- 1Thayer School of Engineering at Dartmouth College, Hanover, NH, United States
- 2Department of Surgery, Geisel School of Medicine at Dartmouth College, Lebanon, NH, United States
Introduction: Radiation therapy is a mainstay of treatment for numerous gastrointestinal (GI) malignancies, where our ability to deliver dose to tumors is limited by acute GI toxicity. Ultra-high dose-rate (UHDR) ‘FLASH’ irradiation can spare normal tissue, yet its dependence on physiological variables remains incompletely defined.
Methods: We compared FLASH and conventional dose-rate (CDR) 9 MeV electron total abdominal irradiation (TAI) in C57BL/6 mice anesthetized with either intraperitoneal ketamine/xylazine or inhaled isoflurane in room air, deliberately omitting supplemental oxygen. Single doses of 14 or 16 Gy were delivered, and normal-tissue injury was quantified by time-to-25% body-weight loss.
Results: At 14 Gy, UHDR under K/X produced a marked survival advantage: by day 14, 80% of animals had not reached the weight-loss endpoint versus 40% after CDR K/X; no FLASH benefit was discernible with ISO anesthesia. Raising the dose to 16 Gy accentuated these trends; 40% of UHDR K/X mice were still below the endpoint at study termination, whereas all CDR K/X mice met it by day 7. Again, ISO abolished sparing at both dose rates. To probe mechanism, intraperitoneal oxygen tension was measured with an optical reporter in six mice. ISO anesthesia yielded significantly higher pO2 (62 ± 4 mmHg) than K/X (26 ± 10mmHg), a 2.5-fold difference.
Discussion: These findings identify anesthetic-dependent oxygenation as a reproducible confounder in pre-clinical FLASH studies: elevated pO2 under ISO negates abdominal sparing, whereas K/X preserves it across two clinically relevant doses. Rigorous control and reporting of factors that alter tissue oxygenation are therefore essential when designing experiments and, ultimately, translating FLASH radiotherapy.
Introduction
Radiation therapy (RT) is employed in roughly one-half of all cancer cases and contributes to about 40% of curative treatments worldwide (1). The recent arrival of ultra-high-dose-rate (UHDR), RT has reinvigorated translational efforts to increase the therapeutic ratio by preferentially sparing normal tissue while maintaining tumor control (“FLASH” sparing) (2–4). Elegant proof-of-principle experiments in skin, lung and brain have demonstrated dramatic reductions in toxicity when doses are delivered in micro-second pulses at ≥ 30 Gy/s compared with conventional dose rates (CDR) (5–9). Yet extension of these findings to the abdomen—a site where acute gastrointestinal (GI) syndrome remains a foremost dose-limiting toxicity—has been inconsistent. Whole-abdomen electron FLASH protects crypt stem cells and improves survival in some reports, whereas pencil-beam-scanned proton FLASH and synchrotron proton beams have produced neutral or even worse outcomes relative to CDR irradiation (10, 11). A potential explanation is that the FLASH effect is particularly sensitive to biological context and experimental nuance that is not being controlled for across studies.
A unifying hypothesis for FLASH sparing invokes transient oxygen depletion: extremely intense pulses have been postulated to consume dissolved O2 faster than it can be replenished, driving tissue into a radioprotective hypoxic state for the brief period in which radical-mediated damage is fixed (12–15). Bulk tissue measurements have refuted this idea through systematic study of skin, and tumor, however still debate exists on whether there could be regional or micro-localized hypoxia induced during the irradiation in FLASH. The data also seems to be dependent upon the total dose as well as the tissue oxygen, and so there seems to be both a minimum and a maximum dose threshold to see the FLASH effect (16, 17). It is therefore likely that oxygen may be a factor in this complex range where FLASH tissue sparing can be seen. If it is true that oxygenation level affects the observation, then any variable that alters baseline or dynamic tissue oxygenation could modulate—or even mask—the FLASH effect. Two variables stand out in small-animal work: the choice of anesthetic and the composition of the carrier or supplemental gas.
Intraperitoneal ketamine/xylazine (K/X) and inhaled isoflurane dominate murine RT studies because they are facile and inexpensive. Unfortunately, they create very different physiological milieus. K/X produces profound bradycardia, respiratory depression, and peripheral vasoconstriction, leading to arterial hypoxemia and tissue pO2 values as low as 15 mmHg in skin and brain. Isoflurane, in contrast, raises heart rate, increases tidal volume, and vasodilates vessels; when delivered in room air it maintains skin pO2 near 25–30 mmHg, and when delivered in 100% oxygen it can drive tissue pO2 above 50 mmHg. Carrier-gas oxygenation matters independently of the volatile agent: supplemental oxygenation during irradiation sharply increases murine skin PO2 values and negates the FLASH effect (18–20).
Despite these well-documented effects, anesthetic protocols are rarely reported in pre-clinical FLASH papers, and when they are, oxygen supplementation is common. In a recent systematic review of electron FLASH in vivo studies, fewer than one-third specified the fraction of inspired oxygen, and none quantified tissue pO2. Recent results showed that this omission is not benign: in murine hind-leg skin, breathing 100% oxygen during isoflurane anesthesia abolished FLASH sparing, whereas room-air anesthesia preserved it; moreover, female mice—whose dermis exhibited higher pO2 than males—ulcerated sooner after UHDR irradiation (18, 21). These data strengthened the link between oxygen tension and FLASH, but they also raised a critical question for abdominal irradiation: does the anesthetic itself, independent of inspired O2, dictate whether gut tissues experience protection or injury at UHDR?
