- 1Department of Cardiology, The Fourth Hospital of Hebei Medical University., Shijiazhuang, Hebei, China
- 2Department of Radiation Oncology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 3Hebei Medical University, Shijiazhuang, Hebei, China
- 4Guang’anmen Hospital of Chinese Academy of Chinese Medical Sciences, Beijing, China
Background: Immune checkpoint inhibitors (ICIs) have transformed cancer therapy but may cause immune checkpoint inhibitor-related cardiotoxicities (iRCs). Blood-derived inflammatory ratios may serve as practical prognostic tools for these life-threatening complications.
Methods: We conducted a retrospective study of 105 iRC patients treated with ICIs between 2020 and 2023. Patients were classified by Common Terminology Criteria for Adverse Events (CTCAE) severity grades. We identified the most important blood-derived ratio indexes at iRC diagnosis associated with the severity of iRCs, 40-day major adverse cardiovascular events (MACEs), and long-term mortality using receiver operating characteristic (ROC) analyses and restricted cubic spline (RCS) curves. Kaplan–Meier survival curves, Cox regression, and subgroup analysis were also performed to evaluate them.
Results: High-grade iRCs patients (n=40) showed a significantly higher system inflammation response index (SIRI) (8.21 vs. 2.21, p<0.001) and neutrophil-to-lymphocyte ratio (NLR) (11.46 vs. 5.81, p=0.001) than low-grade cases. SIRI >3.07 was strongly associated with 40-day MACEs [hazard ratios (HR)=6.56, p<0.001], whereas NLR >7.88 was associated with increased long-term mortality risk (HR = 2.33, p=0.003). Both SIRI and NLR remained significant after adjusting for cardiac biomarkers and clinical variables and were found associated with iRC severity-related cardiac biomarkers.
Conclusion: SIRI and NLR are effectively associated with iRC severity and could stratify the risk of developing poor short- and long-term prognoses. These readily available inflammatory indexes could improve risk assessment and guide clinical decision-making for iRC patients. However, further prospective studies should validate their utility in diverse populations.
Introduction
Immune checkpoint inhibitors (ICIs) mainly target T-cell activation in order to eradicate tumor cells efficiently (1). ICIs have transformed cancer therapies in recent years. They have demonstrated clear advantages in the immunotherapy of advanced cancers, including renal cell carcinoma, non-small-cell lung cancer (NSCLC), colon cancer, and melanoma (2). Nonetheless, with cancer treatments addressing tumor immune checkpoints advanced, physicians progressively identified immune-related adverse events (irAE). Immune checkpoint inhibitor-related cardiotoxicities (iRCs), the most lethal adverse effect, are the main cause for concern (3).
There have been numerous reports of iRCs, such as myocarditis, cardiomyopathy, pericardial disease, arrhythmia, stable angina or acute coronary syndrome, valvular dysfunction, and stress cardiomyopathy (4). It has been established that cytotoxic T cells, which directly destroy myocytes, fibroblasts, endothelium, and mesothelial cells, may cause damage to the heart. This damage is linked to elevated levels of pro-inflammatory cytokines from activated immune cells (5). Relevant research, however, has demonstrated that a variety of factors influence the exact process behind myocardial T-cell injury (6, 7). Thus, there is still much to learn about the mechanism underlying iRCs (8).
Patients with iRCs have a poor prognosis and would bear heavy financial costs (9). Clinicians should thus identify patients with iRCs who could be more likely to develop a poor clinical outcome. Clinical biomarkers that can predict prognosis and illness severity should be used in conjunction with risk stratification to provide more accurate clinical advice (8). Recently, blood-derived ratio indexes have been explored and showed prognostic value of iRCs for cancer patients receiving ICI therapy. For example, an association between increased neutrophil-to-eosinophil ratio (NER) at iRC onset and the development of severe iRCs was demonstrated (9). In addition, Haj-Yehia et al. have suggested that a neutrophil-to-lymphocyte ratio (NLR) was associated with overall cancer therapy-related cardiovascular toxicity in cancer patients with preexisting cardiovascular disease under ICI therapy (10). However, these studies all have certain limitations, such as a small sample size of 47 and 88 cases, respectively, and inadequate assessment of major adverse cardiovascular event (MACE) occurrence and mortality.
In recent years, many blood-derived ratio indexes have been evaluated for the prognosis of cardiovascular diseases (11, 12). For example, increasing evidence has suggested a link between the system inflammation response index (SIRI) and the prognosis of cardiovascular illnesses such as congestive heart failure and acute coronary syndrome (13–15). Given that patients with iRCs may experience acute coronary syndrome or right heart failure, it is reasonable to hypothesize that part of blood-derived ratio indexes are relevant to the MACEs following the occurrence of iRCs. Moreover, some ratio indexes, such as NLR, are associated with increased long-term all-cause mortality among community-dwelling individuals, which are also associated with the long-term survival outcomes among patients who received the ICI therapy (16–18). However, there is still a lack of systematic analysis to explore the prognostic value of these ratio indexes toward patients with iRCs. As a result, we conducted a retrospective study to evaluate the performance of ratio indexes to affect the short or long-term prognosis of those who were diagnosed with iRCs.
Materials and methods
Study design and population
This retrospective study was conducted in the Fourth Hospital of Hebei Medical University (Shijiazhuang, China). Between January 2020 and December 2023, a total of 105 patients diagnosed with iRCs underwent at least one cycle of immune-based therapies in our institution. Inclusion criteria are as follows: (1) patients aged 18 years and older; (2) patients received at least one cycle of ICI monotherapy or immunotherapy in combination with other antitumor treatments; (3) patients diagnosed with iRCs according to the European Society of Cardiology (ESC) Cardio-Oncology 2022 and International Cardio-Oncology Society (IC-OS) guidelines. Consensus statement (19, 20); (4) all iRC cases were confirmed by a senior cardiovascular specialist and an oncologist. Exclusion criteria included the following: (1) patients who could not be followed up; (2) patients who had received other antitumor treatments without undergoing immunotherapy.
