- 1Department of Internal Medicine, Inova Fairfax Hospital, Falls Church, VA, United States
- 2Department of Dermatology, Semler Dermatology Inc., Landsdowne, VA, United States
- 3Department of Pathology, Inova Fairfax Hospital, Falls Church, VA, United States
- 4Genitourinary (GU) Medical Oncology, Inova Schar Cancer Institute, Fairfax, VA, United States
- 5Inova Melanoma and Skin Cancer Center, Inova Schar Cancer Institute, Fairfax, VA, United States
Cutaneous metastasis of prostate adenocarcinoma is exceedingly rare, accounting for less than 1% of all skin metastases. It typically occurs late in the disease course and often mimics benign or primary skin malignancies. An elderly man with a history of benign prostatic hypertrophy presented for a routine dermatologic examination, during which an ulcerated nodule was identified on his upper back. Histopathology of the lesion revealed a dermal-based malignant glandular tumor. Immunohistochemistry was negative for CK20, CK5/6, PAX-8, TTF-1, PSA, and PIN4, but positive for PSAP and NKX3.1, consistent with mucin-producing prostate adenocarcinoma. Although his recent digital rectal exam was normal and prostate cancer screening had been deferred due to age, further work-up showed elevated serum PSA (30.49 ng/mL) and widespread metastatic disease on PET/CT imaging. He was started on androgen deprivation therapy with relugolix. This case underscores the diagnostic importance of skin biopsies in evaluating atypical lesions and highlights a rare presentation of metastatic prostate adenocarcinoma in an elderly patient with minimal systemic symptoms. It also raises awareness of prostate cancer presenting as solitary cutaneous metastasis and calls attention to the potential value of PSA screening in select elderly patients. Recognizing uncommon cutaneous manifestations may lead to earlier diagnosis and improved management of advanced internal malignancies.
Introduction
