- 1Central Laboratory, The Affiliated Panyu Central Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
- 2Geriatric Medicine Institute of Panyu District, The Affiliated Panyu Central Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
- 3Rehabilitation Medicine Institute of Panyu District, The Affiliated Panyu Central Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
- 4Intensive Care Unit Ward 1, The Affiliated Panyu Central Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
Post-translational modifications (PTMs) represent a pivotal regulatory mechanism in cellular processes, wherein the addition or removal of specific functional groups to amino acid residues dynamically modulates protein activity, subcellular localization, expression levels, and interactions with other biomolecules. Key PTMs, including phosphorylation, acetylation, methylation, glycosylation, ubiquitination, and emerging types like succinylation and crotonylation, exponentially diversify the proteome’s functional landscape. In lung cancer, PTMs orchestrate critical pathological processes, such as EGFR phosphorylation-driven proliferation, H3K27me3-mediated epigenetic silencing, and KEAP1 succinylation-regulated redox homeostasis. Recent advances in mass spectrometry (MS), phosphoproteomics, and epigenomic profiling have enabled systematic mapping of PTM networks, revealing their potential as diagnostic biomarkers, therapeutic targets, and predictors of drug response. This review synthesizes the mechanistic roles of PTMs in lung cancer pathogenesis and their translational applications, highlighting multi-omics integration and PTM-targeted therapies as future frontiers in precision oncology.
Introduction
Primary bronchopulmonary carcinoma, commonly referred to as lung cancer, represents one of the most prevalent and lethal malignancies worldwide, including in China (1). Epidemiological data from 2022 revealed that lung cancer constituted 18.06% of all newly diagnosed malignant tumors in China, ranking as the most frequently occurring cancer. Furthermore, it accounted for 23.9% of total cancer-related mortality, maintaining its position as the leading cause of cancer deaths (2). The insidious nature of early-stage lung cancer often results in asymptomatic progression, with the majority of cases being diagnosed at advanced stages upon clinical presentation. Notably, the overall 5-year survival rate for advanced lung cancer patients remains dismal at approximately 20% (3). Consequently, deciphering the molecular pathogenesis of lung cancer and identifying novel therapeutic targets to enhance patient survival carry profound clinical and scientific implications.
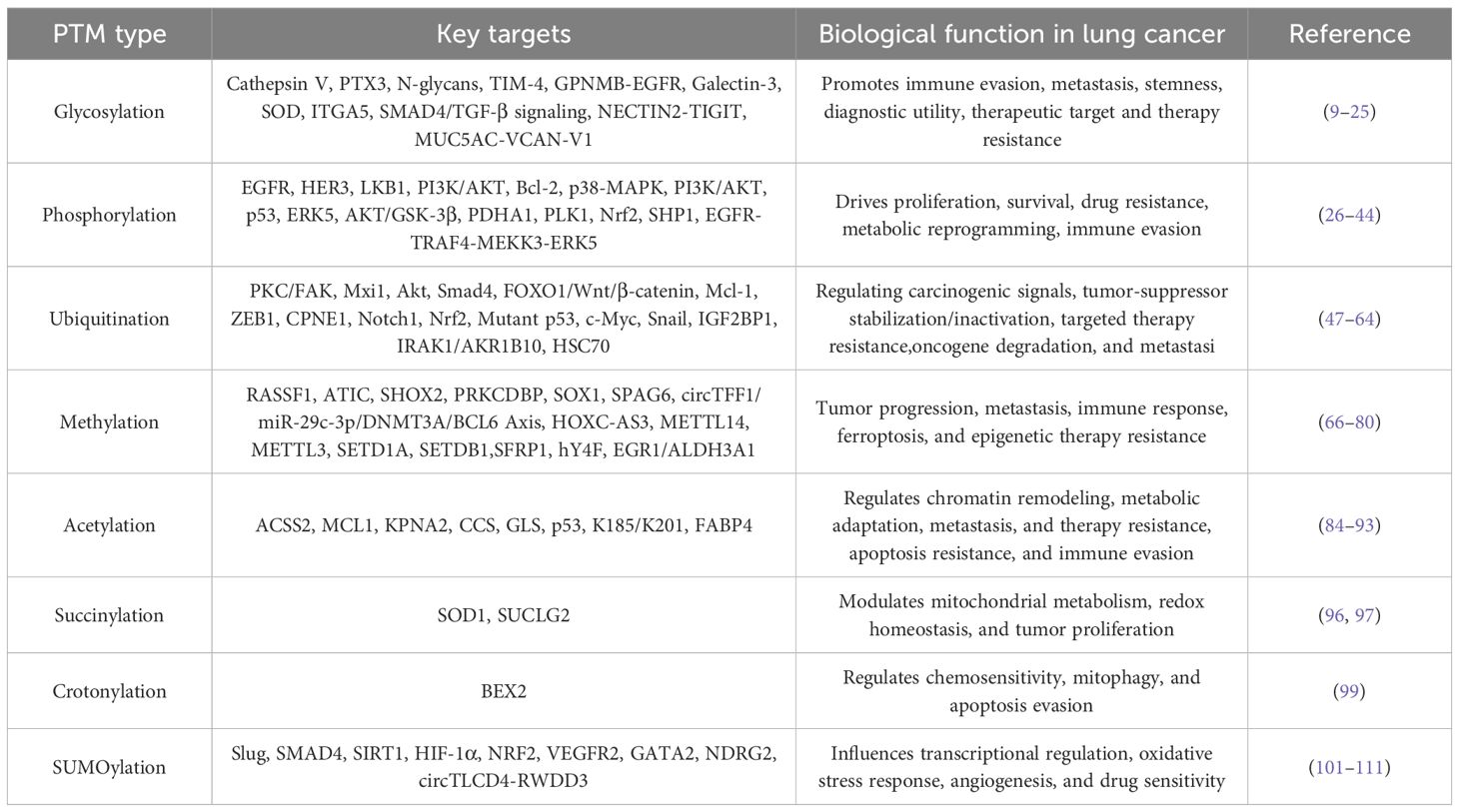
PTMs represent essential biochemical processes involving covalent alterations of amino acid residues that occur co- or post-translationally. These modifications, mediated by specialized enzymatic machinery, dynamically modulate protein structure and function, thereby regulating stability, subcellular localization, and molecular interactions (4). Current estimates suggest that more than 5% of the human proteome comprises enzymes capable of catalyzing over 200 distinct PTM types, including kinases, phosphatases, transferases, ligases, and proteases (5). PTMs can occur throughout the protein lifecycle, with many proteins undergoing combinatorial modifications through sequential proteolytic processing and functional group additions during maturation and activation (6, 7). The expanding repertoire of >400 documented PTM types has dramatically enhanced proteomic complexity and functional diversity (8). Key PTM classes, encompassing phosphorylation, glycosylation, acetylation, methylation, ubiquitination, SUMOylation, succinylation, and crotonylation, regulate fundamental hallmarks of cancer through their unique structural and biochemical characteristics. This review synthesizes their specific roles in lung cancer, providing a systematic analysis of their applications in diagnostics and targeted therapy, alongside their prognostic value (Table 1).
Literature search strategy
Searches were performed on PubMed and Web of Science using the following key terms: “post-translational modification”, “lung cancer”, “cell signaling”, “signal transduction”, “pathway”, “prognosis” and “diagnosis”, covering publications from 2015 to 2025. The search was limited to articles published in English. The initial search yielded 264 records from PubMed and 141 from Web of Science. After removing 85 duplicates and 60 articles with inaccessible full texts, a total of 260 publications were included in this review (Figure 1).
Glycosylation and lung cancer
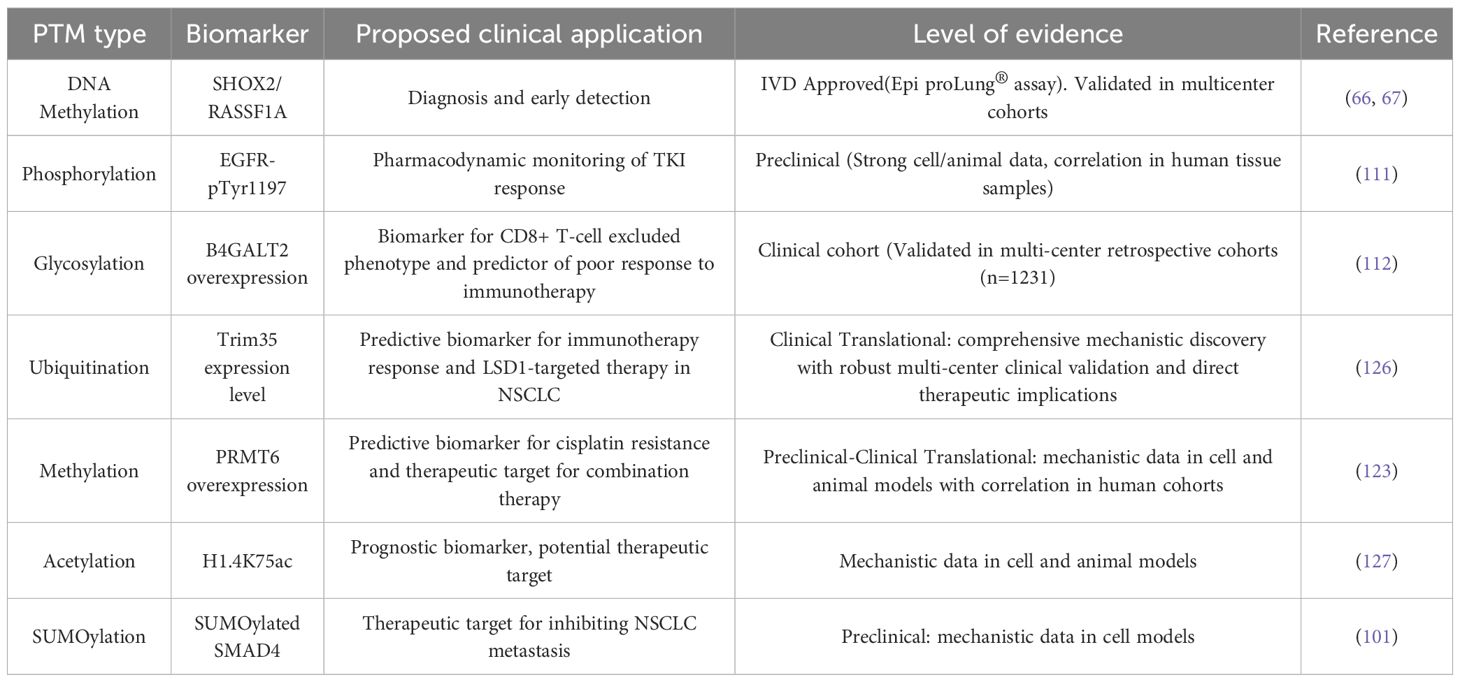
Glycosylation, an enzymatically-driven process initiating in the endoplasmic reticulum (ER) and maturing in the Golgi apparatus, represents a fundamental and ubiquitous protein modification with profound implications in lung cancer pathogenesis and clinical management (9) (Figure 2). Its functional diversity is exemplified through a complex repertoire of modifications: N-glycosylation of cathepsin V (at N221/N292) promotes lymph node metastasis and serves as a serum biomarker (10), while distinct N-glycan signatures on extracellular vesicles enable histological subtyping (11), and aberrant mucin O-glycosylation drives immune evasion (12). Beyond diagnostic utility, glycosylation actively regulates therapeutic response, as evidenced by tunicamycin chemosensitization through PTX3 modification and integrin β1 collaboration with glycosylated collagen in determining cancer stem cell fate (13, 14). Mechanistically, disease-specific haptoglobin beta chain N-glycosylation offers discriminative power while MS reveals elevated α1,6-/α1,2-/α1,3-linked fucosylation and sialylated fucosylated N-glycans mediating adhesion (15–17). Critically, O-GlcNAcylation opposes oncogene-induced senescence to promote transformation, whereas N-glycosylation appears to stabilize TIM-4 at Asn291 to enhance motility] and enables GPNMB-EGFR interaction at Asn134 to propel progression (18–20). Therapeutically, engineered sGal-3 exploits glycosylation for selective cytotoxicity, core-fucosylated SOD resists proliferation, and GALNT-mediated O-glycosylation of ITGA5 co-activates PI3K/AKT and MAPK/ERK pathways (21–23). Furthermore, O-GlcNAcylationhas been shown to stabilize SMAD4, thereby modulating TGF-β signaling (24), and ST6GalNAc-I-mediated sialylation dually orchestrates immune suppression (via NECTIN2-TIGIT) and matrix remodeling (via MUC5AC-VCAN-V1) (25). Collectively, these findings suggest glycosylation not as isolated events but as an interconnected regulatory network central to lung cancer malignancy, offering a compelling framework for novel diagnostic and therapeutic strategies.
