- 1Department of Biomedical Sciences, School of Medicine, Nazarbayev University, Astana, Kazakhstan
- 2Department of Multidisciplinary Surgery, National Research Oncology Center, Astana, Kazakhstan
- 3Department of Science, National Research Oncology Center, Astana, Kazakhstan
- 4Department of Medicine, School of Medicine, Nazarbayev University, Astana, Kazakhstan
Introduction: Gastric cancer remains a major global health burden, with Central Asia, Kazakhstan in particular, exhibiting high incidence and mortality. There is a lack of recent national data providing a detailed clinical picture of gastric cancer. Most reports have been limited to summary statistics on incidence or mortality, without stratification by tumor stage, histological subtype, survival, or associated comorbidities. This study addresses gaps by using national registry data to evaluate 12-year gastric cancer trends, patient characteristics, and outcomes. We aim to investigate gastric cancer epidemiology, survival, and associated risk factors in Kazakhstan for the prevention and control strategies.
Methods: This retrospective cohort study analyzed 33,992 patients with gastric cancer using the Unified National Electronic Healthcare System in Kazakhstan from 2012 to 2023. Cases were identified via ICD-10 codes (C16.0-C16.9). Demographic, staging, histological, treatment and comorbidity data were extracted. Outcomes included incidence, mortality, and survival. Kaplan-Meier analysis and Cox regression were used to evaluate survival differences and predictors. Population-based rates were age-standardized using WHO standards.
Results: The age-standardized incidence rate declined from 17.46 to 13.63 per 100,000; mortality dropped from 16.16 to 8.74. Prevalence doubled over 12 years. Most cases (33.5%) were diagnosed at Stage III, closely followed by Stage II and Stage IV. One- and five-year survival rates were 38.1% and 17.1%, respectively. Men and patients aged 60–69 had the highest incidence. Survival declined sharply with stage: Stage I (49.1%) vs Stage IV (3.5%, P < 0.001). The most common tumor site was the cardia, and adenocarcinoma was the predominant histology. Cox regression identified older age (HR 1.17 per decade), advanced stage (HR 3.48 for Stage IV), recurrence, metastases and cancer complications as significant mortality predictors (all P < 0.001). Cardiovascular and gastrointestinal diseases were the most common comorbidities.
Conclusion: Gastric cancer in Kazakhstan shows late-stage diagnosis and poor survival. Targeted screening, earlier diagnosis, and improved management of comorbidities are essential to improve outcomes and reduce mortality.
1 Introduction
Gastric cancer (also called stomach cancer) is one of the most widespread cancer types worldwide. According to the GLOBOCAN (2022) data stomach cancer was 5th by incidence (968,350 cases) and 5th by mortality (659,853 deaths) (1). Globally, the incidence of gastric cancer increased from 883 thousand cases to 1.3 million between 1990 and 2019. In 2017, global age-standardized incidence rate (ASIR) was 15.36 while age-standardized mortality rate (ASMR) was 10.98 per 100,000 people. In 2017, Central Asia reported an ASIR of 14.12 and a nearly equivalent ASMR of 14.34 per 100,000 population, indicating not only a high disease burden but also a high case-fatality rate (2).
According to the Global Cancer Observatory, Asia reported the highest incidence, mortality, and prevalence of gastric cancer for both sexes in 2022 (3). Globally, the age-standardized incidence rate (ASIR) for gastric cancer was 9.2 per 100,000 population (12.8 in men and 6.0 in women), while the corresponding mortality rate was 6.1 (8.6 in men and 3.9 in women) (4). In Kazakhstan, the ASMR decreased from 16.4 per 100,000 in 2009 to 9.4 in 2018, reflecting progress in disease control (5). However, a 2016 predictive model anticipated a continued increase in gastric cancer burden in the country (6). Between 2014 and 2022, gastric cancer had the second-highest prevalence after respiratory cancers, representing 11.4% of all cancer types (7).
Known risk factors for gastric cancer are: lifestyle and diet, genetic predisposition, medical conditions, applied treatment, demographic characteristics, occupational exposures and radiation (8). Late-stage diagnosis and poor early detection remain key drivers of high mortality.
While previous studies have described general cancer trends in Kazakhstan (7, 9), there is a lack of recent national data providing a detailed clinical picture of gastric cancer as the latest one published covers the period from 2005 till 2014 (10) and 2009-2018 (5)Recent reports have been limited to summary statistics in local journals, newsletters on incidence or mortality, without stratification by tumor stage, histological subtype, survival, or associated comorbidities (11). No study to date has used large-scale electronic health records to analyze long-term trends alongside clinical outcomes at the population level. This study addresses that gap by providing the first comprehensive nationwide analysis of gastric cancer in Kazakhstan using individual-level data over a 12-year period (2012-2023). By combining demographic, clinical, and survival information, we aim to identify diagnostic delays, characterize high-risk patient groups, and support the development of targeted screening and treatment strategies.
2 Methods
2.1 Study design and database
This retrospective, population-based study aimed to investigate the epidemiology, comorbidities, histological subtypes, and staging of gastric cancer in Kazakhstan. Data were sourced from the UNEHS’s Electronic Registry of Inpatients; a national digital database launched in late 2013 to consolidate inpatient medical records across Kazakhstan’s healthcare institutions (13). Historical data from prior years were manually integrated into the system, with the 84 most complete and consistent coverage beginning in 2012. Also, data was taken from the Electronic Registry of Oncological Patients (EROP) in particular, between 2012 and 2023, which records clinically relevant encounters (inpatient admissions, outpatient visits, and official follow-up) across 3 participating public facilities (12). Patients in the registry are assigned unique, lifelong population registry numbers (RpnIDs), ensuring that no identifiable information is available.
Population denominators for rate calculations were obtained from the Statistics Committee under the Ministry of National Economy of the Republic of Kazakhstan (14).
2.2 Participants and eligibility criteria
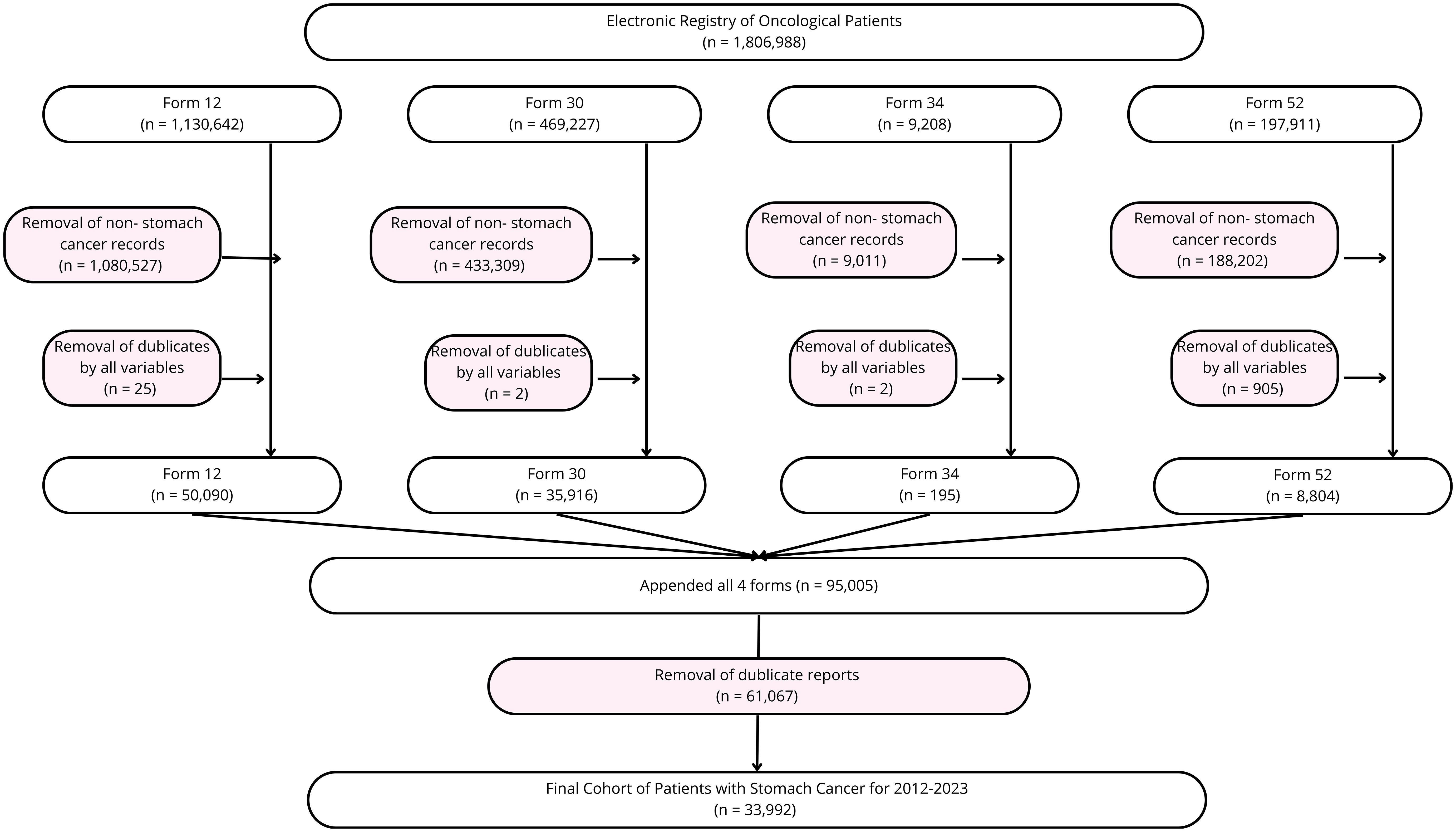
Gastric cancer cases were identified using ICD-10 codes C16, C16.0-C16.9. A total of 95,005 hospital admission records from 2012 to 2023 were initially extracted. After data cleaning and de-duplication based on unique registry personal numbers (RpnID), 33,992 unique patients were retained for analysis.
2.3 Exposures and covariates
Individual patient data included RpnID, demographic and clinical characteristics.
There were more than 100 nationalities; therefore, ethnicity was grouped as Kazakhs, Russians, Ukrainians, and Others. The year of registration was determined based on the patient’s initial admission. Age was categorized into eight groups (0-19, 20-29,…, 80+ years), adapted from the Centers for Disease Control and Prevention for epidemiological analysis (15).
Occupational status was divided into 19 groups. This structured approach ensures clarity and consistency in the dataset while allowing for comprehensive epidemiological analysis.
The timing of comorbidity diagnosis was determined using the admission date associated with each ICD-10-coded diagnosis in the UNEHS inpatient registry. “Before cancer” refers to comorbidities recorded in any inpatient admission prior to the date of the first gastric cancer admission. “Simultaneous” was defined as comorbidities recorded during the same hospitalization as the first gastric cancer diagnosis. “After cancer” refers to comorbidities first recorded in subsequent admissions following the initial cancer admission. Due to the lack of outpatient data and treatment timelines, this classification may be affected by reporting delays or hospitalization priorities.
2.4 Tumor characteristics
Tumor staging followed the TNM classification system. Due to inconsistencies in the national clinical protocol (now under revision), staging was cross-validated using the Russian Federation’s guidelines, which align with the American Cancer Society standards (16–18). In Kazakhstan, gastric cancer staging is standardized nationally and follows the Russian Federation clinical protocol, which is aligned with the TNM 8th edition. As a result, staging was consistently applied across institutions during the study period, and no retrospective restaging or conversion using ICD-O or other systems was required. Stage migration was not anticipated, given the uniform use of the TNM-based classification system throughout. For analysis cancer stages were grouped into bigger ones (In Situ, I, II, III, IV) due to lack of precise diagnosis written in database. Histological types of tumors were presented in more than 100 types and were grouped into 10 categories. Comorbidities were collected by the RPN IDs from all databases and included into the analysis. Overall, 49 comorbidities were identified.
We calculated and report the proportion of missing values for key variables including histology, stage, and comorbidity status. No imputation was performed; analyses were conducted using complete case data, with “unknown” categories retained as separate groups where applicable. Distribution of missing data is also provided in the results.
