- 1Imperial College School of Medicine, London, United Kingdom
- 2Department of Surgery and Cancer, Imperial College, London, United Kingdom
Apelin is widely expressed in the body. Apelin is secreted as a propeptide and is proteolytically cleaved into six main isoforms. As an adipokine, apelin has a cardio- and neuroprotective role and is involved in angiogenesis in healthy tissue. Recently, numerous roles of endothelial cell-derived apelin on cancer cells have been uncovered, including angiogenesis, cell proliferation, and cellular invasion. Studies have reported elevated apelin levels in tumor tissue of patients with cancer compared to healthy controls, highlighting its role in determining prognosis. Research suggests that inhibiting apelin reduces tumor invasiveness and angiogenesis and further mechanistic studies are required to realize the full potential of therapeutics targeting apelin in malignancy.
Introduction
Apelin (APLN) is an adipokine that is ubiquitously expressed in healthy tissues and plays an integral role in homeostasis through regulating angiogenesis, cellular metabolism, and proliferation, among many others (1–3). These functions of APLN are critical to its contribution to tumorigenesis, but recent studies have revealed contradictory results in regard to the effect of APLN on recruiting immune cells into the tumor microenvironment in murine models of different cancers (4, 5). Interestingly, such contrary effects were also seen for migration and metastasis, where APLN either promoted or reduced tumor growth depending on the cancer type (6, 7). Because of the role of APLN in cancer, tissue and serum APLN have both been studied as prognostic biomarkers, and the protein shows promise as a potential anti-tumorigenic agent (3). Several studies have shown reduced tumor growth when compounds targeting APLN are used in conjunction with other therapeutic agents (7). While the effect of APLN varies significantly based on the cancer type, studies highlight the potential application of APLN in the prognostic and therapeutic settings, which is the subject of this narrative.
Structure of apelin
The APLN gene is located on chromosome Xq25-26.1 and contains three exons, which result in two transcriptional products (8). APLN encodes APLN, an adipokine, or cytokine produced by adipose tissue, and is involved in various processes including energy metabolism and angiogenesis (9). Apelin is secreted as a propeptide of 77 amino acids, which is proteolytically cleaved, resulting in biologically active C-terminal peptides (10). The main isoforms of apelin include APLN-36, APLN-17, APLN-13, pyroglutamated-13, and pyroglutamated-12 (11) (Figure 1). The amino acid sequence is evolutionarily conserved, with APLN-17 and APLN-13 being 100% homologous between human, rat, mouse, and bovine (14). APLN is the ligand for the apelin receptor (APLNR), a seven-transmembrane G protein-coupled receptor. APLNR is encoded on chromosome 11q12, and its amino acid sequence is highly conserved between species. It shares its sequence with the angiotensin type-1 receptor but is not activated by angiotensin II (8).
