Abstract
In patients with plasma cell dyscrasias presenting with kidney injury, renal biopsy usually displays the presence of a single histological damage. However, the coexistence of heterogeneous damages has been occasionally described and is associated with worse renal outcomes. In this report, we describe the case of a patient with a concomitant diagnosis of multiple myeloma and lymphoplasmacytic lymphoma, presenting with acute kidney injury and with renal biopsy revealing the unexpected concurrent presence of several different renal damages, who achieved a good hematologic response and dialysis independence after anti-myeloma therapy.
Introduction
Plasma cell (PC) dyscrasias and other lymphoproliferative disorders may be associated with heterogeneous types of renal involvement. The most frequent cause of kidney failure in patients affected by multiple myeloma (MM) is represented by light chain cast nephropathy (LCCN), which is commonly associated with high serum-free light chain (sFLC) and monoclonal proteinuria and is regarded as a myeloma-defining event (MDE) (1). Conversely, light chain amyloidosis (AL) and other monoclonal gammopathies of renal significance (MGRS) are generally related to underlying small clones (2, 3). Each condition presents distinctive histological features and may be associated with specific clinical and laboratory findings. In the majority of cases, renal biopsy reveals the presence of a single renal damage, although the coexistence of different patterns in the same patient has been occasionally described, with the combination of LCCN and light chain deposition disease (LCDD) being reported as the most common (4–8). The pathogenic mechanisms of renal injury are heterogeneous and have been classified as either direct or indirect based on the presence or absence of monoclonal immunoglobulin in the kidneys. These mechanisms involve physicochemical properties of the monoclonal immunoglobulin, immunological mechanisms, and features of the renal microenvironment (2).
Herein, we report on the case of a patient diagnosed with both MM and lymphoplasmacytic lymphoma (LPL) unexpectedly associated with the concurrent presence of multiple morphological renal damages, leading to dialysis-requiring acute kidney injury (AKI) as the main onset feature, and who achieved a good hematologic response and dialysis independence after anti-myeloma therapy.
Case description: clinical presentation and diagnostic assessment
In August 2023, a 41-year-old man without relevant medical history had asymptomatic documentation (during routine laboratory exams) of severe anemia (hemoglobin = 6.8 g/dl) and AKI [creatinine = 7 mg/dl, estimated glomerular filtration rate (eGFR) = 9 ml/min]. Further laboratory tests (Table 1) revealed the presence of nephrotic-range proteinuria of 3.6 g/day (2.9 g/day of monoclonal kappa light chain, κ-LC), κ-sFLC of 32,970 mg/L (κ/λ ratio = 1,490), and a positive serum immunofixation for κ-LC (not quantifiable by serum protein electrophoresis), leading to suspicion of MM, likely with LCCN. Bone marrow biopsy confirmed the diagnosis of MM with clonal PCs 35% (CD138+PAX5−) and showed the co-presence of an indolent non-Hodgkin’s B-cell lymphoma (NHL) (B-lymphoid cells 15%, CD20+PAX5+CD19+CD5−CD138−; PC differentiated lymphocytes 5%, CD138+PAX5+) harboring the MYD88 L265P mutation (identified via Sanger sequencing). Fluorescence in situ hybridization (FISH) analysis of the PCs was negative for chromosome 14 translocations, chromosome 17 deletion, or chromosome 1 abnormalities, defining a cytogenetically standard-risk disease (9). The International Staging System (ISS) was III (β2-microglobulin = 22 mg/L), the Revised ISS (R-ISS) was II, and R2-ISS was III. Skeletal assessment through 18F-FDG-PET/CT documented bone marrow diffuse uptake without osteolytic or focal lesions (FLs) and did not show lymphadenopathies or organomegaly. Magnetic resonance imaging (MRI) of the axial skeleton showed diffuse hypointense signal on T1-weighted sequences without FLs. Despite the high probability of LCCN, renal biopsy was performed to confirm the etiology of AKI and to assess the entity of the kidney injury and the interstitial compartment. A histological picture of diffuse acute and chronic interstitial nephritis was found, associated with moderate fractured, metachromatic with giant cell reaction casts restricted for κ-LC on the immunofluorescence studies consistent with LCCN and Congo red positivity suggestive of amyloid. In addition, diagnostic features of LCDD were found in the glomeruli, with mild mesangial matrix expansion, Congo red negativity, and κ-LC restriction on immunofluorescence. Furthermore, a mixed interstitial infiltrate of lymphocytes and PCs, both exhibiting κ-LC restriction, was observed (Figure 1). A transthoracic echocardiogram described mild thickening of the ventricular walls [interventricular septum (IVS), thickness of 1.2 cm] and mild pericardial effusion, without kinetic alterations (62% ejection fraction, EF). Cardiac MRI arose suspicion of infiltrative disease; however, endomyocardial biopsy to confirm AL was not performed in order to allow rapid treatment initiation. There were neither laboratory or imaging signs of liver AL nor clinical symptoms of gastrointestinal or neurological involvement.
