- 1Department of Hepatobiliary Surgery, Second Hospital of Hebei Medical University, Shijiazhuang, China
- 2Ophthalmology, Hebei Medical University Third Hospital, Shijiazhuang, China
Splenic angiosarcoma (SA) is an rare malignant tumor in clinical practice, characterized by high malignancy, atypical early symptoms, and a dismal prognosis. In recent years, growing attention has been focused on its diagnosis and treatment. This report describes a patient who was admitted to the hospital due to left upper abdominal pain. Contrast-enhanced CT indicated unevenly enhanced tumors in the spleen, liver, and right atrium. The patient was treated with laparoscopic splenectomy + partial hepatectomy and was diagnosed based on postoperative pathology and immunohistochemistry (positive for CD31 and CD34). After the operation, anlotinib + toripalimab targeted immunotherapy was used, and the patient recovered well. This case suggests that SA is prone to metastasis in the early stage. Clinically, the diagnosis of SA requires a combination of pathological diagnosis and imaging diagnosis, and postoperative targeted immunotherapy may improve the prognosis.
Introduction
SA is a malignant mesenchymal tumor that originates from the endothelial cells of the splenic sinusoids (1). It accounts for less than 2% of all sarcomas. It is a rare disease with an incidence rate of only 0.15 to 0.26 per million people (2). Since Langhans first reported the disease in 1879, there have been only about 300 related reports worldwide up to now, so SA remains a poorly understood entity.
SA is clinically rare and has an extremely high metastasis rate. Moreover, most patients are diagnosed with multiple metastases at the time of diagnosis. The incidence of SA is extremely low, and its early clinical symptoms are atypical. Patients usually present with atypical signs such as abdominal pain, fatigue, anemia, and thrombocytopenia (3). Therefore, SA is usually discovered at an advanced stage and has a poor prognosis.
The etiology of this disease remains unclear and serum-specific biomarkers are lacking. Diagnosis requires a combination of imaging examinations and histopathological examinations, supplemented by immunohistochemical detection of vascular differentiation markers (4).