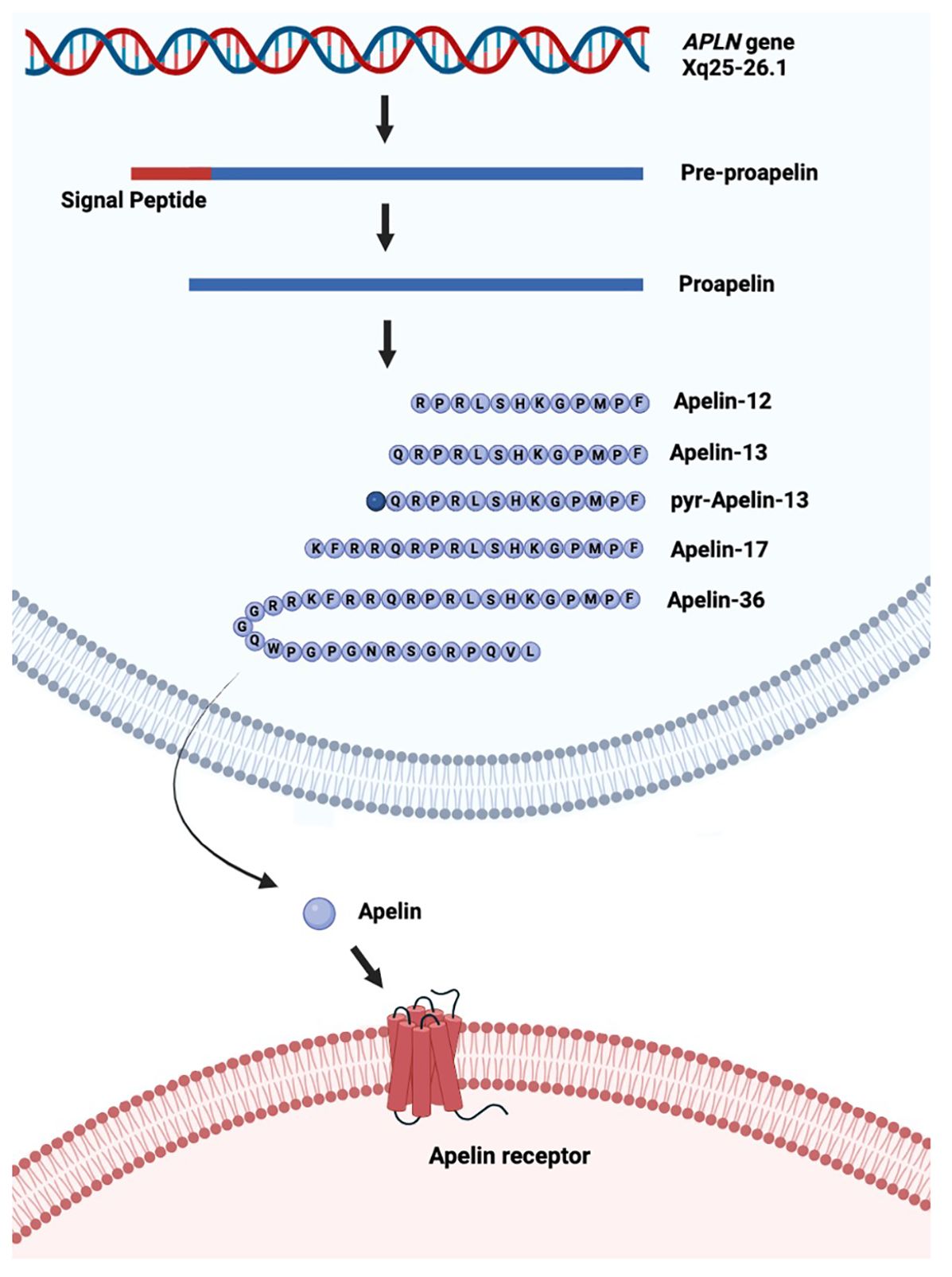
Figure 1. Schematic of production of the different isoforms of apelin from the APLN gene. The APLN gene is transcribed into pre-proapelin from which the signal peptide is cleaved to produce proapelin. Proapelin is subsequently modified into one of five different isoforms of apelin (APLN), which leave the cell and bind to the apelin receptor (APLNR). Pyr: pyroglutamated. Created in Biorender.com and based on Melgar-Lesmes et al. (12) and Murali and Aradhyam (13) under CC BY 4.0.
Function of apelin in healthy tissue
APLN/APLNR mRNA is widely expressed, with the highest expression observed in cardiac tissue, lung, stomach, and central and peripheral nervous systems, among other areas (3). APLN has a physiological role in homeostasis including cellular migration and proliferation, angiogenesis, inflammation, and metabolism (1, 2). The APLN/APLNR system is critical to the regulation of blood pressure as APLN opposes the effect of the angiotensin II–renin system (15). Receptors of apelin and angiotensin are co-expressed and may have regulatory effects on each other (16). As an adipocytokine, APLN is released by adipose tissue and is implicated in metabolism. Plasma concentrations are increased in obese patients and patients with type II diabetes mellitus (17). APLN reduces plasma glucose concentration through insulin-dependent and -independent pathways endogenously (18), but the effect of administering APLN on diabetic control externally remains unknown.
