- 1Department of Molecular Medicine, Mayo Clinic, Rochester, MN, United States
- 2Department of Oncology, Mayo Clinic, Rochester, MN, United States
- 3Department of Radiation Oncology, Mayo Clinic, Phoenix, AZ, United States
- 4Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, United States
- 5Department of Orthopedic Surgery, Mayo Clinic, Rochester, MN, United States
Introduction: Ewing sarcoma (ES) is a malignancy that mostly affects adolescents and young adults, with relapse or refractory cases posing major therapeutic challenges. Its unique transcriptional profile offers multiple targetable pathways, including the insulin-like growth factor-1 (IGF-1) receptor (IGF-1R) pathway.
Case Report: We present the case of a 42-year-old female with recurrent ES with pulmonary metastases who, after progressing on anti-IGF-1R monotherapy with figitumumab (CP-751,871, NCT00560235), achieved complete remission in a phase I clinical trial (NCT00976508) that combined figitumumab IGF-1R-inhibition with growth hormone receptor antagonist pegvisomant. The patient has remained in long-term remission (>10 years) since the discontinuation of both agents and has not received any additional therapeutic interventions.
Literature Review: We reviewed PubMed and the ClinicalTrials.gov database to identify clinical trials employing IGF-1R-targeted therapies in patients with ES and identified 24 relevant studies treating 723 patients with anti-IGF-1R therapy.
Conclusion: This case represents the first report to our knowledge of patient outcomes following IGF-1R and growth hormone inhibition combination. The impressive response observed highlights the clinical synergy of this combination which warrants further clinical exploration as well as the potential of IGF-1R inhibition for ES. Additionally, this case suggests that targeted therapy discontinuation might be an option for select patients with long-term complete remission.
Introduction
A better understanding of the physiology of cancer growth and progression has led to the identification of molecular targets and the development of effective targeted therapies, revolutionizing the treatment of solid and hematological malignancies (1). This progress extends to sarcomas, as an increasing proportion of these tumors appear to be driven by specific signaling pathways that could serve as novel therapeutic targets (1–6).
Ewing sarcoma (ES), the second most common malignant bone tumor in adolescents and young adults, is characterized by a high recurrence rate, frequent development of multi-drug resistance, and therefore poor survival following relapse (7, 8). ES is caused by pathognomonic translocations juxtaposing the EWS RNA binding protein 1 (EWSR1) gene with one of E26 transformation-specific (ETS) genes, with EWSR1 being most commonly fused with friend leukemia integration 1 (FLI1) or ETS-related gene (ERG) (9, 10). These typical translocations can alter the transcription of multiple gene activating pathways critical for oncogenesis and metastasis (11).
The insulin-like growth factor-1 (IGF-1) receptor (IGF-1R) pathway is among the dysregulated pathways affected by the EWS-FLI1 fusion. Its persistent activation has been demonstrated in ES cell lines and clinical samples, suggesting a key role in disease pathogenesis (12–14). Consequently, multiple clinical attempts have been made to inhibit IGF-1R signaling (15–33) with collective analysis of data from phase I and II clinical trials targeting this pathway in ES, having demonstrated a response rate of 10-14% (34, 35). Figitumumab (previously known as CP-751,871), a fully humanized monoclonal antibody (mAb) targeting IGF-1R, has been investigated as monotherapy and in combination with other agents in several phase I-III clinical trials (28, 31, 36–39).
We present a case of a patient with recurrent, refractory ES who achieved a complete response following treatment with dual IGF-1R and growth hormone receptor inhibition and review published literation on IGF-1R inhibition in ES. Additionally, we briefly present the efficacy and toxicity data of the NCT00976508 trial, which has not been published in a prior manuscript. This case is significant for multiple reasons. First, our patient’s response highlights the therapeutic potential of IGF-1R inhibition in ES. Second, the fact that the patient had previously progressed on figitumumab monotherapy underscores the importance of addressing the compensatory upregulation of growth hormone caused by the loss of negative IGF-1R feedback in the pituitary and hypothalamus during anti-IGF-1R therapy (15, 40). Finally, the patient’s long-term sarcoma remission, sustained nearly a decade after discontinuation of both agents, contributes to the ongoing discussion regarding the optimal duration of targeted therapy in patients who achieve long-term complete response.
Case description
We report the case of a 42-year-old female with recurrent ES who achieved complete remission following treatment with figitumumab and pegvisomant and has remained progression-free for >10 years after therapy cessation. A timeline of the different therapeutic interventions since the initial diagnosis is described below and presented schematically in Figure 1.
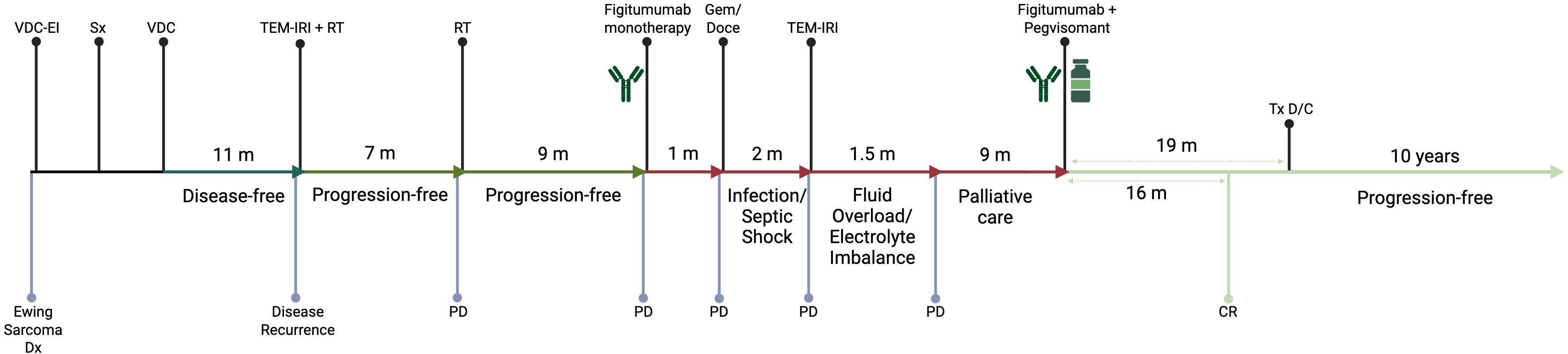
Figure 1. Timeline of different therapeutic interventions since the diagnosis of the primary Ewing Sarcoma tumor. CR, Complete Response; D/C, Discontinuation; Dx, Diagnosis; Gem/Doce, Gemcitabine and Docetaxel; m, month(s); PD, Progressive Disease; RT, Radiotherapy; Sx, Surgical Resection; TEM-IRI, Temozolomide and Irinotecan; Tx, Treatment; VDC, Vincristine, Doxorubicin, and Cyclophosphamide; VDC-EI, Vincristine, Doxorubicin, Cyclophosphamide, Etoposide/Ifosfamide.