In terms of treatment, splenectomy is the most important treatment method, but it rarely achieves a radical cure. Recent studies have shown that adjuvant chemotherapy has shown promise in improving survival, with median overall survival (OS) extending from 4 months to over 12 months in treated patients, even in those with large tumors (>5 cm) or metastases (5). In recent years, some studies have also explored the potential application of new treatment methods such as combined therapy with anti-PD-1 inhibitors and anti-VEGF tyrosine kinase inhibitors, which have achieved complete remission in individual cases, bringing new hope for the treatment of SA (6).
Herein, we report a SA case with concurrent liver and cardiac metastases—an extremely rare presentation. We discuss the diagnostic and therapeutic decision-making, and review relevant literature.
Case description
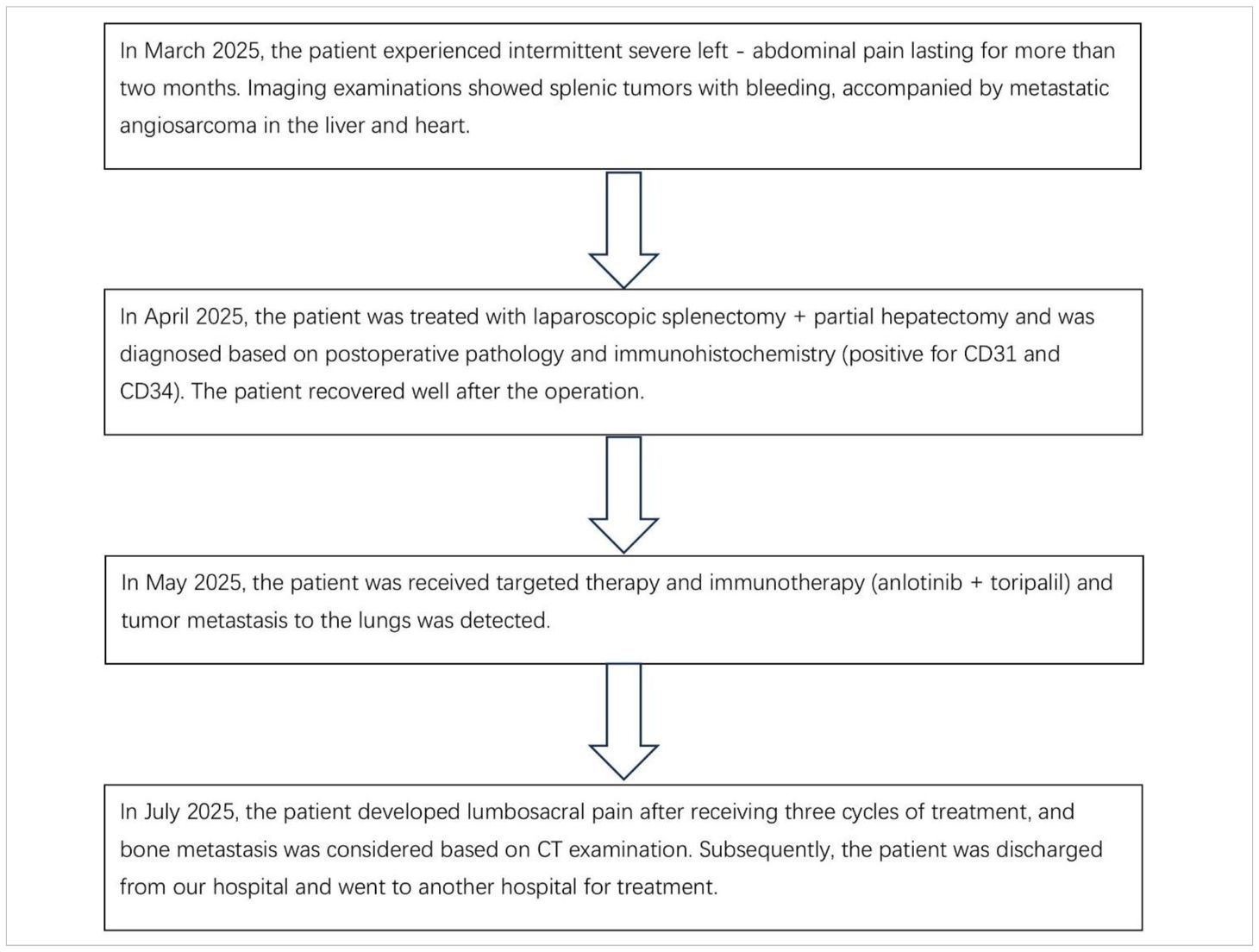
The patient is a 35-year-old male who was admitted to the hospital due to intermittent severe pain in the left abdomen for more than two months. Regarding the tumor, vascular computed tomography angiography (CTA) (Figure 1) revealed multiple circular mixed-density lesions in the spleen and the splenic tumor was compressing the left kidney. The tumor in the inferior pole protrudes locally outside the spleen contour, with a size of approximately 6.3 cm×7.3 cm×13.6 cm. Besides, CTA showed multiple circumscribed lesions with circular and heterogeneous enhancement in the liver. The larger one was located in segment VII of the liver, with a size of approximately 4.1 cm×4.0 cm×3.5 cm. CTA showed an inhomogeneous enhancement tumor in the right atrial region, with a maximum diameter of 7.1 cm. Due to the presence of surface ulcers and perisplenic hemorrhage in the patient’s splenic tumor, there is an extremely high risk of life-threatening spontaneous rupture. Additionally, the patient has asymptomatic cardiac metastasis without acute heart failure or hemodynamic instability. We performed laparoscopic partial hepatectomy and laparoscopic splenectomy on the patient.
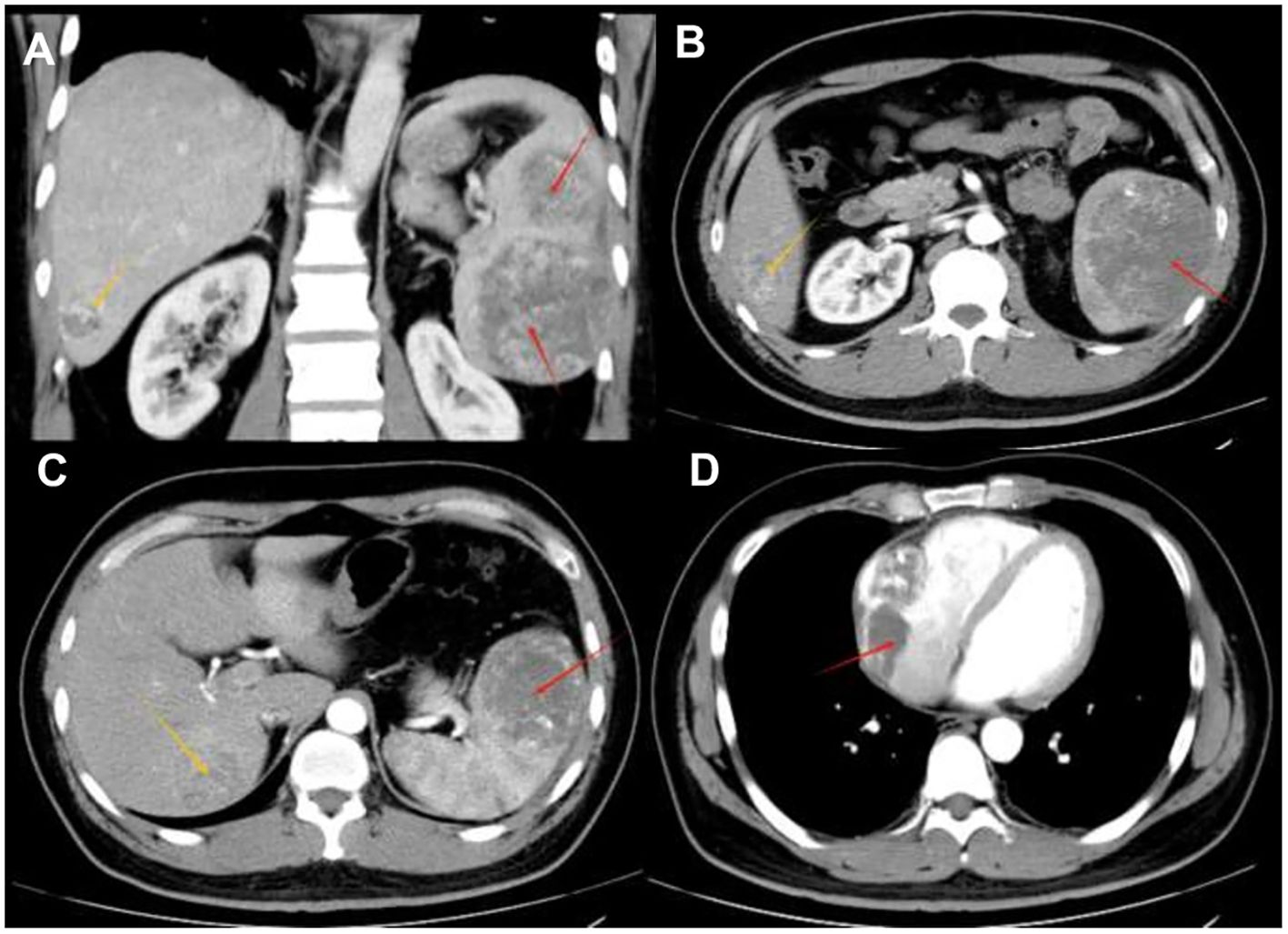
Figure 1. Radiological findings (A) A tumor within the spleen can be seen compressing the left kidney in the coronal position. (B) Contrast-enhanced CT shows multiple circular mixed-density lesions in the spleen. The size of the lesion in the inferior pole of splenic area is approximately 7.3 cm×6.3 cm×13.6 cm (indicated by the red arrow). Unevenly enhanced lesions can be seen in the V segment of the liver, approximately 3.7 cm×4.1 cm×4.5 cm in size (indicated by the yellow