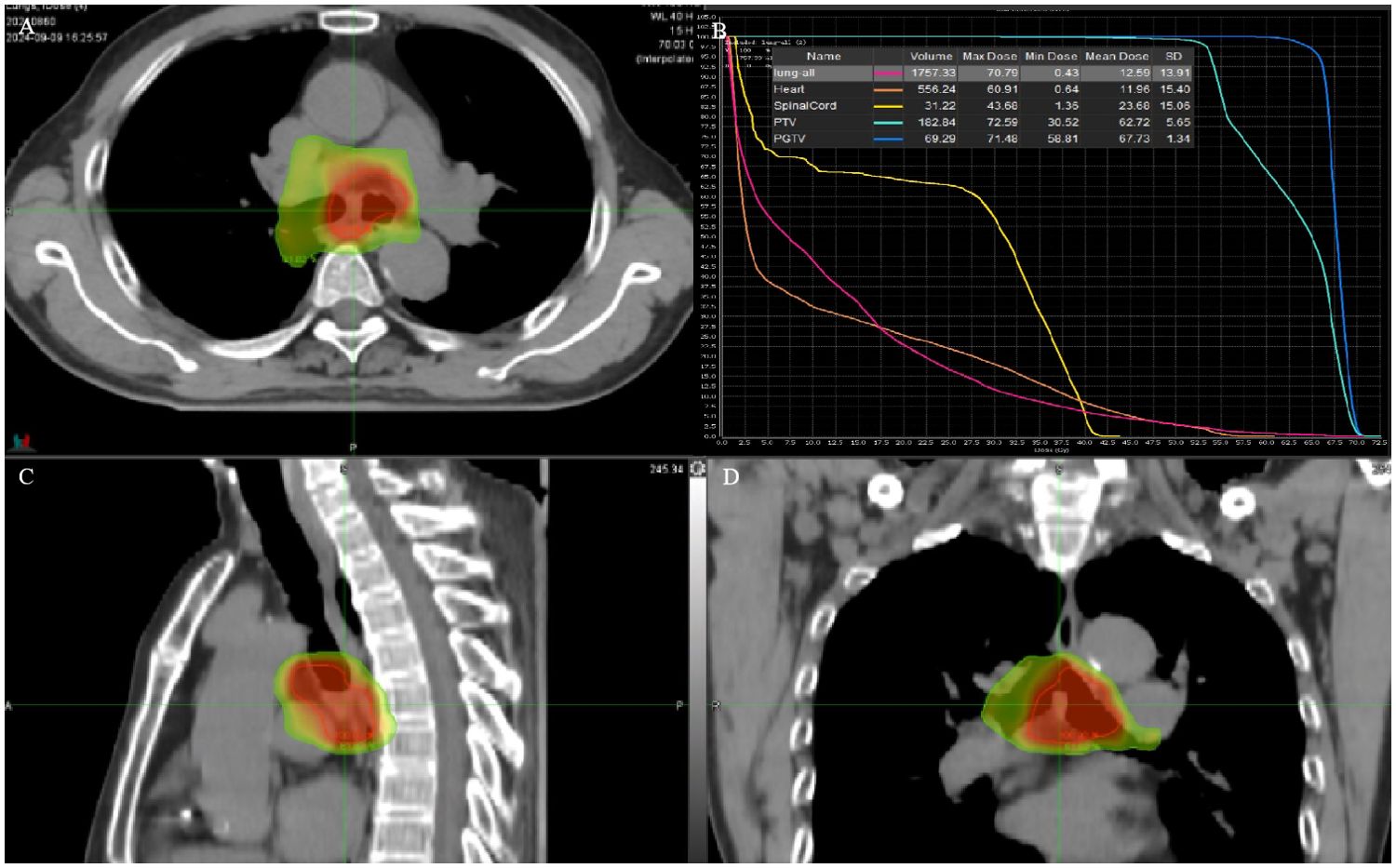
Figure 2. (A, C, D) RT target; area of red line: 66 Gy; and area of yellow line: 54 Gy. (B) DVH of case 1 patient.
The patient had no obvious side effects during RT, only mild nausea and fatigue. He recovered well after RT without chemotherapy. At 2 months later, the CT result showed that the trachea was unobstructed and that there was no recurrence (Figures 1C, D). At 6 months after RT, he reported no blood-tinged sputum, and his body movement had returned to normal. The authors confirm that written informed consent was provided by the patient for the publication of this case report and the inclusion of accompanying images.