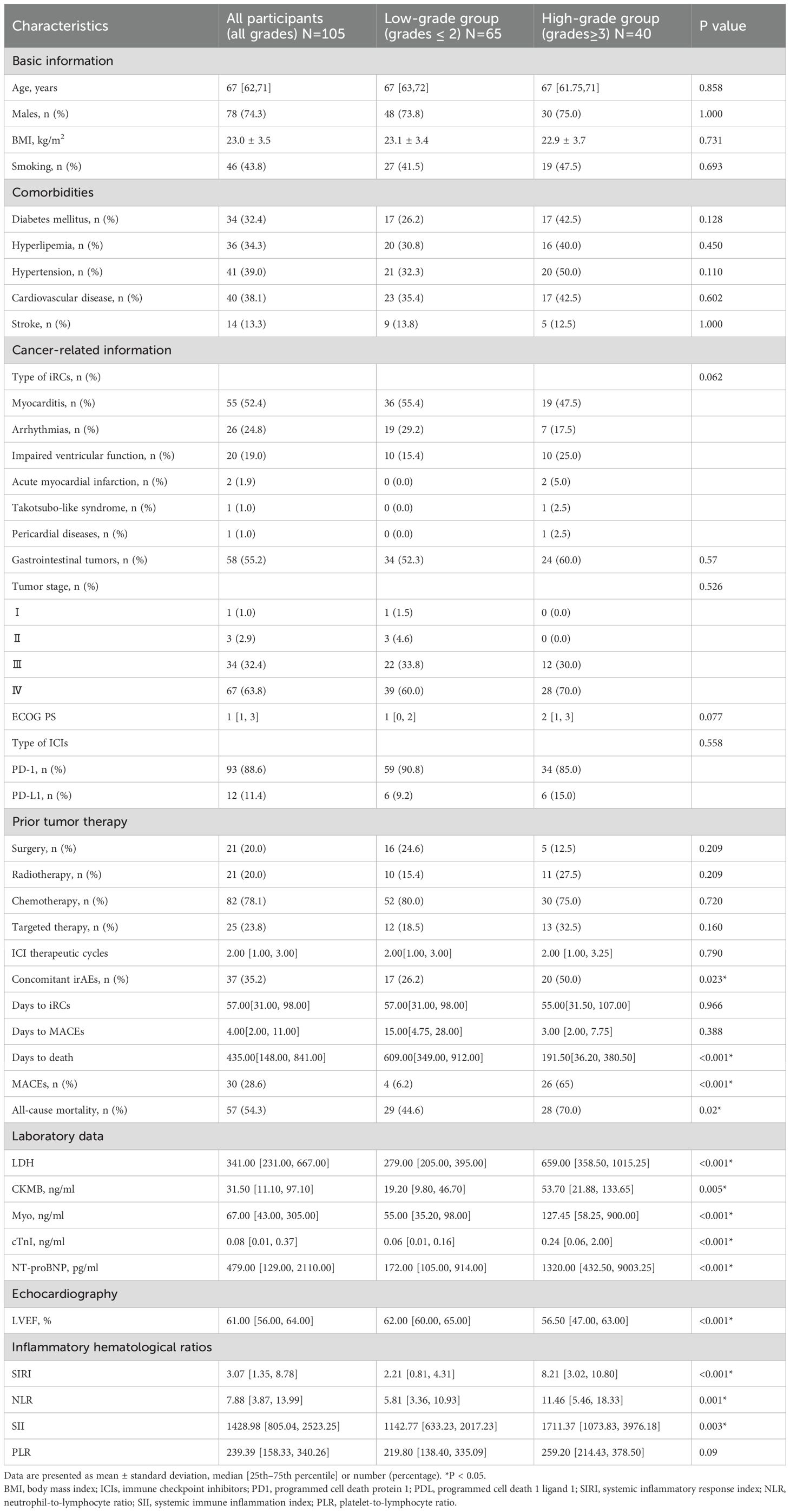
The study included 105 patients with iRCs after excluding those who met the exclusion criteria. Patient information included demographics, smoking habits, and medical history including hypertension, diabetes, and hyperlipidemia. Tumor category, stage, the time from the start of immunotherapy to the onset of iRCs (abbreviated as days to iRCs), the time from the diagnosis of iRCs to the occurrence of MACEs within 40 days (abbreviated as days to MACEs), the time from the diagnosis of iRCs to all-cause mortality (abbreviated as days to death), the number of cycles of immunotherapy until iRCs occurred, history of previous tumor treatments, Eastern Cooperative Oncology Group (ECOG PS) performance status, types of ICIs, the values of left ventricular ejection fraction (LVEF), N-terminal pro-brain natriuretic peptide (NT-proBNP), cardiac troponin I (cTnI), myoglobin (Myo), creatine kinase myocardial band (CKMB), lactate dehydrogenase (LDH), SIRI, NLR, systemic immune inflammation index (SII), and platelet-to-lymphocyte ratio (PLR) were recorded and calculated when iRCs occurred. Standard methods were used to measure hematological parameters in the central laboratory of the Fourth Hospital of Hebei Medical University.
Definition of iRCs and inflammatory hematological markers
The cardiotoxicity caused by ICIs spreads to almost all parts of the heart and includes both inflammatory cardiotoxicity and non-inflammatory cardiotoxicity. The former includes myocarditis, perimyocarditis, pericarditis, and left ventricular dysfunction without myocarditis. The latter includes asymptomatic non-inflammatory left ventricular dysfunction, Takotsubo-like syndrome, coronary vasospasm, arrhythmias, and myocardial infarction. Cardiotoxicities associated with immune checkpoint inhibitors include myocarditis which is the most common cardiotoxic reaction (4, 21). The values used to calculate SIRI, NLR, PLR, and SII formulas were as follows: SIRI = (neutrophil count × monocyte count)/lymphocyte count. NLR = neutrophil count/lymphocyte count; PLR = platelet count/lymphocyte count; SII = (neutrophil count × platelet count)/lymphocyte count.
Severity stratification strategy
Patients were stratified into a low-grade group and a high-grade group using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 criteria published by the US Department of Health and Human Services in 2017. CTCAE grades ≤2 were classified as a low-grade group, and grades ≥3 belonged to the high-grade group.
Follow-up and outcome
The main focus of this study was to assess the clinical prognosis of the iRC patients, which included the occurrence of MACEs during the 40-day follow-up and all-cause mortality during the long-term follow-up. MACEs are defined as cardiovascular death, cardiac arrest, cardiogenic shock, and hemodynamically significant complete heart block that occurred after the initial diagnosis of iRCs by referencing relevant articles (11, 22). Participants included were followed up for both short-term (40 days after the occurrence of iRCs) and long-term periods. MACE incidence and all-cause mortality data were obtained through observation during hospitalization and subsequent routine telephone follow-up.
Statistical analysis
Continuous data were evaluated for normality using Shapiro–Wilk tests. Continuous variables were presented as the mean with standard deviation (SD) or the median with interquartile range (IQR) according to whether a variable had a normal distribution, Categorical variables were presented as numbers and proportions (%). Appropriate statistical tests, such as independent-sample t-test, Mann–Whitney U-test, chi-square test, or Fisher’s exact test, were employed to compare the two groups. The univariate logistic regression analysis was used to identify the ratio indexes significantly associated with the grade level of iRCs. Spearman correlation coefficients were utilized to investigate the associations between the ratio indexes and the grade level of iRCs as well as the associations between the ratio indexes and cardiac biomarkers (23). The receiver operating characteristic (ROC) curves of four ratio indexes were utilized to discriminate the occurrence of 40-day MACEs and assisted in finding the ratio index with the highest area under the curve (AUC). The time‐dependent ROC curve analysis was performed to identify the ratio indexes most associated with long-term survival outcomes. The medians of ratio indexes were initially assigned as the cutoff value to discriminate iRC patients into the low- and high-risk groups (24–26). Restricted cubic spline (RCS) curves, with the medians of ratio indexes as the reference values, were then used to assess the relationship between the ratio indexes and hazard ratios (HR) for 40-day MACEs and the long-term survival outcomes. The Kaplan–Meier method was utilized to derive survival curves among the low- and high-risk groups, which were then compared using the log-rank test. The Cox regression analysis was further conducted to confirm the significance of selected ratio indexes associated with clinical prognosis. Subgroup analyses of Kaplan–Meier analysis and Cox regression analysis were also performed to test the efficiency of selected ratio indexes. The level of statistical significance was determined by P < 0.05 (two-sided). R software (version 4.4.1) was utilized for conducting data analysis.
Results
Baseline characteristics
This study included 105 individuals diagnosed with iRCs, with a median age of 67 years, of which 74.3% were men. The disparities in the variables between patients experiencing low-grade iRCs and high-grade iRCs are presented in Table 1. In contrast to patients who did not experience high-grade iRCs, there were no significant differences in the type of iRCs. Likewise, comorbidities, tumor stage, ECOG PS, type of ICIs, ICI therapeutic cycles, and even previous tumor therapy did not significantly differ between patients with low-grade iRCs and high-grade iRCs. The median interval from initiation of immunotherapy to iRCs and from iRCs to MACEs was respectively 57 days (IQR: 31–98 days) and 4 days (IQR: 2–11 days), but both showed no significant difference between the two groups. However, patients in the high-grade group developed more frequently concomitant irAEs (26.2% vs. 50%; p=0.023) and were associated with all-cause mortality (44.6% vs. 70%; p=0.002). Furthermore, patients who encountered high-grade iRCs demonstrated a significantly shorter duration from iRCs to death compared with those with low-grade iRCs (191.5 days vs. 609 days; P<0.001). Patients who encountered high-grade iRCs demonstrated significantly worse LVEF. In contrast to low-grade iRCs patients, individuals with high-grade iRCs exhibited significantly increased levels of CKMB, Myo, cTnI, and NT-proBNP. Patients with high-grade iRCs had significantly higher SIRI, NLR, and SII values than individuals with low-grade iRCs when iRCs occurred. High-grade iRCs patients showed significantly higher SIRI (8.21 vs. 2.21, p<0.001) and NLR (11.46 vs. 5.81, p=0.001) than low-grade cases.