Our patient originally presented at the age of 35 with right shoulder pain. An x-ray revealed a proximal humerus mass which was biopsied and diagnosed as ES. She received neoadjuvant chemotherapy with vincristine, doxorubicin, and cyclophosphamide alternating with etoposide and ifosfamide followed by resection of the proximal right humerus. The patient subsequently received 11 cycles of adjuvant vincristine, doxorubicin and cyclophosphamide and remained disease-free for 11 months.
She developed metastatic disease, with left pulmonary mass, which was managed with 10 cycles of temozolomide, and irinotecan chemotherapy combined with radiotherapy, leading to the resolution of the mass. However, 7 months later the patient presented with Horner syndrome and left scapular pain, with imaging showing a left paravertebral/upper thoracic mass that was managed again with radiotherapy.
A follow-up computerized tomography (CT) scan 9 months later revealed bilateral pulmonary nodules biopsied positive for recurrent ES. She then participated in phase II arm of the phase I-II trial (NCT00560235/A4021020) with figitumumab monotherapy (4-week cycles, 30 mg/kg intravenously [IV] on days 1 and 2 first cycle subsequently, 30 mg/kg IV on day 1 for subsequent cycles). However, the patient developed disease progression during the first cycle and was switched to gemcitabine and docetaxel. After receiving a single cycle, she developed neutropenic fever complicated by septic shock. Following her recovery, she was found to have progressive disease and rechallenged with irinotecan and temozolomide. Due to rapid disease progression and decline in performance status, she was transitioned to palliative care.
The patient had recurrent pulmonary infections but gradually improved over the ensuing 9 months. Remarkably, several of her lung nodules were either improved or stable, except for a growing right lung nodule (Figure 2A). Given her dramatic clinical improvement, she consented to participate in a phase I study (NCT00976508/A40201040) of combination therapy of figitumumab and pegvisomant. The treatment plan was structured in 3-week cycles, with the patient receiving 20 mg/kg of the anti-IGF-1R mAb figitumumab IV on day 1 and 20 mg/kg of the growth hormone receptor antagonist pegvisomant subcutaneously daily.
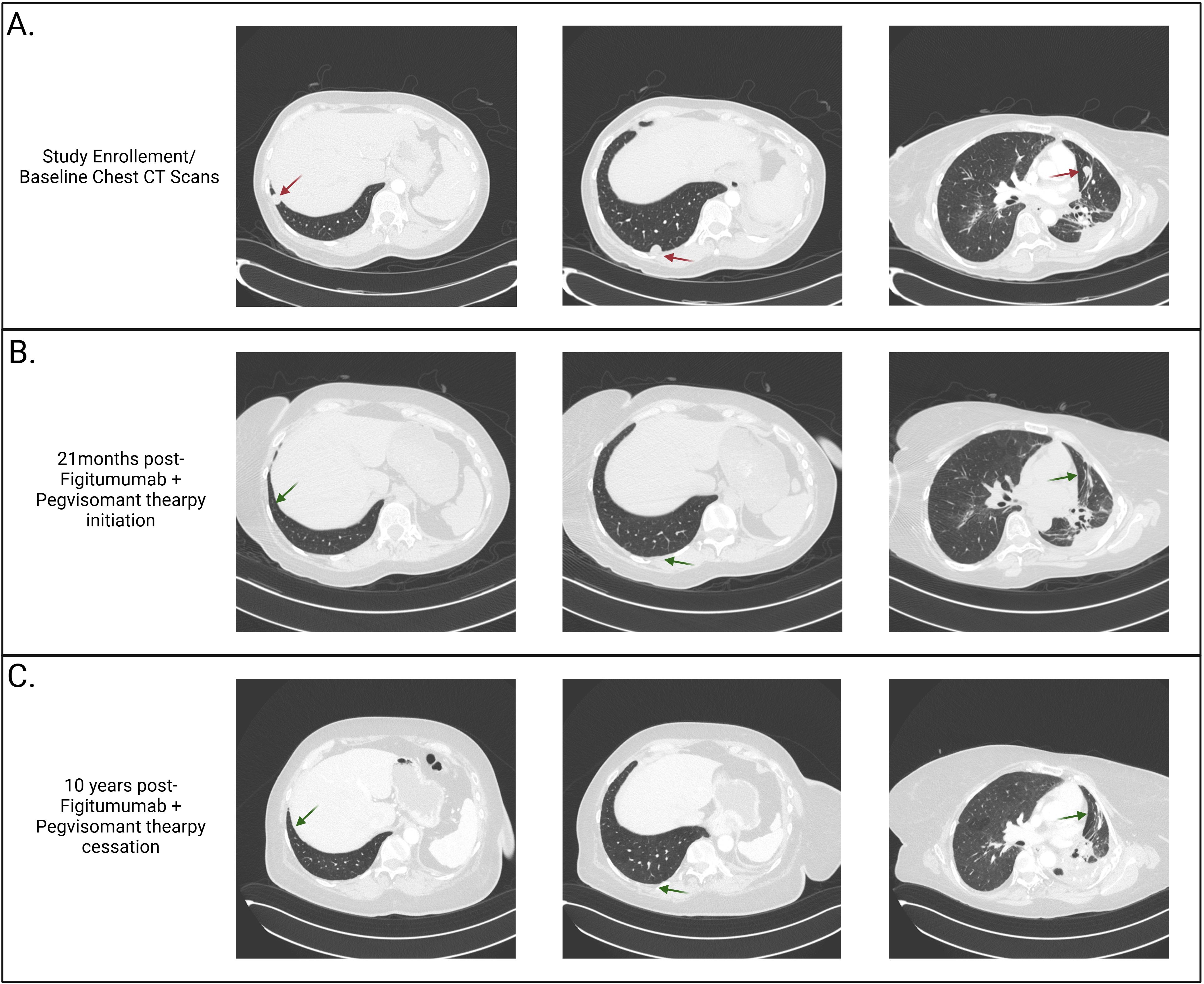
Figure 2. Computerized tomography (CT) scans. (A) Baseline CT scans of the lung demonstrating three intraparenchymal lung nodules (1.3 cm in diameter in the posterior and lateral right lower lobe, 1.4 cm in the subpleural right lower lobe, and 1.4 cm in the left mid-lung lobe) and post-radiation fibrosis (due to previous lines of therapy) prior to enrollment in the NCT00976508 clinical trial. (B) CT scans taken twenty-one months following clinical trial enrollment and two months following the completion of figitumumab and pegvisomant therapy, demonstrating complete resolution of the three lung nodules. (C) CT scans obtained ten years after discontinuing figitumumab and pegvisomant therapy, demonstrating sustained resolution of the lung nodules.
