- 1Division of Plastic and Reconstructive Surgery, Department of Surgery, Yale School of Medicine, New Haven, CT, United States
- 2Department of Plastic and Reconstructive Surgery, University of Mississippi, Jackson, MS, United States
- 3Division of Plastic and Reconstructive Surgery, Department of Surgery, University of Rochester, Rochester, NY, United States
- 4Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States
- 5Department of Dermatology, Yale School of Medicine, New Haven, CT, United States
- 6Yale School of Medicine Department of Pharmacology, Database Management, Yale University School of Medicine, New Haven, CT, United States
- 7Division of Surgical Oncology, Department of Surgery, Yale University School of Medicine, New Haven, CT, United States
Introduction: The treatment of positive margins following primary resection of cutaneous melanoma remains controversial with the current standard of care being repeat surgical resection. 5% topical imiquimod therapy has been proposed as an alternative treatment for positive in-situ surgical margins. This study revisits the use of 5% topical imiquimod therapy as a method of clearing positive surgical margins following primary excision of cutaneous melanoma.
Methods: A retrospective chart review of all Yale Melanoma Registry Database patients with positive melanoma in situ margins after excision of primary melanoma between January 2008 and December 2021 was conducted. Patients were included if they received 5% topical imiquimod therapy for treatment of positive melanoma in situ surgical margins. Demographics, treatment duration, treatment response, complications, follow-up time, and associated costs were recorded.
Results: A total of 69 patients with positive margins post wide local excisions were treated with topical imiquimod therapy. 38 of these patients had histological diagnosis of melanoma at primary excision while the remainder, 31, had MIS. Five patients were excluded due to follow-up with an outside dermatologist, leaving 64 patients in the final cohort. Fifty-two percent of patients were female with a median age of 73 years old in the entire cohort of patients. Treatment duration with imiquimod ranged from four to twelve weeks of therapy with a median duration of 12 weeks. Clinical response rate after final biopsies was 84% over an average follow-up duration of 36 months. Six patients (8.7%) had recurrences in follow-up after negative scouting biopsies following treatment with an average follow-up duration of 1086 days or approximately 36 months.
Conclusion: The use of 5% topical imiquimod therapy is a safe, cost-effective, and reasonable alternative approach in the management of positive surgical margins. The degree of inflammation around the site of disease may be used as a reliable predictor of outcome.
Introduction
The incidence rates of malignant melanoma have been increasing over the past decade (1–4). Last year, it was estimated that over 200,000 melanomas were diagnosed in the United States (5). The current standard of care remains to be wide local excision with margins dependent on the depth or invasiveness of the melanoma, ranging from 5mm for in-situ to 2cm for greater than 1.0mm invasion (6). The goal of taking margins of normal appearing tissue is to ensure histopathologic disease control by securing negative, disease-free margins.
Despite taking adequate tissue margins, securing histologic margins that are disease-free continues to be a challenge that practitioners must face. The rates of positive margins have been reported in the literature to be as high as 15-25% (2, 7–11). Risks of positive margins on final surgical pathology of melanoma increase in cosmetically sensitive areas, anatomic regions such as the head/neck, MIS histological subtype, presence of regression, and increasing age of the patients (10, 12).
Management of positive margins remains a subject of debate. In some instances, re-excision of residual melanoma may suffice; however, this subjects the patient to an additional operation and potential for further disfigurement. Topical immunotherapy with imiquimod has become increasingly popular as a treatment adjuvant for melanoma in situ. We previously reported our successful experience with imiquimod for the topical treatment of positive histologic margins after definitive surgical resection of melanoma in a 22 patient cohort (13). The National Comprehensive Cancer Network’s Clinical Practice Guidelines for melanoma now include the recommendation that imiquimod be considered as a treatment option for selected patients with positive MIS margins after optimal surgery (14). Imiquimod is an immunomodulator that works by inducing a TH1 immune response, thereby leading to infiltration of cytotoxic T-cells that result in apoptosis of melanoma cells (15).