Table 1
| Laboratory tests | At diagnosis | Post-induction therapy (prior to ASCT) | Prior to maintenance therapy |
|---|---|---|---|
| Hemoglobin (g/dl) | 6.8 | 9.3 | 11.1 |
| Platelets (×109/L) | 97 | 113 | 113 |
| Leukocytes (×109/L) | 5.5 | 4.8 | 2.4 |
| Neutrophils (×109/L) | 3.1 | 3.7 | 1.5 |
| Creatinine (mg/dl) | 7 | 4 | 3.3 |
| eGFR (ml/min) | 9 | 17 | 22 |
| Calcium (mg/dl) | 9.7 | 8.3 | 9.2 |
| β2-microglobulin (mg/L) | 22 | 11.2 | 8.8 |
| Albumin (g/dl) | 4.3 | 4 | 4.1 |
| LDH (IU/L) | 209 | 238 | 166 |
| Serum immunofixation | κ-LC | κ-LC | Negative |
| κ-sFLC (mg/L) | 32,970 | 1,220 | 130 |
| κ/λ ratio | 1,490 | 80 | 22 |
| Urine immunofixation | κ-LC | κ-LC | κ-LC |
| Total urine protein (g/24 h) | 3.6 | 0.9 | 0.3 |
| Urine M-protein (g/24 h) | 2.9 | 0.6 | 0.08 |
Main laboratory findings at diagnosis, before autologous stem cell transplant (ASCT), and before maintenance.
eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; LC, light chain; sFLC, serum free light chain; M-protein, myeloma protein
Figure 1
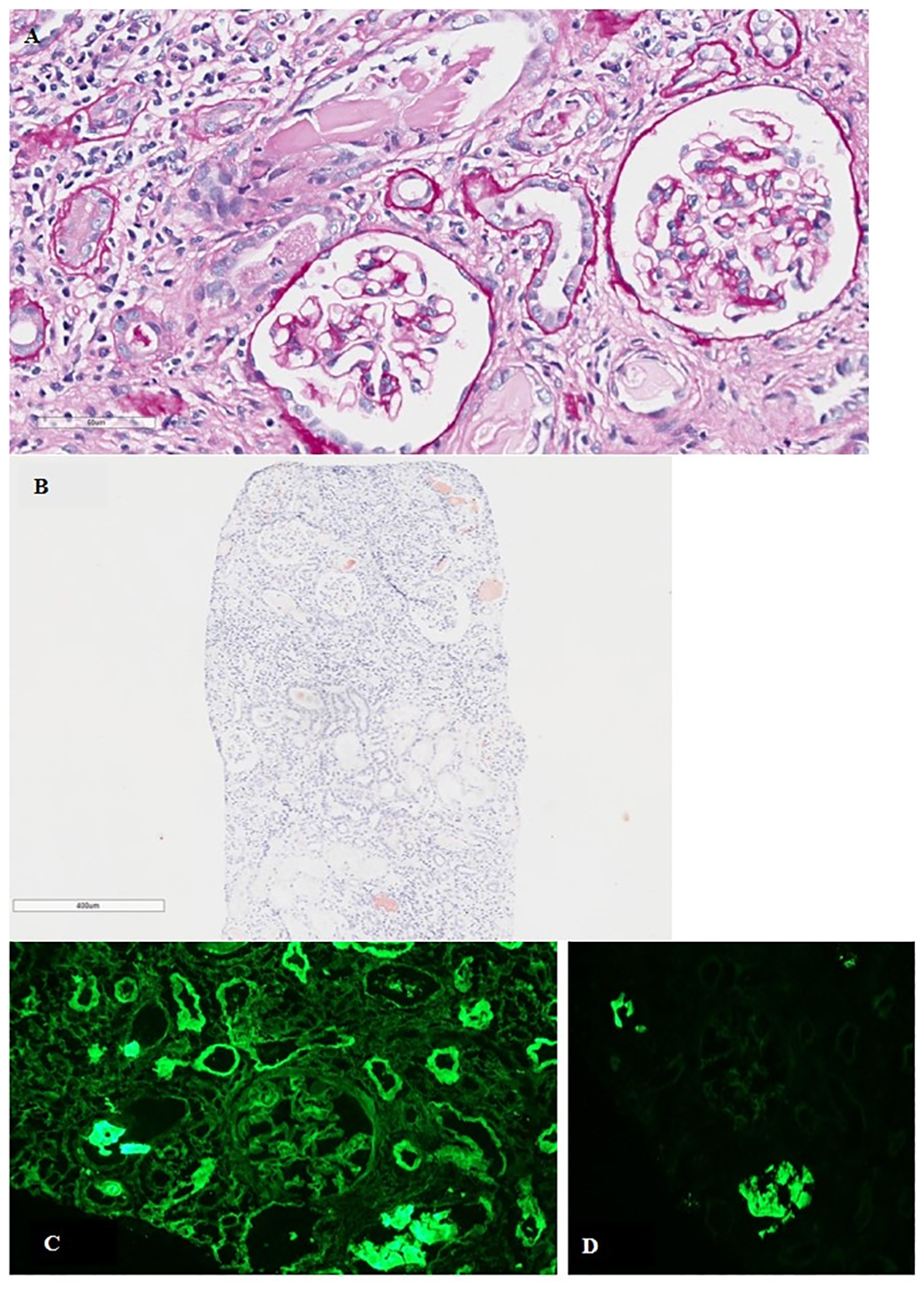
(A) Tubulo-interstitial nephritis with fractured intratubular cast surrounded by granulomatous inflammatory reaction. Mild mesangial matrix expansion in the glomeruli (PAS; original magnification, ×400). (B) Tubular casts positive for Congo red staining (original magnification, ×400). (C) Direct immunofluorescence for κ- and λ-LC showing κ-LC restriction along the glomerular basement membranes and tubular basement membranes (original magnification, ×200). (D) Polyclonal reaction in tubular casts (original magnification, ×200). PAS, periodic acid–Schiff stain; LC, light chain.
Case description: treatment and outcome
As the severe renal damage was attributable to the PC dyscrasia, despite the concomitant presence of indolent NHL, a quadruplet anti-MM induction regimen based on daratumumab, bortezomib, thalidomide, and dexamethasone (D-VTd) was started. Concurrently, five sessions of extracorporeal removal of sFLC with high-cutoff hemodialysis were initiated in August 2023, followed by hemodialysis three times per week (10, 11).
