- Department of Hematology and Oncology, Ningbo University Affiliated Yangming Hospital, Yuyao, China
Background: The use of totally implantable venous access devices (TIVADs) and peripherally inserted central catheters (PICCs) are the two options for patients receiving chemotherapy for hematologic malignancies. However, it remains unclear which approach yields superior patient outcomes. This meta-analysis aimed to compare the efficacy of TIVAPs and PICCs in patients undergoing chemotherapy for hematologic malignancies.
Methods: A comprehensive literature search was conducted in PubMed, Embase, Cochrane Library, Wanfang, and China National Knowledge Infrastructure (CNKI) to identify available articles comparing the effect of TIVADs and PICCs. Statistical analyses were performed using RevMan 5.3 and STATA 12.0, with odds ratios (OR) and 95% confidence intervals (CI) used as effect indicators.
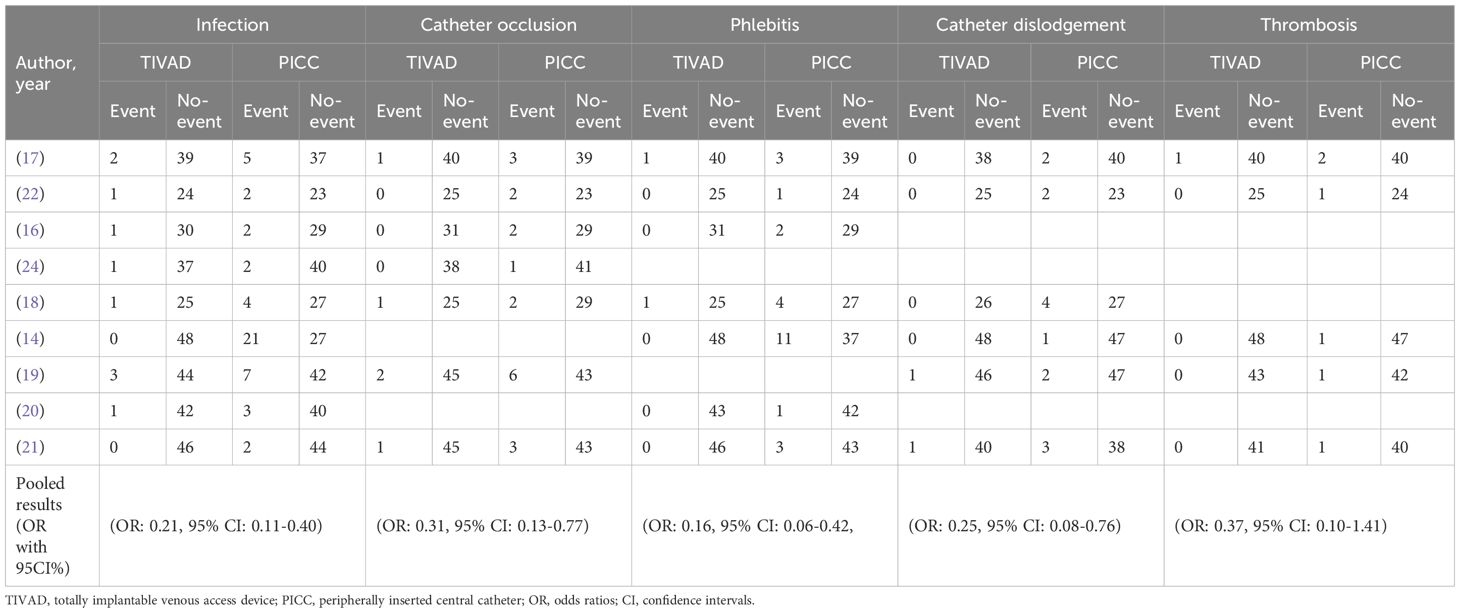
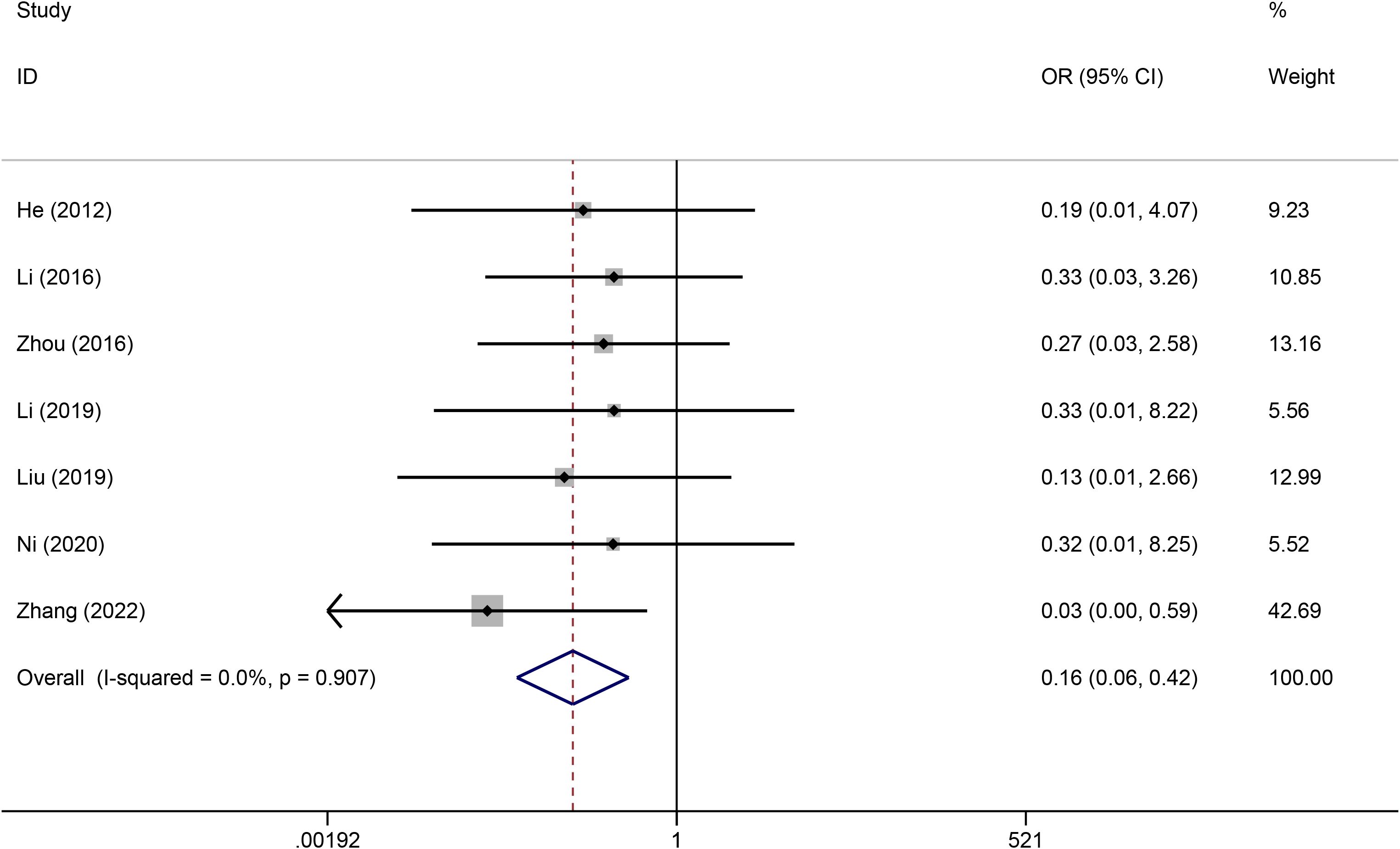
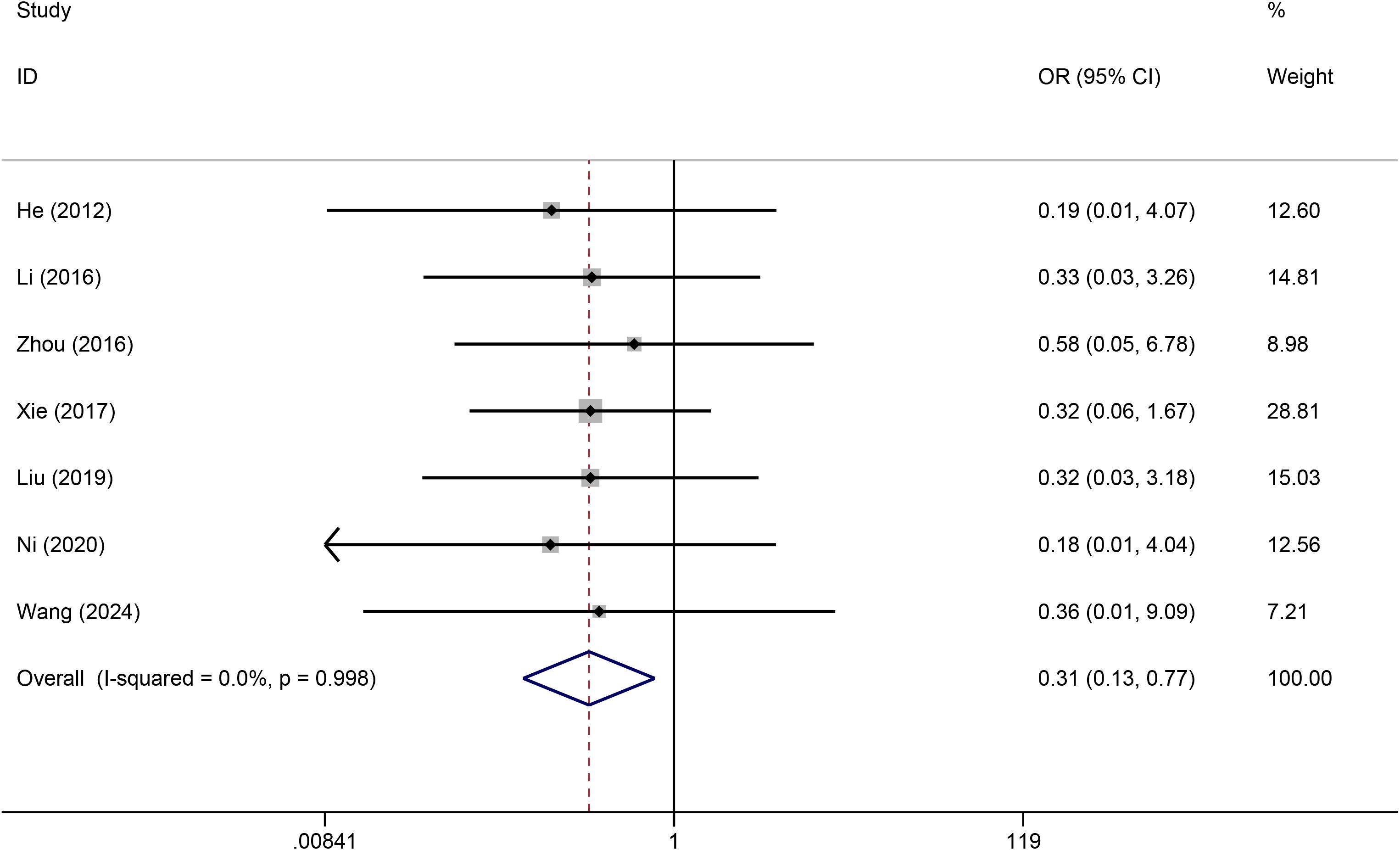
Results: A total of 10 studies, including 784 patients (386 in the TIVAD group and 398 in the PICC group), met the eligibility criteria. The meta-analysis results demonstrated that compared with PICCs, TIVAPs were associated with lower significantly risks of infection (OR: 0.21, 95% CI: 0.11-0.40), catheter occlusion (OR: 0.31, 95% CI: 0.13-0.77), phlebitis (OR: 0.16, 95% CI: 0.06-0.42), and catheter dislodgement (OR: 0.25, 95% CI: 0.08-0.76) compared to PICCs. However, there was no significant difference between the two devices in terms of thrombosis risk (OR: 0.37, 95% CI: 0.10-1.41).
Conclusion: This meta-analysis suggests a potential association between TIVAPs and a lower risk of complications compared with PICCs in patients with hematologic malignancies undergoing chemotherapy.
Introduction
Patients with hematologic malignancies, such as leukemia, lymphoma, and multiple myeloma, often require prolonged and recurrent intravenous chemotherapy, anti-infective treatment, and blood product transfusions (1, 2). Since these treatment procedures involve the infusion of chemotherapy drugs, peripheral venipuncture can not only cause phlebitis but also vein damage, thereby making long-term treatment more difficult (3). To ensure safe and effective drug administration while minimizing the discomfort of repeated venipunctures, a central venous access device (CVAD) is widely utilized in clinical practice (4).