Few studies have expanded on the management of melanoma positive margins using topical immunotherapy. Ellis et al.’s review of the literature described a plethora of encouraging data through case reports, case series, and open-label studies; however, they highlighted the need for more comprehensive studies to develop reliable and reproducible treatment regimens (16). In this present study, our objective was to provide additional detailed assessment of results, complications, and patient outcomes of medical management of positive margins with 5% topical imiquimod therapy in a larger group of patients with in-situ and invasive melanoma.
Methods
After receiving institutional review board approval, a retrospective chart review of all Yale Melanoma Registry Database patients with positive margins after excision of melanoma in situ or primary melanoma between January 2008 and December 2021 was conducted. Subjects were identified based on Current Procedural Terminology codes and included if they had ever had a melanoma excision with subsequent positive margins. Patients with acral lentiginous melanoma were excluded as we do not offer Imiquimod routinely to these patients. Patients were found to have varying thicknesses of melanoma through histopathologic analysis of surgical specimens. Patients with residual disease at margins after definitive surgical resection were offered a course of topical application of 5% Imiquimod or re-excision. Those who opted for treatment with Imiquimod were included in our investigation. Patients were excluded if their charts were incomplete or were lost to follow-up.
Patients began imiquimod therapy, typically starting at a frequency of five applications per week. The patients were monitored closely with regular follow-up, typically at four-week intervals. During each visit, the treatment region was gradually tailored based upon the patient’s clinical response (labeled as no response, mild, moderate, or exuberant response). A goal of 12 weeks of therapy was made. Posttreatment histological scouting biopsies were performed at 6–8 weeks following the last application of topical 5% imiquimod cream.
Results
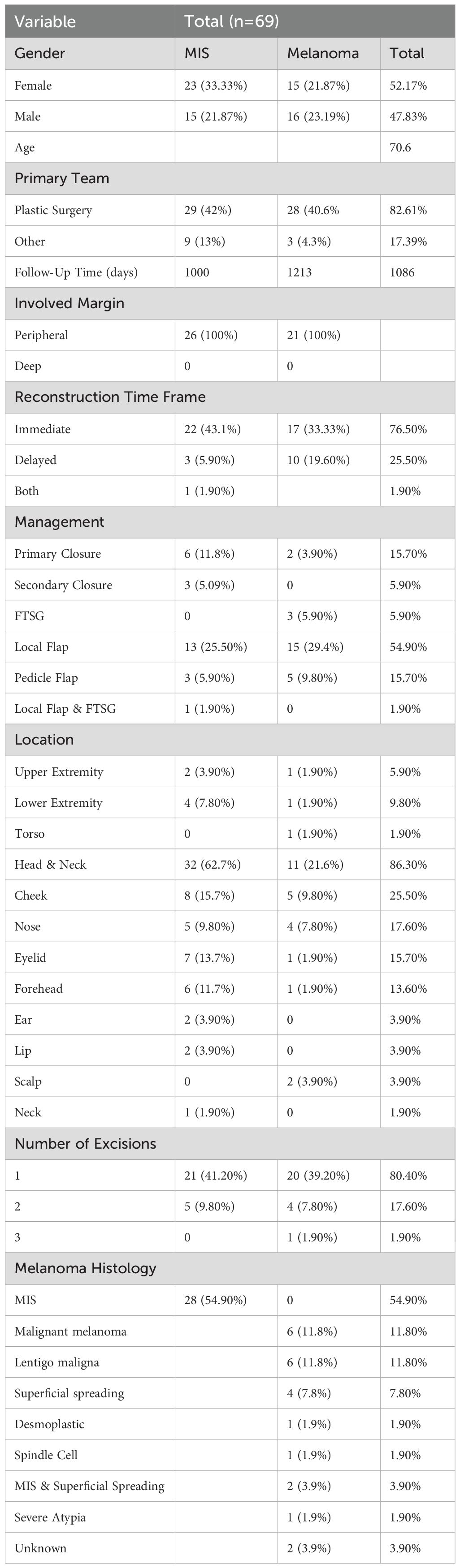
A total of 69 patients met initial inclusion criteria. Of these patients, five were lost to follow-up, leaving 64 patients in our final cohort. Patient demographics and details of primary lesions are described in Table 1. Thirty-three patients in the cohort were male and 36 were female. There was a median age of 73 years old at the initial excision of melanoma. At primary excision, 38 patients had the histological diagnosis of melanoma in situ (MIS) while the remainder (31) had invasive melanoma. Average follow-up time was 1086 days or 36 months. All 69 patients had MIS at the margin, and all affected margins were peripheral in location. No patients had invasive melanoma at the margin.
