- 1Nervous System Medical Oncology Department, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto delle Scienze Neurologiche di Bologna, Bellaria Hospital, Bologna, Italy
- 2Department of Radiotherapy, Azienda Unità Sanitaria Locale (AUSL)-IRCCS Scienze Neurologiche, Bologna, Italy
- 3Unit of Neurosurgery, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bellaria Hospital, Bologna, Italy
- 4Department of Biomedical and Neuromotor Sciences (DIBINEM)- Alma Mater Studiorum- University of Bologna, Bologna, Italy
- 5Programma Neuroradiologia Con Tecniche Ad Elevata Complessità, IRCCS Istituto Delle Scienze Neurologiche Di Bologna Bellaria Hospital, Bologna, Italy
- 6Department of Experimental, Diagnostic and Specialty Medicine, University of Bologna, Bologna, Italy
- 7Programma Diagnostica Neuropatologica dei Tumori Cerebrali, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy
- 8Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna, Italy
- 9Solid Tumor Molecular Pathology Laboratory, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
Purpose: Available prognostic scores for adult-type diffuse glioma with isocitrate dehydrogenase (IDH) mutant were validated before the evaluation of biomolecular features. The selection of patients who did not receive postoperative radiotherapy and chemotherapy would provide an ideal setting to describe the natural history of these tumors.
Methods: We investigated the clinical outcomes of patients with adult-type diffuse glioma with isocitrate dehydrogenase IDH mutation approached with active surveillance after primary surgery.
Results: We evaluated 61 patients consisting of 35 patients with IDH-mutant astrocytomas and 26 patients with IDH-mutant 1p19q oligodendrogliomas. The median follow-up was 13.1 years (95% CI 11.4–17.7). A total of 56 progression-free survival events were available at the time of analysis. The median age was 32.2 years, higher in IDH-mutant 1p19q oligodendrogliomas (39.5 years) compared to IDH-mutant astrocytomas (31.4 years; p = 0.003). Residual tumor [hazard ratio (HR) 2.63, 95% CI −1.23 to 5.58, p = 0.007], post-surgical diameter product (HR 1.11, 95% CI 1–1.22, p = 0.03), and midline crossing (HR 6.79, 95% CI 1.5–30.4, p = 0.005) were the only factors directly influencing progression-free survival in univariate analyses. No variables confirmed their predictive role in multivariate models. At the time of data analysis, we registered 22 overall survival (OS) events. In a multivariate Cox regression model, histo-molecular diagnosis (oligodendroglioma vs. astrocytoma; HR 0.28, 95% CI 0.10–0.8, p = 0.02) and initial tumor area assessed as continuous variables (HR 1.82, 95% CI 1.01–3.3, p = 0.05) independently affected the survival of patients (p = 0.01).
Conclusions: In our series, the presence and dimension of residual tumors and midline crossing were the only independent variables predicting progression-free survival after primary surgery in grade 2 diffuse glioma.
1 Introduction
Among primary brain tumors, adult-type diffuse glioma isocitrate dehydrogenase (IDH) mutant is a group of slow-growing, rare neoplasms (incidence of one to two cases per 100,000/year), with highly variable clinical outcomes reflecting histological and molecular heterogeneity. Over the past decade, the unequivocal impact of molecular profiling in the definition of histological tumor types has led to more accurate diagnosis and prognostic evaluation, thus raising clinical questions about appropriate management. The 2021 WHO classification of central nervous system tumors integrated IDH 1/2 mutation status as a mandatory disease-defining marker for adult diffuse gliomas (1).
IDH mutation is inversely associated with tumor grade, with the highest prevalence (~80%–85%) in adult-type diffuse glioma IDH mutant. In addition, 1p19q codeletion is a defining feature of oligodendroglioma (IDH mutant, 1p19q codeleted) compared to the astrocytoma subtype (IDH mutant, 1p19q non-codeleted) (1, 2).
In addition to clinical characteristics, both molecular features and morphological grading are associated with disease behavior. In particular, adult-type diffuse gliomas IDH mutant are characterized by indolence and favorable survival outcomes (median overall survival 10–18 years) (1–4). Nevertheless, the majority of these tumors will recur over time. A balanced consideration of the benefits expected from treatment in terms of progression-free and overall survival is necessary, considering young median age at diagnosis (30–40 years), patient long-life expectancy, and long-term side effects of adjuvant therapies. Current guidelines recommend either post-surgical surveillance or radiotherapy with a sequential PCV (procarbazine, CCNU, lomustine) regimen, calibrated on the patient's individual “risk” (5). In this context, two distinct prognostic indexes are currently being employed in clinical practice: the European Organization for Research and Treatment of Cancer (EORTC) and the Radiation Therapy Oncology Group (RTOG) criteria. Molecular typing is not incorporated within either of the two scoring systems, primarily because, at the time they were developed, the WHO classification scheme did not contemplate the integration of molecular typing with histologic diagnosis.
Whether the EORTC and RTOG risk scores can be replicated in the era of molecularly defined IDH-mutant grade 2 gliomas remains a subject of considerable debate (5–11). To date, retrospective studies have explored several prognostic indicators in adult-type diffuse glioma IDH-mutant cohorts, with inconsistent results and limitations due to heterogeneity in patient selection, diagnostic criteria adopted, and the extent of post-surgical treatment (7, 12–22). In this context, the recent results of the phase 3 practice-changing INDIGO trial provide evidence for the favorable impact of IDH1/2 inhibitor vorasidenib on progression-free survival in untreated adult-type diffuse glioma IDH-mutant patients (23). The optimal subset of patients and the appropriate timing for this new kind of treatment are matters of debate. This is because the phase 3 trial allowed randomization between 1 and 5 years after surgery. In contrast, Food and Drug Administration (FDA) approval was granted without any recommendation regarding the time from surgical treatment. Given the paradigm shift in disease profiling and the future impact of targeted therapy, it is essential to achieve a more precise definition of clinical and molecular features that affect prognosis and eventually identify tumors with different propensities to progress. With this aim, we explored prognostic factors in a cohort of adult-type diffuse glioma IDH mutant on active surveillance after primary surgery to characterize their natural history and to identify those features that could represent a rational basis for risk stratification and personalized treatment forms.
2 Patients and methods
2.1 Patients
This is a retrospective cohort study evaluating clinical and molecular variables influencing the clinical outcomes of patients with adult-type diffuse glioma IDH mutant on active surveillance after primary surgery. The primary endpoint of interest was progression-free survival (PFS), defined as the time between primary surgery and first evidence of tumor progression. The secondary endpoint of interest was overall survival (OS), defined as the time between primary surgery and death due to disease progression.
We selected patients assessed in our institution (Nervous System Medical Oncology Department, IRCCS Istituto Scienze Neurologiche di Bologna, Italy) between January 2000 and December 2021.
We selected only patients i) with a diagnosis of IDH-mutant astrocytoma (IDH-mutated/1p19q non-codeleted) and grade 2 oligodendroglioma IDH-mutated 1p19q codeleted according to the WHO 2021 classification scheme (4), ii) without evidence of contrast enhancement on the first magnetic resonance imaging (MRI), and iii) who underwent active surveillance after primary surgery. We excluded patients with known CDKN2A/B deletion, as well as those who were able to undergo oncological treatment following primary surgery, with an Eastern Cooperative Oncology Group (ECOG) score ranging from 0 to 1.
The diagnosis of diffuse astrocytoma and oligodendroglioma was confirmed after next-generation sequencing (NGS) molecular assessment and reviewed by an expert neuropathologist. Homozygous deletion of CDKN2A is defined as the simultaneous absence of signals related to the target genes in the presence of signals marking the reference chromosome in at least 30% of lesion cells.
MRIs were reviewed by an expert neuro-radiologist, who recorded the pre-surgical and post-surgical tumor/residue dimensions. MRI examination was performed, including perfusion-weighted imaging (PWI), diffusion-weighted imaging (DWI), T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence, and post-contrast sequences. The extent of resection (EOR) was defined through quantitative assessment of maximal cross-sectional T2-weighted FLAIR diameters to determine the size of non-contrast-enhancing lesions. The immediate postoperative MRI scan was performed using advanced MRI techniques, within 48 hours of surgery, to evaluate EOR. Patients with gross total/complete resection were those without measurable disease, defined as lesions with clearly defined margins by MRI scan, with both perpendicular diameters on a single slice of at least 10 mm. Disease assessment was determined by the investigators according to low-grade glioma Response Assessment in Neuro-Oncology (RANO) criteria (24).
