- 1Department of Nuclear Medicine, Ganzhou Cancer Hospital, Ganzhou, China
- 2Department of Gynecological Oncology, Ganzhou Cancer Hospital, Ganzhou, China
- 3Department of Medical Technology, Gannan Healthcare Vocational College, Ganzhou, China
Background: The clinical utility of postoperative radioiodine therapy in patients with low-risk differentiated thyroid cancer (DTC) remains a subject of ongoing debate. Although radioiodine has been widely employed to reduce the risk of recurrence, its necessity in low-risk populations is increasingly questioned, given the favorable outcomes observed with surgery alone. To address this issue, we conducted a meta-analysis exclusively based on randomized controlled trials (RCTs) to comprehensively evaluate the efficacy and safety of radioiodine therapy in this specific patient population.
Methods: We systematically searched 6 databases for eligible phase 3 RCTs comparing surgery with or without radioiodine in patients with low-risk DTC. Primary outcomes included recurrence and recurrence-free survival (RFS); secondary outcomes included adverse events (AEs), structural events, and biological events. Risk ratios (RRs) or hazard ratios (HRs) with 95% confidence intervals (CIs) were pooled and analyzed.
Results: Two phase 3 RCTs (the ESTIMABL2 and IoN trials), encompassing 1280 patients, were included. Compared to the non-radioiodine group, radioiodine therapy did not significantly reduce recurrence rates (RR: 0.78 [0.36-1.70], P = 0.53) or improve RFS (HR: 0.96 [0.80-1.15], P = 0.68). The total number of structural events (RR: 0.83 [0.68-1.02], P = 0.07) and biological events (RR: 0.88 [0.71-1.08], P = 0.23) were also similar between the two groups. In the safety analysis, the two groups exhibited comparable rates of AEs (RR: 0.97 [0.79-1.20], P = 0.80), grade 3–5 AEs (RR: 0.25 [0.03-2.20], P = 0.21), death (RR: 1.28 [0.48-3.41], P = 0.62), and second primary cancers (RR: 1.26 [0.58-2.73], P = 0.55).
Conclusion: Radioiodine therapy did not confer significant benefits in reducing recurrence or improving RFS in patients with low-risk DTC after thyroidectomy, and the safety profiles were comparable between the two groups.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD420251105509.
Introduction
Differentiated thyroid cancer (DTC) is the most prevalent endocrine malignancy, with papillary thyroid carcinoma accounting for approximately 85-90% of all cases (1). Over the past several decades, the global incidence of DTC has markedly increased, largely due to the widespread use of high-resolution imaging and enhanced diagnostic practices (2). Despite this rising incidence, the majority of patients—especially those with low-risk DTC—have an excellent prognosis, with disease-specific survival rates exceeding 95% at 10 years (3).
For low-risk DTC, which is typically defined by small, intrathyroidal tumors without lymph node involvement or distant metastasis, thyroidectomy alone is often considered curative. However, the postoperative use of radioiodine therapy in this population remains a matter of ongoing debate (4). Historically, radioiodine was widely administered to ablate remnant thyroid tissue, facilitate follow-up with serum thyroglobulin, and potentially eliminate microscopic residual disease (5). Yet, with the evolution of risk stratification systems and improved surveillance methods, the necessity of radioiodine in low-risk patients has been increasingly questioned (6).
Several studies and retrospective analyses have suggested that radioiodine provides little to no benefit in reducing recurrence or improving survival in low-risk DTC (7, 8). Moreover, radioiodine is not without risks. Acute and chronic adverse effects such as sialadenitis, taste alterations, and, in rare cases, second primary malignancies have raised concerns about overtreatment (9). These potential harms, combined with the excellent baseline prognosis of low-risk patients, have led to more conservative guideline recommendations. Notably, the 2015 American Thyroid Association (ATA) guidelines advise against the routine use of radioiodine in patients with low-risk DTC (10). To provide high-level evidence, two recent phase 3 randomized controlled trials—ESTIMABL2 and IoN—were conducted specifically in low-risk DTC populations to assess whether radioiodine therapy offers any additional clinical benefit (11, 12). These trials used modern diagnostic tools and long-term follow-up protocols, enabling a more accurate evaluation of recurrence and adverse outcomes.
In light of this, we performed a meta-analysis of available phase 3 RCTs comparing radioiodine therapy versus non-radioiodine in patients with low-risk DTC following thyroidectomy. Our objective was to clarify the impact of radioiodine on recurrence, recurrence-free survival (RFS), adverse events (AEs), mortality, and secondary cancer risk, and to provide comprehensive evidence to guide individualized treatment decisions for this growing patient population.
Materials and methods
Search strategy
A systematic search approach was applied using terms including “Radioiodine”, “Thyroid Cancer”, and “Randomized” (Supplementary Table S1), and relevant trials were retrieved from major databases such as PubMed, EMBASE, ScienceDirect, Cochrane Library, Web of Science, and Scopus, covering studies published until June 20, 2025.
Selection criteria
The inclusion standards were:
(1) Patients confirmed with low-risk DTC (defined according to the WHO Classification of Endocrine and Neuroendocrine Tumours, 5th Edition [2022], Thyroid Tumours chapter: Non-invasive follicular thyroid neoplasms with papillary-like nuclear features, tumors of uncertain malignant potential, and hyalinizing trabecular tumors — all originating from follicular epithelial cells — are characterized by encapsulation or well-circumscribed borders and the absence of lymph node or distant metastasis [EX0, N0, M0]) (13);
(2) Comparisons of radioiodine and non-radioiodine after thyroidectomy;
(3) Documentation of at least one outcome: recurrence, RFS, AEs, structural events, and biological events.
(4) Phase 3 RCTs published in English;
Studies were excluded if they met any of the following criteria: (1) review articles, meta-analyses, or case-based descriptions; (2) experiments conducted on animals or non-human models; (3) data unavailable or insufficient for analysis.
Data extraction
Two reviewers independently used a standardized data sheet to extract trial-related details. Information included study profiles (patients, histology, etc), recurrence, RFS, AEs, mortality, structural events, and biological events. Structural events in both cohorts referred to suspicious imaging results on neck ultrasound, such as abnormal nodes or thyroid remnants. Biological events were defined as elevated Tg or TgAb levels without structural confirmation. Any conflicting interpretations were discussed, and unresolved differences were adjudicated by a third investigator.
Quality assessment
Assessment data were extracted from peer-reviewed publications and, when available, official trial registries. Methodological quality and risk of bias were evaluated using the Cochrane risk-of-bias tool and the Jadad scale (14, 15). Studies with Jadad scores ranging from 4 to 7 were considered to have high methodological quality. Additionally, the certainty of evidence was assessed using the GRADE framework, with evidence categorized from high to very low certainty (16).
Statistical analysis
Statistical evaluations were conducted using RevMan 5.4 and STATA 17.0. Pooled estimates of hazard ratio (HR) and risk ratio (RR) were derived to examine event-time and dichotomous outcomes. Between-study variability was measured using the I² statistic and Cochran’s Q test, with heterogeneity deemed considerable when I² exceeded 50% or the p-value was below 0.10. A random-effects model addressed substantial heterogeneity, whereas fixed-effects were chosen for lower inconsistency. Funnel plots were utilized to assess reporting bias. Statistical significance was established at p < 0.05.
