- 1Department of Gastroenterology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Nephrology, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 3National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 4Department of General Medicine, Eighth Medical Center, Chinese People's Liberation Army (PLA) General Hospital, Beijing, China
The worldwide health and economic burden of cancer is substantial, necessitating urgent, focused prevention and treatment strategies. The investigation of cancer animal modeling techniques is particularly critical. N-methyl-N’-nitro-N-nitrosoguanidine (MNNG), a nitrosamine carcinogen, is extensively utilized in the development of several tumor animal models due to its ability to replicate the natural onset of cancer. Nonetheless, MNNG exhibits a propensity for multi-organ carcinogenesis; yet, this aspect remains undiscussed. The MNNG model exhibits distinct characteristics depending on the route of administration, yet it also presents inherent limitations such as toxicity, environmental contamination, and inconsistent modeling outcomes. These issues necessitate standardized protocols to refine the model, ensuring it meets the criteria for efficient and precise tumor induction while adhering to animal welfare principles. This study examines the current applications of MNNG in gastric cancer models and models of other organs, its carcinogenic mechanisms, translational relevance to human tumors, and practical application features, with a particular focus on its use in gastric contexts. Furthermore, it summarizes and compares the advantages and disadvantages of various MNNG administration routes, as well as contrasts its carcinogenic properties with those of other chemical inducers.Through the examination of drug administration routes, dosage effects, combined modeling strategies, and model specificity, we endeavored to identify effective methods to enhance the specificity of target organs by optimizing the administration approach (local exposure, integration of advanced detection technologies with auxiliary factors). Furthermore, we encourage researchers to disclose negative results, as this practice helps improve model stability and accuracy, reduces research costs, and aligns with animal welfare guidelines.Experimental animals are crucial in scientific study. Future investigations must develop standardized protocols to minimize non-target organ damage and examine the interaction mechanisms between these animals and the tumor microenvironment.
1 Introduction
Cancer continues to pose a significant global public health challenge (1). Examining cancer pathophysiology and formulating prevention measures are fundamental objectives in oncology research, indicating the essential requirement for suitable tumor models. N-Methyl-N’-nitro-N-nitrosoguanidine (MNNG), a nitrosoamine carcinogen, is extensively utilized as a chemical mutagen in animal cancer models (2). It accurately replicates the high-risk factor associated with excessive nitrite consumption in daily life and induces tumors through direct contact. Adenocarcinoma is the predominant tumor type associated with mutagenesis, commonly utilized in animal models of stomach adenocarcinoma, hence offering an optimal experimental framework for investigating the cancer mechanisms and therapeutic target development induced by nitrite (3).
In accordance with the 3R principles of animal ethics (4), Replacement should be the first and foremost consideration when planning related studies. As a widely used chemical carcinogen for establishing tumor models, MNNG often involves complex in vivo microenvironments and systemic disease progression, which are difficult to fully replicate using in vitro cell cultures. Although recent years have seen attempts to induce tumors using MNNG in 3D organoid models (5), the high cost and technical immaturity of these systems mean that animal studies remain one of the primary approaches for investigating nitrite-induced primary tumors. Therefore, under current technological constraints, upholding animal ethics relies critically on the implementation of Reduction and Refinement.By adopting more scientific experimental designs, researchers can maximize the value derived from each animal, reduce the total number of animals used, and minimize the pain and stress experienced by animals throughout the study.
Nonetheless, a significant limitation of MNNG in tumor model establishment is its relatively low specificity. Previous studies have shown that MNNG promotes carcinogenesis not only in target organs but also in non-target sites (6), which increases experimental cost and uncertainty. However, its broad systemic effects across various organ systems remain poorly characterized. Moreover, MNNG administration protocols vary considerably across different tumor models, and even within the same animal species, standardized methodologies are lacking. MNNG is an extremely potent carcinogen, and its use entails significant exposure risks as well as potential harm to the environment.It is therefore essential to comprehensively evaluate the strengths and limitations of various modeling approaches, promote adherence to the Reduction and Refinement principles, and ensure that ethical considerations for animal welfare are fully integrated without compromising scientific objectives.
2 Research landscape of MNNG and its implications for cancer development
MNNG, a nitrosourea compound, mimics dietary nitrite intake in humans and is widely used to model gastric mucosal carcinogenesis (7). N-nitrosamines are strongly associated with various cancers. Recent studies have extensively utilized MNNG-induced animal models to investigate tumorigenesis.MNNG enables the establishment of both in vitro and in vivo models for esophageal, uterine, lung, and colon cancers (8). Furthermore, MNNG drives tumor development by dysregulating multiple signaling pathways, including cellular immunity, oxidative stress, inflammatory response, glycolysis, apoptosis, autophagy, and proliferation (9–11). Through its multi-mechanism, multi-stage complex network, MNNG recapitulates key molecular events in human carcinogenesis and provides a valuable experimental model for clinical translation.Based on the MNNG model, numerous phytochemicals with potential for cancer prevention have been screened, and their therapeutic targets have been explored, serving clinical cancer treatment (12).
3 Mechanisms of tumorigenesis induced by MNNG
The incidence of MNNG-induced tumors is closely associated with its carcinogenic mechanism. The carcinogenic properties of MNNG were initially documented by Sugimura and Fujimura, who effectively induced glandular stomach tumors in rats via prolonged exposure to MNNG in drinking water (13, 14). Since then, research into the tumorigenic processes of MNNG has advanced dramatically. It can induce carcinogenic effects directly, without the need for bioenzyme metabolism, indicating that exposure to MNNG elevates cancer risk and is more likely to affect non-target organs. Figures 1, 2 illustrates the schematic diagram of its mechanism.
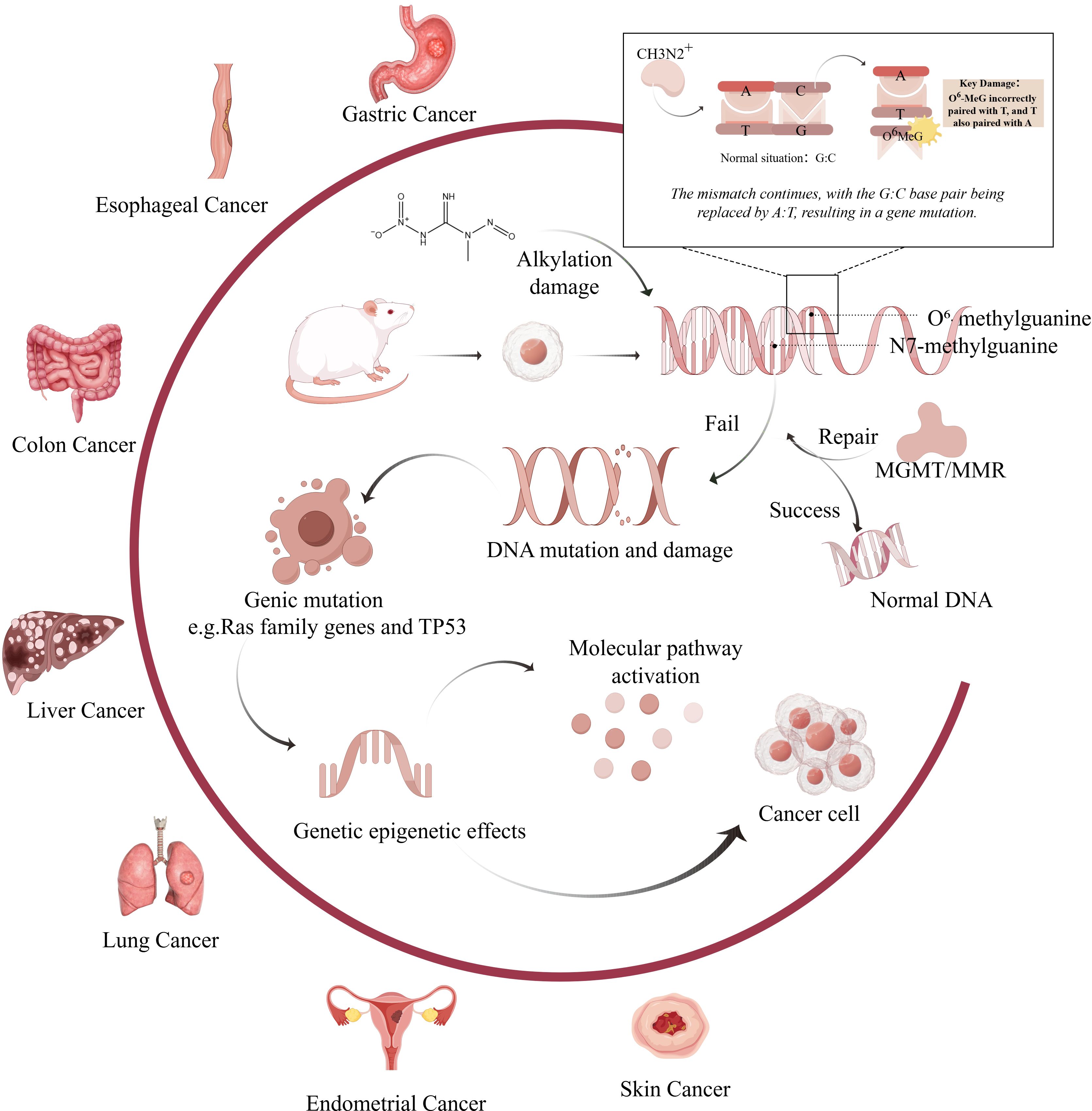
Figure 1. Mechanism of carcinogenic effects of MNNG on various organs.(Created by figdraw,ID : WPIWUff28f). MNNG directly alkylates DNA bases, primarily forming mutagenic adducts such as O6-methylguanine (O6-MeG) and N7-methylguanine (N7-MeG). Misincorporation of thymine opposite O6-MeG during replication results in G→A transition mutations. Inadequate repair of these lesions by mechanisms such as O6-methylguanine-DNA methyltransferase (MGMT) or mismatch repair (MMR) systems leads to persistent DNA damage. Chronic damage contributes to the activation of oncogenes (e.g., Ras family genes) and inactivation of tumor suppressors (e.g., TP53), ultimately promoting tumor development in various organs including the stomach, esophagus, colon, liver, lung, and endometrium.
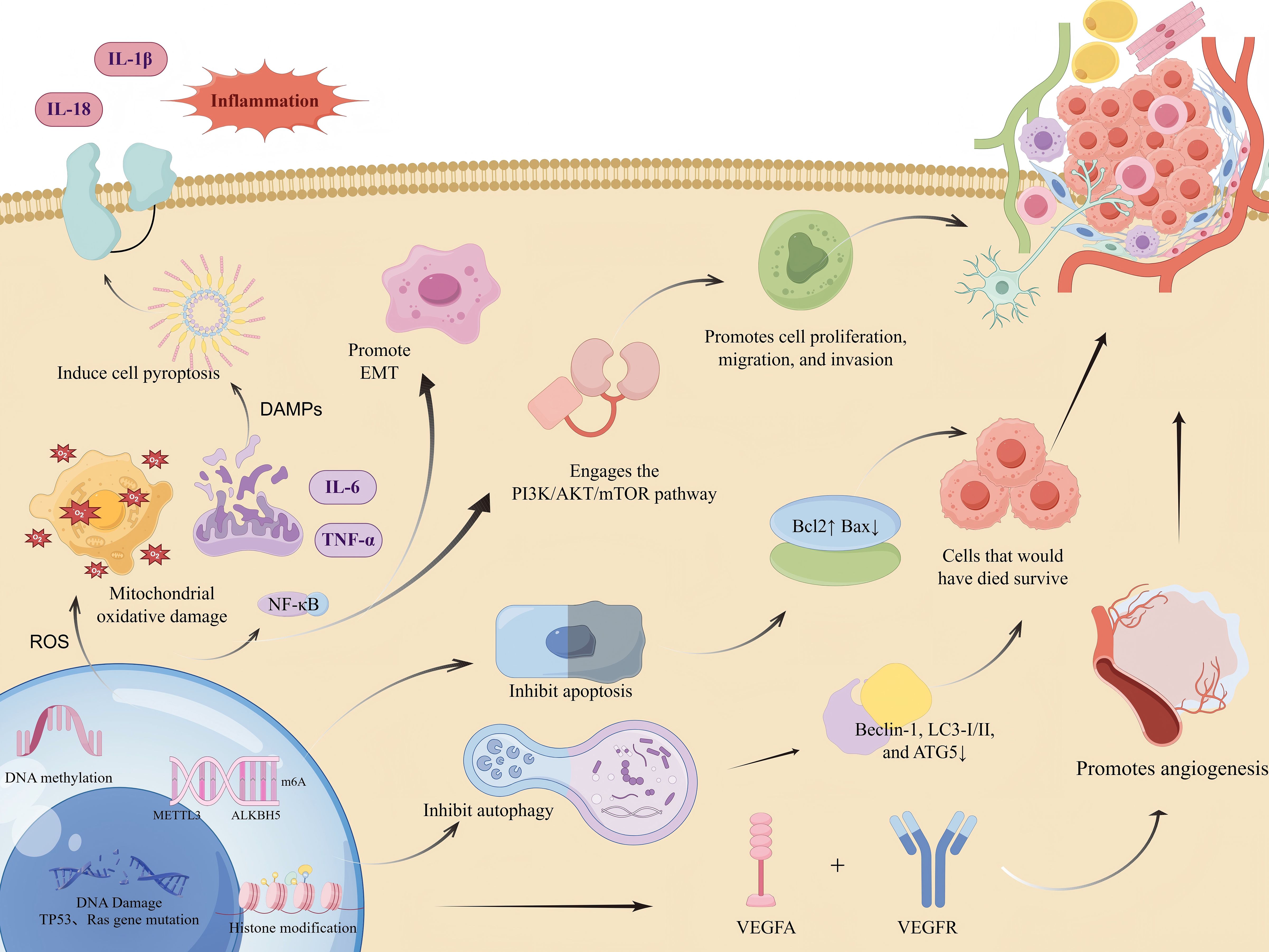
Figure 2. Molecular mechanisms underlying MNNG-promoted tumorigenesis.(Created by figdraw,ID : IPAYSa222a). MNNG induces DNA damage and mutations in key genes such as TP53 and Ras. It also modulates gene expression through epigenetic mechanisms including DNA methylation, histone modifications, and m6A RNA methylation. MNNG induces a sharp increase in reactive oxygen species (ROS), leading to oxidative stress and mitochondrial damage. This, in turn, triggers the release of damage-associated molecular patterns (DAMPs). The DAMPs then activate the NLRP3 inflammasome, which promotes caspase-1-mediated pyroptosis and the cleavage and maturation of pro-inflammatory cytokines, resulting in the secretion of mature IL-1β and IL-18.Concurrently, elevated ROS activates the NF-κB signaling pathway. NF-κB activation drives the transcription of pro-inflammatory genes (including those encoding pro-IL-1β and pro-IL-18) and epithelial-mesenchymal transition (EMT)-related genes, thereby further amplifying the inflammatory response and promoting EMT.These effects collectively cause dysregulation of critical signaling pathways, including PI3K/AKT/mTOR, resulting in suppressed apoptosis and autophagy. Key markers such as Bcl-2, Bax, Beclin-1, LC3, and ATG5 are altered. Moreover, MNNG enhances cell proliferation, migration, invasion, and angiogenesis via VEGFA.
3.1 DNA alkylation injury
MNNG is a powerful, direct-acting mutagenic nitroso chemical. Its reactive metabolites (e.g., methyldiazonium ions, CH3N2+) directly interact with DNA bases, resulting in alkylation damage and subsequent gene alterations that promote carcinogenesis.The primary targets of DNA alkylation are the O6 and N7 positions of guanine, as well as the N3 position of adenine.Following MNNG exposure, the primary adduct identified in double-stranded DNA was N7-methylguanine (N7-MeG) at 67%, along with the minor adducts N3-methyladenine (N3-MeA) (12%) and O6-methylguanine (O6-MeG) (7%) (15). The O6-methylguanine (O6-MeG) adduct formed at the O6 position is the most mutagenic lesion and represents a key form of DNA damage induced by MNNG (16). During DNA replication, DNA polymerase misincorporates thymine (T) opposite O6-MeG, rather than cytosine (C), which is the correct partner for guanine.Normal cells typically contain a greater number of G-C base pairs than malignant cells, a phenomenon attributed to the mispairing of O6-MeG with thymine, which leads to a reduction in methylatable cytosine residues. Subsequently,O6-MedG can induce sister chromatid exchanges(SCE), chromosomal aberrations, and further lead to double-strand breaks, thereby promoting genetic mutations and tumorigenesis (17, 18). Furthermore, the key MNNG-induced DNA adduct O6-MeG contributes to mutagenesis by activating proto-oncogenes or inactivating tumor suppressor genes.Mutations in Ras family genes, such as H-Ras at codons 12 and 13, result in persistent proliferative signaling (9, 10). Mutations in tumor suppressor genes, including TP53 ans P53, can lead to the inactivation of tumor suppression,hence promoting carcinogenesis (19, 20).