Patients were also assessed according to the prognostic risk proposed and validated by the RTOG (25) and the EORTC (26). Variables included in the RTOG and EORTC risk scores are age > 40, regardless of EOR, and age < 40 with incomplete resection for the RTOG risk score and at least three of the following: age ≥40, tumor diameter ≥6 cm, tumor crossing the midline, astrocytoma histology, and preoperative neurological deficit for the EORTC risk score.
The study was approved by the Ethical Committee of Azienda Sanitaria Locale di Bologna (protocol number CE09113, Bologna, Italy). All information regarding the human material was managed using anonymous numerical codes, and all samples were handled in compliance with the Declaration of Helsinki.
2.2 Molecular analysis
Molecular typing was performed on formalin-fixed and paraffin-embedded (FFPE) samples. Fluorescence in situ hybridization (FISH) was used to analyze 1p and 19q chromosomal regions. FISH was performed on 4-μm-thick sections from the most representative paraffin-embedded blocks using standard sets of 1p and 19q locus-specific identifier probes (1p36.32/1q25.2 Vysis and 19p13.2/19q13.33 Vysis) (Abbot Laboratories, Chicago, IL, USA) and following established protocols (27), as previously described (28). In cases with equivocal FISH results, 1p19q codeletion was confirmed using a single-nucleotide polymorphism (SNP) NGS panel covering the entire chromosomes 1 and 19 (28). NGS was used to identify IDH1 and IDH2 mutations. DNA for NGS was extracted after the manual dissection of tumor material under microscopic guidance from 10-μm-thick FFPE sections obtained from the same paraffin block used for FISH analysis, as previously described (28), using a laboratory-developed solid tumor multi-gene panel that includes exon 4 of both IDH1 and IDH2, where mutational hotspots are located (29). Briefly, NGS was performed using the Gene Studio S5 sequencer (Thermo Fisher Scientific), according to the manufacturer’s instructions. For amplicon library preparation, the AmpliSeq Plus LibraryKit 2.0 (Thermo Fisher Scientific) was used, starting from approximately 50 to 100 ng of input DNA. Templates were prepared using an Ion Chef Machine and sequenced using an Ion 530 chip. Sequences were analyzed with the Ion Reporter tool (Thermo Fisher Scientific). Only nucleotide variations detected in both strands and at least 5% of the total number of reads analyzed were considered for the mutational call (29). The same DNA used for NGS was also utilized to evaluate methylated–DNA–protein–cysteine methyltransferase (MGMT) promoter methylation. MGMT promoter methylation status was analyzed by pyrosequencing following established protocols (30), with a methylation cut-off at 10%. Our molecular biology laboratory employed available kits to assess the methylation status of five CpG islands located within the promoter region of exon 1 of the MGMT gene. The methylation percentage reported corresponds to the average methylation level across the analyzed CpG sites.
2.3 Statistical methods
Continuous variables were reported as mean with standard deviation, median, and range. Quantitative variables were reported as frequencies and percentages. Comparison between quantitative variables was performed using the chi-square test, while the t-test or Wilcoxon test was employed for the comparison of quantitative variables with a normal or skewed distribution.
Time to event outcomes (PFS and OS) were estimated using the Kaplan–Meier method, and the log-rank test was adopted to compare survival within different subgroups.
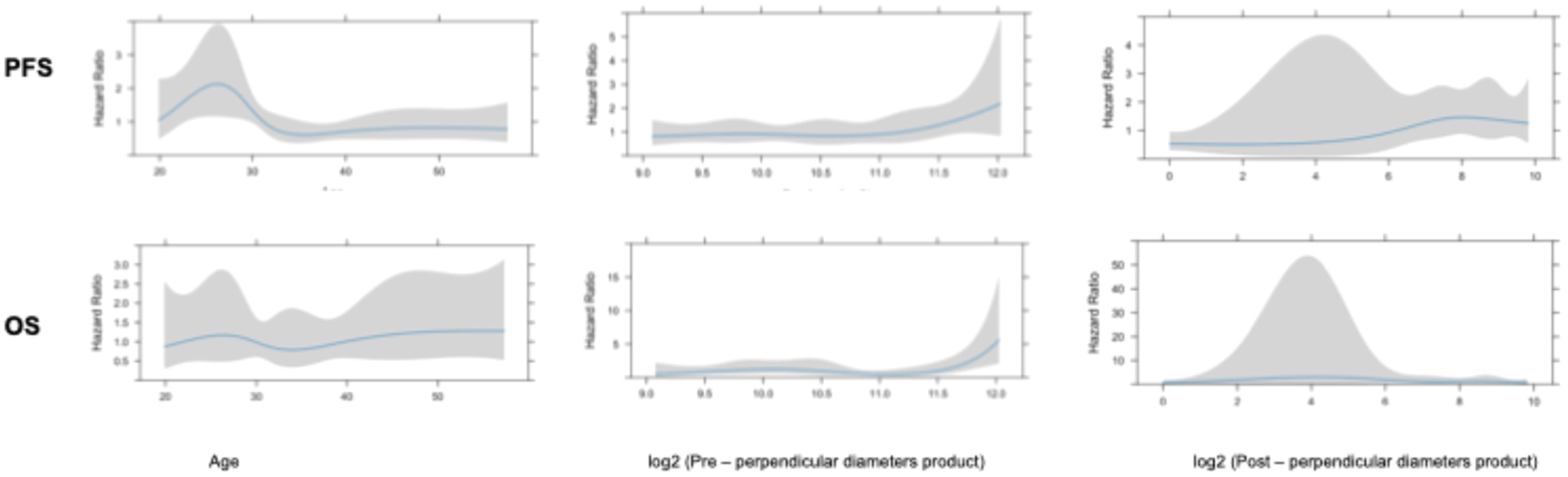
The impact of continuous variables on PFS and OS was further investigated using the restricted cubic spline method. Of note, as previously reported, some variables of interest, such as post-surgical residual area and initial volume area, present a skewed distribution (21, 22). To switch them to a normal distribution, base 2 logarithmic conversion was performed, as previously described (21). Multivariate analysis was performed employing a Cox regression model. The proportional hazards assumption was verified with the use of Schoenfeld residuals.
The Bayesian information criterion (BIC) and the Akaike information criterion (AIC) were used to evaluate the EORTC and RTOG predictive score (31–33). BIC and AIC are two tools that estimate predictive model performance and are based (in part) on the likelihood function. The lower the BIC and AIC detected, the better the predictive value of the model of interest. All analyses were performed using the R software version 4.3.1.
