- 1Department of Cardiothoracic Surgery, Shenzhen Hospital, Southern Medical University, Shenzhen, China
- 2Department of Thoracic Surgery, The Seventh Affiliated Hospital of Sun Yat-Sen University, Shenzhen, China
Castleman’s disease (CD) is a rare, benign lymphoproliferative disorder of unknown aetiology. CD occurring in the oesophageal region is exceedingly rare and may be misdiagnosed as oesophageal carcinoma or lymphoma, thus posing challenges for subsequent treatment selection. A 54-year-old male with a one-month history of chest pain was admitted to our hospital. Barium oesophagography and contrast-enhanced computed tomography (CT) revealed stenosis in the lower oesophagus, accompanied by wall thickening at the gastroesophageal junction. Positron emission tomography-computed tomography (PET-CT) revealed increased glucose metabolism in the oesophageal region and lymph nodes, which was suspicious for malignancy. However, a gastroscopic biopsy revealed only inflammatory granulation tissue without evidence of malignancy. Following partial oesophagectomy with intrathoracic oesophagogastric anastomosis, pathology revealed onion-skin hyperplasia of lymphoid follicles with hyalinized vessels. Combined with immunohistochemistry, these features confirmed hyaline vascular type Castleman’s disease (HV-CD). The patient exhibited good postoperative recovery. We described a rare case of oesophageal unicentric Castleman’s disease (UCD) and highlighted the significant diagnostic challenge in distinguishing oesophageal CD from oesophageal tumours preoperatively. Furthermore, we emphasized the dual significance of complete surgical resection for UCD, achieving both a definitive diagnosis and curative treatment.
Introduction
Castleman’s disease (CD), also known as giant lymph node hyperplasia or angiofollicular lymphoid hyperplasia (1), was first described by Castleman in 1956 (2). It is a rare benign lymphoproliferative disorder of unknown aetiology (3). Clinically, CD can be classified into unicentric type (UCD) and multicentric type (MCD) (4). UCD is typically asymptomatic, whereas MCD has a poorer prognosis and is often associated with systemic symptoms such as fever, night sweats, weight loss, and anaemia. CD commonly involves lymph nodes in the mediastinum, neck, and abdomen (5). Oesophageal involvement is exceedingly rare, with only a few cases reported. Consequently, oesophageal masses are rarely considered to be CD and are often misdiagnosed as lymphoma or other tumours, which may impact subsequent clinical management decisions.
Herein, we describe a complex case of oesophageal UCD. The contradictory findings between endoscopic biopsy and imaging studies, such as positron emission tomography-computed tomography (PET-CT) and contrast-enhanced computed tomography (CT), underscore the significant diagnostic challenge in distinguishing oesophageal CD from oesophageal tumours preoperatively. Furthermore, we highlighted the dual significance of complete surgical resection for UCD, achieving both a definitive diagnosis and curative treatment.
Case presentation
A 54-year-old male presented with chest pain that had persisted for over one month. This patient did not exhibit fever or other inflammatory symptoms. The laboratory reports indicated elevated CD-related inflammatory markers, including: C-reactive protein 8.66 mg/L (normal range 0.00-5.00mg/L); erythrocyte sedimentation rate 24 mm/h (normal range < 15 mg/L); Immunoglobulin G 23.4% (normal range 9.2-18.2%); Interleukin-6 (IL-6) 7.28 pg/ml (normal range 0.00-5.30 pg/ml). Test results for the infections of human herpesvirus 8 (HHV-8) and human immunodeficiency virus (HIV) were all negative. CT revealed wall thickening at the gastroesophageal junction (Figure 1A). Oesophagography revealed stenosis in the lower oesophageal lumen (Figure 1B), whereas PET-CT revealed increased glucose metabolism at the oesophageal lesion site (Figure 1C). No distant metastases were detected in the left paraesophageal, bilateral cervical, bilateral supraclavicular, or axillary lymph node regions. These imaging findings suggested possible malignancy in the distal oesophagus.