Prostate cancer is the most common cancer in men in the United States (excluding skin cancer) and the second-leading cause of cancer-related death following lung cancer (1). Patients with advanced prostatic adenocarcinoma (PAC) typically present with genitourinary or musculoskeletal symptoms due to tumor burden in the pelvis and bones. PAC is associated with distinct immunologic profiles (2). Metastatic PAC to the skin is rare and can present as an ulcerated skin nodule, typically on the trunk. We report a case of a metastatic mucin-producing PAC initially presenting as an ulcerated skin nodule.
Case report
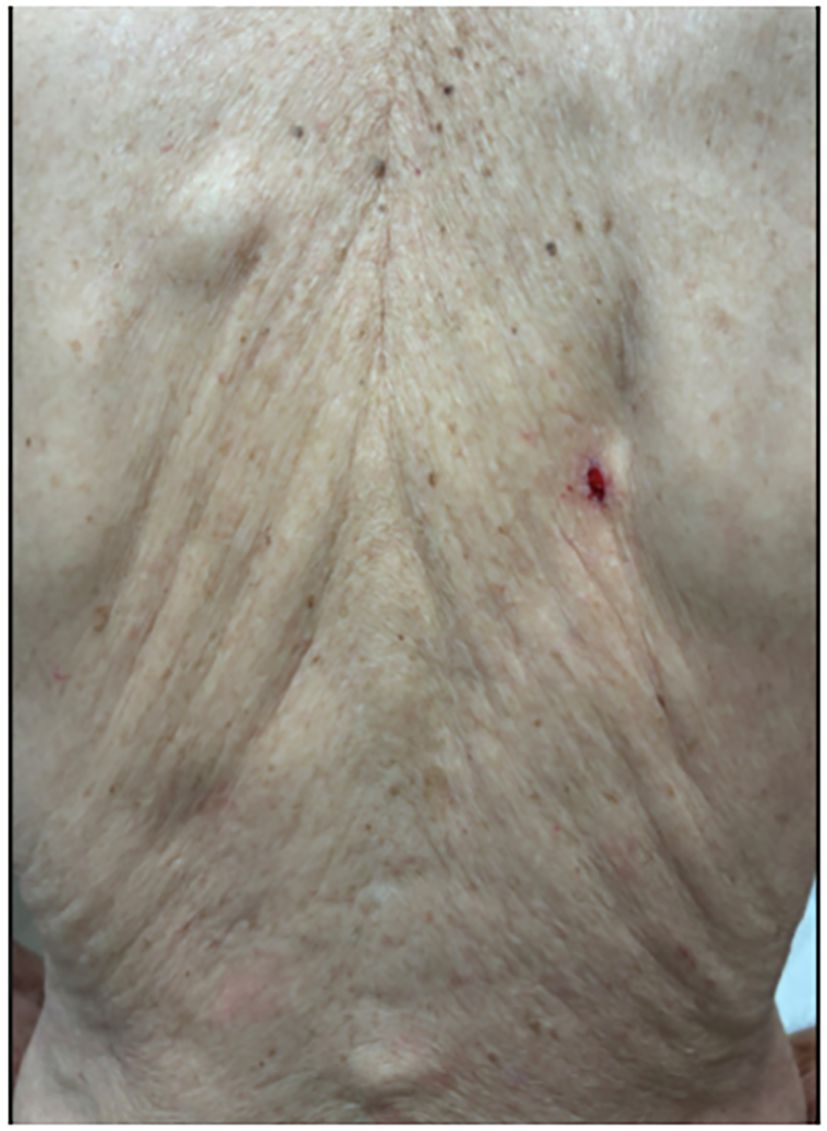
A 91-year-old white man with a history of benign prostatic hypertrophy (BPH) presented to his dermatologist for a skin examination for an unrelated complaint. On exam, an ulcerated pink-to-skin-colored nodule was noted on the right upper back (Figure 1). Histology from a punch biopsy from the edge of the nodule revealed a dermal-based malignant glandular tumor (Figure 2). Immunohistochemistry (IHC) was negative for CK20, CK5/6, PAX-8, and TTF-1, as well as the prostate specific markers, prostate-specific antigen (PSA) and PIN4. Lesional cells were positive for the prostate markers PSAP and NKXC3, consistent with a mucin-producing adenocarcinoma (Figure 3). Two months earlier, he had undergone a digital rectal exam (DRE), which was reported as normal. Based on his age, prostate cancer screening with a PSA test was initially deferred, and he resumed tamsulosin for his BPH. Metastatic workup included a whole-body positron emission tomography/computed tomography (PET/CT) scan, which showed multiple hypermetabolic lesions, including the left scapula, axial spine, mediastinal lymph nodes, and an enlarged prostate. Serum PSA level was obtained and found to be elevated to 30.49 ng/mL. These findings were compatible with a metastatic, mucin-producing PAC. The patient began androgen deprivation therapy with relugolix, a GnRH antagonist, with plans to add on an androgen receptor pathway inhibitor (ARPI) with the primary goal of symptom palliation and preservation of his quality of life given his advanced age.
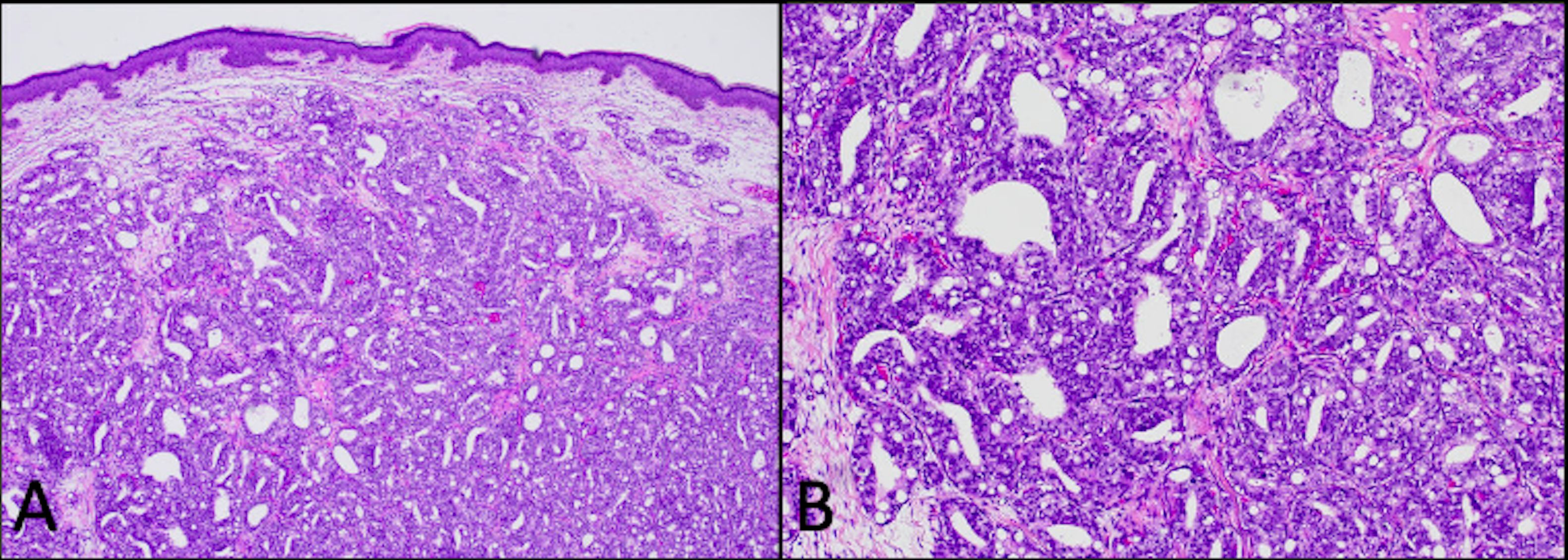
