- 1Department of Clinical Oncology, Queen Mary Hospital, Hong Kong, Hong Kong SAR, China
- 2Department of Clinical Oncology, Li Ka Shing (LKS) Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
Introduction: Two trastuzumab biosimilars have been introduced into the local public healthcare system since April 2021 as alternatives for treatment of human epidermal growth factor receptor (HER2) positive breast cancer. While their oncological efficacy compared to reference trastuzumab (Herceptin®) is well established, local comparative data on cardiotoxicity among the three formulations remain limited. This study evaluates the cardiac safety of Herzuma® and Kanjinti® compared with Herceptin® in a single-center cohort.
Methods: We conducted a retrospective review of 90 patients with HER2-positive early-stage breast cancer treated with adjuvant trastuzumab (Herceptin®, Herzuma®, or Kanjinti®) between January 2020 and January 2025 at Queen Mary Hospital, Hong Kong. Cardiac monitoring was performed via multi-gated acquisition (MUGA) scans every three months. Cardiotoxicity was defined as a ≥10% absolute decline in left ventricular ejection fraction (LVEF), LVEF <50%, or symptomatic heart failure. The primary study endpoint was time to cardiac event.
Results: Cardiac events occurred in 19 patients (Herceptin®:18.6%, Herzuma®: 19.4%, Kanjinti®: 36.4%). No statistically significant differences in time to cardiac event were observed (log-rank p > 0.20). The odds ratio for events was highest in the Kanjinti® group (vs. Herceptin®, OR = 2.50, p = 0.24). Median time to event was shortest in the Kanjinti® group. No significant associations between cardiac events and cardiovascular risk factors identified.
Conclusion: Trastuzumab biosimilars demonstrated comparable short-term cardiac safety to reference trastuzumab. While a higher event rate was observed in the Kanjinti® group, the small sample size limits interpretation. Prospective studies with longer follow-up and larger patient number are warranted to better characterize long-term cardiac outcomes.
1 Introduction
Trastuzumab (Herceptin®) was first approved by the U.S. Food and Drug Administration (FDA) in 1998 for the treatment of human epidermal growth factor receptor (HER2) positive breast cancer (1). While its clinical efficacy is well established through landmark trials (2–4), the high cost of this monoclonal antibody has created challenges to healthcare sustainability, particularly in publicly funded health systems. To improve treatment accessibility while maintaining oncological outcome, trastuzumab biosimilars have been developed as alternatives over past years. Trastuzumab CT-P6 (Herzuma®) (5) and Trastuzumab ABP-980 (Kanjinti®) (6), both FDA-approved in 2018 and 2019, respectively, have demonstrated comparable safety and efficacy to Herceptin® (7, 8). These biosimilars have been available at Queen Mary Hospital, Hong Kong since April 2021, as part of hospital policy to optimize resource allocation within the public healthcare system.
Cardiotoxicity is a well-recognized concern in trastuzumab-based therapy, as declines in left ventricular ejection fraction (LVEF) can lead to treatment modification or discontinuation, potentially compromising oncological outcomes. In the phase III CT-P6 equivalence trial, a ≥10% drop in LVEF was observed in 12.2% of patients receiving the biosimilar CT-P6 and in 11.5% of those receiving reference trastuzumab. Overall 1.8% in the CT-P6 group and 1.1% in the reference group experienced LVEF reduction ≥10% to below 50%, supporting cardiac safety equivalence. Separately, the LILAC trial evaluating ABP 980 reported LVEF decline of ≥10% and to below 50% in 2.8% of patients on the biosimilar versus 3.3% on the reference product, again demonstrated comparable safety. While these trials provide reassuring data, real-world evidence remains limited. Our study aims to evaluate the cardiac safety of trastuzumab biosimilars in a real-world setting by assessing treatment-related LVEF decline, providing practical insights into HER2-positive early breast cancer management, especially in balancing cost and toxicity considerations.
2 Materials and methods
This retrospective study was conducted in the Department of Clinical Oncology at Queen Mary Hospital, utilizing data extracted from the Clinical Management System and Electronic Patient Record system. A total of 90 patients with HER2-positive early-stage breast cancer who received adjuvant chemotherapy with Herceptin®, Kanjinti®, or Herzuma® between January 2020 and January 2025 were included in the analysis. Patient characteristics such as age at trastuzumab initiation, cardiovascular risk factors including smoking history, hypertension, diabetes mellitus, hyperlipidemia, and history of coronary artery disease were retrieved. Tumor laterality, tumor and nodal staging by the 8th American Joint Committee on Cancer (AJCC) staging, were recorded.
Patients were treated with one of the two standard adjuvant chemotherapy regimens. The first regimen consisted of weekly paclitaxel (80 mg/m²) plus trastuzumab (loading dose 4 mg/kg, then 2 mg/kg) for 12 weeks, followed by trastuzumab (6 mg/kg) every three weeks for an additional 13 cycles (TH) (9). The second regimen comprised docetaxel (75 mg/m²) plus carboplatin (AUC = 6) plus trastuzumab (loading dose 8 mg/kg, followed by 6 mg/kg) every three weeks for 6 cycles, followed by trastuzumab every three weeks for 12 additional cycles (TTCH) (10). The brand of trastuzumab received, and exposure to radiotherapy and/or hormonal therapy, were documented.