2.5 Outcome assessment
Incidence proportion, prevalence and mortality rates based on the admission and discharge status were calculated based on the following formulas (1–3):
Age-standardized incidence (ASIR) per 100,000 people =
Age-standardized mortality rate (ASMR) per 100,000 people =
Age-standardized prevalence per 100,000 people =
where, i = age group, t = current year, StandardPop = WHO standard population per age group.
Standard population age-group data was taken from the WHO official report for standard population numbers from 2000 to 2025.
In the survival analysis, the start date was defined as the day of the first admission, and the follow-up was until October 11, 2024 (day of registry download), or until the date of death.
2.6 Statistical analysis
Data cleaning and statistical analysis were performed using Stata 18.5 MP version (19). Descriptive statistics summarized patient demographics and clinical features. Continuous variables were reported as means with standard deviations, and categorical variables as frequencies and percentages. Kaplan–Meier survival curves were used to estimate overall survival by cancer stage at diagnosis. To identify factors associated with mortality, we applied Cox proportional hazards regression. Variables for the multivariable Cox regression model were selected using a stepwise backward elimination approach, based on Akaike Information Criterion (AIC). Candidate variables included those with clinical relevance and statistical significance (P < 0.05) in univariate analysis. Multicollinearity was assessed using variance inflation factors (VIFs), and no variables exceeded accepted thresholds. Because treatment variables (surgery, chemotherapy, radiotherapy) were >60% missing and lacked timing, we did not include them as baseline covariates to avoid selection and immortal-time bias. To partially account for prognosis that influences treatment selection, the multivariable Cox model adjusted for stage, metastasis/recurrence status, comorbidity burden and timing (relative to cancer), age, sex, and histology. Although some variables - such as comorbidities and complications - may be interrelated, their inclusion was justified based on their clinical importance and distinct coding in the registry data. Both univariable and multivariable models were constructed, with results reported as hazard ratios (HRs) and adjusted hazard ratios (AHRs) with 95% confidence intervals. Statistical significance was set at ɑ < 0.05.
3 Results
Figure 1 shows the data cleaning and data collection process. Overall, 1,806,988 patient records were obtained and 33,992 used for further analysis.
3.1 Demographic data
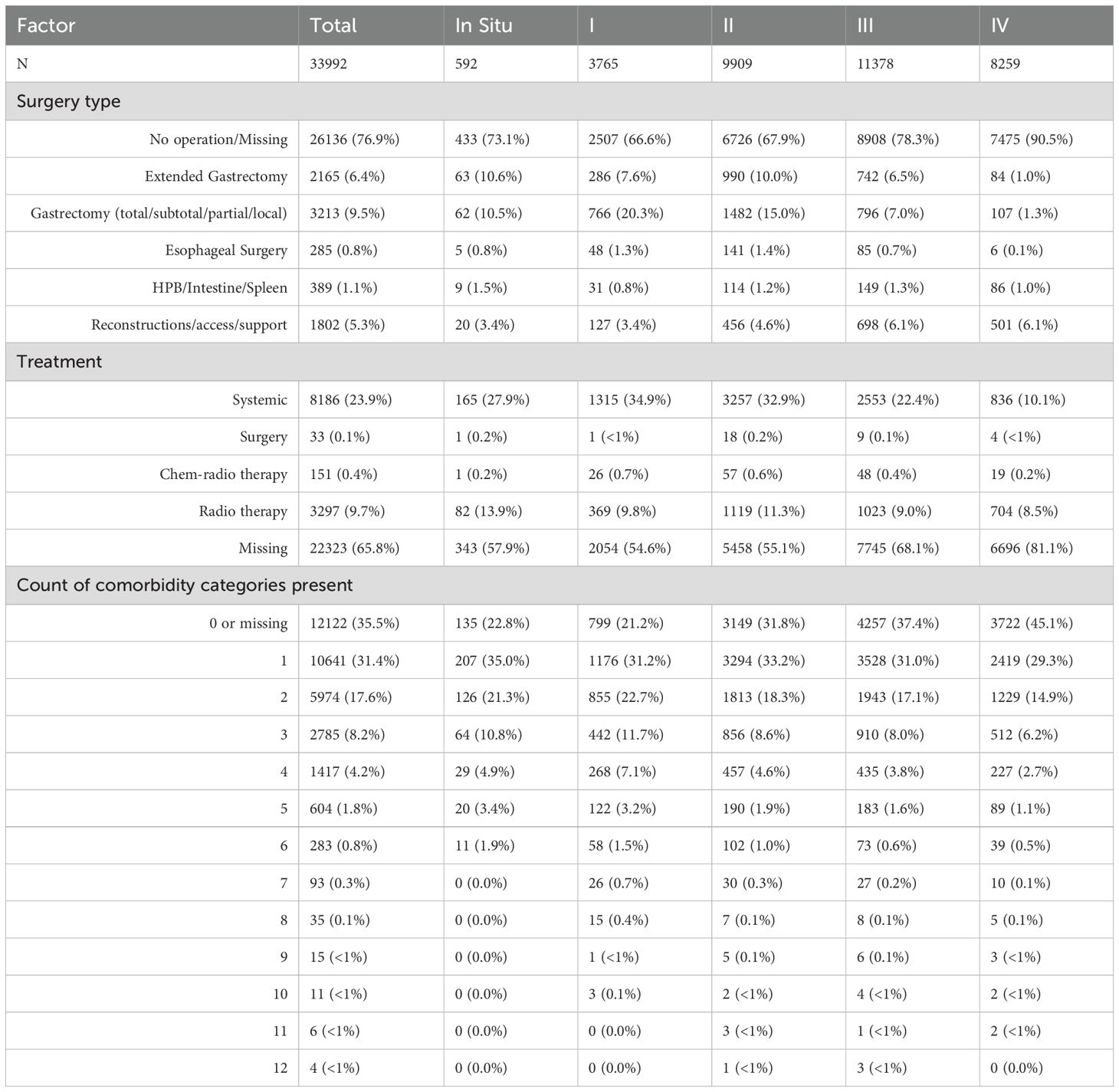
The demographic data is summarized in Table 1. In total 33,992 (35.9% women and 64.1% men) gastric cancer patient records were identified and analyzed in the national electronic database for the period 2012-2023, from all Kazakhstani regions. The mean age (± SD) was 64.2 ± 11.5 years. Of the patients, 59.3% were Kazakhs, 24.5% were Russians, 3.7% were Ukrainians, and 12.6% were listed as other nationalities.
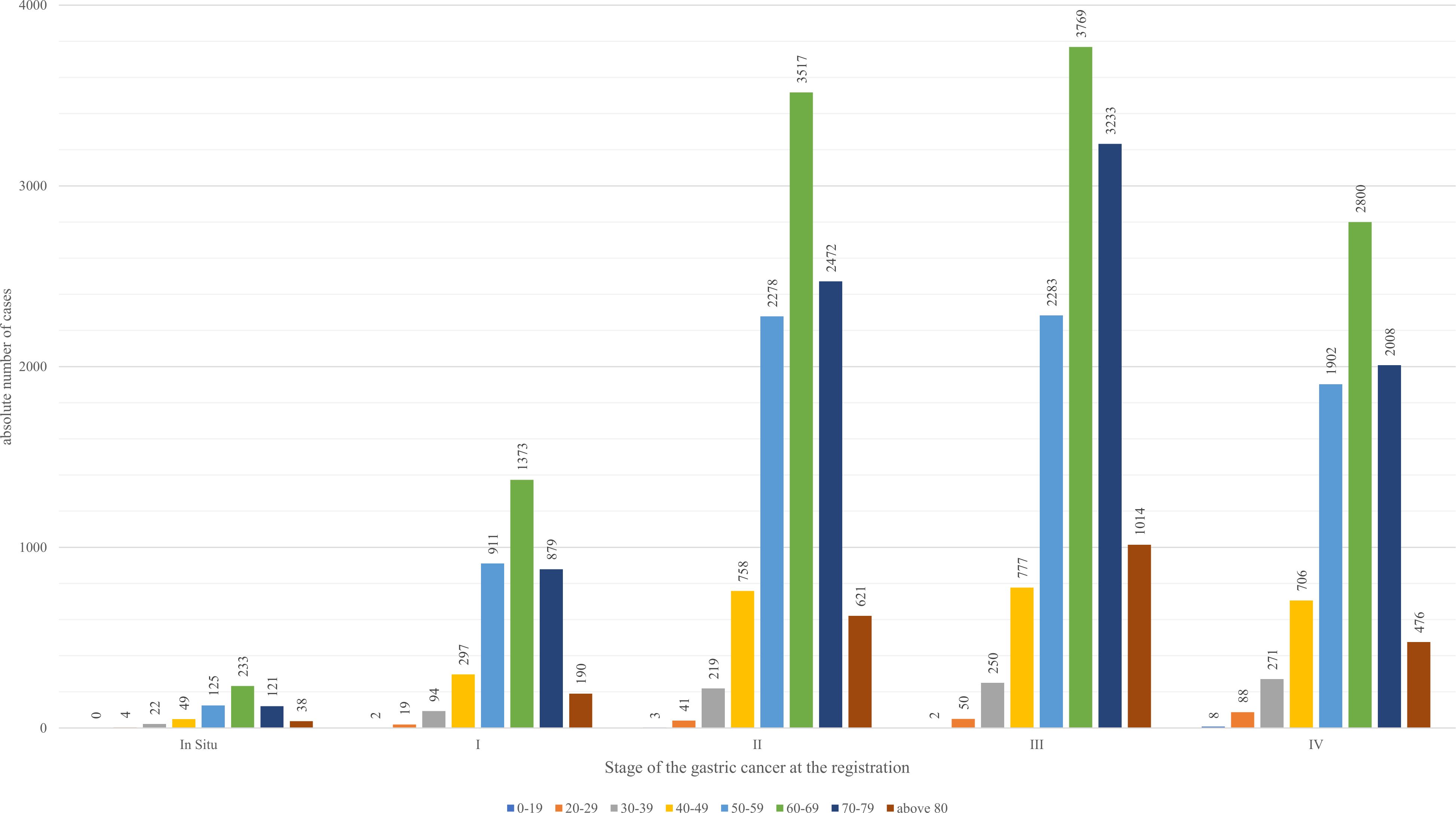
Among the age groups, as seen from Figure 2 60-69-year-olds had the highest registered cases of gastric cancer within the period, and most of the registrations occurred at Stage III of the disease (33.47%).