arrow) —consistent with typical SA liver metastasis. (C) An unevenly enhanced lesion with a size of approximately 4.1 cm×4.0 cm×3.5 cm (indicated by the yellow arrow) can be seen in segment VII of the liver. The lesion in the superior pole of the spleen is approximately 7.3cm×6.8cm×7.0cm in size (indicated by the red arrow). (D) Contrast-enhanced CT shows an unevenly enhanced lesion in the right atrium, with a maximum diameter of 7.1 cm (as indicated by the red arrow) —a rare metastatic site in SA, reported in only 1% of cases.
Intraoperative observations: We found that the patient had a tumor of approximately 3 cm3×2 cm3×2 cm3visible in the V segment of the liver. In addition, a tumor could be seen near the lower edge of the patient’s spleen, with ulceration visible on the surface. Furthermore, a small amount of blood could be seen around the patient’s spleen. After we examined that there were no other metastases in the patient’s abdominal cavity, we removed the tumor in the V segment of the liver 2 cm along the tumor edge by using the forceps method. Then, we completely remove the spleen. The patient received regular dressing changes, and the patient recovered well.
Postoperative pathology showed that the tumor was composed of atypical epithelioid cells, and no cortical or medullary structures were observed in the spleen. A large number of proliferating tumor cells and some cells showing necrosis (indicated by red arrows) could be seen in the field of vision, which was consistent with angiosarcoma (Figure 2). The liver metastatic lesions also conform to the characteristics of angiosarcoma, and the resection margins are negative. The immunohistochemistry results were as follows: Scavenger Receptor Cysteine-Rich Family Member 1(CD163) (scattered focal +), vascular endothelial marker (CD31) (+), (CD34) (+), CD68 (scattered +)、CD8 (scattered +), pancytokeratin (CKpan) (–), transmembrane glycoprotein (D2-40) (-), Epithelial Membrane Antigen (EMA) (-), ETS-Related Gene (ERG) (+), Factor VIII-related antigen (FVIII-Rag) (partial +), Human Herpesvirus 8(HHV-8) (-), Proliferating Cell Nuclear Antigen Ki-67(Ki-67) (approximately30% +), and smooth muscle actin (SMA) (-). More than one month after the operation, the patient visited the oncology department of our hospital and received targeted immunotherapy with anlotinib + toripalimab. Then the patient received treatment at another hospital, so we lost follow-up (Table 1).