The small intestine is among the most radiosensitive organs, and weight-loss kinetics as well as crypt-regeneration assays are gold-standard read-outs for acute GI toxicity. Several groups have attempted to exploit FLASH in this setting, yet outcomes have ranged from substantial sparing to overt harm. None of these studies compared different anesthetic regimens head-to-head or measured intraperitoneal oxygenation. Given that intestinal perfusion is highly responsive to vasoactive and respiratory changes, and that K/X and isoflurane exert opposite effects on both, anesthetic selection may be a hidden confounder driving inter-study variability.
Here we directly address this gap by evaluating FLASH sparing in the mouse total abdominal irradiation model, anesthetized with either KX or isoflurane in room air. To mechanistically link any observed differences to oxygen availability, a parallel cohort received intravenous PdG4 Oxyphor and real-time phosphorescence lifetime imaging (OxyLED) was used to record intra-abdominal pO2 immediately after laparotomy (22).
Methods
Animals and irradiation
All animal experiments were approved by the Dartmouth College Institutional Animal Care and Use Committee (IACUC). Eighty (N=80) male C57BL/6 mice were ordered from Jackson Laboratories and allowed to acclimate to the vivarium for at least 2 weeks. Following the acclimation period, mice were weighed for 3 days to establish baseline weight. On the day of irradiation, mice were anesthetized using either an IP (intra-peritoneal) injection of Ketamine/Xylazine (ketamine 100 mg/kg, xylazine 10 mg/kg; n=10) or inhaled isoflurane anesthesia delivered in room air (SomnoFlo by Kent Scientific, USA) through a non-rebreather mask (3% induction for 3 minutes, then maintained on 1.5%; n=10). A Mobetron linear accelerator (LINAC; IntraOp, Inc, USA) was used to deliver 14 or 16 Gy of 9 MeV electron CDR and UHDR radiation to a 3 cm x 4 cm area centered on the mouse abdomen with a 1 cm air gap. CDR irradiation was delivered at a dose rate of 0.1 Gy/s, while UHDR was delivered at an average dose rate of 420 Gy/s. For the 14 Gy UHDR treatment, the source-to-surface distance (SSD) was set to 37 cm, and each pulse had a width of 3.10 µs delivered at a pulse repetition frequency (PRF) of 90 Hz. A total of four pulses were administered, with each pulse depositing 3.5 Gy, resulting in the prescribed 14 Gy dose. For the 16 Gy UHDR treatment, the SSD was set to 37 cm, and each pulse had a width of 3.54 µs delivered at a PRF of 90 Hz. A total of four pulses were administered, with each pulse depositing 4 Gy, resulting in the prescribed 16 Gy dose. These UHDR beam parameters are consistent with those previously reported in literature for abdominal electron FLASH and meet or exceed the prescribed thresholds for observing FLASH sparing, namely a dose rate > 100 Gy/s (10). CDR treatments were delivered using a PRF of 30 HZ, pulse width of 1.2 us, and the integrated ion chambers were utilized to deliver the prescribed dose.
Dosimetry verification
Prior to the day of live animal irradiation, on “verification day”, an in-house beta treatment planning program was used to estimate the necessary treatment parameters for 14 and 16 Gy dose delivery. Briefly, the planning software utilizes cutout specific output data from water-tank measurements and daily output data from LINAC quality assurance procedure to calculate an estimated pulse width, repetition rate, and number of pulses needed for a given dose. These parameters were then used to deliver both CDR and UHDR irradiation to (1) radiochromic film on solid water (EBT-XD, Ashland Inc, USA) (2) pre-calibrated FlashDiamond (uD) detector (PTW Inc., USA) placed at a depth of 1 mm in solid water, and (3) radiochromic film on the abdomen of a mouse phantom. The dose delivered was within 3% of the expected dose. The cutout depth-dose profile is presented in Figure 1.
