- 1Department of Radiation Oncology, City of Hope, Duarte, CA, United States
- 2Department of Medical Oncology, City of Hope, Duarte, CA, United States
- 3Department of Dermatology, City of Hope, Duarte, CA, United States
Introduction: Immune checkpoint inhibitors (ICI) effectively treat advanced cutaneous squamous cell carcinoma (cSCC), yet some patients continue to have disease progression. Combining radiation therapy (RT) with ICI represents a potential therapeutic option, yet limited data exist regarding oncologic outcomes and safety profile.
Methods: This retrospective cohort study examined patients treated with concurrent ICI and RT between April 2019 and November 2022 and stratified by locally advanced or metastatic status. Outcomes included locoregional control (LRC), freedom from distant metastases (FFDM), progression-free survival (PFS), overall survival (OS), and toxicity. Statistical analysis was performed using Kaplan-Meier or Fine-Gray competing risk survival analyses.
Results: Thirteen patients (median age 77 years) with locally advanced (53.8%) or metastatic (46.2%) cSCC on cemiplimab (84.6%) or pembrolizumab (15.4%) received concomitant RT using intensity-modulated radiotherapy (69.2%) or stereotactic body radiotherapy (30.8%). With median follow-up of 15.4 months, overall 1-year and 2-year outcomes were OS: 75.2% and 62.7%; PFS: 59.8% and 25.6%; FFDM: 83.8% and 62.4%; LRC 100% and 84.3%, respectively. Locally advanced patients had significantly greater LRC than metastatic patients (100% vs. 56.3%; p<0.001), but no significant difference in PFS, FFDM, or OS. Only one patient experienced grade 3 radiation dermatitis, with no grade 4+ toxicities.
Conclusion: Radioimmunotherapy demonstrated favorable oncologic outcomes with minimal toxicity. Addition of consolidative RT to ICI therapy may represent a safe and effective approach for this challenging patient population, warranting further prospective investigation.
Introduction
With an estimated incidence of 1.8 million cases per year, cutaneous squamous cell carcinoma (cSCC) afflicts significant morbidity on the US population (1). The National Comprehensive Cancer Center (NCCN) stratifies mortality risk of a primary cSCC lesion into low, high, or very high-risk based on clinicopathologic characteristics including size, location, depth of invasion, perineural invasion (PNI), and others (2). An estimated 4000–8000 patients die of cSCC annually, and the majority of these deaths arise from patients with high-risk or very high-risk disease (3). Historically, cSCC patients with local lymph node involvement exhibit 2-year overall survival (OS) of 69% to 83%, and increasing nodal burden correlates with poorer outcomes (4). Patients with distant metastases, arising in 2% of cSCC patients, harbor a range of survival outcomes at 2-years of 38% to 64%, whereby mortality may be 2-fold higher in immunocompromised patients (4–6).
When possible, surgical resection to clear margins remains the gold standard treatment for cSCC (7). Radiation therapy (RT) is typically reserved for primary treatment of unresectable tumors, or RT can be delivered adjuvantly following surgery to reduce risk of recurrence in tumors with high-risk features, such as PNI, positive margins, tumor stage of T3 or higher, parotid gland involvement, or in the setting of immunosuppression (7–9). Furthermore, adjuvant radiation may improve survival in tumors with extensive PNI, lymph node positivity, or metastatic disease (10).
Since 2018, immune checkpoint inhibitors (ICI) have demonstrated significant activity in the treatment of locally advanced and metastatic cSCC no longer amenable to curative local therapy (11). Both cemiplimab and pembrolizumab are approved by the United States Federal Drug Administration (FDA) in advanced cSCC, and there is now evidence that ICI therapy may benefit patients if used neoadjuvantly for earlier stage, resectable disease (12, 13).
While ICI treatment of advanced cSCC can result in dramatic improvement, approximately 50% of patients with advanced cSCC do not respond to ICI (14, 15). These patients may still benefit from RT, yet few series have thus far reported on the outcomes of concurrent use of RT and ICI therapy in locally advanced and metastatic cSCC, and their combined use is not well understood (16). Given the concern of additive toxicities among these two modalities, we set out to review 13 cases of advanced cSCC with clinical progression on ICI monotherapy, to which RT was added to enhance disease control while maintaining an overall acceptable toxicity profile. In this study, we examine a cohort of patients with cSCC treated with RT and ICI and report rates of oncologic control, survival, and toxicity.
Materials and methods
Study design
We examined a retrospective cohort of patients at a single institution who received concurrent ICI and RT between April 2019 and November 2022. All patients were at least 18 years of age with tissue diagnosis confirming cSCC. RT was delivered using either intensity modulated radiotherapy (IMRT) or stereotactic body radiotherapy (SBRT) techniques. Concurrent radioimmunotherapy was defined as ICI infusion within three weeks before or after RT delivery. Deidentified patient data was collected from electronic medical records (EMR), and the study was approved by the Institutional Review Board and conducted following the ethical tenets of the Helsinski Declaration.
Data collection
The EMR provided information regarding patient demographic, clinical, oncologic, and treatment data. Subjects were characterized as having locally advanced or metastatic cSCC based on disease extent during initial staging work-up and multidisciplinary tumor board discussion. Tumor (T) stage was determined using the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 8th edition. Nodal (N) stage for patients with cSCC of the head and neck (H&N) was defined based on non-HPV-associated H&N cancers. For cSCC arising from the body, patients were deemed either node-positive (N1) or node-negative (N0). Patients were considered metastatic (M1) by distant spread to organs or non-regional lymph nodes. Toxicity data was collected at each visit using the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0). Tabulated toxicity data incorporated the highest grade acute and late toxicity documented in the EMR for each specific patient.
Study endpoints and statistical analysis
Patients were monitored with clinical examination and imaging, either with computed tomography (CT), positron emission tomography (PET), and/or magnetic resonance imaging (MRI), performed at minimum every 12 weeks, or as clinically indicated. Radiographic response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 criteria for patients with measurable cSCC.
Endpoints were analyzed at 1 year and 2 years following completion of RT. Locoregional control (LRC) was defined as the absence of disease progression or recurrence in the radiation field, first echelon draining lymph node basin, or in-transit tissue. Similarly, freedom from distant metastases (FFDM) was attributed to distant progression, either from new or enlarging metastatic tumor deposits noted on physical examination or imaging. Progression-free survival (PFS) was defined as any disease progression, or any death event. Patients without progression were censored at last follow-up. Finally, OS was calculated using death from any cause after completion of RT. Statistical analyses and comparison between groups were performed using Kaplan-Meier or Fine-Gray competing risk survival analyses, as appropriate. 95% confidence interval (CI) is also reported for each endpoint. The date of last follow-up was January 30, 2023. Given the small sample size, the statistical comparisons between groups should be considered exploratory in nature, and p-values interpreted with appropriate caution.
