- 1Department of Onco-Hematology, Cell and Gene Therapy, Bambino Gesù Children’s Hospital, Scientific Institute for Research, Hospitalization and Healthcare Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 2Neuroradiology Unit, “Bambino Gesù” Children’s Hospital Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 3Ophthalmology Unit, Bambino Gesù Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Children’s Hospital, Rome, Italy
- 4Pathology Unit, Department of Laboratories, Bambino Gesu Children’s Hospital, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
High-dose chemotherapy with autologous stem cell rescue has improved outcomes in patients with metastatic retinoblastoma (RB). However, significant short- and long-term toxicities—especially in very young children with a constitutional RB1 gene mutation—highlight the need for alternative therapeutic strategies. Monoclonal antibodies targeting tumor-associated antigens such as GD2 have emerged as promising agents in this setting. We report the case of a 2-year-old child diagnosed with extensive left-eye retinoblastoma and massive extraocular dissemination at presentation. The patient was treated with systemic conventional and high-dose chemotherapy combined with intrathecal Topotecan. As consolidation therapy, the child received three courses of the anti-GD2 monoclonal antibody Dinutuximab beta over a 10-day schedule. The patient achieved complete remission and remains disease-free six years after the initial diagnosis. This case suggests that anti-GD2 immunotherapy, used as consolidation treatment, may improve the prognosis of patients with advanced retinoblastoma and potentially reduce the toxicity associated with standard therapies. Further clinical investigation is warranted to validate these findings.
Introduction
Retinoblastoma (RB) is a highly aggressive tumor and the most common primary intraocular malignancy in children. Metastatic RB is particularly challenging, as it requires intensive, multimodal therapies that often come with substantial toxicities, especially in very young children and even more critically in those with a constitutional Rb1 mutation. A recent study on patients with metastatic RB treated with high-dose chemotherapy followed by autologous hematopoietic stem cell transplant (HDC-ASCT) reported an overall survival rate grater then 70% for those without CNS metastases. Survival dropped less than 10% for patients with CNS involvement (1, 2). Complications associated with metastatic RB and its treatments include treatment related mortality, hearing loss, neurocognitive deficits and the development of second malignant neoplasms (SMNs), especially in patients carrying genetic predisposition (3–6). This highlights the urgent need for alternative treatment strategies that are both effective and less toxic. In this regard, RB cells, like other neuroectodermal-derived tissues—including neuroblastoma (NB)—highly express GD2, a cell surface disialoganglioside that plays a key role in malignant transformation and is a well-recognized therapeutic target (7, 8). Anti-GD2 monoclonal antibodies (mAbs) have markedly improved outcome in patients with high-risk neuroblastoma (HR-NB) (9–13). Dinutuximab (ch14.18) and Dinutuximab beta (ch14.18/CHO) had been approved by the United States Food and Drug Administration and the European Medicines Agency, respectively, for the treatment of HR-NB. Clinical trials of GD2-targeting therapy are currently ongoing for other GD2-expressing tumors apart from NB (14). According to recent literature, only seven patients with metastatic RB treated with anti-GD2 mAb have been reported (15–17). Anti-GD2 therapy was used as consolidation treatment for minimal residual disease (MRD) following HDC; however, in 2/7 patients, it was administered in the context of active disease. Here, we report a case of metastatic RB, with orbital and multifocal bulky bone disease at diagnosis, managed with anti-GD2 mAb Dinotuximab beta (Db) as consolidation after HDC-ASCT.