Currently, two primary types of CVADs are commonly employed for chemotherapy in patients with hematologic malignancies: peripherally inserted central catheters (PICCs) and totally implantable venous access devices (TIVADs) (5, 6). PICCs are inserted through peripheral veins, such as the basilic, cephalic, or median cubital veins, and advanced into the central circulation (7). This method is relatively simple and does not require surgical intervention; furthermore, the device can be used immediately after insertion. However, the external catheter component increases the risk of infection and thrombosis. Additionally, patients with PICCs require regular maintenance, including flushing and dressing changes, to minimize complications (8, 9). In contrast, TIVADs are fully implanted venous access systems, with the port placed subcutaneously and the catheter positioned in the superior vena cava (10). Because TIVADs do not have an external component when not in use, they carry a lower risk of infection and require minimal maintenance. Furthermore, TIVADs can remain in place for extended periods, thus reducing the inconvenience of frequent catheter replacement during long-term treatment. The subcutaneous placement of the port also allows patients greater mobility and improved quality of life (11). However, the implantation procedure requires surgery, involves higher initial costs, and may be associated with complications such as subcutaneous infections, thrombosis, or catheter-related issues (12).
Although both PICCs and TIVADs are widely used in clinical practice, their respective advantages and limitations remain debated (13). Some studies suggest that TIVADs are superior in reducing infection and thrombosis rates, prolonging catheter retention, and enhancing patient satisfaction (14). Conversely, other studies argue that PICCs offer greater flexibility and eliminate surgical risks. Therefore, this study aimed to conduct a meta-analysis to compare the efficacy and safety of TIVADs versus PICCs in patients receiving chemotherapy for hematologic malignancies, thus providing evidence-based guidance for clinical decisions.
Methods
The meta-analysis is conducted in accordance with the Preferred Reporting Items for Systematic Evaluation and Meta‐Analysis (PRISMA) (15).
Search strategies
A comprehensive literature search was conducted across multiple databases, including PubMed, Embase, Cochrane Library, Wanfang, and China National Knowledge Infrastructure (CNKI), to identify studies published up to February 2025 on the application of peripherally inserted central catheters (PICC) and totally implantable venous access devices (TIVAD) in patients undergoing chemotherapy for hematologic malignancies. The search strategy was consisted of free text terms and Medical Subject Headings, such as “PICC”, “peripherally inserted central catheter”, “totally implantable vascular access device”, “PORT”, “TIVAD”, “hematologic malignancies”, and “chemotherapy”. No language limitation was set during the literature search. An additional relevant search was performed by manually searching the references of eligible studies or reviews.