Cardiac function was monitored using multi-gated acquisition (MUGA) scans at baseline and every three months throughout the treatment course, in accordance with departmental protocol and with reference to both product information (11) and the European Society for Medical Oncology (ESMO) guidelines (12). Collected data included baseline LVEF, intervals between MUGA scans, and changes in LVEF over time. Cardiac events were defined as an absolute LVEF decline of ≥10% from baseline, a reduction in LVEF to <50%, or the development of symptomatic heart failure. This definition was based on the European Society of Cardiology Position Statement (13), ESMO Clinical Practice Guidelines on cardiotoxicity (14) and the National Cancer Research Institute traffic light system for cardiac monitoring (15). These guidelines underpin our departmental protocol and were selected for their clinical relevance and applicability to local practice. Treatment modifications or discontinuations due to cardiac concerns were also documented.
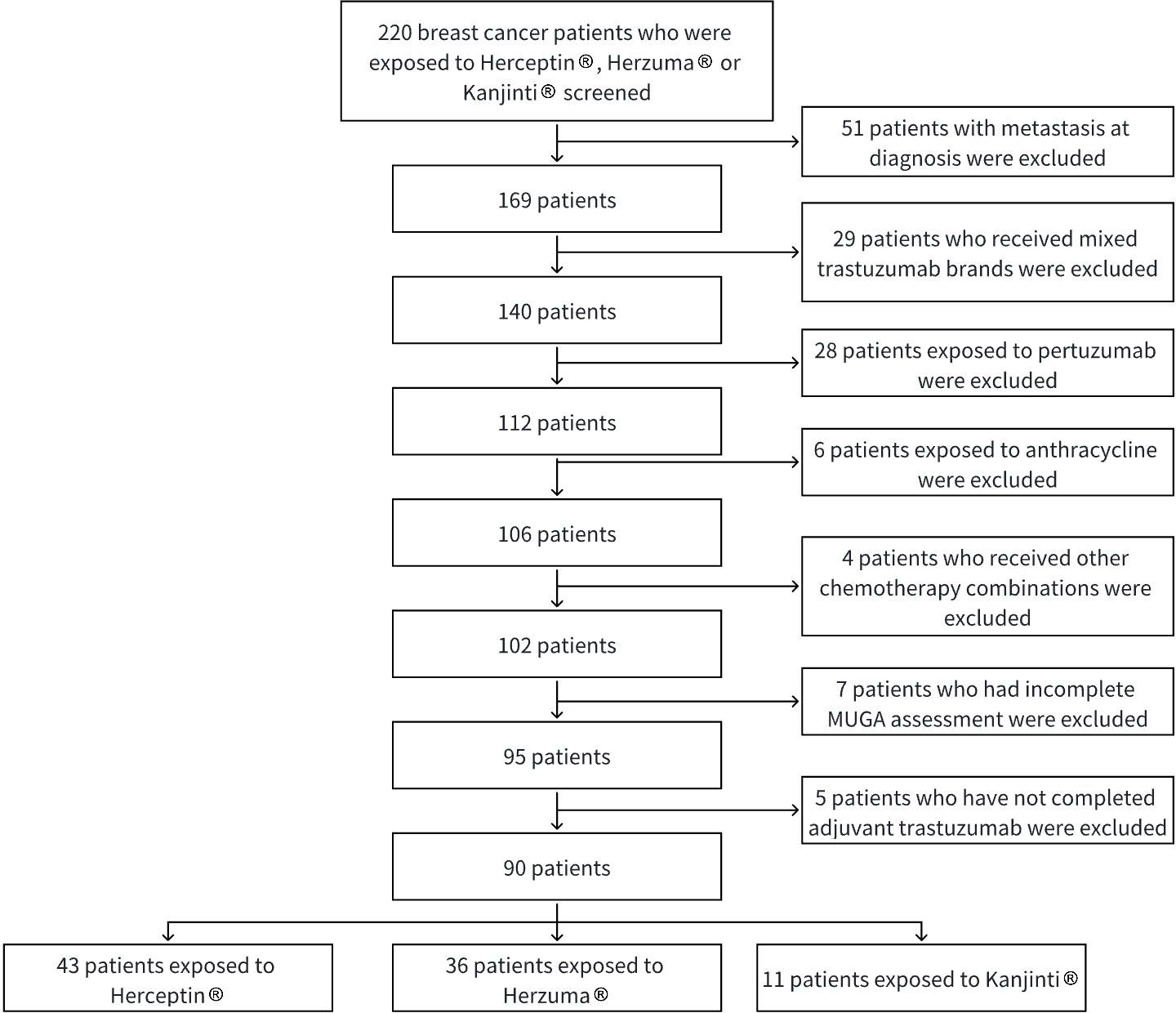
Patients were excluded if they had distant metastases at diagnosis, incomplete clinical follow-up data related to cardiac outcomes, or had received more than one brand of trastuzumab during their treatment course. Those with prior or concurrent use of pertuzumab, prior exposure to anthracyclines, or those who did not complete trastuzumab treatment (except in cases of discontinuation due to a cardiac event) were also excluded. Patients who have not completed adjuvant trastuzumab by study end date were excluded.