Results
Search results
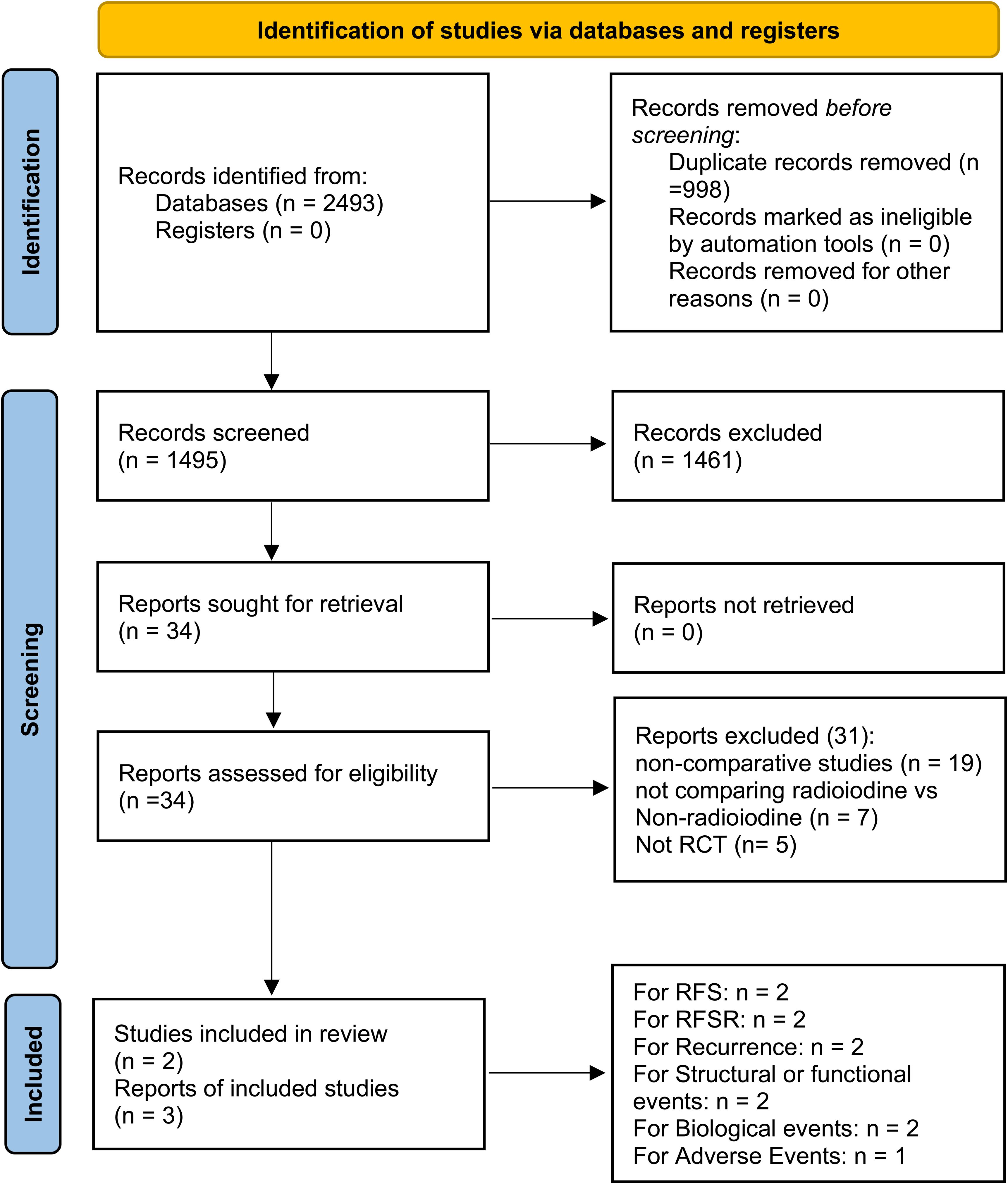
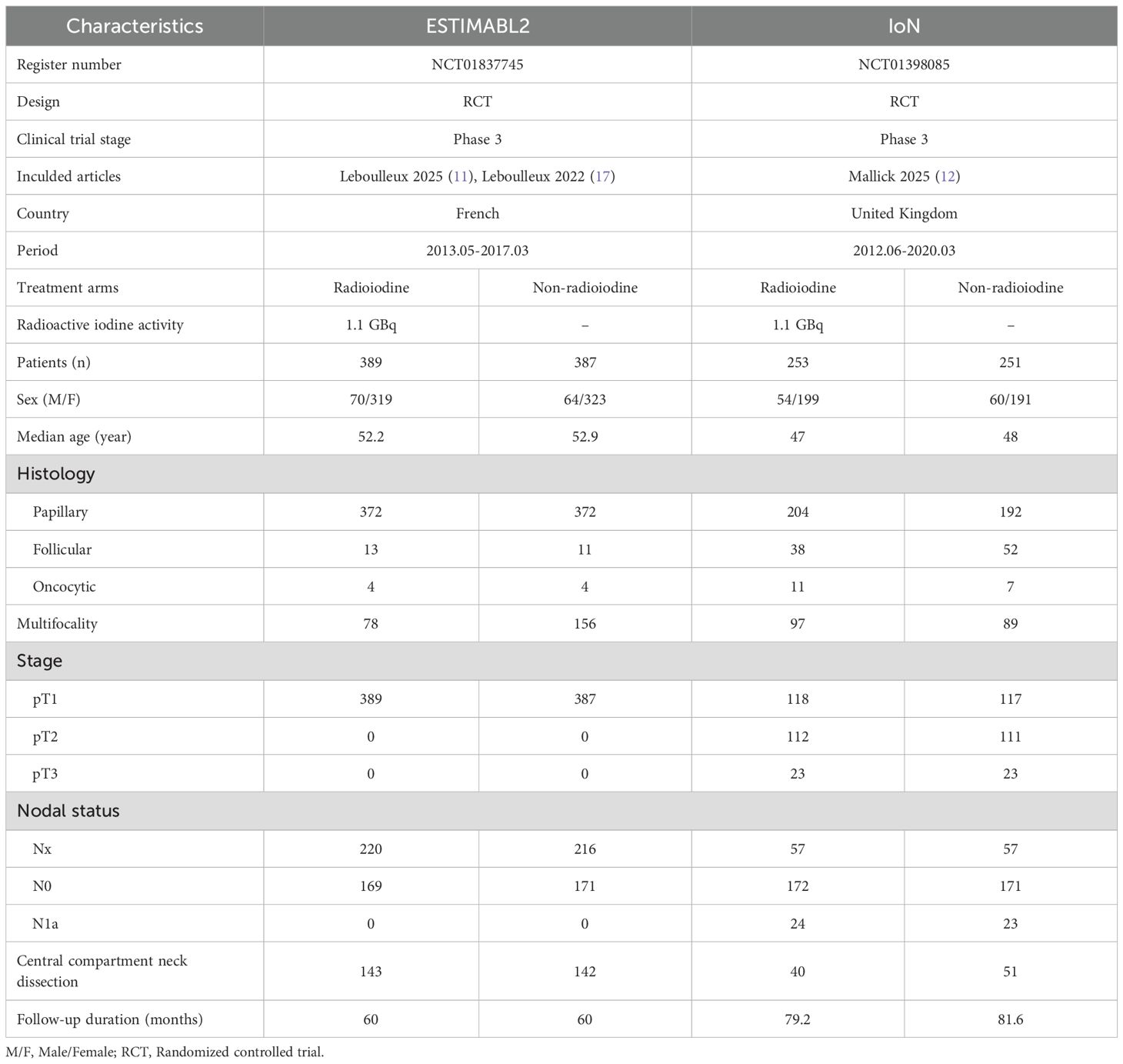
This pooled review incorporated three reports derived from two phase 3 RCTs—ESTIMABL2 and IoN—encompassing 1280 individuals diagnosed with low-risk DTC (11, 12, 17). Study selection steps, detailed in Figure 1, adhered to PRISMA 2020 criteria. Supplementary Figure S1 and Supplementary Table S2 reveal both trials employed rigorous methodology and exhibited minimal bias. According to the GRADE framework, the certainty of evidence ranged from moderate to high (Supplementary Table S3). The ESTIMABL2 study was performed in France (11), while the IoN investigation took place in the United Kingdom (12). Table 1 presents core trial structures and baseline participant characteristics.