3 Results
3.1 Patient selection
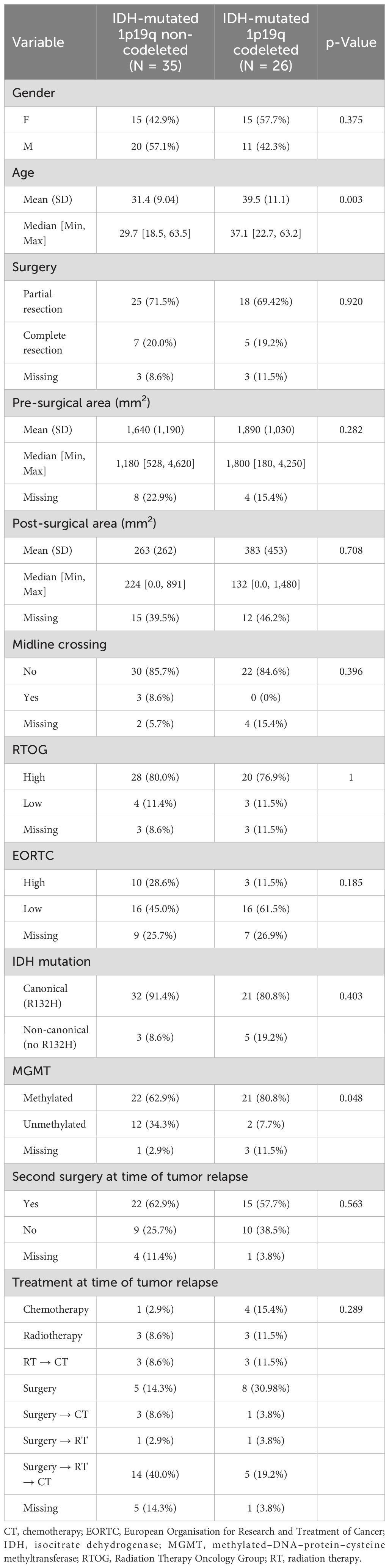
Overall, we evaluated 61 patients according to inclusion/exclusion criteria (Table 1). This cohort consisted of 35 patients (57.4%) with IDH-mutant astrocytoma and 26 (42.6%) oligodendrogliomas IDH mutant 1p19q codeleted. The median age was 32.2 years. Patients with oligodendrogliomas IDH mutant 1p19q codeleted were significantly older (39.5 years) compared to those with IDH-mutant astrocytoma (31.4 years; p = 0.003). MGMT promoter methylation was more frequent in oligodendrogliomas IDH mutant 1p19q codeleted (80.8%) as compared to IDH-mutant astrocytomas (62.9%; p = 0.048). There were no significant differences between the distribution of the other clinical and molecular variables between the two subgroups (Table 1). Notably, as regards baseline disease extension, three patients with astrocytoma diagnosis had tumors crossing the midline.
At the time of data analysis, the median follow-up was 13.1 years (95% CI 11.4–17.7).
3.2 Progression-free survival
Complete data on progression-free survival were available for 56 patients. In five patients, we were unable to obtain information about the time of progression; we thus decided to remove these patients from the PFS analyses but included them in the OS assessment. Median PFS was 3.96 years (95% CI 3.22–4.48). All patients with complete data of PFS (n = 56) experienced progression at the time of analysis.
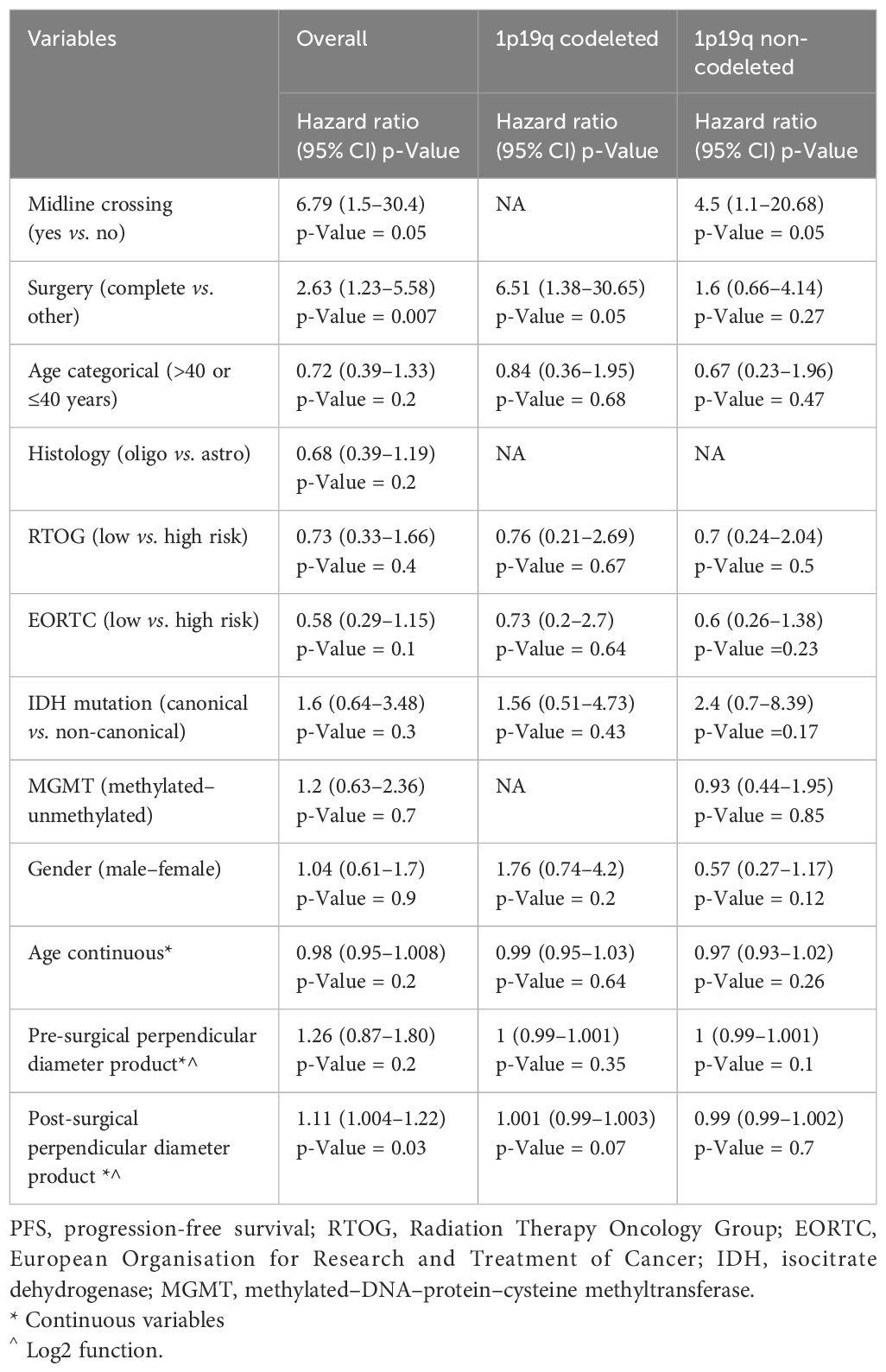
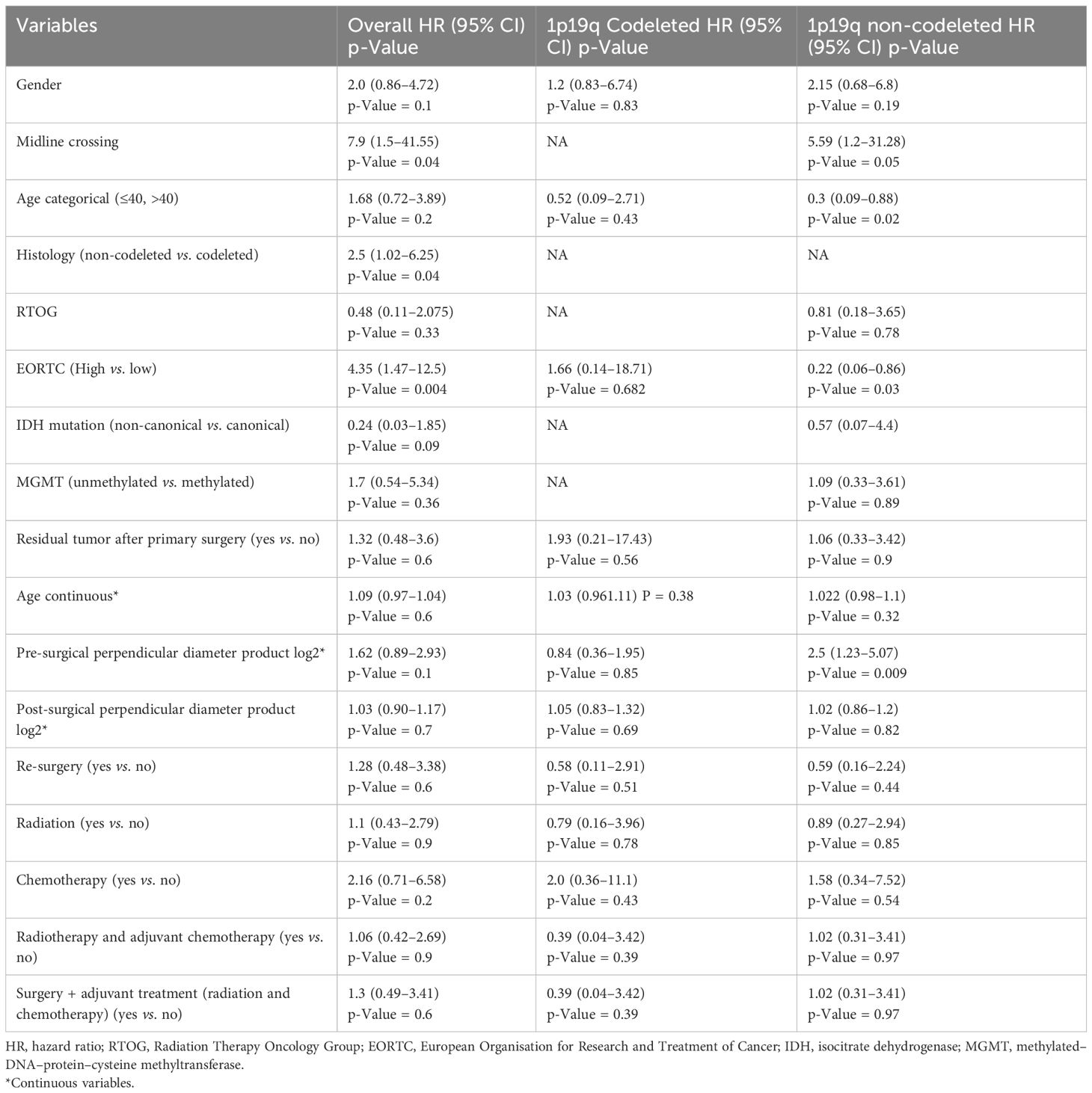