Statistical analyses were performed to compare baseline characteristics and assess the association between cardiac events and trastuzumab treatment groups. The association between cardiac events and categorical baseline characteristics were analyzed using Chi-Square tests or Fisher’s Exact test where appropriate; Continuous variables were analyzed using analysis of variance (ANOVA) or Kruskal-Wallis test if data is not normally distributed. The time to cardiac event across treatment groups was analyzed using Cox proportional hazards model, and compared using the log-rank tests. A significance level of α ≤ 0.05 (two-sided) was considered statistically significant. All statistical analyses were performed using SPSS version 29.0 (IBM).
This research was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster, Hong Kong (Ref No.: UW25-169). The requirement for patient consent was waived by the Committee due to the retrospective nature of the research.
The selection of study subjects is illustrated in Figure 1.
3 Results
3.1 Patient characteristics
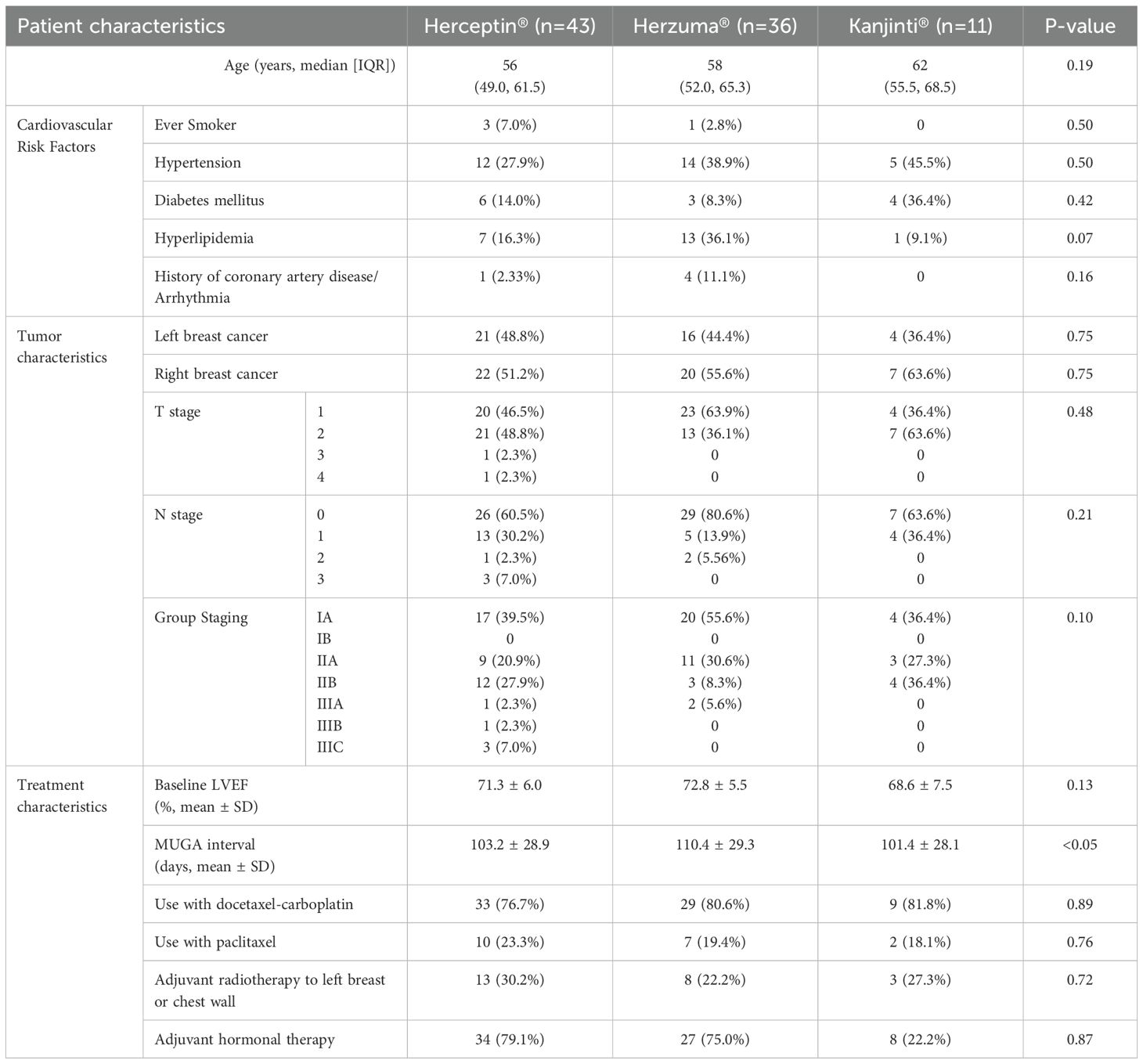
A total of 90 patients with HER2-positive early-stage breast cancer who received adjuvant trastuzumab were included in the analysis. Among them, 43 received Herceptin®, 36 received Herzuma®, and 11 received Kanjinti®. Baseline demographic and clinical characteristics were generally balanced across treatment groups (Table 1).