Identification of indicators most associated with severity of iRCs patients
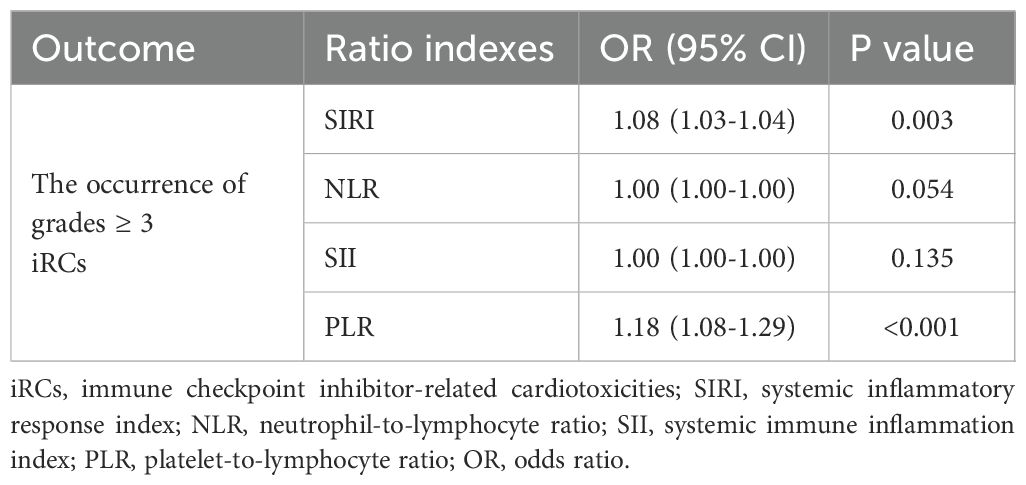
In this part, we explored the association between ratio indexes and cardiac biomarkers that may be associated with the severity of iRCs. Initially, significant positive correlations and differences were observed between ratio indexes (NLR, SIRI, SII) and iRC grades, among which SIRI (R = 0.47, P = 0.00052) and NLR (R = 0.33, P = 5.1×10−7) showed moderate correlations with iRC grades (Figure 1). Meanwhile, the univariate logistic regression analysis (Table 2) revealed that a higher NLR [odds ratio (OR)=1.08, 95% confidence interval (CI): 1.03-1.04, P = 0.003] and SIRI (OR = 1.18, 95% CI: 1.08-1.29, P<0.001) at onset of iRCs were significantly associated with an increased risk of developing ≥3 grade iRCs. In contrast, no significant associations were found for SII (P = 0.054) or PLR (P = 0.135). As shown in Supplementary Figures S2A, B, SIRI and NLR exhibit relatively significant correlations with LVEF, NT-proBNP, and various serum myocardial enzymology indices including LDH, CKMB, Myo, and cTnI.
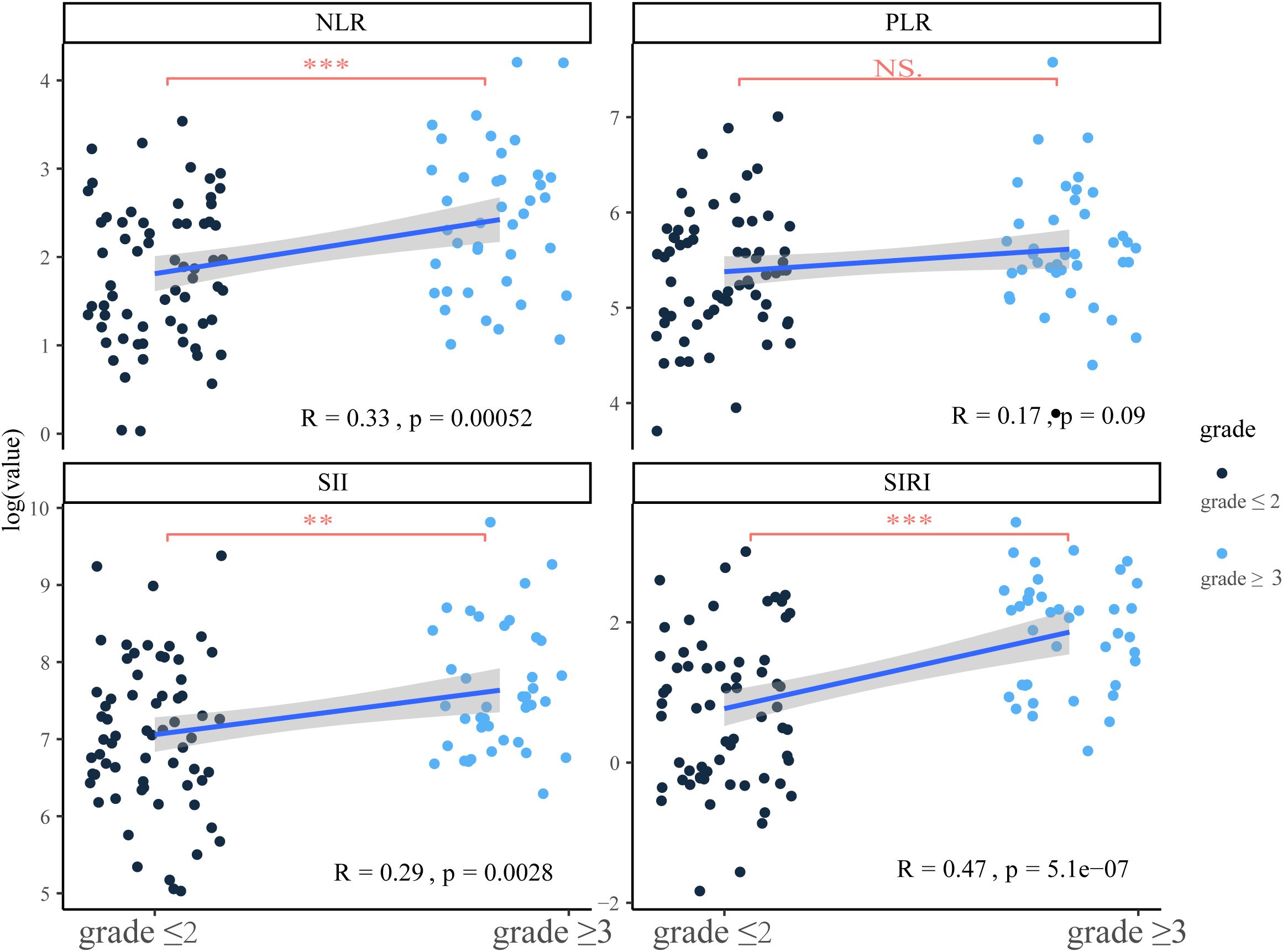
Figure 1. Correlation between ratio indexes at onset and the severity of immune checkpoint inhibitor-related cardiotoxicities and the comparison of ratio indexes in different severity groups. SIRI, systemic inflammatory response index; NLR, neutrophil-to-lymphocyte ratio; SII, systemic immune inflammation index; PLR, platelet-to-lymphocyte ratio; NS, no significance. **P<0.01; ***P<0.001.