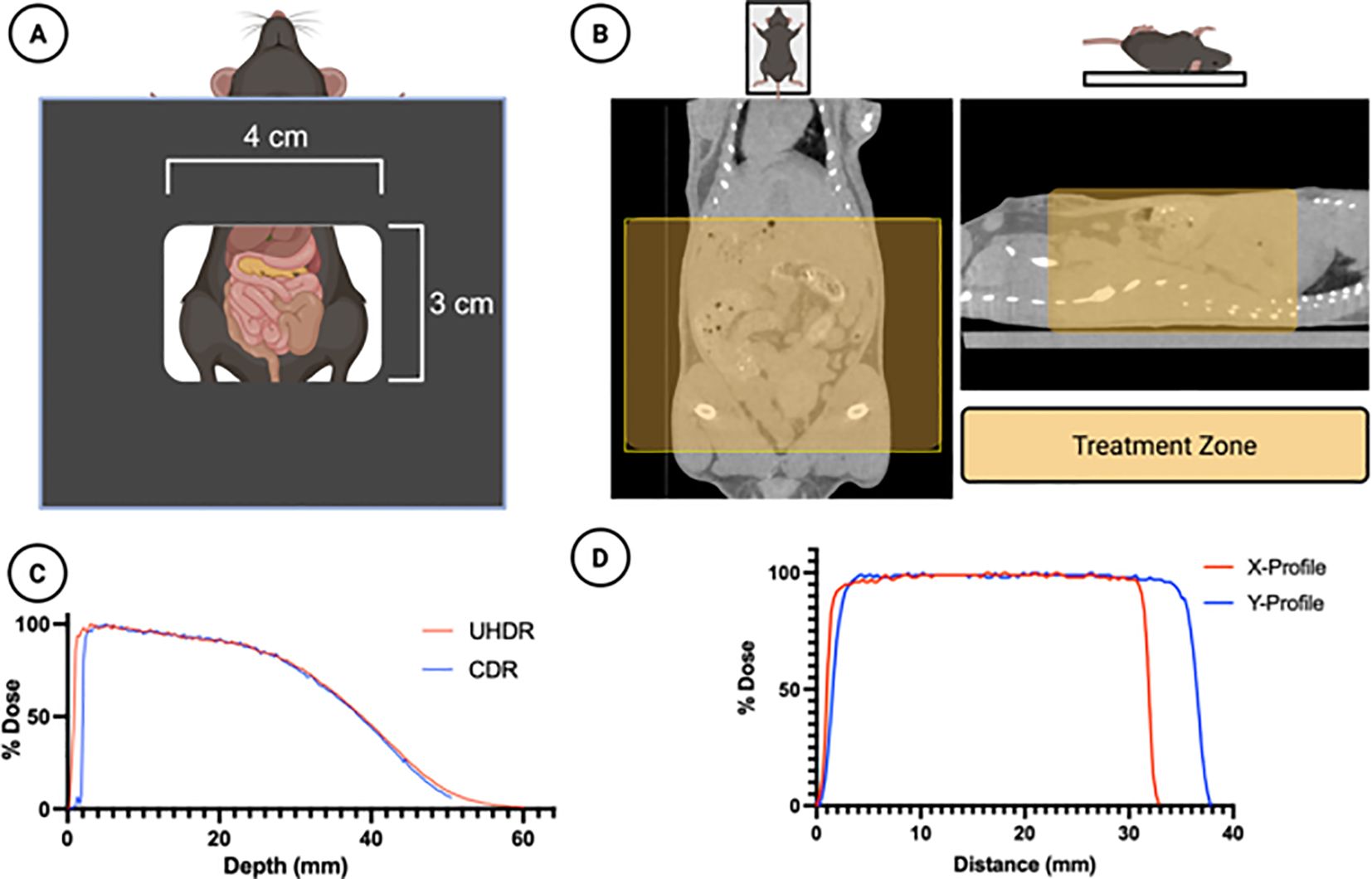
Figure 1. Treatment Design. (A) A 4x3 cm rectangular cutout was used to irradiate the mouse abdomens. (B) A micro-CT scan of the mice demonstrates the treatment zone (shaded yellow) superimposed in two planes. (C) The dose-depth characteristics of the cutout for both UHDR and CDR beams are shown in solid water for a 14Gy prescribed dose, obtained using the PTW FLASH diamond detector (D) The horizontal and vertical dose profiles of the irradiation field are shown, obtained using EBT-XD radiochromic film. Figure created using Biorender.com.
On the day of live animal irradiation, quality assurance was conducted to verify machine output in CDR and UHDR mode was within 3% of verification day. The calculated output was then inputted into the treatment planning program to determine the final treatment parameters. The final treatment parameters were delivered to the pre-calibrated uD detector at a depth of 1 mm in solid water and dose delivered was once again verified to be within 3% of the expected dose. Finally, individual dose delivery was monitored by placing the uD at the edge of the irradiation field.
Radiation damage assay
Following irradiation, mice were weighed daily and percent weight loss was calculated from the pre-irradiation baseline weight. Time to 25% weight loss was used as the primary endpoint for time to event (survival) analysis.
Oxygen measurements
Six (N=6) additional male C57BL/6 mice were used as (1) a non-irradiated control group and (2) for abdominal oxygen measurements. Following the acclimation period, mice were weighed for 3 days to establish baseline weight. They were then weighed daily for an additional 14 days, mirroring the irradiated mice. Following this monitoring period, mice received IV injections of PdG4 Oxyphor and were anesthetized using either IP injection of Ketamine/Xylazine (ketamine 100 mg/kg, xylazine 10 mg/kg, n=3) or inhaled isoflurane anesthesia delivered in room air through a non-rebreather mask (3% induction for 3 minutes, then maintained on 1.5%, n=3). Upon verification of the surgical plane of anesthesia, a terminal laparotomy was done to expose the abdominal cavity. Oxygen measurements were taken using the OxyLED (Oxygen Enterprises Inc, USA), with the optical fiber centered at the abdominal cavity.
Statistical analysis
Time to event data were analyzed using log-rank tests. Mice were censored in analyses if death occurred prior to 25% weight loss or if the weight loss was not achieved by 14 days post-irradiation. Oxygen measurements were analyzed using two sample t-tests. All analyses were conducted in Prism (GraphPad Software LLC., USA) with the significance level being α < 0.05.
Results
Weight loss
Mice were anesthetized with ketamine/xylazine (K/X) or isoflurane, and irradiated with 14 Gy or 16 Gy of either UHDR or CDR. We used time to 25% weight loss as our primary survival endpoint. Following 14 Gy abdominal irradiation, 14 animals (8 in the UHDR K/X arm, 4 in the CDR K/X arm, and 2 in the CDR ISO arm) never reached the weight-loss threshold before the 14-day study cut-off and were therefore censored. Kaplan–Meier analysis showed that UHDR K/X mice maintained body weight significantly longer than UHDR ISO mice. Median time to endpoint was 6 days for UHDR-ISO and CDR-ISO, whereas it was 7.5 days for CDR-K/X and was not reached for UHDR-K/X, with 80% of those animals still on study at day 14. This is demonstrated in Figure 2.