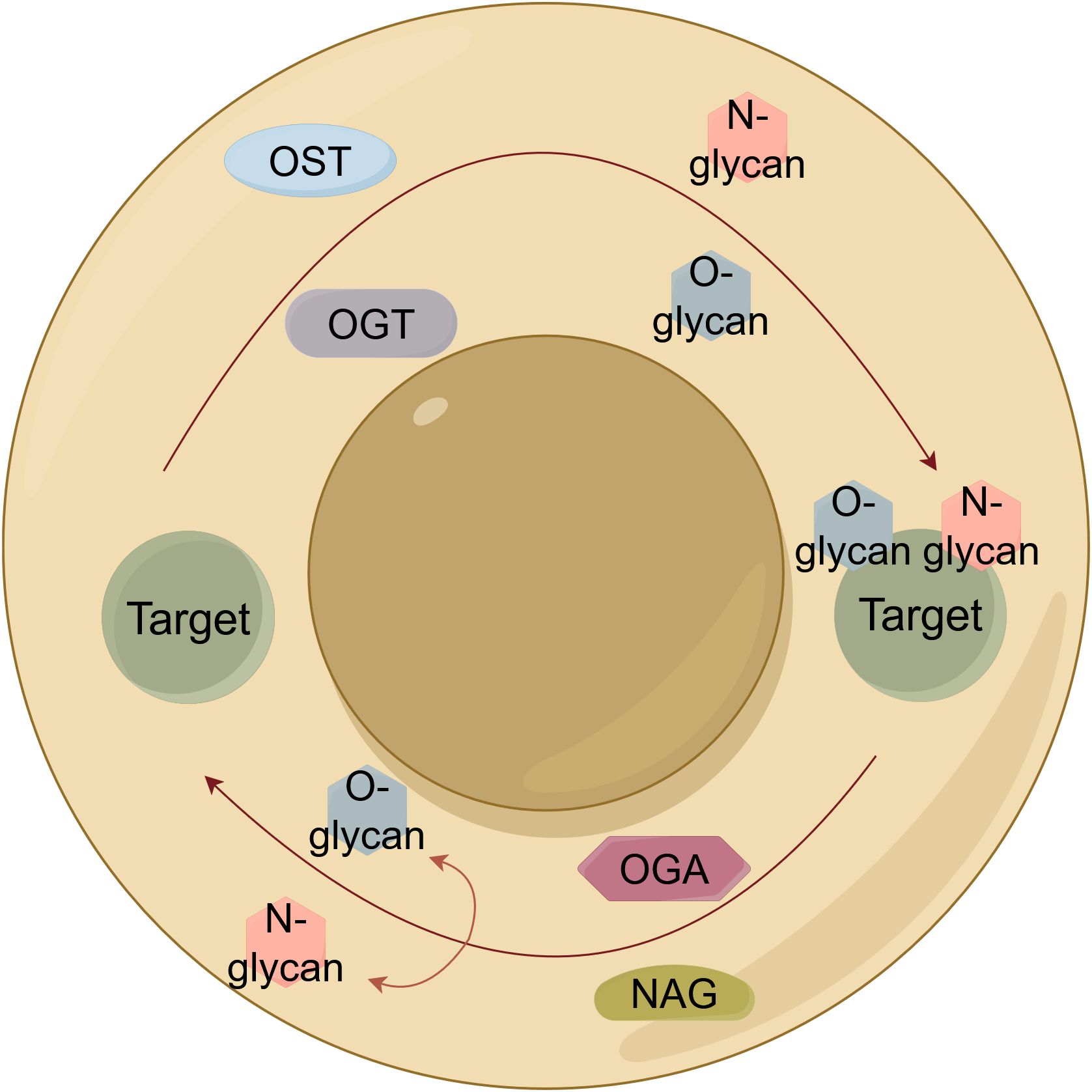
Figure 2. Schematic illustration of protein glycosylation mechanisms in lung cancer. Core pathways of protein glycosylation: N-linked (OST-mediated) and O-GlcNAcylation (OGT/OGA cycle). OST, Oligosaccharyltransferase; OGT, O-linked GlcNAc transferase; OGA, O-GlcNAcase.
Phosphorylation and lung cancer
As the most prevalent and evolutionarily conserved PTM, protein phosphorylation serves as the principal mechanism for cellular signal transduction across prokaryotic and eukaryotic organisms, functioning as a reversible covalent modification that centrally governs protein activity (Figure 3). This dynamic process involves kinases transferring phosphate groups to substrate proteins and phosphatases catalyzing their removal, collectively regulating lung cancer pathogenesis and therapeutic response. Mechanistically, diverse phosphorylation-mediated pathways contribute to oncogenesis: neurotensin receptor 1 (NTSR1) activation induces tyrosine phosphorylation of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 3 (HER3), driving proliferation in non-small cell lung cancer (NSCLC) (26, 27), tobacco carcinogen NNK promotes liver kinase B1 (LKB1) hyperphosphorylation via β-adrenergic receptor/protein kinase A (β-AR/PKA) signaling (28), and hepatocyte nuclear PI3K/AKT activation to suppress malignant growth (29). Factor 1B (HNF1B)/protocadherin-α13 (PCDHα13) overexpression attenuates Chemoresistance emerges through JNK-mediated Bcl-2 phosphorylation impairing autophagy-dependent death (30), while curcumin restores apoptotic sensitivity via reactive oxygen species (ROS)-dependent p38 mitogen-activated protein kinase (MAPK) activation (31). Cell cycle regulation occurs through protein kinase C (PKC) substrate phosphorylation induced by bisindolylmaleimide derivatives (32), and pleckstrin homology domain-containing family H member 2 (PLEKHH2) enhances focal adhesion kinase (FAK) phosphorylation to activate PI3K/AKT signaling and promote invasion (33). Radiation resistance develops through kinesin light chain 2 (KLC2)-mediated reduction of p53 phosphorylation (34), while proliferation is driven by oncogenic EGFR-TNF receptor-associated factor 4 (TRAF4)-MAP kinase kinase kinase 3 (MEKK3)-extracellular signal-regulated kinase 5 (ERK5) axes (35). Therapeutic strategies include p53 reactivation through Ser392 phosphorylation targeting (36) and nickel chloride (NiCl2) promotes lung cancer invasion and metastasis by activating the IL-6/STAT3 pathway, which upregulates the E3 ligase TRIM31 to drive ubiquitination and degradation of the tumor suppressor TP53 (37), while chromium-induced carcinogenesis involves polo-like kinase 1 (PLK1) phosphorylation of pyruvate dehydrogenase E1 subunit alpha 1 (PDHA1) at Thr57, inducing mitochondrial dysfunction and mitophagy (38). Additional mechanisms include HORMA domain-containing protein 1 (HORMAD1)-mediated β-catenin stabilization through sequential AKT (Ser473) and glycogen synthase kinase-3β (GSK-3β) (Ser9) phosphorylation (39), zinc finger E-box binding homeobox 1 (ZEB1)-orchestrated PLK1-dependent kinetochore phosphorylation (40). RhoQ inhibition was found to enhance transforming growth factor-β (TGF-β)-Smad3 phosphorylation (41), tumor cells expressing SUR1 promote the transformation of normal fibroblasts into cancer-associated fibroblasts (CAFs) and tumor progression by reducing the delivery of tumor-suppressive let-7a-5p miRNA via exosomes (42), and long non-coding RNA LINC00473-mediated nuclear factor erythroid 2-related factor 2 (Nrf2) phosphorylation suppression inducing apoptosis (43). Immunologically, TGF-β blockade potentiates interferon-γ (IFN-γ) resistance to anti-programmed death ligand 1 (PD-L1) therapy by dysregulating Src homology region 2 domain-containing phosphatase 1 (SHP1) activity (44). This comprehensive phosphorylation network highlights promising diagnostic and therapeutic targets for lung cancer precision medicine.
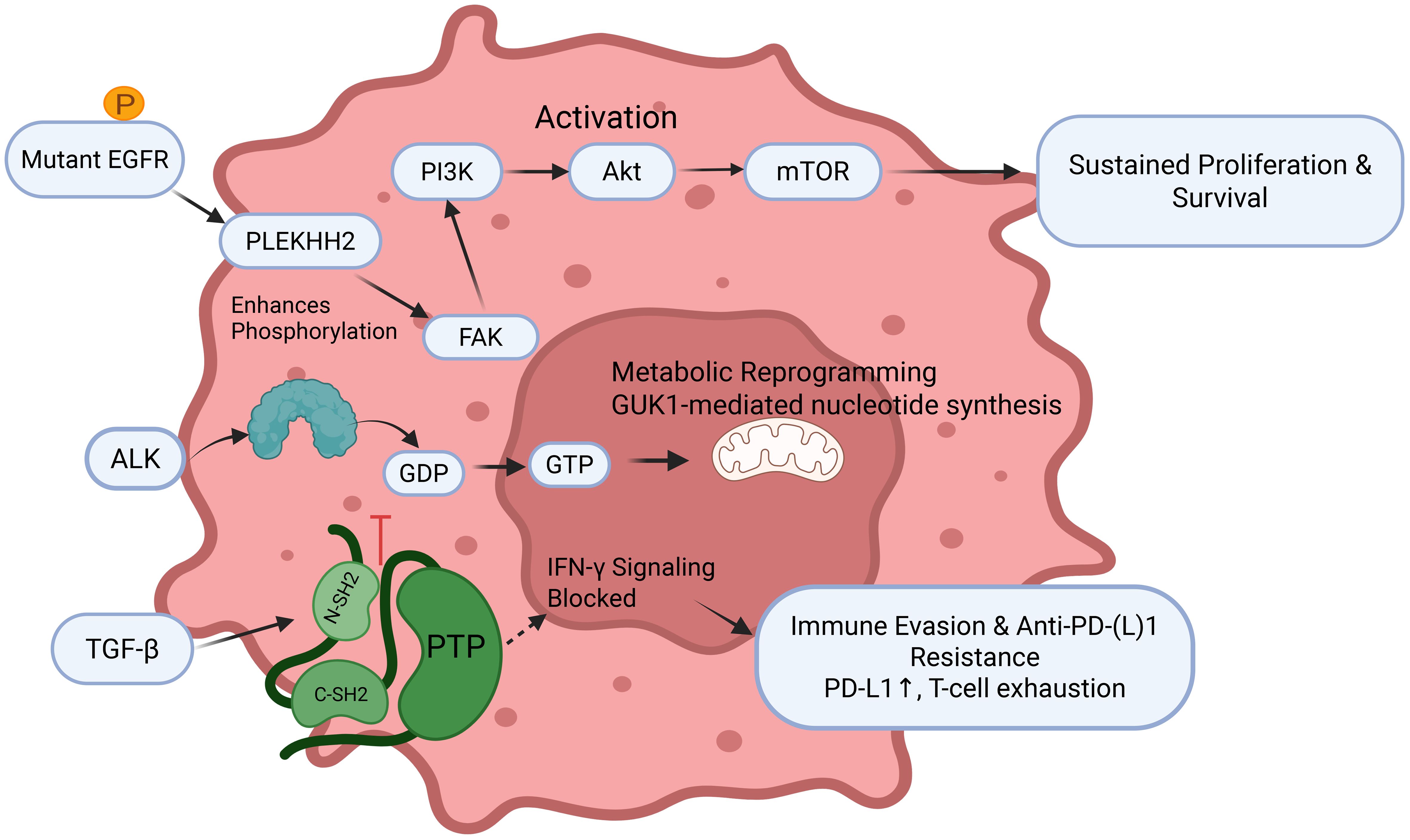
Figure 3. The central role of phosphorylation in driving lung cancer pathogenesis and therapeutic resistance EGFR, Epidermal Growth Factor Receptor; GDP, Guanosine diphosphate; GTP, Guanosine triphosphate; TGF-β, Transforming growth factor-β; FAK, Focal adhesion kinase.
Ubiquitination and lung cancer
Ubiquitination is a critical PTM process wherein ubiquitin molecules, under the orchestration of specialized enzymatic systems, including ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin-protein ligases (E3), covalently attach to target proteins to regulate their stability, activity, subcellular localization, and interactions, thereby playing a pivotal role in virtually all biological processes including immune regulation, mitophagy, DNA damage repair, cell cycle control, epigenetic modulation, proliferation, and apoptosis (45, 46)(Figure 4). In lung cancer, ubiquitination drives carcinogenesis through diverse mechanisms: the aPKC inhibitor DNDA promotes Cbl-b-mediated ubiquitination and degradation of PKC/FAK to suppress metastasis (47), UBE2O catalyzes degradation of Mxi1 to drive tumorigenesis (48), RNF-8 mediates K63-linked ubiquitination to activate Akt and promote chemoresistance (49), HECW1 catalyzes K48-linked polyubiquitination of Smad4 to facilitate NSCLC progression (50), UBE2T targets FOXO1 for degradation and activates Wnt/β-catenin signaling (51), FBW7 ubiquitinates Mcl-1 to inhibit anti-apoptotic signaling (52), USP51 stabilizes ZEB1 to confer cisplatin resistance (43). NEDD4L regulates CPNE1 degradation to modulate oncogenic signaling (54). MIB2 degrades Notch1 to exert tumor-suppressive effects (55). KLHL18 promotes p85α degradation to inhibit PI3K/AKT and PD-L1 signaling (56). Ablation of AdipoR4 enhances Keap1-mediated Nrf2 ubiquitination and degradation to increase chemosensitivity (57). SIRT3 modulates ubiquitin-dependent degradation of mutant p53 (58). TRIM2 catalyzes K48-linked ubiquitination of Snail1 to enhance invasion (59). lncRNA AFAP1-AS1 stabilizes c-Myc by inhibiting its ubiquitination to drive metastasis (60). USP37 deubiquitinates and stabilizes Snail to promote migration (61). circNDUFB2 facilitates ubiquitin-dependent degradation of IGF2BP1 and activates anti-tumor immunity (62). RNF152 catalyzes K48-linked ubiquitination of IRAK1 to downregulate AKR1B10 and suppress malignancy (63), and ROS-induced o8G modification of circPLCE1 enhances HSC70 ubiquitination to inhibit autophagy and tumor progression (64).
Figure 4. Ubiquitin-proteasome system and therapeutic targeting in lung cancer. The canonical E1-E2-E3 ubiquitination cascade; Ub, Ubiquitin; E1, Ubiquitin-activating enzymes; E2, Ubiquitin-conjugating enzymes; E3, Ubiquitin-protein ligases; DUB, Deubiquitinating enzyme.