Identification of the ratio indexes most associated with prognosis of iRCs patients
In the present study, ROC analysis evaluated the ability of different blood-derived ratio indexes to distinguish the occurrence of MACEs at the end of the 40-day follow-up and the occurrence of death at different time points during the follow-up period (Figures 2A, B, Supplementary Figure S1). Notably, SIRI exhibited the highest AUC value of 0.824 when it comes to the occurrence of short-term MACEs whereas NLR all exhibited the highest AUC value as for the occurrence of long-term death (at the of 1-, 2-, and 3-year time points). We initially selected the median of SIRI/NLR as the cutoff value to discriminate iRC patients into the low- and high-risk groups. Subsequently, when regarding the medians as references, RCS curves were utilized to detect the non-linear relationship between SIRI/NLR and short-term MACEs/long-term death. As Figures 2C, D displays, we found a J-shaped association between SIRI and short-term MACEs and an S-shaped association between NLR and long-term death. The RCS curve showed a relatively slow increase of HR by the increase of SIRI before the median and a substantial increase when over the median value (Figure 2C). As for the NLR, the RCS curve displayed the highest increasing rate at the point of the median of NLR (Figure 2D). Thus, the median value of the onset SIRI/NLR value owned a certain clinical value to serve as the cutoff value in order to identify the high-risk group (> median value) and low-risk group (< median value) for further prognostic analyses.
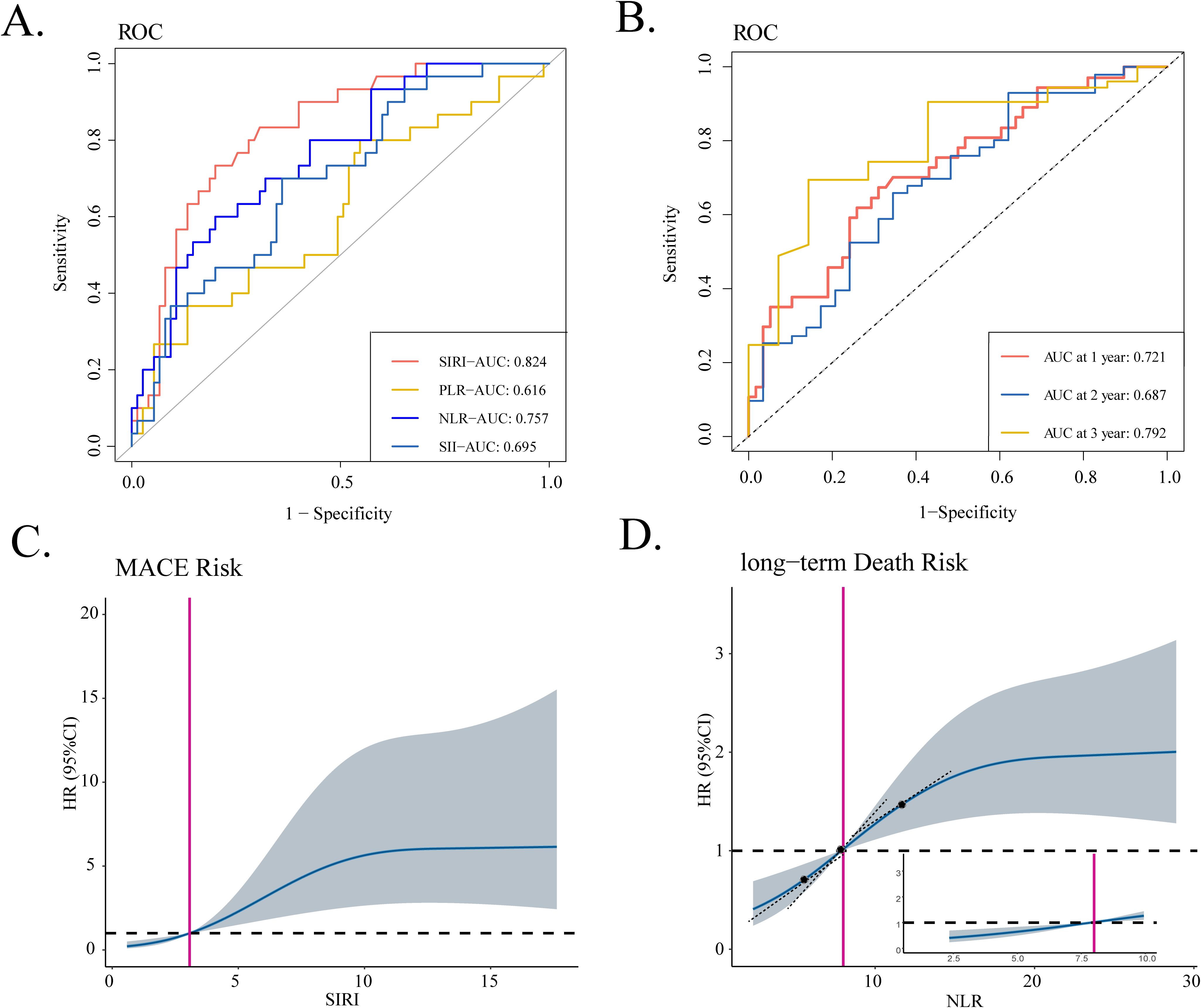
Figure 2. Identification of blood-derived ratio indexes most associated with prognosis of iRC patients. (A) The ROC curves of four ratio indexes to discriminate the occurrence of 40-day MACEs. (B) The time-dependent ROC curve of NLR to discriminate the all-cause mortality at of 1-, 2-, and 3-year the time points. (C, D) RCS curves that show the non-linear relationship between SIRI and hazard ratios of 40-day MACEs as well as relationship between NLR and the hazard ratios of long-term death. iRCs, immune checkpoint inhibitor-related cardiotoxicities; ROC, receiver operating characteristic curve; MACEs, major adverse cardiovascular events; SIRI, systemic inflammatory response index; AUC, area under the curve; NLR, neutrophil-to-lymphocyte ratio; SII, systemic immune inflammation index; PLR, platelet-to-lymphocyte ratio; RCS, restricted cubic spline; HR, hazard ratio.
Prognostic value of SIRI during the 40-day follow-up
During the 40-day follow-up period, a total of 30 (28.6%) individuals experienced clinical MACEs. Specifically, there were four patients in the low-grade group and 26 patients in the high-grade group. As displayed in Figure 3A, the high-risk group (SIRI > 3.07) exhibited notably high MACE rates and experienced a shorter duration until MACE than the low-risk group (SIRI ≤ 3.07) in the Kaplan–Meier curve. In three COX regression models adjusted by different variables, SIRI all served as a significant risk factor for the occurrence of MACEs in iRC patients whether being analyzed as a continuous or categorical variable (Table 3). Furthermore, we performed subgroup analysis to confirm the effectiveness of the above analysis. When stratified by the cancer-related variables, the subgroups divided by SIRI >3.07 also displayed significant prognostic differences in the Kaplan–Meier curves, instead of the cohorts who were in stage Ⅰ−Ⅲ and the high-grade iRC cohort (Figure 4A). When performing univariate COX regression models involving only SIRI, the continuous SIRI served as a significant risk factor in different subgroups stratified by different baseline variables, compared with most subgroups for categorical SIRI (Supplementary Figures S3, S4).