Endothelial cells also express APLN, which plays a critical role in angiogenesis. In an in vitro model of human umbilical vein endothelial cells, the addition of vascular endothelial growth factor (VEGF) upregulated APLN and induced both cellular proliferation and increased blood vessel diameter (19). In addition to angiogenesis, APLN also increased cell-to-cell contact, inducing the assembly of endothelial cells (19). ML221, an APLNR antagonist, reduces the formation of neovascular tufts (20), indicating the importance of APLN homeostasis for the regulation of angiogenesis in healthy tissue. APLN has also been found to have neuroprotective effects, playing an integral function in motor neuron survival. In cultured primary rodent cortical neurons, APLN activates both the PI3K/Akt and Raf/ERK cascades to protect against N-methyl-D-asparate excitotoxicity. Similar effects were demonstrated in vivo, with APLNR knock-out (KO) mice exhibiting increased disease progression in amyotrophic lateral sclerosis compared to APLNR-expressing mice (21). These studies show the extensive effects APLN has on healthy tissue, and these pathways are also crucial for the role of APLN in cancer.
Role of apelin in cancer
In malignancy, APLN has been implicated in cancer growth, mortality, and prognosis through a wide range of mechanisms. This is predominantly due to the effect of APLN on the tumor microenvironment through angiogenesis, migration, and invasion (22).
Effect of APLN on angiogenesis and proliferation is dependent on the tumor microenvironment
APLN is integral to tumor development through its role in maintaining angiogenesis and tumor growth. For example, gene expression analysis illustrates significantly elevated APLN levels in non-small cell lung cancer compared to healthy lung tissue (23). However, the effect of APLN on tumor growth differs according to the immune infiltration within the specific tumor microenvironment (see Figure 2). To investigate the effect of APLN on tumor growth, various cancer cell lines were injected into wild-type (WT) and APLN KO mice. In a model of MC38 colon adenocarcinoma, a greater proportion of CD8+ and CD4+ T cells were distributed in the center of tumors of WT mice compared to APLN KO mice. CCL8 is a cytokine important for the recruitment of immune cells, and APLN was shown to induce CCL8 expression in vitro. Based on this, it was hypothesized that APLN enhances intratumoral immune infiltration in a CCL8-dependent manner in the MC38 cancer model (4). However, in a mouse model of glioblastoma (GBM), APLN KO mice had slower tumor growth than WT mice, which contradicts the results for the MC38 cancer model (5). Moreover, mice models of Apln-depleted E0771 mammary carcinoma had significantly increased intra-tumoral NK T cells, and decreased infiltration of polymorphonuclear myeloid-derived suppressor cells compared to WT mice (2). This discrepancy strongly suggests that the effect of APLN is dependent on the tumor microenvironment. Thus, further mechanistic research is required to determine the effect of APLN in different tumor microenvironments.