Overall, six D-VTd induction cycles were administered between August 2023 and January 2024. Adverse events during the induction therapy included grade 1 peripheral sensory neuropathy, episodes of atrial fibrillation (likely due to the presence of a transjugular central venous catheter) requiring both pharmacological and electrical cardioversion, and deep vein thrombosis subsequent to a transfemoral catheter placed to proceed with the dialysis. Due to the thrombotic event, thalidomide was temporarily withheld, and hemodialysis was switched to peritoneal dialysis.
Disease assessment upon induction therapy showed the achievement of hematologic partial response (PR) according to the International Myeloma Working Group (IMWG) criteria (12), with urine M-protein of 0.6 g/day, positive serum immunofixation (κ-LC), and κ-sFLC of 1,220 mg/L (κ/λ ratio = 80). Furthermore, a minor renal response (10 with eGFR equal to 17 ml/min was attained (Table 1). Bone marrow biopsy displayed the presence of a mixed population of PCs (predominant) and lymphoid cells, representing 15% of the overall cellularity.
In the absence of cardiological contraindications, the therapeutic process continued with hematopoietic stem cell mobilization with granulocyte colony-stimulating factor and plerixafor, high-dose chemotherapy (HDT) with melphalan (100 mg/m2, reduced dose for renal function), and autologous stem cell transplant (ASCT) (February 2024). Adverse events of the HDT included mucositis and febrile neutropenia. During hospitalization, peritoneal dialysis was temporarily switched back to hemodialysis in order to reduce the risk of infection.
Subsequently, based on real-life/retrospective data (13), due to the clinical high-risk features, maintenance therapy with bortezomib and lenalidomide was started 3 months after ASCT (May 2024), as the presence of double hematologic disease and renal impairment prevented enrolment in clinical trials.