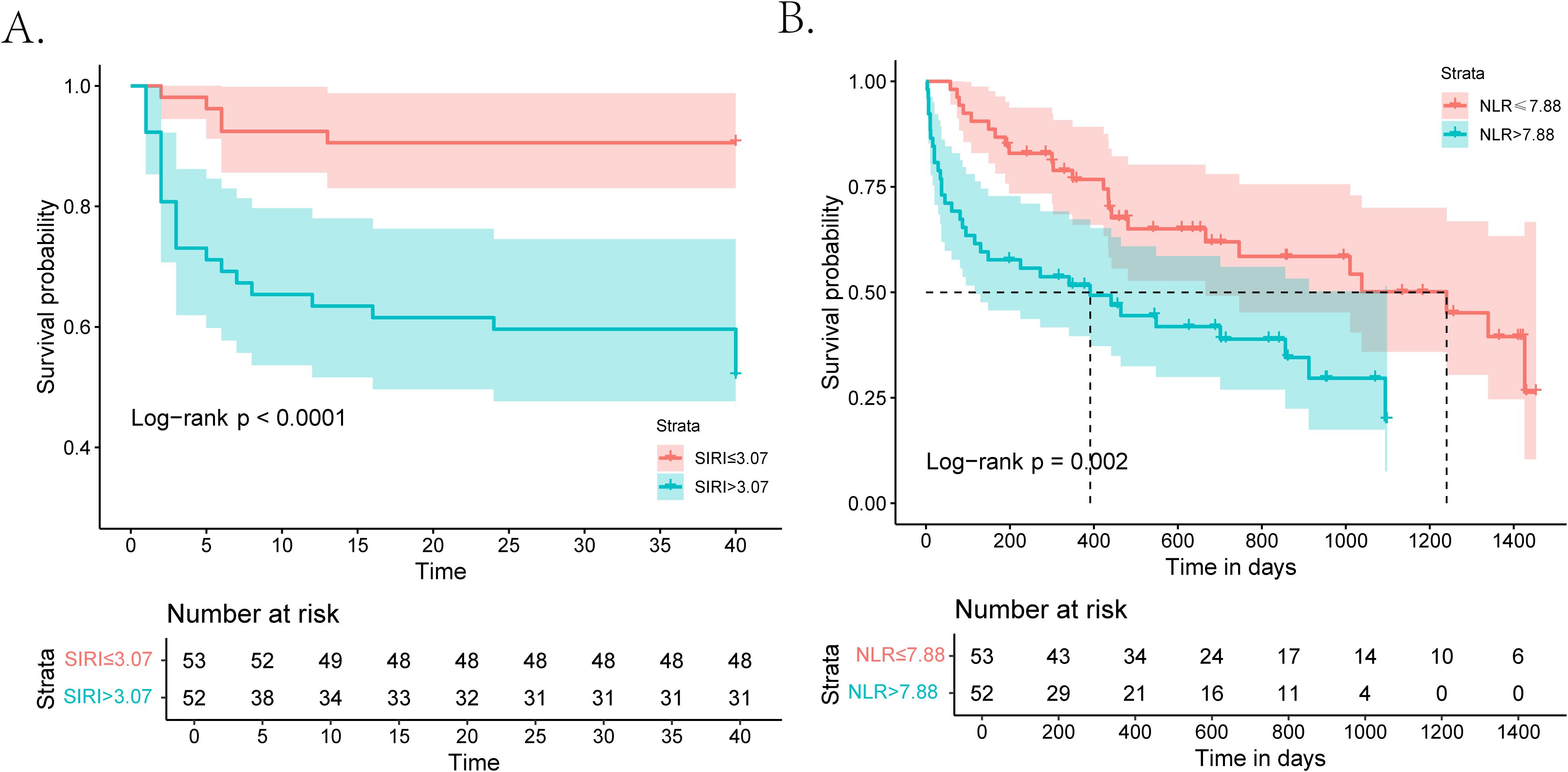
Figure 3. Kaplan–Meier curves for patients with iRCs in terms of 40-day MACEs and long-term mortality. (A) Kaplan–Meier analysis of MACEs during the 40-day follow-up in patients with iRCs classified by the median of SIRI. (B) Kaplan–Meier analysis of all-cause mortality after a longer follow-up period in patients with iRCs classified by the median of NLR. iRCs, immune checkpoint inhibitor-related cardiotoxicities; MACEs, major adverse cardiovascular events; SIRI, systemic inflammatory response index; NLR, neutrophil-to-lymphocyte ratio.
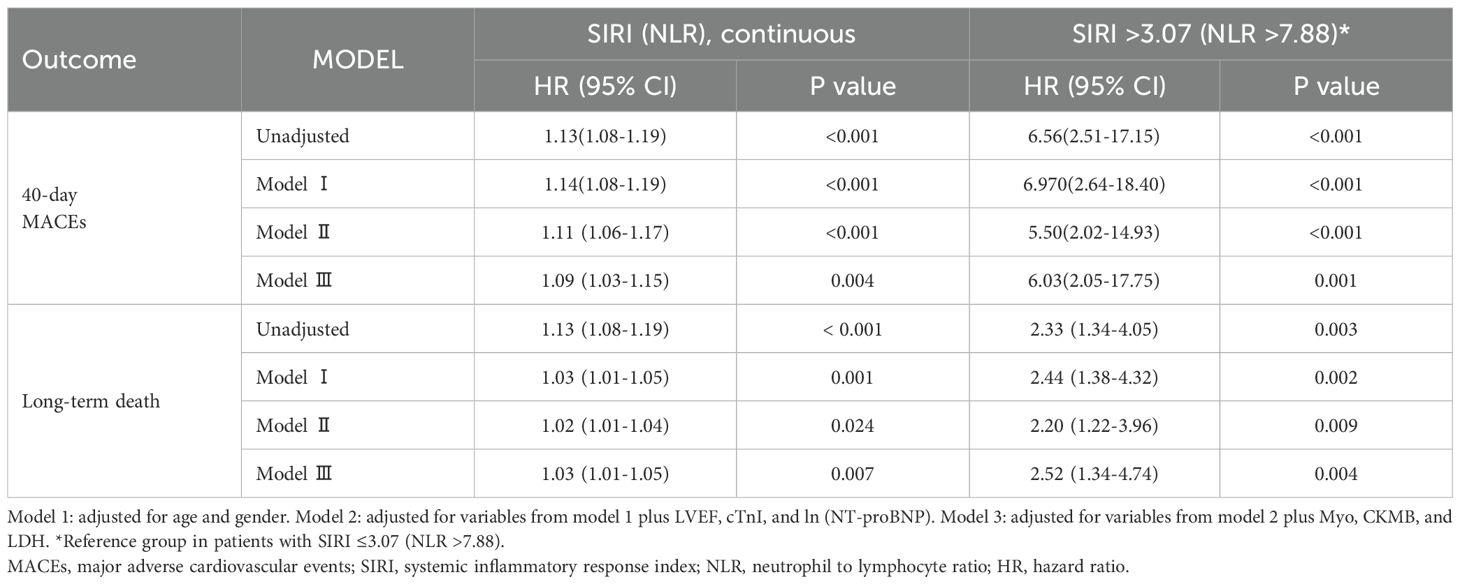
Table 3. Prognostic value of SIRI for 40-day MACEs and NLR for the long-term death among iRC patients.