Case presentation
A 2-year-old boy was referred to our Hospital with a suspected diagnosis of RB in the left eye. Prior to admission, the patient had undergone orbital exenteration. At the clinical visit, the patient was in poor general condition, with severe malnutrition, generalized bone pain, and limb weakness. A total body CT scan revealed massive extraocular disease extension involving the left orbit and multifocal skeletal lesions (Figures 1A–D). A biopsy of a lesion in the right shoulder confirmed the diagnosis of RB. Molecular analysis of the tumor tissue demonstrated a homozygous pathogenic mutation in the Rb1 gene (c.1959dup). FISH analysis showed a normal copy number of the MYCN gene. The bone marrow biopsy showed infiltration by malignant cells. GD2 expression levels in the CD45-negative cells of the fresh tumor biopsy sample were quantified by flow cytometry and found to be 79.7% (Figure 2). Brain and spine MRI showed no evidence of parenchymal or leptomeningeal disease. Cerebrospinal fluid (CSF) analysis was negative for tumor cells. The patient initiated systemic chemotherapy - ICE regimen (Ifosfamide 3gr/mq day 1-2-3, Carboplatin 750mg/mq day 1, Etoposide 150mg/m2 day 1-2-3)-, combined with intrathecal Topotecan (0.4 mg/dose) for CNS prophylaxis. Restaging after four cycles, including whole-body CT and brain and spinal MRI, demonstrated a good partial remission of left orbital and bone lesions. The patient’s general condition progressively improved, including regained mobility and adequate nutritional intake. Constitutional genetic testing for germline Rb1 alterations was negative. Afterwards the patient underwent HDC according to the COG ARET 0321 protocol (Carboplatin 350mg/mq day 1-2-3/Thiotepa 300mg/mq day 4-5-6/Etoposide 250mg/mq day 4-5-6) followed by ASCT (1). No moderate or severe complications during treatment. Post- HDC whole-body CT imaging showed no radiological evidence of residual disease (Figures 1E–H). We did not administer radiotherapy to the sites of bulky disease due to the patient’s young age. Given the high disease burden at diagnosis and the expression of GD2 on tumor cells, anti-GD2 mAb-Db was initiated 60 days after HDC, aiming to replicate the results previously achieved in MRD, following an approach analogous to HR-NB protocols. Three cycles were administered on a compassionate use basis, following informed consent for off-label therapy. The dosing schedule was 100 mg/m² over 10 days, every 5 weeks. Supportive care included morphine and gabapentin. Dinutuximab beta was well tolerated with no serious adverse effects. At the latest follow-up, the patient is in complete remission, with an overall survival of 6 years since diagnosis.
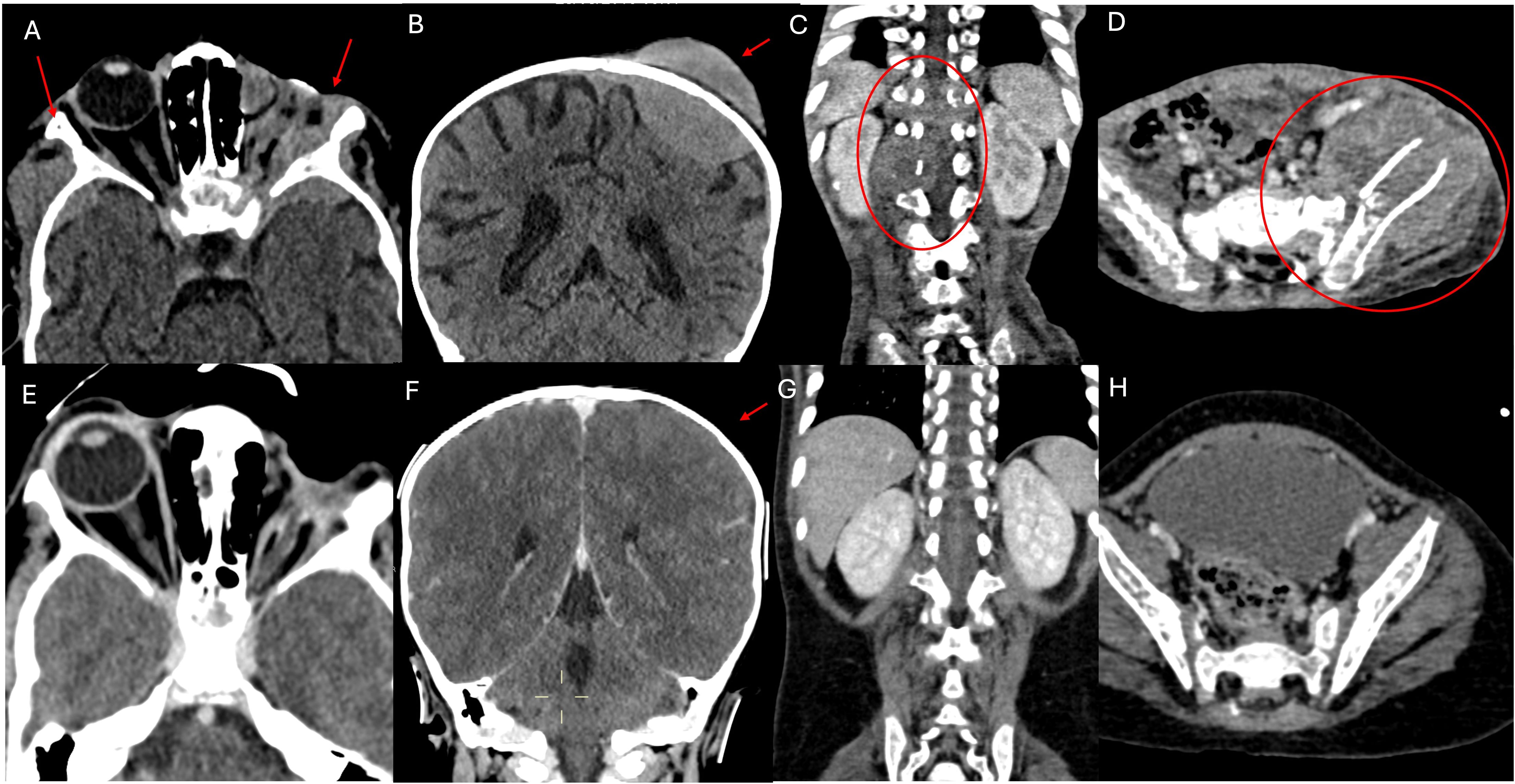