Results
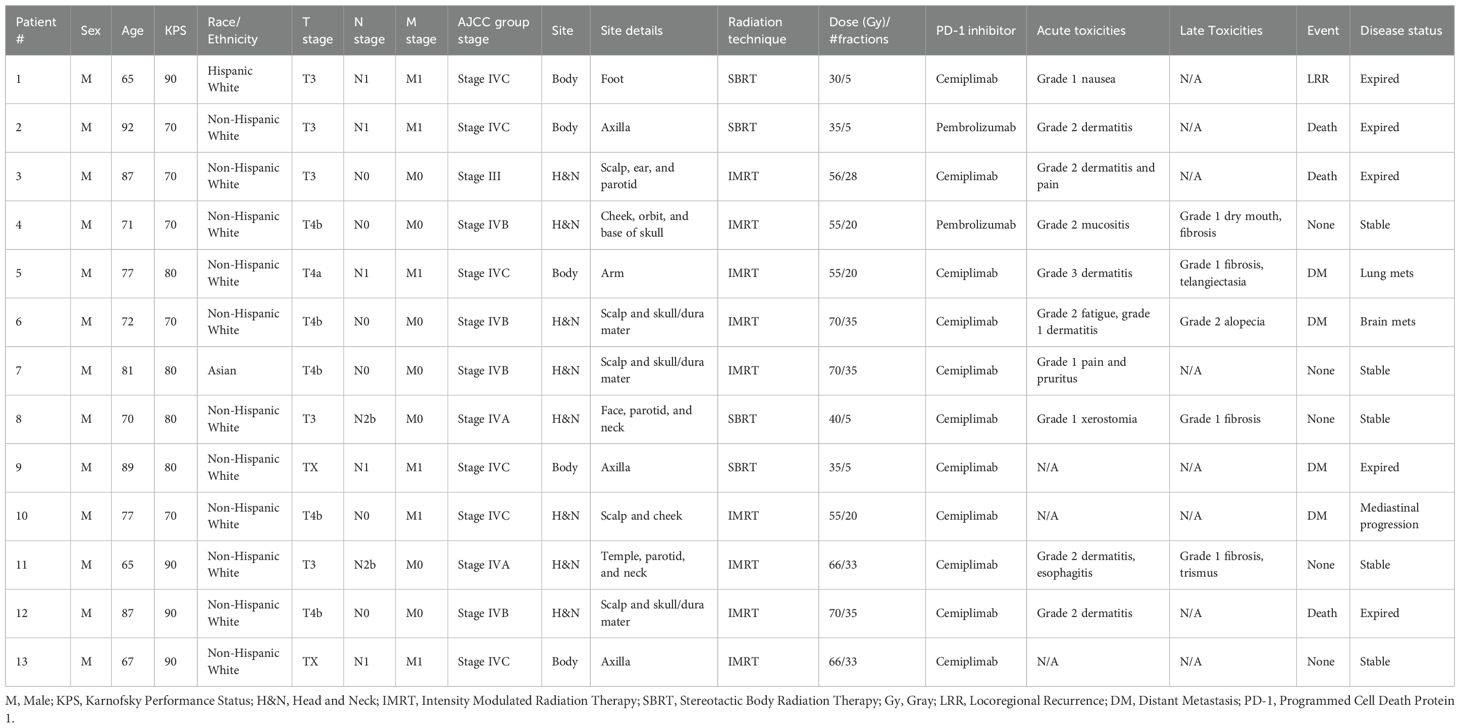
Thirteen patients with either locally advanced (53.8%) or metastatic (46.2%) cSCC received concurrent RT while receiving cemiplimab (84.6%) or pembrolizumab (15.4%) (Table 1). Median age was 77 years (range 65-92), and all patients were male. Most patients (84.6%) were non-Hispanic White. Median Karnofsky performance status (KPS) was 80 (range 70-90). All identified primary cSCC lesions were at least T3, and all patients had very high-risk disease based on the NCCN cSCC guidelines (2). Most patients (61.5%) had lymph node involvement or distant metastases, and almost half of the cSCC lesions (46.2%) were T4a or T4b (Table 2). Radiation dose and fractionation ranged from 55 to 70 Gray (Gy) in 20 to 35 daily fractions for IMRT (69.2%), and 30 to 40 Gy for SBRT (30.8%) in 5 fractions delivered every other day.
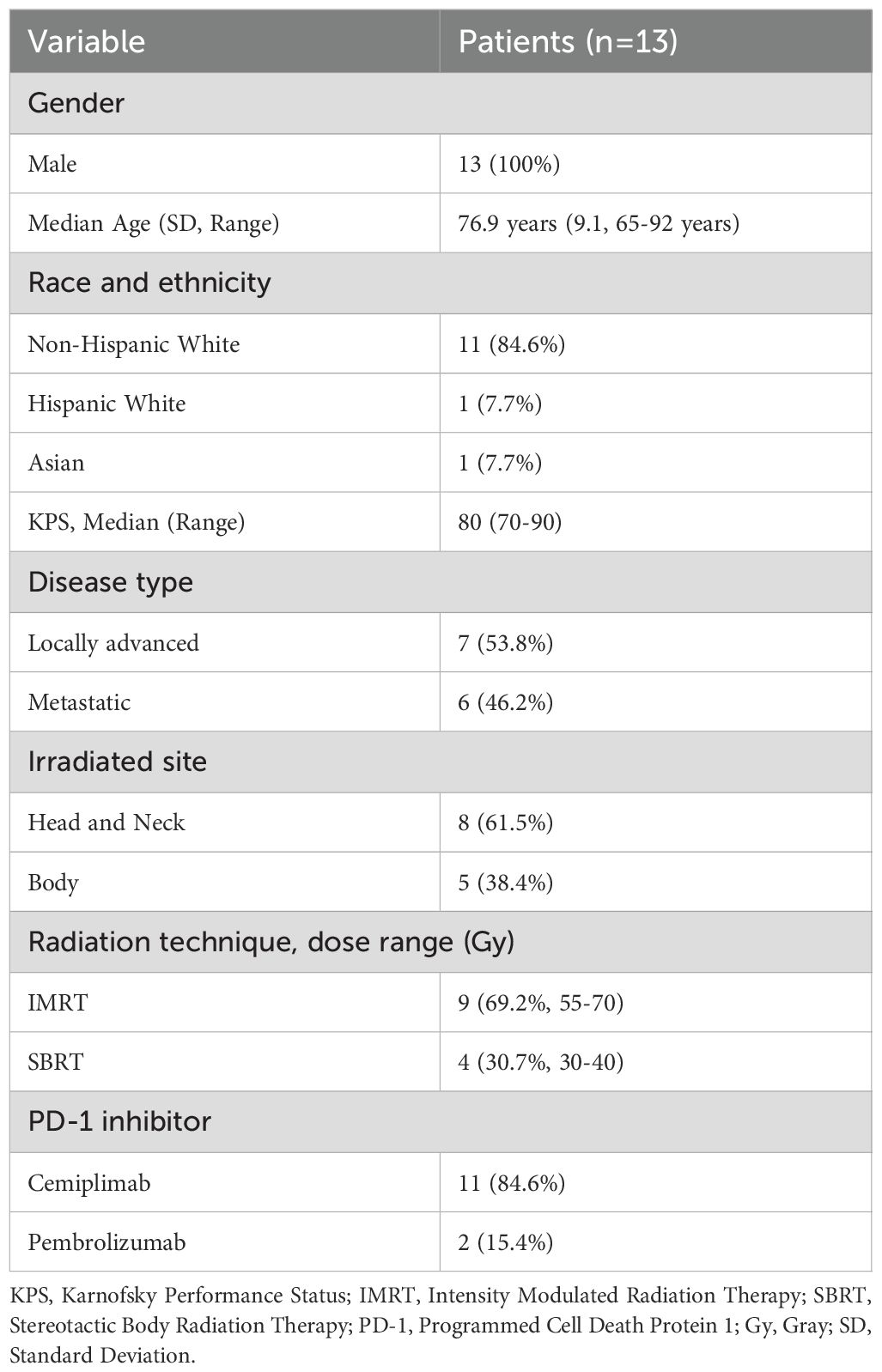
Table 1. Demographic characteristics of overall cohort of patients receiving radiation and immune checkpoint inhibitor therapy.