3.2 Failure of the DNA repair mechanism
Approximately 97% of N7-MeG adducts are eliminated from pyloric mucosa within 48 hours following MNNG exposure, possibly attributable to active base excision repair (BER) (21). Base excision repair (BER) is primarily responsible for repairing base alkylation damage. DNA glycosylases recognize and excise the damaged bases, thereby initiating subsequent cleavage and ligation steps.The repair of O6-MedG is contingent upon the O6-methylguanine-DNA methyltransferase(MGMT) (16, 22). In gastric cancer, early upregulation of MGMT promotes DNA damage repair; however, hypermethylation of its promoter at a later stage leads to reduced MGMT expression (18, 23, 24). Inhibition of MGMT function induces G:C to A:T mutations in the tumor suppressors p53 and PTEN, contributing to human carcinogenesis (25). The mispaired O6-MeG:T lesion is recognized by the mismatch repair (MMR) system. This is supported by the observed upregulation of MMR-related proteins MSH2 and MSH6 in MNNG-treated cells (26). Deficiencies in this essential DNA repair mechanism can lead to the accumulation of mutations, thereby promoting tumor development (27, 28).However, this repair process also could be fatal, as it can lead to DNA double-strand breaks (29).
3.3 Affecting genetic characteristics of genes
MNNG induces not only genotoxic effects (gene mutations) but also profoundly influences gene expression profiles through epigenetic mechanisms, thereby driving tumorigenesis. DNA methylation represents a well-characterized epigenetic feature of MNNG exposure, characterized by the concomitant occurrence of global hypomethylation and localized promoter hypermethylation of specific genes, leading to oncogene activation and tumor suppressor gene silencing (30). For instance, hypermethylation of the tumor suppressor gene p16 promoter and hypomethylation of the oncogene hTERT have been documented in MNNG-induced carcinogenesis (31, 32). Additionally, MNNG can modulate the epigenome by upregulating phosphorylation of histone H3 at serine 10 and 28 (H3S10p, H3S28p) and downregulating acetylation of histone H4 at lysine 16 (H4K16ac) (33). Furthermore, METTL3 can promote gastric carcinogenesis by activating the METTL3/m6A/miR-1184 axis via an m6A-dependent mechanism, thereby interfering with the miR-1184/TRPM2 signaling pathway (34).Recent findings indicate that the demethylase ALKBH5, which regulates ZKSCAN3 expression via N6-methyladenosine (m6A) modification, activates VEGFA transcription and facilitates MNNG-induced gastric cancer cell migration, invasion, cancer stem cell (CSC) generation, vasculogenic mimicry (VM), and ultimately, gastric cancer progression (13).
3.4 Influence the molecular mechanism of tumor
Exposure to MNNG can induce an oxidative stress response. Following MNNG treatment, levels of reactive oxygen species (ROS) increase, while the activity of antioxidant enzymes SOD, CAT, and GSH-Px significantly decreases in both blood and gastric tissues (35). This enhances mitochondrial oxidative damage and promotes cell division, which is one of the primary mechanisms of mutagenesis.Concurrently, this process activates DAMPs, triggering the NLRP3 inflammasome and amplifying the inflammatory response. It is widely recognized that MNNG exposure induces inflammation in the gastric mucosa. In tissues subjected to chronic exposure, the expression levels of inflammatory factors such as IL-6, IL-18, IL-1β, TNF-α, and NF-κB are elevated (36). Similarly, the levels of Gasdermin D (GSDMD), NLR family pyrin domain containing 3 (NLRP3), and Caspase-1 are also upregulated (37), indicating that pyroptosis is indeed involved in the inflammatory burst. This aligns with the progression of inflammation-to-cancer transformation.
O6-MeG secondarily induces DNA double-strand breaks and triggers apoptosis through upregulation of p53 and Fas/CD95/Apo−1, accompanied by decreased Bcl-2 and activation of caspase-9 and caspase-3 (38, 39). However, in the gastric cancer microenvironment, increased expression of Bcl-2 along with reduced levels of Bax, Bim, caspase-8, and caspase-3 indicates suppressed apoptosis (40). The survival of cells that were destined to die,which may accelerate tumor progression.
MNNG also leads to accumulation of p62 and engages the PI3K/AKT/mTOR pathway downstream (41), contributing to malignant processes such as cell proliferation, migration, and invasion. MNNG can suppress normal autophagy and promote the epithelial-mesenchymal transition (EMT) and cell proliferation. Long-term exposure to MNNG reduces the expression of autophagy-related proteins including Beclin-1, LC3-I/II, and ATG5 (42).
Furthermore, following MNNG intervention, regulation of MMP2, MMP9, VEGF, VEGFR1, TIMP-2, and RECK promotes angiogenesis (43), tumor invasion, and metastasis. Many phytochemicals have been found to exhibit preventive effects against tumor formation in MNNG-induced tumor models. Tumor development is multidimensional and involves synergistic activity across multiple pathways; the mechanisms underlying MNNG-induced carcinogenesis are not yet fully elucidated and remain under active investigation.
4 Utilization of MNNG in various tumor models
4.1 MNNG in gastrointestinal models
4.1.1 Animal models associated with gastric cancer
In gastric cancer models, MNNG predominantly induces well-differentiated intestinal-type adenocarcinomas (44, 45). Beyond the previously outlined mechanisms of MNNG carcinogenesis, there are specific characteristics in the mechanism by which MNNG results in gastric adenocarcinoma. It selectively alkylates pyloric gland cells (46), leading to lesions primarily confined to the pyloric region (47, 48), potentially attributable to enhanced carcinogen accessibility to proliferative cells in the gastric antrum (49). Research indicates that the prevalence of O6-MedG-positive cells diminishes systematically from the pylorus to the corpus, forestomach, duodenum, and esophagus (50).
Male rats demonstrate elevated tumor induction rates (up to 88%) compared to female rats (51–53) and are favored. Strains exhibiting increased vulnerability including Wistar-Kyoto, Wistar, Sprague-Dawley (SD), and ACI rats (54–56). Tumor induction rates are often elevated in rats younger than 12 weeks and weighing < 120 g.
4.1.1.1 MNNG-induced models: single-agent approach
MNNG can be delivered through free drinking and intragastric gavage in the development of gastric cancer models. Free drinking refers to rats ingesting MNNG at a certain concentration dissolved in drinking water ad libitum. The concentration of MNNG free drinking water, the administration period and the success rate of modeling are shown in Table 1. Generally, tumors generated by MNNG require an extended period for development. MNNG must be administered in drinking water for a duration of 8-10 weeks to effectively begin adenoma developmen (66). Within 30 weeks of administration, there may be a positive correlation between MNNG induction success and time of administration.Notably, prolonging exposure to 40 weeks at this concentration unexpectedly decreased the incidence to 43.8% (65).
It has been reported that the incidence of MNNG-induced tumors at 32 weeks was actually lower than that at 24 weeks (32). Therefore, we consider 24–30 weeks to be a relatively ideal treatment window when administering MNNG via drinking water.Although high doses of MNNG can induce a higher tumor incidence, increasing its concentration does not lead to a proportional rise in modeling success. This is likely because rats have a sensitive sense of smell, and higher concentrations of MNNG may reduce their water intake.A concentration of 100 μg/mL may be an optimal choice for drinking water administration, though further experimental validation is needed to confirm this hypothesis.
However, we also observed substantial heterogeneity in tumor induction rates even under repeated experiments using the same administration protocol. Although higher doses of MNNG generally lead to relatively higher success rates in tumor induction, significant variability remains across studies.
Certain scholars have noted that the ingestion of MNNG at a concentration of 100 mg/L through drinking water results in an actual cumulative intake of 150 mg to 250 mg, which is insufficient to promote tumor formation; the cumulative MNNG dose required for tumor induction is 300 mg (72). These conflicting observations may indicate considerable instability in the modeling process when MNNG is administered via drinking water, likely related to its susceptibility to degradation under room temperature conditions, which could represent a potential limitation. Furthermore, the lack of reported details in some experimental datasets,such as the age and weight of the animals, housing conditions, diet and water source, manufacturer and storage conditions of MNNG, as well as mortality rates during the experiment,has also hindered a standardized analysis.
Unrestricted drinking modeling more accurately reflects the normal pathological progression and can generate cancer models that align with the adenocarcinoma development pattern observed in humans. It possesses significant reference value and serves as an exemplary modeling method. This method is currently prevalent, and its value resides in its straightforward execution, which can successfully mitigate harm inflicted by mechanical procedures such as surgery or intragastric gavage on rats, hence decreasing the mortality rate. Nevertheless, the free drinking method possesses certain drawbacks. MNNG is light-sensitive and necessitates storage at 2-8°C; while employing shade treatment, elevated interior temperatures may potentially lead to gradual degradation at ambient temperature. Unrestricted access to drinking water does not ensure the daily water consumption of each rat, and elevated concentrations of MNNG inhibit water intake (6, 73). Residual MNNG effluent presents environmental disposal difficulties. Extended modeling durations elevate the possible dangers of operator exposure.
Intragastric gavage is a frequently employed modeling technique. The drug concentration is often established at 150-250 μg/mL and the compound is delivered directly into the stomach. The induction of tumors is positively correlated with the dosage. Elevating gavage concentration from 50 mg/kg to 100 mg/kg augmented the incidence of forestomach squamous cell carcinoma from 21% to 30%, and glandular stomach dysplasia to 52%. The success rate of model construction by the intragastric gavage approach is elevated, and it is occasionally combined with NaCl to enhance the success rate. Two doses of 200 mg/kg/bw resulted in a 100% success rate (74–77). This page compiles data on commonly utilized gavage doses and their high success rates in recent years for researchers’ reference, as illustrated in Table 2.
The acute LD50 for MNNG in 10% DMSO administered via gavage is 90 mg/kg (75), and a dosage of 100 mg/kg resulted in 52% immediate death. It should be noted that gavage administration involves high-dose delivery over a short period, which may lead to acute toxicity and mortality in animals. Although this risk appears to be closely linked to the solvent used, employing aqueous or olive oil solutions can significantly reduce such potential harm.
Intragastric gavage models demonstrate an elevated incidence of forestomach tumors (12%) (84, 85), potentially rendering them suboptimal for studies on glandular gastric adenocarcinoma. This may be attributed to the proximity of the specifications and maximum length of the gastric needle to those of the forestomach, complicating access to the anatomical location of the glandular stomach. The intragastric gavage approach offers the benefit of precise regulation of daily MNNG intake in experimental animals, hence enhancing the model’s success rate. MNNG solutions are freshly created, reducing waste and decreasing environmental and operator exposure. Nonetheless, the drawback of intragastric gavage is that it necessitates a high level of expertise from the experimental team. Prolonged, high-dose gavage administration is likely to have deleterious effects, including esophageal damage and gastrointestinal distension in rats, potentially leading to increased mortality (86).
4.1.1.2 MNNG-induced models: combination approach
Owing to the protracted lengthand inconsistent efficacy of MNNG in isolation, composite modeling techniques have gained prominence in recent years, especially in the investigation of the therapeutic mechanisms of natural medicines targeting stomach precancerous conditions (87). The efficacy of modeling is generally assessed by pathological indicators such as CAG, IM, and Dys identified in stomach mucosa. Table 3 enumerates various composite modeling methodologies and their corresponding model types. Combined modeling approaches enhance model stability and, to some extent, compensate for the limitations of single-factor models. They also take into account animal welfare concerns, making them better aligned with the 3R principles (Replacement, Reduction, and Refinement).Despite variations in modeling cycles and outcomes, potentially due to supplementary modeling techniques, medication compositions, and laboratory settings, the results remain significant references for researchers.
Although numerous studies utilize MNNG-induced precancerous lesions as their endpoint, the specific modeling periods vary significantly—ranging from 12 to 28 weeks. This variation is not merely random inconsistency, but rather a reflection of differences in model design, the dynamic progression of precancerous lesions, variations in experimental protocols, and subtle discrepancies in evaluation criteria. The lack of a standardized approach has led to the use of varying combinations and concentrations of N-nitroso compounds, making it difficult to critically compare conclusions across studies.Furthermore, pathological changes themselves vary in severity. Time points such as 12–16 weeks may capture the initial stages of precancerous pathways, emphasizing “mild” or “early” lesions, while time points around 24–28 weeks may approach the critical transition between precancerous lesions and neoplastic states, highlighting “severe” or “high-grade” pathology. However, most studies only report changes in pathological status without detailing the extent of these alterations, leaving readers confused when confronted with inconsistent results. This underscores an urgent need for greater data standardization.
MNNG is frequently utilized in conjunction with NaCl, ethanol, bile acids, ammonia water, ranitidine, or dietary modifications to reduce modeling duration and enhance efficacy. Zhu Y et al. given MNNG at a dosage of 200 mg/kg, every 15 days, in conjunction with 40% ethanol (1 mL) every 3 days, with a 3-day fasting regimen, successfully created a model of precancerous gastric lesions in rats during a 20-week period (95). ChunYue Yu et al. (99) employed a methodology that included ad libitum consumption of MNNG solution (100 μg/mL) and a diet supplemented with 0.05% ranitidine, alongside irregular feeding and administration of a 2% sodium salicylate solution (0.5 mL per 100 g body weight), to effectively create a gastric cancer model.
4.1.1.2.1 NaCl
While it can not induce cancer, it can amplify the carcinogenicity of MNNG, simulating high-salt diets. Rats provided with 10% sodium chloride in their drinking water consume 1.2 to 1.5 times more than those without sodium chloride (100), and this increase is dose-dependent (101). Alongside the application of MNNG for modeling, the weekly administration of 0.1 mL of 10% sodium chloride solution effectively established the Dys model after 28 weeks (91, 102). Moreover, NaCl consumption can significantly mitigate the decrease in water intake induced by high-concentration MNNG solutions in animals. The factors contributing to NaCl’s role in tumorigenesis can be delineated as follows: 1) Decreases gastric mucus viscosity, compromising the mucosal barrier (99, 103); 2) Elevates ornithine decarboxylase (ODC) activity and replicative DNA synthesis (RDS), indicators of tumor promotion (101); 3) Augments lipid peroxidation associated with gastric epithelial proliferation (103, 104).
4.1.1.2.2 Bile acids
Simulate harm caused by bile reflux. The incorporation of 0.2% taurocholic acid into the diet elevated antral tumor formation from 25% (with MNNG alone) to 72% (105). Various bile salts exert a stimulating influence on the stomach mucosa (106). Bile salts influence the ion channels of gastric mucosal cells, allowing hydrogen ions from the lumen to penetrate the mucosa, so compromising the stomach epithelial barrier and facilitating the absorption of possible carcinogens (107). Sodium deoxycholic acid is currently employed to replicate the irritative effects of bile reflux on gastric mucosa, diminish its barrier function, induce inflammatory responses, and facilitate the development of gastric cancer models.
4.1.1.2.3 Ammonia water
Helicobacter pylori is designated as a Group I carcinogen associated with the onset of stomach cancer. This bacteria exhibits robust urease activity, converting urea in the stomach into ammonia. Intragastric gavage of Hp bacterial water can improve the success rate of model development (108, 109). Nonetheless, as HP is a biological pathogen, it necessitates a high degree of technical proficiency from laboratory professionals and particular environmental conditions. The application of ammonia water in modeling can replicate the high-ammonia conditions and harmful effects associated with H. pylori (Hp) infection, resulting in chronic gastritis. The amalgamation of ammonia water with MNNG can markedly elevate the occurrence of the model (63, 110). In trials, ammonia water is often provided freely at concentrations of 0.05% to 0.1% on fasting days.
4.1.1.2.4 Ranitidine
Moreover, a low-acidic environment can expedite the advancement of stomach cancer (111). An elevation in gastric pH can augment the methylation of MNNG (112). Consequently, it is recommended to utilize the acid-suppressing medication Ranitidine together with modeling. It is essential to recognize that, given the half-life of Ranitidine, to maintain its efficacy in suppressing stomach acid secretion, it is often incorporated at a concentration of 0.03% to 0.05% into rat meal for ad libitum consumption and must be maintained in a dry, cool environment.
4.1.1.2.5 Alcohol
An ethanol solution replicates the actual danger associated with alcohol consumption, a significant risk factor for stomach cancer, and can facilitate the dissolution of MNNG solutions. It is frequently employed in combination to augment MNNG consumption, with 20% glycolic acid markedly elevating both the occurrence and quantity of MNNG (113). Research indicates that ethanol concentrations of 11% can inhibit tumor formation (114). Elevated quantities of 20%-40% ethanol solution are generally delivered via gavage, necessitating vigilant monitoring of the animal’s condition to avert asphyxiation resulting from intoxication.