Eligibility criteria
The following inclusion criteria were adopted: (1) Population: patients receiving chemotherapy for hematologic malignancies; (2) Intervention and comparison: comparing the effect of TIVAD and PICC; (3) Outcome: infection, catheter occlusion, phlebitis, catheter dislodgement, and the thrombosis; (4) Study design: randomized controlled trials (RCTs) or case-controls studies. Articles with the following exclusion criteria were eliminated: (1) duplicate publications; (2) meta-analyses, conference articles, case reports, and reviews; (3) articles without available data.
Data extraction and quality assessment
Data collection and extraction were conducted using a predesigned form. Disagreements were resolved through discussion. The following data were extracted for each included study: first author’s name, publication date, patient age, gender, sample size, and outcomes. The Cochrane risk of bias toll was used to evaluate the methodological quality and risk of bias of included RCTs. The process was conducted by two researchers separately, and differences were resolved through discussion.
Statistical analysis
Data analysis was performed using RevMan 5.3 and STATA 12.0. The dichotomous variables used odds ratio (OR) as the effect indicator. All effect sizes were presented with 95% confidence intervals (CI). The I2 statistic and Cochran’s Q test were used to assess heterogeneity among studies. A I2 statistic values of < 25, 25-75%, and >75% indicate low, moderate, and high levels of heterogeneity, respectively. Substantial heterogeneity (I2>50%) was identified, a random-effects model was used to analysis, and we also conducted a sensitivity analysis to examine the source of heterogeneity. A P value <0.05 is taken to indicate statistical significance.