Pre-maintenance disease assessment documented the achievement of a hematologic very good PR (VGPR): urine M-protein of 0.08 g/day, negative serum immunofixation, and κ-sFLC of 130 mg/L (κ/λ ratio = 22) (Table 1). Histologic re-evaluation through bone marrow biopsy resulted negative for both MM and lymphoma. Minimal residual disease (MRD), assessed using next-generation sequencing (NGS), was positive (10−4 sensitivity). Sanger sequencing of MYD88 resulted wild type for L265P mutation. Cardiologic evaluation showed clinical and echocardiographic stability (IVS thickness = 1.2 cm, EF = 69%). Moreover, a minor renal response was confirmed, with further improvement of kidney function after ASCT (eGFR = 22 ml/min), leading to a progressive reduction of the dialysis weekly sessions until discontinuation 4 months after ASCT.
As of June 2025, the patient has received 12 cycles of maintenance therapy, retaining a hematologic VGPR and dialysis independence.
Discussion and patient perspective
Notably, we herein reported on a rare case of a patient with two lymphoproliferative disorders, MM and LPL, presenting with AKI and with kidney biopsy revealing the unexpected coexistence of Congo red-positive LCCN, LCDD, and interstitial infiltrate of tumor cells.
The co-occurrence of MM and LPL has been previously described in a small number of case reports. A recent case series (14) has underlined that the two entities are not always associated with biclonal M-protein (similarly to our patient), thus emphasizing the importance of integrating clinical, morphologic, immunophenotypic, and genetic data to overcome difficult diagnostic challenges and drive therapeutic decision-making.
The uniqueness of the reported case is the concomitant presence of several different patterns of renal damage revealed by the organ biopsy. At the time of MM diagnosis, renal impairment affects up to 50% of patients, and 2%–4% of these patients require dialysis (10). Moreover, it has been reported as an independent negative prognostic factor, with a significantly higher risk of disease progression and mortality (15). Renal failure (creatinine clearance <40 ml/min or serum creatinine >2 mg/dl) in patients with MM is currently considered a MDE only when caused by LCCN (upon histological confirmation or presumptive diagnosis) as nearly all such cases are associated with a high tumor burden (1). LCCN represents the most common cause of kidney disease in MM as it is documented in 40%–60% of biopsies in MM patients with renal impairment (16). This disease is mainly attributable to the toxic effect of monoclonal sFLCs, which are overproduced and overpass the absorptive and catabolic capacity of proximal tubule cells, hence reaching the distal nephron and forming aggregates with Tamm–Horsfall protein (uromodulin). Such aggregates precipitate and result in the formation of casts determining tubular obstruction and AKI. The massive endocytosis of FLCs in the proximal tubule and the distal obstruction induced by casts also cause oxidative stress, inflammation, apoptosis, and fibrosis, which may lead to chronic damage (10, 11).
Nevertheless, even smaller PC clones and other lymphoproliferative dyscrasias may be causative of renal damage, which are mainly determined by the direct deposition within one or more renal compartments of monoclonal immunoglobulins, their parts, or heterogeneous products of aggregations or by indirect/immunological mechanisms and are currently addressed as MGRS (2). The most frequent patterns are represented by AL (primarily affecting the glomeruli, blood vessels, and, less frequently, the interstitium) and monoclonal immunoglobulin deposition disease (MIDD), particularly its subtype LCDD (involving the glomerular and tubular basement membranes) (3, 17, 18). As most MGRS affect the glomeruli, unlike LCCN, they are typically causative of high albuminuria (19). Renal biopsy represents the only tool for the diagnosis of MGRS and the identification of the specific damage pattern and is recommended in patients with monoclonal gammopathy and unexplained kidney disease, atypical clinical course in the presence of known risk factors for chronic kidney disease, monoclonal gammopathy, and kidney disease in patients <50 years (2). Conversely, renal biopsy is not considered necessary by the current IMWG recommendations in cases of MM with selective proteinuria and high sFLC (≥500 mg/L) without relevant comorbidities, as renal impairment is usually caused by LCCN in such cases (10). However, even though they are often associated with smaller clones, kidney AL and LCDD are documented in 7%–30% and 19%–26% of patients with MM and renal impairment, respectively (16). It has been hypothesized that the diverse physicochemical properties of LCs may be causative of the different renal damages, and it was shown that LCs from patients with LCCN or AL reproduce the same original pattern when injected into mice (20). The concomitant presence of heterogeneous patterns of renal injury has been described in up to 16% of patients affected by PC dyscrasias undergoing organ biopsy for renal impairment (7), with the combination of LCCN and LCDD being reported as the most common. Cases with distinct concomitant damages have been explained by several theories, including the presence of biclonal diseases, acquired mutations within the LC gene, or non-fibrillar proteins serving as precursors for fibrillar ones (6, 21).