Case 2
A 53-year-old woman presented with a history of more than 1 year of cough, coughing up phlegm, and shortness of breath for half a year, which worsened for half a month prior to her current presentation. Notably, she did not experience fever, thoracalgia, or any other relevant symptoms during that time. She denied any history of other diseases. The chest CT revealed the marked thickness of the lower segment and tracheal carina, leading to a locally narrowed trachea (Figures 3A, B). She had been hospitalized at local hospitals and received pharmaceutical treatment aimed at relieving her symptoms before her admission to our hospital. Regrettably, her shortness of breath did not yield with a significant relief. Subsequently, she was referred to our hospital for further medical assessment. After a whole-body examination was completed, there was no distant metastases found. The preliminary diagnosis pointed to an intratracheal mass. A fiberoptic bronchoscope verified a tracheal lesion situated 3 cm above the carina, and the lumen appeared notably narrow (Figures 4A–D). The pathological diagnosis of the biopsy was squamous epithelial high-grade intraepithelial lesion, with a tendency to be cancerous at the focal lesion. The tumor markers showed the following result: squamous cell carcinoma antigen at 2.74 ng/ml. The remaining examinations, as well as the laboratory examination, including routine blood, urine examinations, and those of the biochemistry examination, showed no abnormalities. The patient was diagnosed with tracheal SCC (cT4N1M0, stage IIIA) according to AJCC for lung cancer staging, but according to the classification proposed by Bhattacharyya, the stage was cT2N1M0, stage IIB. The surgery was difficult and risky, and radiation therapy was recommended. The patient received IMRT of true beam (Varian, USA). The target volume and radiation dose were as described in case 1 above combined with four cycles of chemotherapy of carboplatin plus paclitaxel (Figures 3E–H).
Figure 3. CT images and RT target of case 2 patient. (A, B) Computed tomography (CT) revealed a tracheal lesion. (C, D) The lesion was reduced at 1 month after RT. (E–H) RT target and DVH of case 2 patient; area of red line: 66 Gy; and area of yellow line: 54 Gy.
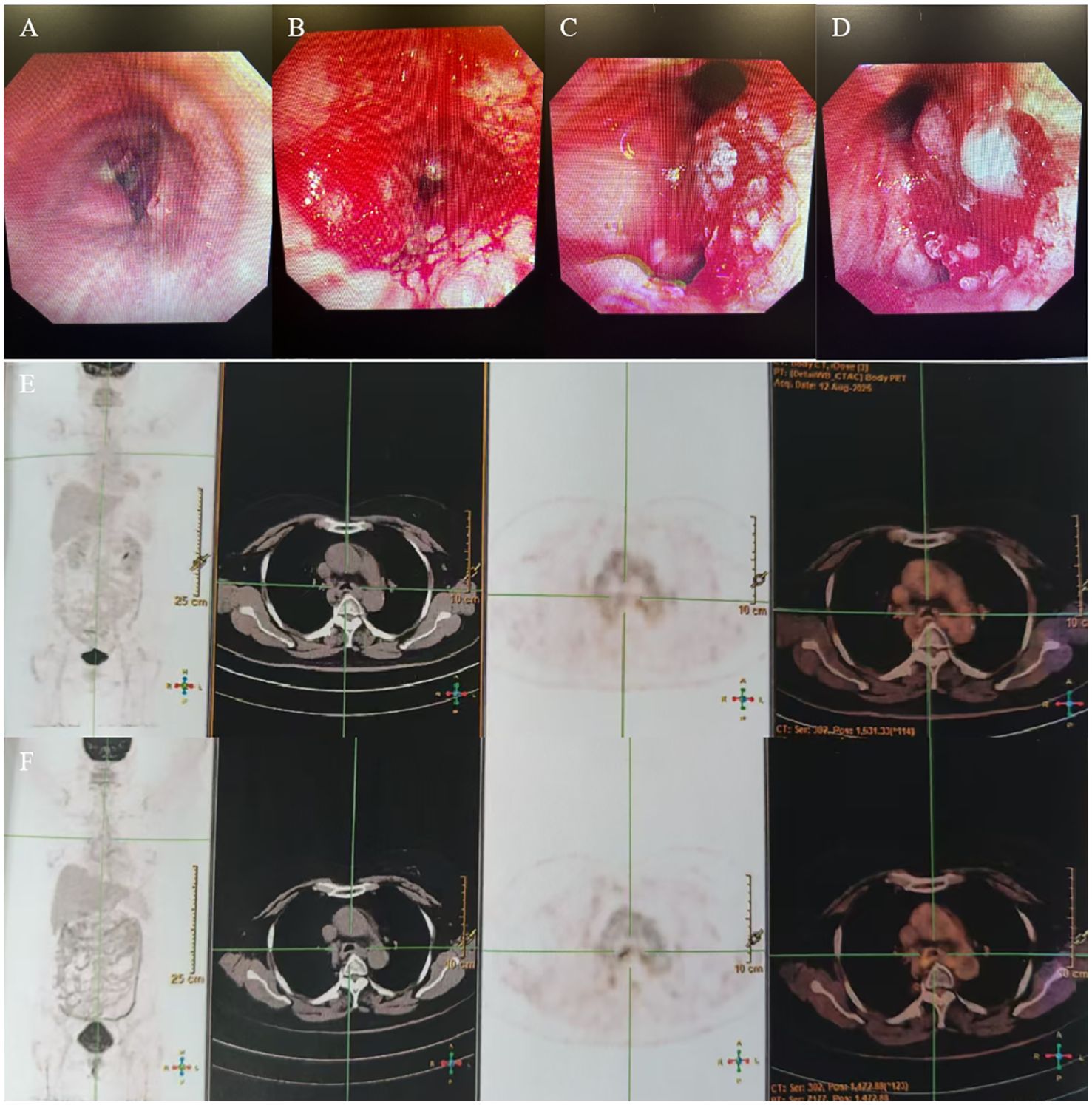
Figure 4. (A–D) Fiberoptic bronchoscopy showed a trachea lesion situated 3 cm above the carina, and the lumen appeared notably narrow. (E, F) PET/CT revealed the thickness of the tracheal carina, showing no 18-FDG uptake and without distant metastasis.