Figure 1. CT scan examination. The first section documents numerous metastatic localizations. (A) shows pathological iperdense tissue with high contrast enhancement involving the soft tissues of the left orbit (site of enucleation surgery for RB), infiltrating the local bony structures (greater wings of the sphenoid, frontal and temporal bones), and extending bilaterally to the middle cranial fossa. The left optic nerve is not recognizable, also due to the limited resolution of the CT scan for soft tissues. (B) shows an osteodural metastatic localization with high contrast enhancement of the cranial vault on the left side (parietal bone). (C, D) respectively show a partially lytic paravertebral mass extending into the spinal canal and a mass centered in the left iliac bone, partially lytic and associated with a fracture, extending to the iliac muscle, both indicative of metastases. (E–H) shown the almost complete disappearance of extraorbital metastatic lesions at the end of the treatment.
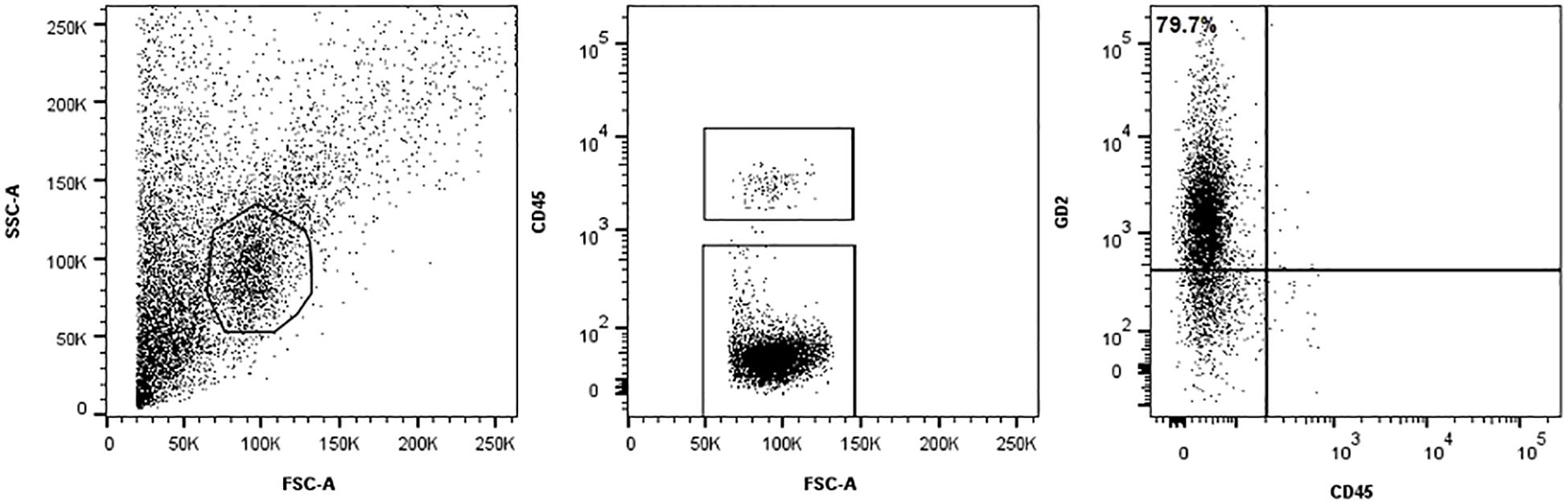
Figure 2. Methods Immunophenotype analysis. The tumor cells were stained with a BV421-conjugated monoclonal mouse anti-human GD2 antibody (clone 14.G2a, BD Biosciences, USA) and anti-human CD45 FITC (BD Biosciences, USA) to identify hematopoietic cells. GD2 expression was evaluated within the CD45-negative single-cell population. Debris was excluded using a morphological gate based on forward and side scatter (FSC/SSC) parameters. To ensure consistency and reliability, isotype controls and peripheral blood mononuclear cells were included as negative controls in flow cytometry run.
Discussion
We present the case of a patient diagnosed with metastatic RB who underwent intensive, multimodal therapy. Given the initially inappropriate management and the massive widespread disease, we opted to consolidate remission following myeloablative therapy with Db. This decision was supported by existing clinical experience with GD2-positive tumors, particularly NB. Notably, our patient’s tumor cells, analyzed from a fresh biopsy, showed high GD2 expression—exceeding 75%—which further supported our rationale for using anti-GD2 mAb in this context. Importantly, published studies indicate that optimal response to dinutuximab is observed in cases where more than 75% of tumor cells express GD2, with a threshold of at least 100,000 GD2 molecules per cell considered significant for clinical benefit (18). The multimodal treatment of extraocular RB has significantly improved prognosis, particularly in cases with extra-CNS metastasis. However, notable treatment-related toxicities have been reported. Among the 60 patients enrolled in the COG ARET 0321 trial (1) there were two therapy-related deaths due to septicemia during induction therapy. Additional relevant complications included: encephalopathy in