When examining cases of melanoma by the anatomical location (Table 1), 58 patients had a lesion on their head/neck, six patients had a lesion on their lower extremity, three patients had a lesion on their upper extremity, and two patients had a lesion on their torso. Thirty-seven patients had one excision performed prior to imiquimod treatment, nine patients had two excisions performed, one patient had three excisions performed, and 22 patients had incomplete surgical records to accurately record the number of initial excisions performed.
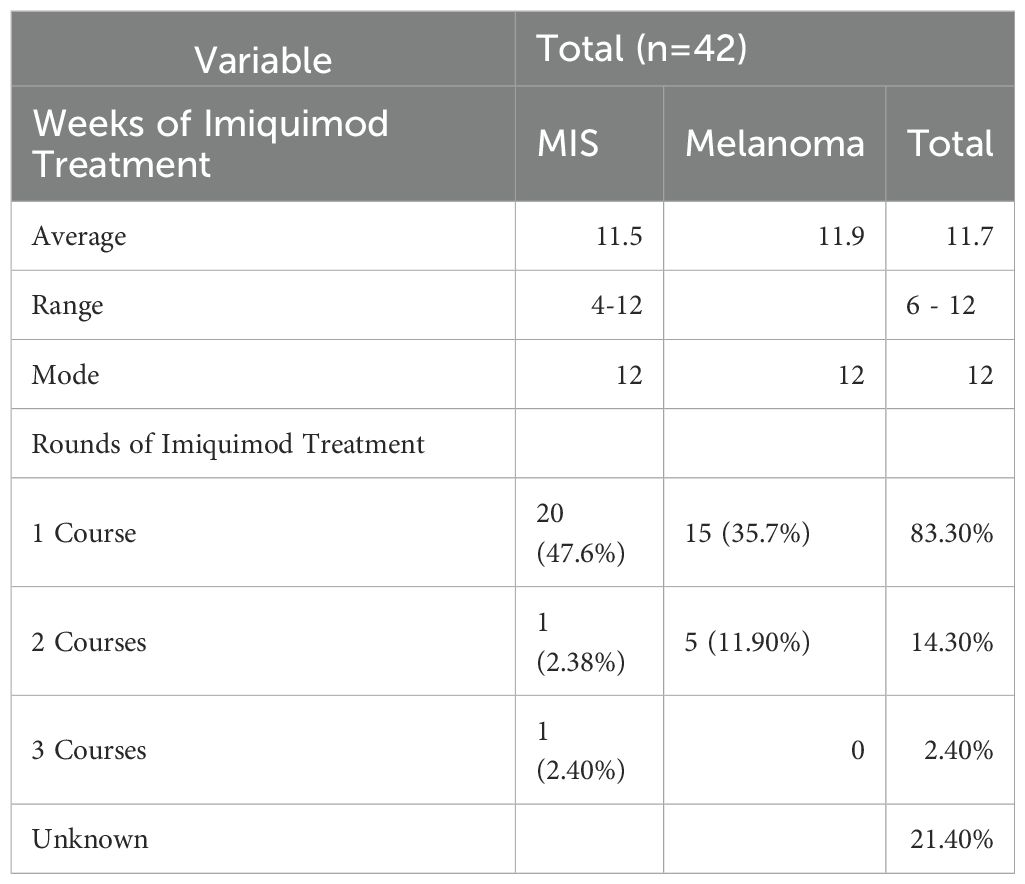
The treatment duration ranged from four to twelve weeks of therapy with a median duration of 12 weeks (Tables 2, 3). Fifty-five patients had one course of therapy, eight patients had two courses of therapy, and one patient had three courses of therapy to achieve clear surgical margins. Another round of therapy was started if margins were still positive on scouting biopsies.