4.1.1.2.6 Sodium salicylate
Prolonged use of non-steroidal anti-inflammatory medicines (NSAIDs) has been extensively researched for its enduring detrimental effects on the gastric mucosa. Sodium salicylate, a frequently utilized pharmaceutical in modeling protocols, can induce harm to vascular endothelial cells, resulting in an inflammatory milieu (115). It also impedes prostaglandin synthesis, compromising the protective barrier of the stomach mucosa. The standard dosage in modeling methods is 2% sodium salicylate. To augment its efficacy, it is frequently paired with an erratic diet. Following a day of fasting, the medication is delivered via gavage to enhance contact duration with the gastrointestinal mucosa.
4.1.1.2.7 Others
Furthermore, the amalgamation of heated saline and intragastric gavage can replicate the irritation induced by high-temperature food on the gastric mucosa and is frequently employed in modeling procedures. An irregular diet, as a primary approach of dietary intervention, can elevate the likelihood of irregular eating patterns. The aforementioned conditions, in conjunction with substances like sodium salicylate and ethanol, can be amplified by doing stomach lavage post-fasting to augment their stimulating effects.
MIWA H et al. (116) documented the dynamic pathological alterations in the gastric mucosa of rats following unrestricted consumption of a 100μg/mL MNNG aqueous solution, which corresponds with the pathological course of gastric cancer as delineated by the Correa model. Kogure K et al. (64) discovered that MNNG delivery resulted in three phases of gastric mucosal alterations. The first stage is predominantly manifested as gastric mucosal injury and atrophy. By the eighth week, superficial erosion and significant atrophy were evident in the antral mucosa. By week 16, significant surface erosion and minor dysplastic glands were noted in the antrum. The second phase is characterized by intestinal metaplasia and dysplasia. Beginning in week 18, irregular atypical glands commenced development near the erosion edges and the pylorus. At week 24, the antral surface epithelium exhibited papillary hyperplasia, characterized by the presence of irregular and atypical glands. The final stage is gastric carcinoma. By week 26, hyperplasia of the surface epithelium and pyloric glands was noted in both the minor and larger curvatures of the antrum, with or without atypia. Between weeks 25 and 32, adenocarcinoma and spindle cell sarcoma were identified, alongside submucosal sarcomas exhibiting atypical gland invasion in the lesser curvature of the antrum. This aligns with prior research (117), which indicated that 12 weeks post-MNNG exposure, the mucosal layer of rat gastric tissue thinned, signifying superficial gastritis; after 24 weeks of MNNG treatment, the gastric mucosa displayed considerable inflammatory cell infiltration, diminished gland size, and reduced gland quantity, leading to a diagnosis of chronic atrophic gastritis (CAG); after 36 weeks of MNNG treatment, the gastric mucosa in the rats revealed inflammatory granulomas or ulcer-like lesions, with glands contracting due to vacuolar goblet cells, and the pathological alteration was atrophic gastritis with intestinal metaplasia (IM). Following 48 weeks of MNNG exposure, the glandular architecture was obliterated, and the pathology exhibited irregular morphologies. The peribasilar membrane is encircled by numerous inflammatory cells of diverse sizes, categorized as dysplasia (Dys). Qiu-yue Li and colleagues (118) discovered that following 12 weeks of MNNG exposure in rats, the stomach mucosal epithelial cells were partially substituted by intestinal-type epithelial cells. After 20 weeks, the stomach mucosa commenced atrophy, progressively deteriorating to moderate or severe stages. The detailed characteristics of the three stages of gastric mucosal alterations induced by MNNG administration are summarized in Table 4.
A comparison of the characteristics of different drug administration methods is presented in Table 5. The selection of a modeling approach involves multi-dimensional trade-offs. In terms of organ specificity, there is currently no ideal tumor model that fully meets all criteria. Compared to other methods, the gavage + combined modeling approach may represent a preferable option. However, from the perspective of animal welfare and simulating the natural progression of disease in humans, free drinking + combined modeling might be a more desirable alternative. The choice of an appropriate modeling strategy should be comprehensively weighed according to research objectives. Given the substantial burden of cancer worldwide, investigating the mechanisms and therapeutic targets of precancerous lesions and states has become a research focus,areas where combined modeling approaches are widely applied.
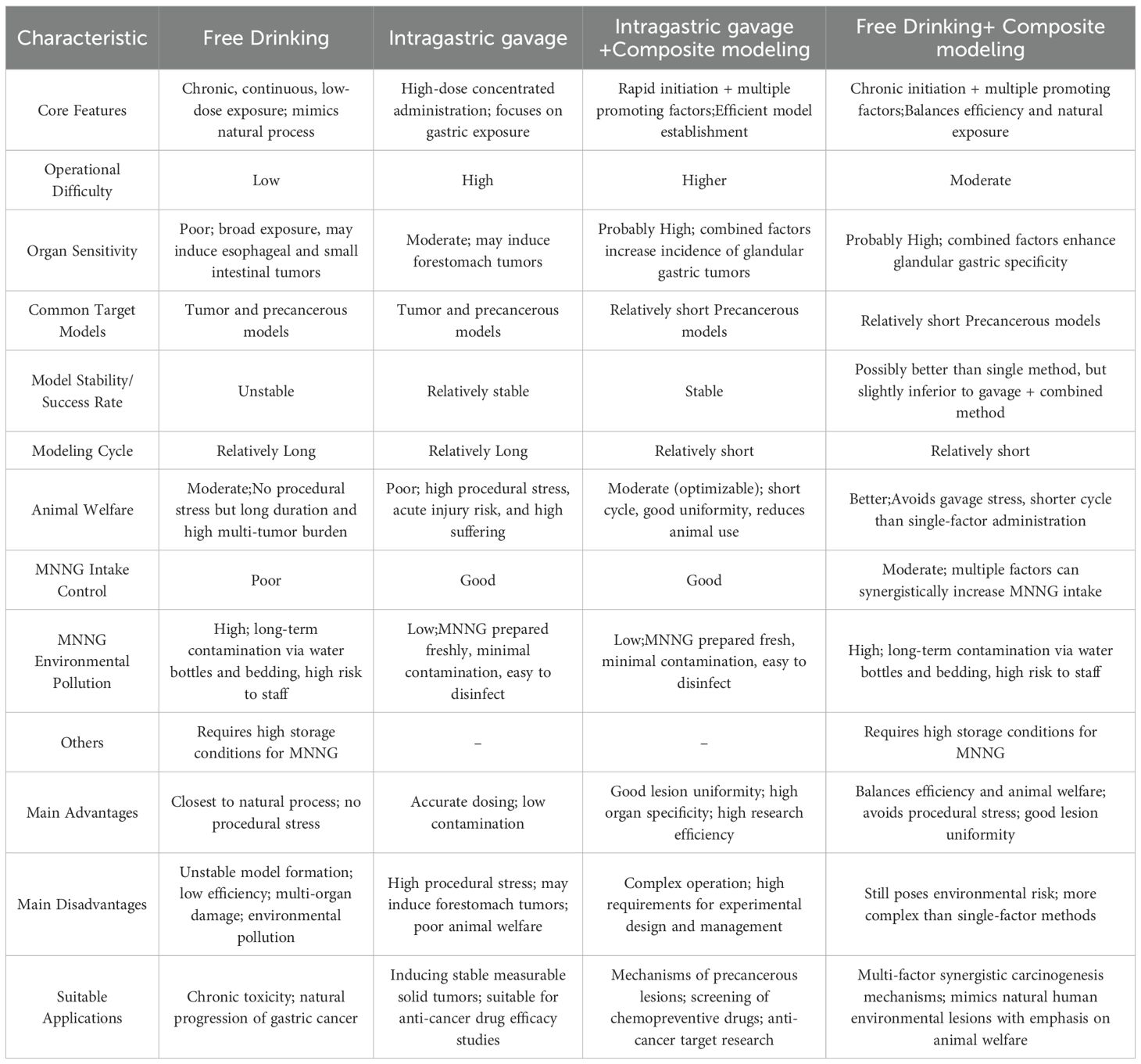
Table 5. Comparison of characteristics of different administration methods in MNNG gastric cancer related models.
4.1.1.3 Similarities and differences between human andMNNG-induced gastric cancer
From an etiological perspective, MNNG can mimic the damage caused by nitrites to the gastric mucosa, which is similar to nitrite-induced human gastric cancer (120). Moreover, when combined with factors such as ethanol, high salt intake, dietary habit changes, nonsteroidal anti-inflammatory drugs (NSAIDs), and Helicobacter pylori infection, MNNG can simulate a more complex environment resembling human tumor development. After MNNG intervention, rats develop inflammatory responses in the gastric mucosa, with pathological manifestations similar to human intestinal-type gastric cancer (116, 117). The histological progression follows the pattern of “chronic superficial gastritis -chronic atrophic gastritis - intestinal metaplasia - dysplasia - tumor,” making it a commonly used model for studying precancerous lesions.
Mutations in genes such as Bcl-2, COX-2, H-ras, and p53 have been documented in both human and MNNG-induced gastric tumors (121). However, the expression of oncogenes such as Ki-ras and β-catenin does not increase in MNNG-induced rats (122), suggesting that these genes are not major drivers in MNNG-induced rat gastric cancer or in human gastric cancer. Additionally, no microsatellite instability (MSI) is observed. Many genes involved in immune responses are upregulated, along with genes related to extracellular matrix (ECM) remodeling, while genes associated with gastric differentiation are downregulated (123). The upregulation of immune/inflammatory response genes is consistent with findings in human gastric cancer. Thus, based on molecular expression profiles, MNNG may serve as a suitable model for differentiated gastric cancer (123).
However, the molecular mechanisms by which MNNG mimics human gastric cancer have certain limitations. While p53 mutations occur in 36–40% of differentiated human gastric cancers, their incidence in rats is very low (122, 124). Moreover, no common human gastric cancer mutations, such as Ki-ras mutations, K-sam amplification, or c-erbB-2 gene amplification, have been detected in MNNG-induced rats (123). The cell cycle regulatory gene Cyclin D1, which is typically upregulated in human gastric cancer, is downregulated in rat models, showing an opposite trend. Genes associated with lymph node metastasis in human gastric cancer (e.g., Rbp4, Igf2, Fn1) are not significantly upregulated in rats, indicating a lower potential for lymph node metastasis in MNNG-induced rat gastric cancer. Additionally, promoter CpG island (CGI) methylation of tumor suppressor genes such as CDH1 (E-cadherin), CDKN2A (p16), MLH1, and RASSF1A (19), which is commonly reported in human gastric cancer, has not been observed in MNNG-induced rats.
Overall, while the disease background of MNNG-induced tumors is relatively simple and may not fully replicate the complex microenvironment of tumor development, MNNG remains a valuable chemically-induced model for studying human differentiated gastric cancer in terms of epigenetics, histopathological features, and molecular immune responses.
4.1.2 Animal models associated with colon cancer
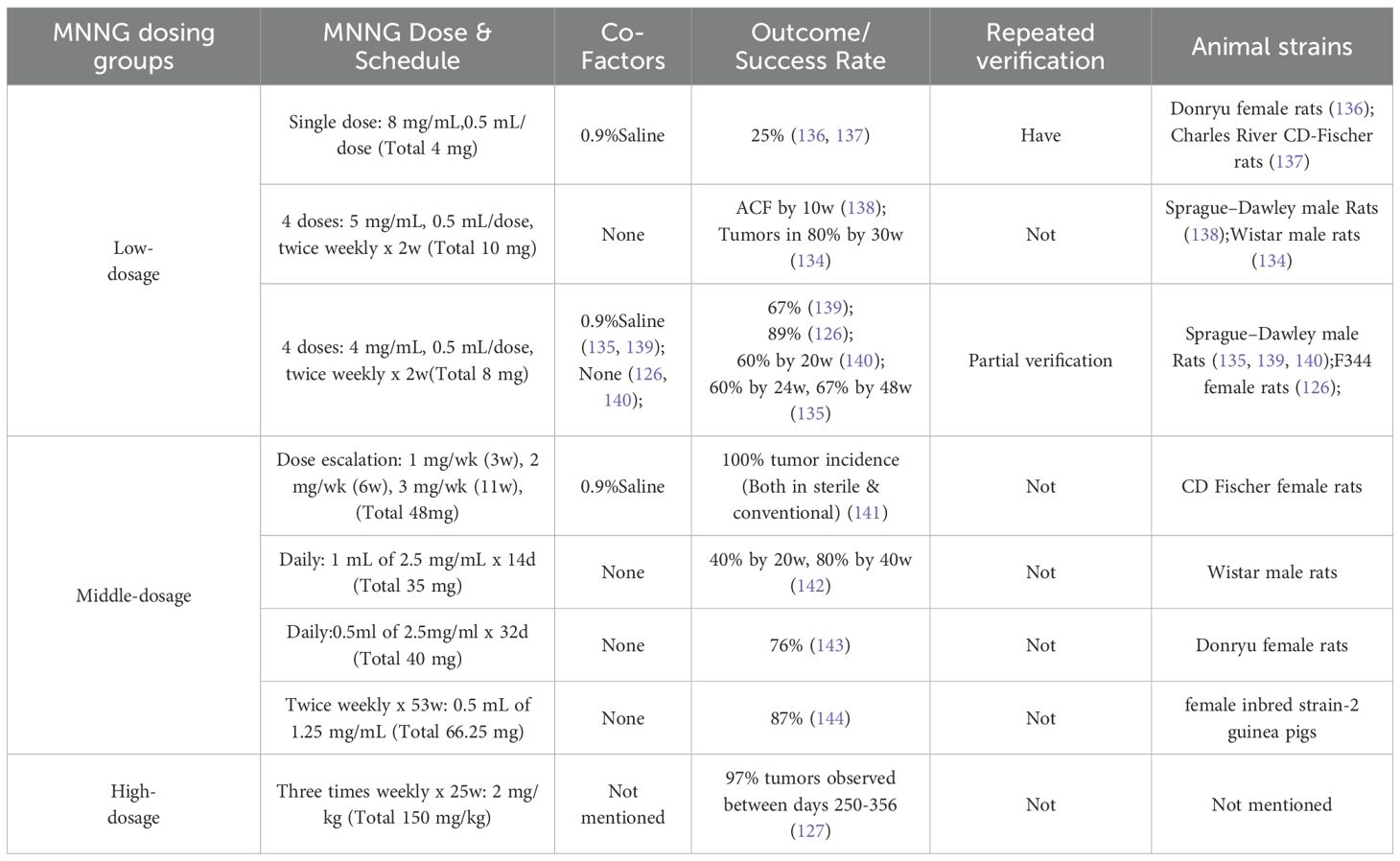
MNNG is a powerful topical carcinogen commonly employed to cause colon cancer, namely well-differentiated adenocarcinomas, situated in the distal colon (125, 126). Intrarectal Instillation: This is the conventional technique, facilitating targeted induction in the distal colon and rectum.Sterile circumstances expedite tumor development and elevate incidence relative to typical surroundings (127, 128). So BT et al. (129) developed colon cancer in all 30 rats with intrarectal administration of MNNG (2 mg/kg) biweekly for 250 days. Kannen V and Frajacomo FT (130, 131) administered four doses of MNNG (5 mg/mL, 0.5 mL/dose) biweekly for two weeks, resulting in the induction of colon cancer within 8-10 weeks. Weekly intrarectal administration of MNNG (1-3 mg/rat) for 20 weeks in male F344 rats resulted in colorectal cancer, comprising 57% adenomas and 43% adenocarcinomas (132, 133). MNNG induces pre-neoplastic lesions such as aberrant crypt foci (ACF) (134). Histologically, it induces goblet cell depletion and lymphocytic infiltration (135). We observed that F433 mice (98) exhibited a significantly higher mutagenesis rate of 89% compared to other models using the same modeling method, which may be directly attributed to differences in animal strains.
MNNG, a direct carcinogen that does not necessitate metabolic activity, elevates p53 protein levels with sustained exposure, signifying active epithelial proliferation (98). Intrarectal injection preferentially promotes cancer at the site of exposure, closely resembling natural development. Intrarectal administration of MNNG represents a stable method for inducing colorectal cancer, with higher total dosage correlating to increased tumor induction rates and correspondingly elevated mutagenesis, as detailed in Table 6. Nonetheless, the modeling cycle is protracted, and precisely determining the dosage for rectal delivery poses difficulties. It is essential to maintain the animals in an inverted position for one minute post-administration to avert the reagent from reverting to the anus and compromising the modeling effect (145, 146).