The median age at trastuzumab initiation was comparable across the three groups (Herceptin®: 56 years, Herzuma®: 58 years, Kanjinti®: 62 years; p = 0.19). Cardiovascular risk factors, including hypertension, diabetes mellitus, hyperlipidaemia, and a history of cardiac disease, showed no significant differences between groups. Baseline LVEF, measured by MUGA scans, was 71.3% in the Herceptin® group, 72.8% in the Herzuma® group, and 68.6% in the Kanjinti® group (p = 0.13). Similarly, tumor characteristics, including laterality and TNM staging (AJCC 8th edition), did not significantly differ among treatment groups (p = 0.10).
The Herzuma® group had the longest mean MUGA monitoring interval at 110.4 ± 29.3 days, while the Kanjinti® group had the shortest at 101.4 ± 28.1 days. A statistically significant difference in monitoring frequency was observed between groups (p <0.05). The shorter interval in the Kanjinti® group is likely attributed to additional cardiac surveillance following an event, in accordance with the Traffic Light System guidelines established by the United Kingdom National Cancer Research Institute (NCRI) for managing trastuzumab-related cardiotoxicity. In the Kanjinti® group, two patients underwent additional MUGA scans following notable LVEF declines: one patient had a repeat scan 35 days after an 18% drop in LVEF, and another had a scan 30 days after LVEF dropped from 58% to 44%. These episodes contributed to the overall shorter scan intervals observed in this group.
3.2 Cardiac events
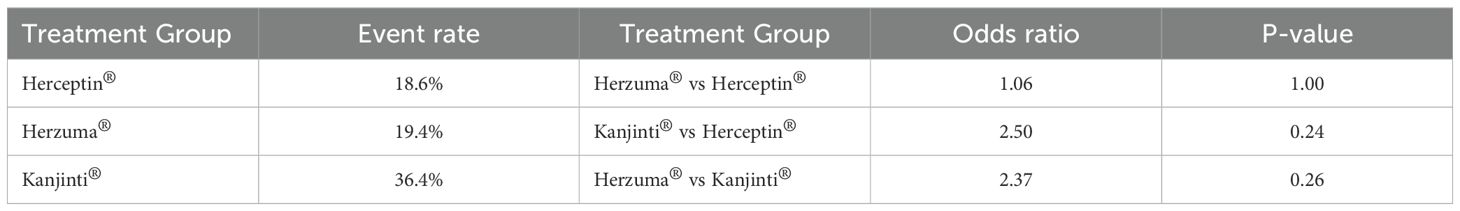
A total of 19 cardiac events were recorded during the study period, with 8 events (18.6%) occurring in the Herceptin® group, 7 (19.4%) in the Herzuma® group, and 4 (36.4%) in the Kanjinti® group. Comparisons of event rates between treatment groups showed that the odds of experiencing a cardiac event were similar between Herzuma® and Herceptin® (OR = 1.06, p = 1.00). While the Kanjinti® group exhibited a numerically higher event rate, statistical analysis did not demonstrate a significant difference when compared to either Herceptin® (OR = 2.50, p = 0.24) or Herzuma® (OR = 2.37, p = 0.26). These findings suggest no statistically significant difference in cardiotoxicity risk among the three trastuzumab formulations, though the small sample size may have limited the power to detect a true difference (Table 2).
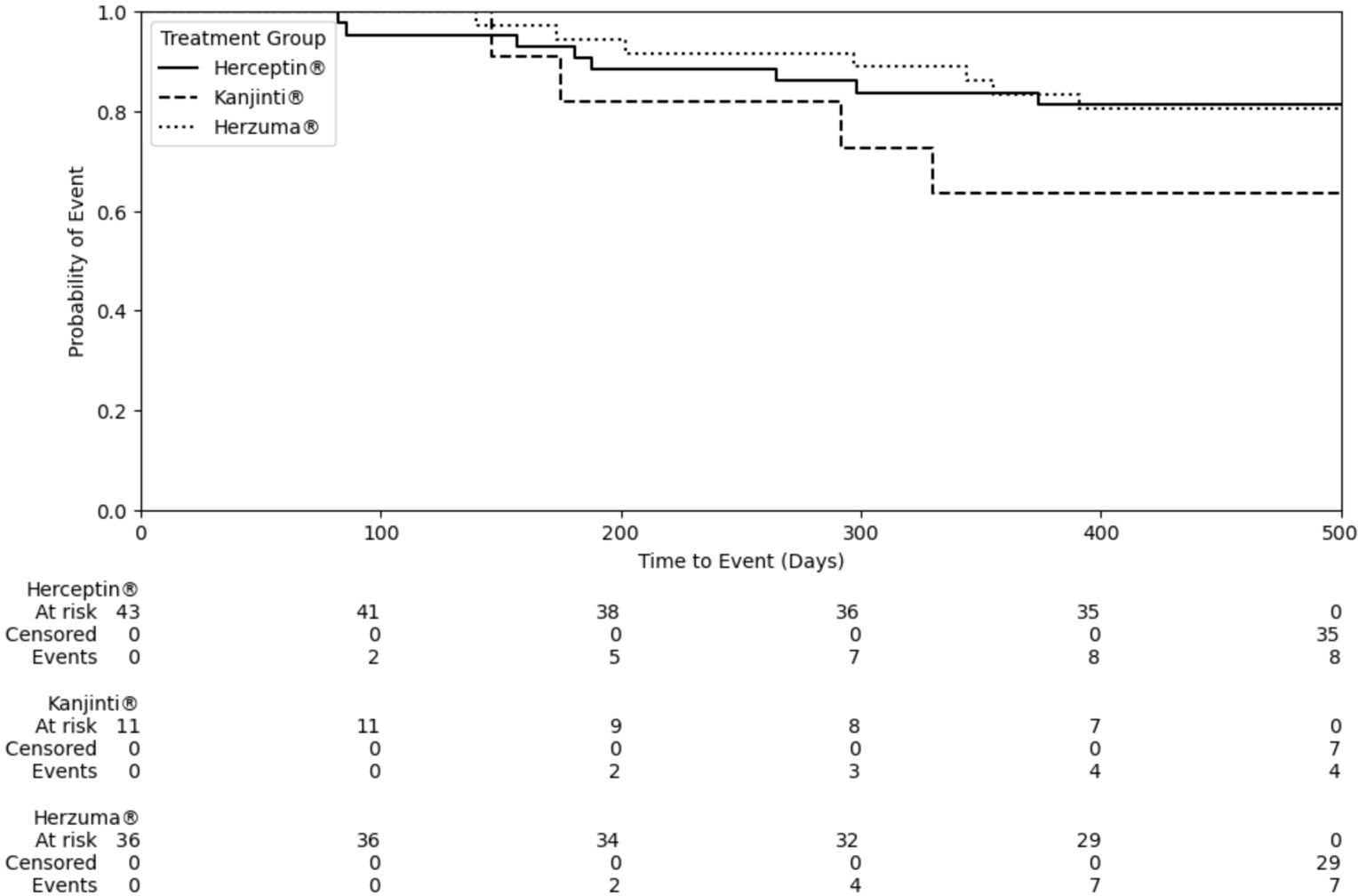