Univariate analysis showed that midline crossing [hazard ratio (HR) 6.65, 95% CI 1.5–29.6, p = 0.004], presence of residual tumor (HR 2.63, 95% CI 1.23–5.58, p = 0.007), and post-surgical perpendicular diameter product (HR 1.11, 95% CI 1.004–1.22, p = 0.03) were the only factors affecting PFS (Table 2, Figure 1). Restricted cubic splines for continuous variables are reported in Figure 2. We used the restricted cubic splines in regression analysis to model non-linear relationships between a continuous predictor variable and an outcome. We investigated the impact of the following continuous variables: age (years), pre-surgical area (with log2 conversion), and residual area (with log2 conversion) on PFS. Pre-surgical area and post-surgical area larger than 2,050 and 100 mm2 appeared associated with a shorter PFS (Figure 2); however, none of these continuous variables were associated with a statistically significant impact on progression-free survival.
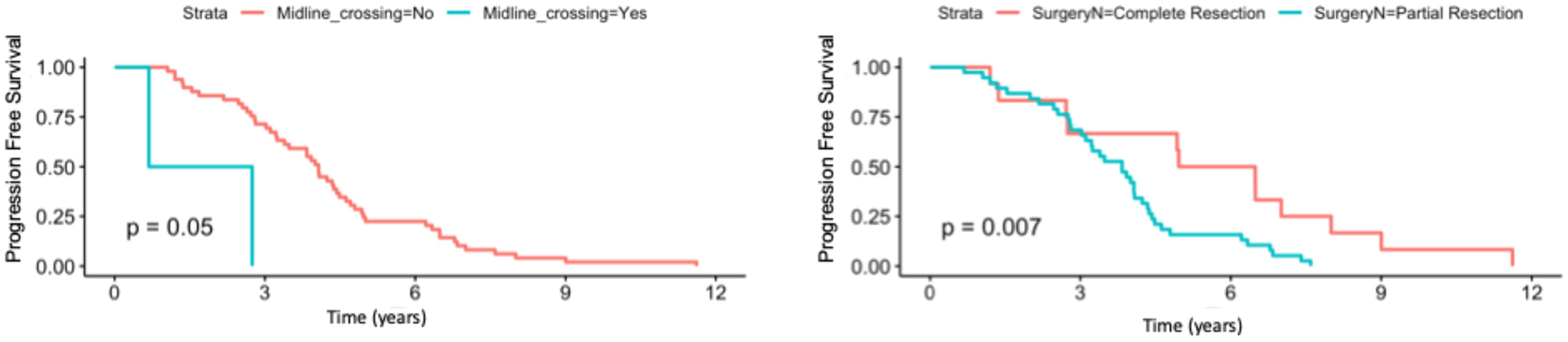
Figure 1. Progression-free survival according to the presence/absence of residual tumor and according to presence/absence of midline crossing.
When the same univariate analyses were carried out according to histology, the prognostic role of surgery was confirmed in IDH-mutant 1p19q codeleted tumors (p = 0.05) but not in 1p19q non-codeleted gliomas (0.27) (Table 2). The dimension of residual tumors seemed to affect the progression-free survival of IDH-mutant 1p19q codeleted tumors more than 1p19q non-codeleted tumors (Table 2).
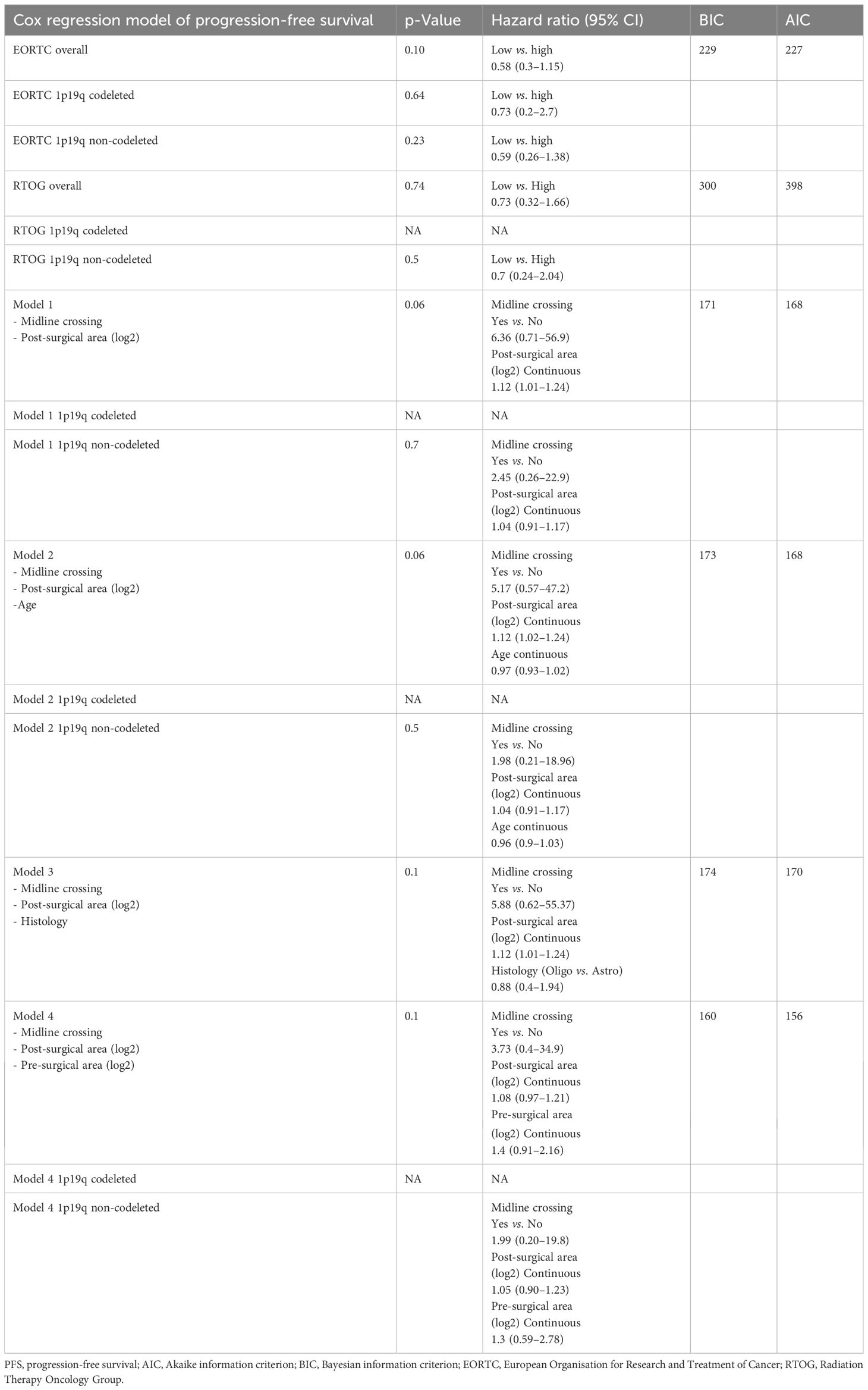
In the Cox regression model for PFS, the EORTC (HR 0.58, 95% CI 0.3–1.15) and RTOG (HR 0.73, 95% CI 0.32–1.66) risk scores did not significantly impact PFS (Table 3).