4.1.3 Animal models associated with esophageal cancer
Esophageal cancer (EC) ranks as the eighth most prevalent cancer worldwide and the sixth in terms of death, with esophageal squamous cell carcinoma (ESCC) being the predominant variant (147). Exposure to nitrosamines constitutes a substantial risk factor for the development of esophageal squamous cell carcinoma (ESCC) (148), and nitrosamine chemicals behave as principal chemical carcinogens in esophageal cancer models. Suizhi Cheng’s team (11) discovered that MNNG can activate NF-κB, leading to the upregulation of inflammatory markers IL-6, IL-8, and TNF-α, which induces esophageal inflammation in SD mice. Moreover, MNNG can induce the proliferation of squamous epithelial cells in the esophageal mucosa of rats and promote the malignant transformation of human esophageal epithelial Het-1A cells (149). MNNG demonstrates heightened sensitivity to adenocarcinoma. N. Yioris et al. (150) injected 5.0 mg/kg of MNNG to the esophagus of 30 mice during a duration of 37 weeks. The research revealed that 11 animals in the cohort acquired stomach adenomas, 2 animals got esophageal squamous cell carcinoma, and 1 animal developed colon adenocarcinoma. Furthermore, five instances of hepatic cystadenoma and one instance of esophageal keratinizing papilloma were noted, indicating that MNNG carcinogenesis may exhibit non-specific traits for target organs.
The manifestation of EC is a complex process that encompasses both environmental and genetic influences. MNNG can replicate the external environmental elements associated with the progression of esophageal cancer. Moreover, while oral administration is the predominant technique for inducing esophageal cancer, MNNG may interact with neighboring organs, including the stomach and small intestine, resulting in carcinogenic effects. Consequently, it may serve as a co-inducer of esophageal and gastric cancer. Research conducted by Mamdooh H Ghoneum et al. demonstrates that MNNG serves as a model for esophageal and gastric adenocarcinoma, revealing that esophageal tissue pathology predominantly presents as squamous cell carcinoma, whereas gastric tissue pathology following carcinogen exposure displays glandular dysplasia and adenocarcinoma (151). Nonetheless, the incidence of esophageal cancer produced by Methyl benzylnitrosamine (NMBA) can attain 100% (152). MNNG exhibits inferior organ specificity compared to the widely utilized chemical inducer NMBA in the EC model. In practical application, it does not precisely trigger particular disease types or target organs, and is primarily utilized to provoke damage and malignant transformation of esophageal epithelial cells.
4.2 MNNG in other system models
4.2.1 Animal models associated with lung cancer
Lung cancer is the malignancy with the greatest global mortality rate, and adenocarcinoma is one of its most common histological subtypes (1). MNNG can be employed to create lung cancer models. Research demonstrates that intravenous administration of MNNG can provoke the onset of lung cancer in animals (153). Lin Deng et al. (154) developed an early-stage lung adenocarcinoma (LAC) model by subcutaneously delivering a 0.4 mg MNNG solution weekly for four weeks. Tumor development was observed in all 10 animals, with micro-CT identifying a total of 231 tumors, all histologically verified as LAC. Additionally, Yan Ping Xie et al. (155) administered 0.4 mg of MNNG subcutaneously into the dorsal area of forty KM mice, once weekly for four successive weeks. The mice were categorized into four groups (n=10 per group), and tissue samples were obtained at 14, 18, 24, and 28 weeks, respectively. The research indicated that the adenomatous proliferation of cancer cells intensified over time, and the overall tumor count had a positive association with time. At week 28, the mean tumor count per mouse was 10.00 ± 5.64. MNNG exhibits significant carcinogenic properties and is frequently employed to produce early forms of lung cancer. Intravenous injection can effectively produce lung cancer in rats, however the mortality rate is elevated. Conversely, subcutaneous injection is more secure. MNNG influences lung tissue by absorption into the bloodstream and systemic circulation. This technique is straightforward to execute, maintains the integrity of lung tissue without harm, and prevents the introduction of confounding variables such as infection. In addition to localized tumors, low-dose repeated administrations can selectively generate lung tumors while sparing other organs from tumor development. Consequently, MNNG functions as a comparatively optimal drug for the induction of lung cancer models.
4.2.2 Animal models associated with endometrial cancer
Endometrial cancer is the predominant malignancy of the female reproductive system, with a persistent upward trend in incidence and a rising prevalence among younger women (156). MNNG, a powerful carcinogenic mutagen, is utilized to create endometrial cancer models (157, 158). MNNG enhances AKT phosphorylation and PI3K activation through TGF-β activation, hence augmenting endothelial cell invasiveness (159). T. Tanaka et al. (158) showed through studies the impact of varying dosages, administration routes, and cycles on the experimental outcomes. It was found that intrauterine injection can induce endometrial adenocarcinoma and other uterine neoplasms. Elevated dosages may induce pronounced local effects leading to tissue damage and hindered tumor development, albeit potentially raising tumor incidence. Cervical and vaginal tumors are the primary focus of vaginal administration, providing a valuable experimental model for investigating the prevalence of uterine cancer. Pakkiri Bhavani et al. (157) employed cotton balls saturated with MNNG (150 mg diluted in 0.2 ml of olive oil) for vaginal retention biweekly in albino female Wistar rats. Currently, intracavitary injection and the placement of absorbent cotton balls into the vagina are the predominant techniques for delivering MNNG. Vaginal delivery simulates a natural infection or local exposure, whereas intrauterine injection may exert a more immediate impact on uterine tissue. Among these methods, the intravaginal approach with saturated pellets is most commonly employed in model establishment.
4.2.3 Animal models associated with liver cancer
MNNG is categorized as a non-hepatocarcinogen and is rarely utilized in the development of liver cancer models. Chemical inducers including aflatoxin B1 (AFB1), carbon tetrachloride (CCl4), and diethylnitrosamine (DEN) are frequently employed in current research. MNNG is sometimes co-administered with several recognized carcinogens to improve the effectiveness of hepatocellular carcinoma induction or to expedite modeling deadlines. Research indicates that MNNG can activate oxidative stress responses, accelerate aberrant cell proliferation, and serve as an inducer of the initial phase of hepatocellular carcinoma (160). Prior studies have shown that administering 80 mg/kg MNNG for seven weeks can precipitate liver cancer when combined with CCL4 or partial hepatectomy (PH), significantly augmenting the quantity of glutathione S-transferase placental (GST-P) positive hepatocyte foci (161). Research by S.L. Herren et al. indicates that prolonged exposure to 0.005% MNNG in drinking water does not exhibit carcinogenic activity specifically affecting the liver (162). T. Ogiso et al. assert that non-hepatocarcinogens can solely generate tumors in certain target organs, shown by EHBN-induced bladder cancer and MNNG-induced gastric adenocarcinoma, without exerting a major promotional influence on the development of hepatocellular carcinoma (163). The origin of this paradoxical behavior may be attributed to variations in dosage and cycles, potentially elucidating why the carcinogenic effects have yet to materialize.
4.2.4 Animal models associated with skin
Skin tumors represent one of the most prevalent malignant neoplasms in humans, encompassing a developmental process characterized by initiation, promotion, and advancement (138, 139). MNNG is frequently employed as an initiator in skin tumor models and can induce irreversible genetic alterations.
Investigation conducted by I. Rehman et al. proposes that MNNG facilitates the development of skin tumors by generating mutations in codon 12 of the Ha-ras and Ki-ras oncogenes (140). J F O’Connell et al. assert that MNNG functions as both a tumor initiator and a tumor promoter (141). The tumorigenicity of MNNG significantly escalates during the dosage range of 0.5 - 5.0 μmol when employed as a full carcinogen. A dose range of 0.1 to 2.0 μmol can induce papilloma formation as an initiator, however elevated doses of the promoter diminish papilloma development. As a promotional agent, MNNG dosage is positively correlated with the incidence of cancers.
G J Patskan et al. (142) administered 2 μmol of MNNG topically to the skin of mice. With the prolongation of the treatment time, the incidence of skin malignancies in the mice progressively escalated, and in the advanced stages, 20% of the mice had lung metastases. The carcinogenic potential of MNNG in eliciting skin cancer seems plausible, and the method of topical delivery is straightforward and expedient. The dose-effect relationship curve of MNNG is intricate, with excessive doses potentially elevating mortality. Additionally, in contrast to the commonly employed two-stage carcinogenesis protocol utilizing DMBA (7,12-dimethylbenz[a]anthracene) as the initiator and TPA (12-O-tetradecanoylphorbol-13-acetate) as the promoter, MNNG exhibits reduced specificity.
4.3 Multi-organ characteristics and optimization strategies of MNNG in different models
4.3.1 The relative specificity of MNNG in gastric cancer-related models
Previous studies have indicated that MNNG exhibits relative specificity in inducing gastric cancer models (164). Following oral administration, the stomach demonstrates the highest level of DNA alkylation. This may be attributed to MNNG’s stability in the acidic gastric environment and the fact that the stomach is exposed to the highest concentration of the compound (165). Studies have shown that the activity of DNA repair enzymes such as MGMT is relatively low in the gastrointestinal tract, leading to the accumulation of alkylation damage (166, 167).
4.3.2 MNNG’s multi-organ properties
As discussed previously, we have examined the application of MNNG in other organs, which sufficiently demonstrates its carcinogenic potential across multiple organs.As a direct alkylating agent, MNNG induces tissue DNA damage without requiring bioactivation (16). It exhibits first-pass effects and localized exposure characteristics, with its organ specificity strongly dependent on the route of administration (168). Currently, MNNG is most commonly used in gastric cancer and pre-gastric cancer models, administered by drinking water and gavage.As a hollow organ connected to multiple parts of the digestive tract, the stomach allows MNNG solution to come into contact with various organs when administered in drinking water. After absorption through the stomach and small intestine, MNNG can be transported to other tissues and organs via systemic circulation and enterohepatic recirculation (169). Studies have reported the development of esophageal sarcomas and tumors in the stomach, liver, and jejunum following MNNG exposure in modeling experiments (150). Research by Mamdooh H. Ghoneum et al. (151) established a comorbid model of esophageal and gastric cancer using MNNG solution, further confirming its ability to induce multi-organ injury.
4.3.3 Organ-specific optimization of MNNG in model applications
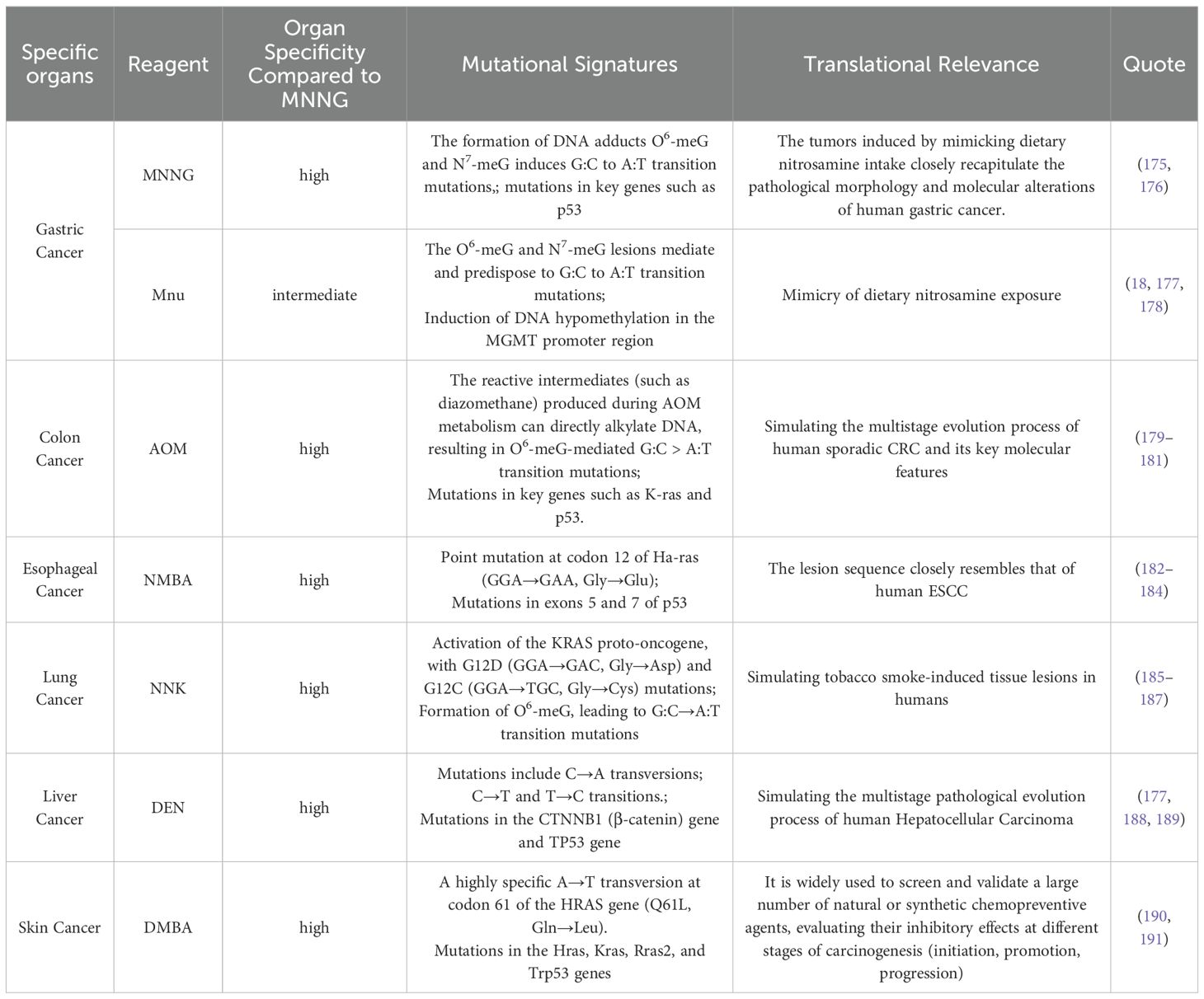
Nonetheless, MNNG is not always the optimal choice for various cancer models. Prioritization of organ-specific carcinogens: When conditions permit, agents with high organotypic specificity should be prioritized. For instance, 4-nitroquinoline 1-oxide (4NQO) and N-nitrosomethylbenzylamine (NMBA) show stronger specificity for esophageal cancer induction (170, 171); diethylnitrosamine (DEN) is more suitable for liver cancer models (172, 173); and 1,2-dimethylhydrazine (DMH) and its metabolite azoxymethane (AOM) are preferred for modeling colon cancer (133, 174). In specific organ contexts, these agents may outperform MNNG in terms of modeling efficiency, duration, and specificity. A comparative summary is provided in Table 7.
In models of tumors outside the digestive tract, although MNNG possesses carcinogenic potential, it is not the most commonly used or specific mutagen. In contrast, MNNG demonstrates a relatively high application rate and specificity in inducing gastric cancer. Nevertheless, off-target tumor development remains a concern (6, 86, 192, 193).Consequently, a significant scientific challenge exists in improving the efficacy of these medicines to selectively produce malignancies in the designated organ.While ensuring modeling efficiency, there is an urgent need to explore how to optimize the modeling approach to achieve higher specificity, better model stability, and more comprehensive animal welfare protection.
When experimental funding allows, the use of gene knockout models in combination with MNNG can be employed to enhance organ sensitivity (194). Optimizing the modeling timeline by selecting MNNG as the initiating factor and other modeling methods as promoting factors can not only more classically simulate different stages of carcinogenesis but also avoid the drawback of long-term MNNG application inducing multi-organ tumors (195). If a tendency for multi-organ tumor development is observed during the process, the promoting factors can be withdrawn to create a window period for further investigation. Furthermore, monitoring during the process should be optimized.Incorporating contemporary technologies is essential. Employing periodic blood tests to monitor early tumor biomarkers and evaluate potential liver damage induced by MNNG (152, 153), facilitating prompt modifications to dosage and scheduling. The endoscopic examination facilitated rapid evaluation, enabling direct visualization to detect model advancement and mitigate excessive exposure (154, 155). Multi-Parametric MRI (MP-MRI) serves as an effective instrument for the detection, localization, and characterization of primary tumors in experimental animals (192).CT and PET are also considered to be effective monitoring methods (196).