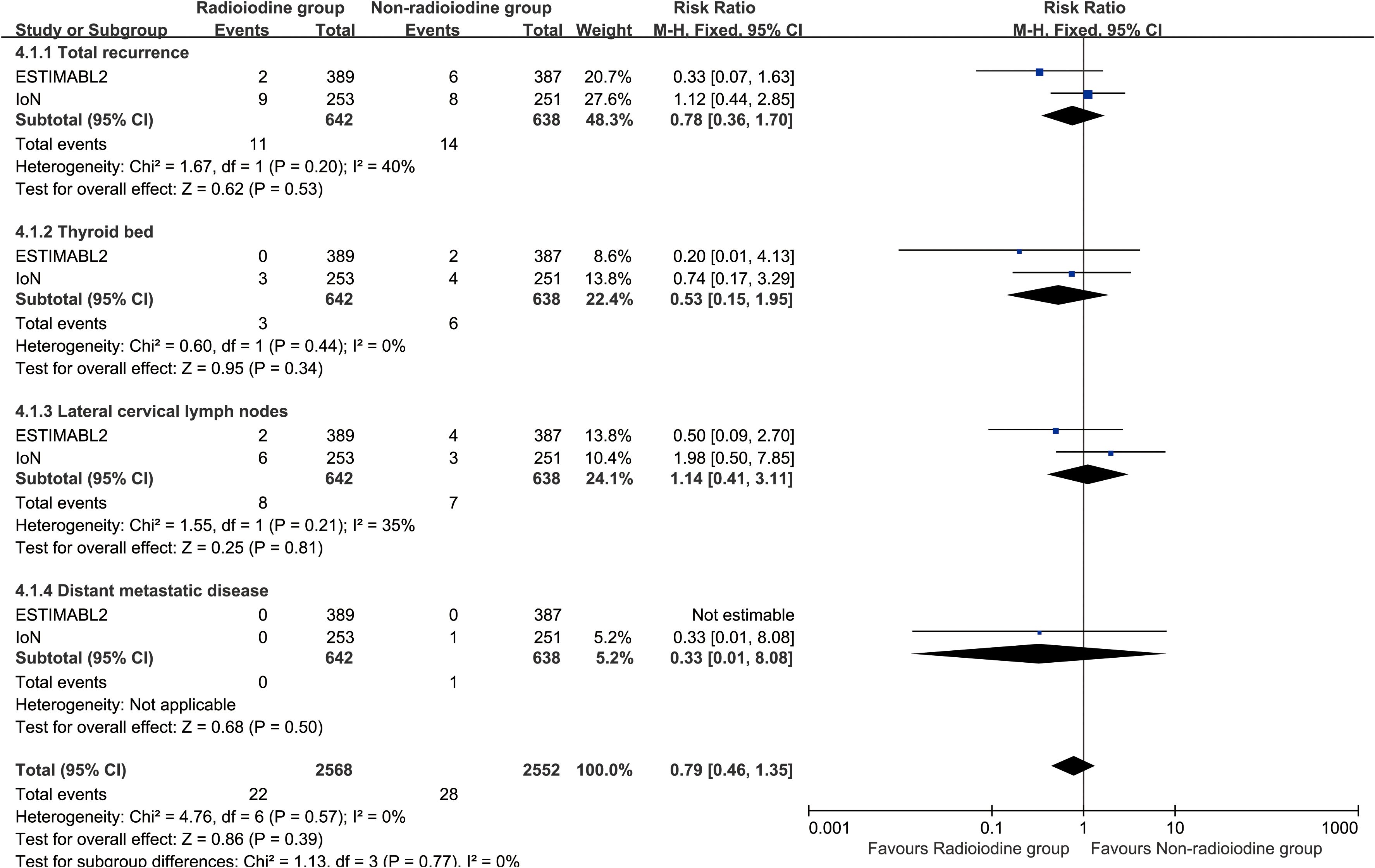
Recurrence
Radioiodine therapy did not significantly improve RFS (HR: 0.96 [0.80–1.15], P = 0.68) (Figure 2). Between 1 and 5 years, the RFS rates (RFSR) were similar in both groups (Supplementary Figures S2, S3). Likewise, the total recurrence rate and site-specific recurrence rates did not differ significantly between the two groups (Figure 3).
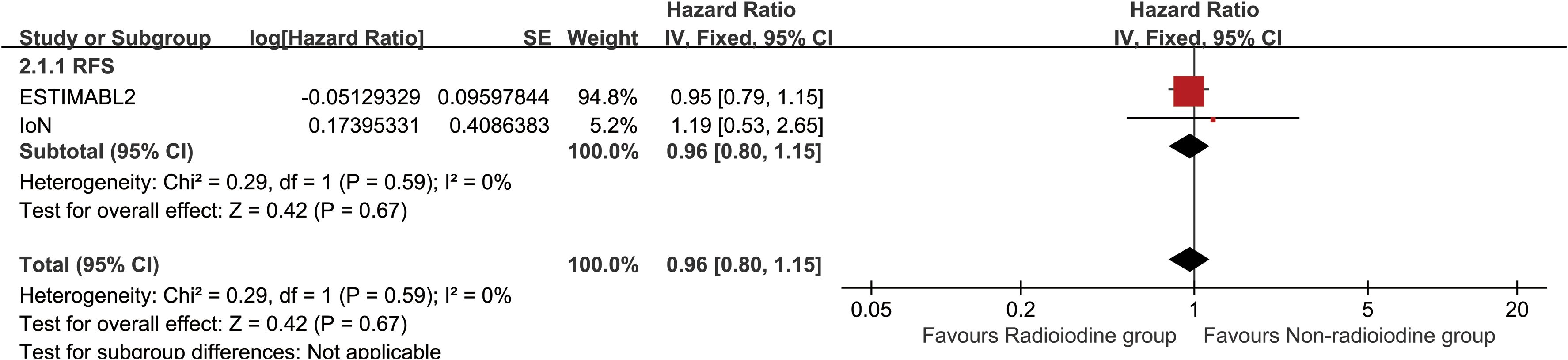
Figure 2. Forest plot of recurrence-free survival associated with radioiodine versus non-radioiodine.