Figure 2. Hematoxylin and eosin (H&E)-stained punch biopsy of the edge of the ulcer revealed a dermal-based malignant glandular tumor [(A) 4×] with mucin production [(B) 100×].
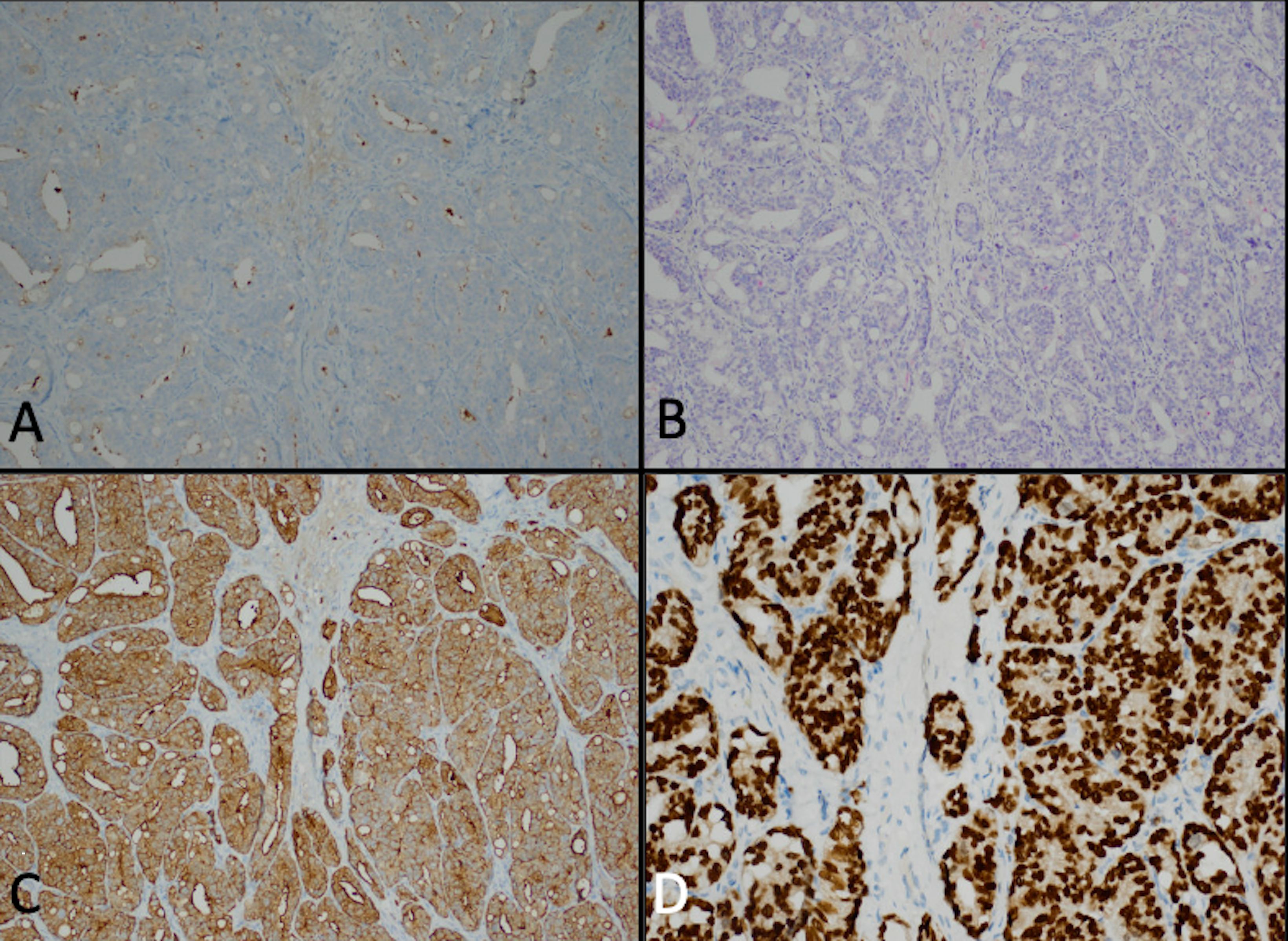
Figure 3. Punch biopsy of the edge of the ulcer revealed weak staining for PSA (A) and PIN4 (B) as well as strong expression of PSAP (C) and NKXC3 (D).
Discussion
In this case, we report a 91-year-old white man with BPH who presented for a routine skin examination and found to have an ulcerated skin nodule that led to the discovery of widely metastatic prostate cancer. The IHC pattern from the skin nodule was compatible with an aggressive subtype of PAC with loss of expression of the prostate markers PSA and PIN4, along with the retention of prostate markers PSAP and NKXC3.
Generally, internal malignancies will involve the skin in 2%–9% of cases (3, 4), and prostate cancers account for less than 1% of all skin metastases (5). Cutaneous metastases from prostate cancer typically involve the lower abdomen, genitals, and thighs (6), likely due to the proximity to the prostate gland via hematogenous dissemination. Additionally, previous case reports indicate that when cutaneous manifestations are present, they are often diagnosed late in the disease course after systemic symptoms or other metastatic sites have appeared (7, 8). This delay may be related to rarity and nonspecific presentations, which may further lead to misdiagnosis or deferred biopsy.
In our patient’s case, his urinary frequency and retention may have been the first symptoms indicative of PAC in spite of a normal DRE 2 months prior. Notably, he did not present with symptoms of widespread metastases disease such as bone pain, weight loss, fatigue, hematuria, constipation, or dry cough. The differential diagnoses of his skin lesion included primary skin cancers such as basal cell carcinoma, squamous cell carcinoma, Merkel cell carcinoma, and amelanotic melanoma. This case highlights the importance of a biopsy for a definitive diagnosis, as it represents a unique presentation of a cutaneous metastases arising from another solid primary malignancy. It also highlights the potential impact of prostate cancer screening in minimally symptomatic elderly patients on early diagnosis and management.