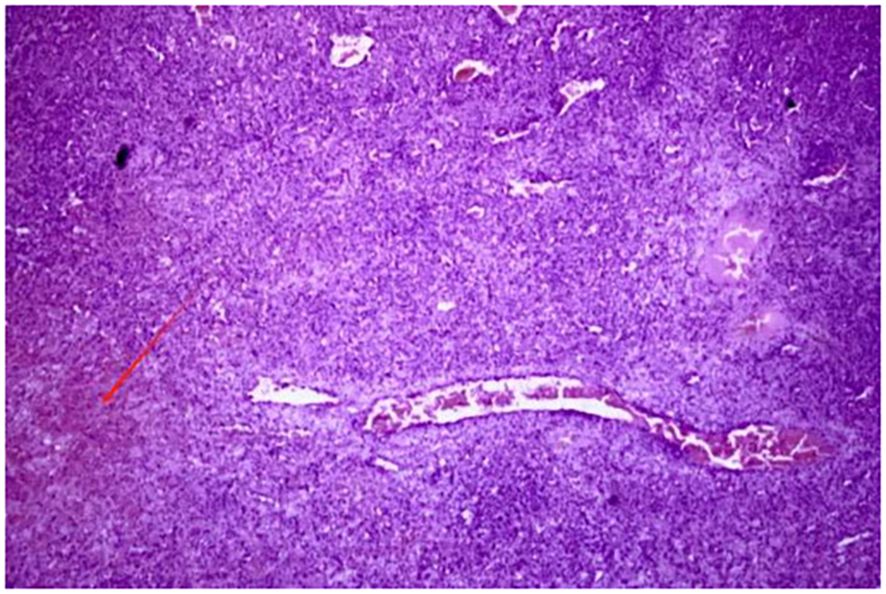
Figure 2. Postoperative pathological examination shows that the tumor was composed of atypical epithelioid cells. No cortical or medullary structures were seen in the spleen. A large number of proliferating tumor cells were observed in the field of view, and some showed necrosis (as indicated by the red arrow) —key histological features distinguishing SA from benign vascular tumors.
Discussions
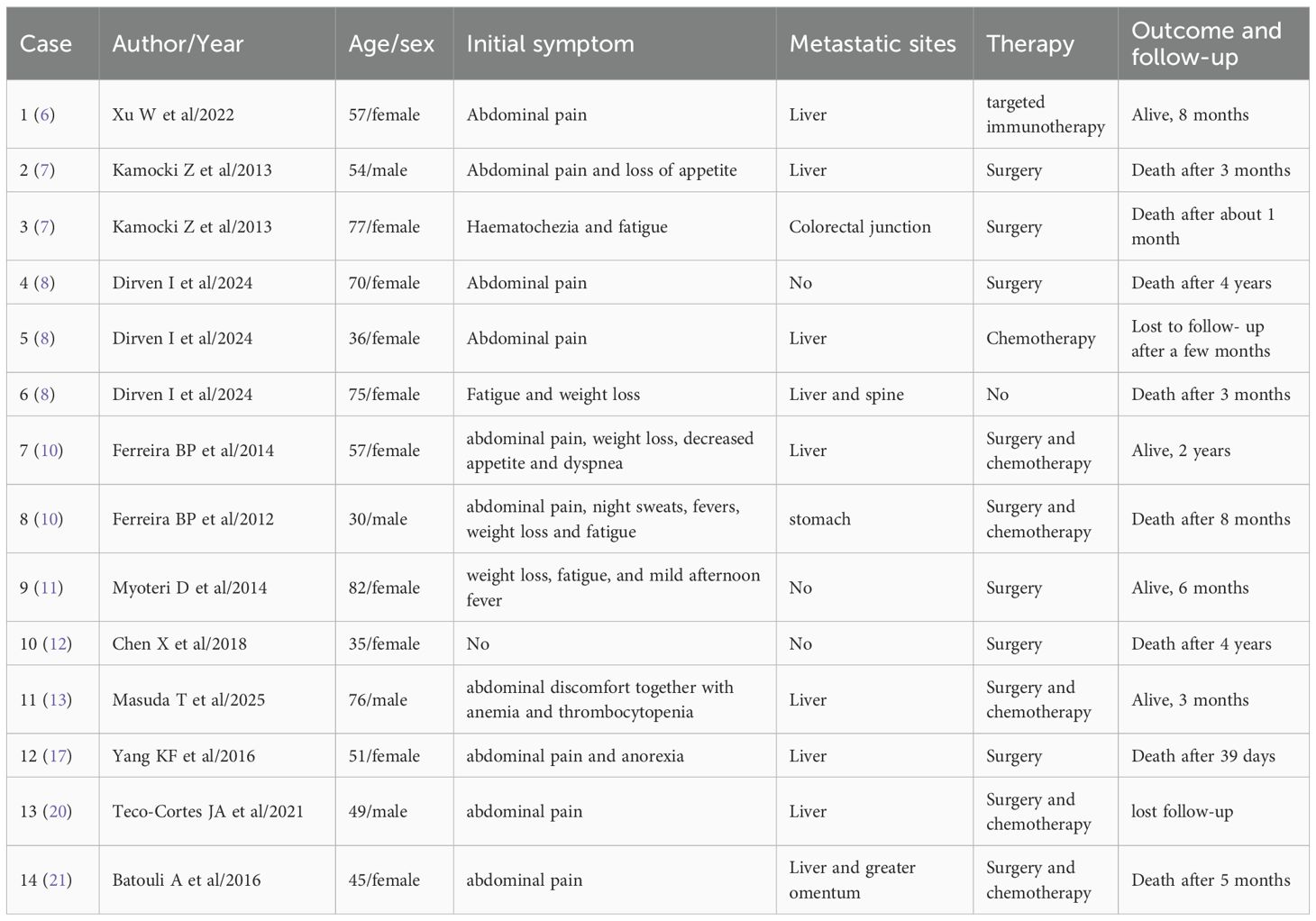
Case reports of SA highlight the complexity and aggressiveness of this rare malignancy. In the context of relevant literature, we summarized the initial symptoms, metastatic sites, therapy, and outcome and follow-up of this disease (Table 2), with the aim of providing valuable insights for clinicians in the diagnosis and management of this rare condition.