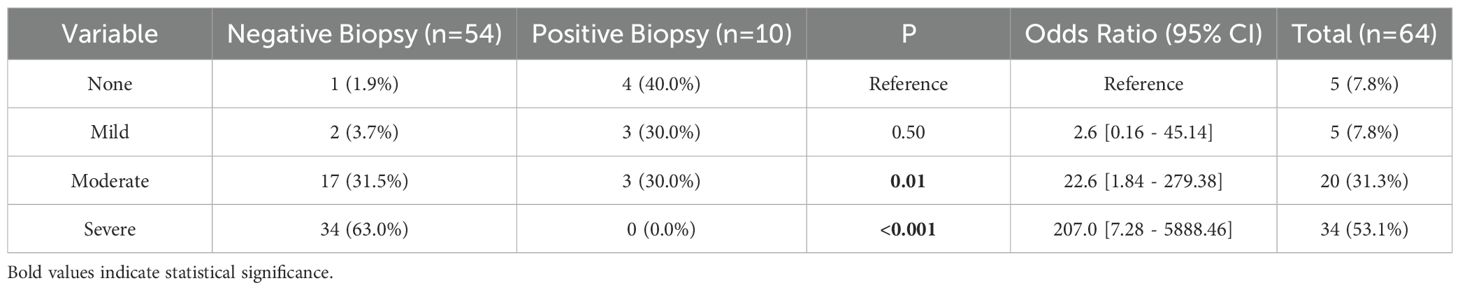
Table 3. Comparison of the severity of cutaneous reaction to imiquimod treatment versus post-treatment biopsy results.
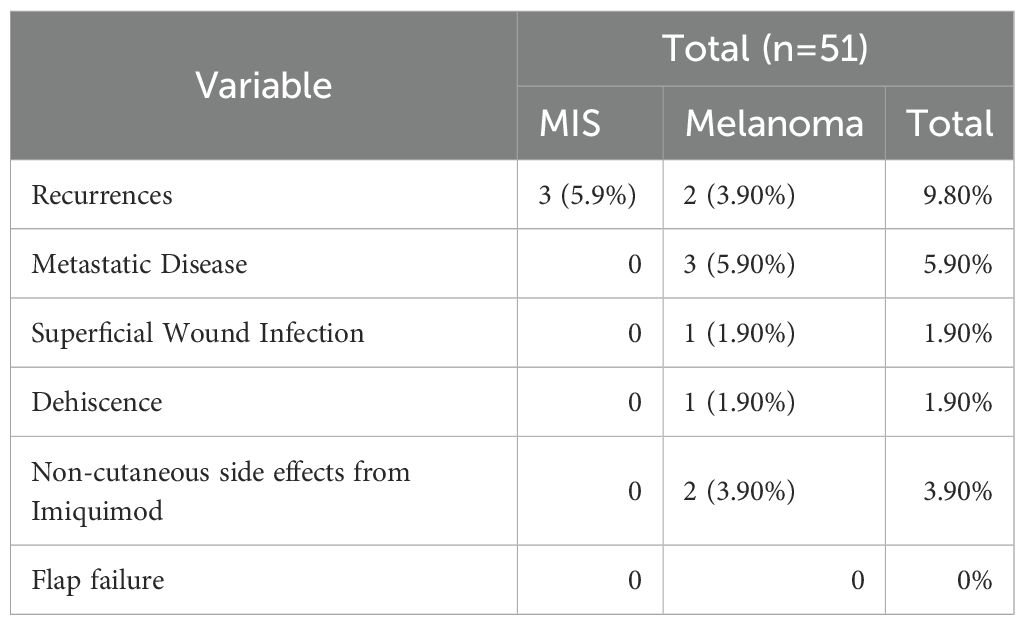
The response to imiquimod treatment was assessed by comparing the severity of the cutaneous reaction to imiquimod to the post-treatment biopsy results. On initial scouting biopsies after completion of the course of topical therapy, 54 patients had no evidence of melanoma on histopathologic examination (Table 4). Of these patients with negative initial scouting biopsies the degree of reactive inflammation to imiquimod varied. One patient had no reaction, two patients had a mild reaction, 11 patients had a moderate reaction, and 21 had a severe reaction. The remaining patients did not have their reaction recorded in the chart. The remaining 10 patients had positive margins on post-treatment biopsy results. Of these patients, three had no reaction to therapy, three had a mild reaction, and three had a moderate reaction. One patient did not have their reaction recorded. After additional rounds of therapy, scouting biopsies were negative and none of these patients experienced a recurrence. Minor complications were minimal in our cohort (Table 4). three patients had non-cutaneous side effects from Imiquimod, which included fatigue, flu-like symptoms, and acute depression. All patients’ side effects eventually resolved. Six patients (8.7%) in the cohort had recurrences after negative margins were achieved through treatment and three patients had metastatic disease. Of these patients with recurrences, two occurred in four years after original excision, one occurred in three years, and the remainder occurred one years following original wide local excision. Two of these patients had MIS on original histopathologic diagnosis while the remainder had melanoma primarily of the lentigo maligna subtype. Four patients had a moderate response to Imiquimod therapy, one had a severe, one mild, and one patient’s response was not documented.