The median time to a cardiac event was 265 days for Herceptin®, 297 days for Herzuma®, and 175 days for Kanjinti®. While no statistical significant difference was observed among the treatment groups (Figure 2; Log-rank test: Herceptin® vs. Herzuma®, p = 0.98; Herceptin® vs. Kanjinti®, p = 0.21; Herzuma® vs. Kanjinti®, p = 0.21), the Kanjinti® group exhibited a numerically shorter time to event. Notably, two patients in the Kanjinti® group required treatment modifications due to cardiotoxicity: one patient temporarily suspended treatment, and another patient permanently discontinued her anticancer therapy after her LVEF dropped below 50%, and developed symptoms of congestive heart failure requiring medical therapy.
Table 3 showed a summary of cardiac events in study population.
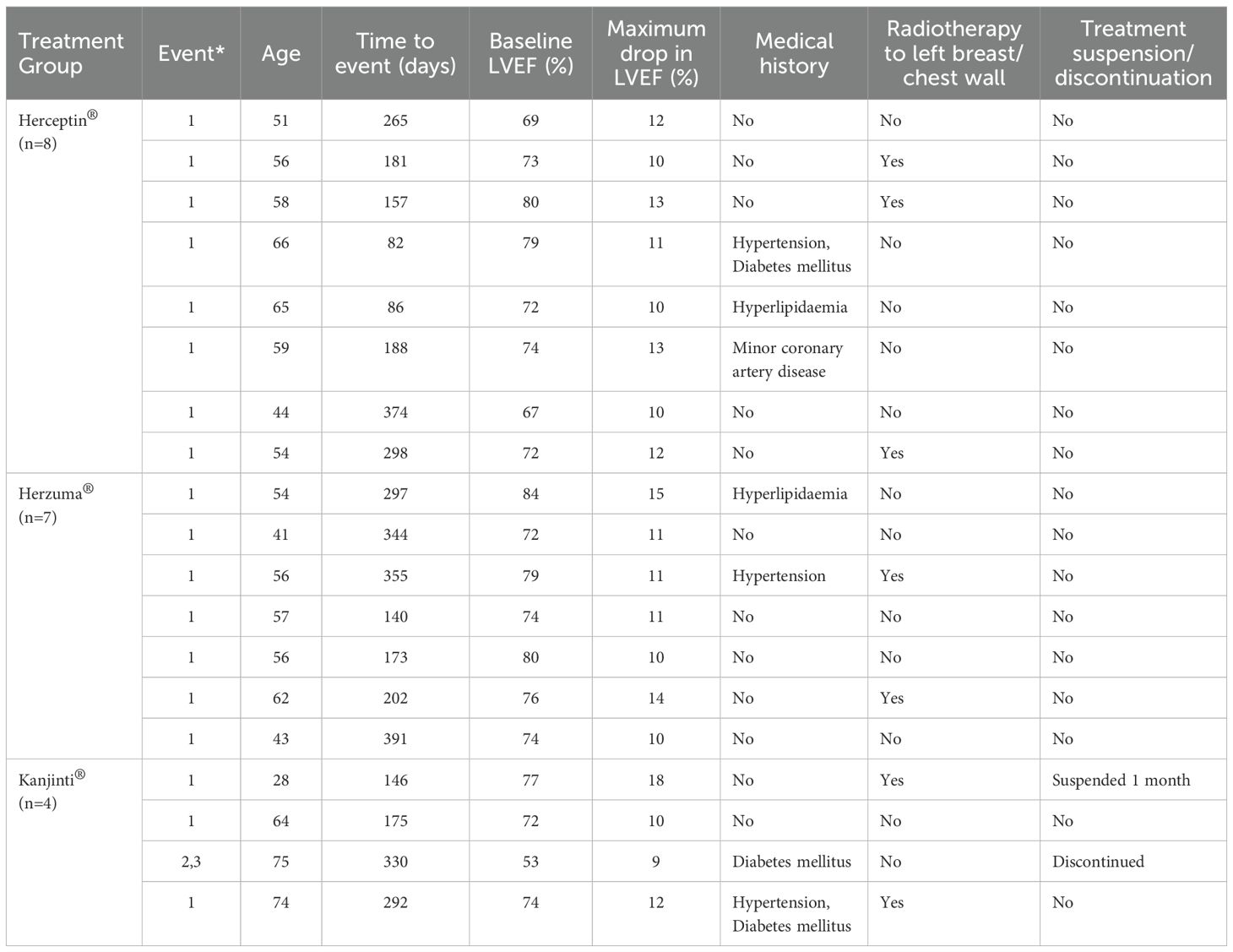
Table 3. Summary of cardiac events (*1: Drop in LVEF >=10%; 2: LVEF <50%; 3: Symptoms of congestive heart failure).
3.3 Correlation between event and patient characteristics
No significant associations were observed between cardiac events and key cardiovascular risk factors, including history of coronary artery disease/arrhythmia (p = 0.62), diabetes mellitus (p = 1.00), hyperlipidaemia (p = 0.24), and prior left-sided breast/chest wall radiotherapy (p = 0.40). Similarly, no significant correlation was found between cardiac events and patient age ≥60 years (p = 0.89), smoking status (p = 1.00), or use of hormonal therapy (p = 0.97). Hypertension was the only characteristic that approached statistical significance (p = 0.10), suggesting a possible trend but not reaching the predefined threshold for significance. Additionally, choice of adjuvant chemotherapy (TTCH vs. TH) was not associated with an increased cardiac risk (p = 1.00). These findings indicate that within the current cohort, traditional cardiovascular risk factors did not appear to independently predict the likelihood of a cardiac event.
4 Discussion
4.1 Comparable cardiac safety of trastuzumab biosimilars in real-world use
HER2-directed therapy has become an integral component of managing HER2-positive breast cancer. A number of approved biosimilars of trastuzumab have become widely available in the past decade, providing a more affordable and comparably effective option to patients who might have financial difficulty of receiving brand-name trastuzumab. However, real-world data on cardiotoxicity of these biosimilars were not widely reported. This retrospective study assessed the cardiac safety of two trastuzumab biosimilars compared to the reference product trastuzumab in the adjuvant treatment of HER2-positive early breast cancer. Among the 90 patients included in this cohort, 19 experienced cardiac events. No statistically significant difference in event rate was found. Our findings are consistent with prior clinical trials and systematic reviews demonstrating that biosimilars exhibit comparable cardiac safety profiles to the reference trastuzumab (16, 17). Although the Kanjinti® group had a numerically higher event rate (36.4%) and two instances of treatment