Abstract
Background:
Hypopharyngeal squamous cell carcinoma (HSCC), an aggressive HNSCC subtype characterized by high metastatic potential and poor prognosis, frequently overexpresses hepatoma-derived growth factor (HDGF), a factor implicated in tumor progression. This study investigates the functional role of HDGF in HSCC and its regulatory mechanisms involving epithelial-mesenchymal transition (EMT) and the AKT/mTOR/VEGF signaling pathway.
Methods:
Bioinformatic analysis of TCGA data revealed elevated HDGF expression in HSCC tissues, significantly correlating with clinical stage. HDGF expression was depleted in the FaDu HSCC cell line using siRNA. Cell proliferation, migration, and invasion were assessed using CCK-8, wound healing, and Transwell assays, respectively. Western blotting evaluated changes in EMT markers (E-cadherin, N-cadherin, Snail, Slug) and key components of the AKT/mTOR/VEGF pathway (p-AKT, p-mTOR, VEGFA).
Results:
Bioinformatics analysis confirmed HDGF overexpression across HNSCC subtypes. In FaDu HSCC cells, siRNA-mediated HDGF knockdown significantly attenuated proliferation, migration, and invasion. Mechanistically, HDGF depletion reversed EMT progression, evidenced by E-cadherin upregulation and concurrent N-cadherin, Snail, and Slug downregulation. Western blotting demonstrated that HDGF knockdown suppressed AKT/mTOR signaling, as indicated by reduced p-AKT and p-mTOR levels, and decreased VEGFA expression.
Conclusion:
Our findings establish HDGF as a key promoter of HSCC progression through dual regulation of EMT and AKT/mTOR/VEGF pathways, suggesting its potential as a therapeutic target. These results provide mechanistic insights for developing HDGF-targeted strategies against this lethal malignancy, warranting further clinical exploration.
Introduction
Head and neck squamous cell carcinoma (HNSCC) ranks as the sixth most common malignancy worldwide, encompassing cancers arising from the squamous epithelium of the oral cavity, oropharynx, larynx, and hypopharynx (1). Among these, hypopharyngeal squamous cell carcinoma (HSCC) is particularly aggressive and represents one of the most lethal HNSCC subtypes (2). Although surgery remains the primary treatment, outcomes are often poor and frequently result in functional impairment. Despite multimodal therapy combining surgery, chemotherapy, and radiotherapy, recurrence rates remain high, with up to 80% of patients developing cervical metastases after initial surgery and neck dissection. Emerging evidence suggests molecular targeted therapy as a promising approach for HSCC, highlighting the crucial need for identifying novel biomarkers.
Hepatoma-derived growth factor (HDGF), an acidic heparin-binding growth factor, has been implicated in the progression of diverse human malignancies, including hepatocellular carcinoma (3, 4), pancreatic cancer, esophageal cancer, gastric cancer (5) gastric cancer (6–8), colorectal cancer (9–11), and gastrointestinal stromal tumor (12). HDGF expression is significantly elevated in tumor tissues compared to adjacent non-tumorous tissues in cancers such as hepatocellular carcinoma and colorectal cancer. Furthermore, high HDGF expression correlates with poor prognosis in patients with hepatocellular carcinoma, pancreatic cancer, cholangiocarcinoma, gallbladder adenocarcinoma, and esophageal cancer (10). HDGF promotes proliferation, migration, and invasion in colorectal cancer, prostate cancer, and bladder cancer (13). However, the role of HDGF in HSCC progression remains unexplored.
This study aimed to investigate the functional role and underlying mechanisms of HDGF in HSCC progression. We performed HDGF knockdown in FaDu HSCC cells and examined its effects on cell proliferation, migration, and invasion in vitro. We further investigated alterations in the epithelial-mesenchymal transition (EMT) process and the AKT/mTOR/VEGF signaling pathway following HDGF depletion to elucidate its regulatory mechanisms in HSCC tumorigenic phenotypes.
Materials and methods
Bioinformatic analysis of HDGF in HSCC
HDGF mRNA expression in head and neck cancer was assessed using The Cancer Genome Atlas (TCGA) datasets. Differential HDGF expression between normal and HSCC tumor tissues was analyzed. Additionally, HDGF mRNA expression levels and their prognostic significance in HNSCC were explored using the Gene Expression Profiling Interactive Analysis (GEPIA) server.
Cell culture
The human FaDu HSCC cell line was obtained from the American Type Cell Collection (ATCC; Manassas, VA, USA). Cells were maintained in RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA), 100 U/ml penicillin (Sigma-Aldrich, St Louis, MO), and 100 μg/ml streptomycin (Sigma-Aldrich, St Louis, MO) at 37°C in a humidified atmosphere with 5% CO2.
Lentiviral transduction
Recombinant lentivirus carrying short hairpin RNA (shRNA) targeting HDGF and a control lentivirus were purchased from Bio-Link (Shanghai, China). Cellular transduction was performed according to the manufacturer’s protocol. Stable transductants were selected using puromycin (Solarbio, Beijing, China). The shRNA-HDGF target sequence was 5’-AACCGGCAGAAGGAGTACAAA-3’, while the scrambled control sequence was 5’-TTCTCCGAACGTGTCACGT-3’ (14).