The patient had no obvious side effects during RT and recovered well. The CT result showed that the trachea lesion was markedly reduced at 1 month after RT (Figures 3C, D). She received four cycles of chemotherapy. At 6 months after RT, the chest CT still showed the thickness of the original tracheal lesion site, so a PET/CT was performed, which revealed the thickness of the tracheal carina and showed no 18-FDG uptake as well as without distant metastasis at nearly 9 months after RT (Figures 4E, F). Since then, she had undergone regular follow-up examinations every 3 months, and no symptom was reported. Her body movement though had returned to normal during the nearly 1-year telephonic follow-up. The patient provided written informed consent for the publication of the case details and the inclusion of accompanying images.
Discussion
Distinct from primary lung carcinoma which is also known as primary bronchial lung carcinoma, tracheal tumor is mainly located in the trachea. The two presented cases showed that the lesions are both located in the trachea: case 1 showed no invading in the left and right bronchi, and case 2 showed the trachea lesion situated 3 cm above the carina; and the combined results of imaging and bronchoscopy support the diagnosis of tracheal malignant mass.
To date, there is no standard TNM staging and treatment guidelines for PTC. The classification proposed by Bhattacharyya seems more suitable compared with the AJCC staging of primary bronchial lung carcinoma (13). For decades, surgical excision combined with airway reconstruction had been considered as the preferred treatment, especially for those with severe life-threatening airway stenosis, and the patients had a good result from surgery (14). A case report described a 74-year-old female patient with PTC who had undergone surgery alone. The pathology results showed that the tumor invaded the adventitia of the trachea and bilateral thyroid. The patient recovered well, and no recurrence was found at 8 months after the operation (15). Another report showed a PTC that was excised using interventional bronchoscopy including an electric snare, electrotomy, cordectomy, and an argon knife. The patient achieved complete recovery for 2 years without any radiotherapy or chemotherapy (16). A retrospective study enrolled 270 patients (135 patients with SCC and 135 with ACC) with PTC. The 5-year OS was 39.1% and 52.4% for resected SCC and ACC patients, respectively. However, it was only 7.3% and 33.3% for unresectable SCC and ACC patients, respectively (8). Despite improved survival, the prognosis for patients with PTC is still poor; the 5-year overall survival (OS) was 31.7% (17). It means that adjuvant therapy combined with resection may delay the patients’ relapse and metastasis.
To date, chemotherapy was reported ineffective, but an increasing number of studies showed that RT was considered as an independent prognostic factor for cases with incomplete excision, with the surgical margin being questionable, or with unresectable lesions. RT could achieve better local control, delay the patients’ relapse and metastasis, and prolong the survival time (Table 1), but postoperative chemotherapy following RT in patients with incomplete resection did not seem to show an additional survival benefit (23, 32). Due to the lack of large randomized clinical trials, the benefit of radiotherapy is not clinically proven, and the total dose and fractionation schedules remain unestablished. Zheng Z et al. (33) analyzed the impact of RT on PTC patients using the data from SEER database. The results showed that the OS was better in patients who received RT compared to those who did not receive RT (median OS: 12 vs. 4 months). Unfortunately, the RT doses were not analyzed and recommended. Yang CJ et al. (34) and Yusuf M et al. (35) retrospectively analyzed the survival value of RT on PTC patients with positive margins and found no significant OS benefit between patients who did or did not receive postoperative RT. Unfortunately, the authors did not give details on the radiotherapy doses.