Following the closure of the clinical trial, she was put on a compassionate-use protocol (MC1212) to continue figitumumab and pegvisomant, and treatment resulted in sustained complete remission 16 months after therapy initiation. After 33 cycles (19 months) of therapy, which was tolerated well, she discontinued both agents due to the unavailability of figitumumab and entered the observation phase (Figure 2B). During treatment, the patient tolerated therapy well, with the main complaints being fatigue, two episodes of upper and lower respiratory tract infections, and a transient elevation in liver function tests. During the observation phase, she was monitored every three months for the first year, every four months for the second year, followed by six-monthly scans. She remains progression-free for >10 years after therapy cessation (Figure 2C).
Literature review
The first preclinical data on IGF-1R inhibition in ES emerged two decades ago (13), however, multiple early-phase trials did not yield encouraging results, leading to the abandonment of single-agent IGF-1R mAbs as an experimental treatment option for ES.
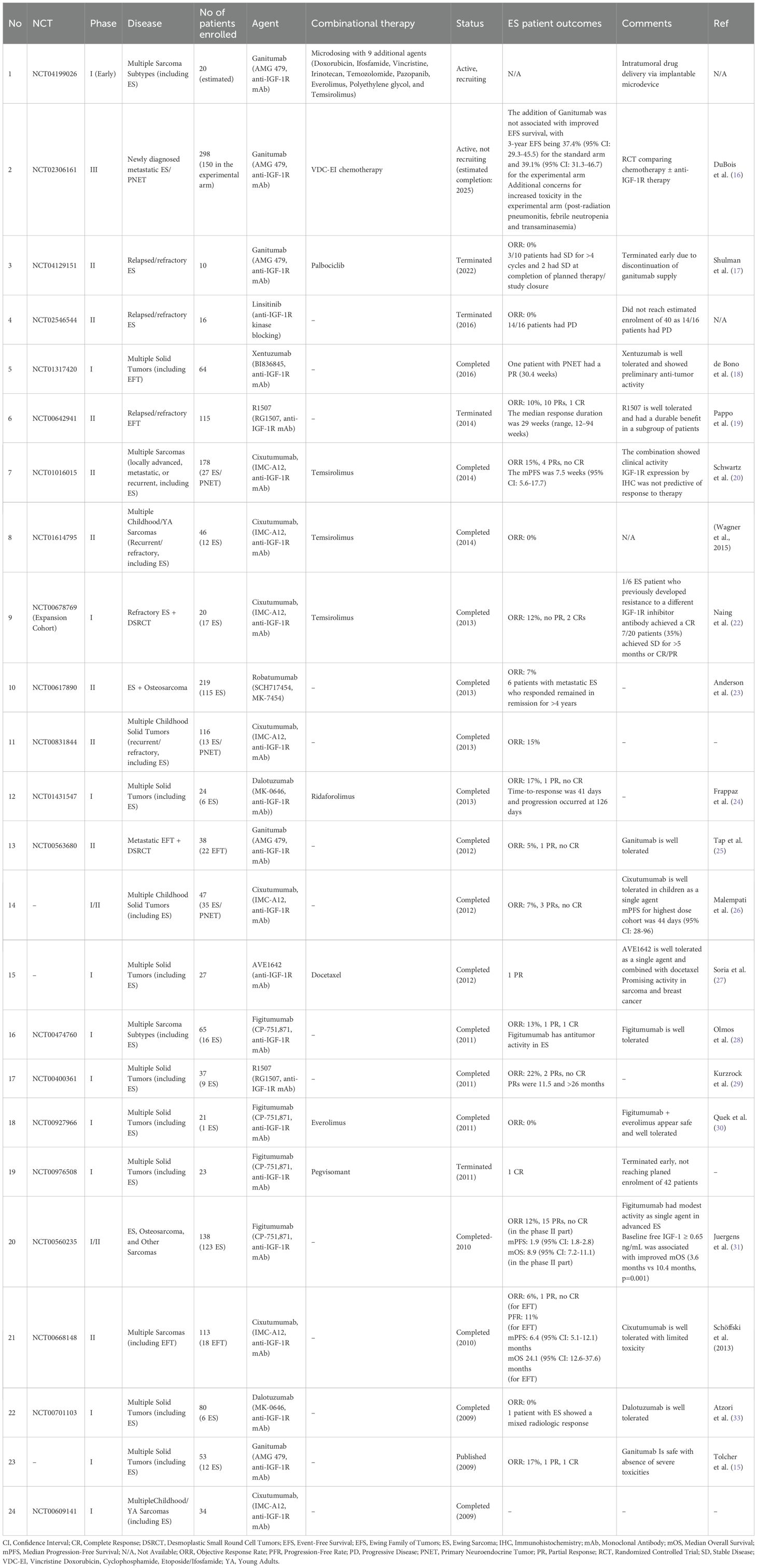
We conducted a targeted literature review to identify clinical trials investigating anti-IGF-1R therapies in patients with ES. PubMed was searched using a combination of Medical Subject Headings (MeSH) and free-text terms for sarcoma and Ewing sarcoma together with variations of IGF-1, IGF-1R, and relevant therapeutic agents (ganitumab, linsitinib, cixutumumab, robatumumab, figitumumab, dalotuzumab). The search was restricted to clinical trial publications. This strategy yielded 34 articles, of which 19 reported the enrollment of at least one patient with ES and were therefore included in our review. In parallel, we searched the ClinicalTrials.gov database using an analogous set of terms to identify ongoing, or recently completed relevant trials that may not have resulted in peer-reviewed publications. This search identified an additional 6 trials. A detailed description of the search keys is provided in the Appendix 1, and the results are summarized in Table 1.