Structural and biological events
Total structural events (RR: 0.83 [0.68, 1.02], P = 0.07), and biological events (RR: 0.88 [0.71, 1.08], P = 0.23) were comparable between the two groups (Figure 4). Notably, Tg > 5ng/ml at any time point was more frequently observed in the non-radioiodine group (Supplementary Figure S4). Subgroup analyses of structural and biological events also revealed no significant differences between the two groups (Supplementary Figure S5).
![Forest plot comparing two subgroups: radioiodine and non-radioiodine. It displays risk ratios with confidence intervals for total structural and biological events. Overall risk ratio favored the non-radioiodine group with 0.85 [0.74, 0.99]. Subgroup analysis showed no significant heterogeneity. The scales range from 0.1 to 10, indicating risk favorability between groups.](https://www.frontiersin.org/files/Articles/1670978/fonc-15-1670978-HTML/image_m/fonc-15-1670978-g004.jpg)
Figure 4. Forest plot of total structural events and biological events associated with radioiodine versus non-radioiodine.
Adverse events
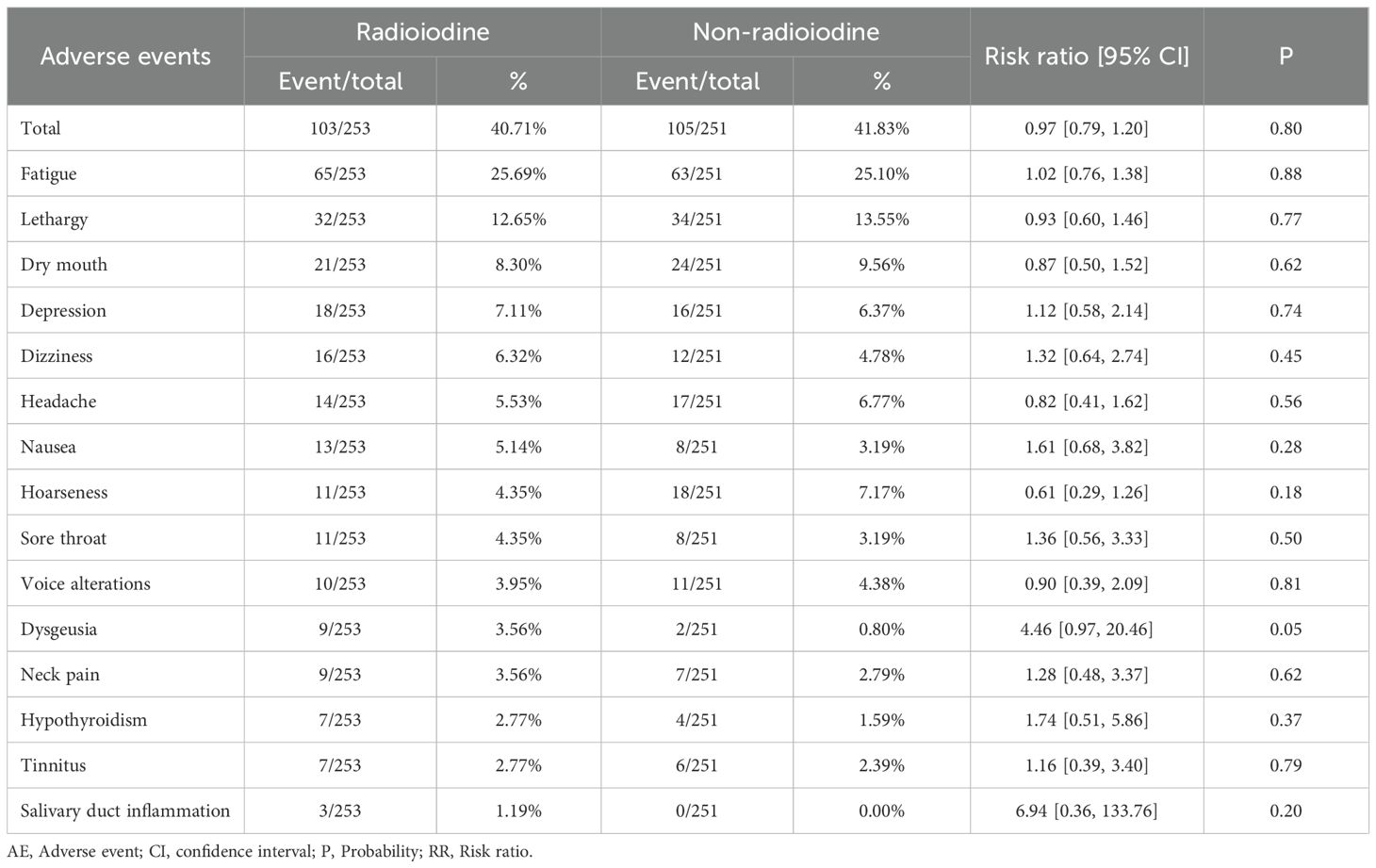
Both any grade AEs (RR: 0.97 [0.79, 1.20], P = 0.80) and grade 3–5 AEs (RR: 0.25 [0.03, 2.20], P = 0.21) were comparable between the two groups. No statistically significant differences were observed for any individual AE (Table 2, Supplementary Table S4). The three most frequently reported AEs in the radioiodine group were fatigue (25.69%), lethargy (12.65%), and dry mouth (8.30%).
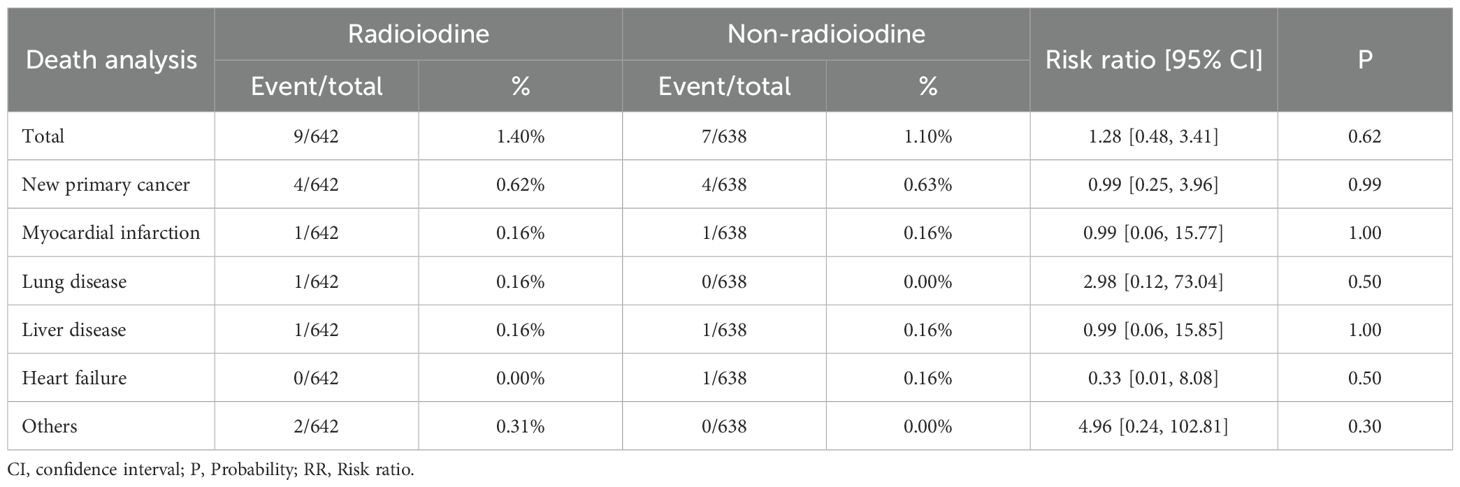
Death analysis
The mortality was similar between the two groups. (RR: 1.28 [0.48, 3.41], P = 0.62). The most common cause of death in both groups was the development of a second new cancer (Table 3).
