- 1Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA, United States
- 2Division of Urology and Urologic Oncology, City of Hope Comprehensive Cancer Center, Duarte, CA, United States
- 3Department of Hematology and Oncology, MD Anderson Cancer Center, Houston, TX, United States
- 4Department of Immunology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, United States
- 5Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, United States
Antibody-drug conjugates (ADCs) are revolutionizing the treatment landscape of advanced urothelial carcinoma (aUC). We systematically reviewed the PubMed and Embase databases for published clinical trials evaluating ADC-combination regimens in aUC. We extracted safety and efficacy outcomes, including objective-response rate (ORR), adverse events (AEs), and ≥ grade 3 AEs. We excluded narrative reviews, retrospective studies, and case reports. Two independent reviewers screened titles and abstracts for relevance, followed by a full-text review for eligibility. A total of 645 patients from 5 trials investigating anti-nectin-4 (enfortumab vedotin, EV), anti-TROP2 (sacituzumab govitecan, SG), and anti-HER2 (disitamab vedotin, DV) ADCs were identified. We recorded a pooled ORR of 65%. We recorded a pooled risk rate for all-grade toxicity of 57%. The most prevalent any-grade AEs were peripheral sensory neuropathy 52% (95% CI, 45%-59%), fatigue 45% (95% CI, 28%-64%), and diarrhea 42% (95% CI, 16%-74%). Peripheral sensory neuropathy, fatigue, and alopecia were more commonly observed in anti-nectin-4 regimens. Gastrointestinal (diarrhea and nausea) and hematologic (anemia and neutropenia) toxicities were more commonly observed in anti-TROP2 regimens. Hepatotoxicity was predominantly found in anti-HER2 regimens. While ADC-based combination regimens show promising responses, they also have high rates of AEs in patients with aUC.
Introduction
Antibody-drug conjugates (ADCs) targeting nectin-4, trophoblast cell-surface antigen 2 (TROP2), and human epidermal growth factor receptor 2 (HER2) have shown efficacy as monotherapy in advanced urothelial carcinoma (aUC) (1–3). Multiple trials have combined immune checkpoint inhibitors (ICIs) and ADCs (NCT06823427, NCT06483334, NCT05911295) (3–7). Notable ADCs in this space include enfortumab vedotin (EV) (anti-nectin-4 antibody with a monomethyl auristatin E (MMAE) payload and a cleavable linker), sacituzumab govitecan (SG) (anti-TROP2 antibody with a SN-38 payload and a cleavable linker), and disitamab vedotin (DV) (anti-HER2 monoclonal antibody with a MMAE payload and cleavable linker). With the approval of EV with pembrolizumab in untreated aUC and several ongoing trials of ADC/ICIs, most patients are likely to receive ADC-based combination therapy as part of their treatment in the future. However, considering the different therapeutic target antigens and payloads (microtubule vs. topoisomerase inhibition) of ADCs, these combinations can have varying efficacy and tolerability. We performed a systematic review and meta-analysis on the efficacy and toxicity of anti-nectin-4, anti-TROP2, and anti-HER2 ADC-based combinations in aUC.
Methods
We searched Embase and PubMed bibliographic databases through May 2025 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Supplementary Figure 1). Prospective clinical trials reporting efficacy and tolerability of ADC combination therapy (ADC + ICI) in patients with aUC were included. We excluded case reports, retrospective cohorts, and narrative reviews. Two independent reviewers (SJC and RBC) examined titles and abstracts for inclusion. Discrepancies were resolved through unanimous agreement (AT). Studies were grouped into anti-TROP2, anti-nectin-4, and anti-HER2 combinations. The pooled objective response rate (ORR) and adverse event (AE) rates with 95% confidence intervals (CIs) were calculated using a random-effects model. Interstudy heterogeneity was analyzed using the Chi-square test and I2 statistics. Heterogeneity was defined as low (I2 < 30%), moderate (30% < I2 < 70%), and high (I2 > 70%). For outcomes with moderate heterogeneity and higher, a random-effects model was used. Otherwise, a fixed-effect model was used. We performed descriptive analysis if data could not be combined. Statistical analysis was conducted using R Statistical Software (v4.3.3; R Core Team 2024). Significance was defined as two-sided (p < 0.05).