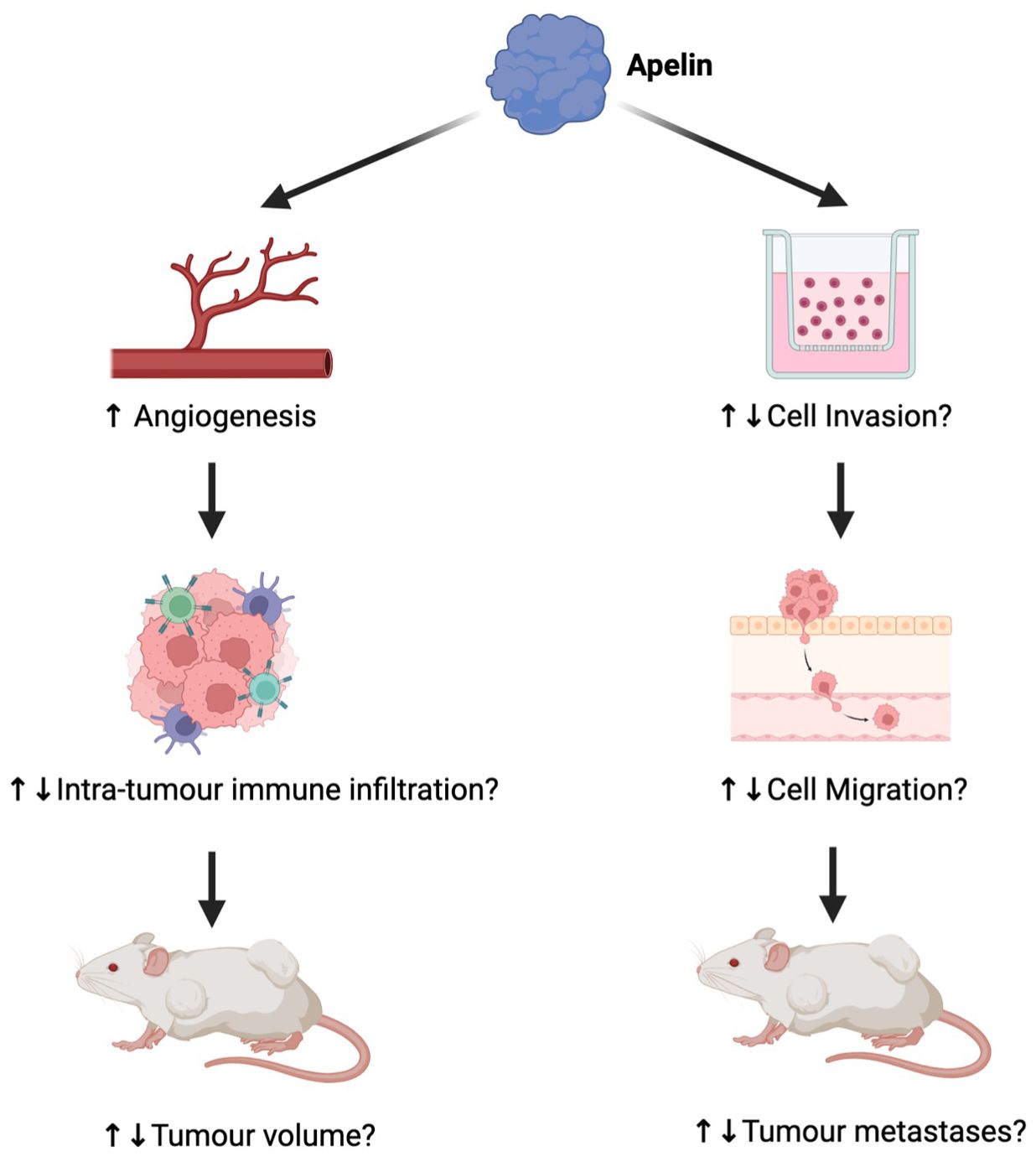
Figure 2. The differential impact of apelin (APLN) on tumors. While APLN increases angiogenesis, in some tumors, the effect has been considered part of functional maturation, causing reduced tumor growth (24), whereas in others, it has contributed to tumor growth (23). As a result, the impact of angiogenesis on intra-tumor immune infiltration and tumor volume varies greatly by tumor. The effect of APLN on cell invasion, migration, and therefore metastases also differs by tumor (6, 7, 25, 26). Figure created in Biorender.com.
APLN has also been implicated in cell invasion and migration. When GBM14, a GBM xenograft cell line, was implanted into APLN KO mice, the invasive tumor volume significantly increased compared to APLN WT mice (7), suggesting a role in reducing cell invasion. In contrast, APLN supplementation stimulated invasion in colon cancer cell models (6) and increased lymphatic vessel densities in melanoma (25), underscoring the differential effects of APLN based on the tumor microenvironment (see Figure 2).