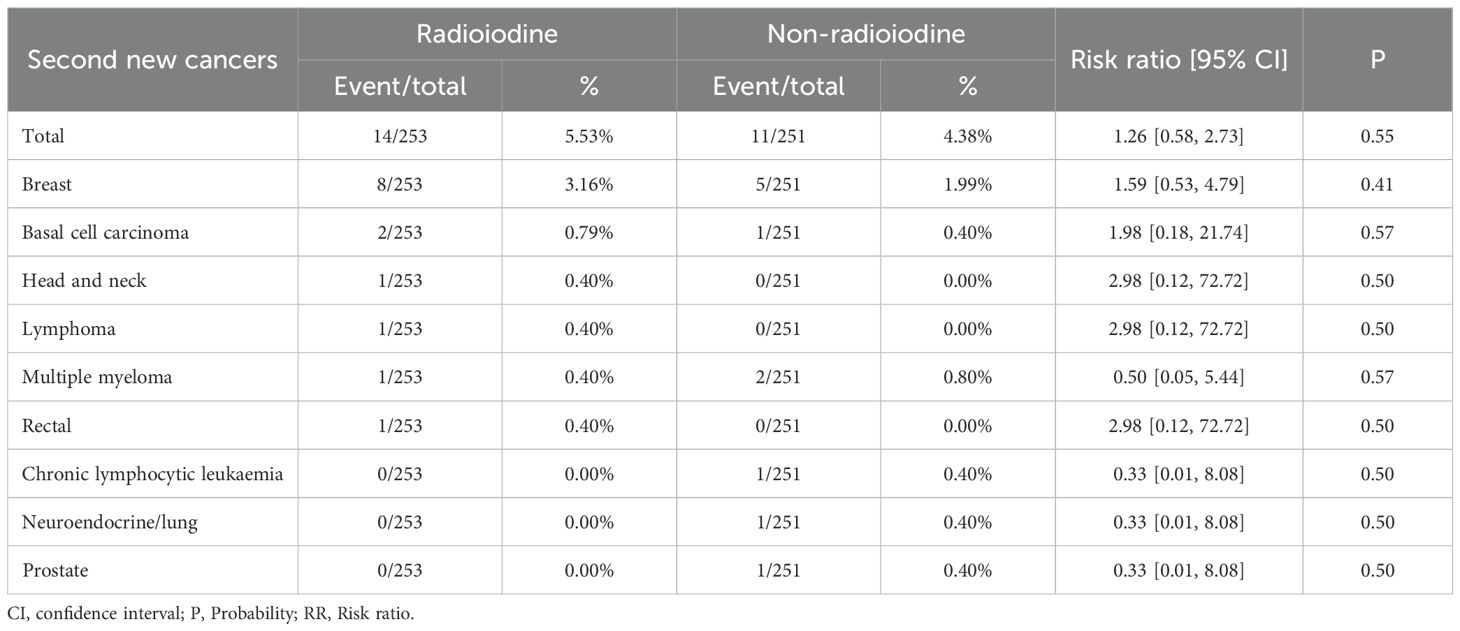
Second new cancers
Total rate of second new cancers was similar between the two groups (RR: 1.26 [0.58, 2.73], P = 0.55). The most common second new cancer in both groups was breast cancer (Table 4).
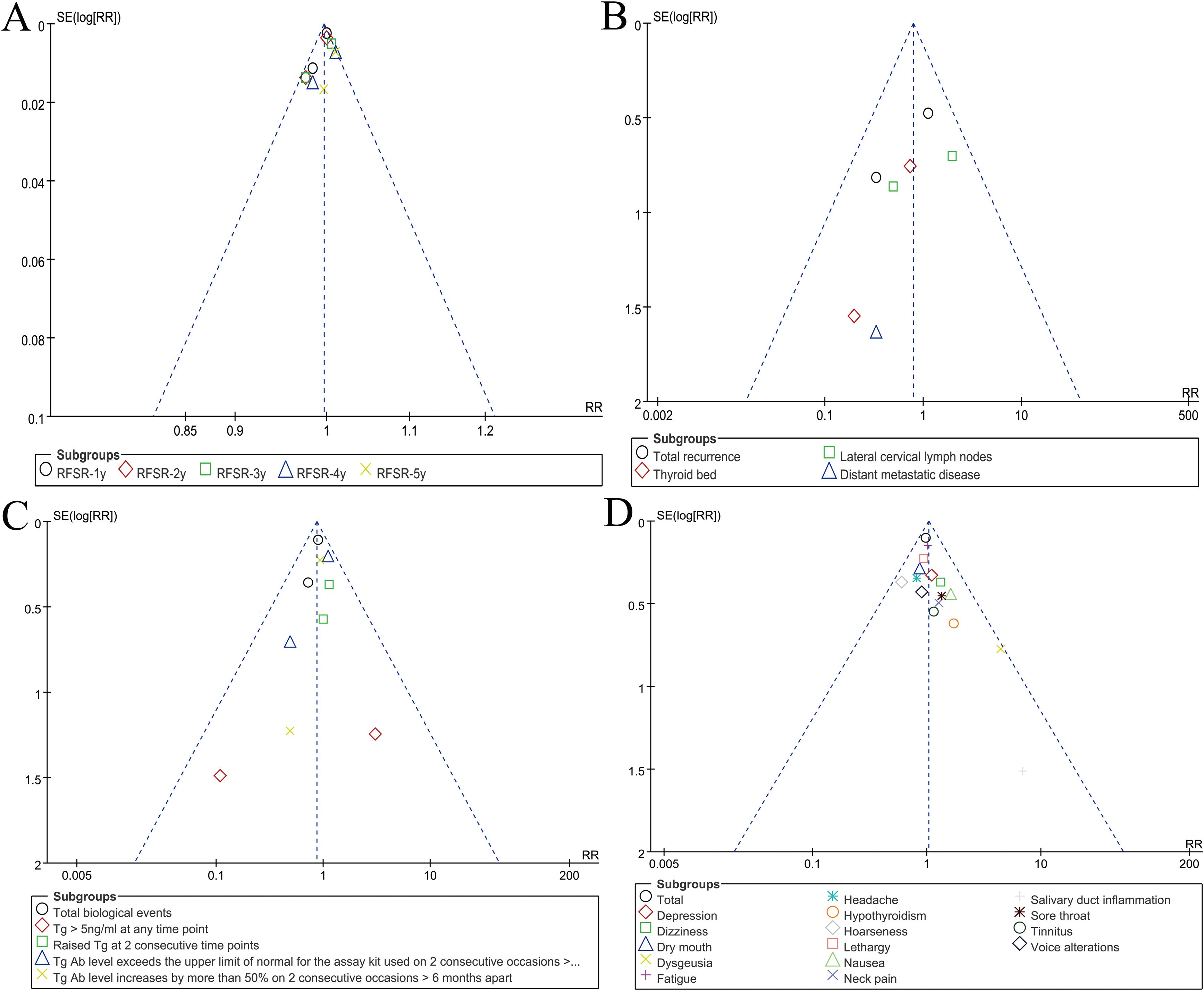
Publication bias
Visual inspection of funnel plots for RFSR, recurrence, biological outcomes, and adverse events indicated low likelihood of reporting bias (Figure 5).