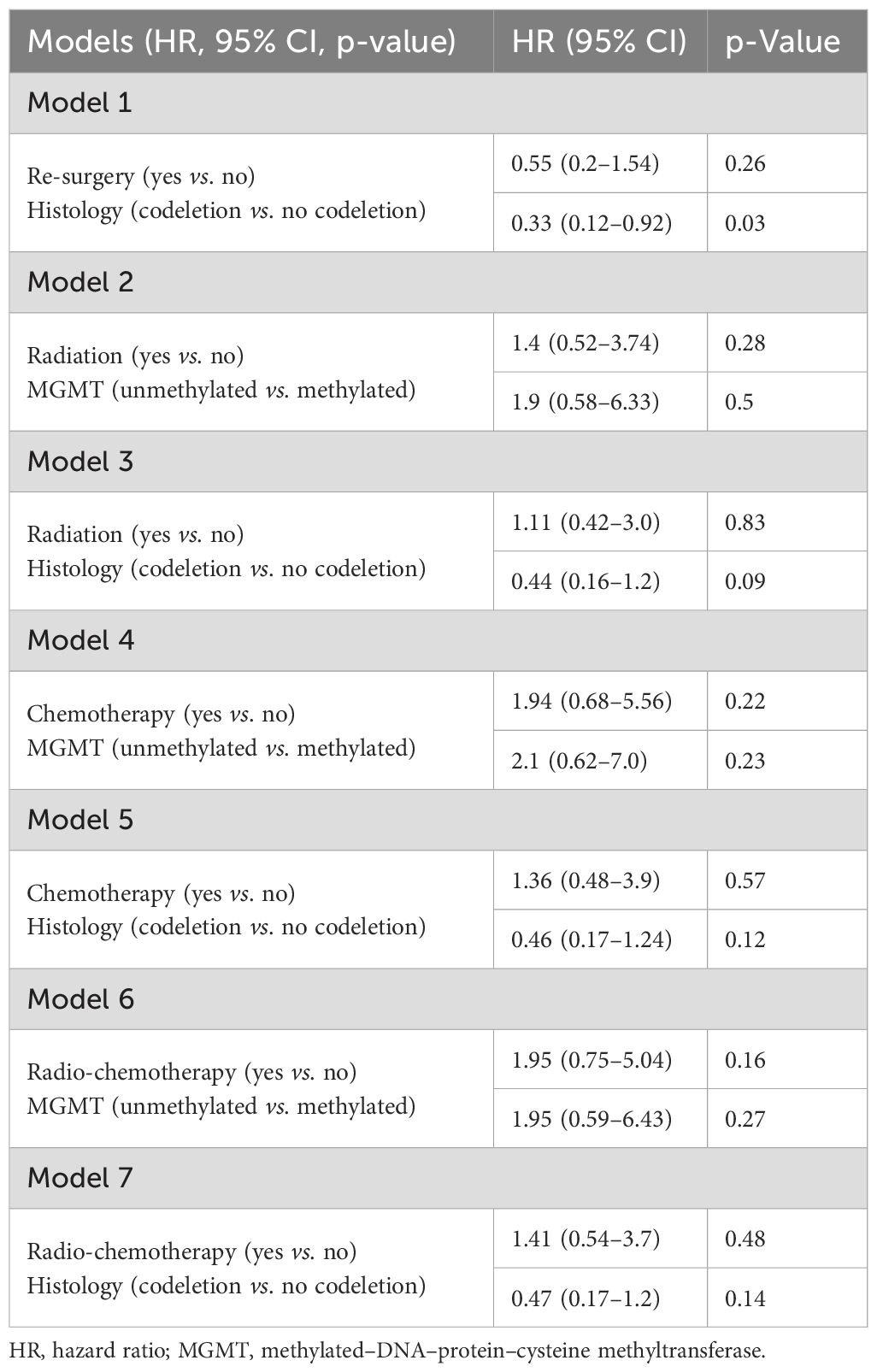
When we consider midline crossing and post-surgical area (log2 converted and reported as a continuous variable) in a Cox regression model, post-surgical area confirmed its prognostic role (HR 1.12, 95% CI 1.01–1.24) on PFS, while midline crossing did not (HR 6.36, 95% CI 0.71–56.9; model 1, Table 3). Even when corrected with age and histology, residual area (log2 converted and reported as a continuous variable) was the only variable associated with PFS (HR 1.12, 95% CI 1.02–1.24 in model 2; HR 1.12, 95% CI 1.01–1.24 in model 3; reported in Table 3). Finally, in a model reporting both pre- and post-surgical areas (log2 converted and reported as a continuous variable) and midline crossing, none of these variables were confirmed to directly impact PFS (model 4, Table 3).
We also performed two additional regression models employing age at diagnosis, pre-/post-surgical area log2 converted (or pre-/post-surgical longer diameter), and histology (Supplementary Table 1).
3.3 Overall survival
At the time of analysis, 39 (63.94%) of 61 patients were alive.
The treatment delivered at the time of progression was surgery alone (n = 13), surgery followed by chemotherapy (n = 4), sequential radio-chemotherapy (n = 19), and radiotherapy (n = 2). Five patients received chemotherapy alone, six patients received radiotherapy alone, and six patients received sequential radio-chemotherapy. The years of diagnosis/study enrolment for each patient in the cohort is reported in the Supplementary Table 2).
In univariate analyses, the presence of midline crossing (HR 7.9, 95% CI 1.5–41.55, p = 0.04), astrocytoma histology (HR 2.5, 95% CI 1.02–6.25, p = 0.04), and high-risk EORTC score (HR 4.35, 95% 1.47–12.5, p = 0.04) significantly correlated with shorter survival (Table 4 and Supplementary Figure 1). The EORTC score confirmed its prognostic value, showing a lower BIC/AIC score compared to the RTOG criteria.
When repeated for both IDH-mutant 1p19q codeleted and IDH-mutant 1p19q non-codeleted tumors, we observed that age, pre-surgical perpendicular diameter product, and EORTC risk score impacted the survival of IDH-mutant 1p19q non-codeleted tumors, while these same variables did not affect the survival of IDH-mutant 1p19q codeleted tumors (Table 4).
In a multivariate Cox regression model, we considered initial tumor area (with logarithmic conversion) and histology as variables of interest. In the composed model, both histo-molecular diagnosis (oligodendrogliomas IDH-mutant 1p19q codeleted vs. IDH-mutant astrocytoma, HR 0.28, 95% CI 0.10–0.8, p = 0.02) and initial tumor area assessed as continuous variables after logarithmic conversion (HR 1.82, 95% CI 1.01–3.3, p = 0.05) independently affected patient survival (p = 0.01). On the contrary, a composed model including histo-molecular diagnosis and post-surgical diameter product did not demonstrate a statistically significant impact on OS. An additional OS model considering histology, treatment received, second surgery, and pre-/post-surgical area (log2 converted) or pre-/post-surgical longer diameter is reported in Table 5.
4 Discussion
Prognostic stratification is crucial for the appropriate clinical management of patients with adult-type diffuse glioma IDH mutant. This study investigated the prognostic hallmarks of adult-type diffuse glioma IDH mutant grade 2 through a correlative analysis of clinical, radiological, histological, and molecular features. The study population was selected according to the following key characteristics: 1) patients underwent an active surveillance approach after initial surgery and 2) included subtypes that met the diagnostic histological and molecular criteria of the WHO classification published in 2021. Improving prognostic tools for low-grade gliomas is a critical need in neuro-oncology to allow the selection of patients more likely to benefit from targeted therapy. Furthermore, the recently updated central nervous system (CNS) tumor classification requires the re-evaluation of clinical prognostic scores for grade 2 IDH-mutant glioma (EORTC and RTOG scores), established in the pre-molecular era (8, 10).