When four different colon cancer models of varying metastatic potential were stimulated with APLN in vitro, invasion increased in all cell lines. APLN increased the ratio of filamentous to monomeric actin, forming migratory protrusions, and also elevated levels of metalloproteinases, which are crucial for the degradation of the extracellular matrix (6). In OVCAR-4 and OCAR-8 high-grade serous ovarian cancer cells, APLN supplementation increased migration and cell invasion compared to control, and this effect was reversed with ML221. The metastatic properties are due to the activation of downstream STAT3. Compared to controls, mice injected with OVCAR-3 cells overexpressing Apln had higher numbers of large tumors on internal organs, indicating the role of APLN on cancer metastasis (26). While there is strong evidence of APLN regulating metastasis through matrix metalloproteinases (MMPs) and actin, the specific properties of certain tumors that may dictate the effects of APLN remain unknown.
Apelin as a biomarker in cancer
Tumor APLN as a prognostic marker
Elevated APLN and APLNR expression have been reported in many cancers including ovarian, prostate, liver, gastric, and lung cancers compared to healthy tissues (3). In a study of 124 patients with high-grade serous ovarian cancer, high tumor APLN expression was associated with a 14.7-month reduction in median overall survival compared to patients with low APLN expression (26). In another study of 60 patients with invasive breast carcinoma, patients with metastatic disease had significantly greater APLN expression in lymph nodes compared to patients without metastasis, suggesting the potential role of APLN as a driver of metastasis (27). High tumor APLN expression, not APLNR, was correlated with reduced overall survival and relapse-free survival in 518 patients with low-grade glioma (3) In contrast, in another study of 163 patients with GBM, neither APLN nor APLNR gene expression was correlated with overall survival or disease-free interval (3), suggesting inter-tumor variation. Therefore, the intra- and inter-tumor heterogeneity of APLN should be characterized if it were to be utilized clinically as a prognostic indicator of cancer.
Serum APLN as a prognostic marker
Several studies have also investigated serum APLN as a non-invasive marker of prognosis. High APLN expression in serum has been correlated with shorter overall survival in renal cell carcinoma (28), gastric cancer (22), and muscle-invasive bladder cancer (29). However, elevated APLN in serum may not always be due to cancer. For example, in gastroesophageal cancer, high serum APLN was correlated with high C-reactive protein, suggesting that the elevated serum APLN could be due to systemic inflammation rather than cancer (30). Moreover, in patients with gastric cancer, both tumor and serum APLN expression were significantly elevated, but in patients with chronic gastritis, APLN expression in serum was also elevated. Together, these studies suggest that tumor APLN expression may be more accurate than serum measurements for prognostic purposes (22).
APLN as a predictive marker
APLN has also been studied as a predictive indicator of treatment response. APLN is an adipokine thought to be increased in cases of obesity as insulin drives APLN expression. In a retrospective exploratory study on 62 patients with breast cancer, APLN expression and obesity were both found to be independently associated with increased aggressiveness of breast cancer and poor response to neoadjuvant chemotherapy (31). Because of the presence of few patients with diabetes in the patient cohort, the study had limited generalizability, but the interaction of obesity and APLN expression and its role in cancer therapies warrant further investigation. In a cohort of 13 patients with colorectal cancer given bevacizumab, non-responders were associated with significantly higher baseline APLN mRNA expression in the cancerous tissue, with poor progression-free survival compared to patients with low baseline APLN expression (32). This suggests that APLN can also be an indicator of treatment response, but further mechanistic research is required before clinical application.