Discussion
The optimal management strategy for DTC following thyroidectomy has remained a point of clinical controversy for years. While total thyroidectomy or lobectomy alone often provides excellent long-term outcomes for low-risk patients, the historical practice of routinely administering postoperative radioiodine to ablate remnant thyroid tissue has persisted in many clinical settings (18). This persistence is partly due to legacy protocols and partly due to uncertainty regarding the true benefits of radioiodine in preventing disease recurrence. Recent advancements in diagnostic surveillance, risk stratification, and molecular understanding of DTC have further challenged the necessity of radioiodine in low-risk settings. Despite evolving guidelines, such as the 2015 ATA recommendation to limit radioiodine use in low-risk patients, practice patterns remain heterogeneous (10). Thus, a high-quality synthesis of the best available randomized evidence was urgently needed. This meta-analysis, focusing exclusively on phase 3 RCTs—ESTIMABL2 and IoN—addresses this gap (11, 12). Our pooled analysis involving 1,280 patients found no significant benefit of radioiodine in reducing recurrence rates, improving RFS, or altering structural and biological event rates. Furthermore, no differences were found in terms of AEs, mortality, or secondary malignancies, reinforcing the argument against routine radioiodine administration in this patient population.
The question of recurrence lies at the heart of the ongoing debate regarding the necessity of radioiodine in low-risk DTC. In our meta-analysis, we found that radioiodine therapy did not confer a statistically significant reduction in recurrence risk, nor did it improve DFS. These findings not only echo the results of the ESTIMABL2 and IoN trials individually but also align with accumulating observational evidence suggesting that the natural course of low-risk DTC is inherently favorable, regardless of adjuvant radioiodine (11, 12). One important consideration is the absolute recurrence rate in both groups, which was below 5%, suggesting that recurrence in this subgroup is a rare event. The implication is that even if radioiodine were to reduce recurrence marginally, the clinical relevance would remain limited due to the already low baseline risk (19). Moreover, the site of recurrence—whether local, regional, or distant—did not differ significantly between the radioiodine and non- radioiodine arms in our analysis. This observation counters the traditional rationale that radioiodine may reduce microscopic distant metastasis that escapes surgical excision. In the current era of high-resolution ultrasound and sensitive serum Tg assays, the early detection of recurrence is more achievable, potentially reducing the clinical need for radioiodine as a prophylactic tool (20). Some experts have also proposed that radioiodine might selectively benefit subgroups of low-risk patients, such as those with multifocality, microscopic lymphovascular invasion, or younger age, but such hypotheses remain unproven in randomized settings (21). Another important angle is the temporal pattern of recurrence. Our data show no difference in RFS over a 5-year follow-up period, suggesting that recurrences, when they do occur, are not only rare but also unlikely to be temporally delayed by radioiodine. In both the ESTIMABL2 and IoN trials, recurrence was primarily defined using surrogate criteria—such as abnormal neck imaging findings or elevated serum thyroglobulin (Tg) or anti-Tg antibodies—rather than histopathologic confirmation. This reflects a well-recognized challenge in low-risk DTC, where many so-called “recurrences” represent indolent or biochemical findings that may not necessitate therapeutic intervention. Accordingly, recurrence in this context should be interpreted as a composite surrogate endpoint rather than a strictly pathological event, which may partly explain the minimal clinical impact observed in our pooled analysis (11, 12). This undermines the argument for radioiodine as a long-term protective measure. Additionally, modern risk-adapted surveillance protocols—such as dynamic risk stratification based on postoperative Tg trends and imaging—allow for more individualized follow-up and delayed intervention strategies, which further diminishes the value of a blanket radioiodine policy (22). Ultimately, these findings reinforce the view that recurrence should not be the principal justification for administering radioiodine in low-risk DTC.
In addition to recurrence, structural and biological events serve as important surrogate endpoints in monitoring patients after thyroidectomy. In our meta-analysis, structural events—defined as radiologic or cytologic findings suggestive of persistent or recurrent disease—were numerically lower in the radioiodine group, but the difference did not reach statistical significance. Similarly, biological events—characterized by elevated levels of serum Tg or TgAb in the absence of structural disease—were also not significantly different between groups. These findings challenge the notion that radioiodine contributes meaningfully to reducing biochemical or structural disease burden in low-risk patients (23). The slightly higher frequency of Tg > 5 ng/mL in the non-radioiodine group may superficially suggest biochemical benefit from radioiodine, but this must be interpreted cautiously. Elevated Tg levels in non-ablated patients may reflect residual normal thyroid tissue rather than recurrent disease. Importantly, this distinction becomes clinically relevant only if the elevated Tg leads to a change in patient management—such as unnecessary imaging, biopsies, or anxiety—which can be mitigated by physician awareness and patient education (23). Moreover, it is worth emphasizing that Tg kinetics over time (e.g., declining or stable trends) are often more informative than absolute values, especially when interpreted alongside imaging findings (24). Another consideration is the effect of radioiodine on diagnostic clarity. While ablation of remnant thyroid tissue may simplify biochemical monitoring, modern assay sensitivity and imaging capabilities allow for effective surveillance even in non-ablated patients. In fact, updated guidelines from the ATA and European Thyroid Association (ETA) now endorse the omission of radioiodine in low-risk patients, partly on the basis of these technological advances (10, 25). In clinical practice, the decision to administer radioiodine should not be driven solely by the desire for biochemical clarity, especially if it comes at the cost of exposing patients to radiation without improving hard outcomes. Furthermore, the absence of significant differences in structural and biological event rates raises questions about the long-term benefits of radioiodine (26). If radioiodine does not appreciably reduce persistent or recurrent structural disease, nor influence biochemical markers in a clinically actionable way, then its utility in this context becomes increasingly tenuous. This is particularly relevant as healthcare systems strive to balance efficacy with cost and safety, and as patient-centered care models prioritize shared decision-making and quality of life.