Employ more precise drug administration methods to avoid off-target effects: Utilize localized administration techniques such as intragastric infusion or other targeted delivery approaches to enhance specificity.Utilizing these strategies can capitalizing on specific agents where feasible, precisely regulating dosage and administration for multi-organ carcinogens, and employing advanced monitoring facilitates the early identification of carcinogenesis and metastasis. This proactive strategy enables researchers to intervene and ultimately reduce the incidence of off-target cancers in other organs.Promote the establishment of standardized model protocols.Animal models exhibit intrinsic limits. Literature reviews indicate considerable variability in model development, with the absence of essential experimental data significantly compromising the integrity of model assessments and study findings. A prior study of more than 250 publications linked to animal models revealed that less than 60% of the articles specifically documented at least three animal characteristics (gender, strain, weight, age) and the number of animals utilized (197). Significantly, blinded experiments were exceedingly uncommon. Document and publicly disclose detailed experimental parameters, including animal age (in weeks), body weight, housing conditions, MNNG concentration, administration method, MNNG supplier and storage conditions, as well as diet and water specifications. Define clear criteria for successful model establishment, using pathological findings as the gold standard and categorizing lesions based on severity (198). Transparently report negative outcomes: Accurately document and publish animal mortality rates, incidents of multi-organ tumors, and cases of acute intoxication during experiments. Avoid redundant experiments by maximizing the utility of existing data, ensuring its value is fully leveraged.
5 MNNG defects and optimization
MNNG is classified as a Group I carcinogen, exhibits both cytotoxic and genotoxic effects (199, 200). Its cytotoxicity may arise through non-mutagenic mechanisms, and the dominant outcome—cell death or mutagenic carcinogenesis—largely depends on exposure concentration and duration (201). Therefore, balancing MNNG-induced mutagenesis and cell death remains a critical challenge. Additionally,MNNG demonstrates dose- and time-dependent hepatotoxicity (202). The severe cytotoxicity, genotoxicity, and hepatotoxicity of MNNG can lead to premature death in experimental animals,for instance, due to acute toxicity rather than cancer development,which complicates the assessment of its carcinogenicity. Additionally, direct exposure to MNNG may pose risks to laboratory personnel and the environment. Optimizing dose-dependent threshold effects is essential to maximizing tumor induction efficiency while minimizing animal mortality.The scientific value of MNNG must be carefully weighed against its potential hazards to ensure it is utilized in a safe and controlled manner, maximizing its research benefits while minimizing harm. This balance remains an important topic for further discussion.
Another limitation of MNNG lies in the difficulty of fully replicating the natural human carcinogenic process through its administration route and dosage. Except for occupational exposures, human contact with carcinogens is typically localized, long-term, and low-dose. In addition to nitrosamines, factors such as Hp infection also contribute to carcinogenesis. Therefore, mechanistic insights or preventive strategies derived solely from MNNG-based models may have limited extrapolation to human contexts.
The localized effects of MNNG contribute to its limited organ specificity, making it challenging to establish pure and organ-specific tumor models. While tumors form in target organs such as the stomach (e.g., gastric adenocarcinoma), concurrent neoplasms or damage often occur in other sites, including forestomach, esophagus, lung adenocarcinoma, liver injury, or colonic lesions. The coexistence of multiple tumor types alters the host’s immune status and metabolic environment, complicating the tumor microenvironment of interest. This complexity compromises the precision, reproducibility, and reliability of intervention studies. Moreover, multi-tumor incidence increases suffering in experimental animals. Nevertheless, optimizing time–dose thresholds and employing combination modeling approaches may enhance its relative specificity and reduce off-target organ damage. Additionally, organoid models—which mimic the complex crosstalk among diverse cell types within tissues—may help overcome the nonspecificity associated with MNNG exposure (5, 199).
Secondly, the environmental and ecotoxicological impact of MNNG represents a critical concern. Improper storage, handling, or disposal during experimental procedures may lead to MNNG leakage into the environment. Highly water-soluble, MNNG can enter water cycles and soil systems through sewage and waste disposal. As a genotoxic and cytotoxic agent, it induces DNA mutations and may bioaccumulate, thereby threatening ecological stability.
Due to the toxic nature of MNNG, its potential for environmental contamination, and the specific requirements of tumor research, it is necessary to optimize animal welfare protection policies during experiments. Rigorous ethical review is required. Prioritizing human safety involves strict toxic waste disposal procedures for MNNG to prevent environmental pollution. Researchers must be fully informed of MNNG’s carcinogenic risks and implement rigorous protective measures to minimize exposure. When handling the agent, personnel must wear masks, gloves, head coverings, and goggles if necessary. They should also be trained in the safe preparation, administration, and disposal of MNNG-contaminated items such as water bottles and cages. Laboratories must classify and label MNNG with the correct hazard identifiers, and experimental waste containing MNNG must be treated as high-risk toxic waste.Personnel must receive advance training to master key techniques including oral gavage, rectal administration, and vaginal inoculation to avoid causing unnecessary suffering to animals due to operational errors (203). Administration strategies should be optimized based on target organs, and the maximum tolerable dose of the carcinogen should be determined. Continuous monitoring and timely adjustments to the experimental protocol are essential. Analgesics must be administered when necessary to alleviate pain, and all observations should be accurately recorded.Humane endpoints must be established: for animals bearing a single tumor, the average diameter should generally not exceed 1.2 cm in mice or 2.5 cm in rats—or 1.5 cm and 2.8 cm, respectively, under certain conditions. If multiple tumors are present (e.g., on contralateral flanks), the size of each should be proportionally smaller and must not exceed the maximum burden of a single tumor (196). Animals showing signs of cachexia or a weight loss exceeding 20% of normal adult body weight require immediate intervention. No animal should be allowed to die naturally from suffering.
6 Discussion
Spontaneous animal tumor models provide a notable advantage: their genesis and course closely resemble human tumors, displaying similar histological complexity and heterogeneity to true human malignancies (192, 204). Despite certain limitations, MNNG remains widely used as a straightforward and effective chemical carcinogen in studies investigating spontaneous tumors and precancerous lesions.Future efforts should focus on enhancing the organ-specific sensitivity and stability of the MNNG-induced model, mitigating associated risks in its application, reducing the number of experimental animals used, and minimizing suffering throughout the experimental process.
Nevertheless, these enhancements are inadequate for progressing model development. Researchers must offer experimental results with thorough and comprehensive information to ensure reproducibility in future studies and optimize model utilization. Considering that laboratory animals bear considerable scientific obligations and utilize important research funding (205), the lack of high-fidelity, repeatable models will hinder contemporary scientific advancement. We can only honor the lifelong commitment of experimental animals to scientific progress by maximizing their value.
Author contributions
XZ: Conceptualization, Writing – original draft, Data curation. YX: Data curation, Methodology, Writing – original draft. JC: Data curation, Formal analysis, Writing – original draft. TL: Investigation, Methodology, Writing – original draft. JW: Supervision, Visualization, Writing – original draft. JT: Supervision, Validation, Writing – review & editing. LZ: Supervision, Visualization, Writing – review & editing. ZL: Funding acquisition, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Grants Number: 82074187), Natural Science Foundation of Beijing Municipality(Grants Number:7232290) and Technology Transfer Fund of Dongzhimen Hospital, Beijing University of Chinese Medicine No. DZMCGZH-2023-002.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Tsukamoto T, Mizoshita T, and Tatematsu M. Animal models of stomach carcinogenesis. Toxicol Pathol. (2007) 35:636–48. doi: 10.1080/01926230701420632
3. Cai Y, Cao Y, Cheng S, Zou L, Yang T, Zhang Y, et al. Study on the mechanism of sancao tiaowei decoction in the treatment of MNNG-induced precancerous lesions of gastric carcinoma through hedgehog signaling pathway. Front Oncol. (2022) 12:841553. doi: 10.3389/fonc.2022.841553
4. Harper L, Herbst KW, and Kalfa N. Ethical issues in research: Human and animal experimentation. J Pediatr Urol. (2018) 14:286. doi: 10.1016/j.jpurol.2017.10.019
5. Li Y, Chen J, Li T, Lin J, Zheng H, Johnson N, et al. Modeling gastric intestinal metaplasia in 3D organoids using nitrosoguanidine. J Mol Cell Biol. (2024) 16:mjae030. doi: 10.1093/jmcb/mjae030
6. Zaidi NH, O’Connor PJ, and Butler WH. N-methyl-N’-nitro-N-nitrosoguanidine-induced carcinogenesis: differential pattern of upper gastrointestinal tract tumours in Wistar rats after single or chronic oral doses. Carcinogenesis. (1993) 14:1561–7. doi: 10.1093/carcin/14.8.1561
7. Zhang Y, Chen Z, Song J, Qian H, Wang Y, and Liang Z. The role of m6A modified circ0049271 induced by MNNG in precancerous lesions of gastric cancer. Heliyon. (2024) 10:e35654. doi: 10.1016/j.heliyon.2024.e35654
8. Wang J, Zhang W, Zhang R, Yang H, Li Y, Wang J, et al. MiR-101-3p promotes tumor cell proliferation and migration via the wnt signal pathway in MNNG-induced esophageal squamous cell carcinoma. Toxics. (2024) 12:824. doi: 10.3390/toxics12110824
9. Tong Y, Wang R, Liu X, Tian M, Wang Y, Cui Y, et al. Zuojin Pill ameliorates chronic atrophic gastritis induced by MNNG through TGF-β1/PI3K/Akt axis. J Ethnopharmacol. (2021) 271:113893. doi: 10.1016/j.jep.2021.113893
10. Liu S, Ji H, Zhang T, Huang J, Yin X, Zhang J, et al. Modified Zuojin pill alleviates gastric precancerous lesions by inhibiting glycolysis through the HIF-1α pathway. Phytomedicine. (2025) 136:156255. doi: 10.1016/j.phymed.2024.156255
11. Cheng S, Chen J, Li Q, Nie Y, Ni T, Peng C, et al. Protective effect of folic acid on MNNG-induced proliferation of esophageal epithelial cells via the PI3K/AKT/mTOR signaling pathway. J Nutr Biochem. (2024) 133:109702. doi: 10.1016/j.jnutbio.2024.109702
12. Lu L, Chen B, Zhang X, Xu Y, Jin L, Qian H, et al. The effect of phytochemicals in N-methyl-N-nitro-N-nitroguanidine promoting the occurrence and development of gastric cancer. Front Pharmacol. (2023) 14:1203265. doi: 10.3389/fphar.2023.1203265
13. Sugimura T, Fujimura S, and Baba T. Tumor production in the glandular stomach and alimentary tract of the rat by N-methyl-N’-nitro-N-nitrosoguanidine. Cancer Res. (1970) 30:455–65.
14. Sugimura T and Fujimura S. Tumour production in glandular stomach of rat by N-methyl-N’-nitro-N-nitrosoguanidine. Nature. (1967) 216:943–4. doi: 10.1038/216943a0
15. Wyatt MD and Pittman DL. Methylating agents and DNA repair responses: Methylated bases and sources of strand breaks. Chem Res Toxicol. (2006) 19:1580–94. doi: 10.1021/tx060164e
16. Kaina B. Mechanisms and consequences of methylating agent-induced SCEs and chromosomal aberrations: a long road traveled and still a far way to go. Cytogenet Genome Res. (2004) 104:77–86. doi: 10.1159/000077469
17. Kaina B, Fritz G, and Coquerelle T. Contribution of O6-alkylguanine and N-alkylpurines to the formation of sister chromatid exchanges, chromosomal aberrations, and gene mutations: new insights gained from studies of genetically engineered mammalian cell lines. Environ Mol Mutagen. (1993) 22:283–92. doi: 10.1002/em.2850220418
18. Chen Y-X, He L-L, Xiang X-P, Shen J, and Qi H-Y. O6-methylguanine DNA methyltransferase is upregulated in Malignant transformation of gastric epithelial cells via its gene promoter DNA hypomethylation. World J Gastrointest Oncol. (2022) 14:664–77. doi: 10.4251/wjgo.v14.i3.664
19. Niwa T, Yamashita S, Tsukamoto T, Kuramoto T, Nomoto T, Wakazono K, et al. Whole-genome analyses of loss of heterozygosity and methylation analysis of four tumor-suppressor genes in N-methyl-N’-nitro-N-nitrosoguanidine-induced rat stomach carcinomas. Cancer Sci. (2005) 96:409–13. doi: 10.1111/j.1349-7006.2005.00068.x
20. Sun Y, Hegamyer G, Nakamura K, Kim H, Oberley LW, and Colburn NH. Alterations of the p53 tumor-suppressor gene in transformed mouse liver cells. Int J Cancer. (1993) 55:952–6. doi: 10.1002/ijc.2910550613
21. Haque K, Cooper DP, and Povey AC. Formation and persistence of N7-methylguanine DNA adducts in the target pyloric tissue following chronic exposure to N-methyl-N’-nitro-N-nitrosoguanidine. Biomarkers. (1999) 4:254–62. doi: 10.1080/135475099230787
22. Yamashita S, Nomoto T, Ohta T, Ohki M, Sugimura T, and Ushijima T. Differential expression of genes related to levels of mucosal cell proliferation among multiple rat strains by using oligonucleotide microarrays. Mamm Genome. (2003) 14:845–52. doi: 10.1007/s00335-003-2299-3
23. Fornaro L, Vivaldi C, Caparello C, Musettini G, Baldini E, Masi G, et al. Pharmacoepigenetics in gastrointestinal tumors: MGMT methylation and beyond. Front Biosci (Elite Ed). (2016) 8:170–80. doi: 10.2741/E758
24. Becker K, Gregel C, Fricke C, Komitowski D, Dosch J, and Kaina B. DNA repair protein MGMT protects against N-methyl-N-nitrosourea-induced conversion of benign into Malignant tumors. Carcinogenesis. (2003) 24:541–6. doi: 10.1093/carcin/24.3.541
25. Soejima H, Zhao W, and Mukai T. Epigenetic silencing of the MGMT gene in cancer. Biochem Cell Biol. (2005) 83:429–37. doi: 10.1139/o05-140
26. Christmann M and Kaina B. Nuclear translocation of mismatch repair proteins MSH2 and MSH6 as a response of cells to alkylating agents. J Biol Chem. (2000) 275:36256–62. doi: 10.1074/jbc.M005377200
27. Kolodner RD. Mismatch repair: mechanisms and relationship to cancer susceptibility. Trends Biochem Sci. (1995) 20:397–401. doi: 10.1016/s0968-0004(00)89087-8
28. Song P, Liu S, Liu D, Keijzers G, Bakula D, Duan S, et al. CNOT6: A novel regulator of DNA mismatch repair. Cells. (2022) 11:521. doi: 10.3390/cells11030521
29. Nowosielska A and Marinus MG. DNA mismatch repair-induced double-strand breaks. DNA Repair (Amst). (2008) 7:48–56. doi: 10.1016/j.dnarep.2007.07.015
30. Chen Y, Feng H, Chen D, Abuduwaili K, Li X, and Zhang H. Protective effects of folic acid on DNA damage and DNA methylation levels induced by N-methyl-N’-nitro-N-nitrosoguanidine in Kazakh esophageal epithelial cells. Hum Exp Toxicol. (2018) 37:1258–67. doi: 10.1177/0960327118769709
31. Bai H, Gu L, Zhou J, and Deng D. p16 hypermethylation during gastric carcinogenesis of Wistar rats by N-methyl-N’-nitro-N-nitrosoguanidine. Mutat Res. (2003) 535:73–8. doi: 10.1016/s1383-5718(02)00288-7
32. Cheng Y-B, Fang D-C, Yao P, Guo L-P, Ning X-Y, and Wang L. Demethylation of the hTERT promoter in normal human gastric mucosal epithelial cells following N-methyl- N’-nitro- N-nitrosoguanidine exposure. Biomed Rep. (2015) 3:176–8. doi: 10.3892/br.2014.398
33. Chen K, Zhang S, Ke X, Qi H, Shao J, and Shen J. Biphasic reduction of histone H3 phosphorylation in response to N-nitroso compounds induced DNA damage. Biochim Biophys Acta. (2016) 1860:1836–44. doi: 10.1016/j.bbagen.2016.05.028
34. Liu T, Feng Y-L, Wang R-Y, Yang S, Ge Y-L, Zhang T-Y, et al. Long-term MNNG exposure promotes gastric carcinogenesis by activating METTL3/m6A/miR1184 axis-mediated epithelial-mesenchymal transition. Sci Total Environ. (2024) 913:169752. doi: 10.1016/j.scitotenv.2023.169752
35. Luo C and Wu X-G. Lycopene enhances antioxidant enzyme activities and immunity function in N-methyl-N’-nitro-N-nitrosoguanidine-enduced gastric cancer rats. Int J Mol Sci. (2011) 12:3340–51. doi: 10.3390/ijms12053340
36. Georgiadis P, Smith CA, and Swann PF. Nitrosamine-induced cancer: selective repair and conformational differences between O6-methylguanine residues in different positions in and around codon 12 of rat H-ras. Cancer Res. (1991) 51:5843–50.