It should be highlighted that the use of effective anti-clone treatment is essential, as the achievement of deep hematologic response is significantly associated with renal response both in MM and MGRS (3, 17, 22, 23). However, the therapies for MM (including cases presenting with LCCN) and AL are currently based on specific guidelines (10, 24–26), whereas there is no consensus regarding the management of other MGRS subtypes due to the lack of data and patients receive heterogeneous treatment options (3, 17).
According to the IMWG criteria, the renal response in MM is based on the best creatinine clearance achieved (10) and is observed in approximately 70% of patients with LCCN, with the rapid reduction of sFLCs representing the most important factor for renal recovery (10, 23). Conversely, the renal responses in AL and other MGRS are based on both the eGFR and the reduction of proteinuria (27, 28) and are achieved in 46%–59% of patients (with discordant data on the differences between AL and non-AL MGRS) (3, 17). A multicenter retrospective study on patients with LCCN demonstrated that the concomitant presence of LCDD or AL (in 6.2% and 2.2% of patients, respectively) is associated with a lower probability of renal response compared with LCCN only (23). Furthermore, another retrospective analysis comparing the clinical and renal outcomes of patients with LCCN, LCDD, and their combination showed inferior overall survival in the latter group compared with LCDD alone, and similar to that of LCCN alone, whereas the death-censored renal survival was similar among the three groups. Half of the patients with both LCNN and LCDD remained dialysis-dependent, and AKI at presentation was documented as the most important renal prognostic factor (29). In our patient, the anti-MM therapy resolved the nephrotic-range proteinuria and slowly improved and stabilized the kidney function, allowing the discontinuation of dialysis after ASCT.
Moreover, renal biopsy revealed the presence of focal intratubular amyloid without evidence of glomerular, vascular, or interstitial amyloid deposits. This scenario was observed in 28% of LCCN cases and resulted significantly associated with the occurrence of systemic AL in a retrospective study (30).
Furthermore, the presence of a mixed infiltrate of lymphocytes and PCs was documented in our patient. MM infiltration into the renal parenchyma has been mainly described in heavily pretreated patients (reported in up to 29% of autopsy series) (31), while it is considered a rare event in newly diagnosed ones, with unclear clinical and renal prognostic significance. However, potential contributes to renal damage (likely due to the compression of tubules and microvasculature, local LC, and cytokine-mediated injury) has been hypothesized (32). Similarly, kidney involvement in NHL has been reported in up to one-third of patients at autopsies, but is considered rarely associated with renal impairment. Nevertheless, a recent retrospective experience regarding patients with both aggressive and indolent NHL, including LPL, undergoing renal biopsy (for AKI, chronic kidney disease, or proteinuria) has documented the presence of lymphomatous renal involvement in 85% of patients, with interstitial infiltrate being the most common lesion and only 38% of patients having radiological anomalies (33).