Discussion
This study revisited and evaluated the use of 5% topical imiquimod therapy for treatment of positive melanoma margins following primary surgical excision. We hypothesized that a treatment duration of 12 weeks of imiquimod therapy, tailored based on clinical response and patient’s tolerance to treatment, would be sufficient to clear positive margins. We were successfully able to achieve an 84% initial clinical response rate in our cohort of 64 patients. Achieving negative margins can be challenging with narrower excisions at anatomical regions such as the head/neck (10). It is likely that smaller than necessary margins are taken near cosmetically sensitive areas such as the lip, eyelid, and face leading to an increase in persistently positive margins in these areas. Our data further supports the clinical use of topical imiquimod therapy as a nonsurgical alternative for the treatment of positive margins after primary melanoma excision. To our knowledge, this is the largest cohort of patients treated with imiquimod for management of positive margins in the literature to date.
At our institution, patients with primary melanoma who have positive margins for melanoma in situ are offered two treatment options: Imiquimod therapy or surgical excision. We ensure that patients are thoroughly informed about Imiquimod treatment, including its 12-week duration and the reliance on scouting biopsies to confirm treatment success. Following the completion of therapy, we perform six circumferential biopsies 6–8 weeks after completion of Imiquimod treatment. By this time, all the inflammation has resolved ensuring more accurate histopathologic evaluation of biopsy specimens.
Patients are counseled that, unlike surgical excision where all margins are examined, biopsies only allow us to confirm clearance within the sampled areas. It remains possible for malignant cells to persist between biopsy sites, which could lead to recurrence. For this reason, most patients opt for re-excision after the first experience with a positive margin, valuing its more definitive nature. However, if margins remain positive after a second excision, we again offer Imiquimod as a treatment option. At this stage, more patients tend to choose Imiquimod to evaluate its therapeutic effectiveness.
The use of imiquimod for treatment of MIS can be traced back to 2000 (16). Yet, there is still no standardized treatment protocol for frequency of applications and duration of therapy. Steinmann et al. reported a use of three applications/weekly for a total duration of 12 weeks to successfully clear cutaneous melanoma metastasis at the breast (17). Naylor et al.’s open label trial of imiquimod for treatment of 30 patients with lentigo maligna demonstrated a 93% clinical response rate following three months of daily therapy (18).
As mentioned by previous studies, the degree of clinical response may be used as a reliable predictor of outcome and therefore guide treatment duration (13, 19, 20). Ly et al.’s trial showed that there was a statistically significant association between visible association and histopathologic clearance (19). In our present series of 64 patients, five patients had no visible inflammatory response to imiquimod and four had mild inflammatory responses. Four out of the five patients with no visible inflammatory response had surveillance biopsies showing persistently positive surgical margins despite treatment for 12 weeks, and 3 out of the five patients with mild inflammatory responses had surveillance biopsies showing persistently positive surgical margins. Our data further supports the observation that more exuberant inflammatory reactions signal better clinical outcomes.