Among the 24 identified studies, 13 (54%) were phase I, 2 (8%) were phase I/II, 9 (38%) were phase II, and 1 (4%) was phase III. Most of these were safety and dose-finding studies enrolling patients with multiple advanced solid tumors and sarcomas, while only 4 (17%) studies focused exclusively on ES. The most common therapeutic modality targeting IGF-1R was mAbs, although one study (4%) investigated linsitinib, an orally administered small molecule that selectively targets IGF-1R and the insulin receptor. A total of 10 studies (42%) combined anti-IGF-1R mAbs with other agents, including mTOR inhibitors (n = 5, 21%), chemotherapy (n = 2, 8%), cyclin-dependent kinase (CDK) 4/6 inhibitors (n = 1, 4%), pegvisomant (n = 1, 4%), and one ongoing trial (4%) is exploring microdosing of 10 anti-tumor agents using an intratumoral device.
Across all trials, 723 patients with ES/Ewing Family of Tumors (EFT)/Primitive Neuroectodermal Tumor (PNET) were treated with anti-IGF-1R therapy. Of the 24 studies, 18 (75%) reported objective response rates (ORR) in patients with ES/EFT/PNET, covering approximately 573 patients. The reported ORR ranged from 0% to 17%, with a mean of 9%. Among the 52 responses described, 40 were partial responses, 5 were complete responses, and the remainder were unspecified.
Of note, clinical interest in the development and testing of these agents peaked during the previous decade, with 18 of the 24 studies (88%) conducted before 2016, and has since shown a marked decline in efforts to further evaluate their efficacy.
Regarding the only phase III clinical trial; it was run by the Children’s Oncology Group study and was terminated early in March 2019 after ganitumab (AMG479) failed to synergize with standard-of-care cytotoxic chemotherapy in newly diagnosed metastatic ES (16).
Despite these setbacks, the favorable safety profile and occasional complete and partial responses suggest that combination strategies warrant further exploration. One of the most promising approaches is the combination of IGF-1R with mTOR inhibition (16, 22, 34). This approach was designed following preclinical evidence of upregulation of IR-alpha, IRS-1, STAT3, MSTR1, and other proteins throughout the IGF-1R/PI3K/mTOR signaling cascade in xenografts that had adapted to IGF-1R-targeted therapy (41–44).
Notably, the first clinical trial combining the anti-IGF-1R mAb, cixutumumab, with the mTOR inhibitor, temsirolimus, demonstrated tumor regression of more than 20% in approximately 29% of the patients and a sevenfold increase in median response duration (>14 months) in patients with ES (22). Subsequent trials, however, failed to replicate an improvement in survival, with the results scrutinized due to concerns about the dosing of the mTOR inhibitor and the lack of comparative monotherapy arms in these single-arm studies (20). Nevertheless, a recent meta-analysis showed that combination of IGF-1R and mTOR inhibition improved progression-free survival (PFS) compared to IGF-1R inhibition alone in patients with ES (34).
By contrast, the available data on combining anti-IGF-1R therapy with CDK4/6 inhibitors remain limited and less promising. In one study exploring this combination, the ORR was 0%, with 3 of 10 patients (30%) achieving stable disease for more than 4 cycles and a 6-month PFS rate of 30% (17).
Finally, although biologically relevant, the combination of anti-IGF-1R mAbs with growth hormone inhibitors such as pegvisomant remains underexplored, with only one trial to our knowledge exploring this direction.
Discussion
Compensatory growth hormone increase has been observed in patients treated with anti-IGF-1R mAbs since the introduction of these agents in the clinic (15). This is expected as anti-IGF-1R therapy disrupts the negative feedback loop at the hypothalamic–pituitary axis, leading to elevated circulating growth hormone which can upregulate IGF-1R expression and other downstream pathways that sustain tumor growth (45). Nonetheless, the combination of IGF-1R and growth hormone inhibitors, such as pegvisomant, has only been attempted in the clinical trial our patient was enrolled in (NCT00976508/A40201040).
This trial included two treatment arms: one with 10 mg/kg figitumumab plus 10 mg daily pegvisomant (arm A), and another with 10 mg/kg figitumumab plus 20 mg daily pegvisomant (arm B). Interestingly, none of the patients in the lower-dose pegvisomant group (n=0/17, 0%) achieved an objective response, whereas half of the patients in the higher-dose group (n=3/6, 50%) did, suggesting that a higher pegvisomant dose may be necessary to achieve therapeutic benefit.
Although our patient did not experience significant toxicity, serious adverse events were reported in 52.94% of patients in arm A (n=9/17) and 66.67% in arm B (n=4/6), underscoring the importance of carefully balancing efficacy with the risk of adverse effects. Reported toxicities included disease progression (n=5), gastrointestinal hemorrhage (n=1), death (n=1), pelvic infection (n=1), elevated blood uric acid (n=1), increased CRP (n=1), dehydration (n=1), back pain (n=1), flank pain (n=1), cauda equina syndrome (n=1), headache (n=1), and pneumonitis (n=1).