37. Sha Z-G, Lin S, Fan Z-L, Yang Z-C, Gong Y-T, Qin T, et al. Efficacy and potential mechanisms of jatrorrhizine on MNNG-induced chronic atrophic gastritis in rats based on serological metabolomics and molecular docking. Sci Rep. (2025) 15:21018. doi: 10.1038/s41598-025-05502-2
38. Roos W, Baumgartner M, and Kaina B. Apoptosis triggered by DNA damage O6-methylguanine in human lymphocytes requires DNA replication and is mediated by p53 and Fas/CD95/Apo-1. Oncogene. (2004) 23:359–67. doi: 10.1038/sj.onc.1207080
39. Ochs K and Kaina B. Apoptosis induced by DNA damage O6-methylguanine is Bcl-2 and caspase-9/3 regulated and Fas/caspase-8 independent. Cancer Res. (2000) 60:5815–24.
40. Velmurugan B, Mani A, and Nagini S. Combination of S-allylcysteine and lycopene induces apoptosis by modulating Bcl-2, Bax, Bim and caspases during experimental gastric carcinogenesis. Eur J Cancer Prev. (2005) 14:387–93. doi: 10.1097/00008469-200508000-00012
41. Zhang Y, Ma Y, Zhao C, Zhang H, Pu Y, and Yin L. Synergistic Carcinogenesis of HPV18 and MNNG in Het-1A Cells through p62-KEAP1-NRF2 and PI3K/AKT/mTOR Pathway. Oxid Med Cell Longev. (2020) 2020:6352876. doi: 10.1155/2020/6352876
42. Liang Z, Song J, Xu Y, Zhang X, Zhang Y, and Qian H. Hesperidin reversed long-term N-methyl-N-nitro-N-nitroguanidine exposure induced EMT and cell proliferation by activating autophagy in gastric tissues of rats. Nutrients. (2022) 14:5281. doi: 10.3390/nu14245281
43. Manikandan P, Murugan RS, Priyadarsini RV, Vinothini G, and Nagini S. Eugenol induces apoptosis and inhibits invasion and angiogenesis in a rat model of gastric carcinogenesis induced by MNNG. Life Sci. (2010) 86:936–41. doi: 10.1016/j.lfs.2010.04.010
44. Chen J, Kähne T, Röcken C, Götze T, Yu J, Sung JJY, et al. Proteome analysis of gastric cancer metastasis by two-dimensional gel electrophoresis and matrix assisted laser desorption/ionization-mass spectrometry for identification of metastasis-related proteins. J Proteome Res. (2004) 3:1009–16. doi: 10.1021/pr049916l
45. Zhu X-D, Lin G-J, Qian L-P, and Chen Z-Q. Expression of survivin in human gastric carcinoma and gastric carcinoma model of rats. World J Gastroenterol. (2003) 9:1435–8. doi: 10.3748/wjg.v9.i7.1435
46. Ohgaki H, Ludeke BI, Meier I, Kleihues P, Lutz WK, and Schlatter C. DNA methylation in the digestive tract of F344 rats during chronic exposure to N-methyl-N-nitrosourea. J Cancer Res Clin Oncol. (1991) 117:13–8. doi: 10.1007/BF01613190
47. Tatematsu M, Furihata C, Hirose M, Shirai T, and Ito N. Changes in pepsinogen isozymes in stomach cancers induced in Wistar rats by N-methyl-N’-nitro-N-nitrosoguanidine and in transplantable gastric carcinoma (SG2B). J Natl Cancer Inst. (1977) 58:1709–16. doi: 10.1093/jnci/58.6.1709
48. Yasui W, Sumiyoshi H, Hata J, Mandai K, and Tahara E. Gut endocrine cells in rat stomach carcinoma induced by N-methyl-N’-nitro-N-nitrosoguanidine. J Cancer Res Clin Oncol. (1986) 111:87–92. doi: 10.1007/BF00400742
49. Sørbye H, Kvinnsland S, and Svanes K. Penetration of N-methyl-N’-nitro-N-nitrosoguanidine to proliferative cells in gastric mucosa of rats is different in pylorus and fundus and depends on exposure time and solvent. Carcinogenesis. (1993) 14:887–92. doi: 10.1093/carcin/14.5.887
50. Zaidi NH and O’Connor PJ. Identification in rat stomach mucosae of a cell population characterized by a deficiency for the repair of O6-methyldeoxyguanosine from DNA. Carcinogenesis. (1995) 16:461–9. doi: 10.1093/carcin/16.3.461
51. Furukawa H, Iwanaga T, Koyama H, and Taniguchi H. Effect of sex hormones on the experimental induction of cancer in rat stomach - a preliminary study. Digestion. (1982) 23:151–5. doi: 10.1159/000198722
52. Ketkar M, Reznik G, and Green U. Carcinogenic effect of N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) in European hamsters. Cancer Lett. (1978) 4:241–4. doi: 10.1016/s0304-3835(78)94842-5
53. Wakui S, Motohashi M, Muto T, Takahashi H, Hano H, Jutabha P, et al. Sex-associated difference in estrogen receptor β expression in N-methyl-N’-nitro-N-nitrosoguanidine-induced gastric cancers in rats. Comp Med. (2011) 61:412–8.
54. Ohgaki H, Kusama K, Hasegawa H, Sato S, Takayama S, and Sugimura T. Sequential histologic changes during gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in susceptible ACI and resistant BUF rats. J Natl Cancer Inst. (1986) 77:747–55. doi: 10.1093/jnci/77.3.747
55. Ohgaki H, Kawachi T, Matsukura N, Morino K, and Sugimura T. Genetic control of sensitivity of rats to gastrocarcinogenesis by N-methyl-N’-nitro-N-nitrosoguanidine. IARC Sci Publ. (1982) 41:603–9.
56. Tatematsu M, Aoki T, Inoue T, Mutai M, Furihata C, and Ito N. Coefficient induction of pepsinogen 1-decreased pyloric glands and gastric cancers in five different strains of rats treated with N-methyl-N’-nitro-N-nitrosoguanidine. Carcinogenesis. (1988) 9:495–8. doi: 10.1093/carcin/9.3.495
57. Yano H, Tatsuta M, Iishi H, Baba M, Sakai N, and Uedo N. Attenuation by d-limonene of sodium chloride-enhanced gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Int J Cancer. (1999) 82:665–8. doi: 10.1002/(sici)1097-0215(19990827)82:5<665::aid-ijc8>3.0.co;2-e
58. Tatsuta M, Iishi H, Baba M, Hirasawa R, Yano H, Sakai N, et al. Attenuation by all-trans-retinoic acid of sodium chloride-enhanced gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Br J Cancer. (1999) 79:732–6. doi: 10.1038/sj.bjc.6690117
59. Barten M. The effects of different MNNG (N-methyl-N’-nitro-N-nitrosoguanidine) doses on the stomach and the upper small intestine of the rat. I. The frequency and histopathology of the induced tumours. Exp Pathol. (1987) 31:147–52. doi: 10.1016/s0232-1513(87)80097-x
60. Iishi H, Tatsuta M, Baba M, Mikuni T, Yamamoto R, Iseki K, et al. Enhancement by monochloramine of the development of gastric cancers in rats: a possible mechanism of Helicobacter pylori-associated gastric carcinogenesis. J Gastroenterol. (1997) 32:435–41. doi: 10.1007/BF02934080
61. Tatsuta M, Iishi H, Baba M, Yano H, Iseki K, Uehara H, et al. Promotion by substance P of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. (1995) 96:99–103. doi: 10.1016/0304-3835(95)03917-l
62. Johnson FE, Awad EM, Doerr DE, LaRegina MC, Tolman KC, Stoutenger WA, et al. Effect of cyclosporine on carcinogenesis induced in rats by N-methyl-N’-nitro-N-nitrosoguanidine. J Surg Res. (1984) 37:180–8. doi: 10.1016/0022-4804(84)90178-1
63. Tsujii M, Kawano S, Tsuji S, Nagano K, Ito T, Hayashi N, et al. Ammonia: a possible promotor in Helicobacter pylori-related gastric carcinogenesis. Cancer Lett. (1992) 65:15–8. doi: 10.1016/0304-3835(92)90207-c
64. Kogure K, Sasadaira H, Kawachi T, Shimosato Y, and Tokunaga A. Further studies on induction of stomach cancer in hamsters by N-methyl-N’-nitro-N-nitrosoguanidine. Br J Cancer. (1974) 29:132–42. doi: 10.1038/bjc.1974.49
65. Lu B, Yu L, Xu L, Chen H, Zhang L, and Zeng Y. The effects of radix curcumae extract on expressions of VEGF, COX-2 and PCNA in gastric mucosa of rats fed with MNNG. Curr Pharm Biotechnol. (2010) 11:313–7. doi: 10.2174/138920110791111915
66. Okazaki K, Ishii Y, Kitamura Y, Maruyama S, Umemura T, Miyauchi M, et al. Dose-dependent promotion of rat forestomach carcinogenesis by combined treatment with sodium nitrite and ascorbic acid after initiation with N-methyl-N’-nitro-N-nitrosoguanidine: possible contribution of nitric oxide-associated oxidative DNA damage. Cancer Sci. (2006) 97:175–82. doi: 10.1111/j.1349-7006.2006.00162.x
67. Nishikawa A, Furukawa F, Mitsui M, Enami T, Kawanishi T, Hasegawa T, et al. Inhibitory effect of calcium chloride on gastric carcinogenesis in rats after treatment with N-methyl-N’-nitro-N-nitrosoguanidine and sodium chloride. Carcinogenesis. (1992) 13:1155–8. doi: 10.1093/carcin/13.7.1155
68. Wang X, Liu H, Wang X, Zeng Z, Xie L-Q, Sun Z-G, et al. Preventive effect of Actinidia valvata Dunn extract on N-methyl-N’-nitro-N-nitrosoguanidine-induced gastrointestinal cancer in rats. Asian Pac J Cancer Prev. (2014) 15:6363–7. doi: 10.7314/apjcp.2014.15.15.6363
69. Tatsuta M, Iishi H, Baba M, Mikuni T, and Taniguchi H. Effects of propranolol and cimetidine on cysteamine inhibition of gastric carcinogenesis induced in Wistar rats by N-methyl-N’-nitro-N-nitrosoguanidine. Int J Cancer. (1989) 43:464–7. doi: 10.1002/ijc.2910430320
70. Xu Q, Yang CH, Liu Q, Jin XF, Xu XT, Tong JL, et al. Chemopreventive effect of epigallocatechin-3-gallate (EGCG) and folic acid on the N-methyl-N’-nitro-N-nitrosoguanidine (MNNG)-induced gastrointestinal cancer in rat model. J Dig Dis. (2011) 12:181–7. doi: 10.1111/j.1751-2980.2011.00494.x
71. Tsukamoto H, Mizoshita T, Katano T, Hayashi N, Ozeki K, Ebi M, et al. Preventive effect of rebamipide on N-methyl-N’-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in rats. Exp Toxicol Pathol. (2015) 67:271–7. doi: 10.1016/j.etp.2015.01.003
72. Siu KF, Wei WI, and Wong J. Chronic gastric ulcers are not predisposed to tumor formation when exposed to a low dose of carcinogen. J Surg Res. (1986) 41:58–64. doi: 10.1016/0022-4804(86)90009-0
73. Kodama M, Kodama T, and Kodama M. Interaction between N-methyl-N’-nitro-N-nitrosoguanidine and 2 steroid hormones in the glandular stomach of mouse. I. Acceleration of hydrocortisone turnover by use of a salt-rich diet and the carcinogen. BioMed Pharmacother. (1989) 43:197–205. doi: 10.1016/0753-3322(89)90215-1
74. Ekambaram G, Rajendran P, Magesh V, and Sakthisekaran D. Naringenin reduces tumor size and weight lost in N-methyl-N’-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in rats. Nutr Res. (2008) 28:106–12. doi: 10.1016/j.nutres.2007.12.002
75. Habs M, Deutsch-Wenzel R, Preussmann R, and Schmähl D. Induction of gastric tumors in BD-VI rats by single application of N-methyl-N’-nitro-N-nitrosoguanidine in dimethylsulfoxide (DMSO). Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. (1978) 91:183–8. doi: 10.1007/BF00284024
76. Suleyman H, Cadirci E, Albayrak A, Halici Z, Gundogdu C, and Hacimuftuoglu A. Occurrence of anticancer activity of prednisolone via adrenalectomy and inhibition of adrenaline in rats. Int J Cancer. (2010) 126:1740–8. doi: 10.1002/ijc.24869
77. Zhang L, Jia B, Velu P, and Wu H. Corilagin induces apoptosis and inhibits HMBG1/PI3K/AKT signaling pathways in a rat model of gastric carcinogenesis induced by methylnitronitrosoguanidine. Environ Toxicol. (2022) 37:1222–30. doi: 10.1002/tox.23478
78. Balansky RM, Blagoeva PM, Mircheva ZI, Stoitchev I, and Chernozemskí I. The effect of antioxidants on MNNG-induced stomach carcinogenesis in rats. J Cancer Res Clin Oncol. (1986) 112:272–5. doi: 10.1007/BF00395922
79. Velmurugan B, Bhuvaneswari V, and Nagini S. Antiperoxidative effects of lycopene during N-methyl-N’-nitro-N-nitrosoguanidine-induced gastric carcinogenesis. Fitoterapia. (2002) 73:604–11. doi: 10.1016/s0367-326x(02)00216-2
80. Arivazhagan S, Velmurugan B, Bhuvaneswari V, and Nagini S. Effects of aqueous extracts of garlic (Allium sativum) and neem (Azadirachta indica) leaf on hepatic and blood oxidant-antioxidant status during experimental gastric carcinogenesis. J Med Food. (2004) 7:334–9. doi: 10.1089/jmf.2004.7.334
81. Velmurugan B and Nagini S. Combination chemoprevention of experimental gastric carcinogenesis by s-allylcysteine and lycopene: modulatory effects on glutathione redox cycle antioxidants. J Med Food. (2005) 8:494–501. doi: 10.1089/jmf.2005.8.494
82. Manikandan P, Vinothini G, Vidya Priyadarsini R, Prathiba D, and Nagini S. Eugenol inhibits cell proliferation via NF-κB suppression in a rat model of gastric carcinogenesis induced by MNNG. Invest New Drugs. (2011) 29:110–7. doi: 10.1007/s10637-009-9345-2
83. Murugan RS, Mohan KVPC, Uchida K, Hara Y, Prathiba D, and Nagini S. Modulatory effects of black tea polyphenols on oxidant-antioxidant profile and expression of proliferation, apoptosis, and angiogenesis-associated proteins in the rat forestomach carcinogenesis model. J Gastroenterol. (2007) 42:352–61. doi: 10.1007/s00535-007-2018-z
84. Proctor DM, Gatto NM, Hong SJ, and Allamneni KP. Mode-of-action framework for evaluating the relevance of rodent forestomach tumors in cancer risk assessment. Toxicol Sci. (2007) 98:313–26. doi: 10.1093/toxsci/kfm075
85. Wang X-Y, Wang L-L, Zheng X, Meng L-N, Lyu B, and Jin H-F. Expression of p-STAT3 and vascular endothelial growth factor in MNNG-induced precancerous lesions and gastric tumors in rats. World J Gastrointest Oncol. (2016) 8:305–13. doi: 10.4251/wjgo.v8.i3.305
86. Eichler G, Habs M, and Schmähl D. Induction of tumors of the forestomach in rats by oral application of N-methyl-N’-nitro-nitrosoguanidine. J Cancer Res Clin Oncol. (1983) 105:194–6. doi: 10.1007/BF00406933
87. Yin J, Yi J, Yang C, Xu B, Lin J, Hu H, et al. Chronic atrophic gastritis and intestinal metaplasia induced by high-salt and N-methyl-N’-nitro-N-nitrosoguanidine intake in rats. Exp Ther Med. (2021) 21:315. doi: 10.3892/etm.2021.9746
88. Li D, Zhao L, Li Y, Kang X, and Zhang S. Gastro-protective effects of calycosin against precancerous lesions of gastric carcinoma in rats. Drug Des Devel Ther. (2020) 14:2207–19. doi: 10.2147/DDDT.S247958