Results
Search results and study characteristics
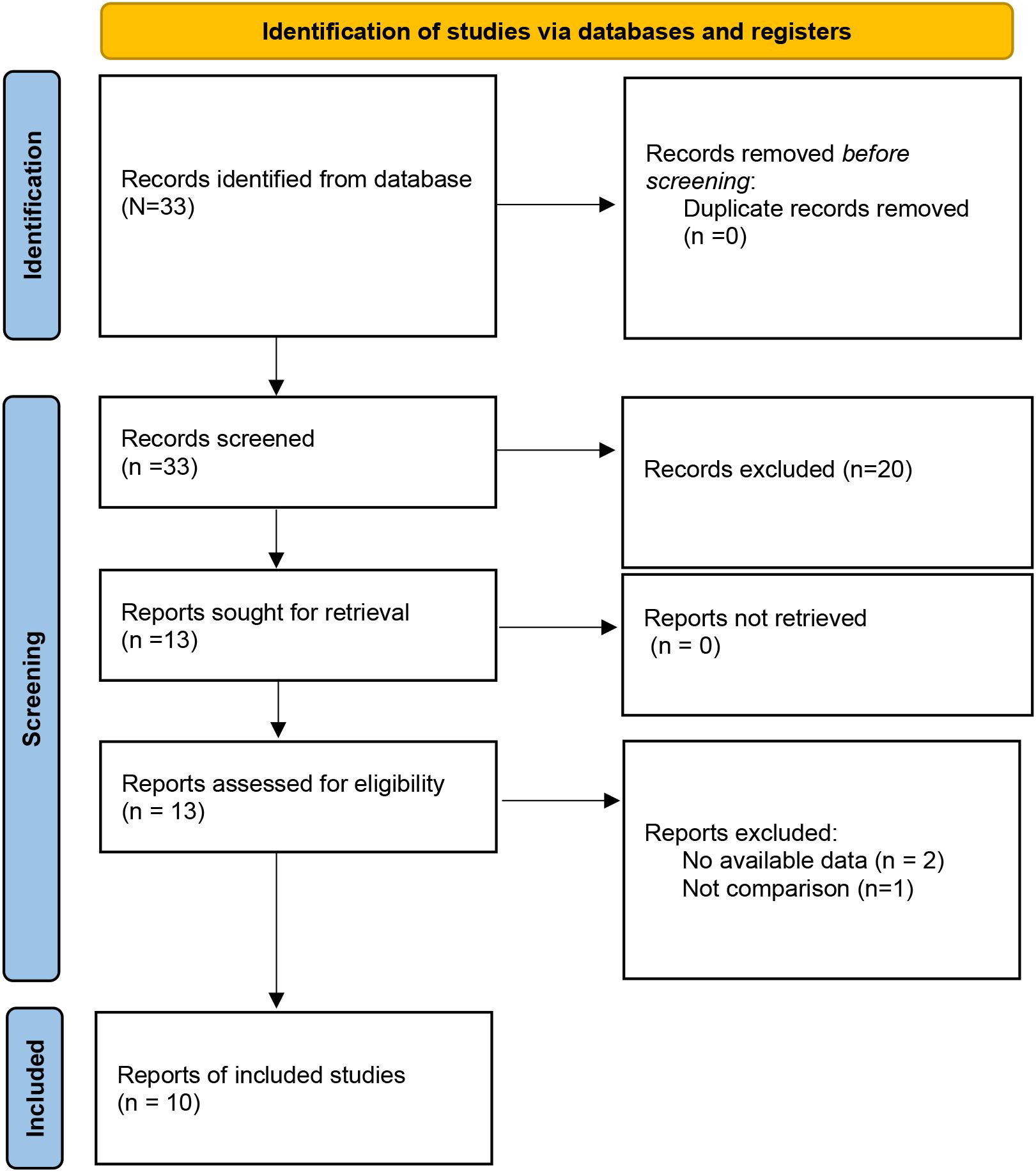
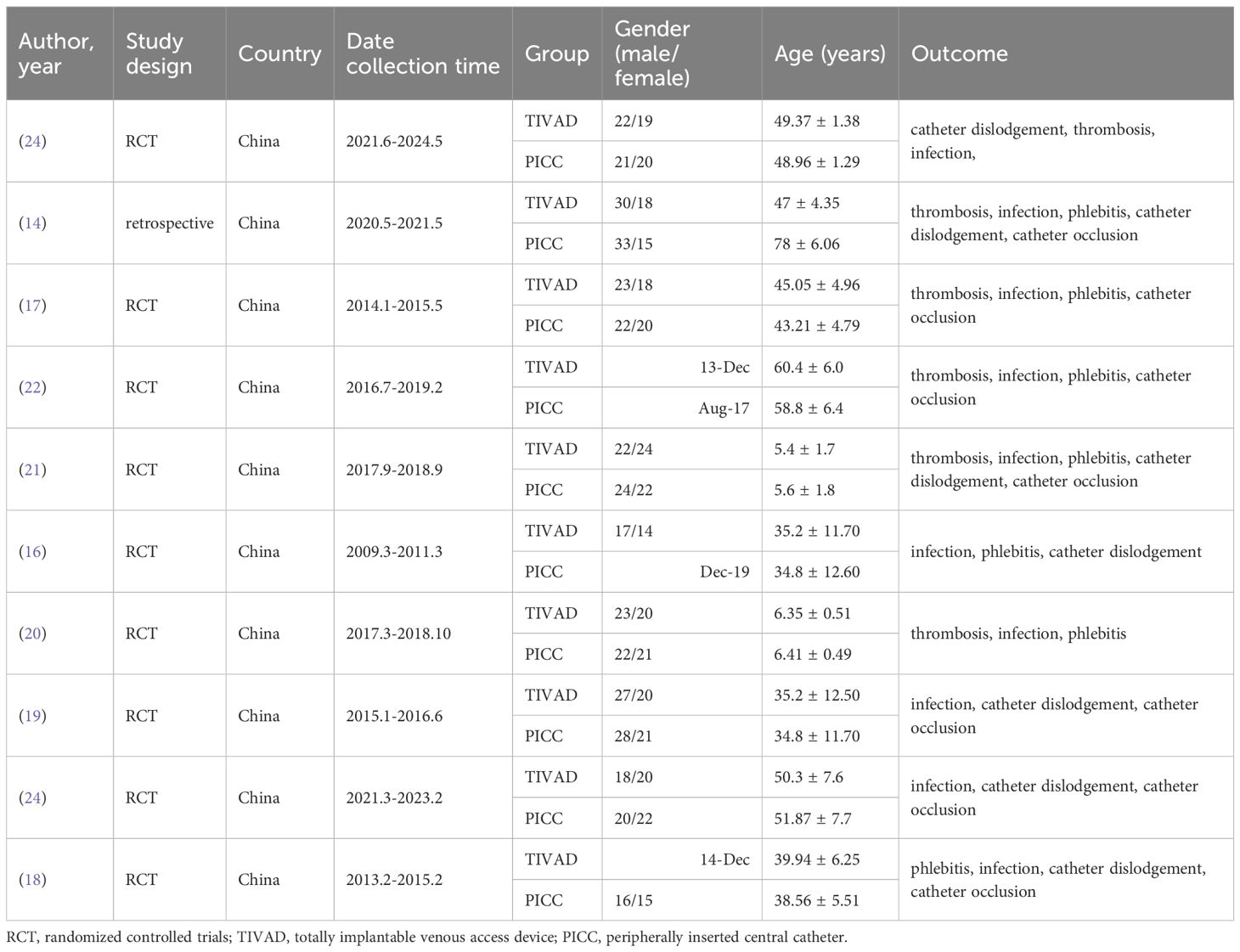
A total of 33 studies were initially retrieved. After the removal of duplicates, 33 studies remained for title and abstract screening. Of these, 20 studies were excluded because they were irrelevant or did not meet the eligibility criteria. The remaining 13 articles were subjected to full-text review, and 3 additional articles were excluded because of a lack of available data and comparison groups. Ultimately, 10 studies (14, 16–24) were included in this meta-analysis (Figure 1). Among the 10 studies, 9 studies were RCTs, one study was a case–control study, and all the studies were from China. Overall, 784 patients were included: 386 in the TIVAD group and 398 in the PICC group. The median ages in the studies ranged widely (range: 5–60 years). The outcome indices mainly included infection, catheter occlusion, phlebitis, catheter dislodgement, and thrombosis (Table 1).
Quality of the studies
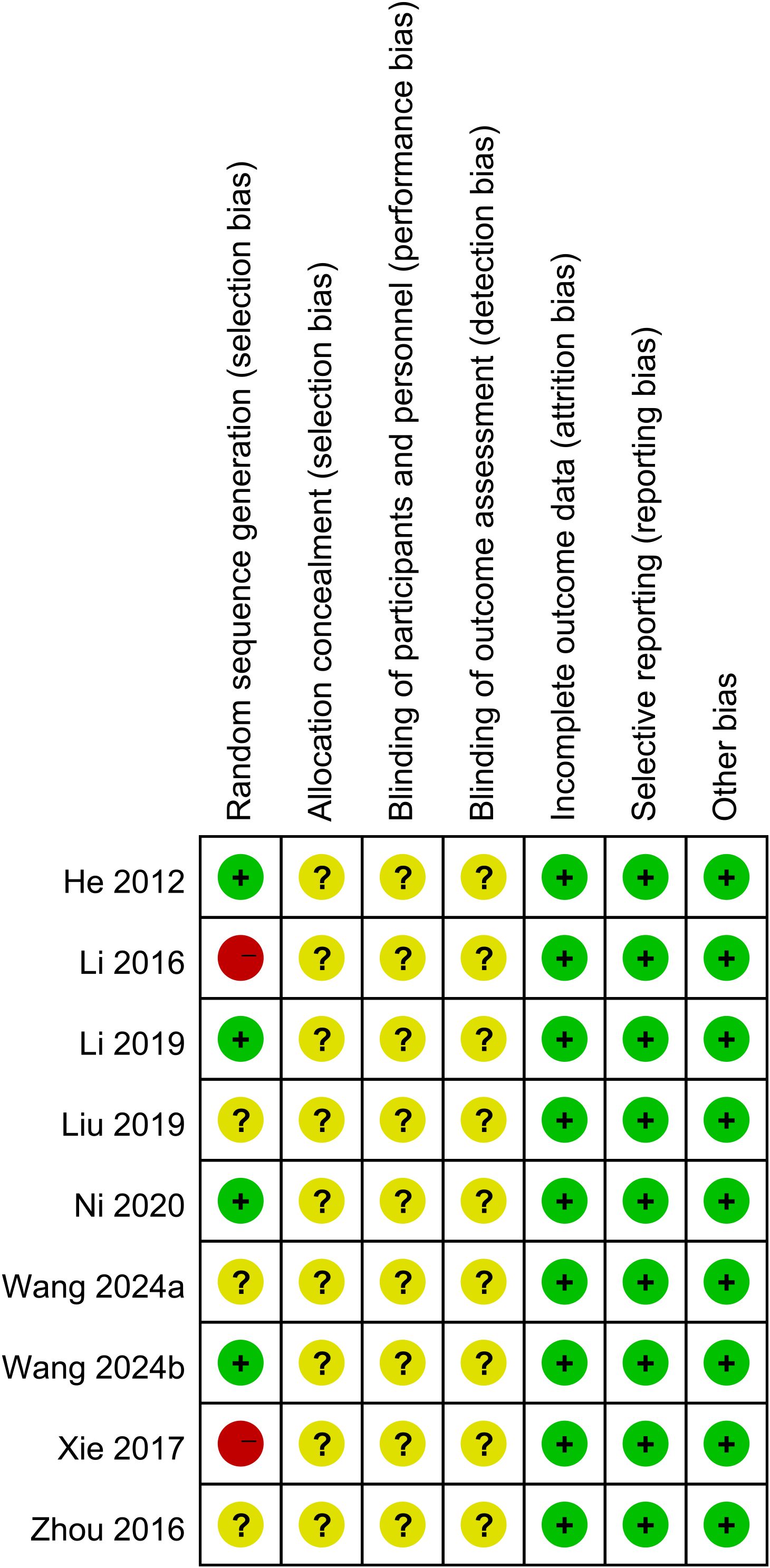
Nine studies were RCTs, and the risk of bias was assessed with the Cochrane Risk of Bias tool. Two studies did not report random sequence generation. Nine studies did not describe allocation concealment, blinding of participants and personnel, or blinding of outcome assessment (Figure 2). One retrospective study was included, and a score of 6 was given according to the Newcastle–Ottawa Scale (NOS) score.