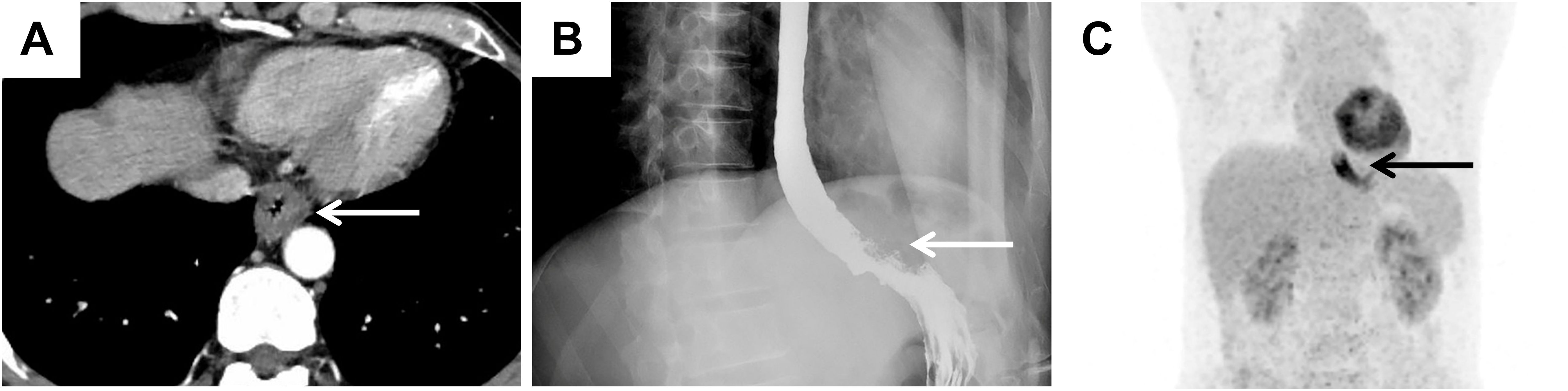
Figure 1. Imaging findings suggested the possibility of malignancy in the oesophagus. (A) CT image suggesting significant thickening of the wall of the lower oesophagus at the gastroesophageal junction (white arrow). (B) The results of oesophagography revealed narrowing of the distal oesophageal lumen (white arrow). (C) PET–CT image showing significant thickening of the wall of the lower thoracic oesophagus with increased glucose metabolism (black arrow).
However, gastroscopic examination and biopsy indicated inflammatory granulation tissue at the lesion site, with no evidence of malignancy (Figures 2A, B). Imaging and biopsy results complicated the preoperative diagnosis. To establish a definitive diagnosis and initiate treatment, the patient underwent partial oesophagectomy with intrathoracic oesophagogastric anastomosis and additional intraoperative biopsy.
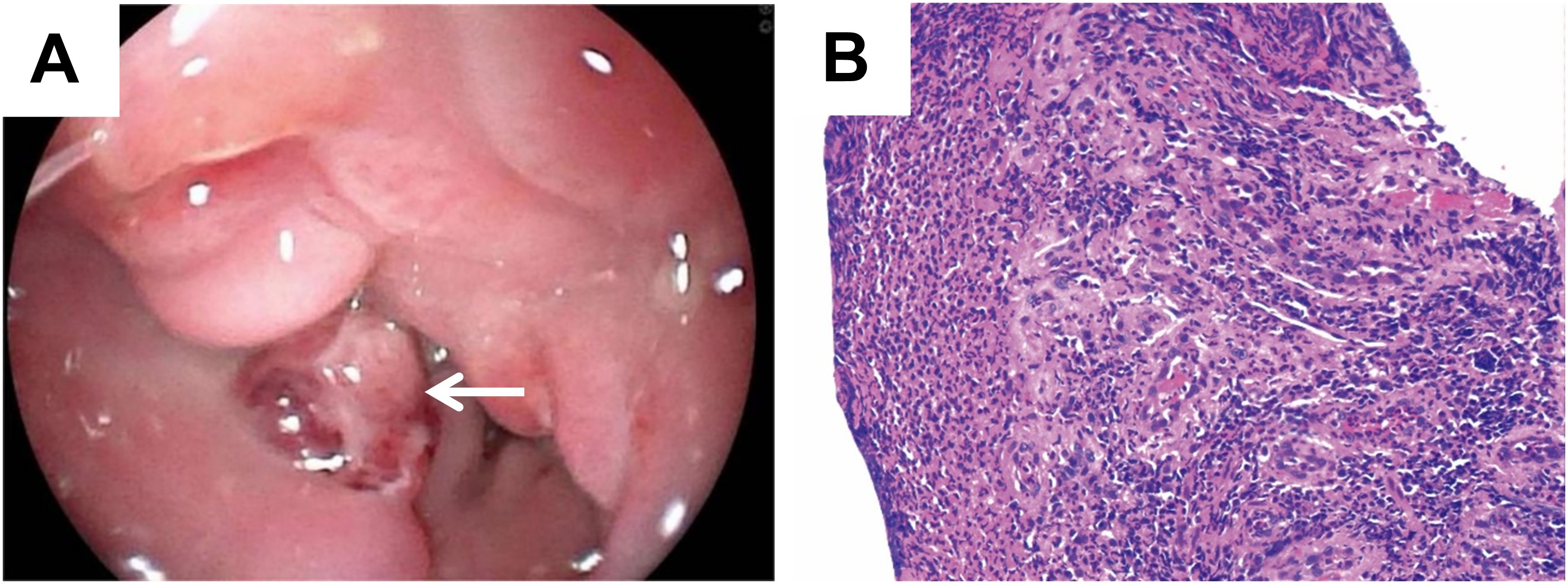
Figure 2. Gastroscopic examination and biopsy showed benign changes. (A) Gastroscopy revealed a protruding mass with luminal narrowing in the lower oesophagus (white arrow). (B) Pathology of gastroscopic biopsy revealed inflammatory lesions (H&E, magnification: ×10).