The pathways of prostate cancer metastasis to the skin are not well-understood due to its rarity. Possible routes include lymphatic or hematogenous spread for distant disease and direct extension to the skin from an underlying prostate cancer (9). It has been theorized that serine proteases, specifically human tissue kallikreins, may facilitate dermal extravasation by disrupting epidermal adhesions (10). We surmise that in our patient’s case, he developed spine and dermal metastases via hematogenous spread, as his fluorodeoxyglucose (FDG) PET/CT scan did not reveal any other metastatic lesions that would have extended directly to the dermis of his right mid-back.
Patients with widespread metastatic prostate cancer typically present with pain or systemic symptoms like fatigue or weight loss. In some instances, metastatic prostate cancer is diagnosed after routine PSA screening. Our case is notable for a solitary cutaneous lesion in a patient with no prior history of prostate cancer. In contrast, another reported case of prostate cancer skin metastases was a 67-year-old man with a 4-year history of castration-resistant prostate cancer who developed extensive cutaneous involvement on the lower abdomen, inguinal area, and left flank areas. Despite aggressive treatments, including surgery, chemotherapy, and androgen deprivation therapy, he was ultimately referred to hospice care (7). The 5-year survival of advanced prostate cancer is approximately 30% (11), consistent with the disease course observed in the aforementioned case. Both of these cases underscore the reality that advanced prostate cancer, like other malignancies, can manifest in unusual and diverse ways. A more comprehensive understanding of the variable presentations of cutaneous manifestation in prostate carcinomas is warranted.