Quantitative synthesis
Infection
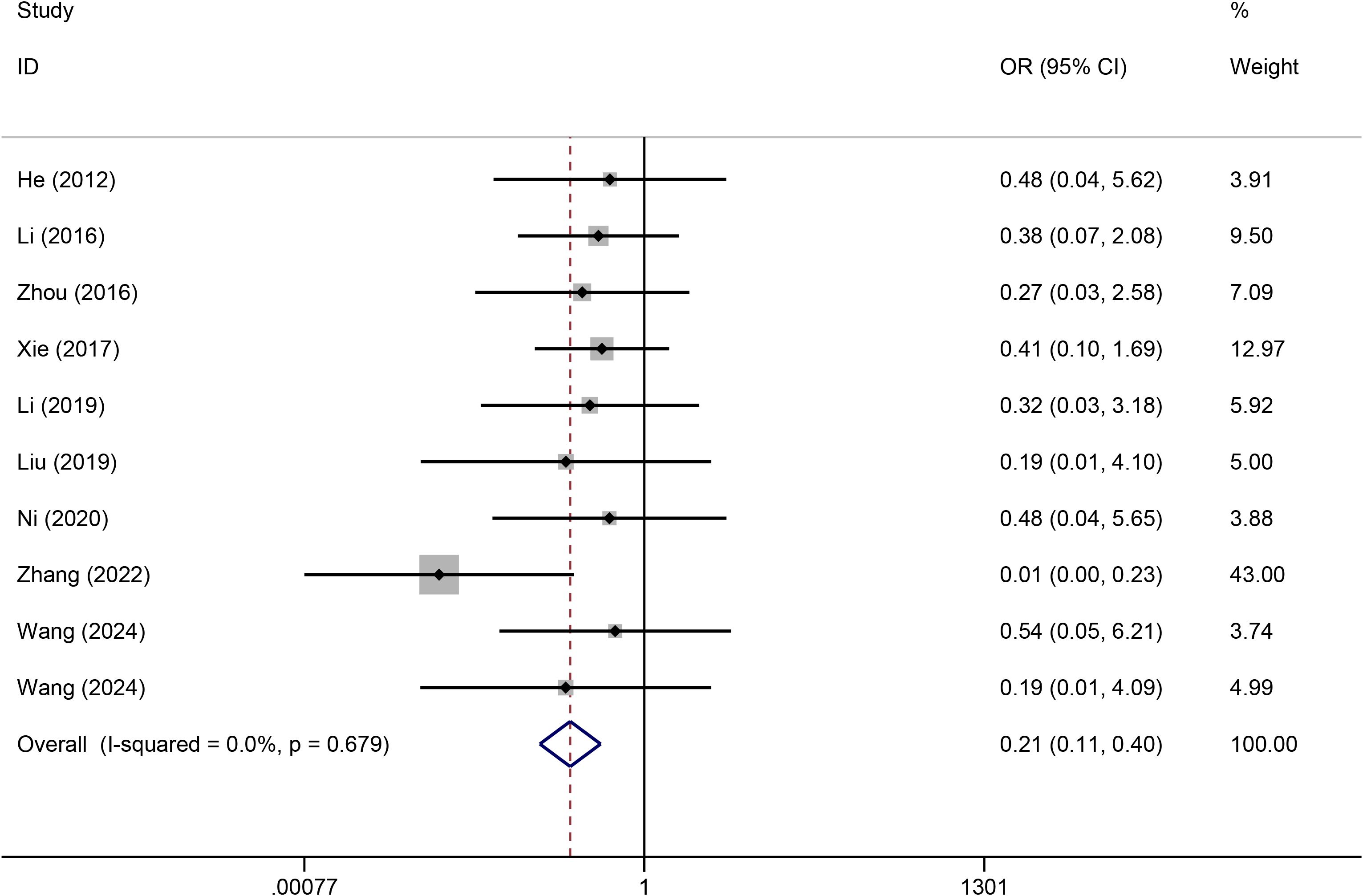
A heterogeneity test was conducted on the 10 included articles, and the results revealed no significant heterogeneity among the studies (I2 = 0%). Thus, a fixed effects model was used for the analysis. Meta-analysis revealed that compared with PICCs, TIVADs significantly decreased the infection risk (OR: 0.21, 95% CI: 0.11-0.40, p < 0.001) (Figure 3) (Table 2).
Catheter occlusion
Seven studies reported the effects of TIVADs and PICCs on catheter occlusion. No statistical heterogeneity was observed among the studies (I2 = 0%). Pooled analysis revealed that the risk of catheter occlusion was significantly lower for TIVADs than for PICCs (OR: 0.31, 95% CI: 0.13-0.77, p < 0.001) (Figure 4; Table 2).
Phlebitis
Seven studies reported phlebitis data. A fixed effects model was used to pool the data because heterogeneity across the included studies was low (I2 = 0.0%). The results revealed that compared with PICCs, TIVADs were associated with a lower phlebitis rate (OR: 0.16, 95% CI: 0.06-0.42, p < 0.001) (Figure 5; Table 2).
Catheter dislodgement
A total of six studies reported catheter dislodgement data. No statistical heterogeneity was observed among the studies (I2 = 0.0%). The forest plot revealed that the rate of catheter dislodgement was significantly lower for TIVADs than for PICCs (OR: 0.25, 95% CI: 0.08-0.76, p < 0.001) (Figure 6; Table 2).
Thrombosis
Five studies reported the effects of TIVADs and PICCs on thrombosis. No statistical heterogeneity was observed among the studies (I2 = 0.0%). The results revealed that thrombosis risk (OR: 0.37, 95% CI: 0.10-1.41, p = 0.144) was similar between the two groups (Table 2).
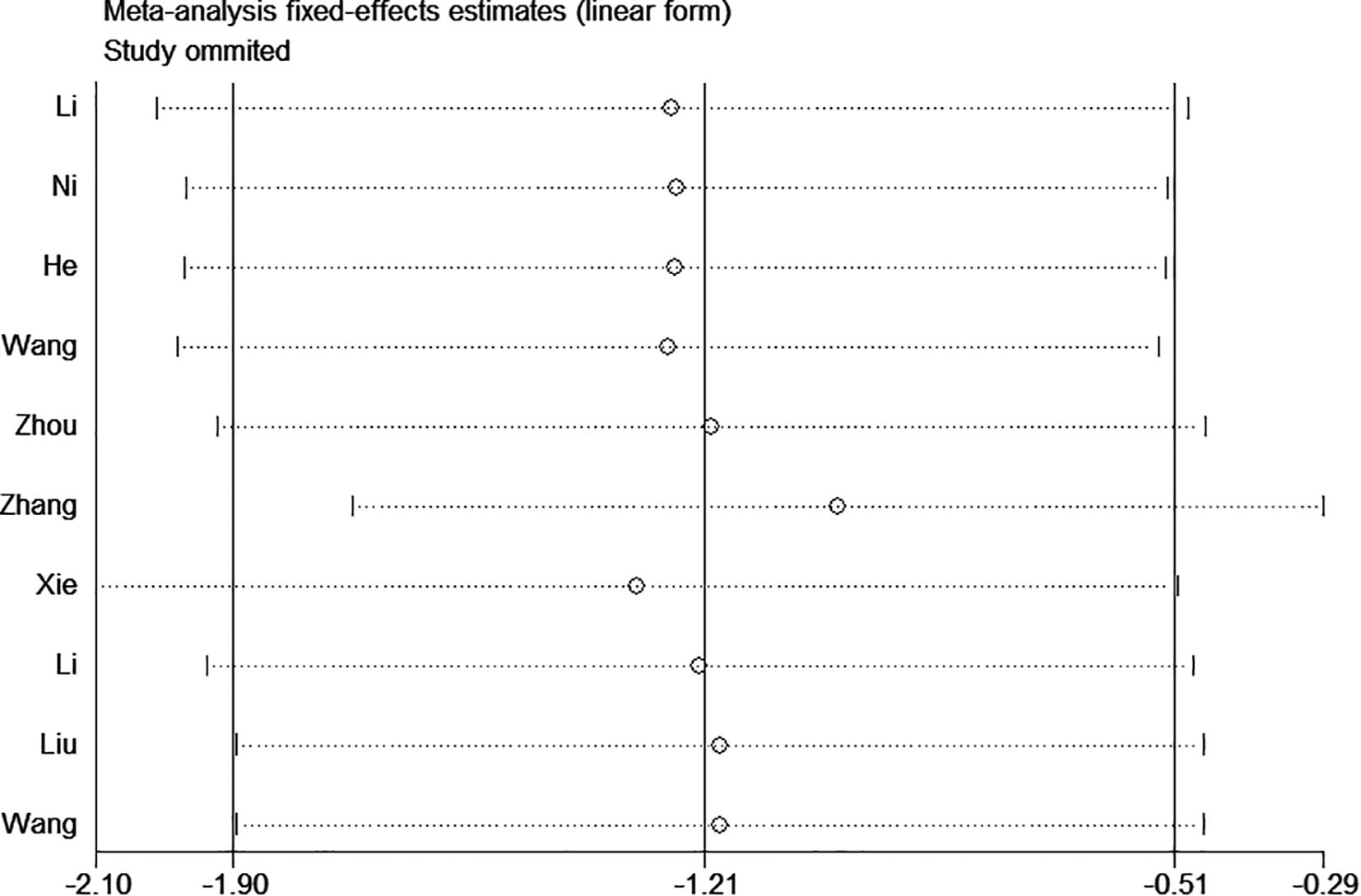
Sensitivity analysis
Sensitivity analyses for infection, catheter occlusion, phlebitis, catheter dislodgement, and thrombosis were performed, which demonstrated that excluding any one study did not affect the reliability of the results and suggested stability and reliance (Figure 7).