Methylation and lung cancer
Methylation, a fundamental epigenetic and PTM, involves methyltransferases catalyzing the covalent transfer of methyl groups from S-adenosylmethionine (SAM) to specific residues on DNA, RNA, or proteins, dynamically regulating gene expression, protein function, and RNA metabolism (Figure 5) (65). Dysregulated methylation is mechanistically implicated in lung cancer pathogenesis, diagnosis, and therapy: DNA hypermethylation of tumor suppressors including RASSF1, ATIC, SHOX2 (mSHOX2) and PRKCDBP serves as a clinically validated biomarker for early detection, lymph node metastasis, and poor prognosis (66–68), promoter hypermethylation of SOX1 and SPAG6 contributes to transcriptional silencing and activates oncogenic pathways such as JAK/STAT (69, 70), non-coding RNAs including circTFF1 (via miR-29c-3p/DNMT3A/BCL6 axis), hsa_circ_0077837 (PTEN silencing), and HOXC-AS3 (activated by SETD1A-mediated H3K4me2) promote proliferation, invasion, and ferroptosis resistance (71–73). RNA methylation regulators METTL14 and METTL3 enhance stability and translation of oncogenes, such as PLAGL2 and DDX23, through m6A modification, activating β-catenin and PI3K/AKT pathways (74, 75). Histone methyltransferases SETD1A and SETDB1 catalyze H3K4 trimethylation and modulate SPG20 methylation to drive tumor progression and metastasis (76, 77), and the microRNA miR-26a-5p attenuates Wnt signaling by inhibiting DNMT3A-mediated SFRP1 promoter methylation (78). Additionally, YRNA fragment hY4F is secreted via methylated YBX1-packaged EVs, attenuating its tumor-suppressive function (79). Critically, NNMT upregulation depletes methyl donors, reducing global H3K9me3/H3K27me3 levels and establishing feedback loops via EGR1/ALDH3A1/lactate that sustain EGFR-TKI resistance, highlighting therapeutic targeting potential (80).
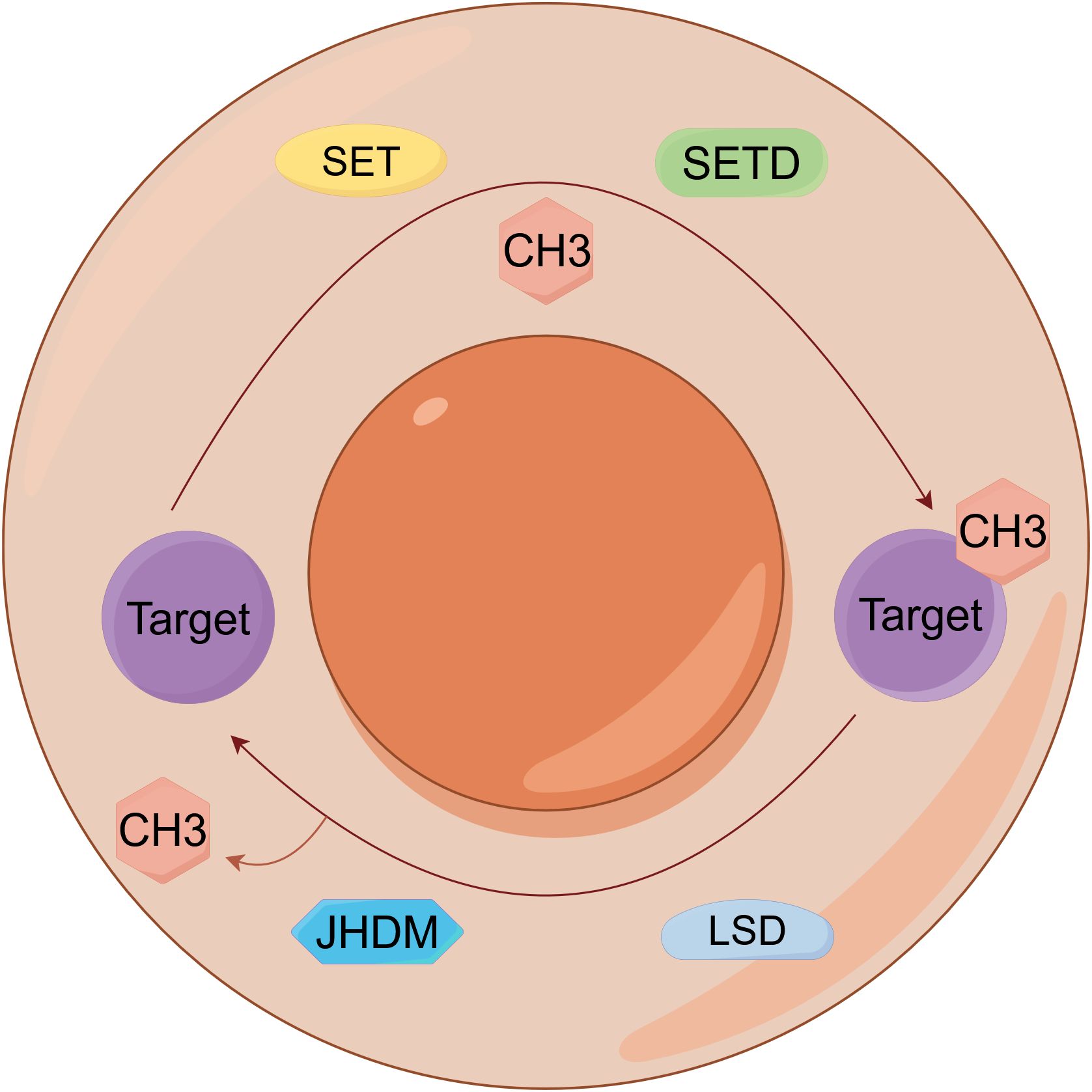
Figure 5. Epigenetic regulation by DNA and histone methylation in lung cancer. Dynamic regulation of epigenetic signaling through antagonistic protein methylation and demethylation on histone targets. SET, Su(var)3-9, Enhancer-of-zeste, Trithorax; SETD, SET Domain-containing; JHDM, Jumonji C-domain-containing histone demethylase; LSD, Lysine specific demethylase.
Acetylation and lung cancer
Acetylation, a reversible PTM catalyzed by acetyltransferases (HATs/KATs), involves the transfer of acetyl groups to lysine ϵ-amino groups, dynamically regulating protein function, chromatin architecture, and gene expression by modulating histone tail modifications and transcription factor accessibility (Figure 6) (81). This versatile modification orchestrates cellular processes through multiple mechanisms: modulating DNA-protein interactions via histone acetylation, fine-tuning protein interactions via non-histone protein conformational changes, allosterically regulating enzymatic activity, and directing subcellular localization through nuclear localization signal masking (82, 83). In lung cancer, acetylation plays pivotal roles in pathogenesis and therapy: miR-15a-5p targets ACSS2 to inhibit acetyl-CoA production, suppressing lipid metabolism and histone acetylation-mediated transcription to attenuate metastasis (84). Acetylated α-tubulin stabilizes MCL1 by shielding it from ubiquitin ligase recognition and inhibiting K48-linked polyubiquitination, determining paclitaxel sensitivity (85). CBP/p300-mediated acetylation of KPNA2 promotes its nuclear export and attenuates oncogenicity (86). HDAC4 deacetylates GLS at K311 to activate glutaminolysis, while H3K27 acetylation epigeneticallyis correlated with the activation of CCS transcription, enhancing ROS scavenging and cytoprotective autophagy (87, 88). Suberoylanilide hydroxamic acid (SAHA) induces radiosensitization in lung cancer cells by promoting K120 acetylation of p53, which regulates mitochondrial apoptosis, and this effect requires specific p53 status (89). AKR1C1 acetylation at K185/K201 enhances enzymatic activity and metastatic potential (90). Shikonin inhibits c-Myc-mediated HDAC1 recruitment to ATF3, promoting local histone acetylation (91). SIRT5 promotes the progression of non-small cell lung cancer by inducing deacetylation and reducing the expression of FABP4 (92). Additionally, ACAT1-mediated hypersuccinylation elevates ROS and impedes tertiary lymphoid structure formation, promoting anti-PD1 resistance, highlighting acetylation-related pathways as promising therapeutic targets (93).
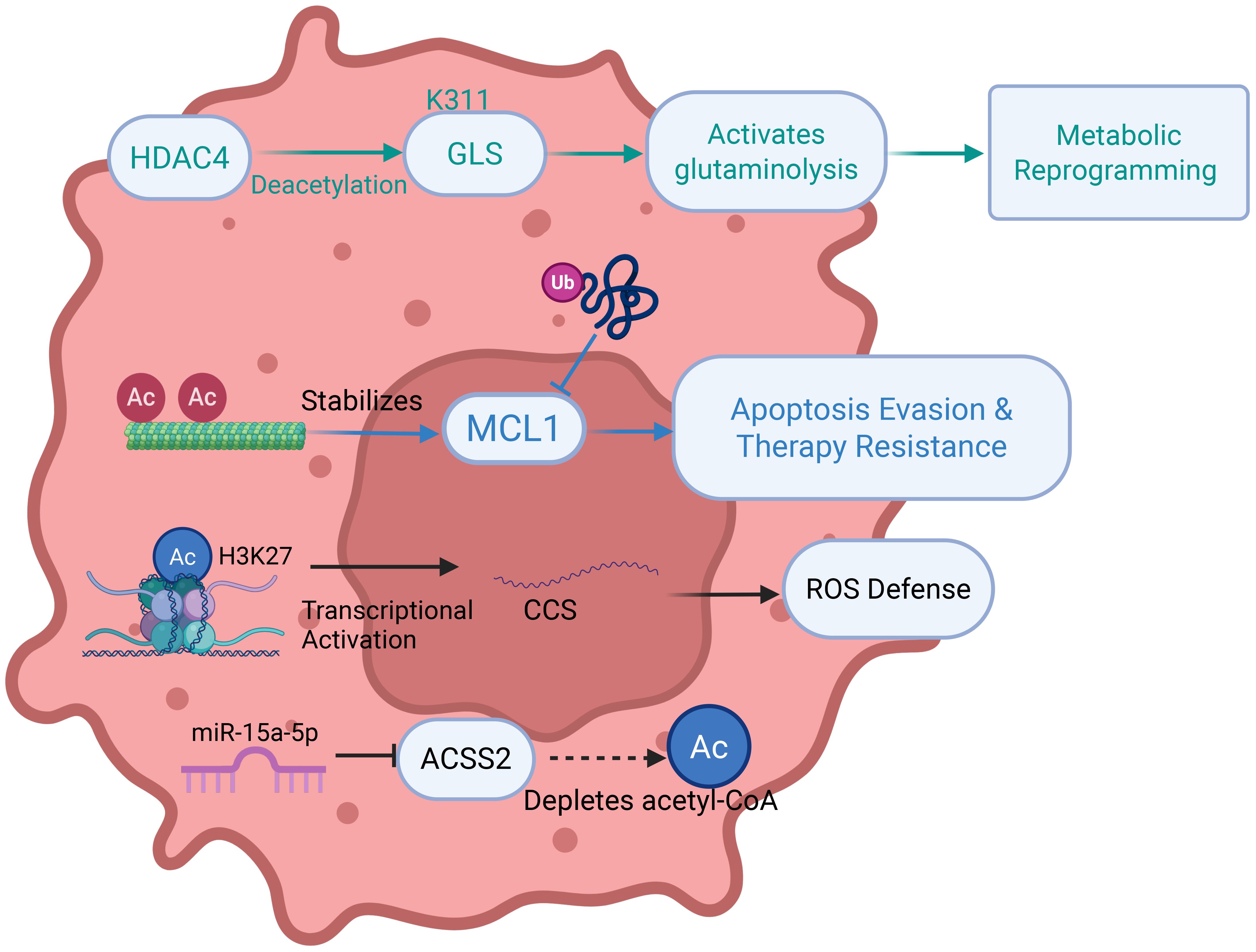
Figure 6. Mechanisms of acetylation in regulating metabolic reprogramming, cell death, and immune microenvironment in lung cancer. HDAC, Histone Deacetylase; GLS, Glutaminase; MCL1, Myeloidcell leukemia 1; AC, Acetyl group; CCS, Copper Chaperone for Superoxide dismutase; ACSS2, Acyl-CoA Synthetase Short-Chain Family Member 2.
Succinylation, crotonylation and lung cancer
Protein succinylation and crotonylation represent emerging and critically important PTMs that significantly contribute to lung cancer pathogenesis by dysregulating metabolic signaling and cell death pathways (Figure 7A). Succinylation involves the enzymatic transfer of a succinyl group (-CO-CH2-CH2-COO-) from succinyl-CoA to lysine ϵ-amino groups, playing key regulatory roles in the tricarboxylic acid (TCA) cycle, amino acid metabolism, and fatty acid metabolism (94), whereas crotonylation entails crotonyl-CoA-mediated transfer of crotonyl groups to histone residues, crucially influencing gene expression and other biological processes (Figure 7B) (95). Specifically, succinylation of superoxide dismutase 1 (SOD1) diminishes its enzymatic activity, and mutation at the SOD1 succinylation site suppresses lung tumor growth, underscoring its therapeutic potential (96). Additionally, succinylation at lysine 93 (K93) stabilizes succinate-CoA ligase subunit beta (SUCLG2), enhancing its abundance and promoting LUAD proliferation (97). Furthermore, crotonylation of brain expressed X-linked 2 (BEX2) promotes its interaction with nuclear dot protein 52 (NDP52), augmenting mitophagy and attenuating chemotherapy-induced apoptosis (98). Collectively, these findings highlight the intricate and multifaceted roles of succinylation and crotonylation in lung cancer, revealing novel mechanistic insights into metabolic reprogramming, drug response, and survival pathways, thereby offering promising targets for therapeutic intervention.