Apelin as an anti-tumorigenic therapeutic target
Considering the role of APLN in driving cell proliferation and angiogenesis, Hall et al. (33) demonstrated a dose-dependent response to APLN in the growth of cholangiocarcinoma. Treatment with ML221 using mouse models of cholangiocarcinoma demonstrated decreased tumor volume, decreased gene expression of proliferation (Ki-67), VEGF, and MMP compared to the untreated control (33). ML221 also inhibited migration and invasion in high-grade serous ovarian cancer (26), and MM54, another APJ antagonist, reduced the tumorigenic effects of APLN on melanoma (34).
Currently used GBM therapies such as the anti-VEGF antibody bevacizumab have been shown to decrease APLN mRNA expression and increase tumor volume in GBM murine models. Therefore, it was hypothesized that VEGF-A treatment resistance may be due to increased cell invasiveness from APLN reduction (7). Mice with orthotopic p53KO platelet-derived growth factor receptor-B GBM were synergistically given bevacizumab and APLN-F13A, an APLNR antagonist with partial agonist properties. Results showed decreased invasive volume and vascular density, and prolonged survival in the combination group compared to monotherapy (7). Similar results were seen in GL261 glioma murine models (5). APLN-F13A is a poorly understood compound, and the effects in the study may have been observed due to the partial agonistic properties of APLN-F13A, indicating that, in GBM, a complete antagonist of APLNR may be detrimental.
The studies show that the effect of APLNR modulation is different based on the tumor microenvironment, an important area for future research. Furthermore, the tumor heterogeneity of APLN and APLNR, as well as the percentage of patients who express increased APLN should be investigated as therapeutic efficacy targeting APLN will heavily depend on its expression. APLN affects multiple cancer pathways, and while initial data on its therapeutic efficacy alone or in combination are promising, further mechanistic studies are required to determine its efficacy.
Conclusions
APLN was originally known for its role in angiogenesis in healthy tissues but has been found to significantly affect immune infiltration, epithelial–mesenchymal transition, tumor invasiveness, and a variety of other pathways in different cancers. Studies have shown that APLN can affect cancers differently according to the nature of their tumor microenvironment. Understanding their underlying mechanisms will be crucial to the application of APLN as a prognostic and predictive marker for different cancers. Mechanistic insight is also integral for shedding light on the potential for APLNR modulation for anti-cancer therapy.
Author contributions
YA: Writing – review & editing, Investigation, Conceptualization, Writing – original draft. RS: Conceptualization, Supervision, Project administration, Investigation, Writing – original draft, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Doğan A. Apelin receptor (Aplnr) signaling promotes fibroblast migration. Tissue Cell. (2019) 56:98–106. doi: 10.1016/j.tice.2019.01.003
2. Uribesalgo I, Hoffmann D, Zhang Y, Kavirayani A, Lazovic J, Berta J, et al. Apelin inhibition prevents resistance and metastasis associated with anti-angiogenic therapy. EMBO Mol Med. (2019) 11:1–19. doi: 10.15252/emmm.201809266
3. Lv S, An Y, Dong H, Xie L, Zheng H, Cheng X, et al. High APLN expression predicts poor prognosis for glioma patients. Oxid Med Cell Longev. (2022) 2022:1–16. doi: 10.1155/2022/8393336