Quantitative real-time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Subsequently, 1 μg of RNA was reverse-transcribed into complementary DNA (cDNA) using the ReverTra Ace qPCR RT Kit (TOYOBO, Osaka, Japan). qRT-PCR was performed using SYBR Green Realtime PCR Master Mix (TOYOBO) on a QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). Primers were as follows: HDGF forward 5’-CTCTTCCCTTACGAGGAATCCA-3’, reverse 5’-CCTTGACAGTAGGGTTGTTCTC-3’; β-actin forward 5’-CATGTACGTTGCTATCCAGGC-3’, reverse 5’-CTCCTTAATGTCACGCACGAT-3’. HDGF mRNA expression levels were calculated using the 2-ΔΔCt method normalized to β-actin.
CCK-8 cell proliferation assay
Stably transduced FaDu cells were seeded into 96-well plates at 2000 cells/well. Cell viability was assessed after 1, 2, 3, or 4 days using the CCK-8 solution (Dojindo, Kumamoto, Japan). Absorbance was measured at 450 nm using a Microplate Reader (Bio-Rad Laboratories Inc, Hercules, CA, USA) after 1.5 hours of incubation. Experiments were performed in quintuplicate and repeated three times.
Colony formation assay
Stably transduced FaDu cells were seeded in 6-well plates at 800 cells/well. After 14 days, colonies were fixed with 4% paraformaldehyde for 30 minutes and stained with 0.1% crystal violet for 15 minutes. Colonies containing >50 cells were counted. Experiments were performed in triplicate and repeated three times.
Wound healing assay
Cells were seeded in 6-well plates and cultured to 70%-80% confluency. A linear scratch was created using a 200 μl pipette tip. After washing with PBS to remove debris, wound closure was monitored at 0, 12, and 24 hours using an inverted microscope. The wound area was quantified using ImageJ software to calculate the migration ratio. Experiments were performed in triplicate.
Transwell assay
Cell migration and invasion were assessed using Transwell chambers (8-μm pore size, Costar, New York, NY) (15). For migration, cells (3 × 104/well) were seeded in the upper chamber in serum-free medium; the lower chamber contained medium with 20% FBS. For invasion, the upper chamber was pre-coated with Matrigel (1:8 dilution; BD Bioscience, San Jose, CA, USA). After 24 hours at 37°C with 5% CO2, migrated/invaded cells on the lower membrane surface were fixed, stained with crystal violet, and counted in five random high-power fields (×200). Experiments were performed in triplicate and repeated three times.
Western blot assay
Total cellular protein was extracted using RIPA buffer (Beyotime, Shanghai, China) supplemented with 1% 100 mM PMSF (Solarbio, Beijing, China). Western blotting was performed as described previously (16). Primary antibodies used were: β-actin (1:1000, Abcam, Cambridge, MA, USA), HDGF (17), E-cadherin, N-cadherin, Snail, Slug (all 1:1000, CST, Boston, USA), VEGFA, p-AKT, p-mTOR (all 1:1000, Abcam). Membranes were incubated with HRP-conjugated secondary antibodies (1:1000, CST) and visualized using Western Blotting Luminol Reagent (Santa Cruz, CA, USA). Experiments were performed in triplicate.
Statistical analysis
Data are presented as mean ± SD. Statistical analyses were performed using SPSS 22.0 (IBM, USA) and GraphPad Prism 9 (GraphPad Software, USA). Two-way ANOVA was used for CCK-8 assay data. Student’s t-test was used for qRT-PCR, colony formation, migration, and invasion assays. A p-value < 0.05 was considered statistically significant.
Results
HDGF is overexpressed in hypopharyngeal squamous cell carcinoma
Analysis of TCGA data revealed that HDGF mRNA expression was significantly higher in HSCC tumor tissues compared to normal tissues in both unpaired and paired samples (Figures 1A, B). HDGF expression increased with higher pathological grade (Figure 1C) and advanced clinical stage (Figure 1D). Database correlation analysis showed elevated HDGF expression in males and individuals with a history of alcohol abuse (Figures 1E, F). Furthermore, HDGF expression was increased in cases with lymphovascular invasion and following radiation therapy (Figures 1G, H). GO annotation analysis of the top 30 proteins most correlated with HDGF in HSCC implicated these proteins in telomeric DNA binding (molecular function), telomere maintenance (biological process), and chromosomal regions (cellular component) (Figures 1I–K) (Supplementary table 1). HDGF showed strong gene expression correlations in both normal and HSCC tissues (Figure 1L).