one patient, hearing impairment in four cases, gastric hemorrhage in one. Furthermore, the incidence of SMNs was high: six patients developed second cancers (2/6 received radiotherapy). Radiotherapy is generally reserved for bulky disease not in complete remission after induction chemotherapy. Its use is associated with an increased risk of SMNs within the irradiated field, particularly in patients with an underlying genetic predisposition (3–6). Moreover, due to the typically young age of these patients, radiotherapy is often not recommended. In this context, there is an increasing need for new therapeutic strategies that enhance treatment efficacy while reducing side effects. Dinutuximab is a chimeric monoclonal antibody that targets the disialoganglioside GD2, a glycolipid highly expressed on the surface of several tumor types, including neuroblastoma, small cell lung cancer, and certain sarcomas. By binding to GD2, dinutuximab induces tumor cell lysis through antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Clinically, it has proven effective primarily in high-risk neuroblastoma, where it is used as maintenance therapy post-consolidation to improve event-free and overall survival. Emerging evidence supports its potential utility in other GD2-expressing malignancies, such as osteosarcoma and triple-negative breast cancer, although these indications are still under active investigation (18, 19). Dinutuximab is administered intravenously, typically as a continuous infusion over 4 consecutive days per treatment cycle, with a standard dose of 17.5 mg/m²/day. Dinutuximab beta, a similar formulation, can be given as a continuous infusion over up to 10 days, which has been associated with reduced pain toxicity compared to shorter infusions. Administration is usually through a central intravenous line to minimize extravasation risk and facilitate management of adverse events. Common side effects include neuropathic pain, fever, hypersensitivity reactions, capillary leak syndrome, and hypotension. Pain management with analgesics, such as opioids and gabapentin, is critical during treatment. Close monitoring for allergic reactions, including anaphylaxis, is mandatory during infusion (20). Recently, seven patients with recurrent extraocular RB treated with anti-GD2 mAb have been reported in the literature (15–17). (Table 1) Unlike our case, these patients initially presented with intraocular disease and later developed distant metastases following the failure of conservative treatment. All patients received HDC-ASCT. Patients 1 and 2 were treated with a reduced-intensity myeloablative regimen excluding Carboplatin, due to previous ototoxicity from platinum-based compounds, aiming to preserve hearing in light of their significant visual impairment. Patients 3, 