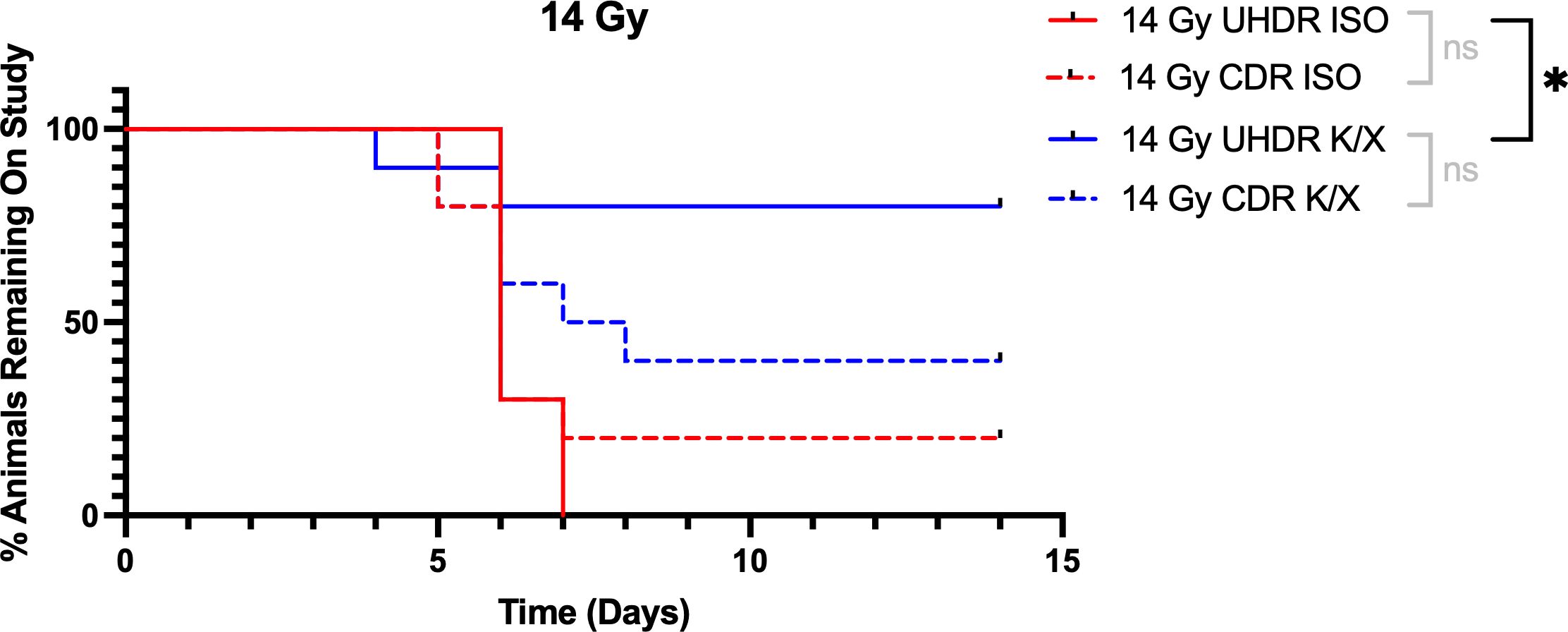
Figure 2. Kaplan-Meier curves for mice irradiated with 14 Gy. Mice (N=40) were anesthetized with either isoflurane (ISO) or ketamine/xylazine (K/X) and irradiated with 14 Gy of conventional (CDR) or ultra-high dose rate (UHDR) irradiation (n=10). Mice were removed from study when they reached 25% weight loss from baseline. NS = not significant, *p <0.05.
Following 16 Gy irradiation, 4 animals in the UHDR K/X arm did not reach the weight loss threshold before the study cut off and were censored. Kaplan–Meier analysis showed that UHDR K/X mice maintained body weight significantly longer than CDR K/X mice, whereas the ISO mice did not differ in weight. Median time to endpoint was 6 days for UHDR-ISO, CDR-ISO, and CDR K/X. UHDR K/X mice had a median time to endpoint of 7 days, but 40% remained on study at day 15. This is demonstrated in Figure 3. No mice in the non-irradiated control group show weight loss in the study period.