Figure 1
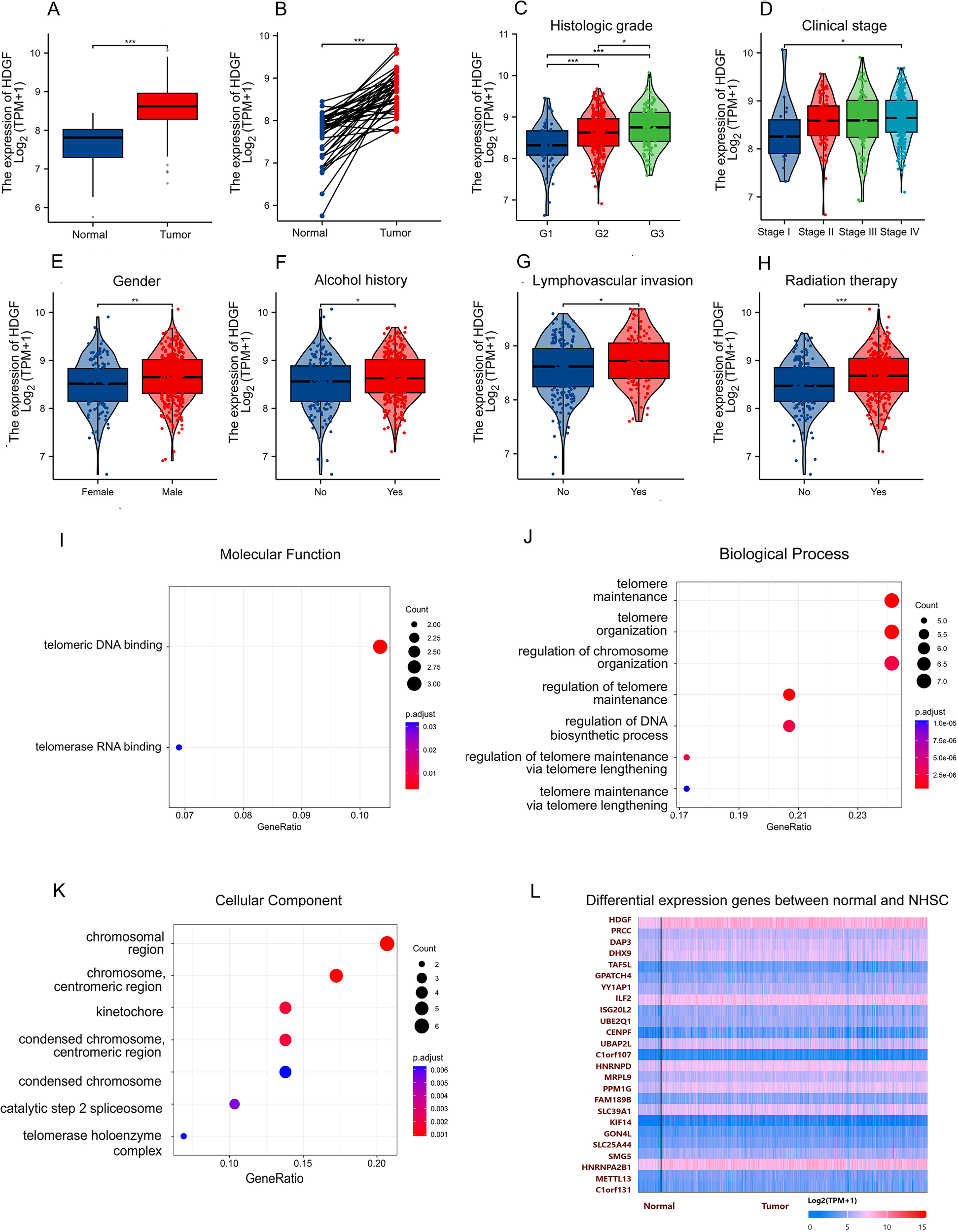
Expression characteristics and functional enrichment of HDGF in hypopharyngeal squamous cell carcinoma (HSCC). (A) HDGF expression was significantly higher in HSCC tissues than in normal tissues (P < 0.001). (B) Paired analysis confirmed HDGF upregulation in tumors compared with matched normal samples (P < 0.001). (C) HDGF expression increased with histologic grade and was highest in G3 tumors (P < 0.001). (D) HDGF levels elevated with clinical stage and were higher in stage IV than stage I (P < 0.05). (E) HDGF expression was higher in male patients. (G) HDGF levels were elevated in patients with lymphovascular invasion (P < 0.05). (H) Patients with a history of radiotherapy showed higher HDGF expression (P < 0.05). (I) GO Molecular Function analysis revealed enrichment in telomeric DNA binding and telomerase RNA binding. (J) GO Biological Process terms included telomere maintenance, telomere organization, and regulation of chromosomal organization. (K) GO Cellular Component terms included chromosomal region, centromeric region, kinetochore, condensed chromosome, and telomerase holoenzyme complex. (L) Heatmap of differentially expressed genes (DEGs) between normal and HSCC tissues. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
HDGF knockdown suppresses FaDu cell proliferation and colony formation
HDGF knockdown in FaDu cells was achieved using lentiviral shRNA. Transduction efficiency exceeded 80%, confirmed by GFP expression (Figure 2A). Both HDGF mRNA (Figure 2B) and protein (Figure 2C) levels were significantly reduced. The CCK-8 assay demonstrated that HDGF knockdown markedly suppressed FaDu cell proliferation compared to control cells (Figure 2D). Similarly, the colony formation assay revealed a significant reduction in colony-forming capacity following HDGF knockdown (Figures 2E, F). These results indicate that HDGF critically regulates FaDu cell proliferation and clonogenicity.