Microscopic examination of the surgical specimens showed extensive patchy necrosis and exfoliation of the oesophageal epithelium, resulting in tissue defects. The surface was covered with inflammatory necrotic exudate and exhibited granulation tissue formation. Postoperative pathological results revealed increased lymphoid follicles, regressed germinal centres, expanded mantle zones of lymphocytes, and a proliferated interfollicular vasculature, forming an onion-skin configuration, as shown in Figures 3A, B. Immunohistochemical staining further demonstrated positive expression of CD20 and CD79a in B cells, whereas CD3 was positively expressed in T cells. Additionally, CD21 (Figure 3C) and CD23 were positively expressed in follicular dendritic cells. Ki-67 expression was significantly elevated, exceeding 20%. Based on these findings, the patient was diagnosed with hyaline vascular type Castleman’s disease (HV-CD). The postoperative course was uneventful; the patient recovered well and was discharged. A one-year follow-up examination revealed no signs of recurrence. We conducted long-term follow-up examinations to monitor the patient’s subsequent recovery and recurrence status.
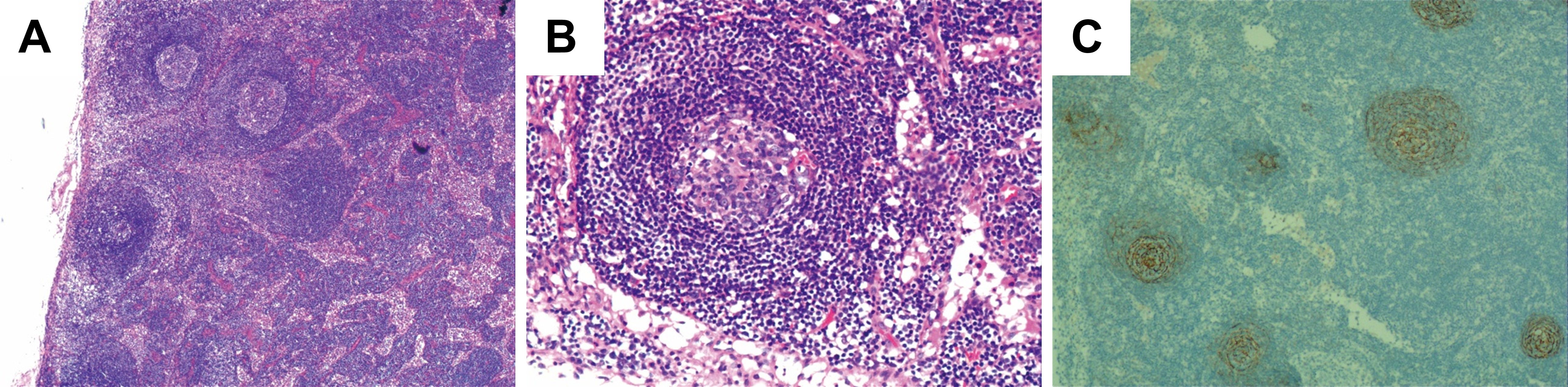
Figure 3. Postoperative pathologic findings demonstrated typical lymph node manifestations in CD. (A, B) Conventional pathological microscopic results of the postoperative biopsy. H&E, A, Magnification: 400×; B, Magnification: 400×. (C) Follicular dendritic cells of the germinal centres stained with CD21 antibodies.