The safety profile of radioiodine has historically been a concern, particularly regarding both acute side effects and long-term sequelae. In our analysis, we found no statistically significant difference in the overall incidence of AEs between the radioiodine and non- radioiodine groups, nor in the incidence of grade 3–5 AEs. The most frequently reported AEs in the radioiodine group were fatigue (25.7%), lethargy (12.7%), and dry mouth (8.3%)—all of which are consistent with transient, non-life-threatening symptoms associated with salivary gland irradiation. Dysgeusia also tended to occur more frequently in the radioiodine group (3.6% vs 0.8%), with a relative risk of 4.46 (p = 0.05). This trend is biologically plausible, as radioiodine uptake by the salivary glands and gustatory epithelium can transiently disrupt taste perception through localized radiation-induced inflammation or ductal damage. Importantly, dysgeusia is generally mild and self-limited, resolving within weeks to months after treatment, and does not typically require medical intervention. Nevertheless, this observation underscores the need for patient counseling regarding temporary sensory disturbances following RAI (27). These findings are clinically important, as they support the assertion that radioiodine, when used judiciously, is a well-tolerated therapy in most patients (28). However, the absence of significant differences in AEs should not necessarily be interpreted as justification for routine use. Even mild or moderate symptoms can adversely affect patient quality of life, particularly when they occur in patients who are unlikely to derive measurable benefit from the intervention (29). Additionally, the possibility of rare but serious complications—such as sialadenitis, lacrimal gland dysfunction, or transient infertility—remains a theoretical concern, especially in younger patients or those receiving repeat doses of radioiodine (30). Mortality was also comparable between groups, and no thyroid cancer-related deaths were reported. This reinforces the well-established notion that low-risk DTC has an exceedingly favorable prognosis and that death is a rare event, further calling into question the need for aggressive adjuvant therapy (31). Notably, the most common cause of death in both arms was second primary malignancy, which leads to the next point of discussion. The incidence of second primary cancers, a key long-term safety concern with radioactive exposure, was not significantly increased in the radioiodine group. Previous retrospective studies have raised alarm over possible associations between radioiodine and subsequent hematologic or solid tumors (32). However, our findings, derived from prospective RCTs, are more reassuring. The most commonly observed second cancer was breast cancer—likely reflecting baseline population prevalence rather than treatment-induced risk. It is plausible that modern radioiodine dosing strategies, which typically involve lower activities and risk-adapted indications, mitigate the potential for carcinogenicity (33). Nevertheless, given the latency of radiation-induced malignancies, ongoing long-term surveillance in this population remains essential. In conclusion, our analysis indicates that radioiodine does not meaningfully increase the risk of acute or chronic toxicity, death, or second malignancy in low-risk DTC. While this supports the safety of radioiodine in appropriately selected patients, it also underscores the lack of compelling justification for its routine use in a population unlikely to benefit. Clinicians should weigh these findings against patient-specific factors and preferences when discussing adjuvant treatment options.
Despite the strengths of this meta-analysis, several limitations warrant consideration. First, the number of included RCTs remains limited to two, although they represent the highest quality evidence currently available. Second, the follow-up durations in both trials, while adequate to detect early recurrence, may be insufficient to capture very late recurrences or long-term sequelae such as chronic toxicity or secondary cancers. Third, subgroup analyses based on tumor histology, patient age, gender, or surgical extent were not possible due to lack of granular data, which may limit the generalizability of the findings across diverse patient populations. Additionally, patient adherence to follow-up protocols, variability in Tg assay sensitivity, and institutional differences in imaging thresholds could all introduce confounding biases. Another limitation relates to the definition of recurrence in the included RCTs, which was largely based on imaging or biochemical criteria rather than surgical or pathological confirmation. As a result, some recurrences may reflect indolent biochemical findings without clinical relevance, potentially leading to an overestimation of event rates. Lastly, while statistical heterogeneity was low, clinical heterogeneity cannot be entirely excluded given differing national practices, healthcare settings, and patient expectations across the French and UK cohorts.
Conclusion
The radioiodine therapy does not significantly reduce recurrence or improve RFS in patients with low-risk DTC following thyroidectomy. Structural and biological event rates were comparable between groups, suggesting that modern surveillance methods are effective even without radioiodine. There were no meaningful differences in AEs, mortality, or secondary malignancies, reinforcing the safety of omitting radioiodine in appropriately selected patients. These findings support a risk-adapted, individualized approach and advocate for de-escalation of radioiodine use in the management of low-risk DTC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
CY: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DL: Conceptualization, Data curation, Formal Analysis, Writing – original draft. JX: Conceptualization, Data curation, Formal Analysis, Writing – original draft. YZ: Conceptualization, Data curation, Formal Analysis, Writing – original draft. FC: Conceptualization, Data curation, Formal Analysis, Writing – original draft. WY: Conceptualization, Data curation, Formal Analysis, Writing – original draft. LZ: Conceptualization, Data curation, Formal Analysis, Writing – original draft. JL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was Project supported by Scientific and Technological Projects of Jiangxi Provincial Health Commission (Grant number: 202212553). The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
The authors appreciate all the public health workers who participated in the databases (PubMed, ScienceDirect, the Cochrane Library, Scopus, EMBASE, and Web of Science).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1670978/full#supplementary-material
Supplementary Figure 1 | Cochrane Risk Assessment.
Supplementary Figure 2 | Forest plots of RFSR at 1–5 years associated with radioiodine versus non-radioiodine.
Supplementary Figure 3 | Comparisons of RFSR associated with radioiodine versus non-radioiodine. (A) RFSR at 1–5 years; (B) Trend of risk ratios in RFSR.
Supplementary Figure 4 | Forest plots of biological events associated with radioiodine versus non-radioiodine.
Supplementary Figure 5 | Forest plots of structural events associated with radioiodine versus non-radioiodine.
Abbreviations
AE, Adverse event; ATA, American Thyroid Association; CI, Confidence interval; DTC, Differentiated thyroid cancer; ETA, European Thyroid Association; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HR, Hazard ratio; M/F, Male/Female; P, Probability; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, Randomized controlled trial; RFS, Recurrence-free survival; RFSR, Recurrence-free survival rate; RR, Risk ratio; Tg, Thyroglobulin; TgAb, Thyroglobulin antibodies.