SA, as a malignant hemangioendothelioma of the spleen, is a highly malignant tumor derived from mesenchymal cells. SA differentiates from the splenic sinuses into endothelial cells and is extremely rare in clinical practice. At present, the etiology of SA in clinical research is not clear. Previous research report has found that SA was associated with a history of exposure to chemicals (vinyl chloride, thorium dioxide, and arsenic), radiation exposure, and benign splenic hemangioma (7). The main group affected by SA is men aged 50 to 70, and there is no genetic predisposition (8). Due to the abundance of blood sinuses in the spleen, SA is prone to spread through the bloodstream in the early stage, especially flowing back to the portal vein via the splenic vein. Therefore, approximately 70% of SA cases will develop liver metastasis. Cardiac metastasis is exceptionally rare: a review of 205 global SA cases (9) showed only 3 cases with cardiac involvement (1 in left ventricle, 2 in right atrium), and none with concurrent liver and right atrial metastases. The patient presented with metastatic tumors in both the liver (segments V/VII) and the right atrium, which expands the known metastatic spectrum of SA and highlights the unpredictable invasiveness of SA.
The average survival time of SA patients ranges from 4.4 months to 14 months, and only about 20% of patients can survive for more than 6 months (10). Therefore, the early diagnosis and treatment of SA are of great significance. However, SA patients usually lack specific clinical features. Most of them are admitted to the hospital with abdominal pain, accompanied by signs such as fatigue, splenomegaly, and weight loss. As the tumor progresses further, some patients may experience spontaneous splenic rupture, which can be life-threatening (11). The patient in this article was also admitted to the hospital for treatment due to spontaneous splenic rupture and bleeding. Decreased hemoglobin is the main manifestation of SA. In addition, some patients also show symptoms such as decreased white blood cells and platelets, and increased erythrocyte sedimentation rate (12). Previous report has indicated that thrombocytopenia in some patients was due to extensive liver metastasis of SA (Kasabach-Merritt phenomenon) (13). However, the manifestations such as pancytopenia caused by SA were very similar to those of hematological diseases. Moreover, the tumor markers of the majority of patients are within the normal range. Therefore, most SA patients are easily missed or misdiagnosed in clinical practice at the beginning.
Most SA patients do not have typical clinical symptoms and need to be differentiated from lymphoma, splenic metastatic tumors, benign splenic vascular tumors, splenic abscesses, and other splenic diseases Hence, imaging examinations (ultrasound, CT, and MRI) are of great significance in the diagnosis of SA. Ultrasound examination is the first choice for the initial examination of SA. Some patients showed solid heterogeneous tumors with anechoic spaces under ultrasound, which represented the characteristic vascular spaces of the tumors (14). However, most patients might only see mild splenomegaly in most cases. Therefore, ultrasound examination does not have specificity.
CT has high value in evaluating the general characteristics and complications of SA. Heterogeneous splenic tumors can be observed in 60% cases by CT. They presented as low-density shadows of varying sizes and densities, with irregular shapes and unclear boundaries from the surrounding lesions. Some lesions might fuse with each other (15). High attenuation areas shown by CT might indicate acute bleeding or hemosiderin deposition. Contrast-enhanced CT scans revealed significant differences between the enhanced and non-enhanced areas of the spleen immediately after the injection of contrast agent. The areas that have not been enhanced were mostly lesions with poor blood supply or ischemic necrosis. The lesions started to enhance after 10 min to 50 min, which was a sign of delayed enhancement of SA. These CT manifestations can be used to differentiate lymphoma from metastases. Nodules with enhanced margins could be seen in the lesion within the liver parenchyma, and necrosis in the center of the lesion presents as low-density shadows when liver metastasis occurs in the lesion (16).