modification or discontinuation, the small sample size of this subgroup (n=11) substantially limits the statistical power to draw firm conclusions. Furthermore, neither univariate nor multivariable analyses identified statistically significant associations between cardiac events and conventional cardiovascular risk factors. These findings suggest that baseline comorbidities alone may not fully account for individual susceptibility to trastuzumab-related cardiotoxicity, or that the study may have been underpowered to detect such relationships due to small sample size.
4.2 Cardiotoxicity surveillance tools: current tools vs emerging strategies
In this study, cardiotoxicity was assessed primarily by evaluating declines in LVEF and symptomatic heart failure, which remain the most widely used monitoring parameters in local oncological practice. These metrics are practical and more accessible, especially in resource-limited public healthcare settings. Compared to landmark clinical trials such as HERA (4) and NSABP B-31 (2), our study observed higher rates of cardiotoxicity, which may be attributed to variability in definitions of cardiac events across different guidelines and countries (18–20). Moreover, other cardiac manifestations such as arrhythmias, myocardial infarction, and myocarditis were not evaluated. The omission of these events, coupled with reliance on LVEF alone, may limit the sensitivity of our analysis in detecting subclinical or early-stage cardiotoxicity.
Emerging evidence suggests that a multimodal approach of integrating circulating biomarkers and advanced imaging techniques, which can enhance the early detection of trastuzumab-related cardiotoxicity. Several studies have demonstrated that biomarkers such as N-terminal pro–B-type natriuretic peptide (NT-proBNP), high-sensitivity troponin, Creatine kinase–MB isoenzyme (CK-MB), and Micro-ribonucleic acids (microRNAs) can identify myocardial injury prior to detectable LVEF reduction. For instance, Pillai et al. showed that microRNA-based panels could detect cardiotoxic changes even in asymptomatic HER2-positive breast cancer patients (21). Sawaya et al. and Díaz-Antón et al. further supported the utility of combining echocardiographic strain imaging with biomarkers to predict cardiac dysfunction with greater accuracy (22, 23). A systematic review by Thavendiranathan et al. reinforced these findings, highlighting the prognostic value of myocardial strain imaging in cardiac oncology (24). While promising, these tools are not routinely implemented in local settings due to cost and limited availability. Future prospective studies should consider incorporating these novel modalities to optimize cardiac monitoring in patients undergoing HER2-targeted therapy.