Most patients underwent RT to the H&N (61.5%) Three patients had parotid gland involvement, which was included in the radiation field. Of those, two had N2b disease with multiple pathologic ipsilateral neck lymph nodes, while the third was N0. In the other five H&N cSCC patients, all had T4b lesions with skull bone invasion, and none had positive neck lymph nodes. Seven out of eight total H&N patients received IMRT while the eighth received SBRT. One patient who received H&N RT had M1 disease with mediastinal lymphadenopathy.
Regarding the patients receiving RT to the body (38.5%), four underwent radiation to a proximal upper extremity and/or axilla while one had treatment of a lower extremity. All five patients who received RT to the body had node-positive and metastatic disease. Two patients who underwent RT to axillary lesions had unknown primary cutaneous sites and were designated TX. Three patients underwent SBRT while the other two received IMRT.
Median follow-up was 15.4 months overall and 16.7 months for living patients. At final follow-up, eight patients remained alive, and five were without disease progression following radioimmunotherapy. Outcomes were analyzed at 1 and 2 years (Figure 1). Median OS was 25 months [95% CI: 17.6 months to not reached (NR)], and 1-year and 2-year median OS were 75.2% [95% CI: 54.2%-100%] and 62.7% [95% CI: 38.6%-100%], respectively (Table 3). Median PFS was 21.5 months [5.2 months to NR], and 1-year median PFS was 59.8% [95% CI: 37.8%-94.7%] while 2-year median PFS decreased to 25.6% [95% CI: 8.3%-78.9%]. There was also a drop in median FFDM from 83.8% [95% CI: 65.6%-100%] at 1 year compared to 62.4% [95% CI: 39.0%-99.8%] at 2 years. Finally, median LRC among the entire patient cohort was 100% [95% CI: 100%-100%] at 1 year and 84.3% [95% CI: 60.6%-100%] at 2 years. In-field local control was 100% in both groups at 1-year and 2-years, and there were no in-field local failures following RT.
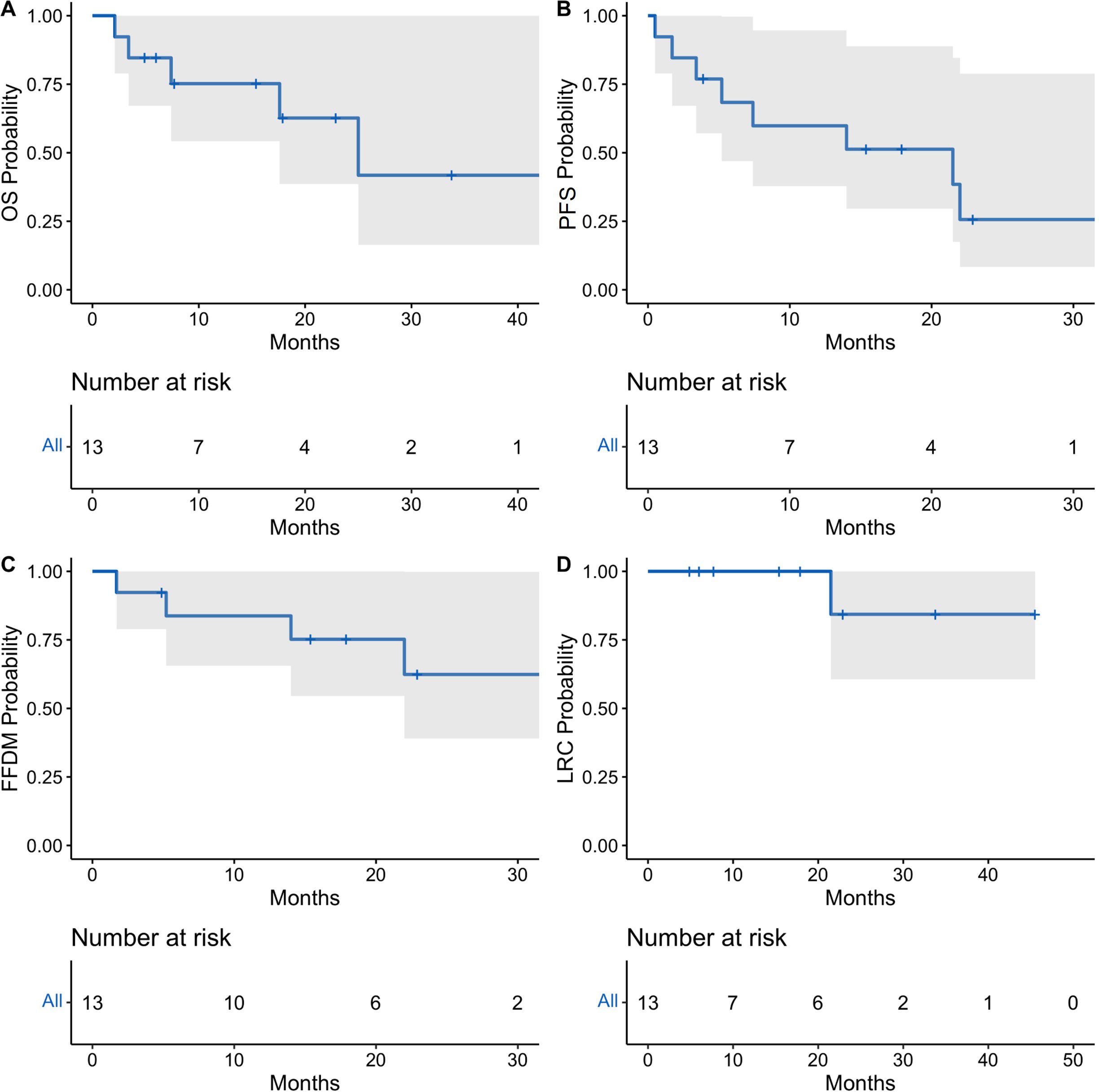
Figure 1. Oncologic outcomes for total cohort of patients receiving concurrent radioimmunotherapy. Kaplan-Meier estimates showing probability of overall survival (A), progression-free survival (B), freedom from distant metastasis (C), and locoregional control (D). Tick-marks indicate censored data. Data was analyzed at 12 and 24 months. 95% confidence intervals denoted by shaded areas.