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
2. Hartl DM. Practice-changing evidence for low-risk differentiated thyroid cancer. Lancet. (2025) 406:5–6. doi: 10.1016/S0140-6736(25)00781-0
3. Steinschneider M, Pitaro J, Koren S, Mizrakli Y, Benbassat C, and Muallem Kalmovich L. Differentiated thyroid cancer with biochemical incomplete response: clinico-pathological characteristics and long term disease outcomes. Cancers (Basel). (2021) 13:5422. doi: 10.3390/cancers13215422
4. Haymart MR, Esfandiari NH, Stang MT, and Sosa JA. Controversies in the management of low-risk differentiated thyroid cancer. Endocr Rev. (2017) 38:351–78. doi: 10.1210/er.2017-00067
5. Liu Y, Wang J, Hu X, Pan Z, Xu T, Xu J, et al. Radioiodine therapy in advanced differentiated thyroid cancer: Resistance and overcoming strategy. Drug Resist Updat. (2023) 68:100939. doi: 10.1016/j.drup.2023.100939
6. Tang M, Li J, Sun M, Song X, Zheng K, Luo X, et al. Global, regional, and national trends in thyroid cancer burden (1990-2021): Insights from the GBD 2021 study. Biomol BioMed. (2025) 25(12):2680–95. doi: 10.17305/bb.2025.12503
7. Wadsley J, Ainsworth G, Coulson AB, Garcez K, Moss L, Newbold K, et al. Results of the SEL-I-METRY phase II trial on resensitization of advanced iodine refractory differentiated thyroid cancer to radioiodine therapy. Thyroid. (2023) 33:1119–23. doi: 10.1089/thy.2022.0707
8. Dong P, Qu Y, Yang L, Xiao L, Huang R, and Li L. Outcomes after radioiodine ablation in patients with thyroid cancer: Long-term follow-up of a Chinese randomized clinicaltrial. Clin Endocrinol (Oxf). (2021) 95:782–9. doi: 10.1111/cen.14563
9. Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. (2012) 366:1674–85. doi: 10.1056/NEJMoa1109589
10. Pitoia F and Miyauchi A. 2015 American thyroid association guidelines for thyroid nodules and differentiated thyroid cancer and their implementation in various care settings. Thyroid. (2016) 26:319–21. doi: 10.1089/thy.2015.0530
11. Leboulleux S, Bournaud C, Chougnet CN, Lamartina L, Zerdoud S, Do Cao C, et al. Thyroidectomy without radioiodine in patients with low-risk thyroid cancer: 5 years of follow-up of the prospective randomised ESTIMABL2 trial. Lancet Diabetes Endocrinol. (2025) 13:38–46. doi: 10.1016/S2213-8587(24)00276-6
12. Mallick U, Newbold K, Beasley M, Garcez K, Wadsley J, Johnson SJ, et al. Thyroidectomy with or without postoperative radioiodine for patients with low-risk differentiated thyroid cancer in the UK (IoN): a randomised, multicentre, non-inferiority trial. Lancet. (2025) 406:52–62. doi: 10.1016/S0140-6736(25)00629-4
13. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. (2022) 33:27–63. doi: 10.1007/s12022-022-09707-3
14. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
15. Higgins JP, Altman DG, Gøtzsche PIC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
16. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, and Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) 64:380–2. doi: 10.1016/j.jclinepi.2010.09.011
17. Leboulleux S, Bournaud C, Chougnet CN, Zerdoud S, Al Ghuzlan A, Catargi B, et al. Thyroidectomy without radioiodine in patients with low-risk thyroid cancer. N Engl J Med. (2022) 386:923–32. doi: 10.1056/NEJMoa2111953
18. Chandekar KR, Satapathy S, and Bal C. Impact of radioiodine therapy on recurrence and survival outcomes in intermediate-risk papillary thyroid carcinoma -A systematic review and meta-analysis. Clin Endocrinol (Oxf). (2024) 100:181–91. doi: 10.1111/cen.15001
19. Dehbi HM, Mallick U, Wadsley J, Newbold K, Harmer C, and Hackshaw A. Recurrence after low-dose radioiodine ablation and recombinant human thyroid-stimulating hormone for differentiated thyroid cancer (HiLo): long-term results of an open-label, non-inferiority randomised controlled trial. Lancet Diabetes Endocrinol. (2019) 7:44–51. doi: 10.1016/S2213-8587(18)30306-1
20. Pryma DA and Mandel SJ. Radioiodine therapy for thyroid cancer in the era of risk stratification and alternative targeted therapies. J Nucl Med. (2014) 55:1485–91. doi: 10.2967/jnumed.113.131508
21. Schott M, Schott-Ohly P, Krieg S, Thomaschky C, Wieltsch JH, Petrovitch A, et al. The prognostic impact of radioiodine therapy in patients with papillary thyroid cancer. Horm Metab Res. (2024) 56:770–8. doi: 10.1055/a-2423-4849
22. Hataya Y, Fujishima Y, Fujimoto K, Iwakura T, and Matsuoka N. Association between serum thyroglobulin levels and glycemic control in patients with thyroid cancer after radioiodine therapy. Endocr J. (2025) 72:637–43. doi: 10.1507/endocrj.EJ24-0417
23. Giovanella L, Milan L, Roll W, Weber M, Schenke S, Kreissl M, et al. Postoperative thyroglobulin as a yard-stick for radioiodine therapy: decision tree analysis in a European multicenter series of 1317 patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. (2023) 50:2767–74. doi: 10.1007/s00259-023-06239-8
24. van Velsen EFS and Verburg FA. Adjuvant radioiodine for intermediate-risk papillary thyroid cancer-to treat or not to treat. J Clin Endocrinol Metab. (2023) 108:e1149–50. doi: 10.1210/clinem/dgad171
25. Durante C, Hegedüs L, Czarniecka A, Paschke R, Russ G, Schmitt F, et al. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. Eur Thyroid J. (2023) 12:e230067. doi: 10.1530/ETJ-23-0067
26. Wang L, Li Y, Yang J, Shen W, Liu Z, Zhu H, et al. Prognostic impact and clinical staging adjustment of tumor deposits in postoperative radioiodine therapy for differentiated thyroid cancer. Endocrine. (2025) 90:215–24. doi: 10.1007/s12020-025-04316-6
27. Baudin C, Bressand A, Buffet C, Menegaux F, Soret M, Lê AT, et al. Dysfunction of the salivary and lacrimal glands after radioiodine therapy for thyroid cancer: results of the START study after 6-months of follow-up. Thyroid. (2023) 33:1100–9. doi: 10.1089/thy.2023.0090
28. James DL, Ryan ÉJ, Davey MG, Quinn AJ, Heath DP, Garry SJ, et al. Radioiodine remnant ablation for differentiated thyroid cancer: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. (2021) 147:544–52. doi: 10.1001/jamaoto.2021.0288
29. Legrand A, Bernier MO, Bressand A, Buffet C, Mandin C, Menegaux F, et al. Health-related quality of life and radioiodine therapy in thyroid cancer patients: a before-and-after study. Qual Life Res. (2024) 33:2721–31. doi: 10.1007/s11136-024-03721-0
30. Auttara-Atthakorn A, Sungmala J, Anothaisintawee T, Reutrakul S, and Sriphrapradang C. Prevention of salivary gland dysfunction in patients treated with radioiodine for differentiated thyroid cancer: A systematic review of randomized controlled trials. Front Endocrinol (Lausanne). (2022) 13:960265. doi: 10.3389/fendo.2022.960265
31. Alshwayyat S, Al-Akhras A, Ghazou A, Alshwayyat TA, Ababneh O, and Alawneh A. Assessing radioiodine therapy long-term outcomes in differentiated thyroid cancer using nomograms. Sci Rep. (2024) 14:23349. doi: 10.1038/s41598-024-72002-0
32. Silva-Vieira M, Carrilho Vaz S, Esteves S, Ferreira TC, Limbert E, Salgado L, et al. Second primary cancer in patients with differentiated thyroid cancer: does radioiodine play a role? Thyroid. (2017) 27:1068–76. doi: 10.1089/thy.2016.0655
Keywords: radioiodine, low-risk differentiated thyroid cancer, thyroidectomy, meta-analysis, randomized controlled trials
Citation: Yang C, Luo D, Xie J, Zou Y, Chen F, Yang W, Zeng L and Liu J (2025) Is radioiodine necessary for patients with low-risk differentiated thyroid cancer after thyroidectomy: a pooled analysis of ESTIMABL2 and IoN trials. Front. Oncol. 15:1670978. doi: 10.3389/fonc.2025.1670978
Received: 22 July 2025; Accepted: 13 October 2025;
Published: 28 October 2025.
Edited by:
Jan Baptist Vermorken, University of Antwerp, BelgiumReviewed by:
Jared Shenson, Orlando Health Cancer Institute, United StatesCongcong Wang, The Affiliated Hospital of Qingdao University, China
Copyright © 2025 Yang, Luo, Xie, Zou, Chen, Yang, Zeng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Liu, bGpreTIwMjZAMTI2LmNvbQ==
 Chuansheng Yang1
Chuansheng Yang1 Jing Liu
Jing Liu