Figure 2
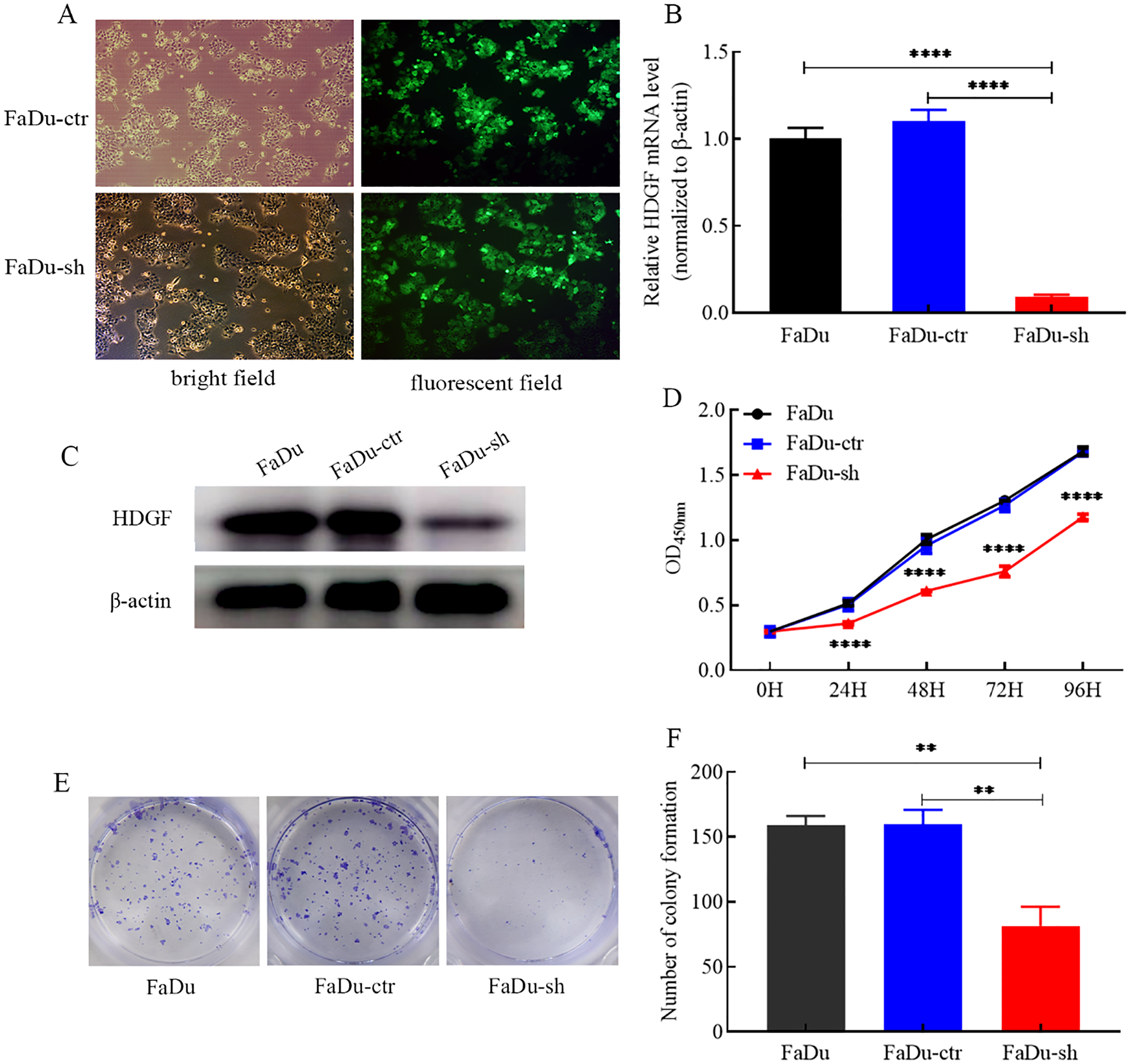
Effects of HDGF knockdown on proliferation and colony formation of FaDu cells. (A) Lentiviral transduction efficiency (>80% GFP-positive cells; magnification, ×200).(B) Relative HDGF mRNA expression in FaDu cells after transduction.(C) HDGF protein expression in FaDu cells after transduction. (D) CCK-8 assay showing reduced proliferation of FaDu cells after HDGF knockdown.(E, F) Colony formation assay demonstrating reduced colony formation capacity after HDGF knockdown. Data are mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001,****P < 0.0001.
HDGF depletion inhibits FaDu cell migration and invasion
Wound healing assays showed a significant reduction in the migration ratio of FaDu cells after HDGF knockdown (Figures 3A, B). Transwell migration assays confirmed a marked inhibition of migratory ability (Figures 3C, D). Furthermore, Transwell Matrigel invasion assays demonstrated that HDGF depletion significantly suppressed the invasive capacity of FaDu cells (Figures 3E, F). These findings highlight the essential role of HDGF in regulating FaDu cell migration and invasion.