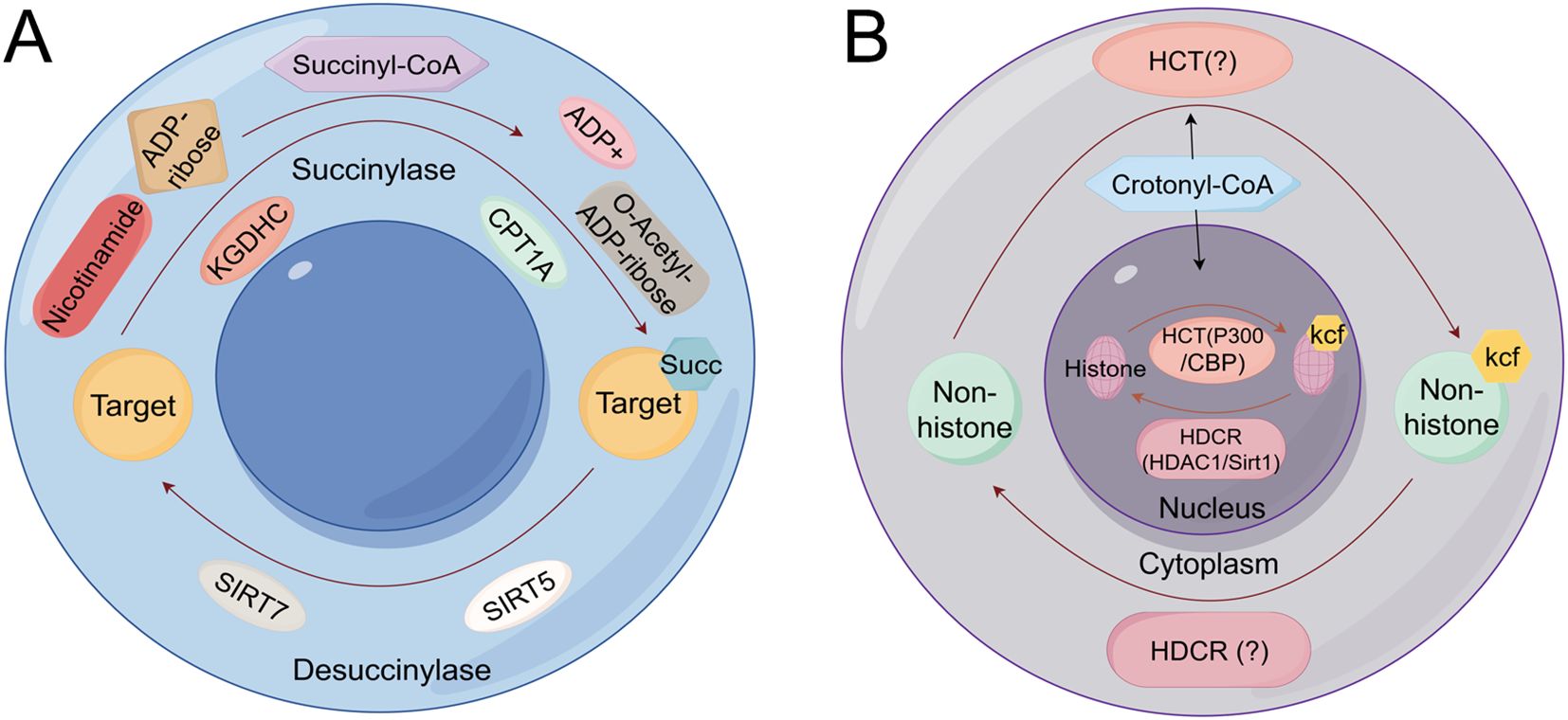
Figure 7. Succinylation and crotonylation mechanism schematic diagram (A) The mitochondrial succinylation-desuccinylation cycle (B) The nuclear crotonylation-decrotonylation cycle catalyzed by HCTs and HDCRs. KGDHC, Ketoglutarate Dehydrogenase Complex; ADP, Adenosine Diphosphate; SIRT5, Sirtuin 5; SIRT7, Sirtuin 7; HDCR, Histone Decrotonylase; HCT, Histone Crotonyltransferase..
SUMOylation and lung cancer
SUMOylation, the covalent attachment of small ubiquitin-related modifier (SUMO) isoforms (SUMO1-4) to target proteins, represents a highly conserved and critical PTM that regulates diverse cellular processes including cell cycle progression, nuclear transcription, protein interactions, DNA damage repair, and differentiation (Figure 8) (99). In lung cancer, SUMOylation contributes centrally to pathogenesis and therapy resistance through multiple mechanisms: Ubc9/PIASy-mediated SUMOylation of Slug promotes NSCLC metastasis by enhancing its transcriptional repression activity through HDAC1 recruitment (100), PIAS1 facilitates SUMO1-SMAD4 complex formation, enhancing vimentin expression and cell migration (101). Hypoxia promotes epithelial-mesenchymal transition and lung cancer metastasis by downregulating SIRT1 expression in a SUMOylation-dependent manner (102). HSP70 promotes HIF-1α SUMOylation under hypoxia, conferring ferroptosis resistance and tumor recurrence after ablation (103). KEAP1 SUMOylation modulates ROS production and disrupts KEAP1-NRF2 interaction to activate antioxidant responses (104). VEGFR2 SUMOylation attenuates downstream signaling, suppressing proliferation, migration, and angiogenesis (105). HSP70 upregulates SUMO1-mediated HIF-1α modification to enhance thermotolerance and induce aberrant immune responses (106). PIASy enhances GATA2 SUMOylation, reducing its transcriptional activity (107). RNF4 enhances the tumor-suppressive function of NDRG2 in lung adenocarcinoma by promoting its SUMOylation (108), and SUMOylation-mediated activation of ALIX upregulates extracellular vesicle-derived circTLCD4-RWDD3 to promote lymphatic metastasis (109). Furthermore, a SUMOylation-related prognostic signature for osimertinib resistance has been identified, with gene expression correlating with immune activation and offering novel biomarkers and therapeutic targets (110).
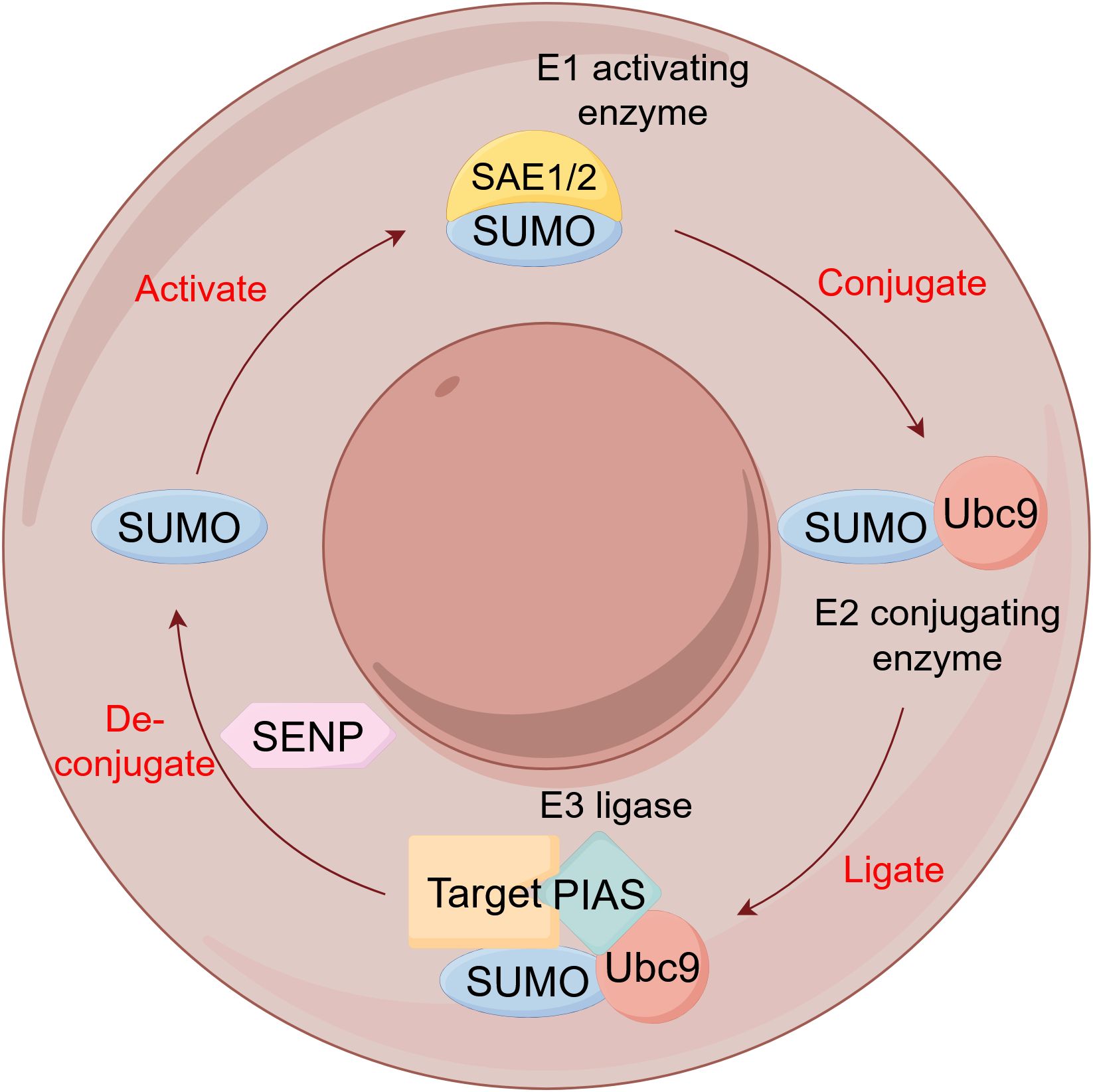
Figure 8. The SUMOylation cycle. SUMO, Small Ubiquitin-like modifier; SAE, SUMO-activating enzyme; Ubc9, Ubiquitin-conjugating enzyme 9; PIAS, Protein Inhibitor of Activated STAT; SENP, SUMO/sentrin-specific protease.
PTM-based diagnostic and prognostic tools in lung cancer
PTMs have emerged as powerful biomarkers for lung cancer detection, stratification, and therapeutic response prediction. Phosphoproteomic profiling of EGFR-mutant lung adenocarcinoma identifies specific phosphorylation events, such as EGFR-pTyr1197, MAPK7-pTyr221, and DAPP1-pTyr139, that serve as quantitative biomarkers of TKI sensitivity, with their inhibition dynamics directly correlating with drug response, offering real-time pharmacodynamic insights (111). Beyond phosphorylation, glycosylation patterns also show clinical relevance: Zhang et al. developed a machine learning-based PTM Learning Signature (PTMLS) from multi-cohort analysis of 1,231 LUAD cases, identifying beta-1,4-galactosyltransferase 2 (B4GALT2) as a key prognostic biomarker within this framework, where elevated B4GALT2 expression correlates with poor survival and CD8+ T-cell exclusion, suggesting an immunoevasive role in LUAD progression (112). Epigenetic modifications further expand the biomarker landscape: EZH2, the catalytic subunit of the PRC2 complex, mediates H3K27 trimethylation (H3K27me3) to drive lung carcinogenesis, and integrated analysis of H3K27me3-nucleosome levels with ctDNA profiling significantly enhances diagnostic accuracy. Notably, elevated H3K27me3 is detected in 25.5% of treatment-naïve patients lacking somatic mutations, and this epigenetic signature improves the detection rate of disease progression from 43.1% to 58.2%, underscoring its dual utility for non-invasive diagnosis and molecular residual disease (MRD) monitoring in lung cancer (113). Supporting the role of glycosylation in immune regulation, a foundational study suggested that glycosylation of PD-L1 stabilizes its expression and inhibits T-cell function, a mechanism that underpins the efficacy of immune checkpoint inhibitors (114). Histone methylation modifications further expand the epigenetic biomarker landscape. EZH2-mediated H3K27 trimethylation (H3K27me3) drives lung carcinogenesis. While direct detection of H3K27me3 in ctDNA is emerging, the analysis of cancer-specific DNA methylationpatterns in ctDNA has become the gold standard for molecular residual disease monitoring (115). The prospective study NCT06358222 demonstrated that combining ctDNA methylation profiling with PET-CT imaging can accurately predict lymph node metastasis in non-small cell lung cancer preoperatively (116). Acetylation markers are also gaining traction as predictors of therapy response, high HDAC1 expression is significantly associated with poor lung cancer differentiation, squamous cell carcinoma subtype, and unfavorable patient prognosis, suggesting its potential as a diagnostic and prognostic marker (117).
Collectively, these findings highlight the multidimensional value of PTM-based biomarkers.The ongoing integration of multi-level PTM profiling, from phosphoproteomics to epigenomics, into large-scale clinical trials promises to further refine lung cancer subclassification, monitor therapeutic efficacy, and guide personalized treatment strategies.
Therapeutic targeting of PTM pathways
Studies showed that the combination of Rapamycin and SAHA enhances radiosensitivity in non-small cell lung cancer by inducing acetylation and autophagy, thereby inhibiting DNA damage repair (118). The combination of vorinostat and cisplatin significantly enhances antitumor efficacy against small cell lung cancer in vitro and in vivo by increasing histone acetylation levels and suppressing thymidylate synthase expression (119). Plant homologous structural domain finger protein 23 (PHF23) promotes tumor proliferation and migration, yet enhances cisplatin/docetaxel sensitivity by facilitating DNA damage repair. PHF23 stabilizes ACTN4 through its PHD domain by inhibiting K48-linked ubiquitination, implicating it as a therapeutic target (120). Emerging therapeutic strategies employ PROTAC-mediated ubiquitination to degrade KRASG12C mutant proteins, offering a viable approach to reduce oncogenic KRAS levels and suppress downstream signaling pathways in cancer cells (121). While when NSCLC cells were treated with norcantharidin (NCTD), a demethylated form of cantharidin, a reduction in both the mRNA and protein levels of YAP, as well as increased YAP phosphorylation were observed, which inhibited the proliferation of NSCLC cells (122). PRMT6 promotes glycolysis and cisplatin resistance through methylation of metabolic enzymes 6PGD (R324) and ENO1 (R9/R372). The PRMT6 inhibitor DCPR049_12 effectively reverses these effects and enhances chemosensitivity (123).