Overall, our case showed the unique co-occurrence of different histological patterns of kidney damage in a patient concomitantly affected by two lymphoproliferative disorders. According to the IMWG recommendations, renal biopsy would not be necessary in the diagnostic workup of our patient as the presentation with AKI, the elevated sFLC burden, and the monoclonal proteinuria would allow a presumptive diagnosis of LCCN. However, kidney biopsy also revealed the concomitant unexpected presence of an extramedullary tumor spread, LCDD, and focal intratubular amyloid within casts. Although these findings did not lead to changes regarding the initial treatment choices, they allowed a more refined prognostic stratification due to the evidence of extramedullary disease and of the co-occurrence of other histological damages beyond LCCN, likely explaining the improvement without full recovery of the renal impairment. Furthermore, renal biopsy unveiled the presence of specific damages potentially associated with extra-renal involvement, particularly cardiac infiltrative disease, although a confirmatory biopsy was not performed. This case highlights the complex heterogeneous etiologies of renal disease in patients with hematologic malignancies and emphasizes the fundamental role of kidney biopsy in improving diagnosis, comprehension of the etiology and physiopathology of the renal impairment, and, hopefully, treatment approaches.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This retrospective review of patient data did not require ethical approval in accordance with local/national guidelines. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MT: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. GV: Writing – original draft, Writing – review & editing, Investigation. SB: Investigation, Writing – original draft, Writing – review & editing. RR: Writing – original draft, Writing – review & editing, Investigation. SM: Investigation, Writing – original draft, Writing – review & editing. IS: Writing – original draft, Writing – review & editing, Investigation. EM: Investigation, Writing – original draft, Writing – review & editing. SG: Writing – original draft, Writing – review & editing, Investigation. MP: Writing – original draft, Writing – review & editing, Investigation. MI: Writing – original draft, Writing – review & editing, Investigation. IR: Writing – original draft, Writing – review & editing, Investigation. LP: Writing – original draft, Writing – review & editing, Investigation. PT: Writing – original draft, Writing – review & editing, Investigation. EB: Writing – original draft, Writing – review & editing, Investigation. VS: Writing – original draft, Writing – review & editing, Formal analysis. CT: Writing – original draft, Writing – review & editing, Investigation. CA: Writing – original draft, Writing – review & editing, Investigation. GL: Writing – original draft, Writing – review & editing, Investigation. MC: Writing – original draft, Writing – review & editing, Resources, Supervision. EZ: Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision. KM: Writing – original draft, Writing – review & editing, Investigation, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
IR has served in advisory role for GlaxoSmithKline and received honoraria from Amgen, GlaxoSmithKline and Sanofi. LP has received honoraria from GlaxoSmithKline, Pfizer and Sanofi. PT has received honoraria from Amgen, Bristol-Myers Squibb/Celgene, Janssen, Takeda, AbbVie, Sanofi, GlaxoSmithKline and Pfizer. MC has received honoraria and has served in a consulting/advisory role for Amgen, AbbVie, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Menarini Stemline, Sanofi, and Karyopharm Therapeutics. EZ has received honoraria and has served in advisory role for Janssen, Bristol-Myers Squibb, Sanofi, Amgen, GlaxoSmithKline, Pfizer, Oncopeptides, Menarini-Stemline. KM has received honoraria from Celgene, Takeda, Amgen, Sanofi and Janssen.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Rajkumar SV Dimopoulos MA Palumbo A Blade J Merlini G Mateos MV et al . International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. (2014) 15(12):e538–48. doi: 10.1016/S1470-2045(14)70442-5
2
Leung N Bridoux F Batuman V Chaidos A Cockwell P D'Agati VD et al . The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. (2019) 15(1):45–59. doi: 10.1038/s41581-018-0102-7