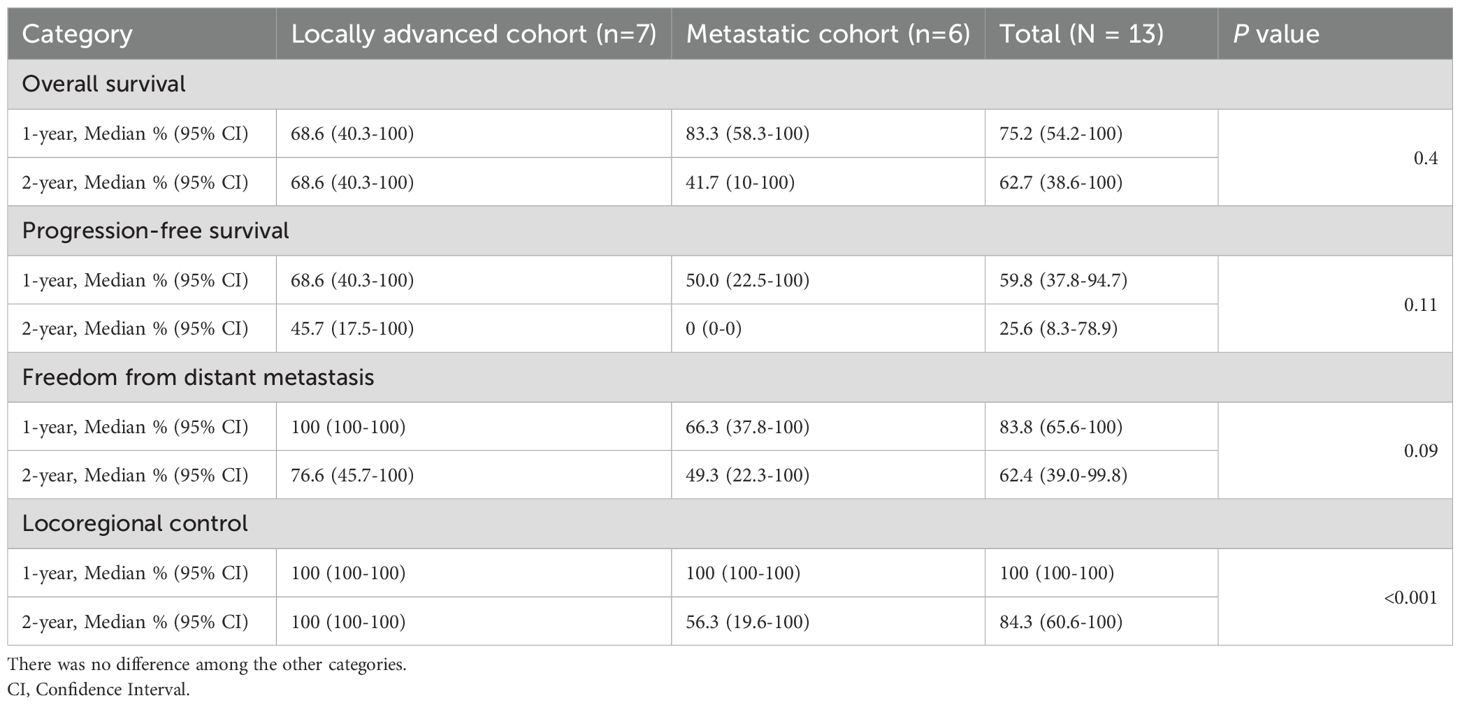
Table 3. Summary of oncologic outcomes after concurrent radioimmunotherapy. There was a statistically significant difference of locoregional control between locally advanced and metastatic cohorts (p<0.001).
Patients were also stratified by locally advanced or metastatic status at the time of radioimmunotherapy (Figure 2). Both locally advanced and metastatic cSCC patients had a median probability of LRC of 100% [95% CI: 100%-100%] at 1 year. At 2 years, median LRC remained 100% [95% CI: 100%-100%] for locally advanced patients, while median LRC for metastatic patients decreased to 56.3% [95% CI: 19.6%-100%]. Patients with locally advanced cSCC had significantly greater LRC compared to patients with metastatic disease (p<0.001).