Clinical translation of PTMs: diagnostic and therapeutic decision-making
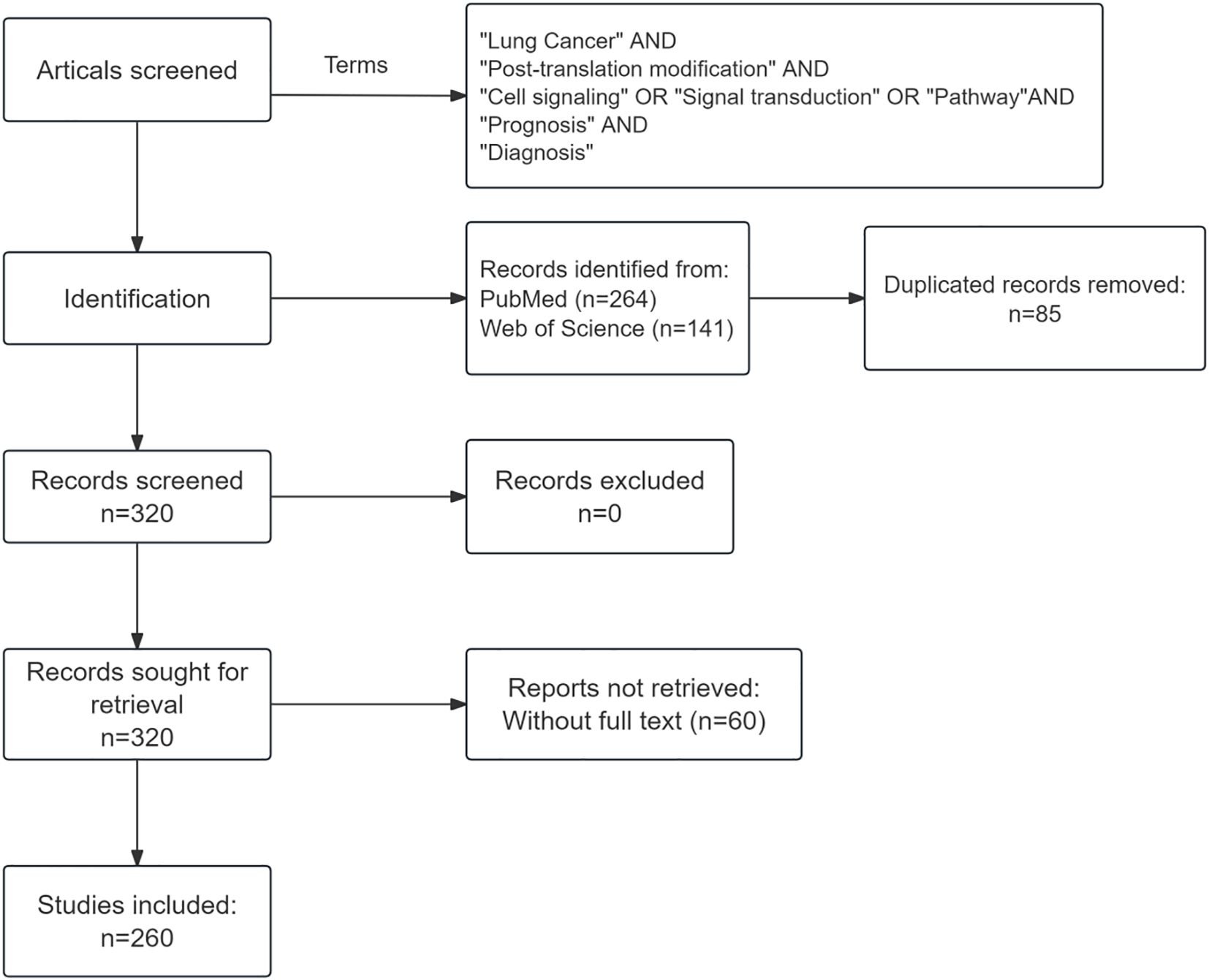
The intricate landscape of PTMs in lung cancer presents both challenges and opportunities for clinical translation (4, 5, 8). We will organize the most promising PTM-related findings around core clinical issues, such as diagnosis, predictive biomarkers, and therapeutic targeting, with the aim of clarifying the role of PTM research advancements in these critical areas (Table 2).
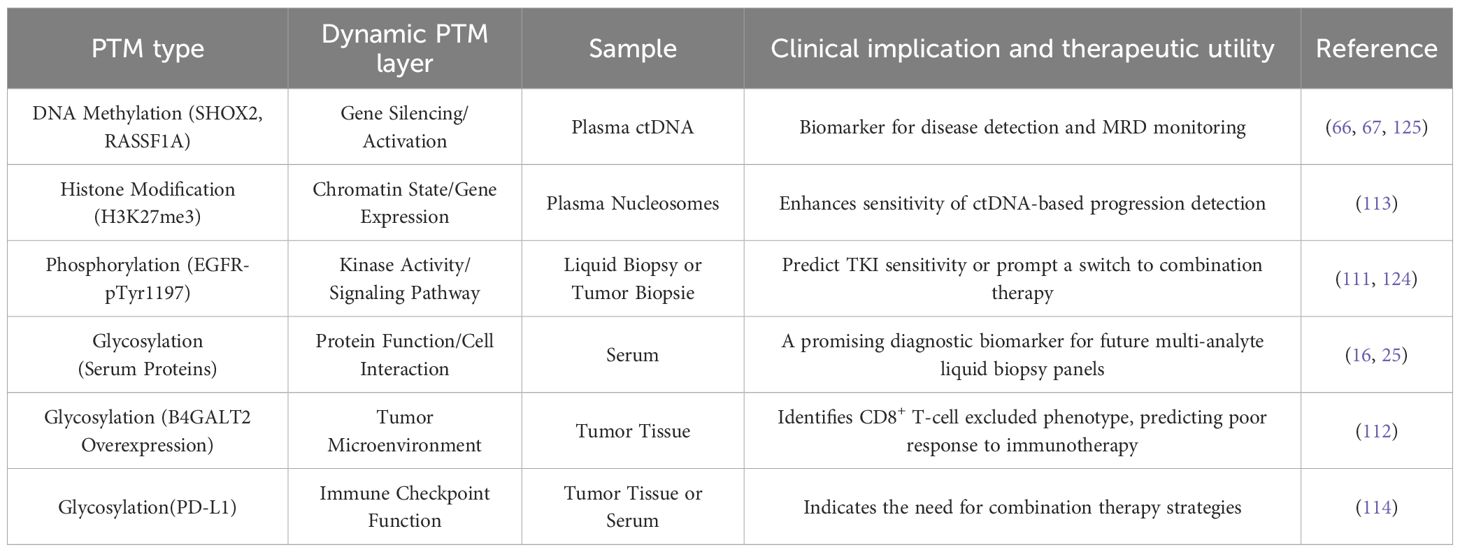
The growing adoption of liquid biopsies has accelerated the search for non-invasive diagnostic biomarkers, and PTMs, owing to their stability and mechanistic relevance to tumor biology, which represent ideal candidates. DNA methylation-based assays, such as the detection of SHOX2 and RASSF1 hypermethylation in plasma ctDNA, currently lead in clinical translation (66, 67), highlighting the validated clinical utility of this PTM type. Beyond DNA modifications, emerging epigenetic and glycomic signatures are showing strong diagnostic potential: for example, H3K27me3-modified nucleosomes in plasma can enhance the sensitivity of ctDNA-based minimal residual disease monitoring (113), while MS-based profiling of serum protein glycosylation patterns, such as sialylation and fucosylation exhibiting high discriminatory power for early-stage detection, suggesting promise for future multi-analyte liquid biopsy panels (16, 25).
The intricate interplay between genomic alterations, dynamic PTM networks, and therapeutic response necessitates a structured framework to guide clinical decision-making in lung cancer. For instance, in EGFR-mutant lung adenocarcinoma, quantitative assessment of EGFR-pTyr1197 phosphorylation via targeted MS of liquid biopsy samples or immunohistochemistry on serial tumor biopsies provides a direct pharmacodynamic readout. A rapid decline in phosphorylation signals effective inhibition and predicts favorable response to tyrosine kinase inhibitors, whereas its rebound heralds acquired resistance, prompting a switch to combination therapies, such as adding a SRC inhibitor upon detecting SRC kinase activation in ALK-positive patients (111, 124). Beyond phosphorylation, glycosylation remodeling offers critical insights into the immune microenvironment. IHC-based detection of B4GALT2 overexpression in tumor tissue identifies patients with CD8+ T-cell excluded phenotypes, who are less likely to respond to immunotherapy (112). Similarly, PD-L1 glycosylation, detectable by IHC or emerging serum MS/ELISA platforms, stabilizes PD-L1 and inhibits T-cell function, underscoring its role as a resistance mechanism to immune checkpoint blockade (114). The feasibility of PTM monitoring is increasingly supported by advancing technologies. Liquid biopsy-based methylation-specific PCR or MS for SHOX2/RASSF1A in ctDNA is already a clinically validated tool for disease detection and MRD monitoring (66, 67, 125). Similarly, immunoaffinity enrichment coupled with MS allows for the detection of H3K27me3-modified nucleosomesin plasma, significantly enhancing the sensitivity of progression detection when combined with ctDNA mutation analysis (113). The integration of dynamic PTM profiling into the clinical workflow, marks a paradigm shift towards a more responsive and precise form of oncology care (Table 3).
Furthermore, we systematically assessed the validation status of the major PTM-based findings discussed herein (Table 4). The applications discussed in this review are therefore organized according to their current level of clinical validation, which spans from clinically implemented assays, such as detection of SHOX2/RASSF1A methylation in liquid biopsies, and biomarkers correlated with outcomes in clinical cohorts, such as Trim35-mediated ubiquitination of LSD1 as a predictor of immunotherapy response, to promising preclinical findings, such as B4GALT2 glycosylation promoting immune exclusion, and novel mechanistic insights, such as the role of histone lactylation that await further investigation. This framework also documents specific limitations, including single-center studies and small sample sizes, aiming to provide a clear benchmark for assessing the maturity of each PTM-driven strategy.
Conclusion and outlook
The intricate involvement of PTMs in lung cancer pathogenesis has unveiled a wealth of novel therapeutic targets and strategies. Targeting the ubiquitin-proteasome system has yielded significant clinical advances. For instance, the E3 ligase Trim35 suppresses LSD1 demethylase activity via K63-linked polyubiquitination at Lys422, serving as a predictive biomarker for immunotherapy response in NSCLC (114). Furthermore, plant homeodomain (PHD) finger protein 23 (PHF23) stabilizes ACTN4 by inhibiting its K48-linked ubiquitination, promoting tumor progression yet paradoxically enhancing cisplatin/docetaxel sensitivity by facilitating DNA damage repair, presenting a complex but exploitable therapeutic node (115).
Beyond ubiquitination, inhibiting specific modifying enzymes represents a mainstream approach. Treatment with norcantharidin (NCTD) reduces YAP expression and promotes its inactivation phosphorylation, effectively inhibiting NSCLC proliferation (128). This aligns with the development of TEAD palmitoylation inhibitors that target the downstream Hippo pathway effector, currently in phase I/II trials for NSCLC (129).Similarly, protein arginine methyltransferase 6 (PRMT6) promotes glycolysis and cisplatin resistance by methylating metabolic enzymes 6PGD and ENO1. The PRMT6 inhibitor DCPR049_12 effectively reverses these effects and enhances chemosensitivity (123). The clinical potential of PRMT inhibition is underscored by the ongoing evaluation of PRMT5 inhibitors in solid tumors (NCT02783300), highlighting the druggability of this enzyme class (130).
Combination therapies targeting PTM-mediated resistance mechanisms are increasingly vital. In ALK-positive NSCLC, phosphorylation-mediated activation of SRC kinase contributes to drug resistance, and combined ALK/SRC inhibition significantly improves therapeutic efficacy (131). Chen et al. identified acetylation of histone H1.4 at K75 as a novel oncogenic mechanism, with the H1.4K75 mutation suppressing malignancy, presenting a compelling rationale for developing inhibitors targeting this acetylation site (127). The clinical success of Valemetostat, a novel dual inhibitor of EZH1 and EZH2, suggested significant clinical efficacy with a 44% objective response rate in a phase 2 trial involving 119 patients with relapsed or refractory peripheral T-cell lymphoma, establishing EZH inhibition as a promising therapeutic strategy for T-cell malignancies (132).
It is worth noting that some studies highlight the emerging significance of cross-talk among PTMs as a pivotal mechanism underlying therapy resistance in lung cancer. Kim et al. (24) revealed that O-GlcNAcylation at Thr63 of SMAD4 impedes its interaction with GSK-3β, thereby suppressing ubiquitin-mediated degradation and stabilizing SMAD4 to enhance TGF-β signaling, which promotes epithelial-mesenchymal transition and metastasis. Complementing this, Wattanathamsan et al. (85) suggested that tubulin acetylation, induced by chemotherapeutic stress, recruits and stabilizes the anti-apoptotic protein Mcl-1 on microtubules, inhibiting its polyubiquitination and conferring resistance to paclitaxel-induced apoptosis. Further expanding this paradigm, Peng et al. (103) showed that HSP70-mediated SUMOylation of HIF-1α after insufficient radiofrequency ablation not only drives tumor recurrence but also suppresses ferroptosis by downregulating key effectors, such as SLC7A11 and ACSL3. Collectively, these studies underscore a synergistic cross-talk between O-GlcNAcylation, acetylation, and SUMOylation pathways, which converge to stabilize oncogenic proteins, such as SMAD4, Mcl-1, HIF-1α, and coordinately inhibit apoptosis. This mechanistic insight advocates for targeting PTM networks as a promising strategy to overcome resistance.
PTMs play a significant role in cell growth, cell signaling regulation, protein localization, and maintaining cellular function by altering protein structure and function. The study of the mechanisms and functions of protein PTMs offers new opportunities in biopharmaceuticals, promising more precise and effective diagnostics and treatments. It can also provide new targets and screening methods for drug discovery and development, potentially accelerating the discovery and development of new drugs.