Discussion and conclusion
CD is a rare chronic lymphoproliferative disorder first reported by Benjamin Castleman in 1956 (2). Its clinical subtypes include UCD and MCD, while the histopathological subtypes are classified as HV-CD, plasma cell type (PC-CD), and mixed type. Previous studies have suggested that dysregulated expression of IL-6, activation of interferon regulatory factor 3 (IRF3), and infections with HHV-8 and HIV contribute to its pathogenesis (6). HHV8 infection is a major driver of HHV8-MCD. The virus replicates in lymph node plasma cells, contributing to systemic inflammatory symptoms and lymph node lesions, as well as a range of other cytokines, including IL-6 (7). However, the exact mechanisms remain unclear.
Currently, cases of idiopathic UCD involving the oesophageal mucosa are exceedingly rare and pose significant challenges in the differential diagnosis from early-stage oesophageal carcinoma (8), critically impacting subsequent treatment selection. This diagnostic difficulty arises from several factors. First, literature reports indicate that lymph node involvement in CD predominantly occurs in the abdomen (35. 7%), cervical (25. 7%), mediastinal (23. 1%), and axillary (6. 4%) regions (9), Some cases also occur in rare areas including the gastrointestinal tract (10) or liver (11), with oesophageal involvement documented in only a few cases (5). Consequently, these rare CD diseases, which manifest in specific regions, can be misinterpreted as malignant changes, thereby impeding clinical evaluation and the selection of subsequent treatment.
Second, the absence of specific clinical symptoms, laboratory markers, or imaging features makes histopathological examination the gold standard for definitive diagnosis, contributing to diagnostic challenges. As reported by Ki Nam Kim et al., a case occurring in the oesophageal submucosal layer further highlights that oesophageal-involved CD can easily be confused with lymphoma or other tumours (8). Furthermore, unlike previously reported cases of UCD in the oesophageal region, this patient exhibited significant wall thickening and luminal stenosis, with imaging manifestations more closely resembling carcinoma, posing a considerable diagnostic challenge. Diagnostic complexity also escalates when CD coexists with oesophageal squamous cell carcinoma (12), frequently leading to diagnostic delays or missed diagnoses.
More importantly, the patient in this case presented unique characteristics. The patient initially sought medical attention for chest pain but exhibited no typical oesophageal cancer symptoms, such as progressive dysphagia. Simultaneously, gastroscopy revealed an inflammatory mass rather than a malignant tumour. However, a preoperative PET–CT scan revealed increased glucose metabolism at the site, suggesting malignancy, which contradicts the results of the gastroscopic biopsy. The final diagnosis of UCD was confirmed only after partial oesophagectomy with intrathoracic oesophagogastric anastomosis and a second biopsy, which demonstrated classic histopathological features of HV-CD. The difference between imaging studies and biopsy findings during this diagnostic process underscores the complexity and uniqueness of this UCD case.