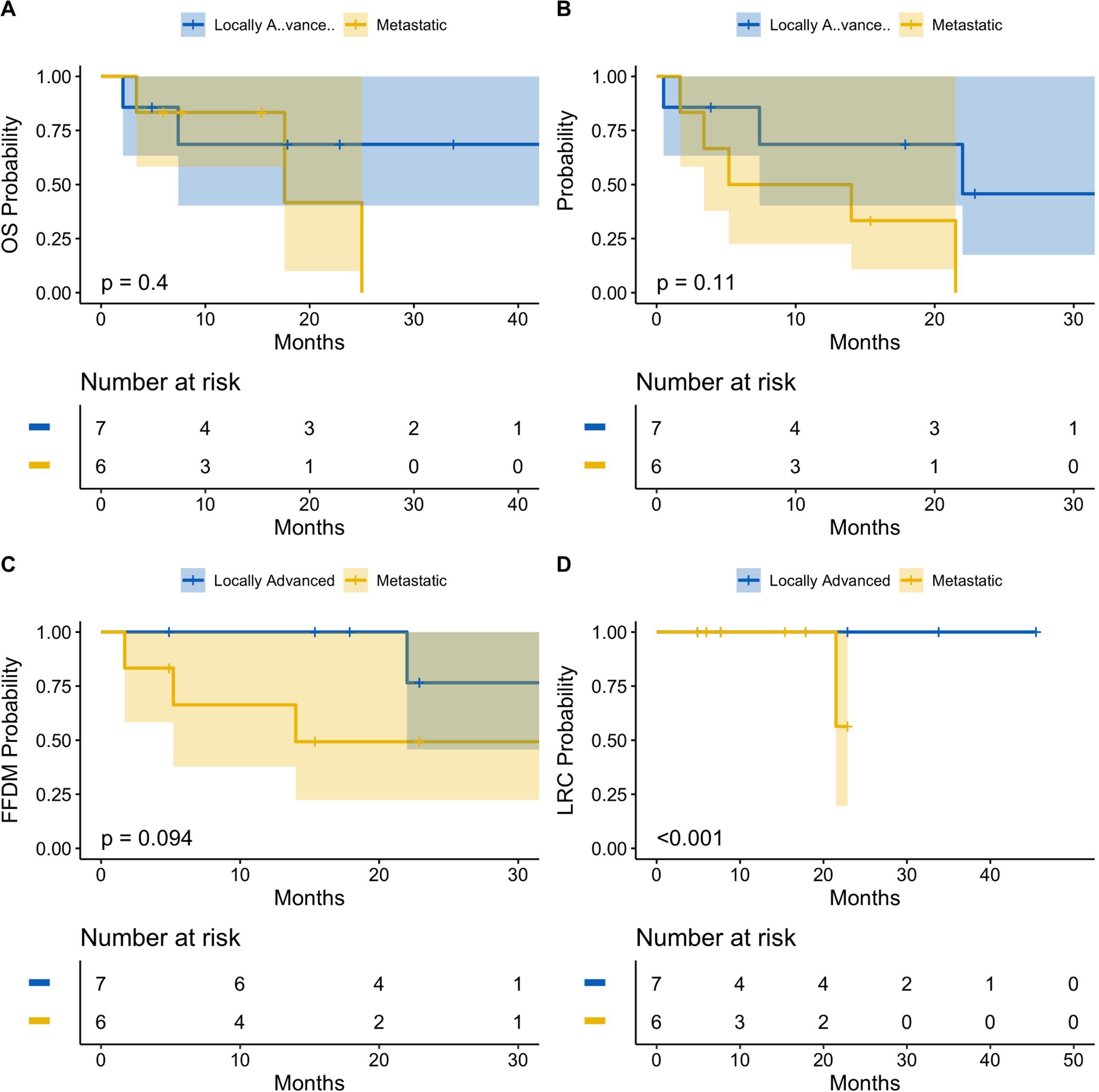
Figure 2. Oncologic outcomes following radioimmunotherapy stratified by locally advanced or metastatic disease status at baseline. Kaplan-Meier estimates showing probability of overall survival (A), progression-free survival (B), freedom from distant metastasis (C), and locoregional control (D). There was a statitstically significant difference of locoregional control between locally advanced and metastatic patients (p<0.001). There was no significant difference between the two cohorts for overall survival, progression-free survival, or freedom from distant metastasis. Tick-marks indicate censored data. Data analyzed at 12 and 24 months. 95% confidence intervals denoted by shaded areas. Blue: Locally advanced. Gold: Metastatic.
Median OS was 68.6% [95% CI: 40.3%-100%] for locally advanced patients at both 1-year and 2-years, versus 83.3% [95% CI: 58.3%-100%] and 41.7% [95% CI: 10.0%-100%] for metastatic patients at 1-year and 2-years, respectively. There was no statistically significant difference in median OS observed between cohorts (p=0.4). Similarly, patients with locally advanced cSCC had a median PFS probability of 68.6% [95% CI: 40.3%-100%] at 1-year and 45.7% [95% CI: 17.5%-100%] at 2-years, compared to patients with metastatic cSCC, who had a median 1-year PFS of 50.0% [95% CI: 22.5%-100%] and 2-year PFS of 0.0% [95% CI: 0%-0%]. Again, there was no statistically significant difference in PFS between locally advanced and metastatic patients (p=0.11). Finally, after 1 year, the median FFDM probability for locally advanced patients was 100% [95% CI: 100%-100%], which decreased to 76.6% [95% CI: 45.7%-100%] at 2-years, compared to 66.3% [95% CI: 37.8%-100%] and 49.3% [95% CI: 22.3%-100%] for metastatic patients at 1-year and 2-years, respectively. There was no statistically significant difference of FFDM between locally advanced and metastatic cSCC patients (p=0.094).
No patient experienced grade 4 or 5 radiation-related toxicities. The complete list of acute and late toxicities is tabulated in Table 2. One patient experienced acute grade 3 radiation dermatitis. Prior to radioimmunotherapy treatment, he presented with a 7 centimeter (cm) ulcerative lesion on his left proximal arm with two satellite nodules located superiorly on the anterior and posterior shoulder (Figure 3). He initially started cemiplimab for 6 months without significant response, and he subsequently underwent IMRT to a total dose of 55 Gy in 20 fractions to improve local control of his left arm ulcerative lesion. About 10 days after delivery of IMRT, he developed moist skin desquamation with superficial bleeding. He underwent regular wound care with medicated foam dressings to facilitate healing via secondary intention, and he did not require any opioids for pain control or negative pressure wound therapy. By his one-month follow-up, his satellite lesions fully regressed and the surrounding radiation dermatitis had also resolved. There was persistent ulceration of his main lesion with granulation tissue and overlying exudate. By his eight-month follow-up, there was complete healing via secondary intention with minor long-term toxicities from radiation, consisting of grade 1 fibrosis and telangiectasia.