Conclusion
This case highlights the importance of including cutaneous metastasis from solid organ malignancies in the differential diagnosis of atypical skin lesions, particularly in the elderly.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
AY: Writing – original draft, Writing – review & editing. DS: Investigation, Resources, Writing – review & editing. JH: Investigation, Resources, Writing – review & editing. E-MY: Investigation, Project administration, Resources, Writing – review & editing. SJ: Investigation, Project administration, Resources, Writing – review & editing. SV: Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
Author DS was employed by the company Semler Dermatology Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BPH, benign prostate hyperplasia; PAC, prostatic adenocarcinoma; PSA, prostate-specific antigen; TAVR, transcatheter aortic valve replacement; digital rectal exam, DRE
References
1. Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2017 (2020). Available online at: https://seer.cancer.gov/csr/1975_2017/ (Accessed August 01, 2025).
2. Mora LB, Buettner R, Ahmad N, Bassel Y, Jove R, and Seigne JD. Prostate adenocarcinoma: cellular and molecular abnormalities. Cancer Control. (2001) 8:551–61. doi: 10.1177/107327480100800612
3. Ayyamperumal A, Tharini G, Ravindran V, and Parveen B. Cutaneous manifestations of internal Malignancy. Indian J Dermatol. (2012) 57:260–4. doi: 10.4103/0019-5154.97657
5. Mueller TJ, Wu H, Greenberg RE, Hudes G, Topham N, Lessin SR, Uzzo RG, et al. Cutaneous metastases from genitourinary Malignancies. Urology. (2004) 63:1021–6. doi: 10.1016/j.urology.2004.01.014
6. Jones C and Rosen T. Multiple red nodules on lower abdomen. Arch Dermatol. (1992) 128:1538–8. doi: 10.1001/archderm.1992.01680210117020
7. Dills A, Obi O, Bustos K, Jiang J, and Gupta S. Cutaneous metastasis of prostate adenocarcinoma: A rare presentation of a common disease. J Investig Med High Impact Case Rep. (2021) 9:2324709621990769. doi: 10.1177/2324709621990769
8. Murali T, Bolick N, Roth K, Royer M, Canevari M, Reyes M, Phillips C, et al. Cutaneous metastasis of an undiagnosed prostatic adenocarcinoma. Cutis. (2025) 115:E7–e9. doi: 10.12788/cutis.1162
9. Venable DD, Hastings D, and Misra RP. Unusual metastatic patterns of prostate adenocarcinoma. J Urol. (1983) 130:980–5. doi: 10.1016/s0022-5347(17)51607-9
10. Mo L, Zhang J, Shi J, Xuan Q, Yang X, Qin M, Lee C, et al. Human kallikrein 7 induces epithelial-mesenchymal transition-like changes in prostate carcinoma cells: a role in prostate cancer invasion and progression. Anticancer Res. (2010) 30:3413–20.
Keywords: prostate adenocarcinoma, mucin-producing, cutaneous, BPH, PSA, PSAP, PIN4, NKXC3
Citation: Yildirim AM, Semler D, Harvell J, Yu E-M, Jang S and Venna S (2025) Case Report: Cutaneous metastatic mucin-producing prostate adenocarcinoma. Front. Oncol. 15:1681839. doi: 10.3389/fonc.2025.1681839
Received: 07 August 2025; Accepted: 22 October 2025;
Published: 10 November 2025.
Edited by:
Tamer Saad Kaoud, The University of Texas at Austin, United StatesReviewed by:
Teodor Florin Georgescu, Carol Davila University of Medicine and Pharmacy, Romaniaİmdat Eroğlu, Gazi University, Türkiye
Copyright © 2025 Yildirim, Semler, Harvell, Yu, Jang and Venna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suraj Venna, c3VyYWoudmVubmFAaW5vdmEub3Jn
 Ahmet M. Yildirim
Ahmet M. Yildirim Douglas Semler2
Douglas Semler2 Sekwon Jang
Sekwon Jang Suraj Venna
Suraj Venna