A study showed that the radiation dose may affect local control and the patients’ OS. The 5-year OS dropped from 12% for patients receiving doses greater than 56 Gy to 5% for lower doses, and it is recommended to administer greater than 60 Gy for primary irradiation in PTC (18). Abbate G et al. (21) reported a 54-year-old male patient diagnosed with primary SCC, with a voluminous tracheal mass of approximately 6 to 7 cm in length, and was treated with palliative RT with a total dose 50.4 Gy. He was given boosts on the trachea up to 61.2 Gy, but recurrence was observed at approximately 4 months after RT. Hetnał M et al. (19) analyzed the role of RT in 50 patients with PTC (24 cases with SCC). The median dose was 64 Gy (range, 56–70 Gy) with radical RT, and 73% of the patients showed PR. Ly V et al. (20) reported a 75-year-old man with primary tracheal SCC who received a total of 66.6 Gy of RT at 1.8 Gy per fraction after electrocautery, argon photocoagulation, and cryotherapy. He achieved CR, and there was no recurrence during the 16-month follow-up period. In a retrospective study, 18 PTC patients received definitive RT. The sub-group univariate analysis indicated that the 5-year progression-free survival (PFS) was better for those who received at least 68 Gy of radiation (28). Agrawal V et al. (24) reported two cases with SCC of PTC; one was treated with 60 Gy RT in 30 fractions, and the other received a total of 66 Gy in 33 fractions, with both achieving complete response (CR). Therefore, 66 Gy of radical RT was recommended for primary SCC.
For ACC, due to its being less radiosensitive, higher doses may be needed. Levy A et al. (26) found that a dose of radiotherapy <60 Gy was associated with a decreased PFS for tracheal ACC. An earlier case report recommended a dose of 70–80 Gy with acceptable toxicities for tracheal ACC patients (22, 27). Krishnasamy S et al. (30) reported a 27-year-old lady who underwent IMRT to the surgical positive margins at 64 Gy/30f. There was no recurrence after 18 months of follow-up. A newly retrospective study evaluated the efficacy of dose-escalated RT for primary tracheobronchial ACC by dividing 48 patients into low (<70.0 Gy, range: 56.3–69.3 Gy) or high (≥70.0 Gy range: 70.0–82.5 Gy) RT dose groups. The results showed that the 5-year OS were 88.2% and 100% in the postoperative RT group (p = 0.230) and 66.7% and 79.0% in the definitive RT group, respectively (p = 0.022). Thus, a radiation of ≥70.0 Gy could be considered a primary treatment option for patients with unresectable lesions for several reasons (31). Je HU et al. (25) analyzed the effect of adjuvant or definitive RT for primary tracheal ACC. The dose was 60–66 Gy of conventional fractionation for definitive RT. Dracham C et al. (29) reported 12 patients who received definitive RT with 54–90 Gy (median dose of 67.8 Gy), and they found that patients receiving a higher RT dose (≥66 Gy) had significantly better survival outcomes. Thus, ≥66 Gy of radical RT was recommended for tracheal ACC.
For the fraction dose, Je HU et al. (25) reported the range from 1.8 to 2.2 Gy. In the study of Yang Y et al., the fraction dose was 2.0–2.14 Gy (36). Another retrospective study showed that it was varied from 1.6 to 3 Gy (median, 2 Gy), with the total dose ranging from 42.5 to 82.6 Gy (median, 66.0 Gy) in the radical RT group (2). Hetnał M et al. (19) reported that the dose per fraction ranged from 1.8 to 2 Gy in radical RT, while it was 3 to 4 Gy in palliative RT. In our center, the two patients both received definitive RT with 66 Gy in 33 fractions of 2 Gy and achieved better local control; the two patients both showed CR. Therefore, we suggest 66.0 Gy for definitive RT as preferable for SCC, but a higher dose for locally advanced ACC patients with acceptable toxicities. It is worth noting that the pathological diagnosis of case 1 was SCC with focal NE marker expression. As we all know, NE markers are important markers to differentiate and diagnose NE tumors, which means relatively high sensitivity with RT and chemotherapy, especially poorly differentiated NE cancers, although exhibiting aggressive characteristics and a higher recurrence rate, such as small cell lung cancer. The study showed that conventional non-small lung cancer (NSCLC), such as adenocarcinoma and SCC, does not exhibit a NE morphology but does express NE marker(s), named NSCLC with NE differentiation (37, 38). For case 1, it was SCC with NE differentiation, which could explain why the effect of RT alone was better and CR was achieved, although the stage was cT1N0M0, stage I according to the classification proposed by Bhattacharyya. Due to his advanced age, the patient did not receive chemotherapy. Future work is needed to understand the role of NE differentiation in PTC, which may help promote a more accurate diagnosis and develop specific treatment strategies.