Despite the promising preliminary data, the study was terminated early on April 18, 2011. Termination was not due to safety concerns but related to timely recruitment in conjunction with the commercial discontinuation of figitumumab.
Our patient, who experienced disease progression on figitumumab monotherapy but achieved a sustained complete response with the combination of figitumumab and pegvisomant, demonstrates that growth hormone upregulation induced by IGF-1R-targeting mAbs can compromise the efficacy of this approach in the clinic. Additionally, clinical data from acromegaly therapy, which also targets this axis, suggest that pegvisomant, in combination with somatostatin analogs, is safe, effective, and can manage refractory acromegaly (46). This approach could also be explored in oncology, as it may offer a novel strategy to enhance treatment efficacy and overcome resistance to anti-IGF-1R therapies.
The above findings must be interpreted in the context of the challenges associated with identifying novel therapeutic targets and agents for ES. A meta-analysis of all phase I and II clinical trials enrolling patients with refractory or recurrent ES reported a median PFS of 1.9 months (range: 1.3–14.7) and an overall survival (OS) of 7.6 months (range: 5–30) (47). Only 18% of published trials were considered positive, with a median PFS of 4.5 months (range: 1.3–10) and OS of 16 months (range: 6.9–30) (47). Acknowledging the risk of publication bias, the actual outcomes may be even less favorable.
Furthermore, in IGF-1R–targeting phase I and II clinical trials, a subset of patients demonstrated significant clinical benefit, including our patient’s extraordinary long-term response, which raises the question of whether specific molecular or clinical features may have contributed. Although detailed molecular characterization of our patient’s tumor was not performed, certain predictive biomarkers have been identified in similar trials. Despite initial expectations, total IGF-1R expression in tumors has not been shown to predict response to IGF-1R therapy (20, 34, 48). By contrast, early data suggest that the absence of phosphorylated IGF-1R (pIGF-1R) (34) or exclusive nuclear staining of total IGF-1R (49) could serve as predictive biomarkers, potentially guiding future clinical trial enrollment. However, given the rarity of ES, implementing a precision medicine approach faces inherent challenges; limited patient populations restrict accrual in biomarker-enriched or stratified trials, underscoring the need for collaborative international consortia, basket trial designs, and adaptive methodologies.
Another critical topic for discussion in this case report is the optimal duration of targeted therapy for sarcomas. While targeted therapies are significantly less toxic than conventional chemotherapy (50, 51), adverse effects remain unavoidable. Common side effects include fatigue, rash, diarrhea, infections, hypertension, bleeding, thyroid dysfunction, proteinuria, and hepatotoxicity, which can range from mild to severe or even fatal (51–54). Additionally, there is often a disconnect between physicians’ perceptions and patients’ lived experiences. Many patients receiving small-molecule therapies deemed “well tolerated” by the medical community still report a significant decline in quality of life (55). Another factor to be acknowledged is the significant cost of these drugs, placing a heavy burden not only on patients themselves but also on social health resources (56, 57). This is only expected to increase in the coming years with the wider implementation of molecular treatments, coupled with the substantial inflation-adjusted price growth of targeted therapies over the past decades (57).
Although cases of long-term remission following discontinuation of targeted therapies have been reported, treatment cessation is rarely attempted due to concerns about disease recurrence. As a result, this field remains largely unexplored. Limited data on targeted therapy discontinuation come from patients with gastrointestinal stromal tumors (GISTs), where tyrosine kinase inhibitors (TKIs) such as imatinib form the cornerstone of treatment (58). Current guidelines recommend indefinite continuation of TKIs in the absence of disease progression (50). This approach is supported by clinical trial data showing that imatinib discontinuation, followed by re-initiation upon disease progression, is associated with decreased time-to-resistance and worse OS (59, 60). However, a significant proportion of patients in the discontinuation arm remained disease-free three years post-treatment cessation, suggesting that some individuals may be at lower risk for recurrence (59). A recent study proposed that minimal tumor burden may be a positive predictive factor for long-term remission following targeted therapy discontinuation—an observation that aligns with the case presented in this manuscript (61).