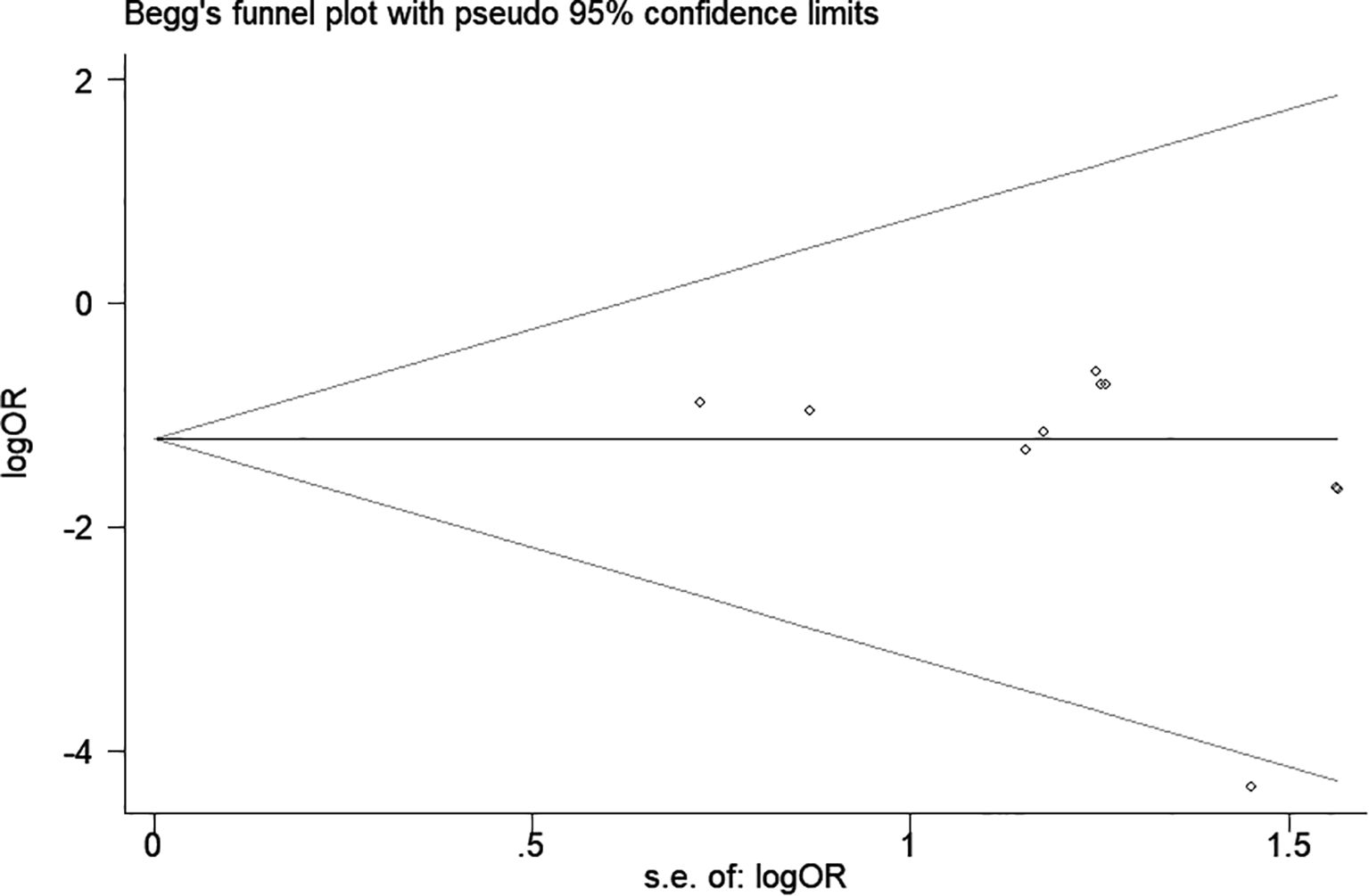
Publication bias
A funnel plot and Egger’s test were used to assess publication bias. As shown in Figure 8, the funnel plot demonstrated a relatively symmetrical distribution of studies, indicating a low likelihood of publication bias. This observation was supported by Begg’s test (p = 0.06) and Egger’s test (p = 0.192).
Discussion
The findings of this meta-analysis provide critical insights into the comparative safety of TIVADs and PICCs in patients undergoing chemotherapy for hematologic malignancies. Our results indicated that compared with PICCs, TIVADs were associated with a lower risk of infection, catheter occlusion, phlebitis, and catheter dislodgement.
One of the most notable findings was the significantly lower infection rate in the TIVAD group. The absence of an external catheter component in TIVAD reduces the risk of contamination, which is particularly important for immunocompromised patients with hematologic malignancies (25). In contrast, PICCs have an external portion that requires frequent handling and maintenance, increasing the risk of microbial colonization and subsequent bloodstream infections (26, 27). Given that infection is a major cause of morbidity and mortality in cancer patients, the lower infection risk associated with TIVAD presents a compelling argument for its preferential use in long-term chemotherapy regimens.
Similarly, the lower rates of catheter occlusion and phlebitis associated with TIVADs may be attributed to their completely implanted nature and the materials used in their construction. TIVADs are typically made of biocompatible materials that reduce thrombotic potential (28), whereas PICCs, which remain in peripheral veins for extended periods, may trigger endothelial irritation, leading to phlebitis and subsequent occlusion (29, 30). This is particularly relevant for patients requiring prolonged chemotherapy, as frequent catheter-related complications can lead to treatment delays and additional medical interventions.
The significant reduction in catheter dislodgement rates further supports the advantages of TIVADs. Since TIVADs are surgically implanted and anchored subcutaneously, they are less likely to be dislodged because of patient movement or accidental traction (31). In contrast, PICCs, which are externally accessible, are more vulnerable to accidental removal, particularly in ambulatory patients who may be at greater risk of catheter displacement (7). This finding suggests that TIVADs may provide greater reliability and continuity in treatment, improving patient compliance and reducing the need for catheter replacement procedures.
Interestingly, our analysis did not find a significant difference in thrombosis risk between the two devices. Although previous studies have suggested that PICCs may be more thrombogenic due to their placement in peripheral veins with slower blood flow (32), the absence of a statistically significant difference in our study suggests that other factors, such as catheter materials, standardized insertion protocols, and thromboprophylaxis practices, may play crucial roles in thrombosis prevention (33). Nevertheless, the lack of detailed thromboprophylaxis data in the included studies limits definite conclusions. Future research should focus on stratifying thrombosis risk by anticoagulation protocols, catheter characteristics, and insertion methods to better inform clinical practice.