Results
After screening, five studies were eligible for data extraction (3, 8–11) (Table 1). These included the TROPHY-U-01 (Cohort 3), EV-302, EV-103 (cohort K), and EV-103 (cohort A), and RC48-C014 trials (3, 8–11). Drugs evaluated across these trials included EV (EV-302, EV-103 cohort K, and EV-103 cohort A), SG (TROPHY-U-01 Cohort 3), and DV (RC48-C014). The pooled ORR was 65% (95% CI, 49%–78%). There was significant heterogeneity noted between trials (I2 = 69%; p = 0.01), largely driven by differences in efficacy between anti-nectin-4, anti-HER2, and anti-TROP2 combinations. For anti-nectin-4 trials, ORR ranged from 73% (EV-103; cohort A: 95% CI, 58%-85%) to 68% (EV-302: 95% CI, 63%-72%) and 64% (EV-103; cohort K: 95% CI, 53%-75%). The anti-HER2 ADC RC48-C014 trial showed an ORR of 73% (95% CI, 57%-86%; Figure 1A) while the TROPHY-U-01 trial showed an ORR of 41% (95% CI, 26%-58%).
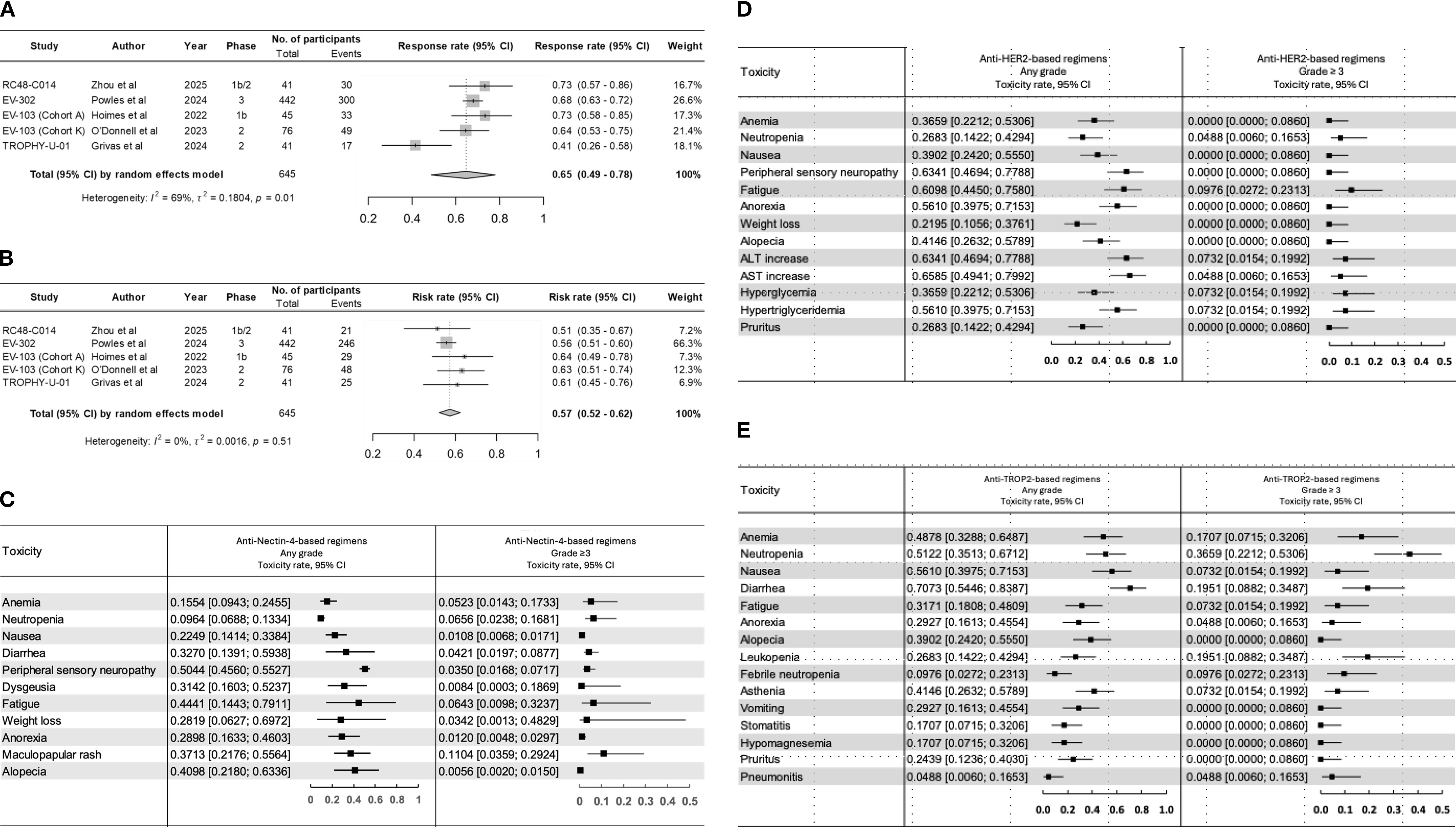
Figure 1. Pooled rate of (A) objective response and (B) toxicity across clinical trials across clinical trials. Forrest plots for all grade and grade ≥3 adverse events observed with (C) anti-nectin-4, (D) anti-HER2, and (E) anti-TROP2 combination regimens.