Topical treatment regimens for melanoma vary greatly across the literature, and there are numerous studies demonstrating the use of imiquimod with other surgical and nonsurgical therapies. These include imiquimod and 5-fluorouracil as dual therapy, imiquimod and tazarotene, and a combination of imiquimod, 5-Fluorouracil, and Tretinoin (21–23). Since 5-FU is an antimetabolite able to induce apoptosis of tumor cells and its effect are enhanced in the presence of some cytokines, INF-α, INF-γ and IL-12, this suggests a synergistic effect when used in combination with imiquimod (23). The use of a keratolytic agent like tazarotene is thought to increase the efficacy of imiquimod by enhancing drug penetration through the skin barrier (24). Treatment with a keratolytic agent can be administered concurrently with imiquimod or weeks prior to the start of therapy. And finally, a retrospective study of patients with cutaneous melanoma metastases demonstrated the use of intralesional IL-2 with topical imiquimod and retinoid cream as a promising therapeutic regimen (25).
Commonly reported side effects of topical imiquimod therapy include local erythema, scaling, edema, burning pain, and tenderness. These mild reactions typically dissipate after discontinuation of treatment. Systemic side effects are rare from topical application but have been reported in the literature. A recent case report described a patient who experienced debilitating fatigue after applying imiquimod to a single cutaneous lesion (26). One patient in our cohort complained of fatigue and diarrhea after the initiation of treatment. Her diarrhea resolved in two weeks; however, the fatigue persisted until the course of imiquimod was complete. Another patient in our cohort developed severe acute depression that did not resolve until cessation of treatment. The only other complication in our series was a patient who experienced flu-like symptoms after two weeks of therapy. The patient self-discontinued imiquimod and scouting biopsies were negative at a follow-up visit.
This study has several limitations that are common to all retrospective reviews. One significant limitation is the relatively small sample size of 64 patients, which limits the precision of risk estimates. Nevertheless, this study represents the largest cohort reported to date in the published literature. Another such limitation would be the interobserver variation in the histological diagnoses and evaluation for positive margins on surgical specimens, although all pathology was examined at the same institution. Secondly, this study had a median follow-up of around 36 months, and thus clinical recurrence could have been missed. However, we have previously reported that 81 percent of all melanoma recurrences were diagnosed by 36 months after the primary melanoma (27). In the MIS literature, a similar median time to recurrence of 36 months has been reported (28). A third limitation was the possibility of variation in physician’s grading of inflammatory response as none, mild, moderate, or severe as this was graded subjectively without inter-rater validation. Furthermore, given the retrospective nature of this review, there were not photographs available for all patients thus we could not standardize the grading of the inflammatory response.
Obtaining multiple physicians’ clinical grades or collecting photographs would have helped decrease variability and improve reproducibility of this study. While post-treatment scouting biopsies can be useful, they are not as reliable as re-excision of the entire surgical margin followed by comprehensive histologic evaluation. Scouting biopsies carry a risk of false negatives due to sampling error. To mitigate this, we obtain multiple histologic samples from the area surrounding the residual scar. Nevertheless, it’s important to recognize the limitations of this approach. As a result, all patients in our cohort are closely monitored for any signs of recurrence, with additional scouting biopsies performed when there is clinical concern.