89. Cai T, Zhang C, Zhao Z, Li S, Cai H, Chen X, et al. The gastric mucosal protective effects of astragaloside IV in mnng-induced GPL rats. BioMed Pharmacother. (2018) 104:291–9. doi: 10.1016/j.biopha.2018.04.013
90. Zheng J, Cai W, Lu X, He W, Li D, Zhong H, et al. Chronic stress accelerates the process of gastric precancerous lesions in rats. J Cancer. (2021) 12:4121–33. doi: 10.7150/jca.52658
91. Zhao Y, Li B, Wang G, Ge S, Lan X, Xu G, et al. Dendrobium officinale Polysaccharides Inhibit 1-Methyl-2-Nitro-1-Nitrosoguanidine Induced Precancerous Lesions of Gastric Cancer in Rats through Regulating Wnt/β-Catenin Pathway and Altering Serum Endogenous Metabolites. Molecules. (2019) 24:2660. doi: 10.3390/molecules24142660
92. Zhao Y, Zhao J, Ma H, Han Y, Xu W, Wang J, et al. High hepcidin levels promote abnormal iron metabolism and ferroptosis in chronic atrophic gastritis. Biomedicines. (2023) 11:2338. doi: 10.3390/biomedicines11092338
93. Yi Z, Jia Q, Wang Y, Zhang Y, Xie T, and Ling J. Elian granules alleviate precancerous lesions of gastric cancer in rats by suppressing M2-type polarization of tumor-associated macrophages through NF-κB signaling pathway. BMC Complement Med Ther. (2023) 23:188. doi: 10.1186/s12906-023-04015-7
94. He R, Ma R, Jin Z, Zhu Y, Yang F, Hu F, et al. Proteomics and Metabolomics Unveil Codonopsis pilosula (Franch.) Nannf. Ameliorates Gastric Precancerous Lesions via Regulating Energy Metabolism. Front Pharmacol. (2022) 13:933096. doi: 10.3389/fphar.2022.933096
95. Zhu Y, Ma R, Cheng W, Qin M, Guo W, Qi Y, et al. Sijunzi decoction ameliorates gastric precancerous lesions via regulating oxidative phosphorylation based on proteomics and metabolomics. J Ethnopharmacol. (2024) 318:116925. doi: 10.1016/j.jep.2023.116925
96. Zhu F, Zhang X, Wen J, Liu Y, and Zhu Y. Celastrus orbiculatus extract reverses precancerous lesions of gastric cancer by inhibiting autophagy via regulating the PDCD4-ATG5 signaling pathway. J Pharm Pharmacol. (2024) 76:257–68. doi: 10.1093/jpp/rgae006
97. Liang L, He C, Han X, Liu J, Yang L, Chang F, et al. Zuojin pill alleviates precancerous lesions of gastric cancer by modulating the MEK/ERK/c-myc pathway: an integrated approach of network pharmacology, molecular dynamics simulation, and experimental validation. Drug Des Devel Ther. (2024) 18:5905–29. doi: 10.2147/DDDT.S487371
98. Xie D, Wu C, Wang D, Nisma Lena BA, Liu N, Ye G, et al. Wei-fu-chun tablet halted gastric intestinal metaplasia and dysplasia associated with inflammation by regulating the NF-κB pathway. J Ethnopharmacol. (2024) 318:117020. doi: 10.1016/j.jep.2023.117020
99. Yu C, Su Z, Li Y, Li Y, Liu K, Chu F, et al. Dysbiosis of gut microbiota is associated with gastric carcinogenesis in rats. BioMed Pharmacother. (2020) 126:110036. doi: 10.1016/j.biopha.2020.110036
100. Tatematsu M, Takahashi M, Fukushima S, Hananouchi M, and Shirai T. Effects in rats of sodium chloride on experimental gastric cancers induced by N-methyl-N-nitro-N-nitrosoguanidine or 4-nitroquinoline-1-oxide. J Natl Cancer Inst. (1975) 55:101–6. doi: 10.1093/jnci/55.1.101
101. Takahashi M, Nishikawa A, Furukawa F, Enami T, Hasegawa T, and Hayashi Y. Dose-dependent promoting effects of sodium chloride (NaCl) on rat glandular stomach carcinogenesis initiated with N-methyl-N’-nitro-N-nitrosoguanidine. Carcinogenesis. (1994) 15:1429–32. doi: 10.1093/carcin/15.7.1429
102. Zhao Y, Sun Y, Wang G, Ge S, and Liu H. Dendrobium Officinale Polysaccharides Protect against MNNG-Induced PLGC in Rats via Activating the NRF2 and Antioxidant Enzymes HO-1 and NQO-1. Oxid Med Cell Longev. (2019) 2019:9310245. doi: 10.1155/2019/9310245
103. Takahashi M, Hasegawa T, Furukawa F, Okamiya H, Shinoda K, Imaida K, et al. Enhanced lipid peroxidation in rat gastric mucosa caused by NaCl. Carcinogenesis. (1991) 12:2201–4. doi: 10.1093/carcin/12.12.2201
104. Tatsuta M, Iishi H, Baba M, Uehara H, Nakaizumi A, and Iseki K. Reduction in NaCl-enhanced gastric carcinogenesis in rats fed a high-protein diet. Cancer Lett. (1997) 116:247–52. doi: 10.1016/s0304-3835(97)00197-3
105. Salmon RJ, Laurent M, and Thierry JP. Effect of taurocholic acid feeding on methyl-nitro-N-nitroso-guanidine induced gastric tumors. Cancer Lett. (1984) 22:315–20. doi: 10.1016/0304-3835(84)90168-x
106. Yoshida Y, Tatematsu M, Takaba K, Shichino Y, and Ito N. Effects of various bile acids and their sodium salts on development of pepsinogen-altered pyloric glands in rats. Teratog Carcinog Mutagen. (1992) 12:179–86. doi: 10.1002/tcm.1770120404
107. Newberne PM, Charnley G, Adams K, Cantor M, Roth D, Supharkarn V, et al. Gastric and oesophageal carcinogenesis: models for the identification of risk and protective factors. Food Chem Toxicol. (1986) 24:1111–9. doi: 10.1016/0278-6915(86)90296-6
108. Kuo C-H, Hu H-M, Tsai P-Y, Wu I-C, Yang S-F, Chang L-L, et al. Short-term celecoxib intervention is a safe and effective chemopreventive for gastric carcinogenesis based on a Mongolian gerbil model. World J Gastroenterol. (2009) 15:4907–14. doi: 10.3748/wjg.15.4907
109. Tokieda M, Honda S, Fujioka T, and Nasu M. Effect of Helicobacter pylori infection on the N-methyl-N’-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in Mongolian gerbils. Carcinogenesis. (1999) 20:1261–6. doi: 10.1093/carcin/20.7.1261
110. Tsujii M, Kawano S, Tsuji S, Takei Y, Tamura K, Fusamoto H, et al. Mechanism for ammonia-induced promotion of gastric carcinogenesis in rats. Carcinogenesis. (1995) 16:563–6. doi: 10.1093/carcin/16.3.563
111. Waldum HL and Rehfeld JF. Gastric cancer and gastrin: on the interaction of Helicobacter pylori gastritis and acid inhibitory induced hypergastrinemia. Scand J Gastroenterol. (2019) 54:1118–23. doi: 10.1080/00365521.2019.1663446
112. Dahm K and Werner B. Susceptibility of the resected stomach to experimental carcinogenesis. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. (1976) 85:219–29. doi: 10.1007/BF00284082
113. Iishi H, Tatsuta M, Baba M, and Taniguchi H. Promotion by ethanol of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Br J Cancer. (1989) 59:719–21. doi: 10.1038/bjc.1989.151
114. Cerar A and Pokorn D. Inhibition of MNNG-induced gastroduodenal carcinoma in rats by synchronous application of wine or 11% ethanol. Nutr Cancer. (1996) 26:347–52. doi: 10.1080/01635589609514490
115. Chu F, Li Y, Meng X, Li Y, Li T, Zhai M, et al. Gut microbial dysbiosis and changes in fecal metabolic phenotype in precancerous lesions of gastric cancer induced with N-methyl-N’-nitro-N-nitrosoguanidine, sodium salicylate, ranitidine, and irregular diet. Front Physiol. (2021) 12:733979. doi: 10.3389/fphys.2021.733979
116. Miwa H, Endo K, Wada R, Hirai S, Hirose M, Misawa H, et al. Cellular proliferation and differentiation in rat atrophic gastric mucosa induced by N’-methyl-N’-nitro-N-nitrosoguanidine. J Clin Gastroenterol. (1997) 25 Suppl 1:S116–121. doi: 10.1097/00004836-199700001-00020
117. Ji R, Zhang X, Qian H, Gu H, Sun Z, Mao F, et al. miR-374 mediates the Malignant transformation of gastric cancer-associated mesenchymal stem cells in an experimental rat model. Oncol Rep. (2017) 38:1473–81. doi: 10.3892/or.2017.5831
118. Li Q-Y, Yang P-H, Huang X, Li X-L, Luo M-Y, Xiao B-J, et al. Gastric mucosa pathology in rats with precancerous lesions of gastric cancer with spleen deficiency and blood stasis. Evid Based Complement Alternat Med. (2022) 2022:1366597. doi: 10.1155/2022/1366597
119. Aoyagi K, Kohfuji K, Yano S, Murakami N, Hori H, Terasaki Y, et al. Morphological change in the MNNG-treated rat gastric mucosa. Kurume Med J. (2000) 47:31–6. doi: 10.2739/kurumemedj.47.31
120. Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J gastrointestinal Oncol. (2012) 4:156–69. doi: 10.4251/wjgo.v4.i7.156
121. Loogna P, Franzén L, Sipponen P, and Domellöf L. Cyclooxygenase-2 and Bcl-2 expression in the stomach mucosa of Wistar rats exposed to Helicobacter pylori, N’-methyl- N’-nitro- N-nitrosoguanidine and bile. Virchows Arch. (2002) 441:77–84. doi: 10.1007/s00428-001-0571-z
122. Hirayama Y, Wakazono K, Yamamoto M, Kitano M, Tatematsu M, Nagao M, et al. Rare mutations of p53, Ki-ras, and beta-catenin genes and absence of K-sam and c-erbB-2 amplification in N-methyl-N’-nitro-N-nitrosoguanidine-induced rat stomach cancers. Mol Carcinog. (1999) 25:42–7. doi: 10.1002/(sici)1098-2744(199905)25:1<42::aid-mc5>3.0.co;2-f
123. Abe M, Yamashita S, Kuramoto T, Hirayama Y, Tsukamoto T, Ohta T, et al. Global expression analysis of N-methyl-N’-nitro-N-nitrosoguanidine-induced rat stomach carcinomas using oligonucleotide microarrays. Carcinogenesis. (2003) 24:861–7. doi: 10.1093/carcin/bgg030
124. Kaneko M, Morimura K, Nishikawa T, Wanibuchi H, Takada N, Osugi H, et al. Different genetic alterations in rat forestomach tumors induced by genotoxic and non-genotoxic carcinogens. Carcinogenesis. (2002) 23:1729–35. doi: 10.1093/carcin/23.10.1729
125. Lindström CG, Rosengren JE, and Ekberg O. Experimental colonic tumours in the rat. III. Induction time, distribution and appearance of induced tumours. Acta Radiol Diagn (Stockh). (1978) 19:799–816. doi: 10.1177/028418517801900510
126. Reddy BS, Narasawa T, Weisburger JH, and Wynder EL. Promoting effect of sodium deoxycholate on colon adenocarcinomas in germfree rats. J Natl Cancer Inst. (1976) 56:441–2. doi: 10.1093/jnci/56.2.441
127. Amberger H. Different autochthonous models of colorectal cancer in the rat. J Cancer Res Clin Oncol. (1986) 111:157–9. doi: 10.1007/BF00400756
128. Balish E, Shih CN, Croft WA, Pamukcu AM, Lower G, Bryan GT, et al. Effect of age, sex, and intestinal flora on the induction of colon tumors in rats. J Natl Cancer Inst. (1977) 58:1103–6. doi: 10.1093/jnci/58.4.1103
129. So BT, Magadia NE, and Wynder EL. Induction of carcinomas of the colon and rectum in rats by intrarectal instillation of N-methyl-N’-nitro-N-nitrosoguanidine. J Natl Cancer Inst. (1973) 50:927–32. doi: 10.1093/jnci/50.4.927
130. Kannen V, Hintzsche H, Zanette DL, Silva WA, Garcia SB, Waaga-Gasser AM, et al. Antiproliferative effects of fluoxetine on colon cancer cells and in a colonic carcinogen mouse model. PloS One. (2012) 7:e50043. doi: 10.1371/journal.pone.0050043
131. Frajacomo FT, Kannen V, Deminice R, Geraldino TH, Pereira-Da-Silva G, Uyemura SA, et al. Aerobic training activates interleukin 10 for colon anticarcinogenic effects. Med Sci Sports Exerc. (2015) 47:1806–13. doi: 10.1249/MSS.0000000000000623
132. Nascimento-Gonçalves E, Mendes BAL, Silva-Reis R, Faustino-Rocha AI, Gama A, and Oliveira PA. Animal models of colorectal cancer: from spontaneous to genetically engineered models and their applications. Vet Sci. (2021) 8:59. doi: 10.3390/vetsci8040059
133. Neto Í, Rocha J, Gaspar MM, and Reis CP. Experimental murine models for colorectal cancer research. Cancers (Basel). (2023) 15:2570. doi: 10.3390/cancers15092570
134. Maurin N, Forgue-Lafitte M-E, Levy P, Zimber A, and Bara J. Progression of tumors arising from large ACF is associated with the MUC5AC expression during rat colon MNNG carcinogenis. Int J Cancer. (2007) 120:477–83. doi: 10.1002/ijc.22302
135. Zusman I, Zimber A, Madar Z, and Nyska A. Morphological, histochemical and immunohistochemical differences between tumorous and adjacent tissues in chemically induced colon cancer in rats. Acta Anat (Basel). (1992) 145:29–34. doi: 10.1159/000147338
136. Narisawa T, Reddy BS, and Weisburger JH. Effect of bile acids and dietary fat on large bowel carcinogenesis in animal models. Gastroenterol Jpn. (1978) 13:206–12. doi: 10.1007/BF02773665
137. Narisawa T, Magadia NE, Weisburger JH, and Wynder EL. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N’-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. (1974) 53:1093–7. doi: 10.1093/jnci/53.4.1093
138. Che TCH, François S, Bouchet S, Chapel A, and Forgue-Lafitte M-E. Early lesions induced in rat colon epithelium by N-methyl-N’-nitro-N-nitrosoguanidine. Tissue Cell. (2010) 42:190–4. doi: 10.1016/j.tice.2010.04.002
139. Zusman I, Zimber A, and Nyska A. Role of morphological methods in the analysis of chemically induced colon cancer in rats. Acta Anat (Basel). (1991) 142:351–6. doi: 10.1159/000147215
140. Zusman I, Madar Z, and Nyska A. Individual variability of pathological parameters in chemically induced rat colon tumors. Acta Anat (Basel). (1992) 145:106–11. doi: 10.1159/000147350
141. Weisburger JH, Reddy BS, Narisawa T, and Wynder EL. Germ-free status and colon tumor induction by N-methyl-N’-nitro-N-nitrosoguanidine. Proc Soc Exp Biol Med. (1975) 148:1119–21. doi: 10.3181/00379727-148-38700
142. Yamaguchi A, Ishida T, Nishimura G, Katoh M, and Miyazaki I. Investigation of colonic prostaglandins in carcinogenesis in the rat colon. Dis Colon Rectum. (1991) 34:572–6. doi: 10.1007/BF02049897
143. Nakano H. Histopathological studies on rat colo-rectal carcinoma induced by N-methyl-N’-nitro-N-nitrosoguanidine. Tohoku J Exp Med. (1973) 110:7–21. doi: 10.1620/tjem.110.7
144. Narisawa T, Wong CQ, and Weisburger JH. Large bowel carcinoma in strain-2 Guinea pigs by intrarectal instillation of N-methyl-N’-nitro-n-nitrosoguanidine. Gan. (1976) 67:41–6.