Conclusions
Although clinical testing of IGF-1R-targeted therapies in ES has faced challenges, including the failure of single-agent approaches and the discontinuation of key studies, its potential remains significant, especially when combined with other agents. The combination of IGF-1R inhibitors with mTOR inhibitors or growth hormone receptor inhibitors, such as pegvisomant, represents an exciting avenue for further exploration, as demonstrated by the outstanding clinical response in our patient. Additionally, while the optimal duration of targeted therapy remains uncertain, the possibility of therapy discontinuation in select patients could be considered, as the long-term remission can sometimes be sustained. Refining patient selection through predictive biomarkers and exploring combination therapies could help maximize the efficacy of IGF-1R-targeted treatment and provide potential therapeutic options for patients with refractory or recurrent ES as well as other tumors. Notably, among the antibodies tested in ES, only ganitumab remains in active clinical development. Its pharmacological profile, which is comparable to figitumumab, suggests that it could be combined with growth hormone receptor antagonists to replicate or extend prior observations. While next-generation IGF-1R inhibitors would be valuable, prioritizing the evaluation of combinations using currently available, clinically approved, and safe agents may accelerate the generation of actionable data.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board (IRB). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
GS: Conceptualization, Writing – review & editing, Writing – original draft. BS: Writing – review & editing. SA: Writing – review & editing. JT: Writing – review & editing. MH: Writing – review & editing. TH: Writing – review & editing. SO: Writing – review & editing. SR: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
Approval from Pfizer was obtained to publish this case report. Biorender was used for the generation of Figure 1.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1667628/full#supplementary-material
References
1. Lee YT, Tan YJ, and Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol. (2018) 834:188–96. doi: 10.1016/j.ejphar.2018.07.034
2. Tang F, Tie Y, Wei YQ, Tu CQ, and Wei XW. Targeted and immuno-based therapies in sarcoma: mechanisms and advances in clinical trials. Biochim Biophys Acta Rev Cancer. (2021) 1876:188606. doi: 10.1016/j.bbcan.2021.188606
3. Li S, Zhang H, Liu J, and Shang G. Targeted therapy for osteosarcoma: a review. J Cancer Res Clin Oncol. (2023) 149:6785–97. doi: 10.1007/s00432-023-04614-4
4. Croce S, Devouassoux-Shisheboran M, Pautier P, Ray-Coquard I, Treilleux I, Neuville A, et al. Uterine sarcomas and rare uterine mesenchymal tumors with Malignant potential. Diagnostic guidelines of the French Sarcoma Group and the Rare Gynecological Tumors Group. Gynecol Oncol. (2022) 167:373–89. doi: 10.1016/j.ygyno.2022.07.031
5. M S A, K C, Bhargavan RV, Somanathan T, and Subhadradevi L. An overview on liposarcoma subtypes: Genetic alterations and recent advances in therapeutic strategies. J Mol Histol. (2024) 55:227–40. doi: 10.1007/s10735-024-10195-4
6. Strauss SJ, Berlanga P, and McCabe MG. Emerging therapies in Ewing sarcoma. Curr Opin Oncol. (2024) 36:297–304. doi: 10.1097/CCO.0000000000001048
7. Fayzullina D, Tsibulnikov S, Stempen M, Schroeder BA, Kumar N, Kharwar RK, et al. Novel targeted therapeutic strategies for Ewing sarcoma. Cancers Basel. (2022) 14:1988. doi: 10.3390/cancers14081988
8. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
9. Zucman J, Delattre O, Desmaze C, Plougastel B, Joubert I, Melot T, et al. Cloning and characterization of the Ewing’s sarcoma and peripheral neuroepithelioma t(11;22) translocation breakpoints. Genes Chromosomes Cancer. (1992) 5:271–7. doi: 10.1002/gcc.2870050402
10. Dhir A, Rahul R, Liu Q, Pham D, Kronenfeld R, Koru-Sengul T, et al. Disparities in incidence and survival for patients with Ewing sarcoma in Florida. Cancer Med. (2024) 8):e7151. doi: 10.1002/cam4.7151
11. Tomazou EM, Sheffield NC, Schmidl C, Schuster M, Schönegger A, Datlinger P, et al. Epigenome mapping reveals distinct modes of gene regulation and widespread enhancer reprogramming by the oncogenic fusion protein EWS-FLI1. Cell Rep. (2015) 10:1082–95. doi: 10.1016/j.celrep.2015.01.042
12. Prieur A, Tirode F, Cohen P, and Delattre O. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol. (2004) 24:7275–83. doi: 10.1128/MCB.24.16.7275-7283.2004
13. Scotlandi K, Benini S, Sarti M, Serra M, Lollini PL, Maurici D, et al. Insulin-like growth factor I receptor-mediated circuit in Ewing’s sarcoma/peripheral neuroectodermal tumor: a possible therapeutic target. Cancer Res. (1996) 56:4570–4.
14. Yee D, Favoni RE, Lebovic GS, Lombana F, Powell DR, Reynolds CP, et al. Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation. A potential autocrine Growth factor. J Clin Invest. (1990) 86:1806–14. doi: 10.1172/JCI114910
15. Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin CC, Rodon J, et al. pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. (2009) 27:5800–7. doi: 10.1200/JCO.2009.23.6745
16. DuBois SG, Krailo MD, Glade-Bender J, Buxton A, Laack N, Randall RL, et al. Randomized phase III trial of ganitumab with interval-compressed chemotherapy for patients with newly diagnosed metastatic Ewing sarcoma: A report from the Children’s Oncology Group. J Clin Oncol. (2023) 41:2098–107. doi: 10.1200/JCO.22.01815
17. Shulman DS, Merriam P, Choy E, Guenther LM, Cavanaugh KL, Kao PC, et al. Phase 2 trial of palbociclib and ganitumab in patients with relapsed Ewing sarcoma. Cancer Med. (2023) 12:15207–16. doi: 10.1002/cam4.6208