4. Hu L, Hayashi Y, Kidoya H, and Takakura N. Endothelial cell-derived Apelin inhibits tumor growth by altering immune cell localization. Sci Rep. (2021) 11:14047. doi: 10.1038/s41598-021-93619-5
5. Frisch A, Kälin S, Monk R, Radke J, Heppner FL, and Kälin RE. Apelin controls angiogenesis-dependent glioblastoma growth. Int J Mol Sci. (2020) 21:1–19. doi: 10.3390/ijms21114179
6. Podgórska M, Pietraszek-Gremplewicz K, and Nowak D. Apelin effects migration and invasion abilities of colon cancer cells. Cells. (2018) 7:113. doi: 10.3390/cells7080113
7. Mastrella G, Hou M, Li M, Stoecklein VM, Zdouc N, Volmar MNM, et al. Targeting APLN/APLNR improves antiangiogenic efficiency and blunts proinvasive side effects of VEGFA/VEGFR2 blockade in glioblastoma. Cancer Res. (2019) 79:2298–313. doi: 10.1158/0008-5472.CAN-18-0881
8. Hu G, Wang Z, Zhang R, Sun W, and Chen X. The role of apelin/apelin receptor in energy metabolism and water homeostasis: A comprehensive narrative review. Front Physiol. (2021) 12:632886. doi: 10.3389/fphys.2021.632886
9. Dogra S, Neelakantan D, Patel MM, Griesel B, Olson A, and Woo S. Adipokine apelin/APJ pathway promotes peritoneal dissemination of ovarian cancer cells by regulating lipid metabolism. Mol Cancer Res. (2021) 19:1534–45. doi: 10.1158/1541-7786.MCR-20-0991
10. Podgórska, Diakowska, Pietraszek-Gremplewicz, Nienartowicz, and Nowak. Evaluation of apelin and apelin receptor level in the primary tumor and serum of colorectal cancer patients. J Clin Med. (2019) 8:1513. doi: 10.3390/jcm8101513
11. Janssens P, de Loor H, Decuypere J-P, Vennekens R, Llorens-Cortes C, Mekahli D, et al. On methods for the measurement of the apelin receptor ligand apelin. Sci Rep. (2022) 12:7763. doi: 10.1038/s41598-022-11835-z
12. Melgar-Lesmes P, Perramon M, and Jiménez W. Roles of the hepatic endocannabinoid and apelin systems in the pathogenesis of liver fibrosis. Cells. (2019) 8:1311. doi: 10.3390/cells8111311
13. Murali S and Aradhyam GK. Structure–function relationship and physiological role of apelin and its G protein coupled receptor. Biophys Rev. (2023) 15:127–43. doi: 10.1007/s12551-023-01044-x
14. Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta. (1999) 1452:25–35. doi: 10.1016/s0167-4889(99)00114-7
15. Hus-Citharel A, Bouby N, Frugière A, Bodineau L, Gasc JM, and Llorens-Cortes C. Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int. (2008) 74:486–94. doi: 10.1038/KI.2008.199
16. Chapman FA, Maguire JJ, Newby DE, Davenport AP, and Dhaun N. Targeting the apelin system for the treatment of cardiovascular diseases. Cardiovasc Res. (2023) 119:2683–96. doi: 10.1093/CVR/CVAD171
17. Cavallo MG, Sentinelli F, Barchetta I, Costantino C, Incani M, Perra L, et al. Altered glucose homeostasis is associated with increased serum apelin levels in type 2 diabetes mellitus. PloS One. (2012) 7:e51236. doi: 10.1371/JOURNAL.PONE.0051236
18. Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buléon M, et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. (2008) 8:437–45. doi: 10.1016/j.cmet.2008.10.003
19. Kidoya H, Ueno M, Yamada Y, Mochizuki N, Nakata M, Yano T, et al. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. (2008) 27:522–34. doi: 10.1038/sj.emboj.7601982
20. Ishimaru Y, Shibagaki F, Yamamuro A, Yoshioka Y, and Maeda S. An apelin receptor antagonist prevents pathological retinal angiogenesis with ischemic retinopathy in mice. Sci Rep. (2017) 7:15062. doi: 10.1038/s41598-017-15602-3