For target delineation, GTV included the gross tumor or postoperative tumor bed along with positive lymph nodes. The CTV of postoperative RT had an area of 3 cm above and below the surgical anastomosis including the tumor bed and the draining area of the pathologically positive lymph nodes, while the CTV of definitive RT expanded 3 cm above and below the tumor with the clinically positive nodal regions based on contrast-enhanced CT scans (25, 30). In the report of Dracham C et al. (29), CTV was generated using a 1-cm longitudinal and 1-cm radial margin. In our center, the CTV of definitive RT for these two patients was also the area 2 cm above and below the tumor with the clinically positive nodal regions. PTV was generated with 5 mm margins around the CTV.
For RT techniques, in a retrospective study of 133 cases, 66 patients with positive surgical margins were divided into non-IMRT (two-dimensional RT, 2D-RT, mainly) and IMRT groups. The results showed that the OS of non-IMRT patients showed no significant improvement in comparison with the no-RT patients (the 5-year OS was 70.2% vs. 77%, and the 10-year OS was 45.4% vs. 47.9%), whereas the 5-year (94.7%) and 10-year OS (82.9%) of the adjuvant IMRT group were significantly better than the no-RT group and the non-IMRT group (22). Modern advanced RT techniques such as proton and carbon ion beams have physical advantages and can provide specific better dose distribution and better sparing of normal tissue. In a study that enrolled 18 patients with primary tracheobronchial adenoid cystic carcinoma who received doses of CIRT at 66–72.6 GyE/22–23 fractions, the overall response rate (ORR) was 88.2%, and the 2-year OS and PFS were 100% and 61.4%, respectively. However, considering the expensive fees, it is not suitable for a wide range of clinical applications (39). Nakamura M et al. (40) reported two cases of PTC of the trachea that received proton beam therapy with 74-Gy dose in 37 fractions; both of them achieved long-term survival. Furthermore, brachytherapy may be used for tracheal tumors, even as a boost for external beam irradiation. Carvalho Hde A (41) reported four patients with nonresected primary tracheal tumors who received brachytherapy—two cases of SCC, one case of recurrent ACC, and one case with recurrent plasmacytoma—in three or four fractions of 7.5 Gy, calculated at a depth of 1 cm. All patients presented complete local response at the time of the first bronchoscopic evaluation. Nguyen NT (42) reported eight patients who received brachytherapy alone with 5–7 Gy/fraction, using one to three fractions. The patients experienced symptomatic improvement and good local response, but large randomized clinical trials are needed to prove the role of brachytherapy in PTC.
Chemotherapy was reported ineffective. In recent years, immune checkpoint inhibitors (ICIs), including anti-PD-1, anti-PD-L1, and anti-cytotoxic T lymphocyte antigen 4, have emerged as promising therapeutic agents in those patients with recurrent or metastatic disease. Immunotherapy also seems to have good perspectives in PTC (10). Mikami E et al. (43) and Nakatani Yu et al. (44) both reported one PTC patient who received definitive concurrent chemoradiotherapy followed by immunotherapy (durvalumab) and who achieved successful treatment and prognosis. Immunotherapy may be a promising treatment option for unresectable or recurrent and metastatic PTC patients.
Conclusions
In conclusion, PTC is a rare malignant tumor. For incomplete excision, a positive surgical margin, or unresectable lesions, RT could achieve better local control, delay the patients’ relapse and metastasis, and prolong the survival time. A dose of 66.0 Gy for definitive RT using IMRT or VMAT was preferred for SCC and a higher dose for ACC (such as ≥66.0 Gy) patients with acceptable toxicities. Other RT techniques such as proton and carbon ion beams are not suitable for a wide range of clinical applications. Large randomized clinical trials are urgently needed to prove and recommend the RT dose for patients with PTC. Furthermore, target and immunotherapy may be a promising treatment option for unresectable lesions or recurrent and metastatic PTC patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XC: Writing – original draft. ZT: Writing – original draft. YZ: Data curation, Formal Analysis, Writing – original draft. XG: Writing – review & editing. FL: Data curation, Formal Analysis, Writing – original draft. YY: Data curation, Resources, Writing – original draft. HL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PTC, primary tracheal carcinoma; SCC, squamous cell carcinoma; ACC, adenoid cystic carcinoma; CT, computed tomography; PET/CT, positron emission tomography/computed tomography; 18-FDG, 18F-fluorodeoxyglucose; NE, neuroendocrine; AJCC, American Joint Committee on Cancer; IMRT, intensity-modulated conformal radiation therapy; GTV, gross tumor volume; CTV, clinical target volume; PTV, planning target volume; RT, radiotherapy; OS, overall survival; PFS, progression-free survival; CR, complete response; PR, partial response; ORR, overall response rate.