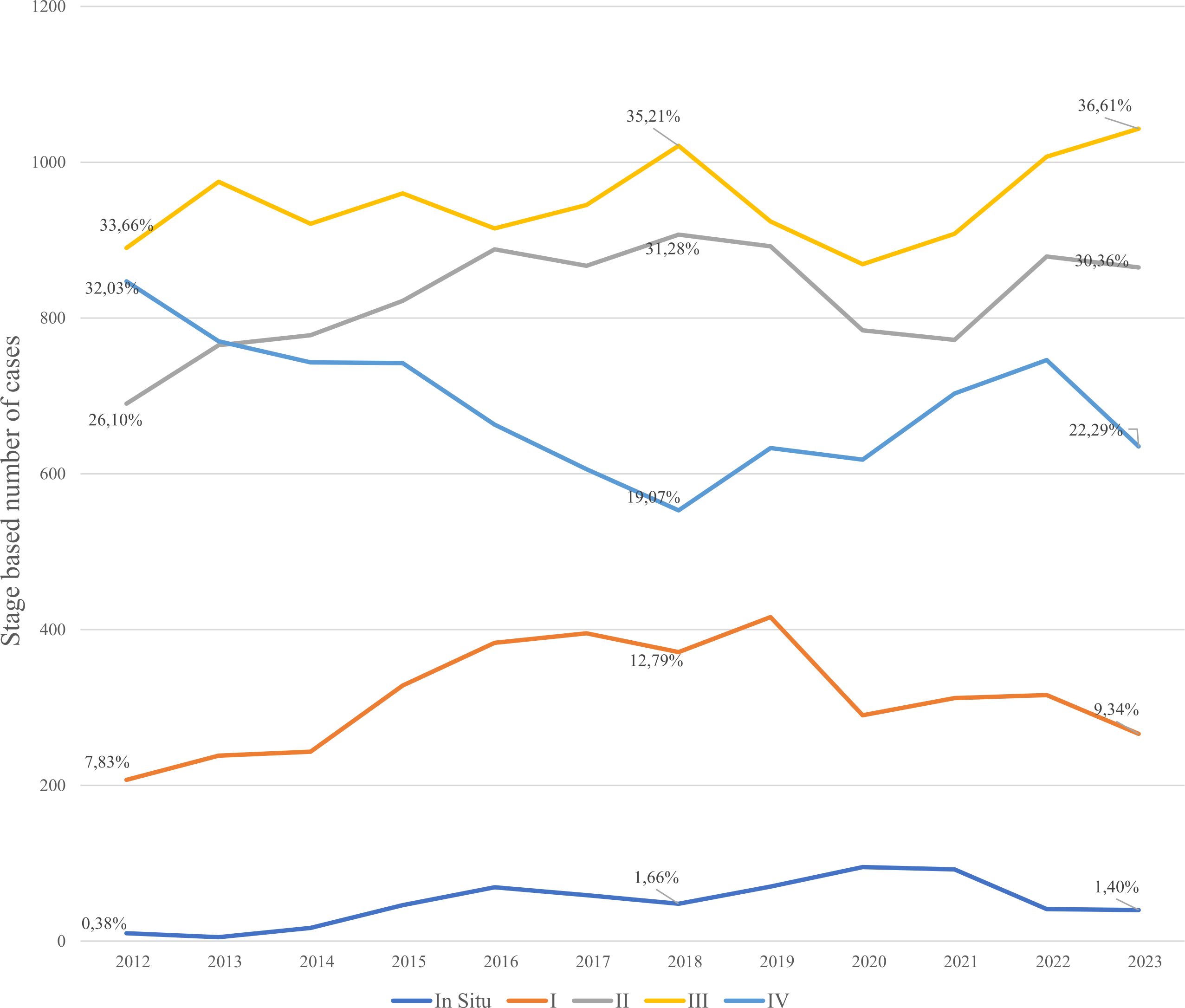
The annual number of registered gastric cancer cases remained relatively stable, ranging from 2,644 cases in 2012 to a peak of 2,989 in 2022, with a temporary decline in 2020 (2,656 cases) likely due to COVID-19–related disruptions in healthcare services (Figure 3). Stage III consistently accounted for the highest proportion of diagnoses, increasing from 33.6% in 2012 to 36.6% in 2023, highlighting the persistent burden of late-stage detection. Stage IV cases declined from 32.0% to 22.3%, while Stage II diagnoses increased from 26.1% to 30.4% over the same period. The proportion of early-stage (Stage I) cases remained lowest throughout, underscoring the ongoing challenge of early detection.
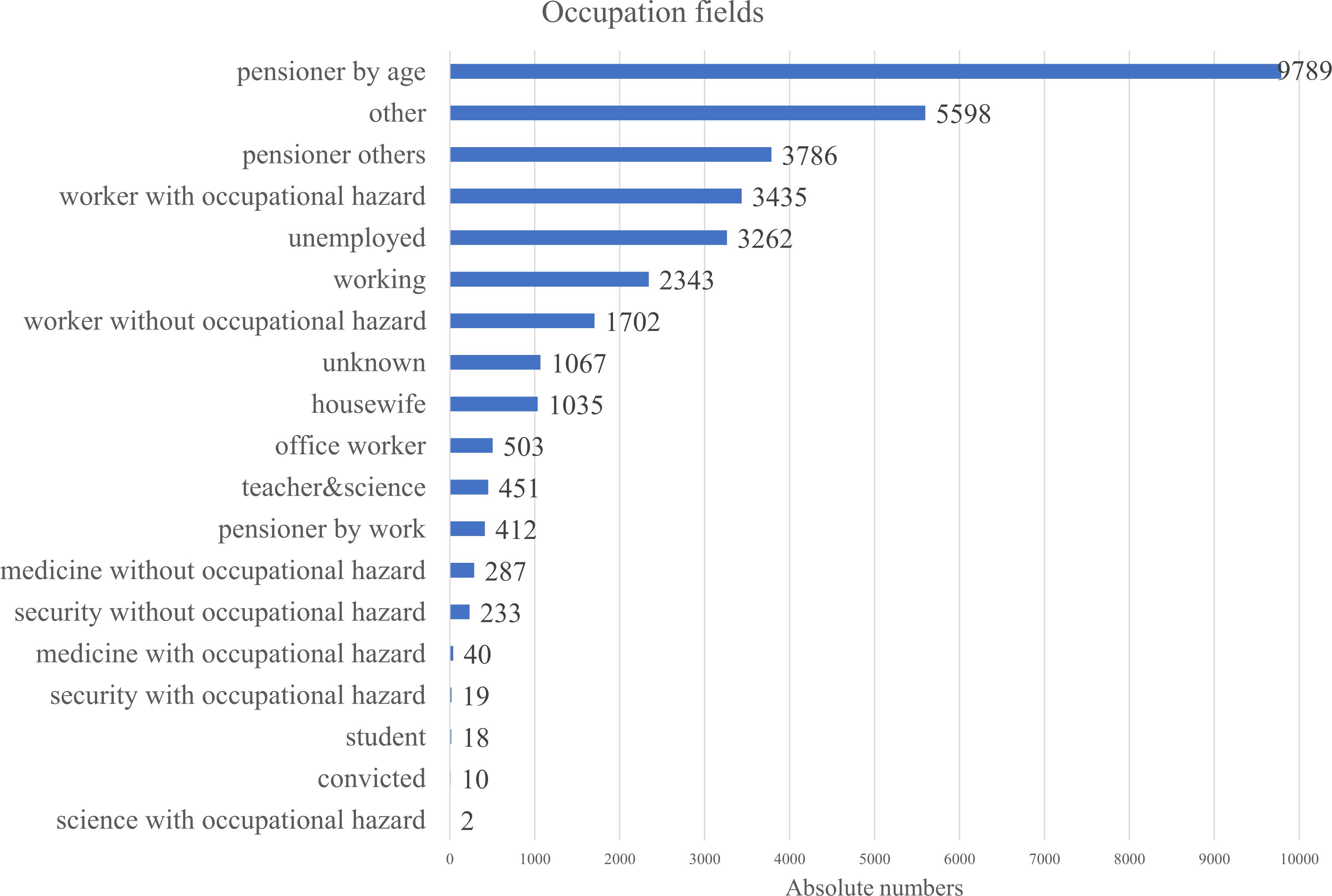
Describing the social status of the cohort, information wasn’t very consistent throughout the database, that is why we merged any information on the person, regarding their occupation into the Figure 4. The analysis of occupational backgrounds among gastric cancer patients revealed that the largest proportion were individuals retired by age, accounting for the majority of cases. This was followed by those categorized under “other” occupations and pensioners from unspecified fields. A notable share of patients were workers exposed to occupational hazards, suggesting potential environmental or workplace-related risk factors. The term worker with occupational hazard included workers from fields of transport, water transport, clerical, woodworking industry, railway worker, industry, construction, leather worker, public utilities and household services worker, forestry, machinery, metallurgy, miner, petrochemical industry, shoemaker, food industry worker, manufacturing, extramural worker, agricultural industry, glass and porcelain industry, building materials, chemical industry, sewing worker. These findings highlight a strong representation of older and retired populations, as well as possible links between occupational exposures and gastric cancer incidence.
3.2 Clinical data
The most common histological type was adenocarcinoma (61.6%), accounting for 62.0% of all deaths. Rare subtypes such as neuroendocrine carcinoma (0.5%) and sarcomas (0.2%) showed varied survival rates due to low case counts.
Metastases were present in 26.1% of cases and associated with a high mortality rate (29.4%, 8,267 cases). Patients with both metastases and recurrence (0.6%) had the highest mortality (94.4%). In contrast, those with no metastases or recurrence had improved outcomes, with 32.5% surviving, while patients with recurrence alone (1.2%) still faced high mortality (86.4%) (Table 1).
3.3 Treatment
Treatment of stomach cancer is based on the Kazakhstan’s protocol of treatment published in 2022 year (17), and also in accordance with NCCN 2025 (20) and Japanese guideline 2022 (21). Based on the Kazakhstani protocol, the sections on stage-based treatment state: for stages 0-II - subtotal/total gastrectomy (EMR for Tis/T1a) with mandatory D2 lymph node dissection; for locally advanced T3-T4 or N1-N2 - curative surgery plus 2–3 cycles of neoadjuvant chemotherapy and adjuvant polychemotherapy; for stage IV - palliative operations only (no lymphadenectomy) and palliative chemotherapy; and for recurrence - individualized surgery/endoscopic options plus palliative chemotherapy (17).
Based on the results obtained from the database (Table 1) most patients had no recorded surgery or data was missing (26,136, or 76.9%). It wasn’t possible to differentiate them, so they are written as one category “no operation/missing”. Among specific procedures, 3 213 (9.5%) underwent gastrectomy (total/subtotal/partial/local), 2 165 (6.4%) extended gastrectomy, 1 802 (5.3%) reconstructive/access procedures, 389 (1.1%) HPB/intestine/spleen procedures, and 285 (0.8%) esophageal surgery. Table 2 shows the distribution of type of surgery done by stages of disease in patients. It was seen that the likelihood of having no operation increased with advancing stage: 73.1% in In Situ and 66.6%–67.9% in stages I–II, rising to 78.3% in stage III and 90.5% in stage IV. Gastrectomy was comparatively concentrated in earlier stages (20.3% of stage I and 15.0% of stage II) and became uncommon in stage IV (1.3%). Reconstructive/access procedures were recorded in 3.4%–4.6% of stages I–II and ~6.1% of stages III–IV. Esophageal and HPB/intestine/spleen operations were rare across all stages (each ~1% per stage). With respect to non-surgical therapy, 8–186 patients (≈24%) had systemic therapy coded, 3 297 (9.7%) radiotherapy, 151 (0.4%) chemoradiotherapy, and 33 (0.1%) surgery-only treatment. Treatment coding was missing in 22 323 (65.8%) overall, and missingness increased with stage (57.9% in In Situ; 54.6% stage I; 55.1% stage II; 68.1% stage III; 81.1% stage IV).
3.4 Comorbidities
Gastrointestinal diseases were the most common comorbidities, accounting for 7,641 cases (22.48%). Cardiovascular diseases followed with 7,142 (21.01%) cases, with hypertension (13.21%), Angina pectoris (4.79%) and cerebrovascular (3.8%) diseases as the most frequent one. Hematologic and renal diseases ranked third with 4,151 cases (12.21%), driven by anemia (2,176 cases, 6.4%). Other notable categories included infectious diseases and immune (12.04%), dermatological and sensory (8.09%) and respiratory (7.21%) disorders (Table 1).
The number of comorbidity categories present was 0 or missing in 12–124 patients (35.5%); 1 category in 10 641 (31.4%); 2 in 5 974 (17.6%); 3 in 2 785 (8.2%); 4 in 1 417 (4.2%); and ≥5 in 1 044 (3.1%) with progressively smaller strata up to 12 (Table 1). Survivors had fewer “0/missing” entries and more recorded multimorbidity than those who died: 13.8% vs 40.1% had 0/missing; 51.6% of survivors vs 29.1% of decedents had ≥2 comorbidity categories, and 27.9% vs 12.8% had ≥3 categories. Comorbidities were right-skewed overall as seen from Table 2. “Zero or missing” made up 12–122 patients (35.5%) and became more common with advancing stage (from 22.8% in in-situ to 45.1% in stage IV). One category was recorded in 10 641 (31.4%) and stayed fairly stable across stages (~29–35%). Two categories were seen in 5 974 (17.6%), falling from 22.7% in stage I to 14.9% in stage IV. Higher counts were progressively less frequent: 3 categories in 2 785 (8.2%), 4 in 1 417 (4.2%), 5 in 604 (1.8%), and ≥6 in ≤0.8% each. Taken together, later stages showed fewer documented comorbidity categories and more 0/missing entries - pointing less to truly lower multimorbidity and more to under-ascertainment or under-recording in sicker patients.