In a large series of WHO 2021-defined adult-type diffuse glioma IDH mutant, Harvey-Jumper et al. identified three risk groups for PFS: all IDH-mutant 1p19q codeleted oligodendrogliomas were associated with lower risk, regardless of the extent of surgery (34). Recently, long-term results from the observation arm of the RTOG 9802 (patients with age <40 years and complete resection) confirmed different survival outcomes for IDH-mutant astrocytomas compared to IDH-mutant 1p19q oligodendrogliomas (median PFS 2.8 years vs. 8.3 years, p < 0.001) in a cohort of untreated patients. Compared to this cohort, our study population had less favorable postoperative characteristics, explaining the different median PFS outcomes (11, 35, 36).
The prognostic impact of residual tumor after resection has been addressed by several retrospective studies, and maximal safe resection remains the cornerstone of current practice in adult-type diffuse glioma IDH mutant. In our cohort, the choice of partial resections for most patients was due to tumor location and extension in eloquent areas to avoid permanent functional damage after surgery. In our study, residual tumor after resection had a significant impact on PFS and included the major extent of residual tumor area as a continuous variable in univariate analyses.
While some literature data report a benefit of larger resections in all IDH-mutant gliomas, other retrospective series have reported a benefit only in the IDH-mutant astrocytoma subgroup (21, 37–39). Unequivocal conclusions have long been challenged by the marked heterogeneity in study populations, particularly in patient cohort characteristics (e.g., histological grading, diagnostic assessment, and treatment methodologies). Heterogeneity in study methods (survival endpoints) and in methods to assess tumor extent (tumor area and tumor volume) should also be considered. Following the WHO reclassification of gliomas in 2016, several retrospective studies have attempted to confirm a rationale for a greater extent of resection across molecularly defined subtypes. In a retrospective series of gliomas diagnosed according to the 2016 WHO classification by Wijnenga et al., the authors found that postoperative volume was associated with OS, with a strong detrimental effect of even small tumor remnants only in IDH-mutant astrocytomas, but not in IDH-mutant 1p19q codeleted oligodendrogliomas (22). In another recent large retrospective study, the extent of resection did not affect OS outcomes among IDH-mutant 1p19q codeleted oligodendroglioma patients with non-enhancing disease (18). The hypothesis was that residual tumor would have less impact on outcome in oligodendroglioma due to greater sensitivity to radiotherapy and chemotherapy.
Overall, studies including adult-type diffuse glioma IDH mutants after the 2021 WHO reclassification have suggested that multiple factors interact to shape disease risk. Determining the weight of each of these factors and integrating them into the decision algorithm is the current challenge.
In our series, residual tumor affected PFS but did not appear to affect OS, although we observed a limited number of events for OS analyses. In a recent study, Van der Vaart et al. reported integrated molecular diagnosis of IDH-mutant astrocytoma versus IDH-mutant 1p19q codeleted oligodendrogliomas, with pre- and postoperative tumor volume as independent prognostic factors for survival. Consistent with previous series, the impact of postoperative tumor volume on survival was greater in the IDH-mutant astrocytoma subgroup than in the IDH-mutant 1p19q codeleted oligodendroglioma subgroup. In contrast to our study, all patients with adult-type diffuse glioma IDH mutant were included, regardless of grade. However, pre- and postoperative tumor dimensions had a greater impact on survival than tumor grade or the presence of enhancement (21).
In our study, the immaturity of the OS data made it difficult to draw further conclusions about survival determinants. However, in univariate OS analyses, the EORTC “high-risk” category significantly correlated with shorter survival. We speculate that this may support the greater impact of baseline tumor dimensions.
According to recent literature, it would be possible that tumor dimension may reflect a higher likelihood of developing a more aggressive neoplastic clone through increasing acquisition of genetic alterations (40).
Limiting these observations were the relatively small number of OS events and the inability to obtain a volume estimate of the tumoral mass. In addition, multivariate models employing more than three variables did not allow us to confirm the proportional axiom.
Another limitation of the study was the lack of data regarding the patients' performance status prior to surgery. The patients selected were assessed at our center after primary surgery and were able to undergo oncological treatment, presenting an ECOG score of 0 or 1. The decision not to administer adjuvant therapies after surgery was due to delays in post-surgical evaluation or for historical reasons, e.g., differences in therapeutic approaches based on the year of diagnosis.
Consistent with our hypothesis of the greater impact of baseline tumor dimension, the validation of risk factors from the EORTC prognostic index in a cohort of adult-type diffuse glioma IDH-mutant patients showed that superior PFS and OS in the “low-risk” group were primarily due to the influence of histology and baseline tumor size (26). Similarly, in a cohort of untreated, molecularly characterized adult-type diffuse glioma IDH-mutant patients, tumor diameter >6 cm and midline crossing were identified as independent prognostic factors for PFS among the items included in the EORTC score, highlighting the major impact of tumor size on risk assessment (41).
In a cohort of patients reclassified according to the 2021 WHO, preoperative tumor size was an independent predictor of survival in both the IDH-mutant astrocytoma and IDH-mutant 1p19q codeleted oligodendroglioma subgroups (42). Tom et al. investigated risk factors for tumor progression in patients with adult-type diffuse glioma IDH mutant who underwent a watch-and-wait approach after gross tumor resection: increasing age, larger initial tumor size, and IDH-mutant/1p19q non-codeleted cases demonstrated a detrimental impact on PFS (43). In contrast, age had no effect on survival in our series, which may be explained by the large proportion of elderly patients among IDH-mutant 1p19q mutant oligodendroglioma patients in our cohort. Overall, our data are consistent with previous studies confirming a very limited effect of age and, in particular, of the 40-year cut-off on survival (42).
We expect that future studies will clarify whether tumor extent has a biological significance and, eventually, which biological changes occur in tumors with higher disease burden (44, 45).
A prognostic multidimensional approach is likely to be essential to predict the outcome of adult-type diffuse glioma IDH mutant at an individual level in order to select appropriate management. In a recent integrated summary of recommendations according to WHO 2021, Kotecha et al. proposed the identification of three different risk categories (low, intermediate, and high). In the proposed algorithm, the low-risk group was identified as candidates for a “watch and wait” approach. In this context, long-term follow-up data from the RTOG 9802 observational arm, published in 2022, encouraged a better characterization of patients with extremely favorable prognosis, as almost one-third of patients had no disease progression at 15 years (46). Consistent with data from the INDIGO trial, even patients with residual tumor in the absence of contrast enhancement and clinical risk factors (e.g., functional deficits, uncontrolled seizures, and need for steroid therapy) could benefit from vorasidenib (46, 47).
Although our results are similar to previously published data, our study addresses some important issues: integrated diagnosis according to WHO 2021 is essential to define adult-type diffuse glioma IDH-mutant subgroups and to explore key characteristics of each subtype; also, since patients with grade 2 glioma IDH mutant have a median survival of >10 years, our extended follow-up allows reliable data interpretation. Further follow-up and expansion of the dataset will be important for definitive conclusions.
The main limitation of our study is its retrospective nature; however, ethical and practical issues do not allow a prospective study to enroll patients for a watchful waiting approach without consideration of established risk criteria. In our opinion, retrospective series with detailed clinical and molecular data and long-term follow-up are the best available research strategy. Another important limitation is the inability to assess the presence/absence of cyclin-dependent kinases (CDKN2A/B) in most of the patients in the cohort. To mitigate this limitation, an expert neuropathologist confirmed that all pathological specimens had a molecularly confirmed IDH1 or 2 mutation and low-grade histo-pathological features. However, we did not include in our case series any patient with a known homozygous CDKN2A/2B deletion. According to the previous series, the possibility of finding a CDKN2A/B homozygous deletion is less than 10% in IDH-mutant gliomas (45). In addition, the positive trend in terms of PFS and OS of the whole cohort correlates with tumors without CDKN2A/B deletion.