References
1. Webb BD, Walsh GL, Roberts DB, and Sturgis EM. Primary tracheal Malignant neoplasms: the University of Texas MD Anderson Cancer Center experience. J Am Coll Surg. (2006) 202:237–46. doi: 10.1016/j.jamcollsurg.2005.09.016
2. Napieralska A, Miszczyk L, and Blamek S. Tracheal cancer - treatment results, prognostic factors and incidence of other neoplasms. Radiol Oncol. (2016) 50:409–17. doi: 10.1515/raon-2016-0046
3. Honings J, van Dijck JA, Verhagen AF, van der Heijden HF, and Marres HA. Incidence and treatment of tracheal cancer: a nationwide study in the Netherlands. Ann Surg Oncol. (2007) 14:968–76. doi: 10.1245/s10434-006-9229-z
4. Piórek A, Płużański A, Teterycz P, Tabor S, Winiarczyk K, Knetki-Wróblewska M, et al. Clinicopathological characteristics of patients with primary tracheal tumors: Analysis of eighty-nine cases. Thorac Cancer. (2024) 15:878–83. doi: 10.1111/1759-7714.15231
5. Zhengjaiang L, Pingzhang T, Dechao Z, Reddy-Kolanu G, and Ilankovan V. Primary tracheal tumours: 21 years of experience at Peking Union Medical College, Beijing, China. J Laryngol Otol. (2008) 122:1235–40. doi: 10.1017/S0022215108001710
6. Benissan-Messan DZ, Merritt RE, Bazan JG, D’Souza DM, Abdel-Rasoul M, Moffatt-Bruce SD, et al. National utilization of surgery and outcomes for primary tracheal cancer in the United States. Ann Thorac Surg. (2020) 110:1012–22. doi: 10.1016/j.athoracsur.2020.03.048
7. Hararah MK, Stokes WA, Oweida A, Patil T, Amini A, Goddard J, et al. Epidemiology and treatment trends for primary tracheal squamous cell carcinoma. Laryngoscope. (2020) 130:405–12. doi: 10.1002/lary.27994
8. Gaissert HA, Grillo HC, Shadmehr MB, Wright CD, Gokhale M, Wain JC, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg. (2004) 78:1889–96. doi: 10.1016/j.athoracsur.2004.05.064
9. Schweiger T and Hoetzenecker K. Management of primary tracheal tumors. Thorac Surg Clin. (2025) 35:83–90. doi: 10.1016/j.thorsurg.2024.09.008
10. Marchioni A, Tonelli R, Samarelli AV, Cappiello GF, Andreani A, Tabbì L, et al. Molecular biology and therapeutic targets of primitive tracheal tumors: focus on tumors derived by salivary glands and squamous cell carcinoma. Int J Mol Sci. (2023) 24:11370. doi: 10.3390/ijms241411370
11. Koul R, Alomrann R, Rathod S, Kim J, Leylek A, Ahmed N, et al. Clinical characteristics and prognosis of primary tracheal cancer: A single institution experience. Int J Hematol Oncol Stem Cell Res. (2018) 12:298–302. doi: 10.18502/ijhoscr.v12i4.108
12. Högerle BA, Lasitschka F, Muley T, Bougatf N, Herfarth K, Adeberg S, et al. Primary adenoid cystic carcinoma of the trachea: clinical outcome of 38 patients after interdisciplinary treatment in a single institution. Radiat Oncol. (2019) 14:117. doi: 10.1186/s13014-019-1323-z
13. Bhattacharyya N. Contemporary staging and prognosis for primary tracheal Malignancies: a population-based analysis. Otolaryngol Head Neck Surg. (2004) 131:639–42. doi: 10.1016/j.otohns.2004.05.018
14. Armel K, Stamey T, Maitre J, Cunningham AR, Ju AW, Burke A, et al. Demographic, disease, and treatment characteristics of primary tracheal cancers. Ann Surg Oncol. (2025) 32:841–7. doi: 10.1245/s10434-024-16520-1
15. Wang F, Wu Y, Yao X, Chen S, and Liu H. Surgical treatment of primary tracheal adenoid cystic carcinoma. Ear Nose Throat J. (2025) 104:10S-14S. doi: 10.1177/01455613221111497
16. Huang S, Peng X, Li H, Zhao J, and Hou J. Successful endotracheal intervention for primary tracheal acinic cell carcinoma: A case report and literature review. Med (Baltimore). (2024) 103:e37033. doi: 10.1097/MD.0000000000037033