We recorded a pooled risk rate for all-grade toxicity of 57% (95% CI, 52%-62%) without significant interstudy heterogeneity (I2 = 0%; p = 0.51) (Figure 1B). The highest risk was observed in the EV-103 (cohort A), 64% (95% CI, 49%-78%), and EV-103 (cohort K), 63% (95% CI, 51%-74%) trials. Overall, the most prevalent any-grade AEs were peripheral sensory neuropathy 52% (95% CI, 45%-59%), fatigue 45% (95% CI, 28%-64%), and diarrhea 42% (95% CI, 16%-74%). (Supplementary Table 1). The incidence of AEs varied significantly between regimens. The most common any-grade AEs in anti-nectin-4 regimens included peripheral sensory neuropathy 50% (95% CI, 45%-55%), fatigue 44% (95% CI, 14%-79%), and alopecia 40% (95% CI, 21%-63%). Grade ≥3 AEs included maculopapular rash 11% (95% CI, 3%-29%) and neutropenia 6% (95% CI, 2%-16%). The most common any-grade AEs in anti-TROP2 regimens were diarrhea 70% (95% CI,54%-83%), nausea 56% (95% CI, 39%-71%), and neutropenia 51% (95% CI, 35%-67%). Grade ≥3 AEs included neutropenia 36% (95% CI, 22%-53%) and diarrhea 19% (95% CI, 8%-34%). The most common any-grade AEs in anti-HER2 regimens were AST increase 65% (95% CI, 49%-79%), ALT increase 63% (95% CI, 46%-77%), and peripheral sensory neuropathy 63% (95% CI, 46%-77%). Grade ≥3 AEs included fatigue 9% (95% CI, 2%-23%), ALT increase 7% (95% CI, 1%-19%), and hyperglycemia 7% (95% CI, 1%-19%). (Figures 1C–E).
Discussion
Our study highlights the significant heterogeneity in the efficacy and toxicity profile of ADC-based combination regimens in aUC. Differential expression of the target antigen and the therapeutic design of each drug likely drive the significant heterogeneity in efficacy and toxicity profiles. Peripheral sensory neuropathy, fatigue, and alopecia were more commonly observed in anti-nectin-4 regimens, consistent with other Monomethyl Auristatin E (MMAE) containing ADCs (brentuximab vedotin, glembatumumab vedotin, and polatuzumab vedotin) (12). This on-target off-tumor toxicity is due to the high expression of nectin-4 in the skin and the effect of the MMAE payload (12). Peripheral neuropathy is also associated with ADCs composed of tubulin inhibitor payloads (MMAE, SPP-DM1, and SPDB-DM4) and is caused by the peripheral axonopathy induced by free payload release into the systemic circulation (12). The hepatotoxicity described in the RC48-C014 trial likely reflects HER2-dependent and independent pathways, resulting from the normal expression of HER2 in hepatocytes and the internalization of disitamab vedotin (12). In contrast, gastrointestinal (diarrhea and nausea) and hematologic (anemia and neutropenia) toxicities were more common in the TROPHY-U-01 (Cohort 3) trial and are mediated by the release of SN-38 to the systemic circulation (12). Of note, hematological suppression can be so profound that a black box warning was added to SG for severe or life-threatening neutropenia and severe diarrhea. This echoes the results of the recent TROPiCS-04 study, where SG failed to meet the primary endpoint of overall survival compared to chemotherapy, despite higher ORR (23% vs. 14%) and progression-free survival (4.2 vs. 3.6 months) (13). Notably, the development of severe neutropenia and failure of the TROPiCS-04 study to meet its primary endpoint resulted in Gilead Sciences voluntarily withdrawing SG’s indication for treatment of adult patients with pretreated, locally advanced, or metastatic urothelial carcinoma. This could lead to fewer trials being developed in the pipeline of SG-related combinations and more efforts being made for other anti-TROP2 ADCs. This further underscores the safety signals across all other trials, specifically for anti-nectin-4-based trials, which showed the highest rates of treatment-related adverse events (TRAEs) leading to regimen modifications. For example, the EV-302 trial reported a dose reduction rate of 40.7% and 4 deaths due to TRAEs, and the EV-103 (cohort K) trial reported a treatment discontinuation rate of 47.4% and 3 deaths due to TRAEs.