145. Greene FL, Lamb LS, and Barwick M. Colorectal cancer in animal models–a review. J Surg Res. (1987) 43:476–87. doi: 10.1016/0022-4804(87)90107-7
146. Rosenberg DW, Giardina C, and Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. (2009) 30:183–96. doi: 10.1093/carcin/bgn267
147. Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. (2022) 163:649–658.e2. doi: 10.1053/j.gastro.2022.05.054
148. Sheikh M, Roshandel G, McCormack V, and Malekzadeh R. Current status and future prospects for esophageal cancer. Cancers (Basel). (2023) 15:765. doi: 10.3390/cancers15030765
149. Cheng S, Che L, Yang Q, Sun R, Nie Y, Shi H, et al. Folic acid ameliorates N-methyl-N’-nitro-N-nitrosoguanidine-induced esophageal inflammation via modulation of the NF-κB pathway. Toxicol Appl Pharmacol. (2022) 447:116087. doi: 10.1016/j.taap.2022.116087
150. Yioris N, Ivankovic S, and Lehnert T. Effect of thermal injury and oral administration of N-methyl-N’-Nitro-N-nitrosoguanidine on the development of esophageal tumors in Wistar rats. Oncology. (1984) 41:36–8. doi: 10.1159/000225787
151. Ghoneum MH, Badr El-Din NK, Abdel Fattah SM, Pan D, and Tolentino L. Hydroferrate fluid, MRN-100, provides protection against chemical-induced gastric and esophageal cancer in Wistar rats. Int J Biol Sci. (2015) 11:295–303. doi: 10.7150/ijbs.10586
152. Zhou Y, Xia J, Xu S, She T, Zhang Y, Sun Y, et al. Experimental mouse models for translational human cancer research. Front Immunol. (2023) 14:1095388. doi: 10.3389/fimmu.2023.1095388
153. Stekar J and Gimmy J. Induction of lung tumours in rats by i.v. injection of N-methyl-N’-nitro-N-nitrosoguanidine. Eur J Cancer (1965). (1980) 16:395–400. doi: 10.1016/0014-2964(80)90358-8
154. Deng L, Xiao SM, Qiang JW, Li YA, and Zhang Y. Early lung adenocarcinoma in mice: micro-computed tomography manifestations and correlation with pathology. Transl Oncol. (2017) 10:311–7. doi: 10.1016/j.tranon.2017.02.003
155. Xie YP, Dong ZH, Du JH, Zang XL, Guo HH, Liu M, et al. The relationship between mouse lung adenocarcinoma at different stages and the expression level of exosomes in serum. Math Biosci Eng. (2019) 17:1548–57. doi: 10.3934/mbe.2020080
156. Makker V, MacKay H, Ray-Coquard I, Levine DA, Westin SN, Aoki D, et al. Endometrial cancer. Nat Rev Dis Primers. (2021) 7:88. doi: 10.1038/s41572-021-00324-8
157. Bhavani P, Subramanian P, and Kanimozhi S. Preventive efficacy of vanillic acid on regulation of redox homeostasis, matrix metalloproteinases and cyclin D1 in rats bearing endometrial carcinoma. Indian J Clin Biochem. (2017) 32:429–36. doi: 10.1007/s12291-016-0605-6
158. Tanaka T and Mori H. Experimental induction of uterine cancer in rats by N-methyl-N’-nitro-N-nitrosoguanidine. Pathol Res Pract. (1983) 178:20–6. doi: 10.1016/S0344-0338(83)80081-8
159. Shanmugapriya S, Subramanian P, and Kanimozhi S. Geraniol inhibits endometrial carcinoma via downregulating oncogenes and upregulating tumour suppressor genes. Indian J Clin Biochem. (2017) 32:214–9. doi: 10.1007/s12291-016-0601-x
160. Lee J and Lim K-T. SJSZ glycoprotein (38 kDa) inhibits cell cycle and oxidative stress in N-methyl-N’-nitro-N-nitrosoguanidine-induced ICR mice. Anticancer Agents Med Chem. (2013) 13:647–53. doi: 10.2174/1871520611313040013
161. Kobayashi K, Mutai M, Goto K, Inada K, Tsukamoto T, Nakanishi H, et al. Effects of carbon tetrachloride administration on initiation of liver cell foci by the non-hepatocarcinogens N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) and benzo(a)pyrene (B(a)P). Cancer Lett. (1997) 118:55–60. doi: 10.1016/s0304-3835(97)00222-x
162. Herren SL, Pereira MA, Britt AL, and Khoury MK. Initiation/promotion assay for chemical carcinogens in rat liver. Toxicol Lett. (1982) 12:143–50. doi: 10.1016/0378-4274(82)90177-1
163. Ogiso T, Tatematsu M, Tamano S, Hasegawa R, and Ito N. Correlation between medium-term liver bioassay system data and results of long-term testing in rats. Carcinogenesis. (1990) 11:561–6. doi: 10.1093/carcin/11.4.561
164. Ct G and Pn M. DNA-methylation by nitrosocimetidine and N-methyl-N-nitro-N-nitrosoguanidine in the intact rat. Chemico-biological Interact. (1982) 40:149–57. doi: 10.1016/0009-2797(82)90097-7
165. Mirvish SS. Formation of N-nitroso compounds: chemistry, kinetics, and in vivo occurrence. Toxicol Appl Pharmacol. (1975) 31:325–51. doi: 10.1016/0041-008x(75)90255-0
166. Wu Z, Dai J, Li J, Zhang Z, and Shen X. Exploiting the role of O6-methylguanine-DNA-methyltransferase (MGMT) in gastrointestinal cancers. Naunyn Schmiedebergs Arch Pharmacol. (2025) 398:319–27. doi: 10.1007/s00210-024-03365-4
167. Zaidi NH, Potten CS, Margison GP, Cooper DP, and O’Connor PJ. Tissue and cell specific methylation, repair and synthesis of DNA in the upper gastrointestinal tract of Wistar rats treated with N-methyl-N’-nitro-N-nitrosoguanidine via the drinking water. Carcinogenesis. (1993) 14:1991–2001. doi: 10.1093/carcin/14.10.1991
168. Gichner T and Velemínský J. Genetic effects of N-methyl-N’-nitro-N-nitrosoguanidine and its homologs. Mutat Res. (1982) 99:139–242. doi: 10.1016/0165-1110(82)90057-4
169. Koyama SY, Hollander D, and Dadufalza V. The mechanisms of intestinal absorption of the carcinogen MNNG (N-methyl-N’-nitro-N-nitrosoguanidine). Proc Soc Exp Biol Med. (1988) 188:206–11. doi: 10.3181/00379727-188-42729
170. Bhat N, Al-Mathkour M, Maacha S, Lu H, El-Rifai W, and Ballout F. Esophageal adenocarcinoma models: a closer look. Front Mol Biosci. (2024) 11:1440670. doi: 10.3389/fmolb.2024.1440670
171. Mahmoudian RA, Farshchian M, Golyan FF, Mahmoudian P, Alasti A, Moghimi V, et al. Preclinical tumor mouse models for studying esophageal cancer. Crit Rev Oncol Hematol. (2023) 189:104068. doi: 10.1016/j.critrevonc.2023.104068
172. Galvão FHF, Traldi MCC, Araújo RSS, Stefano JT, D'Albuquerque LAC, and Oliveira CP. PRECLINICAL MODELS OF LIVER CÂNCER. Arquivos gastroenterologia. (2023) 60:383–92. doi: 10.1590/S0004-2803.230302023-58
173. Liu Z-H, Bai M-J, Li J-Q, Yan S-H, Ye X-W, Bu J-G, et al. Correlation between inflammatory cytokines and liver cancer stem cell markers in DEN-induced liver cancer rats. Eur Rev Med Pharmacol Sci. (2021) 25:710–21. doi: 10.26355/eurrev_202101_24632
174. Bürtin F, Mullins CS, and Linnebacher M. Mouse models of colorectal cancer: Past, present and future perspectives. World J Gastroenterol. (2020) 26:1394–426. doi: 10.3748/wjg.v26.i13.1394
175. Nie K, Zheng Z, Li X, Chang Y, Liu F, and Wang X. Explore the active ingredients and potential mechanisms of JianPi QingRe HuaYu Methods in the treatment of gastric inflammation-cancer transformation by network pharmacology and experimental validation. BMC Complement Med Ther. (2023) 23:411. doi: 10.1186/s12906-023-04232-0
176. Tsaalbi-Shtylik A, Mingard C, Räz M, Oka R, Manders F, Van Boxtel R, et al. DNA mismatch repair controls the mutagenicity of Polymerase ζ-dependent translesion synthesis at methylated guanines. DNA Repair (Amst). (2024) 142:103755. doi: 10.1016/j.dnarep.2024.103755
177. Matsumura S, Sato H, Otsubo Y, Tasaki J, Ikeda N, and Morita O. Genome-wide somatic mutation analysis via Hawk-Seq™ reveals mutation profiles associated with chemical mutagens. Arch Toxicol. (2019) 93:2689–701. doi: 10.1007/s00204-019-02541-3
178. Matsumura S, Fujita Y, Yamane M, Morita O, and Honda H. A genome-wide mutation analysis method enabling high-throughput identification of chemical mutagen signatures. Sci Rep. (2018) 8:9583. doi: 10.1038/s41598-018-27755-w
179. Rice PFS, Ehrichs KG, Jones MS, Chen H, Hsu C-H, Abril ER, et al. Does mutated K-RAS oncogene attenuate the effect of sulindac in colon cancer chemoprevention? Cancer Prev Res (Phila). (2018) 11:16–26. doi: 10.1158/1940-6207.CAPR-17-0230
180. Neufert C, Heichler C, Brabletz T, Scheibe K, Boonsanay V, Greten FR, et al. Inducible mouse models of colon cancer for the analysis of sporadic and inflammation-driven tumor progression and lymph node metastasis. Nat Protoc. (2021) 16:61–85. doi: 10.1038/s41596-020-00412-1
181. Shimpo K, Chihara T, Beppu H, Ida C, Kaneko T, Hoshino M, et al. Inhibition of azoxymethane-induced DNA adduct formation by Aloe arborescens var. natalensis Asian Pac J Cancer Prev. (2003) 4:247–51.
182. Liston BW, Gupta A, Nines R, Carlton PS, Kresty LA, Harris GK, et al. Incidence and effects of Ha-ras codon 12 G–>A transition mutations in preneoplastic lesions induced by N-nitrosomethylbenzylamine in the rat esophagus. Mol Carcinog. (2001) 32:1–8. doi: 10.1002/mc.1058
183. Fong LY, Lau KM, Huebner K, and Magee PN. Induction of esophageal tumors in zinc-deficient rats by single low doses of N-nitrosomethylbenzylamine (NMBA): analysis of cell proliferation, and mutations in H-ras and p53 genes. Carcinogenesis. (1997) 18:1477–84. doi: 10.1093/carcin/18.8.1477
184. Lozano JC, Nakazawa H, Cros MP, Cabral R, and Yamasaki H. G–>A mutations in p53 and Ha-ras genes in esophageal papillomas induced by N-nitrosomethylbenzylamine in two strains of rats. Mol Carcinog. (1994) 9:33–9. doi: 10.1002/mc.2940090107
185. Kim Y, Shiba-Ishii A, Ramirez K, Muratani M, Sakamoto N, Iijima T, et al. Carcinogen-induced tumors in SFN-transgenic mice harbor a characteristic mutation spectrum of human lung adenocarcinoma. Cancer Sci. (2019) 110:2431–41. doi: 10.1111/cas.14081
186. Belinsky SA, Devereux TR, White CM, Foley JF, Maronpot RR, and Anderson MW. Role of Clara cells and type II cells in the development of pulmonary tumors in rats and mice following exposure to a tobacco-specific nitrosamine. Exp Lung Res. (1991) 17:263–78. doi: 10.3109/01902149109064417
187. Alanazi M, Weng T, McLeod L, Gearing LJ, Smith JA, Kumar B, et al. Cytosolic DNA sensor AIM2 promotes KRAS-driven lung cancer independent of inflammasomes. Cancer Sci. (2024) 115:1834–50. doi: 10.1111/cas.16171
188. Carotenuto P, Fassan M, Pandolfo R, Lampis A, Vicentini C, Cascione L, et al. Wnt signalling modulates transcribed-ultraconserved regions in hepatobiliary cancers. Gut. (2017) 66:1268–77. doi: 10.1136/gutjnl-2016-312278
189. Li M, Deng Y, Zhuo M, Zhou H, Kong X, Xia X, et al. Demethylase-independent function of JMJD2D as a novel antagonist of p53 to promote Liver Cancer initiation and progression. Theranostics. (2020) 10:8863–79. doi: 10.7150/thno.45581
190. Nassar D, Latil M, Boeckx B, Lambrechts D, and Blanpain C. Genomic landscape of carcinogen-induced and genetically induced mouse skin squamous cell carcinoma. Nat Med. (2015) 21:946–54. doi: 10.1038/nm.3878
191. Rushworth LK, Kidger AM, Delavaine L, Stewart G, van Schelven S, Davidson J, et al. Dual-specificity phosphatase 5 regulates nuclear ERK activity and suppresses skin cancer by inhibiting mutant Harvey-Ras (HRasQ61L)-driven SerpinB2 expression. Proc Natl Acad Sci U.S.A. (2014) 111:18267–72. doi: 10.1073/pnas.1420159112
192. Liu Y, Yin T, Feng Y, Cona MM, Huang G, Liu J, et al. Mammalian models of chemically induced primary Malignancies exploitable for imaging-based preclinical theragnostic research. Quant Imaging Med Surg. (2015) 5:708–29. doi: 10.3978/j.issn.2223-4292.2015.06.01
193. Sugimura T and Terada M. Experimental chemical carcinogenesis in the stomach and colon. Jpn J Clin Oncol. (1998) 28:163–7. doi: 10.1093/jjco/28.3.163
194. Wang Y, Zhang L, Feng W, Liu W, Xue X, and Feng S. Research advancements and evaluation of multifactor-induced murine models for gastric cancer. Anim Model Exp Med. (2025). doi: 10.1002/ame2.70043
195. Odashima S. Overview: N-nitroso compounds as carcinogens for experimental animals and man. Oncology. (1980) 37:282–6. doi: 10.1159/000225453
196. Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. (2010) 102:1555–77. doi: 10.1038/sj.bjc.6605642
197. Robinson NB, Krieger K, Khan FM, Huffman W, Chang M, Naik A, et al. The current state of animal models in research: A review. Int J Surg. (2019) 72:9–13. doi: 10.1016/j.ijsu.2019.10.015
198. Rogers AB. Histologic scoring of gastritis and gastric cancer in mouse models. Methods Mol Biol. (2012) 921:189–203. doi: 10.1007/978-1-62703-005-2_22
199. Wang Z, Chen S, Guo Y, Zhang R, Zhang Q, Jiang X, et al. Intestinal carcinogenicity screening of environmental pollutants using organoid-based cell transformation assay. Arch Toxicol. (2024) 98:1937–51. doi: 10.1007/s00204-024-03729-y
200. Zhang Y-H, Li H-D, Li B, Jiang S-D, and Jiang L-S. Ginsenoside Rg3 induces DNA damage in human osteosarcoma cells and reduces MNNG-induced DNA damage and apoptosis in normal human cells. Oncol Rep. (2014) 31:919–25. doi: 10.3892/or.2013.2914
201. Peterson AR and Peterson H. Differences in temporal aspects of mutagenesis and cytotoxicity in Chinese hamster cells treated with methylating agents and thymidine. Proc Natl Acad Sci U.S.A. (1982) 79:1643–7. doi: 10.1073/pnas.79.5.1643
202. Shertzer HG, Sainsbury M, Graupner PR, and Berger ML. Mechanisms of chemical mediated cytotoxicity and chemoprotection in isolated rat hepatocytes. Chem Biol Interact. (1991) 78:123–41. doi: 10.1016/0009-2797(91)90009-v
203. Kiani AK, Pheby D, Henehan G, Brown R, Sieving P, Sykora P, et al. Ethical considerations regarding animal experimentation. J Prev Med Hyg. (2022) 63:E255–66. doi: 10.15167/2421-4248/jpmh2022.63.2S3.2768
204. Wogan GN, Hecht SS, Felton JS, Conney AH, and Loeb LA. Environmental and chemical carcinogenesis. Semin Cancer Biol. (2004) 14:473–86. doi: 10.1016/j.semcancer.2004.06.010
Keywords: MNNG, cancer, animal models, gastric cancer, multi-organ carcinogenicity
Citation: Zhang X, Xu Y, Cao J, Li T, Wang J, Tao J, Zhang L and Li Z (2025) Mechanisms and applications of N-Methyl-N’-nitro-N-nitrosoguanidine in animal tumor models: current situation and challenges. Front. Oncol. 15:1681270. doi: 10.3389/fonc.2025.1681270
Received: 07 August 2025; Accepted: 30 September 2025;
Published: 23 October 2025.
Edited by:
Daniele Maria-Ferreira, Instituto de Pesquisa Pelé Pequeno Príncipe, BrazilReviewed by:
Danial Khayatan, Columbia University Irving Medical Center, United StatesAya Sayed, Alexandria University, Egypt
Erika Patricia Rendón Huerta, Facultad de Medicina Veterinaria y Zootecnia, Mexico
Luz I. Pascual, Universidad Veracruzana, Mexico
Copyright © 2025 Zhang, Xu, Cao, Li, Wang, Tao, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihong Li, bGl6aGlob25nQGJ1Y20uZWR1LmNu
†These authors have contributed equally to this work
 Xiyan Zhang1†
Xiyan Zhang1† Yupei Xu
Yupei Xu Jiaqi Wang
Jiaqi Wang Jingna Tao
Jingna Tao Liju Zhang
Liju Zhang Zhihong Li
Zhihong Li