17. West D. Invited commentary: Tracheal cancer. A rare and deadly but potentially curable disease that also affects younger people. Eur J Cardiothorac Surg. (2023) 64:ezad251. doi: 10.1093/ejcts/ezad251
18. Mornex F, Coquard R, Danhier S, Maingon P, El Husseini G, and Van Houtte P. Role of radiation therapy in the treatment of primary tracheal carcinoma. Int J Radiat Oncol Biol Phys. (1998) 41:299–305. doi: 10.1016/s0360-3016(98)00073-x
19. Hetnał M, Kielaszek-Ćmiel A, Wolanin M, Korzeniowski S, Brandys P, Małecki K, et al. Tracheal cancer: Role of radiation therapy. Rep Pract Oncol Radiother. (2010) 15:113–8. doi: 10.1016/j.rpor.2010.08.005
20. Ly V, Gupta S, Desoto F, and Cutaia M. Tracheal squamous cell carcinoma treated endoscopically. J Bronchol Interv Pulmonol. (2010) 17:353–5. doi: 10.1097/LBR.0b013e3181f1e809
21. Abbate G, Lancella A, Contini R, and Scotti A. A primary squamous cell carcinoma of the trachea: case report and review of the literature. Acta Otorhinolaryngol Ital. (2010) 30:209.
22. Bonner Millar LP, Stripp D, Cooper JD, Both S, James P, and Rengan R. Definitive radiotherapy for unresected adenoid cystic carcinoma of the trachea. Chest. (2012) 141:1323–6. doi: 10.1378/chest.11-0925
23. Chen F, Huang M, Xu Y, Li T, Xie K, Zhang L, et al. Primary tracheal adenoid cystic carcinoma: adjuvant treatment outcome. Int J Clin Oncol. (2015) 20:686–92. doi: 10.1007/s10147-014-0771-6
24. Agrawal V, Marcoux JP, Rabin MS, Vernovsky I, Wee JO, and Mak RH. Case report of tracheobronchial squamous cell carcinoma treated with radiation therapy and concurrent chemotherapy. Adv Radiat Oncol. (2016) 1:127–31. doi: 10.1016/j.adro.2016.03.003
25. Je HU, Song SY, Kim DK, Kim YH, Jeong SY, Back GM, et al. A 10-year clinical outcome of radiotherapy as an adjuvant or definitive treatment for primary tracheal adenoid cystic carcinoma. Radiat Oncol. (2017) 12:196. doi: 10.1186/s13014-017-0933-6
26. Levy A, Omeiri A, Fadel E, and Le Péchoux C. Radiotherapy for tracheal-bronchial cystic adenoid carcinomas. Clin Oncol (R Coll Radiol). (2018) 30:39–46. doi: 10.1016/j.clon.2017.10.012
27. Spinelli GP, Miele E, Prete AA, Lo Russo G, Di Marzo A, Di Cristofano C, et al. Combined surgery and radiotherapy as curative treatment for tracheal adenoid cystic carcinoma: a case report. J Med Case Rep. (2019) 13:52. doi: 10.1186/s13256-019-1996-9
28. Zeng R, Wang H, Cai X, Guo X, Ping Y, and Yang Q. Radiotherapy for primary tracheal carcinoma: experience at a single institution. Technol Cancer Res Treat. (2021) 20:15330338211034273. doi: 10.1177/15330338211034273
29. Dracham C, Khosla D, Kapoor R, Dey T, Periasamy K, Elangovan A, et al. Expanding role of radiotherapy in adenoid cystic carcinoma of the tracheobronchial tree: a new horizon. Tumori. (2022) 108:347–56. doi: 10.1177/03008916211012461
30. Krishnasamy S, Tang CY, and Tan PH. Tracheal adenoid cystic carcinoma with microscopic positive margin-how we approached with a systematic analysis review of its management. Indian J Thorac Cardiovasc Surg. (2024) 40:332–40. doi: 10.1007/s12055-023-01600-w
31. Lee JH, Jang JY, Noh JM, Yang K, and Pyo H. Dose-escalated radiotherapy for primary tracheobronchial adenoid cystic carcinoma. Cancers (Basel). (2024) 16:2127. doi: 10.3390/cancers16112127
32. Huo Z, Meng Y, Wu H, Shen J, Bi Y, Luo Y, et al. Adenoid cystic carcinoma of the tracheobronchial tree: clinicopathologic and immunohistochemical studies of 21 cases. Int J Clin Exp Pathol. (2014) 7:7527–35.