Limitations and future perspectives
While this review has synthesized the critical roles of diverse PTMs, including phosphorylation, ubiquitination, methylation, acetylation, and SUMOylation, in driving lung cancer pathogenesis and therapy resistance, a fundamental limitation inherent to our synthesis, and to much of the current literature, is its reliance on a static and compartmentalized analytical framework. The majority of evidence discussed herein is derived from single-timepoint analyses of cell lines or clinical samples, captured either at baseline or upon disease progression. This approach, while invaluable for establishing mechanistic links, fails to capture the dynamic evolution and intricate crosstalk of PTM networks throughout the entire therapeutic continuum. To bridge this gap and truly translate PTM biology into clinically actionable strategies, future research must pivot towards longitudinal PTM monitoring, such as the integration of serial liquid biopsy protocols into prospective clinical trial designs.Implementing such a strategy would move the field beyond a static snapshot to a dynamic movie of tumor evolution, which has extremely positive implications for the development of predictive biomarkers and the prevention and treatment of tumors.
The clinical translation of PTM-based biomarkers faces significant technical hurdles across primary detection methodologies. A foremost limitation is the inherent lack of antibody specificity for modified epitopes in immunoassays, where cross-reactivity and sensitivity to adjacent modifications risk yielding false-positive interpretations of PTM abundance and signaling activity. Furthermore, quantitative MS, despite its unbiased discovery power, grapples with substantial technical variability in sample preparation and instrument performance, necessitating robust yet challenging normalization strategies to ensure accurate quantification of low-abundance PTM peptides. Compounding these issues, pre-analytical variability in extracellular vesicle (EV) isolation, driven by methodological differences and sample handling protocols, introduces profound inconsistencies in yield, purity, and detected PTM signatures, severely limiting the reproducibility of liquid biopsy-based approaches. Addressing these multifaceted barriers demands a concerted effort to develop highly specific detection reagents, establish standardized proteomic workflows, and implement international consensus protocols for EV analysis, which are essential prerequisites to realizing the full clinical potential of dynamic PTM profiling.
Author contributions
YZ: Writing – original draft. XS: Data curation, Writing – original draft, Investigation. WL: Resources, Writing – original draft. FX: Writing – original draft, Resources. JS: Investigation, Writing – original draft. JHH: Writing – review & editing, Funding acquisition. ZH: Writing – review & editing, Formal Analysis, Supervision. JJH: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Guangdong Provincial Medical Science and Technology Research Fund Projects (Nos.A2024270), Science and Technology Project of Panyu, Guangzhou (Grant Nos. 2025-Z04-22).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108
3. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveil lanceof trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513–025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
4. Chato-Astrain I, Pronot M, Coppola T, and Martin S. Molecular organization and regulation of the mammalian synapse by the post-translational modification SUMOylation. Cells. (2024) 13:420. doi: 10.3390/cells13050420
5. Xue X, Zhang X, Sun F, and Wang J. Emerging role of protein post-translational modification in the potential clinical application of cancer. NANO Life. (2020) 10:2040008. doi: 10.1142/s1793984420400085
6. Liu J and Zhu P. A novel gene signature associated with protein post-translational modification to predict clinical outcomes and therapeutic responses of colorectal cancer. Mol Biotechnol. (2023) 66:2106–22. doi: 10.1007/s12033-023-00852-6
7. Eisenberg-Lerner A, Ciechanover A, and Merbl Y. Post-translational modification profiling - A novel tool for mapping the protein modification landscape in cancer. Exp Biol Med (Maywood). (2016) 241:1475–82. doi: 10.1177/1535370216651732
8. Heo KS. Regulation of post-translational modification in breast cancer treatment. BMB Rep. (2019) 52:113–8. doi: 10.5483/BMBRep.2019.52.2.017
9. Munkley J and Elliott DJ. Hallmarks of glycosylation in cancer. Oncotarget. (2016) 7:35478–89. doi: 10.18632/oncotarget.8155
10. Yang L, Zeng Q, Deng Y, Qiu Y, Yao W, and Liao Y. Glycosylated cathepsin V serves as a prognostic marker in lung cancer. Front Oncol. (2022) 12:876245. doi: 10.3389/fonc.2022.876245
11. Kondo K, Harada Y, Nakano M, Suzuki T, Fukushige T, Hanzawa K, et al. Identification of distinct N-glycosylation patterns on extracellular vesicles from small-cell and non-small-cell lung cancer cells. J Biol Chem. (2022) 298:101950. doi: 10.1016/j.jbc.2022.101950
12. Lucchetta M, da Piedade I, Mounir M, Vabistsevits M, Terkelsen T, and Papaleo E Distinct signatures of lung cancer types: aberrant mucin O-glycosylation and compromised immune response. BMC Cancer. (2019) 19:824. doi: 10.1186/s12885-019-5965-x
13. Ahmmed B, Khan MN, Nisar MA, Kampo S, Zheng Q, Li Y, et al. Tunicamycin enhances the suppressive effects of cisplatin on lung cancer growth through PTX3 glycosylation via AKT/NF-κB signaling pathway. Int J Oncol. (2018) 54:431–42. doi: 10.3892/ijo.2018.4650
14. Gardelli C, Russo L, Cipolla L, Moro M, Andriani F, Rondinone O, et al. Differential glycosylation of collagen modulates lung cancer stem cell subsets through β1 integrin-mediated interactions. Cancer Sci. (2020) 112:217–30. doi: 10.1111/cas.14700
15. Chen T, He C, Zhang M, Li X, Liu X, Liu Y, et al. Disease-specific haptoglobin-β chain N-glycosylation as biomarker to differentiate non-small cell lung cancer from benign lung diseases. J Cancer. (2019) 10:5628–37. doi: 10.7150/jca.32690
16. Gao Z, Wu Z, Han Y, Zhang X, Hao P, Xu M, et al. Aberrant fucosylation of saliva glycoprotein defining lung adenocarcinomas Malignancy. ACS Omega. (2022) 7:17894–906. doi: 10.1021/acsomega.2c01193
17. Alvarez MR, Zhou Q, Tena J, Barboza M, Wong M, Xie Y, et al. Glycomic, glycoproteomic, and proteomic profiling of philippine lung cancer and peritumoral tissues: case series study of patients stages I-III. Cancers (Basel). (2023) 15:1559. doi: 10.3390/cancers15051559
18. Taparra K, Wang H, Malek R, Lafargue A, Barbhuiya MA, Wang X, et al. O-GlcNAcylation is required for mutant KRAS-induced lung tumorigenesis. J Clin INVEST. (2018) 128:4924–37. doi: 10.1172/JCI94844
19. Chen S, Wang Y, Liu W, Liang Y, Wang Y, Wu Z, et al. N-glycosylation at asn291 stabilizes TIM-4 and promotes the metastasis of NSCLC. Front Oncol. (2022) 12):730530. doi: 10.3389/fonc.2022.730530
20. Han CL, Chen XR, Lan A, Hsu YL, Wu PS, Hung PF, et al. N-glycosylated GPNMB ligand independently activates mutated EGFR signaling and promotes metastasis in NSCLC. Cancer Sci. (2021) 112:1911–23. doi: 10.1111/cas.14872
21. Lee SH, Khwaja Rehman F, Tyler KC, Yu B, Zhang Z, Osuka S, et al. A chimeric signal peptide-galectin-3 conjugate induces glycosylation-dependent cancer cell-specific apoptosis. Clin Cancer Res. (2020) 26:2711–24. doi: 10.1158/1078-0432.CCR-18-3280
22. Ohkawa Y, Kitano M, Maeda K, Nakano M, Kanto N, Kizuka Y, et al. Core fucosylation is required for the secretion of and the enzymatic activity of SOD3 in nonsmall-cell lung cancer cells. Antioxid Redox Signal. (2023) 38:1201–11. doi: 10.1089/ars.2022.0010
23. Hu Q, Tian T, Leng Y, Tang Y, Chen S, Lv Y, et al. The O-glycosylating enzyme GALNT2 acts as an oncogenic driver in non-small cell lung cancer. Cell Mol Biol Lett. (2022) 27:71. doi: 10.1186/s11658-022-00378-w
24. Kim YJ, Kang MJ, Kim E, Kweon TH, Park YS, Ji S, et al. O-GlcNAc stabilizes SMAD4 by inhibiting GSK-3β-mediated proteasomal degradation. Sci Rep. (2020) 10:19908. doi: 10.1038/s41598-020-76862-0
25. Appadurai MI, Chaudhary S, Shah A, Natarajan G, Alsafwani ZW, Khan P, et al. ST6GalNAc-I regulates tumor cell sialylation via NECTIN2/MUC5AC-mediated immunosuppression and angiogenesis in non-small cell lung cancer. J Clin Invest. (2025) 135:e186863. doi: 10.1172/JCI186863
26. Zhang X, Cui Y, Yu M, Su B, Gong W, Baluška F, et al. Phosphorylation-mediated dynamics of nitrate transceptor NRT1.1 regulate auxin flux and nitrate signaling in lateral root growth. Plant Physiol. (2019) 181:480–98. doi: 10.1104/pp.19.00346
27. Moody TW, Ramos-Alvarez I, and Jensen RT. Adding of neurotensin to non-small cell lung cancer cells increases tyrosine phosphorylation of HER3. Peptides. (2022) 156:170858. doi: 10.1016/j.peptides.2022.170858
28. Bian T, Wang Y, Botello JF, Hu Q, Jiang Y, Zingone A, et al. LKB1 phosphorylation and deactivation in lung cancer by NNAL, a metabolite of tobacco-specific carcinogen, in an isomer-dependent manner. Oncogene. (2022) 41:4042–54. doi: 10.1038/s41388-022-02410-x
29. Kang C, Wang L, Wang D, Zhang X, and Chen J. Lung cancer A549 cells suppressed with overexpressed HNF1B or PCDHA13 inhibited PI3K/AKT phosphorylation. Transl Cancer Res. (2020) 9:3819–27. doi: 10.21037/tcr-20-1727
30. Chiu CH, Ramesh S, Liao PH, Kuo WW, Chen MC, Kuo CH, et al. Phosphorylation of Bcl-2 by JNK confers gemcitabine resistance in lung cancer cells by reducing autophagy-mediated cell death. Environ Toxicol. (2023) 38:2121–31. doi: 10.1002/tox.23836
31. Wu MF, Huang YH, and Chiu LY Curcumin Induces Apoptosis of Chemoresistant Lung Cancer Cells via ROS-Regulated p38 MAPK Phosphorylation. Int J Mol Sci. (2022) 23:8248. doi: 10.3390/ijms23158248
32. Liu S, Zhang Y, Yang Q, Zhang Y, Liu H, Huang MH, et al. PKC signal amplification suppresses non-small cell lung cancer growth by promoting p21 expression and phosphorylation. Heliyon. (2022) 8:e10657. doi: 10.1016/j.heliyon.2022.e10657
33. Wang R, Wang S, Li Z, Luo Y, Zhao Y, Han Q, et al. PLEKHH2 binds β-arrestin1 through its FERM domain, activates FAK/PI3K/AKT phosphorylation, and promotes the Malignant phenotype of non-small cell lung cancer. Cell Death Dis. (2022) 13:858. doi: 10.1038/s41419-022-05307-5
34. Qiao S, Jiang Y, Li N, and Zhu X. The kinesin light chain-2, a target of mRNA stabilizing protein HuR, inhibits p53 protein phosphorylation to promote radioresistance in NSCLC. Thorac Cancer. (2023) 14:1440–50. doi: 10.1111/1759-7714.14886
35. He S, Dong D, Lin J, Wu B, Nie X, and Cai G Overexpression of TRAF4 promotes lung cancer growth and EGFR-dependent phosphorylation of ERK5. FEBS Open Bio. (2022) 12:1747–60. doi: 10.1002/2211-5463.13458
36. Li L, Li P, Ma X, Zeng S, Peng Y, and Zhang G. Therapeutic restoring p53 function with small molecule for oncogene-driven non-small cell lung cancer by targeting serine 392 phosphorylation. Biochem Pharmacol. (2022) 203:115188. doi: 10.1016/j.bcp.2022.115188
37. Lu Y, Su F, Cheng Z, Yang J, Dai H, Yang J, et al. Nickel chloride promotes lung cancer invasion and metastasis by up-regulating the expression of E3 ubiquitin ligase TRIM31 through the IL-6/STAT3 signaling axis. Life Sci. (2023) 332:122111. doi: 10.1016/j.lfs.2023.122111
38. Zhang Q, Peng J, Li Z, Rao X, Allison DB, Qiao Q, et al. PLK1-mediated PDHA1 phosphorylation drives mitochondrial dysfunction, mitophagy, and cancer progression in Cr(VI)-associated lung cancer. J Biol Chem. (2025) 301:110406. doi: 10.1016/j.jbc.2025.110406