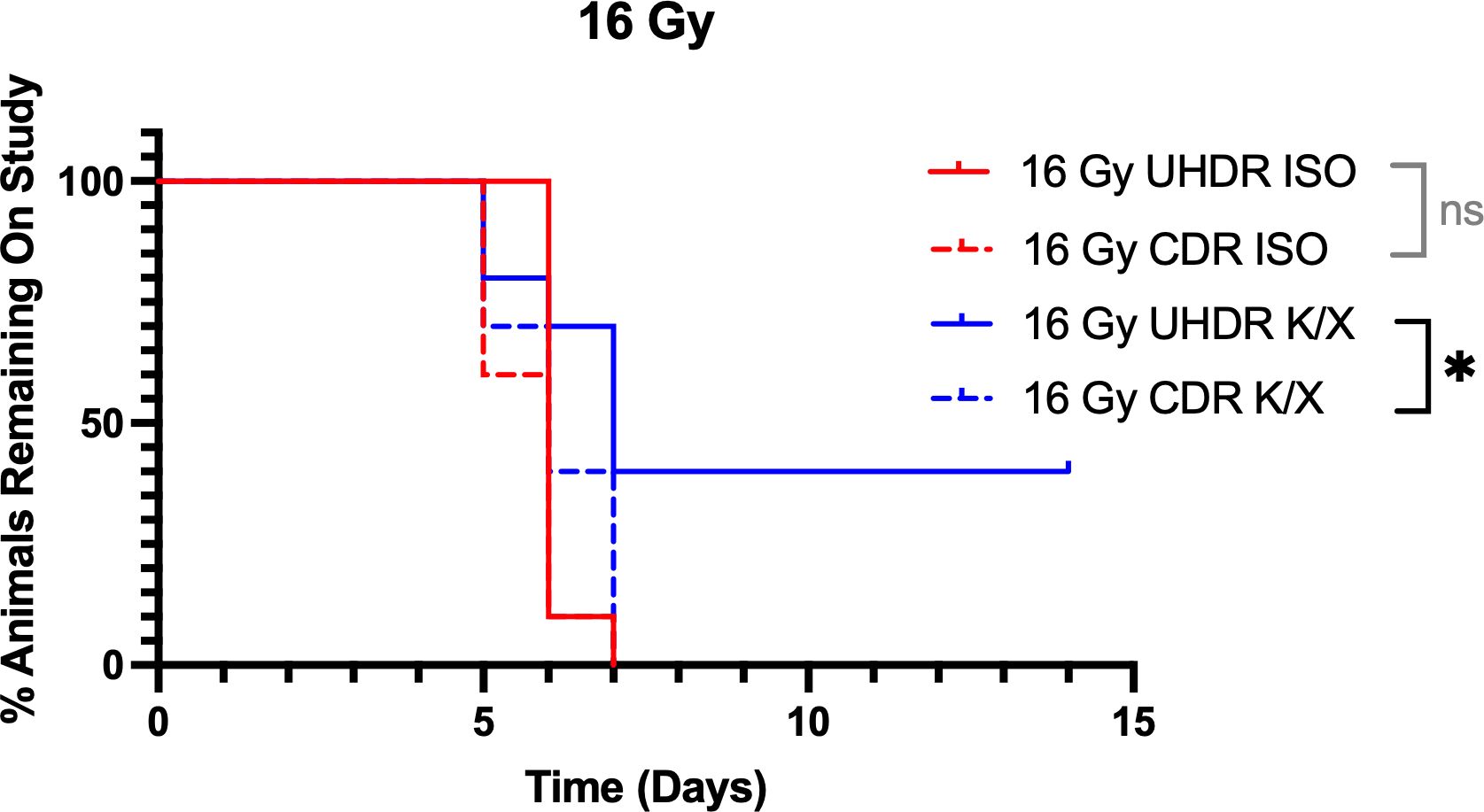
Figure 3. Kaplan-Meier curves for mice irradiated with 16 Gy. Mice (N=40) were anesthetized with either isoflurane (ISO) or ketamine/xylazine (KX) and irradiated with 16 Gy of conventional (CDR) or ultra-high dose rate (UHDR) irradiation (n=10). Mice were removed from study when they reached 25% weight loss from baseline. NS = not significant, *p <0.05.
Oxygen measurements
Bowel oxygen measurements through a laparotomy following systemic administration of PdG4 Oxyphor demonstrated significantly higher oxygen levels in mice anesthetized with isoflurane (mean=62 mmHg, SD=4) compared to mice anesthetized with ketamine/xylazine (mean=27 mmHg, SD=10; Figure 4).
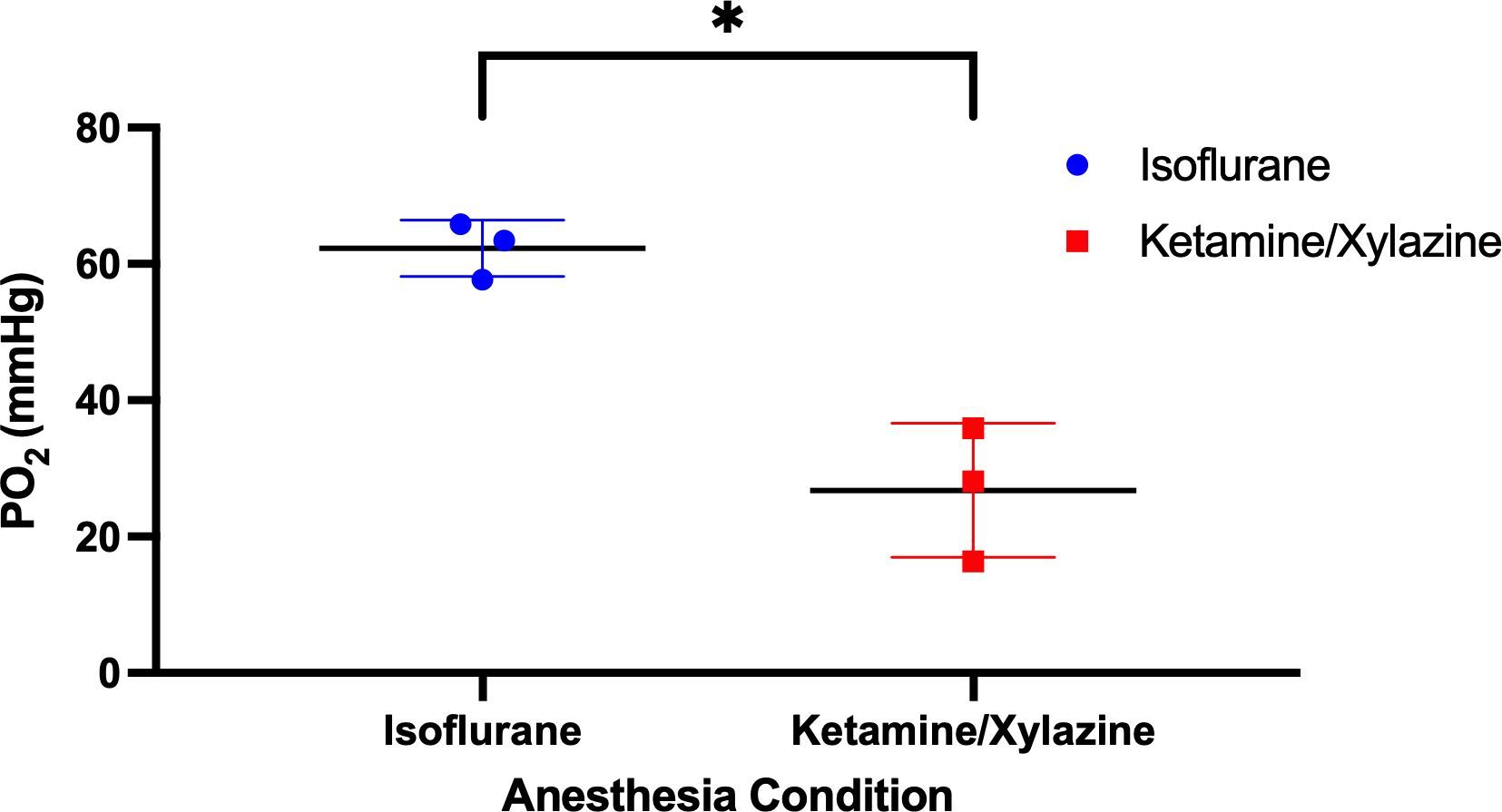
Figure 4. Abdomen oxygen measurements. Mice were anesthetized with either isoflurane or ketamine/xylazine, injected with PdG4 Oxyphor, and a laparotomy was done to expose the abdominal cavity. Oxygen measurements were obtained using the OxyLED system. *p <0.05.
Discussion
Using a 9 MeV electron beam, two previously established doses were delivered, at 14 or 16 Gy, with broad field irradiation to the abdomens of C57BL/6 mice under two commonly interchangeable anesthetics (10): (i) intraperitoneal ketamine/xylazine (K/X, 100/10 mg/kg), and (ii) inhaled isoflurane (ISO, 3% induction, 1.5% maintenance) in room air. Supplemental oxygen was deliberately avoided to isolate anesthetic-specific physiology. Employing time-to-25% weight-loss as an objective endpoint, we asked whether the magnitude of FLASH depended on the anesthetic type.
At the 14 Gy dose, a robust radioprotective effect was seen in UHDR mice anesthetized with K/X compared to ISO. Though a statistically significant sparing effect was not seen between K/X UHDR and CDR mice, it is apparent that this may have been caused by limited statistical power from the few events in the UHDR K/X arm. This is supported by a power analysis showing that a group size (n) of nearly 400 mice is needed to achieve 80% detection power (alpha = 0.05) using the baseline event rate (~0.12) and censoring rate (0.3) observed in the 14 Gy K/X UHDR arm. Regardless, at termination of study (14 days), 80% of UHDR K/X arm had not reached 25% weight loss, compared to 40% in the CDR K/X arm. Importantly though, there is no apparent FLASH effect abdominal sparing in mice anesthetized with ISO, indicating that with ISO anesthesia this would not be seen at this dose level.