33. Zheng Z, Du Z, Fang Z, Shi Y, Chen X, Jin M, et al. Survival benefit of radiotherapy and nomogram for patients with primary tracheal Malignant tumors: a propensity score-matched SEER database analysis. J Cancer Res Clin Oncol. (2023) 149:9919–26. doi: 10.1007/s00432-023-04896-8
34. Yang CJ, Shah SA, Ramakrishnan D, Raman V, Diao K, Wang H, et al. Impact of positive margins and radiation after tracheal adenoid cystic carcinoma resection on survival. Ann Thorac Surg. (2020) 109:1026–32. doi: 10.1016/j.athoracsur.2019.08.094
35. Yusuf M, Gaskins J, Trawick E, Tennant P, Bumpous J, van Berkel V, et al. Effects of adjuvant radiation therapy on survival for patients with resected primary tracheal carcinoma: an analysis of the National Cancer Database. Jpn J Clin Oncol. (2019) 49:628–38. doi: 10.1093/jjco/hyz047
36. Yang Y, Ran J, Wang Y, Zhou Z, Chen D, Feng Q, et al. Intensity modulated radiation therapy may improve survival for tracheal-bronchial adenoid cystic carcinoma: A retrospective study of 133 cases. Lung Cancer. (2021) 157:116–23. doi: 10.1016/j.lungcan.2021.05.006
37. Rekhtman N. Lung neuroendocrine neoplasms: recent progress and persistent challenges. Mod Pathol. (2022) 35:36–50. doi: 10.1038/s41379-021-00943-2
38. Keyhanian K, Phillips WJ, Yeung BS, Gomes M, Lo B, and Sekhon HS. Neuroendocrine differentiation distinguishes basaloid variant of lung squamous cell carcinoma. Diagn Pathol. (2022) 17:46. doi: 10.1186/s13000-022-01223-6
39. Chen J, Mao J, Ma N, Wu KL, Lu J, and Jiang GL. Definitive carbon ion radiotherapy for tracheobronchial adenoid cystic carcinoma: a preliminary report. BMC Cancer. (2021) 21:734. doi: 10.1186/s12885-021-08493-1
40. Nakamura M, Ohnishi K, Nakazawa K, Shimizu K, Miyauchi D, Mizumoto M, et al. Long-term follow-up of unresectable adenoid cystic carcinoma of the trachea and bronchus treated with high-dose proton beam therapy: A report of two cases. Thorac Cancer. (2024) 15:201–5. doi: 10.1111/1759-7714.15158
41. Carvalho Hde A, Figueiredo V, Pedreira WL Jr, and Aisen S. High dose-rate brachytherapy as a treatment option in primary tracheal tumors. Clinics (Sao Paulo). (2005) 60:299–304. doi: 10.1590/s1807-59322005000400007
42. Nguyen NT, Timotin E, Hunter R, Hann C, Puksa S, and Sur RK. Endotracheal brachytherapy alone: An effective palliative treatment for tracheal tumors. Brachytherapy. (2015) 14:543–8. doi: 10.1016/j.brachy.2015.02.193
43. Mikami E, Nakamichi S, Nagano A, Misawa K, Hayashi A, Tozuka T, et al. Successful treatment with definitive concurrent chemoradiotherapy followed by durvalumab maintenance therapy in a patient with tracheal adenoid cystic carcinoma. Intern Med. (2023) 62:2731–5. doi: 10.2169/internalmedicine.1142-22
Keywords: primary tracheal carcinoma, radiotherapy, radiotherapy dose, chemotherapy, surgery
Citation: Chang X, Tian Z, Zhao Y, Ge X, Li F, Yang Y and Liu H (2025) The role of radiotherapy for patients with primary tracheal carcinoma: two case reports of tracheal carina squamous cell carcinoma and literature review. Front. Oncol. 15:1665645. doi: 10.3389/fonc.2025.1665645
Received: 14 July 2025; Accepted: 12 September 2025;
Published: 02 October 2025.
Edited by:
Tao Song, Zhejiang Provincial People’s Hospital, ChinaReviewed by:
Aleksandra Piórek, Maria Sklodowska-Curie National Research Institute of Oncology, PolandMohammad Hadi Mohseni, Shahid Beheshti University of Medical Sciences, Iran
Copyright © 2025 Chang, Tian, Zhao, Ge, Li, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huizhi Liu, bGl1aHVpemhpZmxAMTYzLmNvbQ==
 Xiaojing Chang
Xiaojing Chang Zhesen Tian
Zhesen Tian Yalei Zhao
Yalei Zhao Xiaohui Ge2
Xiaohui Ge2