In MRI scans, T1-weighted MR Images often show low signals in the center and periphery of the lesion, and focal high-signal areas can be seen in the necrotic center of some lesions. T2-weighted MR Images show that the lesions of the spleen and liver present as heterogeneous high-signal lesions with indistinct boundaries. These nodular lesions that show decreased or increased signal intensity are associated with necrosis, hemorrhage or fibrosis within the tumor, which can reflect the content of hemosiderin deposition and the degree of hemorrhage (17). Angiography was more specific in diagnosis. However, angiography is an invasive procedure and can cause certain harm to patients. Moreover, angiography cannot distinguish benign cavernous hemangioma lesions. Therefore, angiography is rarely used in clinical practice for SA examination.
Elhakim et al. (18)reported that endoscopic ultrasound (EUS, with a frequency of 5–10 MHz) has a higher spatial resolution (up to 0.1 mm), which can clearly display the heterogeneity, necrosis, and local infiltration of tumors. Contrast-enhanced endoscopic ultrasound (CE-EUS) using contrast agents such as SonoVue® can evaluate tumor vascular distribution (e.g., rapid regression in malignant tumors) (19), which helps distinguish cardiac metastases of SA from benign cardiac lesions (such as myxomas). Integrating EUS into the multimodal imaging workflow for SA (CTA for initial staging + EUS for cardiac/peri-vascular details + pathological confirmation) can improve diagnostic accuracy and provide a basis for treatment strategies. This is consistent with recent recommendations that EUS should be considered for SA patients with suspected cardiac metastases (18, 19).
The diagnosis of SA in most patients relies on puncture biopsy or postoperative pathological diagnosis. However, spleen biopsy is usually contraindicated because puncture biopsy has a high risk of inducing spleen rupture and tumor metastasis, and the diagnosis rate is not high. Hence, SA can only be diagnosed through the gross morphology, histological morphology and specific histopathological examination of the tumor after splenectomy in most cases. It is visible to the naked eye that SA often shows signs of bleeding and necrosis. Besides, previous report has indicated that SA typically presented as diffuse tumors covering the entire splenic parenchyma, while isolated tumors were less common. The pathological manifestations of SA vary, and there are differences in the same case and among different cases (20). Based on the analysis of previous literature, SA is mainly classified into four types as follows: 1) SA presents as atypical vascular endothelial cells, arranged in a spongy or honeycomb-like pattern (21); 2) Tumor cells are arranged in a porous pattern; 3) Malignant endothelial cells proliferate to form papillary lobes that extend into the vascular space; 4) The proliferation of endothelial cells in patches and the disordered arrangement of polygonal tumor cells form tumors. The stromal component of the tumor exhibits focal or diffuse cavernous hemangioma-like changes, lined by atypical endothelial cells demonstrating solid sarcomatoid and epithelioid growth patterns. The solid component resembles the appearance of fibrosarcoma or malignant fibrous histiocytoma. Immunological tests are usually helpful in confirming histological diagnosis. The common immunohistochemical manifestations of SA are positive for at least two endothelial markers (CD34, FVIIIRAg, VEGFR3 or CD31) and one histiocytic marker (CD68 or lysozyme) (22). The expression of these markers confirmed the endothelial phenotype of this malignant vascular tumor. The immunohistochemical test of this patient showed that both CD34 and CD31 related to vascular differentiation were positive, which was consistent with the reported results.