At the increased 16 Gy dose, K/X administration showed notable radioprotection to the UHDR irradiated mice, as compared to the CDR mice. A significant sparing effect was seen when comparing UHDR to CDR irradiated mice under the K/X condition as well. At the termination of study, 40% of the UHDR K/X mice had not reached the 25% weight loss endpoint, in comparison to CDR mice who all reached the endpoint by 7 days. These results support the conclusion that ISO anesthesia negates the FLASH sparing effect at this dose level.
To investigate a potential mechanism for loss of sparing under ISO anesthesia, intraperitoneal oxygen measurements were performed using an injected optical oxygen reporter and fiber measurement system in six mice. A significant, nearly 2.5-fold difference in oxygen tension was seen between ISO (average ~62 ± 4 mmHg) and K/X (~26 ± 10 mmHg) conditions. Anesthesia affects tissue oxygenation in a variety of organ systems through multi-modal response including respiratory, cardiac, and vessel tone (perfusion) alteration. Still, the GI system, perfused by mesenteric and splanchnic vasculature, are specially responsive anesthetic effects (23). Considering this in the context of recently published work investigating FLASH sparing under varying oxygen conditions in mouse skin (13, 16, 18, 21), this data supports the interpretation that an oxygen-based mechanism affects the observed differences in radiosensitivity between ISO and K/X anesthesia. We see, again, that FLASH sparing is particularly sensitive to tissue oxygenation, beyond what would be expected from our conventional understanding of oxygen radiosensitization and the oxygen enhancement ratio.
There is now a wide body of literature demonstrating that tissue oxygenation is a key determinant of the magnitude of FLAH sparing across organs (5, 17, 20, 24). This data suggests that an oxygenation threshold or spectrum must exist, above which FLASH sparing is not significant. It is widely known that oxygen enhanced damage through peroxyl formation and DNA damage fixation, and so higher oxygen contributes to higher damage, but this is only seen in the range of local pO2 <10 mmHg. Thus, it seems plausible that with UHDR irradiation that some local depletion occurs or local change in reactive oxygen species occur, which only significantly affects the normal tissues with lower initial pO2. Questions remain about what this threshold is, whether it is organ-specific, and if it is modulated by total dose, dose-rate, or fractionation. Once these questions are answered, we can then tackle what we consider to be the key oxygenation question in translational UHDR: Are physiological oxygen values in awake, normally ventilating humans low enough to see a clinically meaningful FLASH sparing effect?