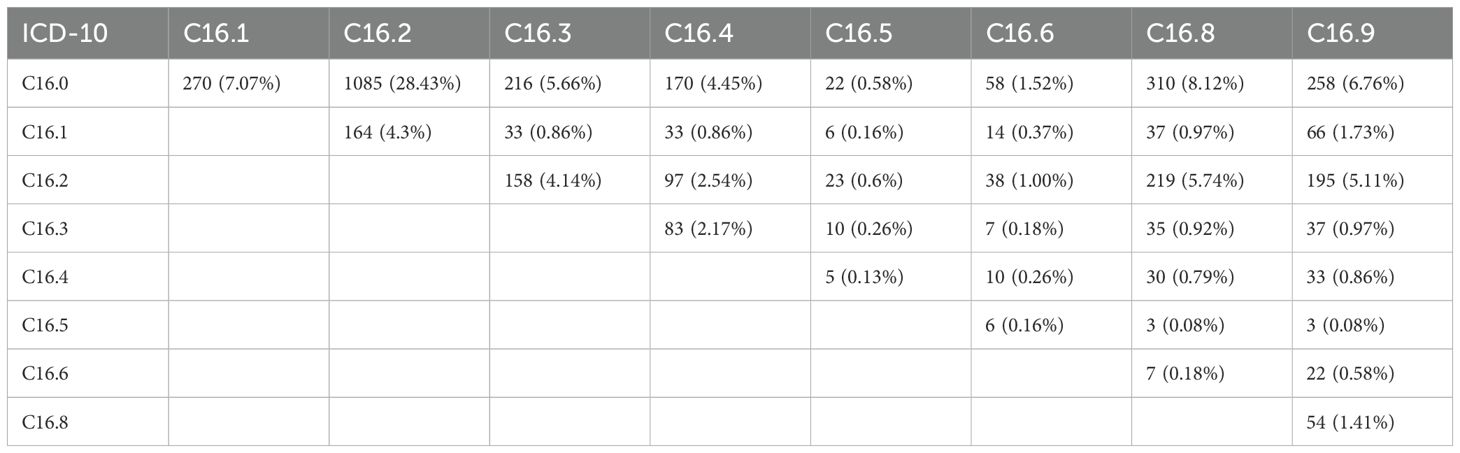
3.5 Tumor location
The most common site was the cardia (C16.0, 30.8%), followed by the body (C16.2, ~25%). Cases involving multiple locations were grouped as mixed. Overlaps were frequent - most notably between cardia and body (28.4%), and between body and overlapping lesions (C16.2 + C16.8) in 219 cases (5.74%), indicating extensive tumor spread (Table 3).
3.6 Incidence, prevalence and mortality rates
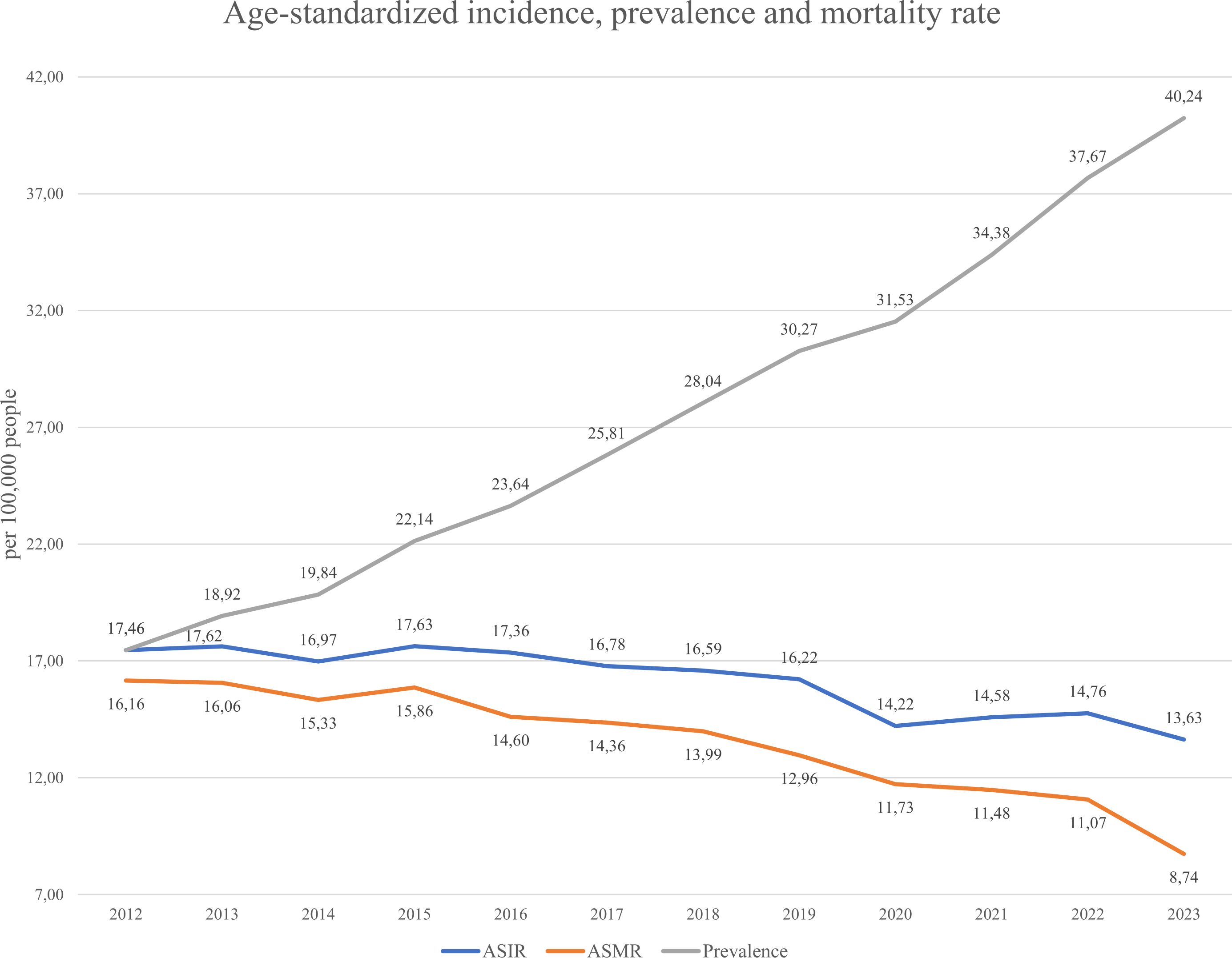
Incidence, prevalence, and mortality are key indicators of disease trends (see Equations 1–3 in Methods). As gastric cancer is considered lifelong, all diagnosed individuals were counted as prevalent cases while alive. Figure 5 shows that the ASIR (Age-standardized incidence Equation 1) remained relatively stable from 2012–2019 (16.06–17.46 per 100,000), then declined to 13.63 in 2023. The ASMR (Age-standardized mortality rate Equation 2) steadily decreased from 16.16 to 8.74, suggesting improvements in management or early detection. In contrast, prevalence (Equation 3) rose consistently from 17.62 to 40.24, indicating an increasing disease burden. Figure 6 displays gender-specific trends. In males, ASIR dropped from 27.51 to 22.17, while prevalence rose sharply (10.87 to 54.19); ASMR declined from 25.87 to 14.56. In females, ASIR declined from 10.87 to 7.91, ASMR from 9.83 to 4.87, and prevalence rose from 10.87 to 27.57. Across all indicators, males bore a higher burden, with a notably sharp rise in prevalence, despite declining incidence and mortality in both sexes.
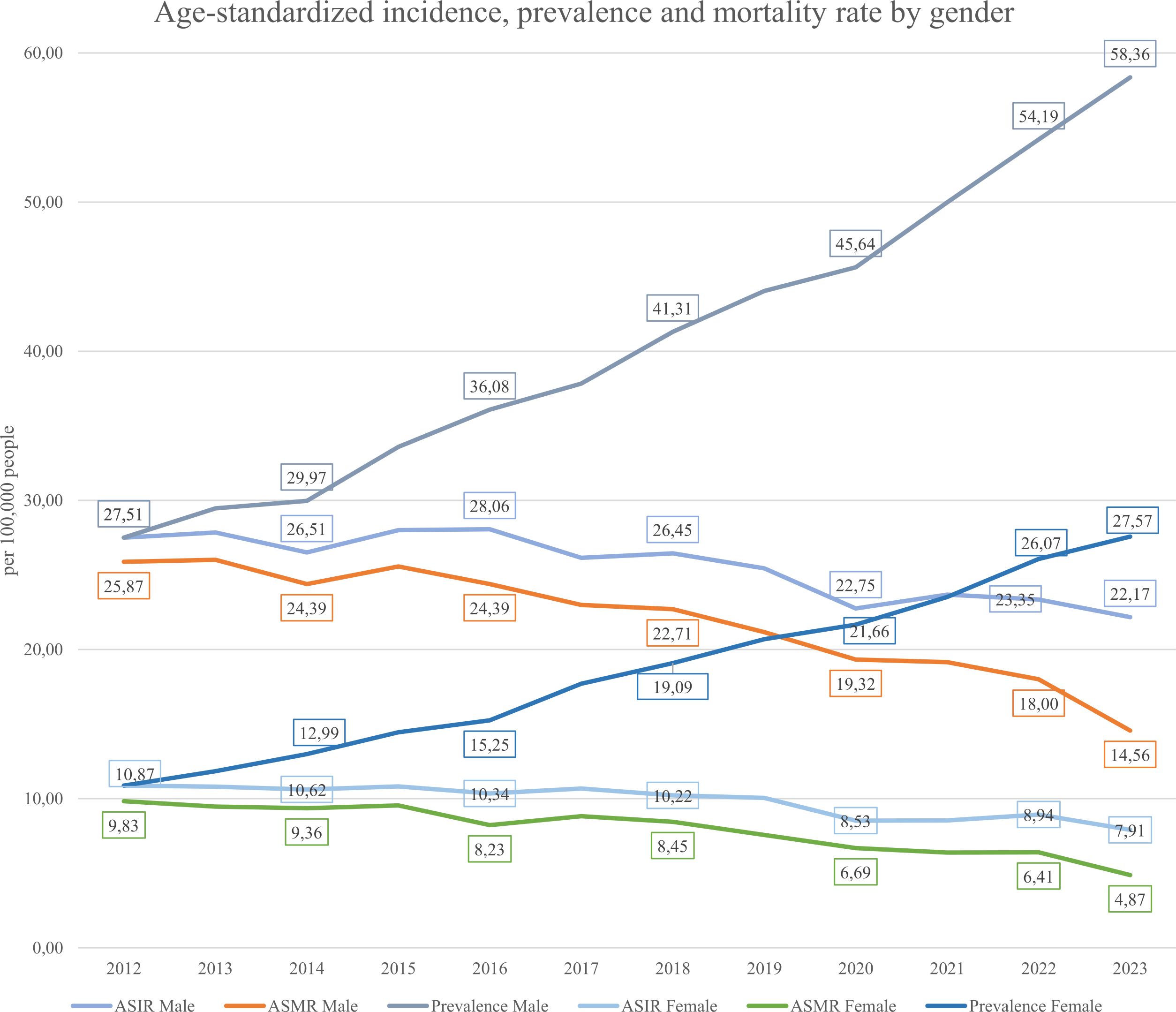
Figure 6. Gender-specific age-standardized incidence (ASIR), prevalence and mortality (ASMR) rate from 2012-2023.