21. Kinjo T, Ebisawa S, Nokubo T, Hashimoto M, Yamada T, Oshio M, et al. Post-translational modifications of the apelin receptor regulate its functional expression. AIMS Neurosci. (2023) 10:282–99. doi: 10.3934/Neuroscience.2023022
22. Feng M, Yao G, Yu H, Qing Y, and Wang K. Tumor apelin, not serum apelin, is associated with the clinical features and prognosis of gastric cancer. BMC Cancer. (2016) 16:794. doi: 10.1186/s12885-016-2815-y
23. Berta J, Kenessey I, Dobos J, Tovari J, Klepetko W, Jan Ankersmit H, et al. Apelin expression in human non-small cell lung cancer: role in angiogenesis and prognosis. J Thorac Oncol. (2010) 5:1120–9. doi: 10.1097/JTO.0b013e3181e2c1ff
24. Kidoya H, Kunii N, Naito H, Muramatsu F, Okamoto Y, Nakayama T, et al. The apelin/APJ system induces maturation of the tumor vasculature and improves the efficiency of immune therapy. Oncogene. (2012) 31:3254–64. doi: 10.1038/onc.2011.489
25. Berta J, Hoda MA, Laszlo V, Rozsas A, Garay T, Torok S, et al. Apelin promotes lymphangiogenesis and lymph node metastasis. Oncotarget. (2014) 5:4426–37. doi: 10.18632/ONCOTARGET.2032
26. Neelakantan D, Dogra S, Devapatla B, Jaiprasart P, Mukashyaka MC, Janknecht R, et al. Multifunctional APJ pathway promotes ovarian cancer progression and metastasis. Mol Cancer Res. (2019) 17:1378–90. doi: 10.1158/1541-7786.MCR-18-0989
27. Baran M, Ozturk F, Canoz O, Onder GO, and Yay A. The effects of apoptosis and apelin on lymph node metastasis in invasive breast carcinomas. Clin Exp Med. (2020) 20:507–14. doi: 10.1007/s10238-020-00635-2
28. Tolkach Y, Ellinger J, Kremer A, Esser L, Müller SC, Stephan C, et al. Apelin and apelin receptor expression in renal cell carcinoma. Br J Cancer. (2019) 120:633–9. doi: 10.1038/s41416-019-0396-7
29. Yang L, Li Y-L, Li X-Q, and Zhang Z. High apelin level indicates a poor prognostic factor in muscle-invasive bladder cancer. Dis Markers. (2019) 2019:1–6. doi: 10.1155/2019/4586405
30. Diakowska D, Markocka-Mączka K, Szelachowski P, and Grabowski K. Serum levels of resistin, adiponectin, and apelin in gastroesophageal cancer patients. Dis Markers. (2014) 2014:1–8. doi: 10.1155/2014/619649
31. Gourgue F, Derouane F, van Marcke C, Villar E, Dano H, Desmet L, et al. Tumor apelin and obesity are associated with reduced neoadjuvant chemotherapy response in a cohort of breast cancer patients. Sci Rep. (2021) 11:9922. doi: 10.1038/s41598-021-89385-z
32. Zuurbier L, Rahman A, Cordes M, Scheick J, Wong TJ, Rustenburg F, et al. Apelin: A putative novel predictive biomarker for bevacizumab response in colorectal cancer. Oncotarget. (2017) 8:42949–61. doi: 10.18632/oncotarget.17306
33. Hall C, Ehrlich L, Venter J, O’Brien A, White T, Zhou T, et al. Inhibition of the apelin/apelin receptor axis decreases cholangiocarcinoma growth. Cancer Lett. (2017) 386:179–88. doi: 10.1016/j.canlet.2016.11.025
Keywords: cancer, biomarkers, cell proliferation, angiogenesis, apelin
Citation: Agarwala Y and Sharma R (2025) The role of apelin in cancer. Front. Oncol. 15:1683865. doi: 10.3389/fonc.2025.1683865
Received: 11 August 2025; Accepted: 03 September 2025;
Published: 03 October 2025.
Edited by:
Mirko Marabese, Mario Negri Institute for Pharmacological Research (IRCCS), ItalyReviewed by:
Fatma Seçer Çelik, Ankara Medipol University, TürkiyeCopyright © 2025 Agarwala and Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rohini Sharma, ci5zaGFybWFAaW1wZXJpYWwuYWMudWs=
 Yuki Agarwala
Yuki Agarwala Rohini Sharma
Rohini Sharma