Our study is not without limitations. First, although we see a potent sparing in in mice who were anesthetized with K/X and received 14 Gy of UHDR radiation compared to CDR, we fail to detect a statistically significant difference using Kaplan-Meier (KM) analysis. This is attributed to the limited number of mice meeting the endpoint (2) in the UHDR arm, which affords very little statistical power to the log-rank test. Still, considering the two dose levels 14 Gy and 16 Gy together, FLASH sparing is most apparent under the K/X condition across doses. The second limitation is the inherent uncertainty in the abdominal compartment that is the primary driver of radiation toxicity/weight loss. In Total Abdominal Irradiation (TAI), numerous organs are irradiated with vastly different radiosensitivities. Though weight loss following TAI is typically attributed to small bowel damage, the more nuanced reality is that damage to many of the intrabdominal structures, aside from small bowel, can lead to weight loss and mortality. Although not quantified in this manuscript, significant hydronephrosis, liver damage, gastric enlargement, and enteritis were noted in post-mortem necropsy of mice that very likely contributed to weight loss. This leads to the third limitation, which is the lack of specifity in the abdominal compartment probed in our oxygen measurements. The wide-field oxygen measurements performed centered on the bowel to maximize signal from this organ of interest. This means that these oxygen values are an average of numerous abdominal structures within the irradiation field, rather than any one organ, but were weighted toward the bowel. Still, the results are clear: intrabdominal oxygen levels under ISO anesthesia are significantly higher than under K/X anesthesia. As oxygen measurement technologies advance, more organ-specific measurements would add value to TAI studies.
In summary, by explicitly integrating anesthetic physiology, dosimetry verified to within 3%, and direct oxygen measurements, the present work identified one of the persistent sources of variability relevant to pre-clinical FLASH research, which is anesthesia method. This is particularly important in the light of pre-clinical animal use refinement techniques moving away from injectables toward gaseous anesthesia. There needs to be practical guidance for experimental design as the field advances toward FLASH clinical trials. While direct measurement of tissue oxygen is challenging, future investigations should consider tighter control and reporting of experimental factors that can alter tissue oxygenation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Dartmouth College Institutional Animal Care and Use Committee (IACUC). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AT: Methodology, Writing – original draft, Writing – review & editing. WD: Data curation, Investigation, Writing – original draft, Writing – review & editing. DH: Writing – review & editing. BA: Writing – review & editing. GC: Writing – review & editing. DG: Conceptualization, Writing – original draft, Writing – review & editing. BP: Funding acquisition, Writing – original draft, Writing – review & editing. PH: Conceptualization, Funding acquisition, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. NCI U01CA260446
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript in editing of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Baskar R, Lee KA, Yeo R, and Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. (2012) 9:193–9. doi: 10.7150/ijms.3635
2. Daugherty E, Zhang Y, Xiao Z, Mascia A, Sertorio M, Woo J, et al. FLASH radiotherapy for the treatment of symptomatic bone metastases in the thorax (FAST-02): protocol for a prospective study of a novel radiotherapy approach. Radiat Oncol. (2024) 19:34. doi: 10.1186/s13014-024-02419-4
3. Matuszak N, Suchorska WM, Milecki P, Kruszyna-Mochalska M, Misiarz A, Pracz J, et al. FLASH radiotherapy: an emerging approach in radiation therapy. Rep Pract Oncol Radiother. (2022) 27:344–51. doi: 10.5603/RPOR.a2022.0038
4. No HJ, Wu Y, ML D, Manjappa R, Skinner L, Ashraf MR, et al. Clinical linear accelerator-based electron FLASH: pathway for practical translation to FLASH clinical trials. Int J Radiat Oncol. (2023) 117:482–92. doi: 10.1016/j.ijrobp.2023.04.011
5. Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci. (2019) 116:10943–51. doi: 10.1073/pnas.1901777116
6. Vorhees CV, Vatner RE, and Williams MT. Review of conventional and high dose rate brain radiation (FLASH): neurobehavioural, neurocognitive and assessment issues in rodent models. Clin Oncol. (2021) 33:e482–91. doi: 10.1016/j.clon.2021.09.002
7. Williams MT, Sugimoto C, Regan SL, Pitzer EM, Fritz AL, Sertorio M, et al. Cognitive and behavioral effects of whole brain conventional or high dose rate (FLASH) proton irradiation in a neonatal Sprague Dawley rat model. Lee J Ed PloS One. (2022) 17:e0274007. doi: 10.1371/journal.pone.0274007