Conclusion
Imiquimod is a safe, cost-effective treatment of positive margins after initial resection of cutaneous melanoma. Patients with positive margins demonstrating MIS should consider Imiquimod as an adjunct or in some cases and alternative to further resection. Patients with more exuberant reactions demonstrate better clinical response to therapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Yale Institutional review board melanoma. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
KL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. SI: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. DA: Writing – original draft, Writing – review & editing. MC: Writing – original draft, Writing – review & editing. JCh: Writing – review & editing. JL: Writing – review & editing. SA: Writing – review & editing, Writing – original draft. RB: Writing – review & editing. KO: Writing – review & editing. JCl: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Quimby AE, Khalil D, and Johnson-Obaseki S. Immediate versus delayed reconstruction of head and neck cutaneous melanoma. Laryngoscope. (2018) 128:2566–72. doi: 10.1002/lary.27250
2. Koolen PGL, Matos TR, Ibrahim AMS, Sun J, Lee BT, Frankenthaler RA, Lin SJ, et al. Recurrence rates over 20 years in the treatment of Malignant melanoma: immediate versus delayed reconstruction. Plast Reconstr Surg Glob Open. (2017) 5:e1378. doi: 10.1097/gox.0000000000001378
3. Wernick BD, Goel N, Zih FS, and Farma JM. A surgical perspective report on melanoma management. Melanoma Manage. (2017) 4:105–12. doi: 10.2217/mmt-2016-0031
4. Karanetz I, Stanley S, Knobel D, Smith BD, Bastidas N, Beg M, Kasabian AK, Tanna N, et al. Melanoma extirpation with immediate reconstruction: the oncologic safety and cost savings of single-stage treatment. Plast Reconstr Surg. (2016) 138:256–61. doi: 10.1097/prs.0000000000002241
5. Siegel RL, Miller KD, Fuchs HE, and Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
6. Swetter SM, Thompson JA, Albertini MR, Barker CA, Baumgartner J, Boland G, et al. NCCN guidelines® Insights: melanoma: cutaneous, version 2.2021. J Natl Compr Canc Netw. (2021) 19:364–76. doi: 10.6004/jnccn.2021.0018
7. Glazer ES, Porubsky CF, Francis JD, Ibanez J, Castner N, Messina JL, et al. Treatment of head and neck melanoma in situ with staged contoured marginal excisions. Ann Plast Surg. (2017) 78:663–7. doi: 10.1097/sap.0000000000000949
8. Hu AC, Lee SA, Clark EG, Yamamoto M, Jakowatz JG, and Evans GRD. Impact of immediate surgical reconstruction following wide local excision of Malignant head and neck melanoma. Plast Reconstr Surg Glob Open. (2020) 8:e2661. doi: 10.1097/gox.0000000000002661
9. Bolshinsky V, Lin MJ, Serpell J, Leung M, Wolfe R, McLean C, et al. Frequency of residual melanoma in wide local excision (WLE) specimens after complete excisional biopsy. J Am Acad Dermatol. (2016) 74:102–7. doi: 10.1016/j.jaad.2015.08.065
10. Namin AW, Welby L, Baker AT, and Dooley LM. Positive margins in cutaneous melanoma of the head and neck: implications for timing of reconstruction. Otolaryngol Head Neck Surg. (2021) 164:1052–7. doi: 10.1177/0194599820969178
11. Jackett LA, Satgunaseelan L, Roper E, Lo SN, Thompson JF, and Scolyer RA. Residual melanoma in wide local excision specimens after ‘complete’ excision of primary cutaneous in situ and invasive melanomas. Pathology. (2022) 54:71–8. doi: 10.1016/j.pathol.2021.05.094
12. Paulson KG, Gupta D, Kim TS, Veatch JR, Byrd DR, Bhatia S, et al. Age-specific incidence of melanoma in the United States. JAMA Dermatol. (2020) 156:57–64. doi: 10.1001/jamadermatol.2019.3353
13. Pandit AS, Geiger EJ, Ariyan S, Narayan D, and Choi JN. Using topical imiquimod for the management of positive in situ margins after melanoma resection. Cancer Med. (2015) 4:507–12. doi: 10.1002/cam4.402
14. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: melanoma. v3.2022. Available online at: https://www.nccn.org/ (Accessed October 1, 2025).