4, 5, and 6 received radiotherapy to sites of bulky disease. Patient 4, developed an alveolar rhabdomyosarcoma of the maxillary bone five years later, located at the periphery of the previous radiation field. The expression of GD2 on tumor sample was assessed only in patients 3, 4, 5, and 6, while for the remaining cases this information was not reported. Patients 3 and 5 had active disease prior to anti-GD2 therapy, allowing for assessment of treatment response: patient 3 showed a complete response of a left mandibular lesion, while patient 5 had a partial response of a left orbital lesion. Our patient also received induction chemotherapy followed by HDC-ASCT, as well as CNS prophylaxis with intrathecal Topotecan. Anti-GD2 therapy was administered during remission after HDC with the intent to eliminate MRD and mitigate the risk of distant relapse, consistent with protocols for HR-NB. Notably, in our patient, the sites of bulky disease identified on imaging after induction chemotherapy were not irradiated, given the patient’s very young age (<3 years). The ability to avoid radiotherapy—along with its well-known long-term toxicities, particularly in very young children—is a significant advantage, highlighting the potential of intensive systemic therapy and targeted immunotherapy to achieve disease control while sparing patients from the harmful effects of radiation. None of the patients reported in the literature, including our case, experienced severe toxicity during anti-GD2 treatment. All adverse effects were effectively managed with appropriate supportive care. Six out of eight patients are alive and in complete remission. Patients 5 and 6 died due to disease relapse in the CNS, occurring 12 and 10 months after anti-GD2 therapy, respectively. This may be attributed to the limited ability of anti-GD2 antibodies to cross the blood-brain barrier, suggesting that CNS disease may require additional therapeutic strategies. These could include cellular immunotherapy employing anti-GD2 Chimeric Antigen Receptor (CAR) T cells (PMID: 37018492) (PMID: 33898999), and/or intrathecal delivery of radiolabeled antibodies, which have already been employed in other solid tumors with CNS metastases (21). All previously reported cases developed distant metastases following the failure of conservative treatments for intraocular tumors. In this context, integrating anti-GD2 mAb into the treatment arsenal for intraocular disease could be a promising strategy. This approach may not only enhance the effectiveness of conservative therapies and improve ocular outcomes, but also help control minimal disseminated disease (MDD) and reduce the risk of extraocular spread. The rationale for such integration is further strengthened by the well-established safety and tolerability profile.