Although the indication for SG in aUC was withdrawn, many other anti-TROP2 ADCs are currently in development as monotherapy and in combination. Datopotamab-deruxtecan (Dato-DXd) demonstrated an ORR of 25% in pre-treated patients, with no grade ≥ 3 hematologic events reported (14). Sacituzumab tirumotecan, an anti-TROP2 ADC with a topo-1 inhibitor payload, is currently being investigated in combination with EV with or without pembrolizumab in the phase 1/2 KEYMAKER-U04 trial (NCT06483334). Advances in the design of ADCs could also improve the safety and tolerability of anti-nectin-4 ADCs. BT8009 is a bicycle toxin conjugate targeting nectin-4 with an MMAE payload linked via a valine-citrulline cleavable linker (15). Preliminary results of the Duravelo-2 study (NCT06225596) showed an ORR of 45% with no grade ≥3 adverse events reported (15).
In the current treatment landscape, EV in combination with pembrolizumab is considered the standard of care for first-line treatment of advanced or metastatic urothelial carcinoma following the promising results of the EV-302 trial, which demonstrated an objective response rate of 67.7% (3). Our pooled analysis of ADC-based combinations (including anti-TROP2, anti-nectin-4, and anti-HER2 ADCs) yielded a similar efficacy to that of EV plus pembrolizumab alone, though at the cost of distinct toxicity profiles. These findings suggest that ADC-based combination regimens may consolidate their role as alternative strategies to EV plus pembrolizumab in the frontline setting, while also remaining alternative options in the salvage setting. However, their definitive role will need to be clarified through ongoing and future randomized trials.
Our study has some limitations. First, the number of eligible trials was small, and pooled subgroup analyses were not feasible for anti-TROP2 and anti-HER2-based regimens, since each was represented by a single study. We therefore reported regimen-level results descriptively, while retaining an overall pooled analysis across all ADC combination regimens. Second, the variability in trial design, patient population, and eligibility criteria may have contributed to interstudy heterogeneity. Moreover, the predominance of anti-nectin-4-based trials limits the generalizability across all ADC drug classes. As the therapeutic landscape in advanced urothelial carcinoma is rapidly evolving, forthcoming trial results are likely to refine the role of ADC-based combinations.
Combination approaches incorporating ADCs have changed the current treatment landscape of aUC, with several studies currently underway. While the non-overlapping toxicity profile of ADCs favors combination strategies, careful assessment of the cumulative risk is crucial for the development and safe clinical adoption of these combinations.
Data availability statement
The raw data supporting the conclusions of this article will only be shared upon reasonable request to the corresponding author.