3.7 Survival analysis
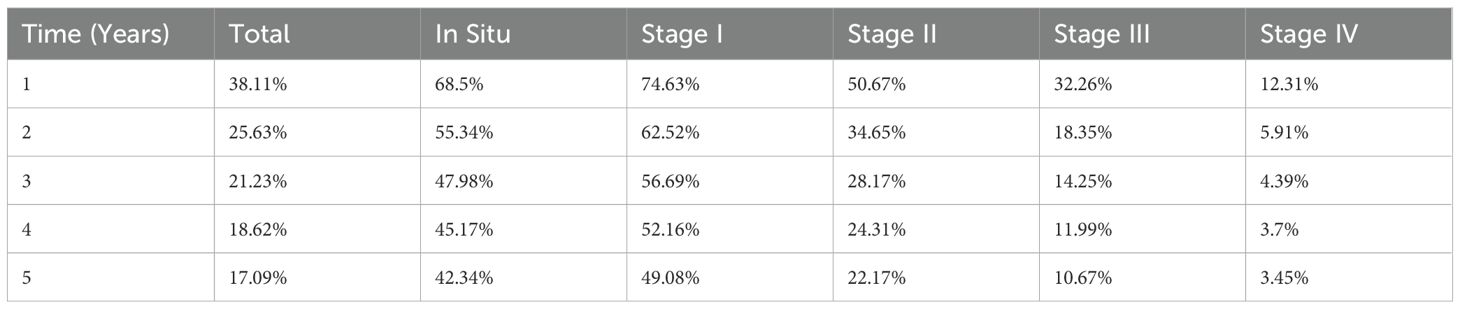
The Kaplan–Meier curves showed in Figure 7 separate early and remain well ordered by stage, with the steepest early decline in stage IV and progressively flatter trajectories for stages III→I. There is no material crossing of curves, underscoring a strong stage gradient. Stage I and in-situ show a visible plateau after ~2–3 years, whereas stages III–IV continue to fall toward very low long-term survival. Five-year survival analysis showed 1-year survival rates declining from 74.6% (Stage I) to 12.3% (Stage IV), and 5-year survival from 49.1% to 3.5%, respectively (Table 4).
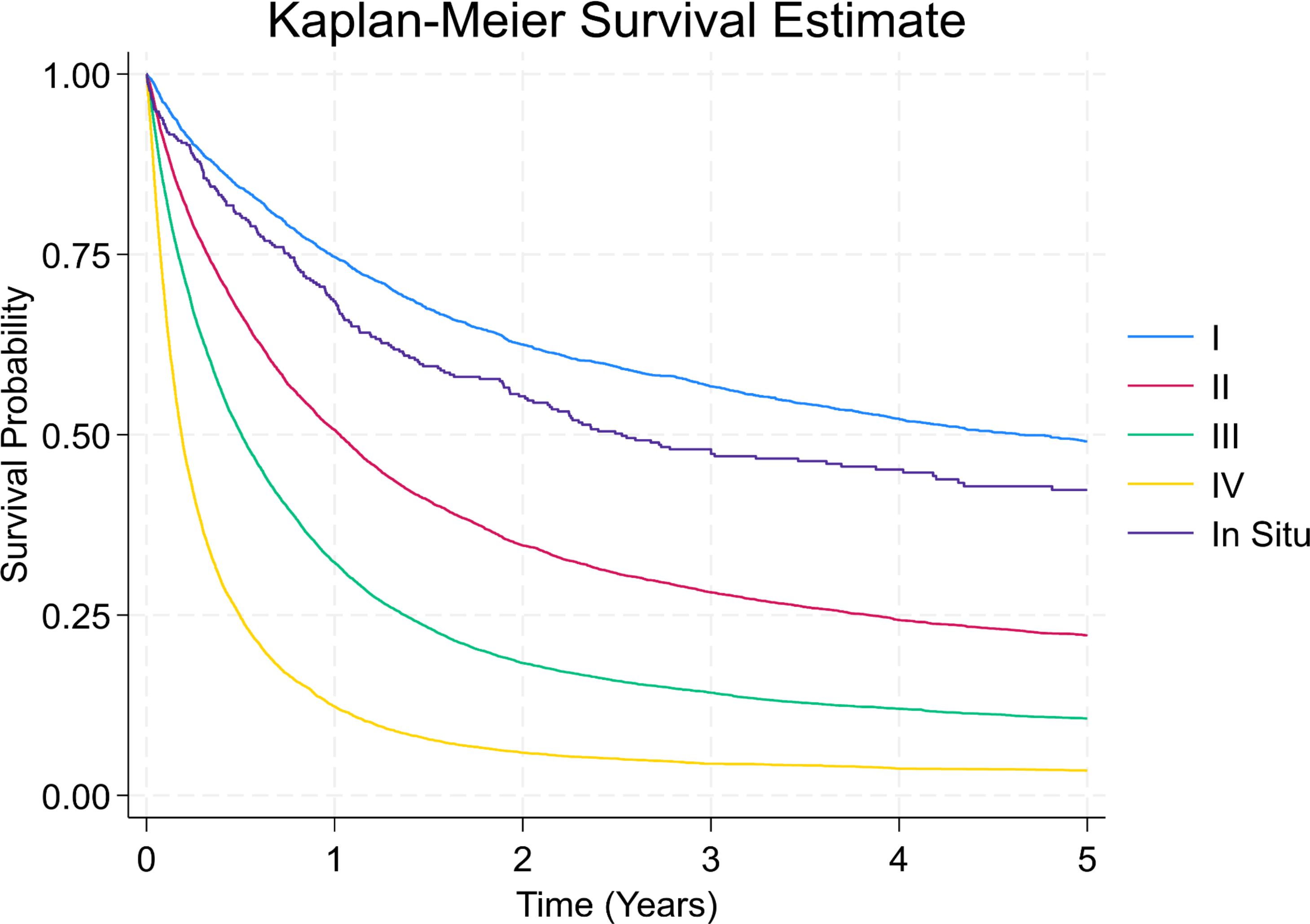
Figure 7. Kaplan-Meier survival curves by stage at diagnosis. This survival plot illustrates overall survival probabilities for patients with gastric cancer based on stage at diagnosis.
3.8 Hazard ratio by predictors
The multivariable Cox model (Table 5) included 31,183 patients and assessed associations between demographic and clinical variables and overall survival. In our dataset, treatment fields (surgery, chemotherapy, radiotherapy) are missing for >60% of patients, with missingness increasing with stage. Because of this extent of missing data, we did not include treatment variables in the multivariable Cox model. Treatment allocation is not random and is correlated with prognosis (confounding by indication): fitter patients are more likely to undergo curative surgery or chemotherapy, leading to downward bias in hazard estimates if treatment is unmeasured. Social status (occupational field) didn’t show any statistically significant results and thus were excluded from the model.
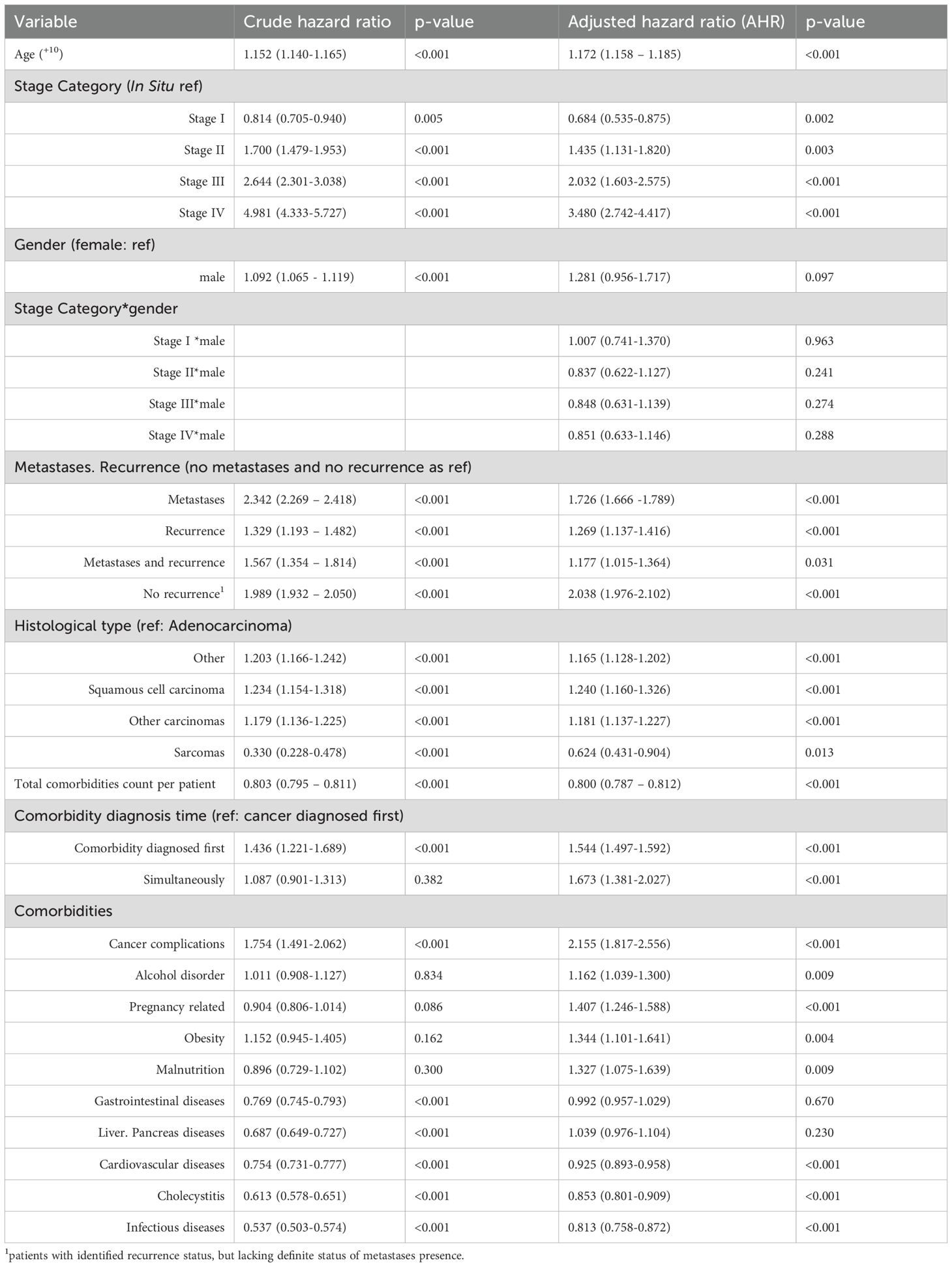
Table 5. Association between clinical variables and hazard ratio for mortality in gastric cancer patients.
3.8.1 Demographics
Age significantly predicted mortality, with an 17.1% increase in hazard per 10-year increase (p < 0.001). Male gender wasn’t independently associated with mortality (AHR = 1.281; p = 0.097), though this effect was modified by cancer stage (described further).
3.8.2 Cancer stage and interactions
Stage at diagnosis was a strong predictor of survival. Using In Situ as a reference AHR for Stage I was 0.684 (p=0.002) showing the lowered risk. Next stages showed higher AHR equal to 1.435 (0.003), 2.032 (p<0.001) and 3.480 (p<0.001) for II, III and IV stages respectively. Stage×sex interaction terms were non-significant (all p>0.24), indicating no evidence that the effect of stage differed by sex.
3.8.3 Metastasis and recurrence
Patients without recurrence/metastasis served as the reference. All other groups showed increased hazard. Notably, a group labelled “no recurrence” (with missing metastasis data) showed a high AHR of 2.038 (p < 0.001), likely due to data gaps in the metastasis data.
3.8.4 Histology
Adenocarcinoma was used as the reference. Other carcinoma types had slightly elevated AHRs, except for the sarcomas group.
3.8.5 Comorbidities and timing of main diagnosis
Timing of non-cancer comorbidities was also informative when compared with “cancer diagnosed first”. Patients whose comorbidity was diagnosed first (AHR = 1.544, p<0.001) or simultaneously with cancer (AHR = 1.673, p<0.001) had higher mortality. At the category level, several comorbidities were independently associated with worse outcomes: cancer complications (AHR = 2.155, p<0.001), pregnancy-related conditions (AHR = 1.407, p<0.001), obesity (AHR = 1.344, p=0.004), malnutrition (AHR = 1.327, p=0.009), and alcohol use disorder (AHR = 1.162, p=0.009). Other categories were not significant (gastrointestinal diseases AHR = 0.992, p=0.670; liver/pancreas diseases AHR = 1.039, p=0.230). Notably, several comorbidity groups showed lower hazards-cardiovascular diseases (AHR = 0.925, p<0.001), cholecystitis (AHR = 0.853, p<0.001), and infectious diseases (AHR = 0.813, p<0.001). Consistent with this pattern, a higher count of comorbidity categories was associated with a lower hazard (per additional category AHR = 0.800, p<0.001), which likely reflects differential recording/ascertainment rather than a protective biological effect.