3
Mancuso K Mina R Rocchi S Antonioli E Petrucci MT Fazio F et al . Clinical presentation and long-term survival outcomes of patients with monoclonal gammopathy of renal significance (MGRS): A multicenter retrospective study. Cancer Med. (2024) 13:e70266. doi: 10.1002/cam4.70266
4
Lin J Markowitz GS Valeri AM Kambham N Sherman WH Appel GB et al . Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J Am Soc Nephrol. (2001) 12(7):1482–92. doi: 10.1681/ASN.V1271482
5
Qian Q Leung N Theis JD Dogan A Sethi S . Coexistence of myeloma cast nephropathy, light chain deposition disease, and nonamyloid fibrils in a patient with multiple myeloma. Am J Kidney Dis. (2010) 56(5):971–6. doi: 10.1053/j.ajkd.2010.06.018
6
Lorenz EC Sethi S Poshusta TL Ramirez-Alvarado M Kumar S Lager DJ et al . Renal failure due to combined cast nephropathy, amyloidosis and light-chain deposition disease. Nephrol Dialysis Transplant. (2010) 25(4):1340–3. doi: 10.1093/ndt/gfp735
7
Ecotière L Thierry A Debiais-Delpech C Chevret S Javaugue V Desport E et al . Prognostic value of kidney biopsy in myeloma cast nephropathy: a retrospective study of 70 patients. Nephrol Dialysis Transplant. (2016) 31(1):64–72. doi: 10.1093/ndt/gfw056
8
Nasr SH Valeri AM Sethi S Fidler ME Cornell LD Gertz MA et al . Clinicopathologic correlations in multiple myeloma: a case series of 190 patients with kidney biopsies. Am J Kidney Dis. (2012) 59(6):786–94. doi: 10.1053/j.ajkd.2011.12.028
9
Avet-Loiseau H Davies FE Samur MK Corre J D'Agostino M Kaiser MF et al . International myeloma society/international myeloma working group consensus recommendations on the definition of high-risk multiple myeloma. J Clin Oncol. (2025) 43(22):2553. doi: 10.1200/JCO-24-01893
10
Dimopoulos MA Merlini G Bridoux F Leung N Mikhael J Harrison SJ et al . Management of multiple myeloma-related renal impairment: recommendations from the International Myeloma Working Group. Lancet Oncol. (2023) 24:e293–311. doi: 10.1016/S1470-2045(23)00223-1
11
Bridoux F Leung N Belmouaz M Royal V Ronco P Nasr SH et al . Management of acute kidney injury in symptomatic multiple myeloma. Kidney Int. (2021) 99(3):570–80. doi: 10.1016/j.kint.2020.11.010
12
Kumar S Paiva B Anderson KC Durie B Landgren O Moreau P et al . International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. (2016) 17:e328–46. doi: 10.1016/S1470-2045(16)30206-6
13
Joseph NS Kaufman JL Dhodapkar MV Hofmeister CC Almaula DK Heffner LT et al . Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. (2020) 38:1928–37. doi: 10.1200/JCO.19.02515
14
Itchaki G Jarhovsky O Castillo JJ Hassan H Gatt ML Leiba M et al . Lymphoplasmacytic lymphoma and multiple myeloma coexisting in the same patient: a case series and literature review. Leukemia Lymphoma. (2024) 65:943–9. doi: 10.1080/10428194.2024.2332499
15
Mohyuddin GR Koehn K Shune L Aziz M Abdallah AO McClune B et al . Renal insufficiency in multiple myeloma: a systematic review and meta-analysis of all randomized trials from 2005–2019. Leukemia Lymphoma. (2021) 62:1386–95. doi: 10.1080/10428194.2020.1867725
16
Korbet SM Schwartz MM . Multiple myeloma. J Am Soc Nephrol. (2006) 17(9):2533–45. doi: 10.1681/ASN.2006020139
17
Gozzetti A Guarnieri A Zamagni E Zakharova E Coriu D Bittrich M et al . Monoclonal gammopathy of renal significance (MGRS): Real-world data on outcomes and prognostic factors. Am J Hematol. (2022) 97(7):877–84. doi: 10.1002/ajh.26566
18
Granina E Henderson J Bertrand KA Sanchorawala V Szalat RE . Epidemiological characteristics and clinical outcomes of patients with monoclonal gammopathy of renal significance (MGRS) diagnosed in a large safety-net hospital in the US. Blood. (2024) 144(1):4680. Available online at: https://www.sciencedirect.com/science/article/pii/S000649712407438X (Accessed November 5, 2024).
19
Leung N Gertz M Kyle RA Fervenza FC Irazabal MV Eirin A et al . Urinary albumin excretion patterns of patients with cast nephropathy and other monoclonal gammopathy-related kidney diseases. Clin J Am Soc Nephrol. (2012) 7(12):1964–8. doi: 10.2215/CJN.11161111
20
Solomon A Weiss DT Kattine AA . Nephrotoxic potential of Bence Jones proteins. New Engl J Med. (1991) 324(26):1845–51. doi: 10.1056/NEJM199106273242603
21