Another limitation of the study is the inability to include growth rate as a variable in our analysis. Growth rate reflects tumor aggressiveness and may correlate with underlying molecular features. Unfortunately, in our study, imaging data and software did not allow for consistent, quantitative measurement of tumor growth. In addition, data on seizure control during the clinical course were not available for most of the patients.
In the future, we expect that the integration of comprehensive clinical profiles and molecular characterization will allow for the accuracy of data interpretation. Moreover, multicenter collaborations would enable the collection of larger and more diverse patient cohorts, thereby increasing the statistical power and generalizability of findings. Such collaborative efforts could also harmonize methodological approaches, reduce biases, and facilitate the validation of biomarkers and prognostic factors.
5 Conclusions
Novel prognostic models should be explored to obtain a better estimation of prognosis and disease-free survival in the molecular era. The presence or absence of 1p19q codeletion influences the survival of these patients.
Our study enforces the impact of clinical parameters on estimating prognosis and raises questions about biological correlates for clinically high-risk patients. In our series of adult-type diffuse gliomas IDH mutant under active surveillance after primary surgery, midline crossing, presence of residual tumor, and extent of residual tumor affected progression-free survival.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was approved by the Ethical Committee of Azienda Sanitaria Locale di Bologna (protocol number CE09113, Bologna, Italy). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because This study was a retrospective analyses.
Author contributions
MA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. VD: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LG: Writing – original draft, Writing – review & editing. AT: Writing – original draft, Writing – review & editing. DB: Writing – original draft, Writing – review & editing. VR: Writing – original draft, Writing – review & editing. AC: Writing – original draft, Writing – review & editing. MoM: Writing – original draft, Writing – review & editing. SlB: Writing – original draft, Writing – review & editing. CA: Writing – original draft, Writing – review & editing. EZ: Writing – original draft, Writing – review & editing. SfB: Writing – original draft, Writing – review & editing. MaM: Writing – original draft, Writing – review & editing. FG: Writing – original draft, Writing – review & editing. GT: Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing. EF: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor AP declared a past co-authorship with the author EF.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1673285/full#supplementary-material
Supplementary Figure 1 | Kaplan Maier curves for overall survival according to (A) presence absence of midline crossing, (B) Histology, and (C) EORTC risk subgroup.
Supplementary Table 1 | Multivariate analyses for progression free survival.
Supplementary Table 2 | Years of diagnosis/study enrolment of patients in study cohort.
References
1. Watanabe T, Nobusawa S, Kleihues P, and Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. (2009) 174:1149–53. doi: 10.2353/ajpath.2009.080958
2. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. (2022) 24(5):v1–v95. doi: 10.1093/neuonc/noac202
3. Mair MJ, Geurts M, van den Bent MJ, and Berghoff AS. A basic review on systemic treatment options in WHO grade II-III gliomas. Cancer Treat Rev. (2021) 92:102124. doi: 10.1016/j.ctrv.2020.102124
4. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
5. Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. (2021) 18:170–86. doi: 10.1038/s41571-020-00447-z
6. Shaw EG, Berkey B, Coons SW, Bullard D, Brachman D, Buckner JC, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg. (2008) 109:835–41. doi: 10.3171/JNS/2008/109/11/0835
7. Ramirez C, Bowman C, Maurage CA, Dubois F, Blond S, Porchet N, et al. Loss of 1p, 19q, and 10q heterozygosity prospectively predicts prognosis of oligodendroglial tumors–towards individualized tumor treatment? Neuro Oncol. (2010) 12:490–9. doi: 10.1093/neuonc/nop071
8. Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. (2002) 20:2076–84. doi: 10.1200/JCO.2002.08.121
9. Franceschi E, Mura A, Lamberti G, De Biase D, Tosoni A, Di Battista M, et al. Concordance between RTOG and EORTC prognostic criteria in low-grade gliomas. Future Oncol. (2019) 15:2595–601. doi: 10.2217/fon-2019-0093
10. Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. (2016) 374:1344–55. doi: 10.1056/NEJMoa1500925
11. Bell EH, Zhang P, Shaw EG, Buckner JC, Barger GR, Bullard DE, et al. Comprehensive genomic analysis in NRG oncology/RTOG 9802: A phase III trial of radiation versus radiation plus procarbazine, lomustine (CCNU), and vincristine in high-risk low-grade glioma. J Clin Oncol. (2020) 38:3407–17. doi: 10.1200/JCO.19.02983
12. Aoki K, Nakamura H, Suzuki H, Matsuo K, Kataoka K, Shimamura T, et al. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. (2018) 20:66–77. doi: 10.1093/neuonc/nox132
13. Carstam L, Latini F, Solheim O, Bartek J Jr., Pedersen LK, Zetterling M, et al. Long-term follow up of patients with WHO grade 2 oligodendroglioma. J Neurooncol. (2023) 164:65–74. doi: 10.1007/s11060-023-04368-6
14. Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. (2015) 372:2499–508. doi: 10.1056/NEJMoa1407279
15. Gorlia T, Wu W, Wang M, Baumert BG, Mehta M, Buckner JC, et al. New validated prognostic models and prognostic calculators in patients with low-grade gliomas diagnosed by central pathology review: a pooled analysis of EORTC/RTOG/NCCTG phase III clinical trials. Neuro Oncol. (2013) 15:1568–79. doi: 10.1093/neuonc/not117
16. Katzendobler S, Niedermeyer S, Blobner J, Trumm C, Harter PN, von Baumgarten L, et al. Determinants of long-term survival in patients with IDH-mutant gliomas. J Neurooncol. (2024) 170:655–64. doi: 10.1007/s11060-024-04826-9
17. Minniti G, Paolini S, Antonelli M, Gianno F, Tini P, Lanzetta G, et al. Long-term treatment outcomes of temozolomide-based chemoradiation in patients with adult-type diffuse IDH-mutant grade 2 astrocytoma. J Neurooncol. (2023) 164:331–9. doi: 10.1007/s11060-023-04418-z
18. Park YW, Kim S, Han K, Ahn SS, Moon JH, Kim EH, et al. Rethinking extent of resection of contrast-enhancing and non-enhancing tumor: different survival impacts on adult-type diffuse gliomas in 2021 World Health Organization classification. Eur Radiol. (2024) 34:1376–87. doi: 10.1007/s00330-023-10125-0
19. Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. (2015) 129:867–73. doi: 10.1007/s00401-015-1438-8
20. Tran S, Thomas A, Aliouat I, Karachi C, Lozano F, Mokhtari K, et al. A threshold for mitotic activity and post-surgical residual volume defines distinct prognostic groups for astrocytoma IDH-mutant. Neuropathol Appl Neurobiol. (2023) 49:e12928. doi: 10.1111/nan.12928
21. van der Vaart T, Wijnenga MMJ, van Garderen K, Dubbink HJ, French PJ, Smits M, et al. Differences in the prognostic role of age, extent of resection, and tumor grade between astrocytoma IDHmt and oligodendroglioma: A single-center cohort study. Clin Cancer Res. (2024) 30:3837–44. doi: 10.1158/1078-0432.CCR-24-0901