8. Soto LA, Casey KM, Wang J, Blaney A, Manjappa R, Breitkreutz D, et al. FLASH irradiation results in reduced severe skin toxicity compared to conventional-dose-rate irradiation. Radiat Res. (2020) 194(6). doi: 10.1667/RADE-20-00090
9. Fouillade C, Curras-Alonso S, Giuranno L, Quelennec E, Heinrich S, Bonnet-Boissinot S, et al. FLASH irradiation spares lung progenitor cells and limits the incidence of radio-induced senescence. Clin Cancer Res. (2020) 26:1497–506. doi: 10.1158/1078-0432.CCR-19-1440
10. Levy K, Natarajan S, Wang J, Chow S, Eggold JT, Loo PE, et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci Rep. (2020) 10:21600. doi: 10.1038/s41598-020-78017-7
11. Bell BI, Velten C, Pennock M, Kang M, Tanaka KE, Selvaraj B, et al. Whole abdominal pencil beam scanned proton FLASH increases acute lethality. Int J Radiat Oncol. (2025) 121:493–505. doi: 10.1016/j.ijrobp.2024.09.006
12. El Khatib M, Van Slyke AL, Velalopoulou A, Kim MM, Shoniyozov K, Allu SR, et al. Ultrafast tracking of oxygen dynamics during proton FLASH. Int J Radiat Oncol. (2022) 113:624–34. doi: 10.1016/j.ijrobp.2022.03.016
13. Clark MA, Tavakkoli AD, Petusseau AF, Scorzo AV, Kheirollah A, Davis SC, et al. Dynamic oxygen assessment techniques enable determination of anesthesia’s impact on tissue. Res Sq. (2024) 3:rs–4751349. doi: 10.21203/rs.3.rs-4751349/v1
14. Jansen J, Knoll J, Beyreuther E, Pawelke J, Skuza R, Hanley R, et al. Does FLASH deplete oxygen? Experimental evaluation for photons, protons, and carbon ions. Med Phys. (2021) 48:3982–90. doi: 10.1002/mp.14917
15. Sunnerberg JP, Zhang R, Gladstone DJ, Swartz HM, Gui J, and Pogue BW. Mean dose rate in ultra-high dose rate electron irradiation is a significant predictor for O2 consumption and H2O2 yield. Phys Med Biol. (2023) 68:165014. doi: 10.1088/1361-6560/ace877
16. Sunnerberg JP, Tavakkoli AD, Petusseau AF, Daniel NJ, Sloop AM, Schreiber W, et al. Oxygen consumption in vivo by ultra-high dose rate electron irradiation depends upon baseline tissue oxygenation. Int J Radiat Oncol Biol Phys. (2025) 121:1053–62. doi: 10.1016/j.ijrobp.2024.10.018
17. Scarmelotto A, Delprat V, Michiels C, Lucas S, and Heuskin AC. The oxygen puzzle in FLASH radiotherapy: A comprehensive review and experimental outlook. Clin Transl Radiat Oncol. (2024) 49:100860. doi: 10.1016/j.ctro.2024.100860
18. Tavakkoli AD, Clark MA, Kheirollah A, Sloop AM, Soderholm HE, Daniel NJ, et al. Anesthetic oxygen use and sex are critical factors in the FLASH sparing effect. Adv Radiat Oncol. (2024) 9:101492. doi: 10.1016/j.adro.2024.101492
19. Blevins CE, Celeste NA, and Marx JO. Effects of oxygen supplementation on injecta ble and inhalant anesthesia in C57BL/6 mice. J Am Assoc Lab Anim Sci JAALAS. (2021) 60:289–97. doi: 10.30802/AALAS-JAALAS-20-000143
20. Adrian G, Konradsson E, Lempart M, Bäck S, Ceberg C, and Petersson K. The FLASH effect depends on oxygen concentration. Br J Radiol. (2020) 93:20190702. doi: 10.1259/bjr.20190702
21. Iturri L, Bertho A, Lamirault C, Brisebard E, Juchaux M, Gilbert C, et al. Oxygen supplementation in anesthesia can block FLASH effect and anti-tumor immunity in conventional proton therapy. Commun Med. (2023) 3:1–13. doi: 10.1038/s43856-023-00411-9
22. Esipova TV, Karagodov A, Miller J, Wilson DF, Busch TM, and Vinogradov SA. Two new “Protected” Oxyphors for biological oximetry: properties and application in tumor imaging. Anal Chem. (2011) 83:8756–65. doi: 10.1021/ac2022234
23. Renard D, Clavier T, Gourcerol G, and Desprez C. Impact of anesthesia drugs on digestive motility measurements in humans: A systematic review. Neurogastroenterol Motil. (2024) 36:e14855. doi: 10.1111/nmo.14855
Keywords: UHDR, flash, anesthesia, oxygen, total abdominal irradiation
Citation: Tavakkoli AD, Daley WW, Hunter DI, Allen BA, Carpenter GC, Gladstone DJ, Pogue BW and Hoopes PJ (2025) Anesthesia is a potent determinant of ultra-high dose rate sparing in the murine total abdominal irradiation model. Front. Oncol. 15:1666489. doi: 10.3389/fonc.2025.1666489
Received: 15 July 2025; Accepted: 13 August 2025;
Published: 01 September 2025.
Edited by:
Poonam Yadav, Northwestern University, United StatesReviewed by:
Mihaela Ghita-Pettigrew, Queen’s University Belfast, United KingdomBeatrice D’Orsi, National Research Council (CNR), Italy
Inna Gertsenshteyn, Novartis, United States
Copyright © 2025 Tavakkoli, Daley, Hunter, Allen, Carpenter, Gladstone, Pogue and Hoopes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Armin D. Tavakkoli, YXJtaW4uZC50YXZha2tvbGkubWVkQGRhcnRtb3V0aC5lZHU=
†These authors have contributed equally to this work
 Armin D. Tavakkoli
Armin D. Tavakkoli William W. Daley1†
William W. Daley1† David I Hunter
David I Hunter Brian W. Pogue
Brian W. Pogue P. Jack Hoopes
P. Jack Hoopes