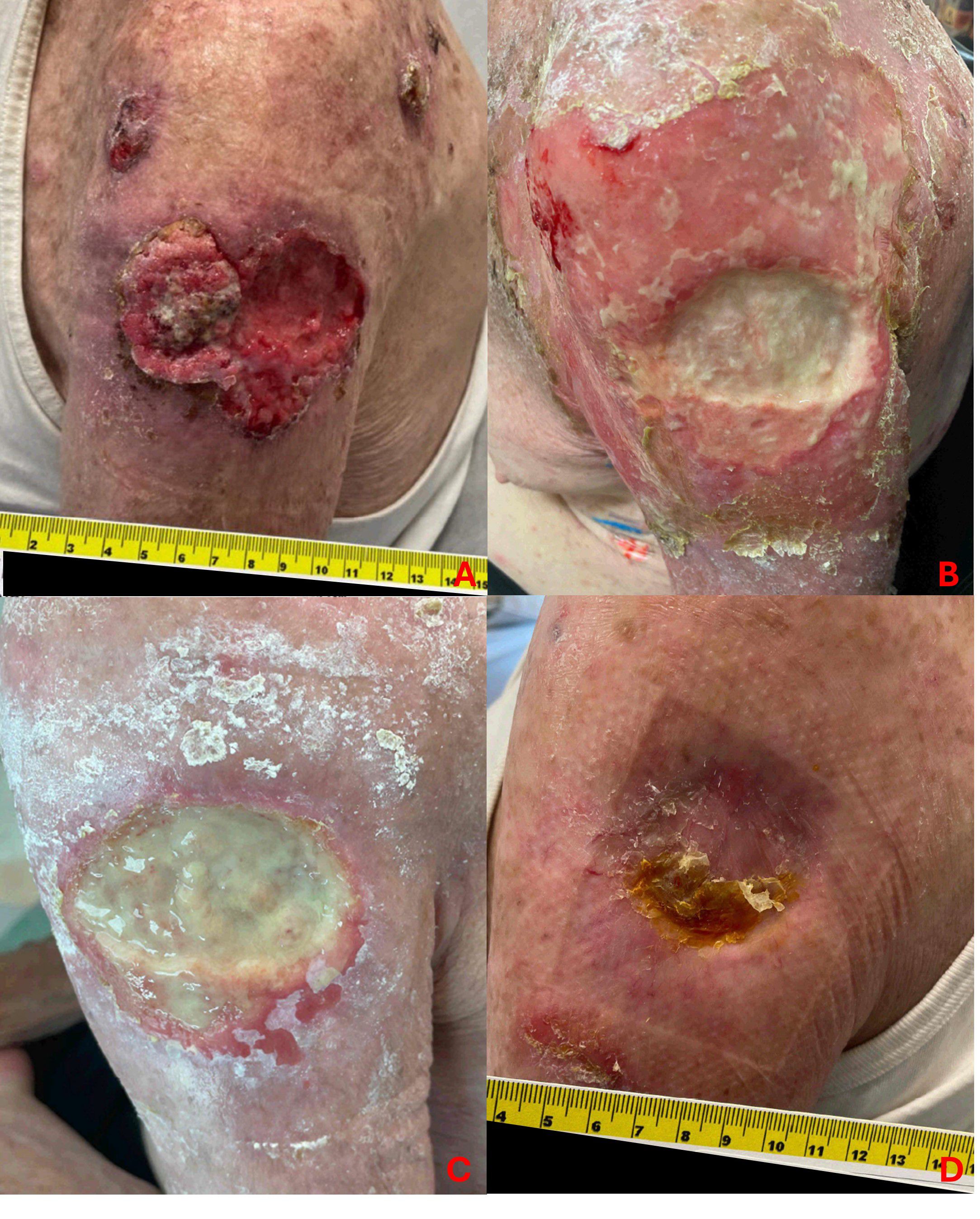
Figure 3. Acute grade 3 radiation dermatitis. One patient experienced acute grade 3 radiation dermatitis following IMRT of a ~7 cm ulcerative lesion with two additional satellite nodules located superiorly on the anterior and posterior left shoulder. Image panels show pre-radiation (A), followed by moist desquamation and minor bleeding at 1-week post-radiation (B). At 1-month (C), there is evidence of granulation tissue and healing by secondary intention. With general wound care and antimicrobial dressing, his lesion healed with expected mild fibrosis and telangiectasia, seen at 8-months post-radiation (D).
Discussion
Historically, the role of radiotherapy in locally advanced and metastatic cSCC is not well defined. Retrospective studies of definitive RT for cSCC have demonstrated that the degree of tumor control correlates with size and stage; T3 and T4 lesions have local control rates anywhere from 75% to 85%, and disease-specific survival at 3 years is about 38% (17, 18). With adjuvant RT, the strongest benefit has been seen in patients with PNI or regional metastatic disease, and addition of RT may improve survival in high-risk patients (10).
Our study examined tumor control, oncologic outcomes, and the toxicity profile of 13 patients receiving RT for either locally advanced or metastatic cSCC with progression on ICI monotherapy. Other than LRC, there were no statistically significant differences in outcomes between locally advanced and metastatic patients. Notably, there were no in-field local failures following RT, and one patient had a regional recurrence along the ankle two years following SBRT to his toe and inguinal lymph nodes. Thus, patients had excellent LRC overall following radioimmunotherapy, suggesting a possible benefit of RT in this population. Importantly, about 50% of patients on ICI monotherapy will fail to respond or have progressive disease (12–15). Under these circumstances, RT may provide a durable LRC benefit of locally aggressive cutaneous lesions causing significant morbidity without significantly increasing toxicity.
Median OS of the entire cohort was 25 months, similar to data from a single-institution study of definitive RT that showed a median OS of 19 months in inoperable stage III and IV cSCC patients (19). After combining operable and inoperable patients, the addition of a PD-1 inhibitor (n=20) yielded a 1-year OS of 84% [95% CI: 67.5-100%] and 2-year OS of 75.7% [54.1-96.9%], compared to OS observed in our cohort of 75.2% [54.2-100%] and 62.7% [38.6-100%] at 1-year and 2-years, respectively. However, the vast majority of patients in our study population had more aggressive tumors with PNI or metastatic disease, and in their study, OS of inoperable patients alone on ICI therapy was not reported.
With cemiplimab monotherapy, 1-year OS has been reported at 93%, and the estimated proportion of patients alive and without disease progression after 1 year is 58% [95% CI: 44-70%] among locally advanced cSCC patients without nodal or distant metastases (15). With the addition metastatic patients, the estimated probability of median OS at 1-year decreases to 81% [95% CI: 68%-89%], and 1-year PFS is 53% [95% CI: 37%-66%] (14). In our overall cohort, 1-year OS was 75.2% [95% CI: 54.1%-100%] and 1-year PFS was 59.8% [95% CI: 37.8%-94.7%]. Importantly, cSCC patients in our population were not well-controlled on ICI therapy, prompting consideration of RT, and progression events almost exclusively consisted of distant metastatic progression and death. RT may represent a feasible option not only to boost local control, but potentially also preserve the patient’s current line of ICI therapy without transitioning to a different systemic agent.
As ICI have become standard treatments for advanced cSCC, radiation oncologists are increasingly consulted to integrate RT for local control of patients with progressive or symptomatic disease. The combination of anti PD-1 agents and RT not only modifies the local tumor immune microenvironment, but it also may impact overall systemic disease control via the abscopal effect (20). By inducing double-stranded DNA breaks and immunogenic cell death, RT propagates release of cytokines and tumor antigens, which subsequently activate cytotoxic CD8+ T-cells (21). Increased cytosolic DNA activates the cyclic GMP-AMP synthase stimulator of interferon genes (cGAS-STING) protein pathway, further propagating a pro-inflammatory tumoricidal response in tumor-draining lymph nodes and regional lymphatics (22). Implementation of ICI sustains this antitumor activity and promotes long-term immune memory, and ultimately promotes both local and systemic disease control (23). Site selection and RT dose become critical, as lower doses may stimulate the immune system, while high dose per fraction to regional lymphatics may induce leukopenia and dampen systemic immunity (24).