4.3 Economic evaluations and local implications
Internationally, several economic evaluations have demonstrated the cost-saving potential of trastuzumab biosimilars. For instance, a Taiwanese cost-effectiveness analysis showed that a trastuzumab biosimilar (not explicitly named in the study) combined with docetaxel was a cost-effective alternative to the reference trastuzumab in metastatic HER2-positive breast cancer, reporting an incremental cost-effectiveness ratio of NTD 811,050 (~USD $28,966) per quality-adjusted life year (QALY) gained (25). Similarly, a budget impact analysis across twenty-eight European countries estimated that using the trastuzumab biosimilar CT-P6 for breast and gastric cancers could lead to cumulative healthcare savings of €0.91 to €2.27 billion over a 5-year period (26).
While these findings explored the economic potential of biosimilars, they originate from healthcare systems with very different drug pricing structures, institutional policies, and patient demographics compared to Hong Kong. In our local public healthcare system, the adoption of trastuzumab biosimilars is still relatively new. As a result, data to support comprehensive cost-effectiveness evaluations, including QALYs and direct cost comparisons with the reference product, remain limited. Moving forward, research in the Hong Kong context should aim to assess cost-effectiveness of biosimilars in local population. This would involve incorporating local drug prices, treatment durations, and patient utility measures to better inform policy decisions and procurement strategies.
4.4 Study limitations and generalizability
This study has several important limitations. Its retrospective design introduces inherent selection bias, as treatment group allocation was determined by institutional policies and/or funding availability rather than randomization. As the two biosimilars were newly introduced, the number of patients is relatively small. The small sample size, particularly in the Kanjinti® group, limits the generalizability and robustness of subgroup comparisons. Besides, the study’s follow-up duration was unable to capture the late-onset cardiotoxic effects, which were previously reported (27, 28). In addition, our results may not be generalizable in the modern era of neoadjuvant HER2-directed therapy before definitive surgery and more widespread use of dual HER2-targeted therapy with pertuzumab and trastuzumab in high-risk patients. Furthermore, we were unable to explore if modification or even early permanent discontinuation of treatment regimen will impact on survival as one recent Japanese study revealed that early discontinuation of trastuzumab treatment resulted in an increased mortality rate secondary to early disease relapse (29).
Future research should prioritize prospective study designs with larger, balanced cohorts, multicenter participation and extended follow-up periods to better evaluate the long-term cardiac safety of biosimilars in various clinical settings.
5 Conclusion
This study provides real-world data demonstrating that trastuzumab biosimilars (Herzuma® and Kanjinti®) exhibit comparable short-term cardiac safety to reference trastuzumab (Herceptin®) in local clinical practice. While no significant differences in cardiac event rates were observed, variations in monitoring intensity and treatment modifications highlight the importance of ongoing cardiac surveillance and adherence to standardized monitoring protocols. Future studies should adopt prospective designs with larger, well-balanced cohorts and longer follow-up to clarify long-term cardiotoxicity risks and support evidence-based use of biosimilars.
Author contributions
LA: Data curation, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing, Conceptualization. ML: Methodology, Supervision, Writing – review & editing, Validation. VL: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing, Formal analysis. KY: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Herceptin® is a registered trademark of Genentech Inc. Herzuma® is a registered trademark of Celltrion Inc., used under license by Teva Pharmaceuticals USA, Inc. Kanjinti® is a registered trademark of Amgen Inc.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. FDA. Approval Letter: Herceptin (1998). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/1998/trasgen092598L.pdf (Accessed January 10, 2025).
2. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. (2005) 353:1673–84. doi: 10.1056/NEJMoa052122
3. Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, et al. Trastuzumab plus adjuvant chemotherapy for HER2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. (2014) 32:3744–52. doi: 10.1200/JCO.2014.55.5730
4. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. (2005) 353:1659–72. doi: 10.1056/NEJMoa052306
5. Celltrion Inc. Herzuma (trastuzumab-pkrb) prescribing information . Incheon (KR): Celltrion Inc. (2024) Available from: https://www.herzuma.com/about-herzuma (Accessed January 10, 2025).
6. Amgen Inc. Kanjinti (trastuzumab-abp) prescribing information. Thousand Oaks (CA): Amgen Inc (2024) Available from: https://www.kanjinti.com/ (Accessed January 10, 2025).