Figure 3
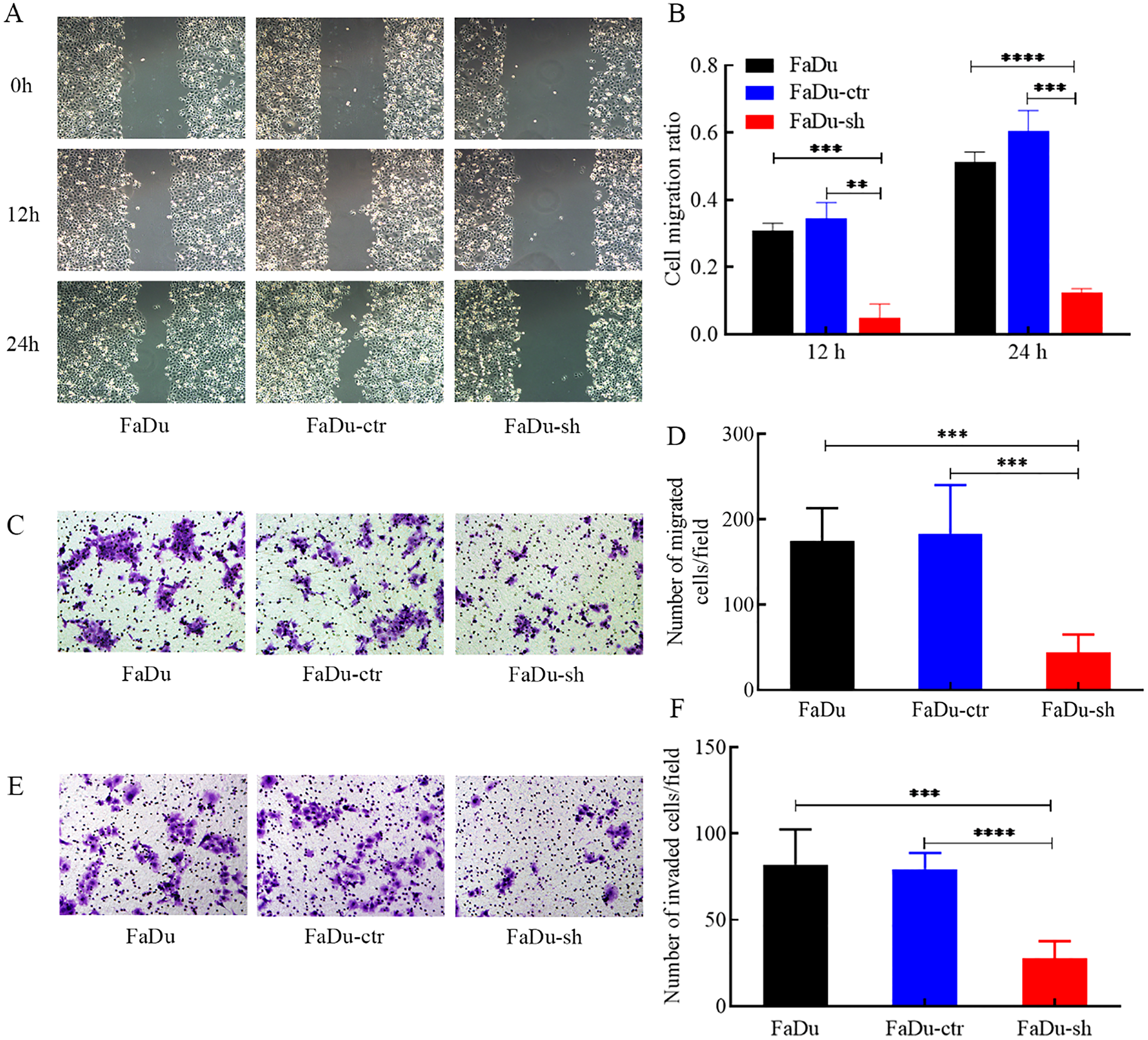
Effects of HDGF depletion on migration and invasion of FaDu cells. (A, B) Wound healing assay (representative images at x100 magnification). (C, D) Transwell migration assay (representative images at x100 magnification). (E, F) Transwell Matrigel invasion assay (representative images at x100 magnification). HDGF knockdown significantly reduced migration (A-D) and invasion (E, F) capacities. Data are mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001,****P < 0.0001.
HDGF knockdown inhibits EMT in FaDu cells
Western blot analysis revealed that HDGF knockdown in FaDu cells significantly decreased the expression of the mesenchymal marker N-cadherin and the EMT transcription factors Snail and Slug, while increasing the expression of the epithelial marker E-cadherin (Figures 4A, B). These changes indicate that HDGF depletion reverses EMT progression.
Figure 4
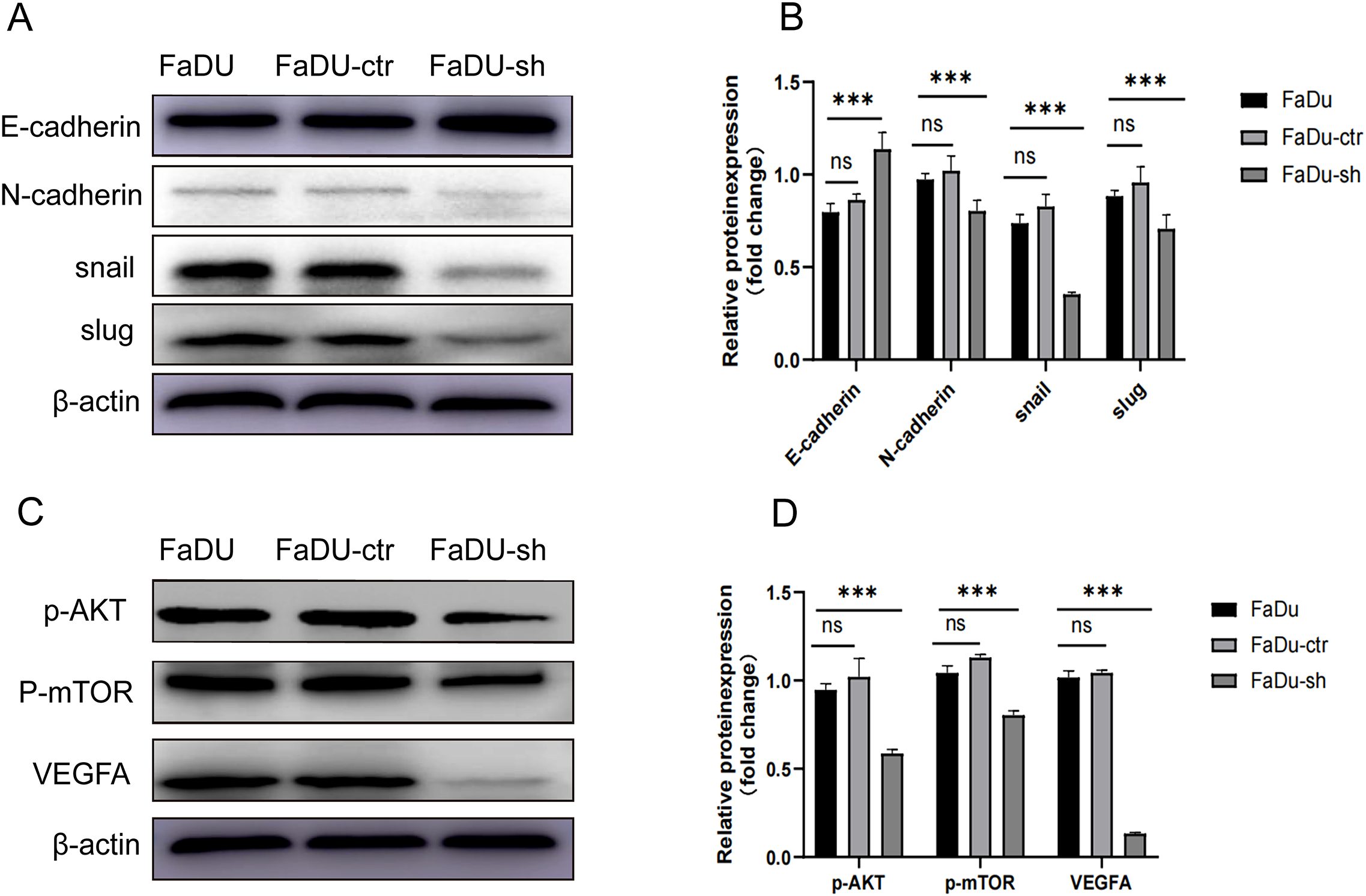
Effects of HDGF knockdown on EMT and AKT/mTOR/VEGF signaling in FaDu cells. (A, B) Western blot analysis of EMT-related proteins: N-cadherin, Snail, and Slug decreased; E-cadherin increased after HDGF knockdown. (C, D) Western blot analysis of AKT/mTOR/VEGF pathway components: p-AKT, p-mTOR, and VEGFA decreased after HDGF knockdown. Data are mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001,****P < 0.0001.
HDGF knockdown suppresses the AKT/mTOR/VEGF pathway in FaDu cells
Western blot analysis demonstrated that HDGF knockdown in FaDu cells significantly decreased the phosphorylation levels of AKT (p-AKT) and mTOR (p-mTOR), and suppressed VEGFA expression (Figures 4C, D). These findings suggest that HDGF promotes tumor progression by activating the AKT/mTOR/VEGF signaling pathway.
Discussion
Hypopharyngeal squamous cell carcinoma (HSCC) is an aggressive HNSCC subtype characterized by high regional metastasis rates and poor prognosis. The molecular mechanisms driving its aggressive behavior remain incompletely understood, hindering effective treatment development. Hepatoma-derived growth factor (HDGF) has emerged as a key oncogenic factor in various cancers (12), promoting tumor growth, angiogenesis, and metastasis. However, its specific role in HSCC progression was previously undefined.
Our bioinformatic analysis of TCGA data confirmed HDGF overexpression in HSCC tissues compared to normal tissues, and revealed associations with higher grade, advanced stage, male gender, alcohol abuse, lymphovascular invasion, and post-radiation status (Figure 1). Functional studies employing siRNA-mediated HDGF knockdown in FaDu HSCC cells demonstrated its critical role in promoting malignant phenotypes. In vitro assays revealed that HDGF depletion significantly attenuated proliferation, migration, and invasion (Figure 3), aligning with reports linking HDGF to poor prognosis in head and neck carcinomas (18) and extending its mechanistic role specifically to HSCC.