Although most studies included in this meta-analysis were RCTs, many lacked sufficient reporting of key methodological features, including randomization procedures, allocation concealment, or blinding. These omissions raise concerns about potential bias, particularly with respect to estimation of complication rates and overall effect sizes. The lack of methodological rigor may have inflated the observed benefits of TIVADs. Therefore, the results should be interpreted cautiously, and further high-quality, multicenter randomized trials are needed to corroborate these finding. However, catheter tip malpositioning is a known risk factor for PICC-related complications. Marano et al. proposed an ultrasonographic method that enables safe and accurate tip positioning without radiation exposure, offering a promising alternative to traditional radiographic confirmation (34). This technique highlights the importance of standardized, precise placement protocols in reducing procedural risks. Additionally, a recent five-year analysis by Abou-Mrad et al. demonstrated the long-term safety and efficacy of TIVADs in oncology patients, underscoring the critical role of optimized implantation techniques and maintenance protocols (35). These findings complement our results and further support the clinical value of TIVADs when used appropriately. Notably, several studies have reported immediate or early-phase benefits of PICCs (such as shorter insertion times, simpler insertion procedures, and lower upfront costs) compared to TIVADs, particularly in patients with shorter chemotherapy regimens (36, 37). However, our meta-analysis could not fully quantify these due to heterogeneous reporting and lack of sufficient data.
In addition to safety-related outcomes, patient-centered aspects such as quality of life (QoL) and ease of use are also important when comparing TIVADs and PICCs. A prospective cohort study in patients with breast or colon cancer found no significant overall difference in global QoL between the two devices, although ports were associated with more pain at insertion, whereas PICCs had a greater negative psychosocial impact (37). More recently, a large observational study in women receiving neoadjuvant chemotherapy reported that overall QoL scores significantly favored PICC-ports over PICCs, particularly among younger patients, with advantages in psychological and social domains, while device-related complication rates were similar between groups (27). Furthermore, retrospective data suggest that TIVADs may offer longer catheter dwell times and fewer removals due to complications, thereby reducing treatment interruptions and potentially improving ease of use and patient satisfaction (38). Taken together, these findings indicate that while complication rates remain essential endpoints, QoL and usability considerations should also be integrated into future comparative studies of vascular access devices.
This meta-analysis has several limitations. First, all included studies were from China, which may limit the generalizability of our results. Differences in health care systems, catheter insertion techniques, maintenance protocols, and complication monitoring practices across regions could influence clinical outcomes. Therefore, our conclusions should be interpreted with caution when applied to non-Chinese populations. Nonetheless, retrospective studies from other countries have similarly reported that TIVADs are associated with fewer complications than PICCs among cancer patients, which provides external support for our findings despite regional limitations. Second, while most included studies were RCTs, the methodological quality of the studies varied. Some studies lacked detailed reporting on randomization, allocation concealment, and blinding, introducing potential bias. Additionally, the inclusion of one retrospective study may have further influenced the reliability of our findings. Future studies with rigorous methodological designs and larger sample sizes are needed to strengthen this evidence. Third, this meta-analysis was limited to five safety-related outcomes because of the availability of data. However, other clinically meaningful factors, such as catheter longevity, patient-reported satisfaction, ease of device management, chemotherapy regimens and cost-effectiveness were rarely reported in the included studies (39). In addition, the absence of CTCAE-based grading across trials may reduce the granularity of our safety assessment and should be considered when interpreting the results. These outcomes are particularly relevant in the context of long-term cancer care, where patient comfort, treatment adherence, and health care resource utilization are critical. Future research should incorporate these dimensions to provide a more comprehensive assessment of central venous access options in oncology.
Conclusion
In summary, our meta-analysis suggests a potential association between TIVAD use and a lower risk of complications compared with PICCs in patients with hematologic malignancies. Nevertheless, given the geographic limitations of the included studies, the absence of cost-effectiveness assessments, and the moderate overall sample size, these results should be interpreted with caution. Additional large-scale, multicenter studies in diverse health care settings are required to validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
MG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. XH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sochacka-Cwikla A, Maczynski M, and Regiec A. FDA-approved drugs for hematological Malignancies-the last decade review. Cancers (Basel). (2021) 14. doi: 10.3390/cancers14010087
2. Lafarge A, Chean D, Whiting L, Clere-Jehl R, Groupe de Recherche en Reanimation Respiratoire du patient, d.O.-H, Clinical Research in Intensive, C, et al. Management of hematological patients requiring emergency chemotherapy in the intensive care unit. Intensive Care Med. (2024) 50:849–60. doi: 10.1007/s00134-024-07454-z
3. Heidenreich D, Hansen E, Kreil S, Nolte F, Jawhar M, Hecht A, et al. The insertion site is the main risk factor for central venous catheter-related complications in patients with hematologic Malignancies. Am J Hematol. (2022) 97:303–10. doi: 10.1002/ajh.26445
4. Boll B, Schalk E, Buchheidt D, Hasenkamp J, Kiehl M, Kiderlen TR, et al. Central venous catheter-related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management, and prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann Hematol. (2021) 100:239–59. doi: 10.1007/s00277-020-04286-x
5. Patel GS, Jain K, Kumar R, Strickland AH, Pellegrini L, Slavotinek J, et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-hematological Malignancies. Supp. Care Cancer. (2014) 22:121–8. doi: 10.1007/s00520-013-1941-1
6. Narita A, Takehara Y, Maruchi Y, Matsunaga N, Ikeda S, Izumi Y, et al. Usefulness of peripherally inserted central catheter port system (PICC-PORT) implantation in the sitting position: a new technique for cases unsuitable for conventional implantation. Jpn J Radiol. (2023) 41:108–13. doi: 10.1007/s11604-022-01317-7
7. Li J, Hu Z, Luo M, Wu Z, Dou X, Wang Z, et al. Safety and effectiveness of tunneled peripherally inserted central catheters versus conventional PICC in adult cancer patients. Eur Radiol. (2024) 34:7776–85. doi: 10.1007/s00330-024-10852-y
8. Larcher R, Barrigah-Benissan K, Ory J, Simon C, Beregi JP, Lavigne JP, et al. Peripherally inserted central venous catheter (PICC) related bloodstream infection in cancer patients treated with chemotherapy compared with noncancer patients: A propensity-score-matched analysis. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15123253
9. Sharp R, Xu Q, Pumpa R, Elliott L, Corsini N, Marker J, et al. Supportive care needs of adults living with a peripherally inserted central catheter (PICC) at home: a qualitative content analysis. BMC Nurs. (2024) 23:4. doi: 10.1186/s12912-023-01614-0
10. Manik Yuniawaty Wetan N, Irawan H, and Adiputra PAT. Spontaneous catheter tip migration in Totally Implantable Venous Access Port (TIVAP) to right atrium. Int J Surg Case Rep. (2024) 123:110122. doi: 10.1016/j.ijscr.2024.110122
11. Guo Y, Wang X, Huang Y, Wu X, and Wang Y. Evaluation of the efficacy of totally implantable venous access port in breast cancer patients undergoing chemotherapy. Discov Oncol. (2025) 16:383. doi: 10.1007/s12672-025-02020-5
12. Thiel K, Kalmbach S, Maier G, Wichmann D, Schenk M, Konigsrainer A, et al. Standardized procedure prevents perioperative and early complications in totally implantable venous-access ports-a complication analysis of more than 1000 TIVAP implantations. Langenbecks Arch Surg. (2022) 407:3755–62. doi: 10.1007/s00423-022-02656-9
13. Werba A, Hennes R, Schuh F, Holze M, Maichle B, Ramouz A, et al. Prospective, monocentric observational study on the clinical use and patient satisfaction of an implantable venous access Port. Langenbecks Arch Surg. (2025) 410:84. doi: 10.1007/s00423-025-03654-3
14. Zhang H, Li Y, Zhu N, Li Y, Fu J, and Liu J. Comparison of peripherally inserted central catheters (PICCs) versus totally implantable venous-access ports in pediatric oncology patients, a single center study. Sci Rep. (2022) 12:3510. doi: 10.1038/s41598-022-07584-8
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
16. He Y, Sun YP, Li N, and Shen JL. Comparison of the effects of PICC and venous access port in patients with hematological Malignancies. Chin J Nurs. (2012) 47:1001–3. doi: 10.3761/j.issn.0254-1769.2012.11.011.015
17. Li J, Wang H, and Ye HY. Comparison of application and key nursing points of PICC and VPA in chemotherapy for patients with leukemia. J Clin Nurs Pract. (2016) 2:39–41. doi: 10.11997/nitcwm.201608013
18. Zhou Y. Evaluation of the application of implantable intravenous infusion port and PICC in patients with Malignant tumor in Department of Hematology. China Health Care Nutr. (2016) 26:286.