15. Imbertson LM, Beaurline JM, Couture AM, Gibson SJ, Smith RM, Miller RL, et al. Cytokine induction in hairless mouse and rat skin after topical application of the immune response modifiers imiquimod and S-28463. J Invest Dermatol. (1998) 110:734–9. doi: 10.1046/j.1523-1747.1998.00174.x
16. Ellis LZ, Cohen JL, High W, and Stewart L. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. (2012) 38:937–46. doi: 10.1111/j.1524-4725.2012.02362.x
17. Steinmann A, Funk JO, Schuler G, and von den Driesch P. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. (2000) 43:555–6. doi: 10.1067/mjd.2000.107954
18. Naylor MF, Crowson N, Kuwahara R, Teague K, Garcia C, Mackinnis C, et al. Treatment of lentigo Maligna with topical imiquimod. Br J Dermatol. (2003) 149 Suppl 66:66–70. doi: 10.1046/j.0366-077x.2003.05637.x
19. Ly L, Kelly JW, O'Keefe R, Sutton T, Dowling JP, Swain S, et al. Efficacy of imiquimod cream, 5%, for lentigo Maligna after complete excision: a study of 43 patients. Arch Dermatol. (2011) 147:1191–5. doi: 10.1001/archdermatol.2011.260
20. Borucki U and Metze D. Topical treatment of lentigo Maligna melanoma with imiquimod 5% cream. Dermatology. (2003) 207:326–8. doi: 10.1159/000073101
21. Florin V, Desmedt E, Vercambre-Darras S, and Mortier L. Topical treatment of cutaneous metastases of Malignant melanoma using combined imiquimod and 5-fluorouracil. Invest New Drugs. (2012) 30:1641–5. doi: 10.1007/s10637-011-9717-2
22. Menzies S, Mc Menamin M, and Barry R. Lentigo Maligna successfully treated with combination therapy of topical tazarotene and imiquimod. Clin Exp Dermatol. (2017) 42:468–70. doi: 10.1111/ced.13053
23. Nahm WJ, Gwillim EC, Badiavas EV, Nichols AJ, Kirsner RS, Boggeln LH, et al. Treating melanoma in situ during a pandemic with telemedicine and a combination of imiquimod, 5-fluorouracil, and tretinoin. Dermatol Ther (Heidelb). (2021) 11:307–14. doi: 10.1007/s13555-020-00473-w
24. Hyde MA, Hadley ML, Tristani-Firouzi P, Goldgar D, and Bowen GM. A randomized trial of the off-label use of imiquimod, 5%, cream with vs without tazarotene, 0.1%, gel for the treatment of lentigo Maligna, followed by conservative staged excisions. Arch Dermatol. (2012) 148:592–6. doi: 10.1001/archdermatol.2012.270
25. Shi VY, Tran K, Patel F, Leventhal J, Konia T, Fung MA, et al. 100% Complete response rate in patients with cutaneous metastatic melanoma treated with intralesional interleukin (IL)-2, imiquimod, and topical retinoid combination therapy: results of a case series. J Am Acad Dermatol. (2015) 73:645–54. doi: 10.1016/j.jaad.2015.06.060
26. Raman J, Bisbee E, Missall TA, and Saikaly SK. A case of topical imiquimod induced fatigue. J Dermatolog Treat. (2022), 1–3. doi: 10.1080/09546634.2022.2125266
27. Fusi S, Ariyan S, and Sternlicht A. Data on first recurrence after treatment for Malignant melanoma in a large patient population. Plast Reconstr Surg. (1993) 91:94–8. doi: 10.1097/00006534-199301000-00014
Keywords: melanoma “in situ”, melanoma, imiquimod, margins, skin cancer
Citation: Lewis K, Islam S, Alper DP, Carney MJ, Choi J, Leventhal J, Ariyan S, Baumann R, Olino K and Clune J (2025) Use of imiquimod topical therapy for management of malignant melanoma positive margins. Front. Oncol. 15:1666463. doi: 10.3389/fonc.2025.1666463
Received: 15 July 2025; Accepted: 15 October 2025;
Published: 29 October 2025.
Edited by:
Xunwei Wu, Hangzhou Medical College, ChinaReviewed by:
Takaya Komori, Saitama Medical University International Medical Center, JapanGabrielle Williams, Melanoma Institute Australia, Australia
Copyright © 2025 Lewis, Islam, Alper, Carney, Choi, Leventhal, Ariyan, Baumann, Olino and Clune. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James Clune, amFtZXMuY2x1bmVAeWFsZS5lZHU=
 Katelyn Lewis
Katelyn Lewis Sara Islam2
Sara Islam2 Jonathan Leventhal
Jonathan Leventhal Stephan Ariyan
Stephan Ariyan Kelly Olino
Kelly Olino