22. Wijnenga MMJ, French PJ, Dubbink HJ, Dinjens WNM, Atmodimedjo PN, Kros JM, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol. (2018) 20:103–12. doi: 10.1093/neuonc/nox176
23. Mellinghoff IK, van den Bent MJ, Blumenthal DT, Touat M, Peters KB, Clarke J, et al. Vorasidenib in IDH1- or IDH2-mutant low-grade glioma. N Engl J Med. (2023) 389:589–601. doi: 10.1056/NEJMoa2304194
24. Wen PY, van den Bent M, Youssef G, Cloughesy TF, Ellingson BM, Weller M, et al. RANO 2.0: update to the response assessment in neuro-oncology criteria for high- and low-grade gliomas in adults. J Clin Oncol. (2023) 41:5187–99. doi: 10.1200/JCO.23.01059
25. Shaw EG, Wang M, Coons SW, Brachman DG, Buckner JC, Stelzer KJ, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. (2012) 30:3065–70. doi: 10.1200/JCO.2011.35.8598
26. Daniels TB, Brown PD, Felten SJ, Wu W, Buckner JC, Arusell RM, et al. Validation of EORTC prognostic factors for adults with low-grade glioma: a report using intergroup 86-72-51. Int J Radiat Oncol Biol Phys. (2011) 81:218–24. doi: 10.1016/j.ijrobp.2010.05.003
27. Smith JS, Perry A, Borell TJ, Lee HK, O'Fallon J, Hosek SM, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. (2000) 18:636–45. doi: 10.1200/JCO.2000.18.3.636
28. de Biase D, Acquaviva G, Visani M, Marucci G, De Leo A, Maloberti T, et al. Next-generation sequencing panel for 1p/19q codeletion and IDH1-IDH2 mutational analysis uncovers mistaken overdiagnoses of 1p/19q codeletion by FISH. J Mol Diagn. (2021) 23:1185–94. doi: 10.1016/j.jmoldx.2021.06.004
29. de Biase D, Acquaviva G, Visani M, Sanza V, Argento CM, De Leo A, et al. Molecular diagnostic of solid tumor using a next generation sequencing custom-designed multi-gene panel. Diagnostics (Basel). (2020) 10. doi: 10.3390/diagnostics10040250
30. Philteos J, Karmur BS, and Mansouri A. MGMT testing in glioblastomas: pitfalls and opportunities. Am J Clin Oncol. (2019) 42:117–22. doi: 10.1097/COC.0000000000000490
31. Dziak JJ, Coffman DL, Lanza ST, Li R, and Jermiin LS. Sensitivity and specificity of information criteria. Brief Bioinform. (2020) 21:553–65. doi: 10.1093/bib/bbz016
32. Selig K, Shaw P, and Ankerst D. Bayesian information criterion approximations to Bayes factors for univariate and multivariate logistic regression models. Int J Biostat. (2020) 17:241–66. doi: 10.1515/ijb-2020-0045
33. Volinsky CT and Raftery AE. Bayesian information criterion for censored survival models. Biometrics. (2000) 56:256–62. doi: 10.1111/j.0006-341x.2000.00256.x
34. Hervey-Jumper SL, Zhang Y, Phillips JJ, Morshed RA, Young JS, McCoy L, et al. Interactive effects of molecular, therapeutic, and patient factors on outcome of diffuse low-grade glioma. J Clin Oncol. (2023) 41:2029–42. doi: 10.1200/JCO.21.02929
35. Fleming JL. Clinical endpoints correlation with molecular features in the NRG oncology/RTOG 9802 low-grade glioma, low-risk observation arm. Int J Radiat Oncology Biology,Physics. (2024) 120:S214.
36. Huang RY, Young RJ, Ellingson BM, Veeraraghavan H, Wang W, Tixier F, et al. Volumetric analysis of IDH-mutant lower-grade glioma: a natural history study of tumor growth rates before and after treatment. Neuro Oncol. (2020) 22:1822–30. doi: 10.1093/neuonc/noaa105
37. Alattar AA, Brandel MG, Hirshman BR, Dong X, Carroll KT, Ali MA, et al. Oligodendroglioma resection: a Surveillance, Epidemiology, and End Results (SEER) analysis. J Neurosurg. (2018) 128:1076–83. doi: 10.3171/2016.11.JNS161974
38. Albuquerque LAF, Almeida JP, de Macêdo Filho LJM, Joaquim AF, and Duffau H. Extent of resection in diffuse low-grade gliomas and the role of tumor molecular signature-a systematic review of the literature. Neurosurg Rev. (2021) 44:1371–89. doi: 10.1007/s10143-020-01362-8
39. Ding X, Wang Z, Chen D, Wang Y, Zhao Z, Sun C, et al. The prognostic value of maximal surgical resection is attenuated in oligodendroglioma subgroups of adult diffuse glioma: a multicenter retrospective study. J Neurooncol. (2018) 140:591–603. doi: 10.1007/s11060-018-2985-3
40. Drucker KL, Jenkins RB, and Schramek D. Switching drivers: epigenetic rewiring to genetic progression in glioma. Cancer Res. (2024). doi: 10.1158/0008-5472.CAN-24-4907
41. Jungk C, Gluszak M, Trong PD, von Deimling A, Herold-Mende C, and Unterberg A. Ncog-25. Revisiting The Pignatti Risk Score In Low-Grade Glioma Patients In The Molecular Era. Neuro-Oncology. (2021) 23:vi157–vi. doi: 10.1093/neuonc/noab196.616
42. Carstam L, Corell A, Smits A, Dénes A, Barchéus H, Modin K, et al. WHO grade loses its prognostic value in molecularly defined diffuse lower-grade gliomas. Front Oncol. (2021) 11:803975. doi: 10.3389/fonc.2021.803975
43. Tom MC, Varra V, Leyrer CM, Park DY, Chao ST, Yu JS, et al. Risk factors for progression among low-grade gliomas after gross total resection and initial observation in the molecular era. Int J Radiat Oncol Biol Phys. (2019) 104:1099–105. doi: 10.1016/j.ijrobp.2019.04.010
44. Ius T, Ng S, Young JS, Tomasino B, Polano M, Ben-Israel D, et al. The benefit of early surgery on overall survival in incidental low-grade glioma patients: A multicenter study. Neuro Oncol. (2022) 24:624–38. doi: 10.1093/neuonc/noab210
45. van den Bent MJ, French PJ, Brat D, Tonn JC, Touat M, Ellingson BM, et al. The biological significance of tumor grade, age, enhancement, and extent of resection in IDH-mutant gliomas: How should they inform treatment decisions in the era of IDH inhibitors? Neuro Oncol. (2024) 26:1805–22. doi: 10.1093/neuonc/noae107
46. Kotecha R, Schiff D, Chakravarti A, Fleming JL, Brown PD, Puduvalli VK, et al. Multidisciplinary management of isocitrate dehydrogenase-mutated gliomas in a contemporary molecularly defined era. J Clin Oncol. (2024) 42:2588–98. doi: 10.1200/JCO.23.02195
Keywords: low grade glioma, prognostic factors, grade 2 oligodendroglioma, grade 2 astrocytoma, progression free survival
Citation: Aprile M, Di Nunno V, Gatto L, Tosoni A, Balestrini D, Ramponi V, Conti A, Maffei M, Battaglia S, Argento CM, Zuliani E, Bartolini S, Margotti M, Gentilini F, Tallini G, Asioli S and Franceschi E (2025) How to predict progression-free survival in patients with grade 2 IDH-mutated diffuse gliomas after surgery: a long-term follow-up analysis. Front. Oncol. 15:1673285. doi: 10.3389/fonc.2025.1673285
Received: 25 July 2025; Accepted: 27 October 2025;
Published: 18 November 2025.
Edited by:
Alessia Pellerino, University Hospital of the City of Health and Science of Turin, ItalyReviewed by:
Francesco Bruno, University and City of Health and Science Hospital, ItalyQazi Zeeshan, University of Pittsburgh Medical Center, United States
Copyright © 2025 Aprile, Di Nunno, Gatto, Tosoni, Balestrini, Ramponi, Conti, Maffei, Battaglia, Argento, Zuliani, Bartolini, Margotti, Gentilini, Tallini, Asioli and Franceschi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincenzo Di Nunno, dmluY2Vuem8uZGludW5ub0Bpc25iLml0
†These authors share first authorship
‡These authors share last authorship
§ORCID: Vincenzo Di Nunno, orcid.org/0000-0003-4441-9834
Alfredo Conti, orcid.org/0000-0002-9408-2662
Monica Maffei, orcid.org/0000-0001-5379-034X
Stella Battaglia, orcid.org/0000-0003-1255-0109
Sofia Asioli, orcid.org/0000-0002-5035-2448
Enrico Franceschi, orcid.org/0000-0001-9332-4677
 Marta Aprile1†
Marta Aprile1† Vincenzo Di Nunno
Vincenzo Di Nunno Lidia Gatto
Lidia Gatto Giovanni Tallini
Giovanni Tallini Sofia Asioli
Sofia Asioli