Splenectomy is the treatment of choice for SA. Regardless of whether SA patients have aggressive dissemination and metastasis or have already experienced splenic rupture, splenectomy can prolong the survival period of patients. In addition to surgery, radiotherapy, chemotherapy, and targeted immunotherapy are also important treatment methods (9). Studies have shown that the survival outcomes SA vary significantly depending on the treatment modality: 1) Surgery alone: The median overall survival (OS) of patients is 3.7–7 months, and whether splenic rupture occurs before surgery is an independent risk factor (5). This is because patients not only face the risk of immediate death due to hemorrhagic shock and disseminated intravascular coagulation, but splenic tumor rupture also promotes peritoneal dissemination and hematogenous metastasis of the tumor; 2) Surgery + chemotherapy: The European Society for Medical Oncology (ESMO) guidelines for soft tissue and visceral sarcoma recommend the use of paclitaxel/gemcitabine for the treatment of angiosarcoma. In addition, the combination of paclitaxel/gemcitabine and docetaxel can also be used as a treatment for angiosarcoma. According to the clinical practice guidelines for soft tissue sarcoma of the Japanese Orthopaedic Association, the combined application of doxorubicin and taxanes is also effective in the treatment of angiosarcoma. According to a study by Li et al. (5), after using a chemotherapy regimen postoperatively, the median overall survival is extended to 12–14 months; 3) Surgery + targeted immunotherapy: receptor tyrosine kinase inhibitors can target and bind to vascular endothelial growth factor (VEGF) receptors, thereby inhibiting tumor angiogenesis and tumor cell proliferation. PD-1 inhibitors can bind to PD-1 receptors and block their interaction with PD-L1 and PD-L2, thus releasing the suppression of immune responses mediated by the PD-1 pathway, including anti-tumor immune responses, and enabling the immune system to better attack and kill tumor cells. The NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma recommends the combination of anti-angiogenic drugs and PD-1 inhibitors for advanced unresectable or metastatic soft tissue sarcomas (especially subtypes such as angiosarcoma and synovial sarcoma) (23), stating that this combination can improve efficacy and has better safety than traditional chemotherapy. Xu et al. (6)reported that 1 patient with metastasis achieved CR after six cycles of targeted immunotherapy. This case confirms that immune checkpoint inhibition and anti-angiogenesis have a synergistic effect. Meanwhile, the Ki-67 index of this patient is approximately 30%, suggesting a high tumor proliferation activity. Therefore, this patient received the targeted immunotherapy regimen of anlotinib + toripalimab. Regrettably, the patient developed lumbosacral pain after receiving three cycles of treatment, and bone metastasis was considered based on CT examination. Subsequently, the patient was discharged from our hospital and went to another hospital for treatment, so we were unable to continue the follow - up. This is a shortcoming of this article.
Conclusion
SA, as an extremely rare malignant tumor, is prone to misdiagnosis and missed diagnosis due to its lack of specific clinical manifestations, and SA has a very poor prognosis. The average survival period of patients is less than 6 months. The diagnosis of SA mainly relies on pathological examination and immunohistochemical analysis. Meanwhile, the clinical manifestations and imaging features of patients need to be comprehensively considered for multi-dimensional assessment. At present, the main approach to improving the survival rate of patients is to perform splenectomy early, supplemented by targeted immunotherapy. However, we still need to explore more effective treatment strategies to increase the survival time of patients in the future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Second Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FC: Software, Writing – review & editing, Conceptualization, Writing – original draft, Investigation. DZ: Conceptualization, Writing – original draft, Software, Investigation. HY: Investigation, Writing – original draft, Conceptualization, Software. SW: Conceptualization, Writing – original draft, Investigation, Software. YW: Writing – original draft, Methodology, Conceptualization, Investigation. LH: Conceptualization, Writing – review & editing, Investigation, Methodology.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Takato H, Iwamoto H, Ikezu M, Kato N, Ikarashi T, and Kaneko H. Splenic hemangiosarcoma with sinus endothelial differentiation. Acta Pathol Jpn. (1993) 43:702–8. doi: 10.1111/j.1440-1827.1993.tb02556.x
2. Falk S, Krishnan J, and Meis JM. Primary angiosarcoma of the spleen. A clinicopathologic study of 40 cases. Am J Surg Pathol. (1993) 17:959–70. doi: 10.1097/00000478-199310000-00001
3. Willcox TM, Speer RW, Schlinkert RT, and Sarr MG. Hemangioma of the spleen: presentation, diagnosis, and management. J Gastrointest Surg. (2000) 4:611–3. doi: 10.1016/S1091-255X(00)80110-9