4 Discussion
This is the first nationwide study in Kazakhstan to assess gastric cancer trends, outcomes, and predictors. Data was taken from national electronic health records from 2012 - 2023. Despite a modest decline in incidence (ASIR 13.6/100,000 in 2023), the 5-year survival remains low at 17.1%, and 82.7% of patients died during the study period. It indicates persistent challenges in detection and treatment.
4.1 Burden and trends of gastric cancer
According to publicly available reports in local websites, journals in 2024th year in Kazakhstan there were 2927 new cases of the stomach cancer, and 67% are of male gender. The highest incidence is identified within the 50–74 years old age group (11). These patterns are broadly consistent with our cohort, supporting the plausibility of our age–sex distributions and suggesting near–population-level coverage. Regionally, North Kazakhstan reported incidence rates of 16.1 per 100,000 in the first nine months of 2022 and 16.2 per 100,000 in 2021 (22), whereas our nationwide estimates for the full year were 14.56 and 14.58 per 100,000, respectively. Given that North Kazakhstan is recognized for a higher oncologic burden than many other regions, the elevated regional rates are expected and align with known geographic variation rather than indicating under-ascertainment in our dataset.
In 2023, Kazakhstan’s age-standardized incidence rate (ASIR) was found to be 13.6 per 100,000, higher than in the U.S. (4.2) and Europe (8.1). However, it was lower than in East Asian countries such as China (20.6), Japan (48.1 for males) and Mongolia (47.2 for males) (23, 24). In Kyrgyz Republic in 2016th year the stomach cancer incidence was 10.9 per 100,000 and 10,0 per 100,000 people in 2017th year (25). While in these years in Kazakhstan incidence was 17.4 and 16.8 per 100,000 people cases respectively, showing higher incidence. In other neighbor country, Uzbekistan, the incidence is even lower, despite the fact it is ranked first out of oncological diseases among men, and fifth among women. In 2020–2023 years, incidence was 5.3 cases per 100,000 individuals, and mortality rate ranging between 3.8 to 4.0 death per 100,000 people (26). In Kazakhstan in 2020–2023 years incidence ranged between 14.2 to 13.6 cases per 100,000 people, and mortality from 11.7 to 8.7 deaths per 100,000 individuals. It is seen that compared with neighborhood countries, Kazakhstan has higher incidence and mortality within the same comparison years. Also, despite regional variation, Kazakhstan’s ASIR remains ~1.5 times higher than the global average that is equal to 9.2 per 100,000 (24). Mortality followed a declining trend, with ASMR falling from 16.6 to 8.7, nevertheless survival outcomes remain poor. These findings highlight significant deficits in early detection, access to care, and treatment effectiveness.
4.2 Late diagnosis and screening gaps
As seen from Figure 8 most patients were diagnosed at Stage III (33.5%), followed closely by Stage II and Stage IV diagnosis. Combined - 87% of patients were diagnosed at Stage II or later, reflecting delayed detection and the main reason was delayed healthcare-seeking behavior. Only 18% of cases were identified through screening, while 72% were detected due to patient’s self-referral to medical practitioners. It indicates low participation in organized screening programs (Figure 9). Nevertheless, the proportion of Stage IV diagnoses decreased by around 10% over the study period – it can be linked to the 2012 implementation of Kazakhstan’s colorectal cancer screening program.
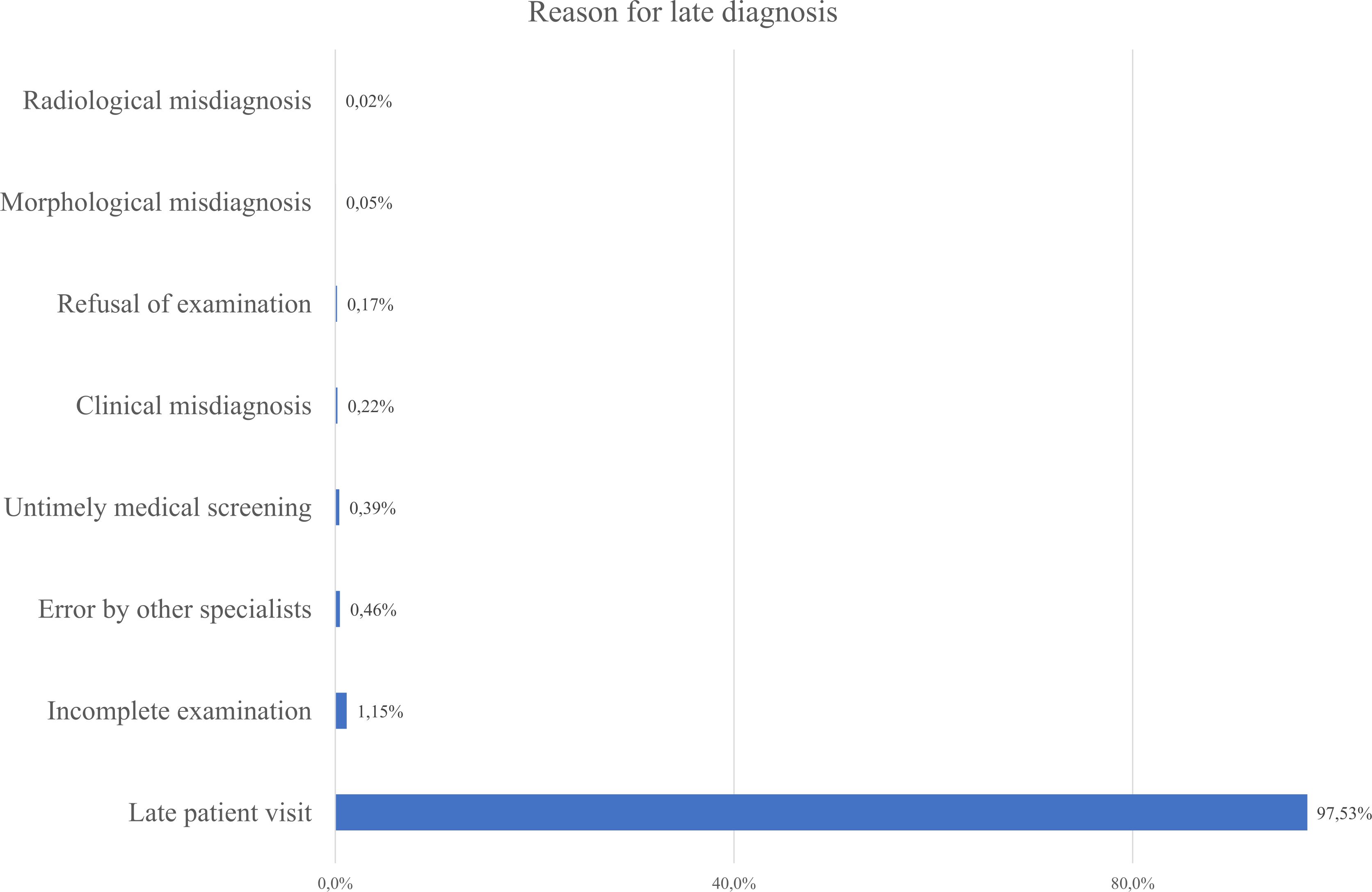
Figure 8. Reported reasons for delayed gastric cancer diagnosis based on the present data in the database.
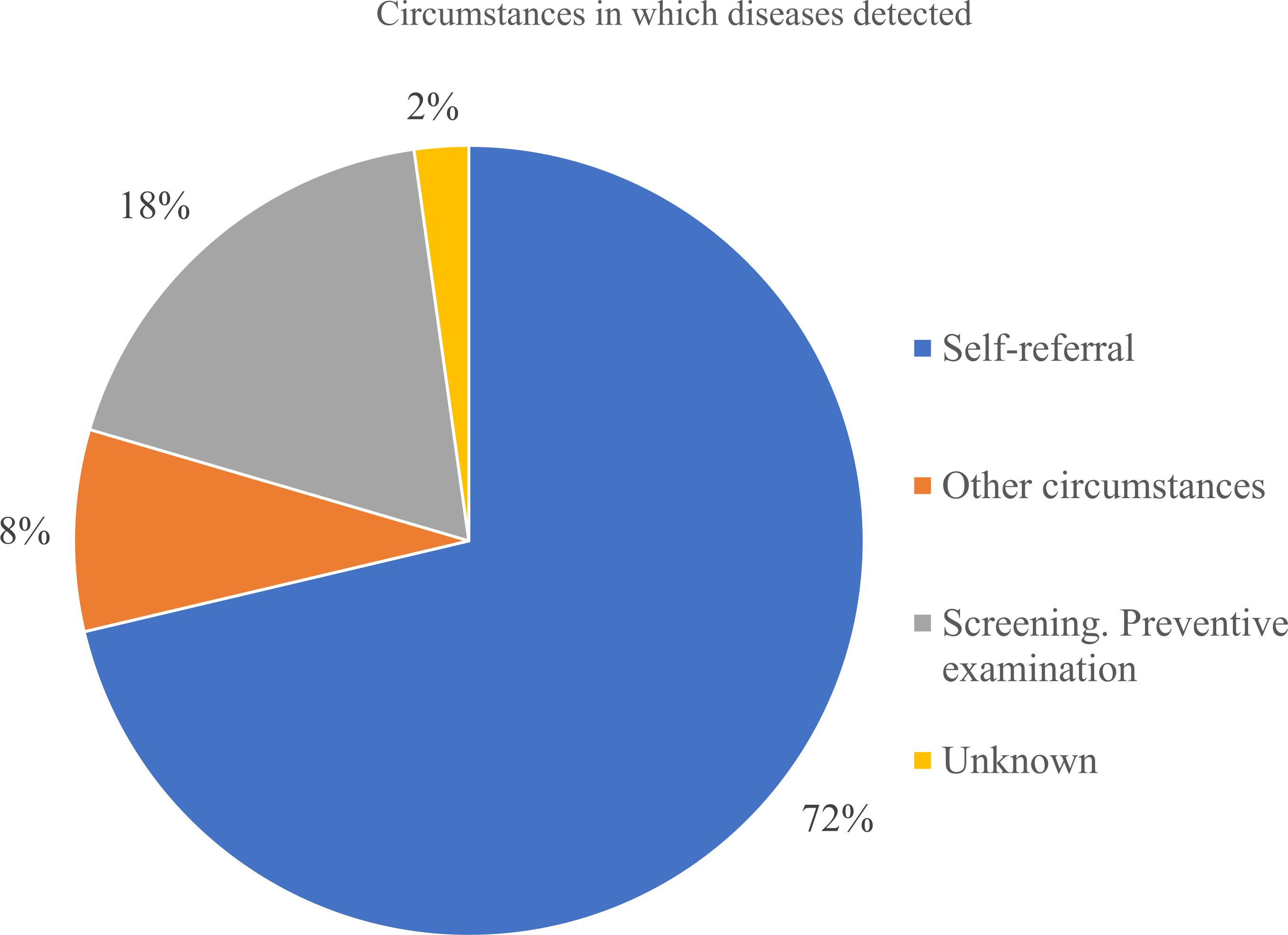
Figure 9. Circumstances in which gastric cancer was detected according to registered reasons in the database.
International comparisons emphasize the value of early detection. Japan and Korea, with long-standing nationwide screening, report 5-year survival rates around 69%, compared to Kazakhstan’s 17.1% (27, 28). Modest screening efforts in China have led to increased survival from 30.2% to 35.9% (29). In Kazakhstan, survival by stage remains much lower than in regional neighbors. For instance, Stage II survival was 22.1% in our cohort, versus 77.6% in Japan and 85.4% in China - showing lack of earlier diagnosis and curative treatment (30, 31).
4.3 Predictors of survival and risk stratification
Multivariable analysis identified several key predictors of survival, including age, gender, cancer stage, metastasis, recurrence, histology, and comorbidities. Hazard ratios rose significantly with advancing stage: Stage IV had an AHR >4.0 compared to In Situ. Presence of recurrence or metastases was associated with nearly 30% mortality, consistent with global data on poor prognosis in advanced disease (29, 32, 33).