Regarding treatment selection for UCD, this case is consistent with the existing literature. Surgical resection remains the primary treatment for UCD (13), with most patients experiencing a favourable prognosis and low recurrence rates (14). Radiotherapy is effective for specific UCD patients (15). For MCD associated with HHV-8 or HIV infection, antiretroviral agents such as interferon (16) and chemotherapy regimens for non-Hodgkin lymphoma (17) are applicable. Recently, targeted therapies, such as siltuximab, a kind of anti-IL-6 monoclonal antibody, have been increasingly utilized in CD treatment (18). Siltuximab (11 mg/kg every 3 weeks) ± corticosteroids may serve as a first-line treatment for all patients with HHV-8-negative/idiopathic MCD cases (iMCD). The additional rounds of combination chemotherapy with or without immunomodulators/immunosuppressants are recommended if insufficient response is achieved (19). Given the localized UCD lesion in this case, complete surgical excision eliminated the need for adjuvant radiotherapy, chemotherapy, or targeted therapy. The patient recovered well during a one-year follow-up but requires ongoing monitoring due to recurrence risk (20).
In summary, this case represents a rare case of oesophageal UCD. The contradictory findings between endoscopic biopsy and imaging studies highlight diagnostic complexities, posing significant challenges in differentiating oesophageal CD from oesophageal carcinoma. We emphasized that oesophageal CD should be considered in the differential diagnosis of oesophageal space-occupying lesions, particularly when imaging and biopsy conclusions conflict. Furthermore, this case demonstrates the dual value of complete surgical resection: establishing a definitive diagnosis while achieving curative treatment in UCD (21).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. The study was ethically approved by the Ethics Committee of Medical Research in the Shenzhen Hospital of Southern Medical University.
Author contributions
QC: Data curation, Writing – original draft. LZ: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. QG: Funding acquisition, Writing – original draft. JK: Funding acquisition, Supervision, Writing – original draft. JZ: Investigation, Methodology, Project administration, Writing – original draft. JT: Data curation, Formal analysis, Software, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Research Fund of the Basic Research Project of Shenzhen (JCYJ20240813145214019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CD, Castleman’s disease; UCD, Unicentric Castleman’s disease; MCD, Multicentric Castleman’s disease; HV-CD, Hyaline vascular type Castleman’s disease; CT, Computed tomography; PET-CT, Positron emission tomography-computed tomography; PC-CD, Plasma cell type Castleman’s disease.
References
1. Gunduz E, Ozdemir N, Bakanay SM, and Karakus S. A rare lymphoproliferative disease: castleman disease. Turk J Haematol. (2021) 38:314–20. doi: 10.4274/tjh.galenos.2021.2021.0440
2. Castleman B, Iverson L, and Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. (1956) 9:822–30. doi: 10.1002/1097-0142(195607/08)9:4<822::aid-cncr2820090430>3.0.co;2-4
3. Pertusa Mataix R, Loaiza Cabello D, and Garcia Morillo JS. Castleman’s disease, pathophysiology, advances in diagnosis and treatment. Med Clin (Barc). (2024) 162:283–90. doi: 10.1016/j.medcli.2023.10.013
4. Dispenzieri A and Fajgenbaum DC. Overview of castleman disease. Blood. (2020) 135:1353–64. doi: 10.1182/blood.2019000931
5. Wong RSM. Unicentric castleman disease. Hematol Oncol Clin North Am. (2018) 32:65–73. doi: 10.1016/j.hoc.2017.09.006
6. Lee H, Jeon H, Park S, and Park C. Castleman’s disease of the spleen. World J Gastroenterol. (2015) 21:1675–79. doi: 10.3748/wjg.v21.i5.1675