Mechanistically, we provide evidence that HDGF depletion suppresses EMT progression, as evidenced by increased E-cadherin and decreased N-cadherin, Snail, and Slug expression (Figure 4A). This reversion from a mesenchymal to a more epithelial state is associated with reduced tumor aggressiveness (19). Furthermore, we identified that HDGF significantly impacts the AKT/mTOR/VEGF signaling pathway (Figure 4B). HDGF knockdown suppressed AKT and mTOR phosphorylation and downregulated VEGFA expression. This pathway is central to promoting cell survival, proliferation, metabolism (20), and angiogenesis, processes crucial for HSCC growth and metastasis. Our findings are consistent with HDGF’s role in activating growth and angiogenic pathways in other cancers (21).
This study has limitations. First, findings are primarily based on in vitro cell line models, which may not fully recapitulate the complex in vivo HSCC microenvironment. Second, while HDGF’s role in the AKT/mTOR/VEGF pathway was established, potential crosstalk with other signaling cascades remains unexplored. Future research should address these aspects for a more comprehensive understanding.
Notably, recent preclinical studies on anti-HDGF antibodies have provided robust support for our conclusion that “HDGF is an important therapeutic target.” Research has demonstrated that in non-small cell lung cancer (NSCLC) xenograft models, HDGF-specific monoclonal antibodies (such as HDGF-C1 and HDGF-H3) significantly inhibit tumor growth. It is particularly noteworthy that in EGFR-mutant NSCLC models, the combination of anti-HDGF antibodies and osimertinib not only achieved complete or near-complete tumor regression but also markedly prolonged progression-free survival (22, 23). The underlying mechanism may involve synergistic inhibition of the AKT/mTOR and MAPK pathways—which were also found to be activated in our study (24, 25). Furthermore, anti-HDGF antibodies exhibited significant efficacy in pancreatic cancer models, with no observable toxicity across all experimental models (25). These cross-cancer evidences robustly validate the therapeutic value of targeting HDGF, laying a solid foundation for developing mono- or combination therapies involving anti-HDGF antibodies for the treatment of HSCC.