Adenocarcinoma accounts for ~90% of gastric cancers (29), but in our dataset it was recorded in only 61.6% of cases. This discrepancy likely reflects incomplete morphology coding: 1,975 records lack morphology, and the broad “other” category aggregates heterogeneous entities - germ cell tumors (n=5), dendritic cell tumors (n=1), leukemia (n=2), lymphomas (n=50), neuroendocrine carcinomas (n=185), “other tumors” (n=6,092), and central nervous system tumors (n=2). The large “other tumors” subgroup (n=6,092) is of uncertain origin; some proportion may in fact be adenocarcinoma, but this cannot be verified from available fields.
So, the most prevalent histology type adenocarcinoma (61.6%) in our database, was associated with worse outcomes. Rarer types like sarcomas and other tumors group (neuroendocrine, leukemia, lymphoma) had lower sample sizes but showed varying prognoses. Although gastric tumors with non-carcinoma histology were staged using the same system in the national registry, we acknowledge that these subtypes follow distinct biological behaviors. Their inclusion may introduce heterogeneity. However, due to their low frequency, we believe the impact on overall survival estimates was limited.
Occupational status was not included in the multivariable Cox regression model due to its lack of statistical significance in univariate analysis. Also, data completeness and heterogeneity across categories was a concern. However, given its relevance to cancer disparities, descriptive analysis of occupation is presented in Figure 4. It highlights socioeconomic patterns in disease distribution. Workers in hazardous industries (e.g., mining, manufacturing) showed the second-highest disease prevalence after pensioners, consistent with findings from other studies (34).
4.4 Role of comorbidities
Comorbidities strongly influenced survival. Malnutrition, obesity, alcohol use disorder, pregnancy-related conditions, and cancer complications were the strongest negative predictors. These findings align with studies from China and Iran, where both malnutrition and obesity increased gastric cancer risk and worsened outcomes (35–37). A recent meta-analysis also linked alcohol use to increased gastric cancer risk (OR = 1.20), consistent with our findings (AHR = 1.23) (35).
Unexpectedly, some comorbidities - including cardiovascular (AHR = 0.925), cholecystitis (AHR = 0.853), and infectious diseases (AHR = 0.813) - were associated with reduced hazard of death. These associations are counterintuitive. Also, it was seen that with increased number of comorbidities registered, the less hazard was identified. These findings justify the issue in registry rather than the biological effect. First, residual confounding may be present if patients with well-documented comorbidities had better healthcare access or were more closely monitored. Second, immortal time bias is a plausible explanation: patients had to survive long enough after their cancer diagnosis to be diagnosed with these comorbidities, artificially lowering their observed hazard. Third, misclassification is possible due to limitations in registry coding, particularly regarding the timing and severity of conditions. Moreover, as seen from Table 1 the “0 or missing” group had the highest percentage of the data with the highest prevalence in later stages (Stage III, IV). The more comorbidities one patient had, the lower was the stage at the registration. This pattern most like is the reason for this so-called protective behavior of the comorbidities.
While we acknowledge these limitations, sensitivity analyses excluding comorbidities diagnosed after the primary cancer event are warranted in future work to clarify these associations.
4.5 Treatment data gaps and clinical practice in Kazakhstan
A major limitation of this study is the absence of full data on treatments such as surgery, chemotherapy, and radiotherapy. The UNEHS does not consistently capture treatment modalities in structured formats suitable for large-scale survival analysis. As a result, we were unable to assess the impact of treatment type, timing, or completion on survival outcomes.
While our analysis could not include treatment variables, it is important to contextualize survival outcomes by considering the national gastric cancer treatment guidelines and potential implementation challenges. The national clinical guidelines for gastric cancer in Kazakhstan largely align with international standards, recommending perioperative chemotherapy (e.g., FLOT, XELOX, or fluorouracil-based regimens), radical surgical resection with D2 lymphadenectomy, and adjuvant therapy based on tumor stage and histology. Standard protocols involve intensive chemotherapy regimens, such as 8 cycles of FLOT or XELOX, and sometimes chemoradiation, with cycles repeated every 2 to 3 weeks over several months.
In comparing with results obtained from the database, the stage pattern (more surgery in I–II; very little in IV) matches the protocol direction. However, the overall rate of “no surgery/missing” is much higher than expected for a curative-intent population and justifies the possibility of “missing” data, rather than “no operation” group purely. The same comes to non-surgical treatment. The protocol expects peri-operative chemotherapy for T3/N+ (many stage II–III) and palliative chemotherapy for most stage IV patients. Table 2 likely understates chemotherapy use because of very high missingness, especially in stage IV where chemo should be common unless patients are unfit. So, overall, we see that even the limited data is consistent with protocol, however due to gaps we consider treatment variables as unreliable for primary modeling.
Importantly, the protocol outlines detailed drug schedules but lacks structured pathways for post-treatment rehabilitation or functional recovery. Most patients transition to lifelong dietary management and basic imaging follow-up, but no formal physical therapy, nutritional counseling, or psychological support is integrated into routine survivorship care.
4.6 Post-treatment outcomes and quality of life
Post-treatment recovery and follow-up remain inadequate. Oncological registry had information on the condition of patients. From 17% of the whole cohort that was alive, only 4.6% of patients returned to full physical activity, while the majority were limited to light or sedentary work. 35.2% were lost to follow-up (Figure 10). System lacks structured survivorship care, with minimal nutritional guidance, psychological support, or recurrence monitoring. Given the known association between nutritional status, lifestyle factors, and gastric cancer outcomes, integrating comprehensive follow-up services is critical to improving quality of life and reducing recurrence (38, 39). Integrating supportive care and rehabilitation into national protocols could help improve long-term performance outcomes and quality of life.
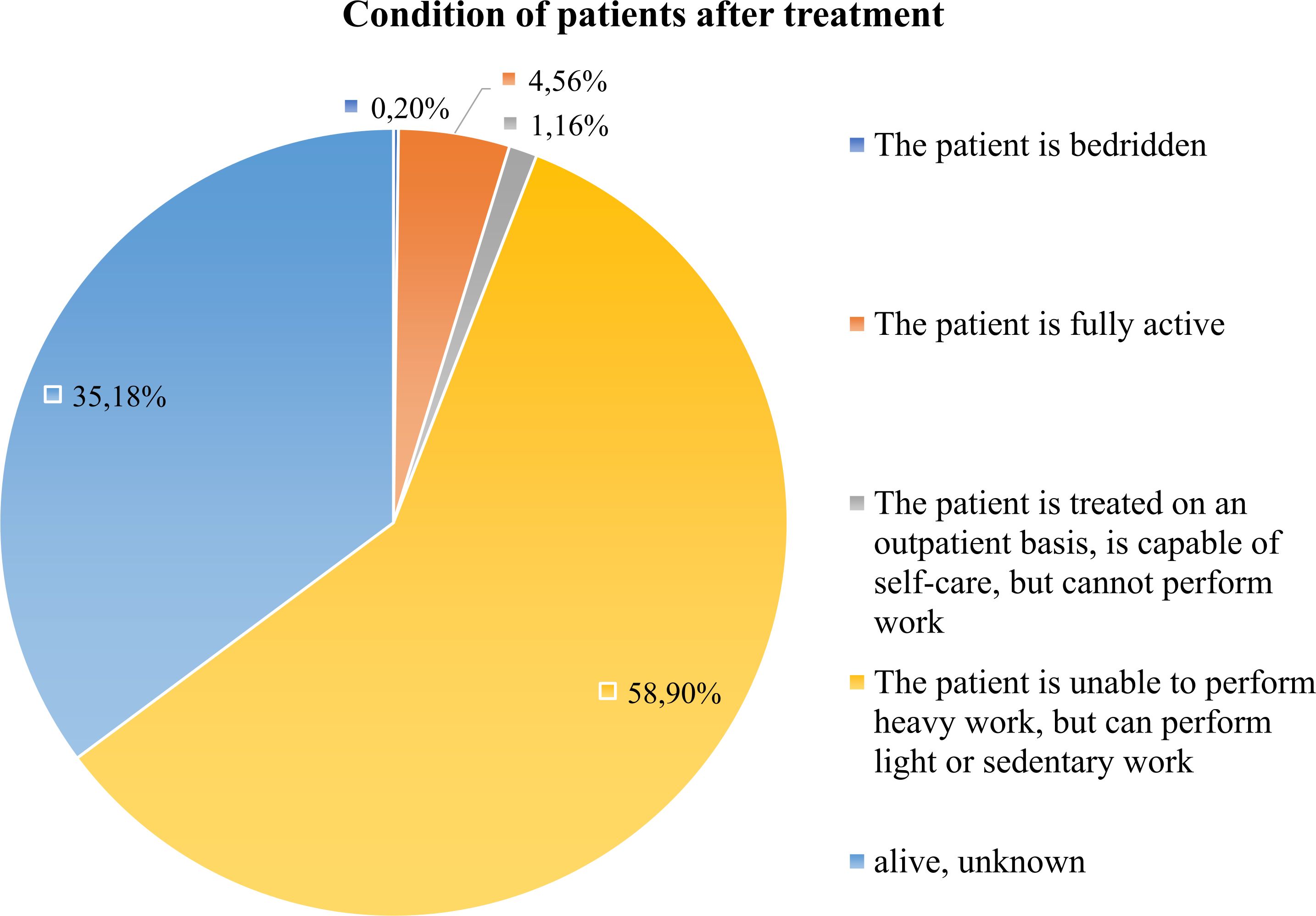
Figure 10. Functional status of gastric cancer post-treatment survivors. This figure shows the distribution of functional/physical status among survived patients after treatment. Most survivors reported limited or sedentary activity, while only a minority regained full physical functioning.
4.7 Strengths and limitations
The strength of this study is the population-based registry, which provides sufficiently large, representative information with detailed staging, histology, and survival data. Also, it provides new statistically significant data on gastric cancer epidemiology for 2012–2023 years essential for further analysis and public health policy. However, limitations include missing information on treatment modalities, H. pylori infection status, smoking, dietary patterns, socioeconomic status (SES), incomplete histology classification, and lack of cause-specific mortality. These unmeasured factors could confound observed associations (e.g., age/stage effects partly mediated by H. pylori or smoking; survival differences influenced by SES or adherence). Leaving out treatment can bias our survival estimates because treatment is tied to baseline fitness and disease severity (classic confounding by indication), and if we treat post-baseline therapy as a baseline covariate, we risk immortal-time bias. UNEHS captures only clinically significant events - hospitalizations, outpatient visits, and official follow-up registrations. As a result, stomach cancer patients with poor access to primary care may never enter the system and could be missing from our dataset. Staging errors and lack of post treatment updates further reduce accuracy for treatment monitoring and epidemiological surveillance.