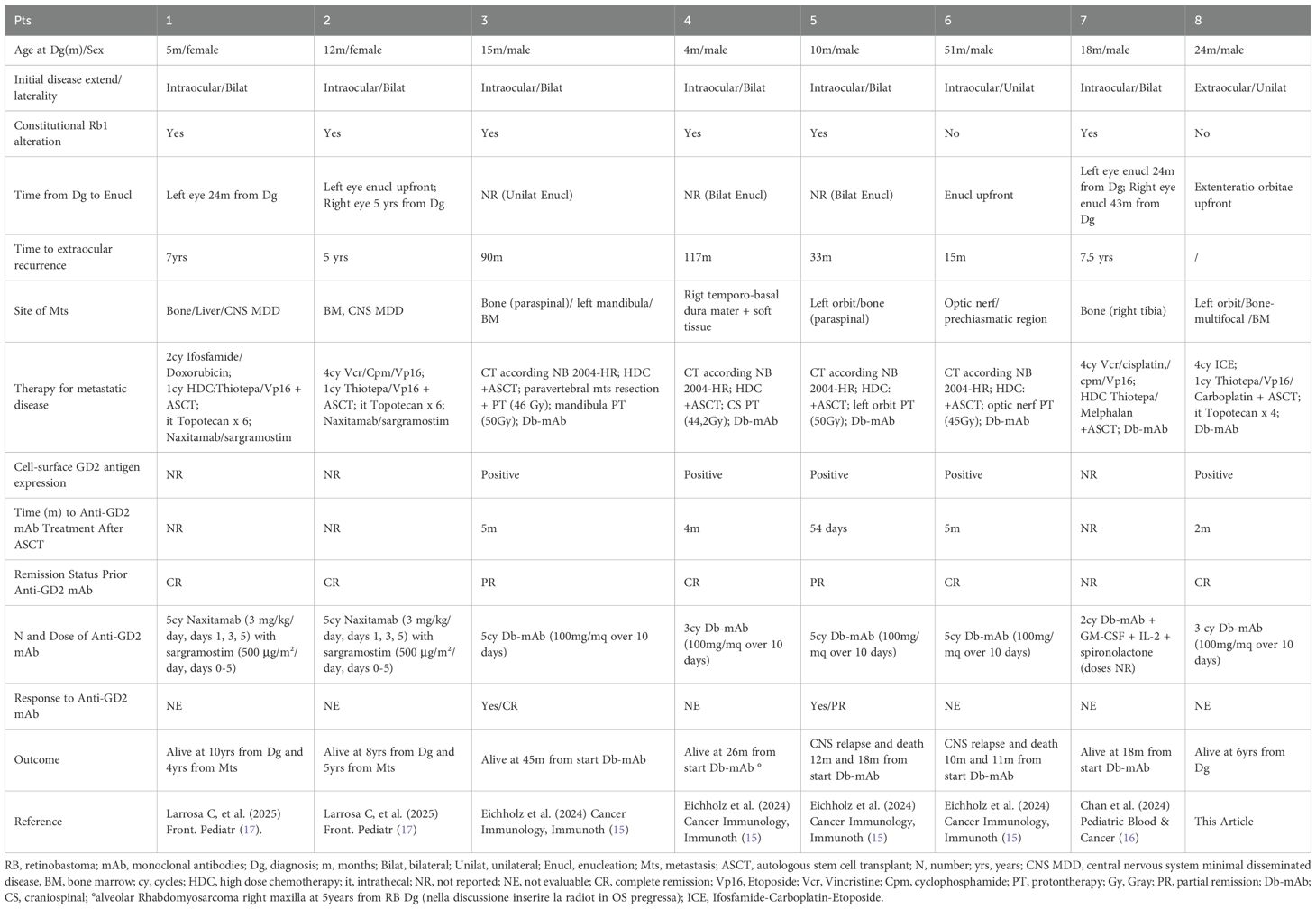
Table 1. The characteristics of patients reported in the literature, including our case affected by metastatic RB and treated with anti-GD2 mAb.
Conclusion
Our case is unique compared with those previously reported in the literature due to the extensive disease burden at diagnosis and the successful use of dinutuximab beta as consolidation therapy without the need for radiotherapy. The challenges we encountered included managing a very young patient with severe initial clinical conditions and balancing treatment intensity with long-term toxicity risks. In conclusion, anti-GD2 therapy appears to be a promising option for the control of both MDD and MRD outside the CNS, contributing to a reduced incidence of distant relapse while maintaining a favorable safety profile. This approach may allow for the avoidance of more toxic treatments in very young patients, particularly those with an underlying genetic predisposition. For optimal use of this therapeutic strategy, preliminary assessment of GD2 expression is advisable. Importantly, anti-GD2 mAb should also be considered as a potential component in the management of advanced and/or refractory intraocular disease, with the aim of improving ocular outcomes and minimizing the risk of extraocular dissemination. These encouraging observations strongly support the rationale for designing prospective multinational collaborative trials that incorporate anti-GD2 therapy into the multimodal treatment of retinoblastoma to validate these findings and standardize future therapeutic approaches.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. All data generated or analyzed during this study are included in this published article.