Author contributions
SJ-C: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. RB-C: Conceptualization, Investigation, Methodology, Visualization, Writing – review & editing. MZ: Conceptualization, Data curation, Investigation, Writing – review & editing. KS: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – review & editing. HE: Conceptualization, Data curation, Investigation, Writing – review & editing. BM: Conceptualization, Data curation, Investigation, Writing – review & editing. DC: Conceptualization, Investigation, Methodology, Writing – review & editing. PZ: Conceptualization, Data curation, Investigation, Writing – review & editing. AL: Conceptualization, Investigation, Writing – review & editing. WY: Data curation, Investigation, Supervision, Writing – review & editing. XL: Formal Analysis, Resources, Visualization, Writing – review & editing. ND: Conceptualization, Data curation, Investigation, Writing – review & editing. NS: Conceptualization, Formal Analysis, Investigation, Writing – review & editing. JH: Conceptualization, Investigation, Writing – review & editing. ZZ: Conceptualization, Investigation, Writing – review & editing. LM: Conceptualization, Investigation, Writing – review & editing. CN: Conceptualization, Investigation, Supervision, Writing – review & editing. AC: Conceptualization, Investigation, Supervision, Writing – review & editing. SP: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. AT: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
AT has received consulting fees from Deka Biosciences, Aadi Biosciences, Seattle Genetics/Astellas, Exelixis, Bayer and Gilead Sciences and research funding from Corvus Pharmaceuticals, EMD Serono and Aravive, Inc. SP reports honoraria and a consulting/advisory role with Novartis, Astellas Pharma, Pfizer, Aveo, Myriad Pharmaceuticals, Roche/Genentech, Exelixis, Bristol-Myers Squibb, Ipsen, and Eisai and honoraria and research funding from Medivation. CN has received travel funding from DAVA Oncology and honoraria from MJH Associates.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1669526/full#supplementary-material
Supplementary Figure 1 | Article selection process (PRISMA).
Supplementary Table 1 | Most common any grade and grade 3 or above adverse events across all studies.
Supplementary Table 2 | Systematic review search syntax for PubMed and Embase online libraries.
References
1. Tagawa ST, Balar AV, Petrylak DP, Rezazadeh Kalebasty A, Loriot Y, Fléchon A, et al. TROPHY-U-01: A phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. (2021) 39:2474–85. doi: 10.1200/JCO.20.03489
2. Sheng X, Wang L, He Z, Shi Y, Luo H, Han W, et al. Efficacy and safety of disitamab vedotin in patients with human epidermal growth factor receptor 2–positive locally advanced or metastatic urothelial carcinoma: A combined analysis of two phase II clinical trials. J Clin Oncol. (2024) 42:1391–402. doi: 10.1200/JCO.22.02912
3. Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. New Engl J Med. (2024) 390:875–88. doi: 10.1056/NEJMoa2312117
4. Lisberg A, Drakaki A, Meric-Bernstam F, Alhalabi O, Kojima T, Kato M, et al. Datopotamab deruxtecan in locally advanced/metastatic urothelial cancer: Preliminary results from the phase 1 TROPION-PanTumor01 study. J Clin Oncol. (2024) 42:603–3. doi: 10.1200/JCO.2024.42.4_suppl.603
5. Grivas P, Tagawa ST, Bellmunt J, De Santis M, Duran I, Goebell PG, et al. TROPiCS-04: Study of sacituzumab govitecan in metastatic or locally advanced unresectable urothelial cancer that has progressed after platinum and checkpoint inhibitor therapy. J Clin Oncol. (2021) 39:TPS498–8. doi: 10.1200/JCO.2021.39.6_suppl.TPS498
6. Powles T, Drakaki A, Teoh JYC, Grande E, Fontes-Sousa M, Porta C, et al. A phase 3, randomized, open-label, multicenter, global study of the efficacy and safety of durvalumab (D) + tremelimumab (T) + enfortumab vedotin (EV) or D + EV for neoadjuvant treatment in cisplatin-ineligible muscle-invasive bladder cancer (MIBC) (VOLGA). J Clin Oncol. (2022) 40:TPS579–9. doi: 10.1200/JCO.2022.40.6_suppl.TPS579