18. de Bono J, Lin CC, Chen LT, Corral J, Michalarea V, Rihawi K, et al. Two first-in-human studies of xentuzumab, a humanised insulin-like growth factor (IGF)-neutralising antibody, in patients with advanced solid tumours. Br J Cancer. (2020) 122:1324–32. doi: 10.1038/s41416-020-0774-1
19. Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Chawla SP, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. (2011) 29:4541–7. doi: 10.1200/JCO.2010.34.0000
20. Schwartz GK, Tap WD, Qin LX, Livingston MB, Undevia SD, Chmielowski B, et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: a multicentre, open-label, phase 2 trial. Lancet Oncol. (2013) 14:371–82. doi: 10.1016/S1470-2045(13)70049-4
21. Wagner LM, Fouladi M, Ahmed A, Krailo MD, Weigel B, DuBois SG, et al. Phase II study of cixutumumab in combination with temsirolimus in pediatric patients and young adults with recurrent or refractory sarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. (2015) 62:440–4. doi: 10.1002/pbc.25334
22. Naing A, LoRusso P, Fu S, Hong DS, Anderson P, Benjamin RS, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clin Cancer Res. (2012) 18:2625–31. doi: 10.1158/1078-0432.CCR-12-0061
23. Anderson PM, Bielack SS, Gorlick RG, Skubitz K, Daw NC, Herzog CE, et al. A phase II study of clinical activity of SCH 717454 (robatumumab) in patients with relapsed osteosarcoma and Ewing sarcoma. Pediatr Blood Cancer. (2016) 63:1761–70. doi: 10.1002/pbc.26087
24. Frappaz D, Federico SM, Pearson ADJ, Gore L, Macy ME, DuBois SG, et al. Phase 1 study of dalotuzumab monotherapy and ridaforolimus-dalotuzumab combination therapy in paediatric patients with advanced solid tumours. Eur J Cancer. (2016) 62:9–17. doi: 10.1016/j.ejca.2016.03.084
25. Tap WD, Demetri G, Barnette P, Desai J, Kavan P, Tozer R, et al. Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol. (2012) 30:1849–56. doi: 10.1200/JCO.2011.37.2359
26. Malempati S, Weigel B, Ingle AM, Ahern CH, Carroll JM, Roberts CT, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol. (2012) 30:256–62. doi: 10.1200/JCO.2011.37.4355
27. Soria JC, Massard C, Lazar V, Ozoux ML, Mery-Mignard D, Deslandes A, et al. A dose finding, safety and pharmacokinetic study of AVE1642, an anti-insulin-like growth factor-1 receptor (IGF-1R/CD221) monoclonal antibody, administered as a single agent and in combination with docetaxel in patients with advanced solid tumours. Eur J Cancer. (2013) 49:1799–807. doi: 10.1016/j.ejca.2013.01.003
28. Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, and Paccagnella ML. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: a phase 1 expansion cohort study. Lancet Oncol. (2010) 11:129–35. doi: 10.1016/S1470-2045(09)70354-7
29. Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjamin R, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. (2010) 16:2458–65. doi: 10.1158/1078-0432.CCR-09-3220
30. Quek R, Wang Q, Morgan JA, Shapiro GI, Butrynski JE, Ramaiya N, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. (2011) 17:871–9. doi: 10.1158/1078-0432.CCR-10-2621
31. Juergens H, Daw NC, Geoerger B, Ferrari S, Villarroel M, Aerts I, et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol. (2011) 29:4534–40. doi: 10.1200/JCO.2010.33.0670
32. Schöffski P, Adkins D, Blay JY, Gil T, Elias AD, Rutkowski P, et al. An open-label, phase 2 study evaluating the efficacy and safety of the anti-IGF-1R antibody cixutumumab in patients with previously treated advanced or metastatic soft-tissue sarcoma or Ewing family of tumours. Eur J Cancer. (2013) 49:219–28. doi: 10.1016/j.ejca.2013.06.010
33. Atzori F, Tabernero J, Cervantes A, Prudkin L, Andreu J, Rodríguez-Braun E, et al. A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. (2011) 17:6304–12. doi: 10.1158/1078-0432.CCR-10-3336
34. Amin HM, Morani AC, Daw NC, Lamhamedi-Cherradi SE, Subbiah V, Menegaz BA, et al. IGF-1R/mTOR targeted therapy for Ewing sarcoma: A meta-analysis of five IGF-1R-related trials matched to proteomic and radiologic predictive biomarkers. Cancers Basel. (2020) 12:1768. doi: 10.3390/cancers12071768
35. Fleuren EDG, Versleijen-Jonkers YMH, Boerman OC, and van der Graaf WTA. Targeting receptor tyrosine kinases in osteosarcoma and Ewing sarcoma: current hurdles and future perspectives. Biochim Biophys Acta. (2014) 1845:266–76. doi: 10.1016/j.bbcan.2014.02.005
36. Calvo E, Soria JC, Ma WW, Wang T, Bahleda R, Tolcher AW, et al. A phase I clinical trial and independent patient-derived xenograft study of combined targeted treatment with dacomitinib and figitumumab in advanced solid tumors. Clin Cancer Res. (2017) 23:1177–85. doi: 10.1158/1078-0432.CCR-15-2301
37. Langer CJ, Novello S, Park K, Krzakowski M, Karp DD, Mok T, et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J Clin Oncol. (2014) 32:2059–66. doi: 10.1200/JCO.2013.54.4932
38. Schmitz S, Kaminsky-Forrett MC, Henry S, Zanetta S, Geoffrois L, Bompas E, et al. Phase II study of figitumumab in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: clinical activity and molecular response (GORTEC 2008-02). Ann Oncol. (2012) 23:2153–61. doi: 10.1093/annonc/mdr574
39. Scagliotti GV, Bondarenko I, Blackhall F, Barlesi F, Hsia TC, Jassem J, et al. Randomized, phase III trial of figitumumab in combination with erlotinib versus erlotinib alone in patients with nonadenocarcinoma nonsmall-cell lung cancer. Ann Oncol. (2015) 26:497–504. doi: 10.1093/annonc/mdu517
40. Weroha SJ and Haluska P. IGF-1 receptor inhibitors in clinical trials–early lessons. J Mammary Gland Biol Neoplasia. (2008) 13:471–83. doi: 10.1007/s10911-008-9104-6
41. Potratz JC, Saunders DN, Wai DH, Ng TL, McKinney SE, Carboni JM, et al. Synthetic lethality screens reveal RPS6 and MST1R as modifiers of insulin-like growth factor-1 receptor inhibitor activity in childhood sarcomas. Cancer Res. (2010) 70:8770–81. doi: 10.1158/0008-5472.CAN-10-1093
42. Garofalo C, Manara MC, Nicoletti G, Marino MT, Lollini PL, Astolfi A, et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing’s sarcoma is dependent on insulin receptor signaling. Oncogene. (2011) 30:2730–40. doi: 10.1038/onc.2010.640