4. Gomez Sanchez J, Lopez-Cantarero Garcia-Cervantes M, Segovia Cornejo E, and Lopez-Cantarero Ballesteros M. Primary splenic angiosarcoma. An aggressive and infrequent pathology. Rev Esp Enferm Dig. (2022) 114:761–2. doi: 10.17235/reed.2022.8976/2022
5. Li R, Li M, Zhang LF, Liu XM, Hu TZ, Xia XJ, et al. Clinical characteristics and prognostic factors of primary splenic angiosarcoma: A retrospective clinical analysis from China. Cell Physiol Biochem. (2018) 49:1959–69. doi: 10.1159/000493656
6. Xu W, Wang K, Gu W, Nie X, Zhang H, Tang C, et al. Case report: complete remission with anti-PD-1 and anti-VEGF combined therapy of a patient with metastatic primary splenic angiosarcoma. Front Oncol. (2022) 12:809068. doi: 10.3389/fonc.2022.809068
7. Kamocki Z, Steward A, Zareba KP, Kuklinski A, and Kedra B. Primary splenic angiosarcoma - the same diagnosis yielding two different clinical pictures. Case report. Contemp Oncol (Pozn). (2013) 17:218–21. doi: 10.5114/wo.2013.34628
8. Dirven I, Leclercq P, D’Hondt L, Delmotte V, Lefesvre P, Reynaert H, et al. Primary splenic angiosarcoma: a case series of a rare oncological entity and diagnostic challenge. Acta Oncol. (2024) 63:192–7. doi: 10.2340/1651-226X.2023.35412
9. Juin Hsien BL and Shelat VG. Spleen angiosarcoma: a world review. Expert Rev Gastroenterol Hepatol. (2021) 15:1115–41. doi: 10.1080/17474124.2021.1945920
10. Ferreira BP, Rodler ET, Loggers ET, Pollack SM, and Jones RL. Systemic therapy in primary angiosarcoma of the spleen. Rare Tumors. (2012) 4:e55. doi: 10.4081/rt.2012.e55
11. Myoteri D, Despoina M, Dellaportas D, Dionysios D, Ayiomamitis G, Georgios A, et al. Primary angiosarcoma of the spleen: an oncological enigma. Case Rep Oncol Med. (2014) 2014:193036. doi: 10.1155/2014/193036
12. Chen X, Li H, Wang F, and Liu H. Early detection and integral resection are keys to extend survival in patients suffered from primary angiosarcoma of the spleen: A care-compliant case report and literature review. Med (Baltimore). (2018) 97:e9718. doi: 10.1097/MD.0000000000009718
13. Masuda T, Beppu T, Nagayama Y, Miyamoto H, Komohara Y, Oda E, et al. Splenic angiosarcoma and numerous liver metastases presenting the kasabach-merritt phenomenon. Anticancer Res. (2025) 45:1777–84. doi: 10.21873/anticanres.17557
14. Hou C, He J, Liao L, and Liang J. Contrast−enhanced ultrasonographic features of primary splenic angiosarcoma. Pol Arch Intern Med. (2021) 131:872–4. doi: 10.20452/pamw.16034
15. Thompson WM, Levy AD, Aguilera NS, Gorospe L, and Abbott RM. Angiosarcoma of the spleen: imaging characteristics in 12 patients. Radiology. (2005) 235:106–15. doi: 10.1148/radiol.2351040308
16. Krol JJ, Krol VV, Dawkins A, and Ganesh HS. Case 213: primary splenic angiosarcoma. Radiology. (2015) 274:298–303. doi: 10.1148/radiol.14110919
17. Yang KF, Li Y, Wang DL, Yang JW, Wu SY, and Xiao WD. Primary splenic angiosarcoma with liver metastasis: A case report and literature review. World J Gastroenterol. (2016) 22:3506–10. doi: 10.3748/wjg.v22.i12.3506
18. Elhakim A, Heinisch F, Elhakim M, Sauter P, Rode M, Seiche M, et al. The role of endosonography in cardiac tumor assessment: a case report. Int J Cardiovasc Imaging. (2025) 41:1621–6. doi: 10.1007/s10554-025-03446-2
19. Elhakim A, Karkour K, Sauter P, Rode M, Elhakim M, Radke PW, et al. The role of endosonography in cardiology: case series and literature review. Eur Heart J - Imaging Methods Pract. (2023) 1. doi: 10.1093/ehjimp/qyad002
20. Teco-Cortes JA, Navarrete-Perez JJ, and Sanchez-Castro OE. Primary splenic angiosarcoma with capsular rupture and disseminated: a case report. Cir Cir. (2021) 89:59–63. doi: 10.24875/CIRU.21000118
21. Batouli A, Fairbrother SW, Silverman JF, Muniz Mde L, Taylor KB, Welnick MA, et al. Primary splenic angiosarcoma: clinical and imaging manifestations of this rare aggressive neoplasm. Curr Probl Diagn Radiol. (2016) 45:284–7. doi: 10.1067/j.cpradiol.2015.07.004
22. Neuhauser TS, Derringer GA, Thompson LD, Fanburg-Smith JC, Miettinen M, Saaristo A, et al. Splenic angiosarcoma: a clinicopathologic and immunophenotypic study of 28 cases. Mod Pathol. (2000) 13:978–87. doi: 10.1038/modpathol.3880178
Keywords: angiosarcoma, cardiac tumor, liver metastasis tumor, splenic tumor, splenic imaging
Citation: Chen F, Zhao D, Yu H, Wang S, Wang Y and Lv H (2025) Splenic angiosarcoma with hepatic and cardiac metastases: a case report and literature review. Front. Oncol. 15:1682054. doi: 10.3389/fonc.2025.1682054
Received: 08 August 2025; Accepted: 13 October 2025;
Published: 24 October 2025.
Edited by:
Carmelo Caldarella, Fondazione Policlinico Universitario A. Gemelli IRCCS, ItalyReviewed by:
Tulika Chatterjee, University of Illinois at Peoria, United StatesToshiro Masuda, Yamaga Shimin Iryo Center, Japan
Copyright © 2025 Chen, Zhao, Yu, Wang, Wang and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haitao Lv, Z2FuZGFubHZoYWl0YW9AMTYzLmNvbQ==
 Fangzheng Chen
Fangzheng Chen Ding Zhao
Ding Zhao Haotian Yu
Haotian Yu Shuai Wang1
Shuai Wang1 Haitao Lv
Haitao Lv