7. Galsky MD, Grande E, Necchi A, Koontz MZ, Iyer G, Campbell MT, et al. Phase 3 open-label, randomized, controlled study of disitamab vedotin with pembrolizumab versus chemotherapy in patients with previously untreated locally advanced or metastatic urothelial carcinoma that expresses HER2 (DV-001). J Clin Oncol. (2024) 42:TPS717–7. doi: 10.1200/JCO.2024.42.4_suppl.TPS717
8. O’Donnell PH, Milowsky MI, Petrylak DP, Hoimes CJ, Flaig TW, Mar N, et al. Enfortumab vedotin with or without pembrolizumab in cisplatin-ineligible patients with previously untreated locally advanced or metastatic urothelial cancer. J Clin Oncol. (2023) 41:4107–17. doi: 10.1200/JCO.22.02887
9. Hoimes CJ, Flaig TW, Milowsky MI, Friedlander TW, Bilen MA, Gupta S, et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J Clin Oncol. (2023) 41:22–31. doi: 10.1200/JCO.22.01643
10. Grivas P, Pouessel D, Park CH, Barthelemy PH, Bupathi MB, Petrylak DP, et al. Sacituzumab govitecan in combination with pembrolizumab for patients with metastatic urothelial cancer that progressed after platinum-based chemotherapy: TROPHY-U-01 cohort 3. J Clin Oncol. (2024) 42:1415–25. doi: 10.1200/JCO.22.02835
11. Zhou L, Yang KW, Zhang S, Yan XQ, Li SM, Xu HY, et al. Disitamab vedotin plus toripalimab in patients with locally advanced or metastatic urothelial carcinoma (RC48-C014): a phase Ib/II dose-escalation and dose-expansion study. Ann Oncol. (2025) 36:331–9. doi: 10.1016/j.annonc.2024.12.002
12. Nguyen TD, Bordeau BM, and Balthasar JP. Mechanisms of ADC toxicity and strategies to increase ADC tolerability. Cancers (Basel). (2023) 15:713. doi: 10.3390/cancers15030713
13. Grivas P, Powles TB, Vulsteke C, Goupil MG, Park SH, Necchi A, et al. LBA9 TROPiCS-04, a randomized phase III study of sacituzumab govitecan (SG) vs chemotherapy (CT) in pretreated advanced urothelial carcinoma (aUC): Overall survival (OS) and safety analysis. Ann Oncol. (2024) 35:S1505–7. doi: 10.1016/j.annonc.2024.10.830
14. Meric-Bernstam F, Alhalabi O, Lisberg A, Drakaki A, Garmezy B, Kogawa T, et al. Datopotamab deruxtecan (Dato-DXd) in locally advanced/metastatic urothelial cancer: Updated results from the phase 1 TROPIONPanTumor01 study. J Clin Oncol. (2025) 43:663–3. doi: 10.1200/JCO.2025.43.5_suppl.663
15. Loriot Y, Siefker-Radtke AO, Friedlander TW, Necchi A, Wei AZ, Sridhar SS, et al. A phase 2/3 study of Bicycle toxin conjugate BT8009 targeting nectin-4 in patients with locally advanced or metastatic urothelial cancer (la/mUC): Duravelo-2. J Clin Oncol. (2024) 42:TPS4619–TPS4619. doi: 10.1200/JCO.2024.42.16_suppl.TPS4619
Keywords: urothelial carcinoma, antibody-drug conjugate, clinical trials, immunotherapy, nectin-4, Trop-2, HER2
Citation: Jaime-Casas S, Barragan-Carrillo R, Zugman M, Shah K, Ebrahimi H, Mercier B, Castro DV, Zang PD, LeVee A, Yip W, Li X, Dizman N, Salgia NJ, Zengin Z, Meza L, Hsu J, Nguyen CB, Chehrazi-Raffle A, Pal SK and Tripathi A (2025) Efficacy and safety of antibody-drug conjugate combination therapy in advanced urothelial carcinoma. Front. Oncol. 15:1669526. doi: 10.3389/fonc.2025.1669526
Received: 19 July 2025; Accepted: 18 September 2025;
Published: 07 October 2025.
Edited by:
Sanja Stifter-Vretenar, Skejby Sygehus, DenmarkReviewed by:
Giandomenico Roviello, University of Firenze, ItalyKhalil Saleh, Gustave Roussy Cancer Campus, France
Copyright © 2025 Jaime-Casas, Barragan-Carrillo, Zugman, Shah, Ebrahimi, Mercier, Castro, Zang, LeVee, Yip, Li, Dizman, Salgia, Zengin, Meza, Hsu, Nguyen, Chehrazi-Raffle, Pal and Tripathi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abhishek Tripathi, YXRyaXBhdGhpQGNvaC5vcmc=
 Salvador Jaime-Casas
Salvador Jaime-Casas Regina Barragan-Carrillo1
Regina Barragan-Carrillo1 Alexander Chehrazi-Raffle
Alexander Chehrazi-Raffle Sumanta K. Pal
Sumanta K. Pal Abhishek Tripathi
Abhishek Tripathi