7. Stebbing J, Baranau Y, Baryash V, Manikhas A, Moiseyenko V, Dzagnidze G, et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: a phase 3 equivalence trial. Lancet Oncol. (2017) 18:917–28. doi: 10.1016/S1470-2045(17)30434-5
8. von Minckwitz G, Colleoni M, Kolberg HC, Morales S, Santi P, Tomasevic Z, et al. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol. (2018) 19:987–98. doi: 10.1016/S1470-2045(18)30241-9
9. Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. (2015) 372:134–41. doi: 10.1056/NEJMoa1406281
10. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. (2011) 365:1273–83. doi: 10.1056/NEJMoa0910383
11. Genentech Inc. Herceptin (trastuzumab) prescribing information. South San Francisco (CA): Genentech Inc (2024). Available online at: http://gene.com/download/pdf/Herceptin_prescribing.pdf (Accessed January 10, 2025).
12. Bovelli D, Plataniotis G, Roila F, and ESMO Guidelines Working Group. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. (2010) 21:v277–82. doi: 10.1093/annonc/mdq200
13. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity. Eur Heart J. (2016) 37:2768–801. doi: 10.1093/eurheartj/ehw211
14. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. (2020) 31:171–90. doi: 10.1016/j.annonc.2019.10.023
15. Jones AL, Barlow M, Barrett-Lee PJ, Canney PA, Gilmour IM, Robb SD, et al. Management of cardiac health in trastuzumab-treated patients: updated UK NCRI recommendations. Br J Cancer. (2009) 100:684–92. doi: 10.1038/sj.bjc.6604909
16. Triantafyllidi E and Triantafillidis JK. Systematic review on the use of biosimilars of trastuzumab in HER2+ breast cancer. Biomedicines. (2022) 10:2045. doi: 10.3390/biomedicines10082045
17. Bae SJ, Kim JH, Ahn SG, Jeung HC, Sohn J, Kim GM, et al. Real-world clinical outcomes of biosimilar trastuzumab (CT-P6) in HER2-positive early-stage and metastatic breast cancer. Front Oncol. (2021) 11:689587. doi: 10.3389/fonc.2021.689587
18. Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. (2022) 43:280–99. doi: 10.1093/eurheartj/ehab674
19. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy. J Am Soc Echocardiogr. (2014) 27:911–39. doi: 10.1016/j.echo.2014.07.012
20. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
21. Pillai SS, Pereira DG, Bonsu G, Chaudhry H, Puri N, Lakhani HV, et al. Biomarker panel for early screening of trastuzumab-induced cardiotoxicity among breast cancer patients in West Virginia. Front Pharmacol. (2022) 13:953178. doi: 10.3389/fphar.2022.953178
22. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. (2012) 5:596–603. doi: 10.1161/CIRCIMAGING.112.973321
23. Díaz-Antón B, Madurga R, Zorita B, Wasniewski S, Moreno-Arciniegas A, López-Melgar B, et al. Early detection of anthracycline- and trastuzumab-induced cardiotoxicity: value and optimal timing of serum biomarkers and echocardiographic parameters. ESC Heart Fail. (2022) 9:1127–37. doi: 10.1002/ehf2.13782
24. Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH, et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. (2014) 63:2751–68. doi: 10.1016/j.jacc.2014.01.073
25. Leung JH, Tai YS, Wang SY, Yip Fion HT, Tsung-Chin H, Chan AL, et al. Cost-effectiveness of trastuzumab biosimilar combination therapy and drug wastage as first-line treatment for HER2-positive metastatic breast cancer. Breast. (2022) 65:91–7. doi: 10.1016/j.breast.2022.07.007
26. Lee SM, Jung JH, Suh D, Lim J, Kim H, Oh DY, et al. Budget impact of switching to biosimilar trastuzumab (CT-P6) for the treatment of breast cancer and gastric cancer in 28 European countries. BioDrugs. (2019) 33:423–36. doi: 10.1007/s40259-019-00359-0
27. Koric A, Chang CP, Mark B, Rowe K, Snyder J, Dodson M, et al. Cardiovascular disease risk in long-term breast cancer survivors: A population-based cohort study. Cancer. (2022) 128:2826–35. doi: 10.1002/cncr.34224
28. Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, and Gammon MD. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. (2016) 27:6–13. doi: 10.1097/EDE.0000000000000394
Keywords: trastuzumab, biosimilar, adjuvant, breast cancer, cardiac function, LVEF (left ventricular ejection fraction)
Citation: Au LSJ, Luk MY, Lee VHF and Yuen KK (2025) Comparative study of the impact of adjuvant trastuzumab and its biosimilars on cardiac function in HER2-positive early breast cancer patients: a single-center study. Front. Oncol. 15:1673372. doi: 10.3389/fonc.2025.1673372
Received: 25 July 2025; Accepted: 29 September 2025;
Published: 22 October 2025.
Edited by:
Agnieszka Jagiełło-Gruszfeld, Maria Sklodowska-Curie National Research Institute of Oncology, PolandReviewed by:
Sneha Pillai, Marshall University, United StatesJane Aboulenein, Mansoura University, Egypt
Copyright © 2025 Au, Luk, Lee and Yuen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lok Sze Joyce Au, YWxzNzE0QGhhLm9yZy5oaw==
 Lok Sze Joyce Au
Lok Sze Joyce Au Mai Yee Luk
Mai Yee Luk Victor Ho Fun Lee
Victor Ho Fun Lee Kwok Keung Yuen1
Kwok Keung Yuen1