39. Liu K, Cheng L, Zhu K, Wang J, and Shu Q The cancer/testis antigen HORMAD1 mediates epithelial-mesenchymal transition to promote tumor growth and metastasis by activating the Wnt/β-catenin signaling pathway in lung cancer. Cell Death Discov. (2022) 8:136. doi: 10.1038/s41420-022-00946-1
40. Ning G, Lu C, Chen Y, Jiang M, Si P, and Zhang R. Transcription factor ZEB1 regulates PLK1-mediated SKA3 phosphorylation to promote lung cancer cell proliferation, migration and cell cycle. Anticancer Drugs. (2023) 34:866–76. doi: 10.1097/CAD.0000000000001477
41. Satoh K, Sakai S, and Nishizuka M. Knockdown of RhoQ, a member of Rho GTPase, accelerates TGF-β-induced EMT in human lung adenocarcinoma. Biochem Biophys Rep. (2022) 32):101346. doi: 10.1016/j.bbrep
42. Chen H, Zhao L, Meng Y, Qian X, Fan Y, Zhang Q, et al. Sulfonylurea receptor 1-expressing cancer cells induce cancer-associated fibroblasts to promote non-small cell lung cancer progression. Cancer Lett. (2022) 536:215611. doi: 10.1016/j.canlet.2022.215611
43. Lin X, Wang C, Qiu M, Lin M, and Wu H Long-chain non-coding RNA LINC00473 antagonist with liposomal nanoparticles as carrier targets for Keap1/Nrf2/ARE signaling pathway to promote lung cancer cell apoptosis. Mater Express. (2022) 12:988–96. doi: 10.1166/mex.2022.2224
44. Ye F, Cai Z, Wang B, Zeng C, Xi Y, Hu S, et al. TGFβ Antagonizes IFNγ-mediated adaptive immune evasion via activation of the AKT-smad3-SHP1 axis in lung adenocarcinoma. Cancer Res. (2023) 83:2262–77. doi: 10.1158/0008-5472.CAN-22-3009
45. Fennell LM, Rahighi S, and Ikeda F. Linear ubiquitin chain-binding domains. FEBS J. (2018) 285:2746–61. doi: 10.1111/febs.14478
46. Dittmar G and Winklhofer KF. Linear ubiquitin chains: cellular functions and strategies for detection and quantification. Front Chem. (2020) 7):915. doi: 10.3389/fchem.2019.00915
47. BommaReddy RR, Patel R, Smalley T, and Acevedo-Duncan M Effects of atypical protein kinase C inhibitor (DNDA) on lung cancer proliferation and migration by PKC-ι/FAK ubiquitination through the cbl-b pathway. Onco Targets Ther. (2020) 13:1661–76. doi: 10.2147/OTT.S224866
48. Huang Y, Yang X, Lu Y, Zhao Y, Meng R, Zhang S, et al. UBE2O targets Mxi1 for ubiquitination and degradation to promote lung cancer progression and radioresistance. Cell Death Differ. (2021) 28:671–84. doi: 10.1038/s41418-020-00616-8
49. Xu Y, Hu Y, Xu T, Yan K, Zhang T, Li Q, et al. RNF8-mediated regulation of Akt promotes lung cancer cell survival and resistance to DNA damage. Cell Rep. (2021) 37:109854. doi: 10.1016/j.celrep.2021.109854
50. Lu C, Ning G, Si P, Zhang C, Liu W, Ge W, et al. E3 ubiquitin ligase HECW1 promotes the metastasis of non-small cell lung cancer cells through mediating the ubiquitination of Smad4. Biochem Cell Biol. (2021) 99:675–81. doi: 10.1139/bcb-2020-0505
51. Yin H, Wang X, Zhang X, et al. Corrigendum to "UBE2T promotes radiation resistance in non-small cell lung cancer via inducing epithelial-mesenchymal transition and the ubiquitination-mediated FOXO1 degradation" [Canc. Lett. 494 (2020) 121-131. Cancer Lett. (2022) 543:215792. doi: 10.1016/j.canlet.2022.215792
52. Kim MJ, Chen G, Sica GL, and Deng X. Epigenetic modulation of FBW7/Mcl-1 pathway for lung cancer therapy. Cancer Biol Ther. (2021) 22:55–65. doi: 10.1080/15384047.2020.1856756
53. Zhou F, Du C, Xu D, Lu J, Zhou L, Wu C, et al. Knockdown of ubiquitin−specific protease 51 attenuates cisplatin resistance in lung cancer through ubiquitination of zinc−finger E−box binding homeobox 1. Mol Med Rep. (2020) 22:1382–90. doi: 10.3892/mmr.2020.11188
54. Zhang R, Zhang W, Zeng Y, Li Y, Zhou J, Zhang Y, et al. The regulation of CPNE1 ubiquitination by the NEDD4L is involved in the pathogenesis of non-small cell lung cancer. Cell Death Discov. (2021) 7:336. doi: 10.1038/s41420-021-00736-1
55. Guo Y, Chi X, Qu S, Sun Y, Liu J, Zhang L, et al. E3 ubiquitin-protein ligase 2 inhibits cell proliferation, migration, and invasion of non-small cell lung cancer through ubiquitination of Notch1. Acta Histochem. (2022) 124:151818. doi: 10.1016/j.acthis.2021.151818
56. Jiang X, Xu Y, Ren H, Jiang J, Wudu M, Wang Q, et al. KLHL18 inhibits the proliferation, migration, and invasion of non-small cell lung cancer by inhibiting PI3K/PD-L1 axis activity. Cell Biosci. (2020) 10:139. doi: 10.1186/s13578-020-00499-9
57. Xu P, Jiang L, Yang Y, Wu M, Liu B, Shi Y, et al. PAQR4 promotes chemoresistance in non-small cell lung cancer through inhibiting Nrf2 protein degradation. Theranostics. (2020) 10:3767–78. doi: 10.7150/thno.43142
58. Tang X, Li Y, Liu L, Guo R, Zhang P, Zhang Y, et al. Sirtuin 3 induces apoptosis and necroptosis by regulating mutant p53 expression in small−cell lung cancer. Oncol Rep. (2020) 43:591–600. doi: 10.3892/or.2019.7439
59. Lin Z, Lin X, Zhu L, Huang J, and Huang Y TRIM2 directly deubiquitinates and stabilizes Snail1 protein, mediating proliferation and metastasis of lung adenocarcinoma. Cancer Cell Int. (2020) 20:228. doi: 10.1186/s12935-020-01316-6
60. Zhong Y, Yang L, Xiong F, He Y, Tang Y, Shi L, et al. Long non-coding RNA AFAP1-AS1 accelerates lung cancer cells migration and invasion by interacting with SNIP1 to upregulate c-Myc. Signal Transduct Target Ther. (2021) 6:240. doi: 10.1038/s41392-021-00562-y
61. Cai J, Li M, Wang X, Li L, Li Q, Hou Z, et al. USP37 promotes lung cancer cell migration by stabilizing snail protein via deubiquitination. Front Genet. (2020) 10:1324. doi: 10.3389/fgene.2019.01324
62. Li B, Zhu L, Lu C, Wang C, Wang H, Jin H, et al. circNDUFB2 inhibits non-small cell lung cancer progression via destabilizing IGF2BPs and activating anti-tumor immunity. Nat Commun. (2021) 12:295. doi: 10.1038/s41467-020-20527-z
63. Zhu D, Nie Y, Zhao Y, Chen X, Yang Z, and Yang Y. RNF152 suppresses fatty acid oxidation and metastasis of lung adenocarcinoma by inhibiting IRAK1-mediated AKR1B10 expression. Am J Pathol. (2023) 193:1603–17. doi: 10.1016/j.ajpath.2023.06.014
64. Zhao Q, Cai D, Xu H, Gao Y, Zhang R, Zhou X, et al. o8G-modified circPLCE1 inhibits lung cancer progression via chaperone-mediated autophagy. Mol Cancer. (2025) 24:82. doi: 10.1186/s12943-025-02283-0
65. Chatterton Z, Lamichhane P, Ahmadi Rastegar D, Fitzpatrick L, Lebhar H, Marquis C, et al. Single-cell DNA methylation sequencing by combinatorial indexing and enzymatic DNA methylation conversion. Cell Biosci. (2023) 13:2. doi: 10.1186/s13578-022-00938-9
66. Mashayekhi M, Asadi M, Hashemzadeh S, Vahedi A, Shanehbandi D, Al-Omar AF, et al. Promoter methylation levels of RASSF1 and ATIC genes are associated with lung cancer in Iranian patients. Horm Mol Biol Clin Investig. (2023) 44:145–52. doi: 10.1515/hmbci-2022-0007
67. Vo TTL, Nguyen TN, Nguyen TT, Pham ATD, Vuong DL, Ta VT, et al. SHOX2 methylation in Vietnamese patients with lung cancer. Mol Biol Rep. (2022) 49:3413–21. doi: 10.1007/s11033-022-07172-z
68. Li J, Qi L, Zhang M, Feng J, Zheng Z, Chen C, et al. PRKCDBP methylation is a potential and promising candidate biomarker for non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. (2022) 25:78–85. doi: 10.3779/j.issn.1009-3419.2022.102.03
69. Kontic M, Jovanovic D, Kern I, Nelson HH, Bojic S, Ognjanovic M, et al. Is hypermethylation of SOX1 gene an independent prognostic marker in surgically resected non-small cell lung cancer? J Cancer Res Ther. (2022) 18:1692–6. doi: 10.4103/jcrt.JCRT_125_20
70. Wu Q, Yan Y, Shi S, Qi Q, and Han J DNMT3b-mediated SPAG6 promoter hypermethylation affects lung squamous cell carcinoma development through the JAK/STAT pathway. Am J Transl Res. (2022) 14:6964–77.
71. Shi Z, Zhang H, Shen Y, Zhang S, Zhang X, Xu Y, et al. SETD1A-mediated H3K4me3 methylation upregulates lncRNA HOXC-AS3 and the binding of HOXC-AS3 to EP300 and increases EP300 stability to suppress the ferroptosis of NSCLC cells. Thorac Cancer. (2023) 14:2579–90. doi: 10.1111/1759-7714.15037
72. Zhao L, Xie H, Li P, Chen H, He J, Wang L, et al. CircTFF1 Promotes Proliferation, Migration and Invasion of Lung Cancer Cells by Facilitating Methylation of BCL6B Promoter via miR-29c-3p/DNMT3A Axis. Mol Biotechnol. (2023) 65:942–52. doi: 10.1007/s12033-022-00594-x
73. Li D, Lv H, Gao H, Wang Z, Wang D, Tian K, et al. Circular RNA hsa_circ_0077837 is upregulated in non-small cell lung cancer to downregulate phosphatase and tensin homolog through methylation. Bioengineered. (2022) 13:6711–8. doi: 10.1080/21655979.2022.2025707
74. Zhou Q, Lai X, Gao Y, Chen Q, Xu Y, and Liu Y. METTL14 regulates PLAGL2/β-catenin signaling axis to promote the development of nonsmall cell lung cancer. J Oncol. (2023) 2023:4738586. doi: 10.1155/2023/4738586
75. Lin C, Li T, Wang Y, Lai S, Huang Y, Guo Z, et al. Correction: METTL3 enhances pancreatic ductal adenocarcinoma progression and gemcitabine resistance through modifying DDX23 mRNA N6 adenosine methylation. Cell Death Dis. (2023) 14:485. doi: 10.1038/s41419-023-05939-1
76. Wang D, Zu Y, Sun W, and Fan X. SETD1A-mediated methylation of H3K4me3 inhibits ferroptosis in non-small cell lung cancer by regulating the WTAPP1/WTAP axis. Curr Med Chem. (2024) 31:3217–31. doi: 10.2174/09298673306662305251432522
77. Liang Z, Zhao B, and Huang J. Correlation and clinical significance of methyltransferases SETDB1 and SPG20 methylation in lung adenocarcinoma SDRP-JCMP. J Cell Mol Physiol. (2022) 4:228–37. doi: 10.25177/jcmp.4.1.ra.10821
78. Yu J, Ge Z, Chen S, Li S, Zhang X, Hu J, et al. miR-26a-5p suppresses wnt/β-catenin signaling pathway by inhibiting DNMT3A-mediated SFRP1 methylation and inhibits cancer stem cell-like properties of NSCLC. Dis Markers. (2022) 2022:7926483. doi: 10.1155/2022/7926483
79. Li C, Wang W, Sun Y, Ni Y, Qin F, Li X, et al. Selective sorting and secretion of hY4 RNA fragments into extracellular vesicles mediated by methylated YBX1 to promote lung cancer progression. J Exp Clin Cancer Res. (2022) 41:136. doi: 10.1186/s13046-022-02346-w
80. Dai J, Lu X, Zhang C, Qu T, Li W, Su J, et al. NNMT promotes acquired EGFR-TKI resistance by forming EGR1 and lactate-mediated double positive feedback loops in non-small cell lung cancer. Mol Cancer. (2025) 24:79. doi: 10.1186/s12943-025-02285-y
81. Xia C, Tao Y, Li M, Che T, and Qu J. Protein acetylation and deacetylation: An important regulatory modification in gene transcription (Review). Exp Ther Med. (2020) 20:2923–40. doi: 10.3892/etm.2020.9073
82. Shimizu K, Gi M, Suzuki S, North BJ, Watahiki A, Fukumoto S, et al. Interplay between protein acetylation and ubiquitination controls MCL1 protein stability. Cell Rep. (2021) 37:109988. doi: 10.1016/j.celrep.2021.109988
83. Kong F, Ma L, Wang X, You H, Zheng K, and Tang R Regulation of epithelial-mesenchymal transition by protein lysine acetylation. Cell Commun Signal. (2022) 20:57. doi: 10.1186/s12964-022-00870-y
84. Ni Y, Yang Y, Ran J, Zhang L, Yao M, Liu Z, et al. miR-15a-5p inhibits metastasis and lipid metabolism by suppressing histone acetylation in lung cancer. Free Radic Biol Med. (2020) 161:150–62. doi: 10.1016/j.freeradbiomed.2020.10.009
85. Wattanathamsan O, Thararattanobon R, Rodsiri R, Chanvorachote P, Vinayanuwattikun C, and Pongrakhananon V Tubulin acetylation enhances lung cancer resistance to paclitaxel-induced cell death through Mcl-1 stabilization. Cell Death Discov. (2021) 7:67. doi: 10.1038/s41420-021-00453-9
86. Feng HP, Liu YC, Wang CL, Liao WC, Yu JS, and Yu CJ Acetylation regulates the nucleocytoplasmic distribution and oncogenic function of karyopherin alpha 2 in lung adenocarcinoma. Biochem BIOPH Res CO. (2023) 659:96–104. doi: 10.1016/j.bbrc.2023.04.014