19. Xie LL. Comparison of the efficacy of implantable intravenous infusion port and PICC in patients with hematologic tumor chemotherapy. Nurs Pract Res. (2017) 14:114–5. doi: 10.3969/j.issn.1672-9676.2017.11.050
20. Li SJ and Lei M. Comparison on the application effects of implanted venous access port and PICC in hematologic Malignancy chemotherapy. Clin Med Eng. (2019) 26:1553–4. doi: 10.3969/j.issn.1674-4659.2019.11.1553
21. Li ZY, Liu FY, and Hu XY. Clinical application and nursing study of completely implantable intravenous infusion port in children with hematologic tumors. Electron. J Pract Clin Nurs Sci. (2019) 4:89–90.
22. Ni LF. Comparison of the mid-term and short-term effects of venous accessport and peripherally inserted central catheter in application of department of hematology. China Med Pharm. (2020) 10:167–169,203. doi: 10.3969/j.issn.2095-0616.2020.23.044
23. Wang JF and Wang L. Comparative analysis of implanted venous access port versus hematologic tumor. Hebei Med J. (2024) 46:2350–2. doi: 10.3969/j.issn.1002-7386.2024.15.025
24. Wang XY. Clinical application and nursing analysis of a fully implantable intravenous infusion port in hematological tumors. Prim Med Forum. (2024) 6. doi: 10.12373/jcyxlt.2024.09.14729
25. Bertoglio S, Cafiero F, Meszaros P, Varaldo E, Blondeaux E, Molinelli C, et al. PICC-PORT totally implantable vascular access device in breast cancer patients undergoing chemotherapy. J Vasc Access. (2020) 21:460–6. doi: 10.1177/1129729819884482
26. Bertoglio S, Faccini B, Lalli L, Cafiero F, and Bruzzi P. Peripherally inserted central catheters (PICCs) in cancer patients under chemotherapy: A prospective study on the incidence of complications and overall failures. J Surg Oncol. (2016) 113:708–14. doi: 10.1002/jso.24220
27. Pinelli F, Barbani F, Defilippo B, Fundaro A, Nella A, Selmi V, et al. Quality of life in women with breast cancer undergoing neoadjuvant chemotherapy: comparison between PICC and PICC-port. Breast Cancer. (2024) 31:945–54. doi: 10.1007/s12282-024-01608-z
28. Blanco-Guzman MO. Implanted vascular access device options: a focused review on safety and outcomes. Transfusion. (2018) 58:558–68. doi: 10.1111/trf.14503
29. Pu YL, Li ZS, Zhi XX, Shi YA, Meng AF, Cheng F, et al. Complications and costs of peripherally inserted central venous catheters compared with implantable port catheters for cancer patients: A meta-analysis. Cancer Nurs. (2020) 43:455–67. doi: 10.1097/NCC.0000000000000742
30. Oza-Gajera BP, Davis JA, Farrington C, Lerma EV, Moossavi S, Sheta MA, et al. PICC line management among patients with chronic kidney disease. J Vasc Access. (2023) 24:329–37. doi: 10.1177/11297298211025897
31. Goltz JP, Scholl A, Ritter CO, Wittenberg G, Hahn D, and Kickuth R. Peripherally placed totally implantable venous-access port systems of the forearm: clinical experience in 763 consecutive patients. Cardiovasc Intervent Radiol. (2010) 33:1159–67. doi: 10.1007/s00270-010-9854-6
32. Chen L, Lu Y, Wang L, Pan Y, and Zhou X. Construction of a nomogram risk prediction model for PICC-related venous thrombosis and its application. Asian J Surg. (2024) 47:107–11. doi: 10.1016/j.asjsur.2023.05.043
33. Chopra V, Kaatz S, Grant P, Swaminathan L, Boldenow T, Conlon A, et al. Risk of venous thromboembolism following peripherally inserted central catheter exchange: an analysis of 23,000 hospitalized patients. Am J Med. (2018) 131:651–60. doi: 10.1016/j.amjmed.2018.01.017
34. Marano L, Izzo G, Esposito G, Petrillo M, Cosenza A, Marano M, et al. Peripherally inserted central catheter tip position: a novel empirical-ultrasonographical index in a modern surgical oncology department. Ann Surg Oncol. (2014) 21:656–61. doi: 10.1245/s10434-013-3391-x
35. Abou-Mrad A, Marano L, and Oviedo RJ. A monocentric analysis of implantable ports in cancer treatment: five-year efficacy and safety evaluation. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16162802
36. Tang TT, Liu L, Li CX, Li YT, Zhou T, Li HP, et al. Which is better for patients with breast cancer: totally implanted vascular access devices (TIVAD) or peripherally inserted central catheter (PICC)? World J Surg. (2019) 43:2245–9. doi: 10.1007/s00268-019-05022-x
37. Burbridge B, Lim H, Dwernychuk L, Le H, Asif T, Sami A, et al. Comparison of the quality of life of patients with breast or colon cancer with an arm vein port (TIVAD) versus a peripherally inserted central catheter (PICC). Curr Oncol. (2021) 28:1495–506. doi: 10.3390/curroncol28020141
38. Yun WS and Yang SS. Comparison of peripherally inserted central catheters and totally implanted venous access devices as chemotherapy delivery routes in oncology patients: A retrospective cohort study. Sci Prog. (2021) 104:368504211011871. doi: 10.1177/00368504211011871
Keywords: peripherally inserted central catheters, totally implantable venous access ports, hematologic malignancies, infection, meta-analysis
Citation: Gu M and Huang X (2025) Safety of totally implantable venous access devices and peripherally inserted central catheters in hematological malignancies patients: a meta-analysis. Front. Oncol. 15:1679363. doi: 10.3389/fonc.2025.1679363
Received: 04 August 2025; Accepted: 16 October 2025;
Published: 31 October 2025.
Edited by:
Andrea Visentin, University of Padua, ItalyReviewed by:
Bilgin Kadri Aribas, Bülent Ecevit University, TürkiyeGiovanni Manfredi Assanto, Sapienza University of Rome, Italy
Copyright © 2025 Gu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaguang Huang, aHVhbmd4aWFndWFuZzExMTNAMTYzLmNvbQ==
 Meier Gu
Meier Gu Xiaguang Huang
Xiaguang Huang