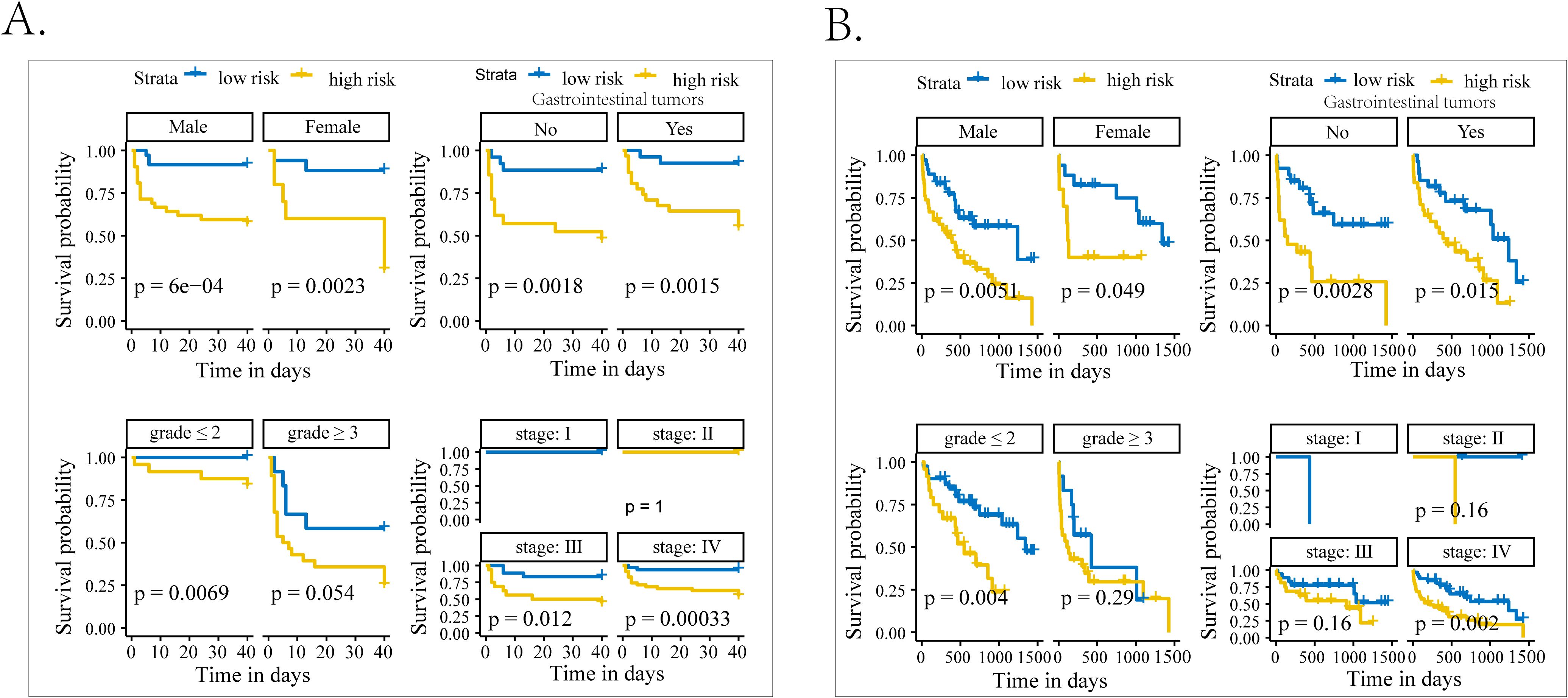
Figure 4. The subgroup analysis of patients with iRCs in terms of Kaplan–Meier analysis. (A) Kaplan–Meier curves of occurrence of 40-day MACEs in patients with iRCs classified by the median of SIRI in subgroups with different characteristics. (B) Kaplan–Meier curves of occurrence of long-term death in patients with iRCs classified by the median of SIRI in subgroups with different characteristics. iRCs, immune checkpoint inhibitor-related cardiotoxicities; MACEs, major adverse cardiovascular events; SIRI, systemic inflammatory response index.
Prognostic value of NLR for the long-term follow-up period
The follow-up deadline was June 30, 2024, and the median follow-up was 857 days (IQR: 44–1665 days). As displayed in Figure 3B, the high-risk group (NLR > 7.88) exhibited notably high all-cause mortality and experienced a shorter duration until death than the low-risk group (NLR ≤ 7.88) in the Kaplan–Meier curve. In three COX regression models adjusted by different variables, NLR all severed as a significant risk factor for the occurrence of all-cause mortality in iRC patients whether being analyzed as a continuous or categorical variable (Table 3). In the subgroup analysis, we further confirmed the effectiveness of the above analysis. When stratified by the cancer-related variables, the subgroups divided by NLR >7.88 also displayed significant prognostic differences in the Kaplan–Meier curves, instead of the cohorts who were in stage Ⅰ−Ⅲ and the high-grade iRC cohort (Figure 4B). When performing univariate COX regression models involving only NLR, the continuous NLR served as a significant risk factor in most subgroups stratified by different baseline variables, compared with approximately half of subgroups for continuous NLR (Supplementary Figures S5, S6).
Discussion
Summary of findings
In this retrospective study, we detected that the SIRI and NLR were associated with the severity of iRCs. As far as is known, there has been no prior investigation into the relationship between SIRI and the iRCs. We found that the SIRI showed a favorable correlation with LVEF, NT-proBNP, LDH, CKMB, Myo, and cTnI, which have been demonstrated to be factors associated with the severity of iRCs (27). It suggests that SIRI and NLR may have the potential in evaluating the severity and prognosis of iRCs like these indicators in clinical practice. Furthermore, our study demonstrated that SIRI functioned as an independent indicator for short-term MACEs, whereas NLR for the long-term all-cause mortality. As a result, SIRI and NLR may be potential biomarkers which own the capacity to transform into a crucial measure for assessing the severity of diseases and the outcomes of clinical prognosis in individuals with iRCs.
The role of inflammation on iRCs
While the exact development of iRCs was complex and varied, recent studies suggested that inflammation played an important role in the development of iRCs. Although immune checkpoint inhibitors have been used primarily to treat cancer by restoring T lymphocyte attack on malignant cells, activated T lymphocytes had no specificity for tumor cells (28). The mechanism of T-cell damage to the myocardium did not exist only in the direct cytotoxic killing of muscle cells, fibroblasts, endothelial cells, and mesothelial cells by T cells, but also the increased levels of proinflammatory cytokines produced by activated immune cells, leading to bystander damage (29). Furthermore, animal studies have shown that programmed death 1 (PD-1)-deficient mice exhibit increased inflammation, increased serum markers of myocardial damage, and increased infiltration of inflammatory cells [including cluster of differentiation (CD) 8T cells], thereby exacerbating inflammation-induced cardiac damage (7).
The short-term prognostic value of SIRI for iRC patients
The role of inflammation has been mentioned above. Thus, it was crucial to assess the inflammatory status while evaluating the severity and prognosis of individuals with iRCs. Recently, Li et al. tried to identified the inflammatory blood ratio indexes and demonstrated the prognostic value of NLR, neutrophils-to-high-density lipoprotein cholesterol ratio (NHR), and lactate dehydrogenase-to-albumin ratio (LAR) for the short-term MACE occurrence among patients with ICI-associated myocarditis (ICI-M), which is one of fatal iRCs among immune-therapy patients (11). Moreover, NLR was also identified as an index related to ICI-M severity in their study. In this study, we evaluated and found SIRI as a significant factor associated with the severity and short-term MACE occurrence. By combining NLR and monocyte-to-lymphocyte ratio (MLR), SIRI overcomes the limitations of traditional markers and inflammatory cells in predicting prognosis, which has been proved as an important prognostic factor among patients receiving ICI therapy (30–32). Meanwhile, several studies identified SIRI as a risk factor in predicting MACEs in patients with acute coronary syndromes (33, 34). It was determined by a compound of neutrophils, monocytes, and lymphocytes in the peripheral blood, which have been demonstrated to play a critical role in the pathogenesis of cardiovascular toxicity (35–37). SIRI integrates these three components into a single metric that captures the balance between the aggressive, pro-inflammatory forces (neutrophils and monocytes) and the regulatory, anti-inflammatory forces (lymphocytes). A high SIRI biologically translates to hyperactive innate immunity (elevated neutrophils and monocytes) and suppresses adaptive immunity (depleted lymphocytes). Particularly, Lou et al. revealed that monocytes were significantly involved in the regulation of innate immunity and inflammation associated with ICI-M by performing a single-cell ribonucleic acid (RNA) sequencing analysis (38). Compared with NLR, the involvement of monocytes may better explain the reason why SIRI is a novel inflammation index for iRC patients. Moreover, the positive correlation between the SIRI and cardiac biomarkers suggests that a higher SIRI may reflect a higher cardio-inflammatory load toward patients who received ICI therapy. Therefore, it was logical to conclude that the SIRI is capable of detecting the severity and short-term prognosis of iRCs.