This study has certain limitations. Firstly, the conclusions are primarily derived from in vitro cell models, which cannot fully recapitulate the complex tumor microenvironment in vivo. Therefore, validating the tumor-promoting role of HDGF using nude mouse xenograft models represents our primary follow-up objective. Secondly, although this study provides key evidence supporting HDGF’s regulation of the AKT/mTOR/VEGF pathway, more comprehensive mechanistic verification, such as pathway rescue experiments, remains to be conducted in the future. Furthermore, exploring the upstream regulators and broader downstream effector networks of HDGF will be essential for a comprehensive understanding of its oncogenic mechanisms.
In conclusion, our study establishes HDGF as a key promoter of HSCC progression by demonstrating its critical role in driving proliferation, migration, invasion, EMT, and activation of the AKT/mTOR/VEGF pathway. These findings strongly suggest HDGF as a promising therapeutic target for HSCC. Developing HDGF-specific inhibitors (e.g., small molecules or antibodies) or incorporating them into combination therapies holds potential for improving the prognosis of patients with this aggressive malignancy.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
FY: Writing – original draft, Investigation, Funding acquisition, Writing – review & editing, Conceptualization. QZ: Methodology, Writing – review & editing, Writing – original draft. JS: Writing – review & editing, Methodology, Data curation, Validation, Visualization. XD: Methodology, Data curation, Software, Writing – review & editing. YH: Validation, Data curation, Writing – review & editing. ZL: Writing – review & editing, Writing – original draft, Validation, Formal Analysis.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by grants from the Natural Science Foundation of Ningxia Hui Autonomous Region (2022AAC05054).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1683145/full#supplementary-material
Abbreviations
cDNA, complementary DNA; EMT, epithelial-mesenchymal transition; GEPIA, Gene Expression Profiling Interactive Analysis; GFP, green fluorescence protein; HNSCC, head and neck squamous cell carcinoma; HSCC, hypopharyngeal squamous cell carcinoma; HDGF, hepatoma-derived growth factor; lncRNAs, long non-coding RNAs; OD, optical density; qRT-PCR, quantitative real-time PCR; shRNA, short hairpin RNA.
References
1
Zhao X Cui L . Development and validation of a m(6)A RNA methylation regulators-based signature for predicting the prognosis of head and neck squamous cell carcinoma. Am J Cancer Res. (2019) 9:2156–69.
2
Wu P Fang X Liu Y Tang Y Wang W Li X et al . N6-methyladenosine modification of circCUX1 confers radioresistance of hypopharyngeal squamous cell carcinoma through caspase1 pathway. Cell Death Dis. (2021) 12:298. doi: 10.1038/s41419-021-03558-2
3
Min X Wen J Zhao L Wang K Li Q Huang G et al . Role of hepatoma-derived growth factor in promoting de novo lipogenesis and tumorigenesis in hepatocellular carcinoma. Mol Oncol. (2018) 12:1480–97. doi: 10.1002/1878-0261.12357
4
Zheng Y Wu J Deng R Lin C Huang Y Yang X et al . G3BP2 regulated by the lncRNA LINC01554 facilitates esophageal squamous cell carcinoma metastasis through stabilizing HDGF transcript. Oncogene. (2022) 41:515–26. doi: 10.1038/s41388-021-02073-0
5
Chen W Zhou Y Wu G Sun P . CCNI2 promotes the progression of human gastric cancer through HDGF. Cancer Cell Int. (2021) 21:661. doi: 10.1186/s12935-021-02352-6
6
Gao W Chen X Chi W Xue M . Long non−coding RNA MKLN1−AS aggravates hepatocellular carcinoma progression by functioning as a molecular sponge for miR−654−3p, thereby promoting hepatoma−derived growth factor expression. Int J Mol Med. (2020) 46:1743–54. doi: 10.3892/ijmm.2020.4722
7
Ma Y Xu XL Huang HG Li YF Li ZG . LncRNA TDRG1 promotes the aggressiveness of gastric carcinoma through regulating miR-873-5p/HDGF axis. BioMed Pharmacother. (2020) 121:109425. doi: 10.1016/j.biopha.2019.109425
8
Chu TH Huang ST Yang SF Li CJ Lin HW Weng BC et al . Hepatoma-derived growth factor participates in Helicobacter Pylori-induced neutrophils recruitment, gastritis and gastric carcinogenesis. Oncogene. (2019) 38:6461–77. doi: 10.1038/s41388-019-0886-3
9
Bao J Bi X Wang J Li X . Long noncoding RNA LINC00649 functions as a microRNA−432−5p sponge to facilitate tumourigenesis in colorectal cancer by upregulating HDGF. Mol Med Rep. (2022) 25:104. doi: 10.3892/mmr.2022.12620
10