Ethics statement
The studies involving humans were approved by Ethics Committee Ospedale Pediatrico Bambino Gesù. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
IR: Data curation, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. VR: Data curation, Writing – original draft. AC: Data curation, Writing – original draft, Writing – review & editing. GB: Writing – review & editing. MP: Writing – review & editing. PV: Writing – review & editing. AG: Writing – review & editing. AS: Writing – review & editing. RV: Writing – review & editing. AM: Writing – review & editing. CQ: Writing – review & editing. BA: Writing – original draft, Writing – review & editing. GM: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dunkel IJ, Piao J, Chantada GL, Banerjee A, Abouelnaga S, Buchsbaum JC, et al. Intensive multimodality therapy for extraocular retinoblastoma: a children’s oncology group trial (ARET0321). J Clin Oncol. (2022) 40:3839–47. doi: 10.1200/JCO.21
2. Hu H, Zhang W, Wang Y, Huang D, Shi J, Li B, et al. Characterization, treatment and prognosis of retinoblastoma with central nervous system metastasis. BMC Ophthalmol. (2018) 18:107. doi: 10.1186/s12886-018-0772-8
3. Friedman DN, Sklar CA, Oeffinger KC, Kernan NA, Khakoo Y, Marr BP, et al. Long-term medical outcomes in survivors of extra-ocular retinoblastoma: the Memorial Sloan-Kettering cancer center (MSKCC) experience. Pediatr Blood Cancer. (2013) 60:694–9. doi: 10.1002/pbc.24280
4. Gombos DS, Hungerford J, Abramson DH, Kingston J, Chantada G, Dunkel IJ, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor? Ophthalmology. (2007) 114:1378–83. doi: 10.1016/j.ophtha.2007.03.074
5. MacCarthy A, Bayne AM, Brownbill PA, Bunch KJ, Diggens NL, Draper GJ, et al. Second and subsequent tumours among 1927 retinoblastoma patients diagnosed in Britain 1951–2004. Br J Cancer. (2013) 108:2455–63. doi: 10.1038/bjc.2013.228
6. Marees T, Moll AC, Imhof SM, De Boer MR, Ringens PJ, and Van Leeuwen FE. Risk of second Malignancies in survivors of retinoblastoma: more than 40 years of follow up. J Natl Cancer Inst. (2008) 100:1771–9. doi: 10.1093/jnci/djn394
7. Nazha B, Inal C, and Owonikoko TK. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front Oncol. (2020) 10:1000. doi: 10.3389/fonc.2020.01000
8. Fredman P, Hedberg K, and Brezicka T. Gangliosides as therapeutic targets for cancer. Biodrugs. (2003) 17:155–67. doi: 10.2165/00063030-200317030-00002
9. Cheung NKV, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocytemacrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. (2012) 30:3264–70. doi: 10.1200/JCO.2011.41.3807
10. Ladenstein R, Potschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. (2018) 19:1617–29. doi: 10.1016/S1470-2045(18)30578-3
11. Mody R, Yu AL, Naranjo A, Zhang FF, London WB, Shulkin BL, et al. Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the children’s oncology group. J Clin Oncol. (2020) 38:2160–9. doi: 10.1200/JCO.20.00203
12. Gray J, Moreno L, Weston R, Barone G, Rubio A, Makin G, et al. BEACONImmuno: Results of the dinutuximab beta (dB) randomization of the BEACONNeuroblastoma phase 2 trial-A European Innovative Therapies for Children with Cancer (ITCC-International Society of Paediatric Oncology Europe Neuroblastoma Group (SIOPEN) trial. (2022). doi: 10.1200/JCO.2022.40.16_suppl.10002