The long-term prognostic value of NLR for iRC patients
In this study, we found that NLR is also related to the severity of iRCs and owns a long-term prognostic value. In the previous studies, elevated NLR was found to be associated with increased long-term all-cause mortality among community-dwelling individuals with heart failure (HF), which was also associated with the long-term survival outcomes among patients who received the ICI therapy (16–18). As for the long-term prognosis of iRC patients, Liang et al. demonstrated that higher NER (>184.3) correlates with higher mortality in a 2-year follow-up (9). In their study, they found that NLR was significantly elevated among severe iRCs patients. However, they did not further explore the long-term prognostic value of NLR as they have explored NER. As we have mentioned above, NLR was also associated with ICI-M severity. Circulating neutrophils contribute to tumor progression and invasiveness by secreting cytokines, vascular endothelial growth factors, and chemokines, whereas lymphocytes could inhibit the proliferation and metastasis of tumors (16). From this perspective, NLR reflects inflammatory and immune responses in peripheral blood after ICI therapy. A lower NLR probably means a better therapeutic effect of immune therapy and may lead to a better long-term prognostic outcome, which may pay the cost of inducing severer cardiotoxicity. Correspondingly, in the present study, we also identified that a high continuous NLR was positively associated with the cardiac biomarkers at the onset of iRCs, which may reflect the damage load of myocardial cells.
The advantages of using SIRI and NLR as prognostic indicators for iRCs
As emerging biomarkers, SIRI and NLR could offer numerous advantages for clinical use. Firstly, these two indexes can be initially calculated by the items of routine blood tests derived from peripheral blood sampling, which is convenient, quick, and inexpensive. Furthermore, our study demonstrated that the SIRI and NLR are capable of independently predicting the severity and future prognosis of patients with iRCs. Particularly, it may assist clinicians processing necessary monitoring for iRC patients with a >3.07 SIRI value (for short-term monitoring) or >7.88 NLR value (for long-term monitoring). Therefore, they may have the great potential to become a valuable tool for assessing the future risks faced by individuals with iRCs. For example, patients with SIRI >3.07 at iRC diagnosis should undergo intensified cardiac monitoring [e.g., serial troponin, electrocardiogram (ECG)] during the first 40 days to intercept MACEs. Those with NLR >7.88 warrant long-term surveillance (e.g., echocardiography). Moreover, high SIRI/NLR at the onset of iRCs may prompt earlier immunosuppression (e.g., corticosteroids) or ICI interruption, especially for ICI-related myocarditis, which is fatal and owns a high mortality rate. However, further research is still needed to investigate its potential in evaluating treatment effectiveness and as a treatment target.
Limitations
There were several limitations to our research. Firstly, this was a single-center, retrospective study. The sample size of the study was limited, and the number of involving factors that could be adjusted is limited. Thus, we propose incorporating SIRI/NLR into iRC management (e.g., risk scores) after collecting enough cases and developing a reliable risk stratification algorithm to personalize monitoring intervals. Secondly, right-censoring existing in our study may introduce potential biases. However, we used standard methods for censored data: both primary statistical methods employed in our study—the Kaplan–Meier estimator for survival probability and the Cox proportional hazards model for multivariate analysis. We also reported the follow-up time using the reverse Kaplan–Meier method. Moreover, outcomes in our study were stratified into short-term (40-day MACEs) and long-term (all-cause mortality) periods, reducing bias from uneven follow-up. Thirdly, during the retrospective follow-up, the causes of death in our study were unable to further distinguish between cardiovascular death and non-cardiovascular deaths such as tumor progression. Further multicenter prospective research is needed to distinguish them for a more specific survival analysis.
Conclusion
In patients with iRCs, we found that SIRI and NLR are effectively associated with iRC severity and could stratify the risk of developing poor short- and long-term prognoses. These readily available inflammatory indexes could improve risk assessment and guide clinical decision-making for ICI-treated patients. However, further prospective studies should validate their utility in diverse populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Fourth Hospital of Hebei Medical University (approval number: 2024KS048). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LG: Conceptualization, Writing – original draft. ZL: Conceptualization, Writing – original draft. GG: Writing – review & editing. JL: Data curation, Formal Analysis, Writing – original draft. ZG: Data curation, Writing – review & editing. TY: Writing – original draft. GL: Writing – original draft. QJ: Writing – original draft. TW: Writing – original draft. YW: Writing – original draft. JM: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Foundation of Hebei Provincial Department of Science and Technology (Grant Numbers 223777114D) and the Government clinical medical talent program (Grant Numbers ZF2024106).
Acknowledgments
We would like to thank author Zhenli Li to provide us with statistical consultation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1676806/full#supplementary-material.
Abbreviations
SIRI, systemic inflammatory response index; iRCs, immune checkpoint inhibitor-related cardiotoxicities; MACEs, major adverse cardiovascular events; irAE, immune-related adverse events; NSCLC, non-small-cell lung cancer; ICIs, immune checkpoint inhibitors; LVEF, left ventricular ejection fraction; NT- proBNP, N-terminal probrain natriuretic peptide; cTnI, cardiac troponin I; Myo, myoglobin; CKMB, creatine kinase myocardial band; LDH, lactate dehydrogenase; SII, systemic immune inflammation index; PLR, platelet-to-lymphocyte ratio; NER, neutrophil-to-eosinophil ratio; NLR, neutrophil-to-to-lymphocyte ratio; ROC, receiver operating characteristic.