Buxbaum J Gallo G . Nonamyloidotic monoclonal immunoglobulin deposition disease. Light-chain, heavy-chain, and light- and heavy-chain deposition diseases. Hematology/Oncology Clinics North America. (1999) 13(6):1235–48. doi: 10.1016/S0889-8588(05)70123-4
22
Courant M Orazio S Monnereau A Preterre J Combe C Rigothier C . Incidence, prognostic impact and clinical outcomes of renal impairment in patients with multiple myeloma: a population-based registry. Nephrol Dialysis Transplant. (2021) 36:482–90. doi: 10.1093/ndt/gfz211
23
Royal V Leung N Troyanov S Nasr SH Écotière L LeBlanc R et al . Clinicopathologic predictors of renal outcomes in light chain cast nephropathy: a multicenter retrospective study. Blood. (2020) 135(21):1833–46. doi: 10.1182/blood.2019003807
24
Dimopoulos MA Terpos E Boccadoro M Moreau P Mateos MV Zweegman S et al . EHA-EMN Evidence-Based Guidelines for diagnosis, treatment and follow-up of patients with multiple myeloma. Nat Rev Clin Oncol. (2025) 22(9):680–700. doi: 10.1038/s41571-025-01041-x
25
Sanchorawala V Boccadoro M Gertz M Hegenbart U Kastritis E Landau H et al . Guidelines for high dose chemotherapy and stem cell transplantation for systemic AL amyloidosis: EHA-ISA working group guidelines. Amyloid. (2022) 29(1):1–7. doi: 10.1097/HS9.0000000000000681
26
Wechalekar AD Cibeira MT Gibbs SD Jaccard A Kumar S Merlini G et al . Guidelines for non-transplant chemotherapy for treatment of systemic AL amyloidosis: EHA-ISA working group. Amyloid. (2023) 30(1):3–17. doi: 10.1080/13506129.2022.2093635
27
Palladini G Hegenbart U Milani P Kimmich C Foli A Ho AD et al . A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. (2014) 124(15):2325–32. doi: 10.1182/blood-2014-04-570010
28
Muchtar E Wisniowski B Geyer S Palladini G Milani P Merlini G et al . Graded organ response and progression criteria for kidney immunoglobulin light chain amyloidosis. JAMA Oncol. (2024) 10(10):1362–9. doi: 10.1001/jamaoncol.2024.2629
29
Zand L Nasr SH Gertz MA Dispenzieri A Lacy MQ Buadi FK et al . Clinical and prognostic differences among patients with light chain deposition disease, myeloma cast nephropathy and both. Leukemia Lymphoma. (2015) 56(12):3357–64. doi: 10.3109/10428194.2015.1040011
30
Gibier JB Gnemmi V Glowacki F Boyle EM Lopez B MacNamara E et al . Intratubular amyloid in light chain cast nephropathy is a risk factor for systemic light chain amyloidosis. Modern Pathol. (2017) 31(3):452–62. doi: 10.1038/modpathol.2017.124
31
Mine S Hagiwara S Hirai R Tanimura A Nakamura M Takeshita M et al . The spectrum of pathological findings of kidney injury in the cases of multiple myeloma: A clinico-pathological study of 93 consecutive autopsy cases. Blood. (2013) 122(21):5321. doi: 10.1182/blood.V122.21.5321.5321
32
Kaur T Krishnaprasad S Shankar R Kumar R Gowrishankar S Padua MD . Plasma cell infiltration of the kidney as a manifestation of myeloma: A report of three cases. Indian J Nephrol. (2017) 27(5):395–8. doi: 10.4103/ijn.IJN_215_16
33
Corlu L Rioux-Leclercq N Ganard M Decaux O Houot R Vigneau C . Renal dysfunction in patients with direct infiltration by B-cell lymphoma. Kidney Int Rep. (2019) 4:688–97. doi: 10.1016/j.ekir.2019.02.008
Summary
Keywords
multiple myeloma, lymphoplasmacytic lymphoma, acute kidney injury, renal damage, renal biopsy
Citation
Talarico M, Vischini G, Barbato S, Restuccia R, Masci S, Sacchetti I, Manzato E, Ghibellini S, Puppi M, Iezza M, Rizzello I, Pantani L, Tacchetti P, Borsi E, Solli V, Terragna C, Agostinelli C, La Manna G, Cavo M, Zamagni E and Mancuso K (2025) Case Report: Uncommon co-occurrence of different renal histopathological entities in a patient with multiple myeloma and lymphoplasmacytic lymphoma. Front. Oncol. 15:1672690. doi: 10.3389/fonc.2025.1672690
Received
24 July 2025
Accepted
17 September 2025
Published
07 October 2025
Volume
15 - 2025
Edited by
Massimo Gentile, Cosenza Hospital, Italy
Reviewed by
Sayalee Potdar, Emory University, United States
Enrica Antonia Martino, Cosenza Hospital, Italy
Updates
Copyright
© 2025 Talarico, Vischini, Barbato, Restuccia, Masci, Sacchetti, Manzato, Ghibellini, Puppi, Iezza, Rizzello, Pantani, Tacchetti, Borsi, Solli, Terragna, Agostinelli, La Manna, Cavo, Zamagni and Mancuso.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Zamagni, e.zamagni@unibo.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.