5 Conclusion
This first nationwide analysis of gastric cancer in Kazakhstan reveals persistently poor survival despite declines in incidence and mortality between 2012 and 2023. Over 87% of cases were diagnosed at Stage II or higher, with survival significantly affected by cancer stage, recurrence, nutritional status, and comorbidities. Cardiovascular and gastrointestinal comorbidities were most common. A key limitation of this study is the lack of data on surgical, chemotherapeutic, or radiotherapeutic treatment. To improve prognosis, future research should incorporate detailed treatment data and refine prognostic models. To reduce this limitation in future work, the registry could (i) when possible or were done add H.Pylori results into the database; (ii) add structured smoking fields, add optional lifestyle modules for alcohol, diet and SES. These enhancements would enable better confounding control and support more causal interpretations. Strengthening registry accuracy, expanding screening - especially for older adults with known risk factors - and ensuring timely diagnosis are essential. Investment in equitable oncology services, implementing screening, and structured post-treatment care is critical to reduce mortality and improve outcomes for gastric cancer patients nationwide.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Nazarbayev University (protocol code: NU-IREC 651/24112022 and date of approval: 28 November 2022). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
AR: Data curation, Formal analysis, Writing – original draft. YS: Data curation, Formal analysis, Writing – review & editing. AK: Conceptualization, Funding acquisition, Writing – review & editing. MM: Writing – original draft. SS: Writing – original draft. DZ: Writing – original draft. AM: Writing – review & editing. ZK: Writing – review & editing. ZB: Writing – review & editing. AG: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan grant number BR24992950 (“Creation and Implementation of Innovative Treatment Methods for Oncological Diseases”) and the APC was funded by the same grant. This research was also partially funded by the grants from the Ministry of Science and Higher Education of the Republic of Kazakhstan 2024–2026 (IRN: AP23484725; Title: Innovative Assessment of Solid Cancers Burden in Kazakhstan and Its 10-year Trend Using Big Healthcare Data and Forecasting Models). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
UNEHS, Unified Nationwide Electronic Health System; ICD, International Classification of Diseases; ASIR, age standardized incidence rate; ASMR, age-standardized mortality rate; WHO, World Health Organization; SD, standard deviation; HR, hazard ratio; AHR, adjusted hazard ratio.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Qin Y, Tong X, Fan J, Liu Z, Zhao R, Zhang T, et al. Global burden and trends in incidence, mortality, and disability of stomach cancer from 1990 to 2017. Clin Transl Gastroenterol. (2021) 10:e00406. doi: 10.1021/jacs.5b01994
3. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74(3):229–63. doi: 10.3322/caac.21834
4. World Cancer Research Fund International. Stomach cancer statistics (2022). Available online at: https://www.wcrf.org/preventing-cancer/cancer-types/stomach-cancer/ (Accessed February 25, 2025).
5. Igissinov N, Taszhanov R, Telmanova Z, Baibusunova A, Rustemova K, Bilyalova Z, et al. Trend in gastric cancer mortality in Kazakhstan. Asian Pac J Cancer Prev. (2022) 23:3779–89. doi: 10.31557/APJCP.2022.23.11.3779
6. Orazova G, Karp L, Rakhimbekova G, and Nogayeva A. Mathematical modeling and forecasting of esophageal and stomach cancer case rate in Kazakhstan. J Clin Med Kazakhstan. (2016) 2:43–9. doi: 10.23950/1812-2892-2016-2-43-49
7. Akhmedullin R, Aimyshev T, Zhakhina G, Yerdessov S, Beyembetova A, Ablayeva A, et al. In-depth analysis and trends of cancer mortality in Kazakhstan: a joinpoint analysis of nationwide healthcare data 2014–2022. BMC Cancer. (2024) 24:1340. doi: 10.1186/s12885-024-13128-2
8. Yusefi AR, Lankarani KB, Bastani P, Radinmanesh M, and Kavosi Z. Risk factors for gastric cancer: a systematic review. Asian Pac J Cancer Prev. (2018) 19:591–603. doi: 10.22034/APJCP.2018.19.3.591
9. Orazova G, Karp L, Yoo KY, Dossakhanov A, Rakhimbekova G, and Gaipov A. Stomach cancer morbidity in the Republic of Kazakhstan: Trends and characteristics. Eur J Gen Med. (2015) 12:282–90. doi: 10.15197/ejgm.01533
10. Zhandossov OK, Kausova GK, Emberdiyev AZ, Lurye AZ, Ivanov SV, Dubovichenko D, et al. Epidemiology of gastric cancer in Kazakhstan in 2005-2014. Ekologiya Cheloveka (Human Ecology). (2017) 24:50–7. doi: 10.33396/1728-0869-2017-6-50-57
11. Admin, & Admin. Stomach cancer: early detection is the key to a full recoveryю (2025). КазНИИОиР. Available online at: https://onco.kz/rak-zheludka-rannee-vyyavlenie-klyuch-k-polnomu-vyzdorovleniyu/ (Accessed April 20, 2025).
12. Beyembetova A, Ablayeva A, Akhmedullin R, Abdukhakimova D, Biniyazova A, and Gaipov A. National electronic oncology registry in Kazakhstan: patient’s journey. Epidemiol Health Data Insights. (2025) 1:ehdi004. doi: 10.63946/ehdi/16385
13. UNEHS. Electronic Registry of Inpatients. Kazakhstan: Republican Center for Electronic Health, Ministry of Health (2024).
14. Statistics Committee, Ministry of National Economy of the Republic of Kazakhstan. , Country statistical report (2024). Available online at: https://stat.gov.kz/en/ (Accessed February 20, 2025).
15. Centers for Disease Control and Prevention (CDC). All types of cancer, United States (2021). Available online at: https://gis.cdc.gov/Cancer/USCS//Demographics/ (Accessed December 20, 2025).
16. American Cancer Society (ACS). Stomach Cancer Stages (2021). Available online at: https://www.cancer.org/cancer/types/stomach-cancer/detection-diagnosis-staging/staging.html (Accessed January 4, 2025).
17. Ministry of Health and National Research Oncology Center of Kazakhstan. Stomach Cancer Protocol (2022). MedElement. Available online at: https://diseases.medelement.com/disease/%D1%80%D0%B0%D0%BA-%D0%B6%D0%B5%D0%BB%D1%83%D0%B4%D0%BA%D0%B0-%D0%BA%D0%BF-%D1%80%D0%BA-2022/17462 (Accessed February 10, 2025).
18. Besova NS, Gamayunov SV, Kalinin AE, Kalinin AE, Kozlov NA, Malikhova OA, et al. Gastric cancer. Malignant tumors. (2024) 14:241–62. doi: 10.18027/2224-5057-2024-14-3s2-1.1-13
20. Guidelines detail. Gastric cancer (2025). NCCN. Available online at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434 (Accessed February 10, 2025).
21. Hirota S, Tateishi U, Nakamoto Y, Yamamoto H, Sakurai S, Kikuchi H, et al. Members of the Systematic Review Team of the Present Guidelines. English version of Japanese Clinical Practice Guidelines 2022 for gastrointestinal stromal tumor (GIST) issued by the Japan Society of Clinical Oncology. Int J Clin Oncol. (2024) 29:647–80. doi: 10.1007/s10147-024-02488-1
22. Multidisciplinary Regional Hospital of the North Kazakhstan Region. Analysis of the key performance indicators of the oncology service for the first 9 months of 2022 compared to the same period in 2021 (2022). Available online at: https://ob.sko.kz/news/read/Analiz_osnovnyh_pokazatelej_deyatelnosti_onkologicheskoj_sluzhby_za_9_mesyacev_2022_goda_po_sravneniyu_s_analogichnym_periodom_2021_goda.html?lang=ru (Accessed April 5, 2025).
23. Xie Y, Shi L, He X, and Luo Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol Rep. (2021) 9:91–104. doi: 10.1093/gastro/goab010
24. Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: a population based modeling study. EClinicalMedicine. (2022) 47:101374. doi: 10.1016/j.eclinm.2022.101404
25. Toigonbekov AK, Akhunbaev S, Umetov M, and Tumanbaev A. Some intense and standardized stomach cancer disease indicators in the Kyrgyz Republic. Bull Sci Pract. (2020) 6:169–75. doi: 10.33619/2414-2948/56/20
26. Abdukodirov A, Tillyashaykhov M, Djanklich S, and Ososkov A. Epidemiological analysis of gastric cancer in Uzbekistan. J Clin Oncol. (2025) 43. doi: 10.1200/jco.2025.43.16_suppl.e13673
27. Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology. (2017) 152:1319–1328.e7. doi: 10.1053/j.gastro.2017.01.029
28. Kim GH, Liang PS, Bang SJ, and Hwang JH. Screening and surveillance for gastric cancer in the United States: is it needed? Gastrointest Endosc. (2016) 84:18–28. doi: 10.1016/j.gie.2016.02.028
29. Ilic M and Ilic I. Epidemiology of stomach cancer. World J Gastroenterol. (2022) 28:1187–97. doi: 10.3748/wjg.v28.i12.1187
30. Kakeji Y, Ishikawa T, Suzuki S, Akazawa K, Irino T, Miyashiro I, et al. A retrospective 5 year survival analysis of surgically resected gastric cancer cases from the Japanese Gastric Cancer Association nationwide registry (2001–2013). Gastric Cancer. (2022) 25:1082–93. doi: 10.1007/s10120-022-01317-6
31. Zhang H, Yang W, Tan X, He W, Zhao L, Liu H, et al. Long term relative survival of patients with gastric cancer from a large scale cohort: a period analysis. BMC Cancer. (2024) 24:1420. doi: 10.1186/s12885-024-13141-5
32. Wang S, Zheng R, Arnold M, Abnet C, Zeng H, Zhang S, et al. Global and national trends in the age specific sex ratio of esophageal cancer and gastric cancer by subtype. Int J Cancer. (2022) 151:1447–61. doi: 10.1002/ijc.34158
33. Supsamutchai C, Wilasrusmee C, Hiranyatheb P, Jirasiritham J, Rakchob T, and Choikrua P. A cohort study of prognostic factors associated with recurrence or metastasis of gastrointestinal stromal tumor (GIST) of stomach. Ann Med Surg (Lond). (2018) 35:1–5. doi: 10.1016/j.amsu.2018.08.010
34. Santibañez M, Alguacil J, de la Hera MG, Navarrete-Muñoz EM, Llorca J, Aragonés N, et al. Occupational exposures and risk of stomach cancer by histological type. Occup Environ Med. (2012) 69:268–75. doi: 10.1136/oemed-2011-100005
35. Deng W, Jin L, Zhuo H, Vasiliou V, and Zhang Y. Alcohol consumption and risk of stomach cancer: a meta-analysis. Chem Biol Interact. (2021) 336:109365. doi: 10.1136/oemed-2011-100071
36. Azizi N, Zangiabadian M, Seifi G, Davari A, Yekekhani E, Safavi-Naini SAA, et al. Gastric cancer risk in association with underweight, overweight, and obesity: a systematic review and meta-analysis. Cancers (Basel). (2023) 15:2778. doi: 10.3390/cancers15102778
37. Yi HC and Hong BZ. Prevalence of malnutrition and associated factors in newly diagnosed upper gastrointestinal cancer patients before treatment. Asian Pac J Cancer Care. (2024) 9:267–75. doi: 10.31557/apjcc.2024.9.2.267-275
38. Orazova G, Karp L, Rakhimbekova G, and Nogayeva A. Identification of esophageal and stomach cancer risk factors among the population of Kazakhstan with the use of QCancer e-base calculator. J Clin Med Kazakhstan. (2016) 2:50–6. doi: 10.23950/1812-2892-2016-2-50-56
Keywords: gastric cancer, survival, epidemiology, risk factors, mortality, cancer stage, comorbidities, Kazakhstan
Citation: Rakhmankulova A, Sakko Y, Kerimkulov A, Mamlin M, Shalekenov S, Zharlyganova D, Manatova A, Kuanysh Z, Burkitbayev Z and Gaipov A (2025) Survival disparities and predictors in gastric cancer: a population-based study from Kazakhstan (2012–2023). Front. Oncol. 15:1670082. doi: 10.3389/fonc.2025.1670082
Received: 21 July 2025; Accepted: 03 November 2025;
Published: 21 November 2025.
Edited by:
Hussain Gadelkarim Ahmed, Prof. Medical Research Consultancy Center -MRCC, SudanReviewed by:
Ildar Fakhradiyev, Kazakh National Medical University, KazakhstanDanish Jamil, University of Santiago de Compostela, Spain
Copyright © 2025 Rakhmankulova, Sakko, Kerimkulov, Mamlin, Shalekenov, Zharlyganova, Manatova, Kuanysh, Burkitbayev and Gaipov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abduzhappar Gaipov, YWJkdXpoYXBwYXIuZ2FpcG92QG51LmVkdS5reg==
†ORCID: Abduzhappar Gaipov, orcid.org/0000-0002-9844-8772
 Aidana Rakhmankulova
Aidana Rakhmankulova Yesbolat Sakko
Yesbolat Sakko Altay Kerimkulov2
Altay Kerimkulov2 Meiram Mamlin
Meiram Mamlin Sanzhar Shalekenov
Sanzhar Shalekenov Zhuldyz Kuanysh
Zhuldyz Kuanysh Abduzhappar Gaipov
Abduzhappar Gaipov