References
1. He M, Yang T, Wang Y, Wang M, Chen X, Ding D, et al. Immune checkpoint inhibitor-based strategies for synergistic cancer therapy. Adv Healthc Mater. (2021) 10:e2002104. doi: 10.1002/adhm.202002104
2. Ribas A and Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. (2018) 359:1350–5. doi: 10.1126/science.aar4060
3. Chan KK and Bass AR. Autoimmune complications of immunotherapy: pathophysiology and management. BMJ. (2020) 369:m736. doi: 10.1136/bmj.m736
4. Guo X, Wang H, Zhou J, Li Y, Duan L, Si X, et al. Clinical manifestation and management of immune checkpoint inhibitor-associated cardiotoxicity. Thorac Cancer. (2020) 11:475–80. doi: 10.1111/1759-7714.13250
5. Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res. (2019) 25:1557–63. doi: 10.1158/1078-0432.CCR-18-2795
6. Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. (2003) 33:3117–26. doi: 10.1002/eji.200324270
7. Tarrio ML, Grabie N, Bu DX, Sharpe AH, and Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. (2012) 188:4876–84. doi: 10.4049/jimmunol.1200389
8. Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. (2019) 115:854–68. doi: 10.1093/cvr/cvz026
9. Liang L, Cui C, Lv D, Li Y, Huang L, Feng J, et al. Inflammatory biomarkers in assessing severity and prognosis of immune checkpoint inhibitor-associated cardiotoxicity. ESC Heart Fail. (2023) 10:1907–18. doi: 10.1002/ehf2.14340
10. Haj-Yehia E, Mincu RI, Korste S, Lampe L, Margraf SM, Michel L, et al. High neutrophil-to-lymphocyte ratio is associated with cancer therapy-related cardiovascular toxicity in high-risk cancer patients under immune checkpoint inhibitor therapy. Clin Res Cardiol. (2024) 113:301–12. doi: 10.1007/s00392-023-02327-9
11. Li Z, Yao T, Liu G, Guan Z, Liu J, Guo L, et al. Nomograms based on ratio indexes to predict severity and prognosis in immune checkpoint inhibitors-related myocarditis: a retrospective analysis. J Cancer Res Clin Oncol. (2024) 150:277. doi: 10.1007/s00432-024-05801-7
12. Stef A, Bodolea C, Bocsan IC, Cainap SS, Achim A, Serban A, et al. The value of biomarkers in major cardiovascular surgery necessitating cardiopulmonary bypass. RCM. (2024) 25. doi: 10.31083/j.rcm2510355
13. Gao L, Zhang S, Zhao Z, Zhao Q, Yang T, Zeng Q, et al. Role of the systemic inflammatory response index in predicting disease severity and prognosis in idiopathic pulmonary arterial hypertension. J Inflammation Res. (2024) 17:447–60. doi: 10.2147/JIR.S434720
14. Urbanowicz T, Michalak M, Komosa A, Olasinska-Wisniewska A, Filipiak KJ, Tykarski A, et al. Predictive value of systemic inflammatory response index (SIRI) for complex coronary artery disease occurrence in patients presenting with angina equivalent symptoms. Cardiol J. (2024) 31:583–95. doi: 10.5603/CJ.a2023.0033
15. Xia Y, Xia C, Wu L, Li Z, Li H, and Zhang J. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: A 20-year follow-up cohort study of 42,875 US adults. J Clin Med. (2023) 12. doi: 10.3390/jcm12031128
16. Booka E, Kikuchi H, Haneda R, Soneda W, Kawata S, Murakami T, et al. Neutrophil-to-lymphocyte ratio to predict the efficacy of immune checkpoint inhibitor in upper gastrointestinal cancer. Anticancer Res. (2022) 42:2977–87. doi: 10.21873/anticanres.15781
17. Parosanu AI, Pirlog CF, Slavu CO, Stanciu IM, Cotan HT, Vrabie RC, et al. The prognostic value of neutrophil-to-lymphocyte ratio in patients with metastatic renal cell carcinoma. Curr Oncol. (2023) 30:2457–64. doi: 10.3390/curroncol30020187
18. Wu CC, Wu CH, Lee CH, and Cheng CI. Association between neutrophil percentage-to-albumin ratio (NPAR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and long-term mortality in community-dwelling adults with heart failure: evidence from US NHANES 2005-2016. BMC Cardiovasc Disord. (2023) 23:312. doi: 10.1186/s12872-023-03316-6
19. Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. (2022) 43:280–99. doi: 10.1093/eurheartj/ehab674
20. Lyon AR, Lopez-Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. (2022) 43:4229–361. doi: 10.1093/eurheartj/ehac244
21. Zhou YW, Zhu YJ, Wang MN, Xie Y, Chen CY, Zhang T, et al. Immune checkpoint inhibitor-associated cardiotoxicity: current understanding on its mechanism, diagnosis and management. Front Pharmacol. (2019) 10:1350. doi: 10.3389/fphar.2019.01350
22. Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. (2020) 141:2031–4. doi: 10.1161/CIRCULATIONAHA.119.044703
23. Cao J and Wang J. Correlation analysis between spontaneous nystagmus characteristics and semicircular canal dysfunction in patients with vestibular neuritis: A quantitative study using electronystagmography and video head impulse test. Life Conflux. (2025) 1:e149. doi: 10.71321/ceerva81
24. Sehgal SA, Malik G, Sachdeva A, Chauhan AK, Kaushal V, Kaur P, et al. Pretreament neutrophil-lymphocyte ratio (NLR): A felicitous prognostic marker in carcinoma lung. J Cancer Res Ther. (2023) 19:S719–23. doi: 10.4103/jcrt.jcrt_688_22
25. Demir H, Demirci A, Eren SK, Beypinar I, Davarci SE, and Baykara M. A new prognostic index in young breast cancer patients. J Coll Physicians Surg Pak. (2022) 32:86–91. doi: 10.29271/jcpsp.2022.01.86
26. Liu X, Bai Y, Liu Y, Li W, Cui Y, Xu J, et al. Correlation between red blood cell distribution width/platelet count and prognosis of newly diagnosed diffuse large B-cell lymphoma. Blood Res. (2023) 58:187–93. doi: 10.5045/br.2023.2023156
27. Power JR, Dolladille C, Ozbay B, Procureur A, Ederhy S, Palaskas NL, et al. Immune checkpoint inhibitor-associated myocarditis: a novel risk score. Eur Heart J. (2025). doi: 10.1093/eurheartj/ehaf315
28. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. (2016) 375:1749–55. doi: 10.1056/NEJMoa1609214
29. Grabie N, Lichtman AH, and Padera R. T cell checkpoint regulators in the heart. Cardiovasc Res. (2019) 115:869–77. doi: 10.1093/cvr/cvz025
30. Gu X, Han X, Shen Y, and Shi Y. Prognostic value of systemic inflammation response index in cancer patients treated with PD-1/PD-L1 immune checkpoint inhibitors: a meta-analysis. Ann Med. (2024) 56:2413415. doi: 10.1080/07853890.2024.2413415
31. Liu C, Zhao H, Zhang R, Guo Z, Wang P, and Qu Z. Prognostic value of nutritional and inflammatory markers in patients with hepatocellular carcinoma who receive immune checkpoint inhibitors. Oncol Lett. (2023) 26:437. doi: 10.3892/ol.2023.14024
32. Zheng F, Meng Q, Zhang L, Chen J, Zhao L, Zhou Z, et al. Prognostic roles of hematological indicators for the efficacy and prognosis of immune checkpoint inhibitors in patients with advanced tumors: a retrospective cohort study. World J Surg Oncol. (2023) 21:198. doi: 10.1186/s12957-023-03077-8
33. Dziedzic EA, Gasior JS, Tuzimek A, Paleczny J, Junka A, Dabrowski M, et al. Investigation of the associations of novel inflammatory biomarkers-systemic inflammatory index (SII) and systemic inflammatory response index (SIRI)-with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23179553
34. Li Q, Ma X, Shao Q, Yang Z, Wang Y, Gao F, et al. Prognostic impact of multiple lymphocyte-based inflammatory indices in acute coronary syndrome patients. Front Cardiovasc Med. (2022) 9:811790. doi: 10.3389/fcvm.2022.811790
35. Murray PJ. Immune regulation by monocytes. Semin Immunol. (2018) 35:12–8. doi: 10.1016/j.smim.2017.12.005
36. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. (2018) 18:134–47. doi: 10.1038/nri.2017.105
37. Zou H, Zhou N, Huang Y, Luo A, and Sun J. Phenotypes, roles, and modulation of regulatory lymphocytes in periodontitis and its associated systemic diseases. J Leukoc Biol. (2022) 111:451–67. doi: 10.1002/JLB.3VMR0321-027RRR
Keywords: immune checkpoint inhibitor-related cardiotoxicities, systemic inflammatory response index, inflammatory ratios, prognosis, severity
Citation: Guo L, Li Z, Gao G, Liu J, Guan Z, Yao T, Liu G, Jiao Q, Wang T, Wang Y and Ma J (2025) Blood-derived ratio indexes associated with severity and prognosis of immune checkpoint inhibitor-related cardiotoxicity: a retrospective analysis. Front. Oncol. 15:1676806. doi: 10.3389/fonc.2025.1676806
Received: 31 July 2025; Accepted: 24 September 2025;
Published: 09 October 2025.
Edited by:
Lily Wang, Cleveland Clinic, United StatesReviewed by:
Peng Xu, Yale University, United StatesChen Cheng, Washington University in St. Louis, United States
Copyright © 2025 Guo, Li, Gao, Liu, Guan, Yao, Liu, Jiao, Wang, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingtao Ma, NDcxMDAyMjRAaGVibXUuZWR1LmNu
 Ling Guo1
Ling Guo1 Zhenli Li
Zhenli Li Jing Liu
Jing Liu Zhengkun Guan
Zhengkun Guan Qian Jiao
Qian Jiao Jingtao Ma
Jingtao Ma