43. Beltran PJ, Chung YA, Moody G, Mitchell P, Cajulis E, Vonderfecht S, et al. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing’s and osteogenic sarcoma models. J Pharmacol Exp Ther. (2011) 337:644–54. doi: 10.1124/jpet.110.178400
44. Lamhamedi-Cherradi SE, Menegaz BA, Ramamoorthy V, Vishwamitra D, Wang Y, Maywald RL, et al. IGF-1R and mTOR blockade: Novel resistance mechanisms and synergistic drug combinations for Ewing sarcoma. J Natl Cancer Inst. (2016) 108. doi: 10.1093/jnci/djw182
45. Clayton PE, Banerjee I, Murray PG, and Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. (2011) 7:11–24. doi: 10.1038/nrendo.2010.171
46. Bianchi A, Valentini F, Iuorio R, Poggi M, Baldelli R, Passeri M, et al. Long-term treatment of somatostatin analog-refractory growth hormone-secreting pituitary tumors with pegvisomant alone or combined with long-acting somatostatin analogs: a retrospective analysis of clinical practice and outcomes. J Exp Clin Cancer Res. (2013) 32:40. doi: 10.1186/1756-9966-32-40
47. Felix A, Berlanga P, Toulmonde M, Landman-Parker J, Dumont S, Vassal G, et al. Systematic review of phase-I/II trials enrolling refractory and recurrent Ewing sarcoma: Actual knowledge and future directions to optimize the research. Cancer Med. (2021) 10:1589–604. doi: 10.1002/cam4.3712
48. Carden CP, Molife LR, and de Bono JS. Predictive biomarkers for targeting insulin-like growth factor-I (IGF-I) receptor. Mol Cancer Ther. (2009) 8:2077–8. doi: 10.1158/1535-7163.MCT-09-0641
49. Asmane I, Watkin E, Alberti L, Duc A, Marec-Berard P, Ray-Coquard I, et al. Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: a predictive biomarker for IGF-1R monoclonal antibody (Ab) therapy in sarcomas. Eur J Cancer. (2012) 48:3027–35. doi: 10.1016/j.ejca.2012.05.009
50. Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:20–33. doi: 10.1016/j.annonc.2021.09.005
51. Abramson RG, Abramson VG, Chan E, Horn L, Keedy VL, Pao W, et al. Complications of targeted drug therapies for solid Malignancies: manifestations and mechanisms. AJR Am J Roentgenol. (2013) 200:475–83. doi: 10.2214/AJR.12.9049
52. Davis JS, Ferreira D, Paige E, Gedye C, and Boyle M. Infectious complications of biological and small molecule targeted immunomodulatory therapies. Clin Microbiol Rev. (2020) 33. doi: 10.1128/CMR.00035-19
53. Wang Z, Yang X, Wang J, Wang S, Mao X, Li M, et al. Risk of serious adverse event and fatal adverse event with molecular target anticancer drugs in cancer patients: A meta-analysis. J Cancer Res Ther. (2019) 15:1435–49. doi: 10.4103/jcrt.JCRT_577_18
54. Liu S and Kurzrock R. Understanding toxicities of targeted agents: Implications for anti-tumor activity and management. Semin Oncol. (2015) 42:863–75. doi: 10.1053/j.seminoncol.2015.09.032
55. Fauske L, Hompland I, Lorem G, Bondevik H, and Bruland ØS. Perspectives on treatment side effects in patients with metastatic gastrointestinal stromal tumour: a qualitative study. Clin Sarcoma Res. (2019) 9:6. doi: 10.1186/s13569-019-0116-3
56. Yu A, Huang E, Abe M, An K, Park SK, and Park C. Cost-effectiveness analyses of targeted therapy and immunotherapy for advanced non-small cell lung cancer in the United States: a systematic review. Expert Rev Pharmacoecon Outcomes Res. (2021) 21:381–93. doi: 10.1080/14737167.2021.1886928
57. Wilson LE, Greiner MA, Altomare I, Rotter J, and Dinan MA. Rapid rise in the cost of targeted cancer therapies for Medicare patients with solid tumors from 2006 to 2015. J Geriatr Oncol. (2021) 12:375–80. doi: 10.1016/j.jgo.2020.11.007
58. Blay JY, Kang YK, Nishida T, and von Mehren M. Gastrointestinal stromal tumours. Nat Rev Primer. (2021) 7:22. doi: 10.1038/s41572-021-00254-5
59. Le Cesne A, Schiffler C, Bouche O, Brahmi M, Duffaud F, Toulmonde M, et al. VP3-2024: A randomized study of 6 vs 3 years of adjuvant imatinib in patients with localized GIST at high risk of relapse. Ann Oncol. (2024) 35:576–7. doi: 10.1016/j.annonc.2024.04.004
60. Blay JY, Devin Q, Duffaud F, Toulmonde M, Firmin N, Collard O, et al. Discontinuation versus continuation of imatinib in patients with advanced gastrointestinal stromal tumours (BFR14): exploratory long-term follow-up of an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. (2024) 25:1163–75. doi: 10.1016/S1470-2045(24)00318-8
61. Hompland I, Boye K, Wiedswang AM, Papakonstantinou A, Røsok B, Joensuu H, et al. Discontinuation of imatinib in patients with oligometastatic gastrointestinal stromal tumour who are in complete radiological remission: a prospective multicentre phase II study. Acta Oncol. (2024) 63:288–93. doi: 10.2340/1651-226X.2024.39851
Keywords: case report, Ewing, IGF-1R, figitumumab, pegvisomant
Citation: Stergiopoulos GM, Siontis BL, Ahmed SK, Thangaiah JJ, Houdek MT, Ho TP, Okuno SH and Robinson SI (2025) Case Report: Should IGF-1R targeted therapy be revisited in Ewing sarcoma? a report of long-term complete response and review of the literature. Front. Oncol. 15:1667628. doi: 10.3389/fonc.2025.1667628
Received: 16 July 2025; Accepted: 04 November 2025; Revised: 21 October 2025;
Published: 19 November 2025.
Edited by:
Angelina Vaseva, The University of Texas Health Science Center at San Antonio, United StatesReviewed by:
Alessandro Gonfiotti, University of Florence, ItalyPanneerselvam Jayabal, The University of Texas Health Science Center at San Antonio, United States
Copyright © 2025 Stergiopoulos, Siontis, Ahmed, Thangaiah, Houdek, Ho, Okuno and Robinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven I. Robinson, cm9iaW5zb24uc3RldmVuQG1heW8uZWR1
 Georgios M. Stergiopoulos
Georgios M. Stergiopoulos Brittany L. Siontis
Brittany L. Siontis Safia K. Ahmed
Safia K. Ahmed Judith Jebastin Thangaiah4
Judith Jebastin Thangaiah4 Scott H. Okuno
Scott H. Okuno Steven I. Robinson
Steven I. Robinson