Clinically, the observation of an abscopal effect (i.e., when RT induces distant tumor control) is elusive in practice. However, combined radioimmunotherapy with ICI and RT may boost LRC in locally advanced and metastatic cSCC without a significant increase in treatment-related toxicity (25, 26). Recent data from Israel demonstrated that RT and PD-1 inhibitors yields durable response rates in locally advanced and metastatic cSCC, with RT delivered before, during, or after ICI (27). Another study from Italy personalized dose and fractionation, employing conventionally fractionated RT over 6–7 weeks when feasible, or delivering a hypofractionated course over 1–2 weeks for older and/or frailer patients (25). Our series employed a similar individualized approach, tailoring dose and fractionation based on performance status, treatment site, and overall disease burden. Prospective trials are needed to further define optimal RT dose and sequencing in combination with ICI.
Overall, limited toxicities were observed in this study. There was one grade 3 acute toxicity following IMRT to a large, ulcerative lesion on the left shoulder with concurrent cemiplimab. Within 1 week following completion of RT, there was evidence of moist desquamation and superficial bleeding in areas other than skin folds, necessitating designation of acute grade 3 dermatitis. Otherwise, there were no other grade 3 or higher acute toxicities, no grade 3 or higher late toxicity, and no patients experienced any treatment-related break. One 87-year-old patient transitioned to hospice and stopped IMRT at 56 Gy instead of 70 Gy due to distant disease progression.
Several limitations exist in the present study. Similar to other retrospective studies of advanced cSCC, patients had an older median age of 77 years old, and almost all patients harbored T4, node-positive, and/or metastatic disease at baseline. Moreover, all patients in the study were male, most were non-Hispanic White, and many had significant medical comorbidities or immunocompromised status. Despite the limited patient number, this was a heterogeneous patient population with stage III or stage IV cSCC involving many different cutaneous H&N or body areas.
Five patients had undergone surgical resection with adverse pathologic features (i.e., positive margins or PNI) or local recurrence, prompting subsequent ICI therapy followed by additional local therapy with RT combined with ICI. Another 5 patients initiated treatment with ICI due to unresectable disease for at least 4–6 cycles, and RT was subsequently added for locoregional control. The remaining patients initiated combined radioimmunotherapy up-front. This heterogeneity, although a limitation of our analysis, represents a real-world application of this approach across different clinical contexts.
Furthermore, follow-up was limited in some instances, resulting in significant censoring of 2-year outcome estimates and larger 95% CI at that timepoint. These 2-year endpoints should be interpreted as exploratory, and additional multi-center prospective trials are needed to better define long-term response rates, oncologic outcomes, and safety profiles of combined RT and ICI therapy. There is a randomized, phase 3 study (NCT03969004) currently enrolling high-risk cSCC patients to receive either cemiplimab or placebo following surgery and adjuvant radiation, which will further evaluate outcomes and toxicity in a similar patient population (28).
Conclusions
In this retrospective study of locally advanced and metastatic patients with cSCC not well-controlled on ICI therapy, the addition of concurrent RT was overall well tolerated, and oncologic outcomes were similar to published endpoints for this challenging patient population. Both acute and late toxicities were overall acceptable, and the addition of RT may provide a benefit to LRC, yet future prospective trials combining the two modalities are necessary to better define the role of RT in advanced cSCC as novel ICI therapies continue to emerge. Overall, our findings suggest there may be a combined benefit of radioimmunotherapy in advanced cSCC without the expense of increased toxicity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by City of Hope Institutional Review Board #23192, IRB exempt. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Retrospective study, IRB exemption.
Author contributions
AK: Formal Analysis, Writing – original draft, Project administration, Methodology, Writing – review & editing, Investigation, Validation, Data curation, Conceptualization. CL: Methodology, Data curation, Validation, Supervision, Investigation, Formal Analysis, Writing – review & editing. AT: Data curation, Writing – review & editing, Validation. SM: Data curation, Methodology, Supervision, Validation, Writing – review & editing. YX: Supervision, Writing – review & editing. RM: Writing – review & editing, Supervision, Validation. BM: Supervision, Conceptualization, Methodology, Investigation, Writing – review & editing, Validation. AA: Conceptualization, Writing – review & editing, Formal Analysis, Supervision, Methodology, Investigation, Data curation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This work was presented as a poster presentation at the American Society for Radiation Oncology (ASTRO) 2024 Annual Meeting in Washington, DC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rogers HW, Weinstock MA, Feldman SR, and Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. (2015) 151:1081–6. doi: 10.1001/jamadermatol.2015.1187
2. National Comprehensive Cancer Network. Squamous cell skin cancer, NCCN guidelines version 2.2022. In: NCCN clinical practice guidelines in oncology Plymouth Meeting, PA: National Comprehensive Cancer Network (NCCN) (2022). Available online at: https://www.nccn.org/guidelines/nccn-guidelines (Accessed March 9, 2025).