Xia C Li Q Cheng X Wu T Gao P . miR-4323 targets hepatoma-derived growth factor (HDGF) to suppress colorectal cancer cell proliferation. Pathol Res Pract. (2021) 225:153544. doi: 10.1016/j.prp.2021.153544
11
Hong YG Huang ZP Liu QZ E JF Gao XH Xin C et al . MicroRNA-95-3p inhibits cell proliferation and metastasis in colorectal carcinoma by HDGF. BioMed J. (2020) 43:163–73. doi: 10.1016/j.bj.2019.03.006
12
Enomoto H Nakamura H Nishikawa H Nishiguchi S Iijima H . Hepatoma-derived growth factor: an overview and its role as a potential therapeutic target molecule for digestive Malignancies. Int J Mol Sci. (2020) 21:4216. doi: 10.3390/ijms21124216
13
Zhang C Chang X Chen D Yang F Li Z Li D et al . Downregulation of HDGF inhibits the tumorigenesis of bladder cancer cells by inactivating the PI3K-AKT signaling pathway. Cancer Manag Res. (2019) 11:7909–23. doi: 10.2147/CMAR.S215341
14
Yang F Yu N Wang H Zhang C Zhang Z Li Y et al . Downregulated expression of hepatoma-derived growth factor inhibits migration and invasion of prostate cancer cells by suppressing epithelial-mesenchymal transition and MMP2, MMP9. PloS One. (2018) 13:e0190725. doi: 10.1371/journal.pone.0190725
15
Wang G Huang Y Yang F Tian X Wang K Liu L et al . High expression of SMYD3 indicates poor survival outcome and promotes tumour progression through an IGF-1R/AKT/E2F-1 positive feedback loop in bladder cancer. Aging (Albany NY). (2020) 12:2030–48. doi: 10.18632/aging.102718
16
Yang F Liu C Zhao G Ge L Song Y Chen Z et al . Long non-coding RNA LINC01234 regulates proliferation, migration and invasion via HIF-2alpha pathways in clear cell renal cell carcinoma cells. PeerJ. (2020) 8:e10149. doi: 10.7717/peerj.10149
17
Yang Y Liang S Li Y Gao F Zheng L Tian S et al . Hepatoma-derived growth factor functions as an unfavorable prognostic marker of human gliomas. Oncol Lett. (2017) 14:7179–84. doi: 10.3892/ol.2017.7180
18
Wu MJ Yang SM Fang WK Chen TJ Wu CY Hsu YJ et al . KDM4C works in concert with GATA1 to regulate heme metabolism in head and neck squamous cell carcinoma. Cell Mol Life Sci. (2025) 82:170. doi: 10.1007/s00018-025-05693-x
19
Li L Zou Q Li B Huang L Wei L . Type XXVIII collagen regulates renal interstitial fibrosis and epithelial-mesenchymal transition by SREBP1-mediated HKDC1 expression. J Renin Angiotensin Aldosterone Syst. (2022) 2022:9582559. doi: 10.1155/2022/9582559
20
Toson B Fortes IS Roesler R Andrade SF . Targeting Akt/PKB in pediatric tumors: A review from preclinical to clinical trials. Pharmacol Res. (2022) 183:106403. doi: 10.1016/j.phrs.2022.106403
21
Yang SM Hu TH Wu JC Yi LN Kuo HM Kung ML et al . Hepatoma-derived growth factor promotes liver carcinogenesis by inducing phosphatase and tensin homolog inactivation. Lab Invest. (2025) 105:104127. doi: 10.1016/j.labinv.2025.104127
22
Zhao J Ma MZ Ren H Liu Z Edelman MJ Pan H et al . Anti-HDGF targets cancer and cancer stromal stem cells resistant to chemotherapy. Clin Cancer Res. (2013) 19:3567–76. doi: 10.1158/1078-0432.CCR-12-3478
23
Ren H Chu Z Mao L . Antibodies targeting hepatoma-derived growth factor as a novel strategy in treating lung cancer. Mol Cancer Ther. (2009) 8:1106–12. doi: 10.1158/1535-7163.MCT-08-0779
24
Li Y Liu H Chen J . Dysregulation of HGF/c-Met signal pathway and their targeting drugs in lung cancer. Zhongguo Fei Ai Za Zhi. (2014) 17:625–34. doi: 10.3779/j.issn.1009-3419.2014.08.08
25
Zhou CQ Li A Ri K Sultan AS Ren H . Anti-HDGF antibody targets EGFR tyrosine kinase inhibitor-tolerant cells in NSCLC patient-derived xenografts. Cancer Res Commun. (2024) 4:2308–19. doi: 10.1158/2767-9764.CRC-24-0020
Summary
Keywords
HDGF, hypopharyngeal squamous cell carcinoma, tumorigenic phenotypes, AKT-mTOR-VEGF signaling, epithelial-mesenchymal transition
Citation
Yang F, Zhang Q, Shan J, Du X, Han Y and Liu Z (2025) Downregulation of HDGF inhibits tumorigenic phenotypes of hypopharyngeal squamous cell carcinoma by suppressing the AKT/mTOR/VEGF pathway. Front. Oncol. 15:1683145. doi: 10.3389/fonc.2025.1683145
Received
10 August 2025
Accepted
24 October 2025
Published
07 November 2025
Volume
15 - 2025
Edited by
Mihai Dumitru, Carol Davila University of Medicine and Pharmacy, Romania
Reviewed by
Nerina Denaro, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, Italy
Yan-Ling Wu, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Updates
Copyright
© 2025 Yang, Zhang, Shan, Du, Han and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziyang Liu, llzy7756@163.com; Feilong Yang, 904100144@qq.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.