87. Wang T, Lu Z, Han T, Wang Y, Gan M, and Wang JB Deacetylation of glutaminase by HDAC4 contributes to lung cancer tumorigenesis. Int J Biol Sci. (2022) 18:4452–65. doi: 10.7150/ijbs.69882
88. Hu Y, Mu H, and Deng Z. H3K27 acetylation activated-CCS regulates autophagy and apoptosis of lung cancer by alleviating oxidative stress. Tissue Cell. (2023) 80:101964. doi: 10.1016/j.tice.2022.101964
89. Wu K, Chen X, Chen X, Zhang S, Xu Y, Xia B, et al. Suberoylanilide hydroxamic acid enhances the radiosensitivity of lung cancer cells through acetylated wild-type and mutant p53-dependent modulation of mitochondrial apoptosis. J Int Med Res. (2021) 49:300060520981545. doi: 10.1177/0300060520981545
90. Zhu H, Hu Y, Zeng C, Chang L, Ge F, Wang W, et al. The SIRT2-mediated deacetylation of AKR1C1 is required for suppressing its pro-metastasis function in Non-Small Cell Lung Cancer. Theranostics. (2020) 10:2188–200. doi: 10.7150/thno.39151
91. Qian X, Zhu L, Xu M, Liu H, Yu X, Shao Q, et al. Shikonin suppresses small cell lung cancer growth via inducing ATF3-mediated ferroptosis to promote ROS accumulation. Chem Biol Interact. (2023) 382:110588. doi: 10.1016/j.cbi.2023.110588
92. Li Z, Yu DP, Wang N, Tao T, Luo W, and Chen H. SIRT5 promotes non-small cell lung cancer progression by reducing FABP4 acetylation level. Neoplasma. (2022) 69:909–17. doi: 10.4149/neo_2022_220107N28
93. Jiao M, Guo Y, Zhang H, Wen H, Chen P, Wang Z, et al. ACAT1 regulates tertiary lymphoid structures and correlates with immunotherapy response in non-small cell lung cancer. J Clin Invest. (2025) 135:e181517. doi: 10.1172/JCI181517
94. Liu J, Shangguan Y, Tang D, and Dai Y. Histone succinylation and its function on the nucleosome. J Cell Mol Med. (2021) 25:7101–9. doi: 10.1111/jcmm.16676
95. Wan J, Liu H, Chu J, and Zhang H. Functions and mechanisms of lysine crotonylation. J Cell Mol Med. (2019) 23:7163–9. doi: 10.1111/jcmm.14650
96. Lin ZF, Xu HB, Wang JY, Lin Q, Ruan Z, Liu FB, et al. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem Biophys Res Commun. (2013) 441:191–5. doi: 10.1016/j.bbrc.2013.10.033
97. Hu Q, Xu J, Wang L, Yuan Y, Luo R, Gan M, et al. SUCLG2 regulates mitochondrial dysfunction through succinylation in lung adenocarcinoma. Adv Sci (Weinh). (2023) 10:e2303535. doi: 10.1002/advs.202303535
98. Mu N, Wang Y, Li X, Du Z, Wu Y, Su M, et al. Crotonylated BEX2 interacts with NDP52 and enhances mitophagy to modulate chemotherapeutic agent-induced apoptosis in non-small-cell lung cancer cells. Cell Death Dis. (2023) 14:645. doi: 10.1038/s41419-023-06164-6
99. Han ZJ, Feng YH, Gu BH, Li YM, and Chen H. The post-translational modification, SUMOylation, and cancer (Review). Int J Oncol. (2018) 52:1081–94. doi: 10.3892/ijo.2018.4280
100. Hung PF, Hong TM, Chang CC, Hung CL, Hsu YL, Chang YL, et al. Hypoxia-induced Slug SUMOylation enhances lung cancer metastasis. J Exp Clin Cancer Res. (2019) 38:5. doi: 10.1186/s13046-018-0996-8
101. Wu C, Zhu X, Dai Q, Chu Z, Yang S, and Dong Z. SUMOylation of SMAD4 by PIAS1 in conjunction with vimentin upregulation promotes migration potential in non-small cell lung cancer. Front Biosci (Landmark Ed). (2023) 28:192. doi: 10.31083/j.fbl2808192
102. Sun L, Li H, Chen J, Dehennaut V, Zhao Y, Yang Y, et al. A SUMOylation-dependent pathway regulates SIRT1 transcription and lung cancer metastasis. J Natl Cancer Inst. (2013) 105:887–98. doi: 10.1093/jnci/djt118
103. Peng B, Ling X, Huang T, and Wan J HSP70 via HIF-1α SUMOylation inhibitsferroptosis inducing lung cancer recurrence after insufficient radio frequency ablation. PloS One. (2023) 18:e0294263. doi: 10.1371/journal.pone.0294263
104. Yang H, Du Y, Fei X, Huang S, Yimiti M, Yang X, et al. SUMOylation of the ubiquitin ligase component KEAP1 at K39 upregulates NRF2 and its target function in lung cancer cell proliferation. J Biol Chem. (2023) 299:105215. doi: 10.1016/j.jbc.2023.105215
105. Wang M and Jiang X. SUMOylation of vascular endothelial growth factor receptor 2 inhibits the proliferation, migration, and angiogenesis signaling pathway in non-small cell lung cancer. Anticancer Drugs. (2020) 31:492–9. doi: 10.1097/CAD.0000000000000896
106. Wan J, Zhou M, Ling X, Ding G, and Wang J HSP70 promotes heat tolerance effect of lung cancer cells through mediating SUMOylation of HIF-1α. J Biomater Tissue Eng. (2020) 10:1077–84. doi: 10.1166/jbt.2020.2378
107. Chen B, Luo J, Zhou Y, Xin X, Cai R, and Ling C PIASy antagonizes Ras-driven NSCLC survival by promoting GATA2 SUMOylation. J Cancer. (2018) 9:1689–97. doi: 10.7150/jca.24137
108. Tantai J, Pan X, and Hu D. RNF4-mediated SUMOylation is essential for NDRG2 suppression of lung adenocarcinoma. Oncotarget. (2016) 7:26837–43. doi: 10.18632/oncotarget.8663
109. Diao X, Guo C, Zheng H, Zhao K, Luo Y, An M, et al. SUMOylation-triggered ALIX activation modulates extracellular vesicles circTLCD4-RWDD3 to promote lymphatic metastasis of non-small cell lung cancer. Signal Transduct Target Ther. (2023) 8:426. doi: 10.1038/s41392-023-01685-0
110. Yang X, Liu Y, Jiang W, Liu X, Zhang X, Liu H, et al. Identification of SUMOylation modifiers involved in lung adenocarcinoma progression and Osimertinib resistance by integrated bioinformatics analysis. Sci Rep. (2025) 15:31130. doi: 10.1038/s41598-025-16615-z
111. Zhang X, Maity T, Kashyap MK, Bansal M, Venugopalan A, Singh S, et al. Quantitative tyrosine phosphoproteomics of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor-treated lung adenocarcinoma cells reveals potential novel biomarkers of therapeutic response. Mol Cell Proteomics. (2017) 16:891–910. doi: 10.1074/mcp.M117.067439
112. Zhang P, Wang D, Zhou G, Jiang S, Zhang G, Zhang L, et al. Novel post-translational modification learning signature reveals B4GALT2 as an immune exclusion regulator in lung adenocarcinoma. J Immunother Cancer. (2025) 13:e010787. doi: 10.1136/jitc-2024-0107877
113. Grolleau E, Candiracci J, Lescuyer G, Barthelemy D, Benzerdjeb N, Haon C, et al. Circulating H3K27 methylated nucleosome plasma concentration: synergistic information with circulating tumor DNA molecular profiling. Biomolecules. (2023) 13:1255. doi: 10.3390/biom13081255
114. Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. (2016) 7:12632. doi: 10.1038/ncomms12632
115. Moding EJ, Liu Y, Nabet BY, Chabon JJ, Chaudhuri AA, Hui AB, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small cell lung cancer. Nat Cancer. (2020) 1:176–83. doi: 10.1038/s43018-019-0011-0
116. Yang H, Gu X, Wang Z, Liu G, Niu Y, Pan X, et al. Predicting non-small cell lung cancer lymph node metastasis: integrating ctDNA mutation/methylation profiling with positron emission tomography-computed tomography (PET-CT) scan: protocol for a prospective clinical trial (LUNon-invasive Study). J Thorac Dis. (2024) 16:6272–85. doi: 10.21037/jtd-24-1033
117. Cao LL, Song X, Pei L, Liu L, Wang H, and Jia M. Histone deacetylase HDAC1 expression correlates with the progression and prognosis of lung cancer: A meta-analysis. Med (Baltimore). (2017) 96:e7663. doi: 10.1097/MD.0000000000007663
118. Wang Y, Liu F, Fang C, Xu L, Chen L, Xu Z, et al. Combination of rapamycin and SAHA enhanced radiosensitization by inducing autophagy and acetylation in NSCLC. Aging (Albany NY). (2021) 13:18223–37. doi: 10.18632/aging.203226
119. Pan CH, Chang YF, Lee MS, Wen BC, Ko JC, Liang SK, et al. Vorinostat enhances the cisplatin-mediated anticancer effects in small cell lung cancer cells. BMC Cancer. (2016) 16:857. doi: 10.1186/s12885-016-2888-7
120. Cheng M, Cao H, Yao P, Guan J, Wu P, Ji H, et al. PHF23 promotes NSCLC proliferation, metastasis, and chemoresistance via stabilization of ACTN4 and activation of the ERK pathway. Cell Death Dis. (2023) 14:558. doi: 10.1038/s41419-023-06069-4
121. Bond MJ, Chu L, Nalawansha DA, Li K, and Crews CM. Targeted degradation of oncogenic KRASG12C by VHL-recruiting PROTACs. ACS Cent Sci. (2020) 6:1367–75. doi: 10.1021/acscentsci.0c00411
122. Guo J, Wu Y, Yang L, Du J, Gong K, Chen W, et al. Repression of YAP by NCTD disrupts NSCLC progression. Oncotarget. (2017) 8:2307–19. doi: 10.18632/oncotarget.13668
123. Sun M, Li L, Niu Y, Wang Y, Yan Q, Xie F, et al. PRMT6 promotes tumorigenicity and cisplatin response of lung cancer through triggering 6PGD/ENO1 mediated cell metabolism. Acta Pharm Sin B. (2023) 13:157–73. doi: 10.1016/j.apsb.2022.05.019
124. Geffen Y, Anand S, Akiyama Y, Yaron TM, Song Y, Johnson JL, et al. Pan-cancer analysis of post-translational modifications reveals shared patterns of protein regulation. Cell. (2023) 186:3945–3967.e26. doi: 10.1016/j.cell.2023.07.013
125. Gao H, Yang J, He L, Wang W, Liu Y, Hu Y, et al. The diagnostic potential of SHOX2 and RASSF1A DNA methylation in early lung adenocarcinoma. Front Oncol. (2022) 12:849024. doi: 10.3389/fonc.2022.849024
126. Tang F, Lu C, He X, Lin W, Xie B, Gao X, et al. E3 ligase Trim35 inhibits LSD1 demethylase activity through K63-linked ubiquitination and enhances anti-tumor immunity in NSCLC. Cell Rep. (2023) 42:113477. doi: 10.1016/j.celrep.2023.113477
127. Chen W, Duan X, Zhu Z, Han Y, and Li Y. Histone H1.4K75 acetylation promotes tumor growth and migration by regulating p53 and ERK1/2 pathway in non-small lung cancer. Biochem Biophys Res Commun. (2025) 768:151880. doi: 10.1016/j.bbrc.2025.151880
128. Witta SE, Gemmill RM, Hirsch FR, Coldren CD, Hedman K, Ravdel L, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. (2006) 66:944–50. doi: 10.1158/0008-5472.CAN-05-1988
129. Edwards AC, Stalnecker CA, Jean Morales A, Taylor KE, Klomp JE, Klomp JA, et al. TEAD inhibition overcomes YAP1/TAZ-driven primary and acquired resistance to KRASG12C inhibitors. Cancer Res. (2023) 83:4112–29. doi: 10.1158/0008-5472.CAN-23-2994
130. Pastore F, Bhagwat N, Pastore A, Radzisheuskaya A, Karzai A, Krishnan A, et al. PRMT5 inhibition modulates E2F1 methylation and gene-regulatory networks leading to therapeutic efficacy in JAK2V617F-mutant MPN. Cancer Discov. (2020) 10:1742–57. doi: 10.1158/2159-8290.CD-20-0026
131. Zhao Y, Yang Y, Xu Y, Lu S, and Jian H. AZD0530 sensitizes drug-resistant ALK-positive lung cancer cells by inhibiting SRC signaling. FEBS Open Bio. (2017) 7:472–476.10. doi: 10.1002/2211-5463.12162
132. Zinzani PL, Izutsu K, Mehta-Shah N, Barta SK, Ishitsuka K, Córdoba R, et al. Valemetostat for patients with relapsed or refractory peripheral T-cell lymphoma (VALENTINE-PTCL01): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. (2024) 25:1602–13. doi: 10.1016/S1470-2045(24)00503-5
Keywords: post-translational modifications, lung cancer, diagnosis, treatment, prognosis, progression
Citation: Zhao Y, Song X, Luo W, Xie F, Shen J, He J, Han Z and Huang J (2025) Post-translational modifications of protein and lung cancer. Front. Oncol. 15:1667200. doi: 10.3389/fonc.2025.1667200
Received: 07 August 2025; Accepted: 29 October 2025;
Published: 11 November 2025.
Edited by:
Maria Rosaria De Filippo, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyReviewed by:
Jonathan Puente Rivera, Hospital Juárez de México, MexicoTheofanis Vavilis, European University Cyprus, Cyprus
Copyright © 2025 Zhao, Song, Luo, Xie, Shen, He, Han and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinju Huang, NDY5ODE2M0BxcS5jb20=; JinHua He, aGVqaW5odWFAcHlob3NwaXRhbC5jb20uY24=; Zeping Han, aGFuemVwaW5nMTk4N0AxMjYuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Ying Zhao
Ying Zhao Xiaoyu Song
Xiaoyu Song WenFeng Luo
WenFeng Luo Fangmei Xie
Fangmei Xie Jian Shen
Jian Shen JinHua He
JinHua He Zeping Han
Zeping Han Jinju Huang
Jinju Huang