7. Fajgenbaum DC and Shilling D. Castleman disease pathogenesis. Hematol Oncol Clin North Am. (2018) 32:11–21. doi: 10.3348/kjr.2006.7.1.73
8. Kim KN, Lee K, Kang MJ, Roh MS, Choi PJ, and Yang DK. Hyaline vascular-type castleman disease presenting as an esophageal submucosal tumor: case report. Korean J Radiol. (2006) 7:73–6. doi: 10.3348/kjr.2006.7.1.73
9. Cesarman E, Chadburn A, and Rubinstein PG. KSHV/HHV8-mediated hematologic diseases. Blood. (2022) 139:1013–25. doi: 10.1182/blood.2020005470
10. Sun T, Sun X, Shan T, Zhao P, Lu Y, Li Q, et al. Castleman disease of stomach treated by endoscopic submucosal dissection: a case report and literature review. Front Oncol. (2025) 15:1563545. doi: 10.3389/fonc.2025.1563545
11. Chen H, Pang X, Li J, Xu B, and Liu Y. Case report: a rare case of primary hepatic castleman’s disease mimicking a liver tumor. Front Oncol. (2022) 12:974263. doi: 10.3389/fonc.2022.974263
12. Tsuboi H, Suzuki H, Akutsu D, Terasaki T, Okamoto S, Kondo Y, et al. Pathologically confirmed oesophageal involvement in idiopathic multicentric castleman disease mimicking early oesophageal cancer. Rheumatol (Oxford). (2021) 60:e50–52. doi: 10.1093/rheumatology/keaa431
13. Kang M, Zhang J, Fang C, Li B, and Su S. Retroperitoneal castlemans disease mimicking a liver cancer: a case report. Front Oncol. (2024) 14:1343157. doi: 10.3389/fonc.2024.1343157
14. Zhang L, Dong Y, Peng H, Li H, Zhang M, Wang H, et al. A national, multicenter, retrospective study of castleman disease in China implementing CDCN criteria. Lancet Reg Health West Pac. (2023) 34:100720. doi: 10.1016/j.lanwpc.2023.100720
15. Mitsos S, Stamatopoulos A, Patrini D, George RS, Lawrence DR, and Panagiotopoulos N. The role of surgical resection in unicentric castleman’s disease: a systematic review. Adv Respir Med. (2018) 86:36–43. doi: 10.5603/ARM.2018.0008
16. Andres E and Maloisel F. Interferon-alpha as first-line therapy for treatment of multicentric castleman’s disease. Ann Oncol. (2000) 11:1613–14. doi: 10.1023/a:1008325114144
17. Imen BI, Zenaidi H, Abdelwahed Y, Sabeur R, and Ayoub Z. Management of isolated retroperitoneal castelman’s disease: a case report. Int J Surg Case Rep. (2020) 70:24–7. doi: 10.1016/j.ijscr.2020.03.048
18. Tanaka T, Narazaki M, and Kishimoto T. Interleukin (IL-6) immunotherapy. Cold Spring Harb Perspect Biol. (2018) 10. doi: 10.1101/cshperspect.a028456
19. Fajgenbaum DC. Novel insights and therapeutic approaches in idiopathic multicentric castleman disease. Blood. (2018) 132:2323–30. doi: 10.1182/blood-2018-05-848671
20. Ren N, Ding L, Jia E, and Xue J. Recurrence in unicentric castleman’s disease postoperatively: a case report and literature review. BMC Surg. (2018) 18:1. doi: 10.1186/s12893-017-0334-7
Keywords: Castleman’s disease, hyaline vascular type, oesophagus, diagnostic dilemma, unicentric Castleman’s disease
Citation: Cai Q, Zhu L, Guo Q, Kuang J, Zhang J and Tan J (2025) Case Report: Diagnostic dilemma: a rare case of oesophageal hyaline vascular unicentric Castleman’s disease mimicking carcinoma. Front. Oncol. 15:1675203. doi: 10.3389/fonc.2025.1675203
Received: 29 July 2025; Accepted: 13 October 2025;
Published: 28 October 2025.
Edited by:
Shuo Chen, Northeastern University, ChinaReviewed by:
Makoto Ide, Takamatsu Red Cross Hospital, JapanMehmet Ağar, Firat University Faculty of Medicine, Türkiye
Copyright © 2025 Cai, Zhu, Guo, Kuang, Zhang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Zhang, empoXzA1MjhAMTI2LmNvbQ==; Jianfeng Tan, dGpmXzYwMUBzbXUuZWR1LmNu
†These authors have contributed equally to this work
 Qingqing Cai1†
Qingqing Cai1† Leqing Zhu
Leqing Zhu Quanwei Guo
Quanwei Guo Jianfeng Tan
Jianfeng Tan