13. Modak S, Kushner BH, Mauguen A, Castaneda A, Varo A, Gorostegui M, et al. Naxitamab-based chemoimmunotherapy for resistant high-risk neuroblastoma: results of “HITS” phase II study. J Clin Oncol. (2022) 40:10028–8. doi: 10.1200/JCO.2022.40.16_suppl.10028
14. Nazha B, Inal C, and Owonikoko TK. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front Oncol. (2020) 10:1000. doi: 10.3389/fonc.2020.01000
15. Eichholz T, Heubach F, Arendt AM, Seitz C, Brecht IB, Ebinger M, et al. Targeted therapies in retinoblastoma: gD2-directed immunotherapy following autologous stem cell transplantation and evaluation of alternative target B7-H3. Cancer Immunol Immunother. (2024) 73:19. doi: 10.1007/s00262-023-03587-0
16. Chan WYK, Fu NW, Fu ECH, Liu APY, Yan CLS, Yau JPW, et al. Autologous hematopoietic stem cell transplantation followed by quadruple immunotherapy with dinutuximab beta, sargramostim, aldesleukin, and spironolactone for relapsed metastatic retinoblastoma. Pediatr Blood Cancer. (2024) 71:e31044. doi: 10.1002/pbc.31044
17. Larrosa C, Simao-Rafael M, Salvador N, Muñoz JP, Lavarino C, Chantada G, et al. Case Report: Successful treatment of metastatic retinoblastoma with CNS involvement with anti-GD2 immunotherapy, intrathecal topotecan and reduced systemic chemotherapy. Front Pediatr. (2025) 12:1509645. doi: 10.3389/fped.2024.1509645
18. Mohd AB, Mohd OB, Alabdallat YJ, Al Dwairy SY, Ghannam RA, Hanaqtah BM, et al. Safety and efficacy of dinutuximab in the treatment of neuroblastoma: A review. J Res Med Sci. (2023) :28:71. doi: 10.4103/jrms.jrms_727_22
19. Ly S, Anand V, El-Dana F, Nguyen K, Cai Y, Cai S, et al. Anti-GD2 antibody dinutuximab inhibits triple-negative breast tumor growth by targeting GD2+ breast cancer stem-like cells. J ImmunoTherapy Cancer. (2021) 9:e001197. doi: 10.1136/jitc-2020-001197
20. Balaguer J, García Hidalgo L, Hladun R, Márquez Vega C, and Pérez Alonso V. Recent evidence-based clinical guide for the use of dinutuximab beta in pediatric patients with neuroblastoma. Target Oncol. (2023) 18:77–93. doi: 10.1007/s11523-022-00930-w
Keywords: retinolastoma, extraocular, high-dose chemotherapy, anti-GD2 monoclonal antibodies, intrathecal topotecan
Citation: Di Ruscio V, Carboni A, Del Baldo G, De Pasquale MD, Valente P, Di Giannatale A, Serra A, De Vito R, Mastronuzzi A, Quintarelli C, De Angelis B, Milano GM and Russo I (2025) Prolonged overall survival in a child with multimetastatic retinoblastoma treated with anti-GD2 monoclonal antibody dinutuximab beta: a case report. Front. Oncol. 15:1665968. doi: 10.3389/fonc.2025.1665968
Received: 14 July 2025; Accepted: 30 September 2025;
Published: 17 October 2025.
Edited by:
Wei Wei, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Sumanth Nagabushan, Children’s Hospital at Westmead, AustraliaSafiye Aktas, Dokuz Eylül University, Türkiye
Copyright © 2025 Di Ruscio, Carboni, Del Baldo, De Pasquale, Valente, Di Giannatale, Serra, De Vito, Mastronuzzi, Quintarelli, De Angelis, Milano and Russo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ida Russo, aWRhLnJ1c3NvQG9wYmcubmV0
 Valentina Di Ruscio
Valentina Di Ruscio Alessia Carboni
Alessia Carboni Giada Del Baldo
Giada Del Baldo Maria Debora De Pasquale
Maria Debora De Pasquale Paola Valente
Paola Valente Angela Di Giannatale
Angela Di Giannatale Annalisa Serra1
Annalisa Serra1 Rita De Vito
Rita De Vito Angela Mastronuzzi
Angela Mastronuzzi Concetta Quintarelli
Concetta Quintarelli Biagio De Angelis
Biagio De Angelis Giuseppe Maria Milano
Giuseppe Maria Milano Ida Russo
Ida Russo