3. Karia PS, Han J, and Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. (2013) 68:957–66. doi: 10.1016/j.jaad.2012.11.037
4. Brunner M, Veness MJ, Ch’ng S, Elliot M, and Clark JR. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head neck. (2013) 35:72–5. doi: 10.1002/hed.22913
5. Tokez S, Wakkee M, Kan W, Venables ZC, Mooyaart AL, and Louwman M. Cumulative incidence and disease-specific survival of metastatic cutaneous squamous cell carcinoma: A nationwide cancer registry study. J Am Acad Dermatol. (2022) 86:331–8. doi: 10.1016/j.jaad.2021.09.067
6. Venables ZC, Autier P, Nijsten T, Wong KF, Langan SM, and Rous B. Nationwide incidence of metastatic cutaneous squamous cell carcinoma in England. JAMA Dermatol. (2018) 155:298–306. doi: 10.1001/jamadermatol.2018.4219
7. Wysong A. Squamous-cell carcinoma of the skin. N Engl Med. (2023) 388:2262–73. doi: 10.1056/NEJMra2206348
8. Ruiz ES, Kus KJB, Smile TD, Murad F, Zhou G, and Ilori EO. Adjuvant radiation following clear margins resection of high T-stage cutaneous squamous cell carcinoma halves the risk of local and locoregional recurrence: a dual center retrospective study. J Am Acad Dermatol. (2022) 87:87–94. doi: 10.1016/j.jaad.2022.03.044
9. Mendenhall WM, Amdur RJ, Hinerman RW, Cognetta AB, and Mendenhall NP. Radiotherapy for cutaneous squamous and basal cell carcinomas of the head and neck. Laryngoscope. (2009) 119:1994–9. doi: 10.1002/lary.20608
10. Harris BN, Pipkorn P, Nguyen KNB, Jackson RS, Rao S, and Moore MG. Association of adjuvant radiation therapy with survival in patients with advanced cutaneous squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg. (2019) 145:153–8. doi: 10.1001/jamaoto.2018.3650
11. Keeping S, Xu Y, Chen CI, Cope S, Mojebi A, and Kuznick A. Comparative efficacy of cemiplimab versus other systemic treatments for advanced cutaneous squamous cell carcinoma. Future Oncol. (2020) 17:611–27. doi: 10.2217/fon-2020-0823
12. Maubec E, Boubaya M, Petrow P, Beylot-Barry M, Basset-Seguin N, and Deschamps L. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J Clin Oncol. (2020) 38:3051–61. doi: 10.1200/JCO.19.03357
13. Gross ND, Miller DM, Khushalani NI, Khushalani NI, Divi V, and Ruis ES. Neoadjuvant cemiplimab for stage II to IV cutaneous squamous-cell carcinoma. N Engl J Med. (2022) 387:1557–68. doi: 10.1056/NEJMoa2209813
14. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, and Lewis KD. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. (2018) 379:341–51. doi: 10.1056/NEJMoa1805131
15. Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, and Hernandez-Aya L. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. (2020) 21:294–305. doi: 10.1016/S1470-2045(19)30728-4
16. Vaidya P, Mehta A, Ragab O, Lin S, and In GK Concurrent radiation therapy with programmed cell death protein 1 inhibition leads to a complete response in advanced cutaneous squamous cell carcinoma. JAAD Case Rep. (2019) 5:763–6. doi: 10.1016/j.jdcr.2019.06.026
17. Locke J, Karimpour S, Young G, Lockett MA, and Perez CA Radiotherapy for epithelial skin cancer. Int J Radiat Oncol Biol Phys. (2001) 51:748–55. doi: 10.1016/S0360-3016(01)01656-X
18. Kim SK and Barker CA. Outcomes of radiation therapy for advanced T3/T4 non-melanoma cutaneous squamous cell and basal cell carcinoma. Br J Dermatol. (2017) 178:e30–2. doi: 10.1111/bjd.15728
19. Amaral M, Osewold D, Presser A, Meiwes A, Garbe C, and Leiter U. Advanced cutaneous squamous cell carcinoma: real world data of patient profiles and treatment patterns. J Eur Acad Dermatol Venereol. (2019) 33:44–51. doi: 10.1111/jdv.15845
20. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, and Kitano S. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. (2012) 366:925–31. doi: 10.1056/NEJMoa1112824
21. Xia WY, Shen YJ, Zhang CC, Qian LQ, Wang H, and Wang K. Combination of radiotherapy and PD-L1 blockade induces abscopal responses in EGFR-mutated lung cancer through activating CD8+ T cells. Trans Oncol. (2024) 48:102074. doi: 10.1016/j.tranon.2024.102074
22. Lynch C, Pitroda SP, and Weichselbaum RR. Radiotherapy, immunity, and immune checkpoint inhibitors. Lancet Oncol. (2024) 25:e352–62. doi: 10.1016/S1470-2045(24)00075-5
23. Wang C, Han L, Zhang J, Ji Q, Guo X, and Li Y. Radiotherapy in combination with PD-1 and TIGIT blockade mediate antitumor abscopal effects and immune memory via CD8+ T cells. Cancer Lett. (2025) 631:217935. doi: 10.1016/j.canlet.2025.217935
24. Bergeron P, Dos Santos M, Sitterle L, Tarlet G, Lavigne J, and Liu W. Non-homogeneous intratumor ionizing radiation doses synergize with PD1 and CXCR2 blockade. Nat Commun. (2024) 15:8845. doi: 10.1038/s41467-024-53015-9
25. Lo Greco MC, Marano G, Milazzotto R, Liardo RL, Finocchiaro I, and La Rocca M The immunomodulatory potential of concurrent high-dose radiotherapy and immune checkpoint inhibitor cemiplimab in advanced squamous cell carcinoma: initial results. J Pers Med. (2024) 14:581. doi: 10.3390/jpm14060581
26. Bailly-Caillé B, Kottler D, Morello R, Lecornu M, Kao W, and Meyer E. Real-life study of the benefit of concomitant radiotherapy with cemiplimab in advanced cutaneous squamous cell carcinoma (cSCC): a retrospective cohort study. Cancers. (2023) 15:495. doi: 10.3390/cancers15020495
27. Averbuch I, Salman S, Shtamper N, Doweck I, Popovtzer A, and Markel G. First-line programmed death-1 inhibitor treatment for locoregionally advanced or metastatic cutaneous squamous cell carcinoma—a real-world experience from Israel. Front Oncol. (2023) 13:1117804. doi: 10.3389/fonc.2023.1117804
28. Rischin D, Fury MG, Lowy I, Stankevich E, Han H, and Porceddu S. A phase III, randomized, double-blind study of adjuvant cemiplimab versus placebo post-surgery and radiation therapy (RT) in patients (pts) with high-risk cutaneous squamous cell carcinoma (CSCC). J Clin Oncol (Abstract Only). (2020) 38:TPS10084. doi: 10.1200/JCO.2020.38.15_suppl.TPS10084
Keywords: cutaneous squamous cell carcinoma (cSCC), immune checkpoint inhibitor (ICI), stereotactic body radiation therapy (SBRT), intensity modulated radiation therapy (IMRT), immunotherapy, concurrent radiation therapy
Citation: Kassardjian AA, Ladbury CJ, Tam A, Maroongroge S, Xing Y, Muddasani R, Modi B and Amini A (2025) Outcomes and toxicity of concomitant radioimmunotherapy following PD-1 blockade for locally advanced and metastatic cutaneous squamous cell carcinoma. Front. Oncol. 15:1679699. doi: 10.3389/fonc.2025.1679699
Received: 05 August 2025; Accepted: 04 November 2025; Revised: 21 October 2025;
Published: 20 November 2025.
Edited by:
Johnny Kao, Good Samaritan Hospital Medical Center, United StatesReviewed by:
Rafal Becht, Chemotherapy and Cancer Immunotherapy Pomeranian Medical University, PolandMaria Chiara Lo Greco, European Organization for Research and Treatment of Cancer, Belgium
Copyright © 2025 Kassardjian, Ladbury, Tam, Maroongroge, Xing, Muddasani, Modi and Amini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ari A. Kassardjian, YWthc3NhcmRqaWFuQGNvaC5vcmc=
†These authors share senior authorship
 Ari A. Kassardjian
Ari A. Kassardjian Colton J. Ladbury
Colton J. Ladbury Andrew Tam
Andrew Tam Sean Maroongroge1
Sean Maroongroge1 Arya Amini
Arya Amini