- 1School of Nursing, Jilin University, Changchun, China
- 2School of Nursing, Changchun University of Chinese Medicine, Changchun, China
- 3China-Japan Union Hospital of Jilin University, Changchun, China
- 4Department of Gastrointestinal and Colorectal Surgery, The First Hospital of Jilin University, Changchun, China
- 5Nursing Department, The Bethune Hosptial Stomatology Jilin University, Changchun, China
Aim: To explore the relationship between gastric cancer and sarcopenia and review the underlying mechanisms.
Method: A systematic search was conducted across the Web of Science, PubMed, Cochrane, CNKI, Wanfang, and VIP databases following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines. Literature describing the relationship between gastric cancer and sarcopenia was included in this study, with methodological quality assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Tools.
Results: Among the 1,518 identified publications, 33 cohort studies involving 10,679 participants were ultimately included. The results revealed a sarcopenia prevalence ranging from 6.8% to 72.22% in gastric cancer patients. Most studies indicated that reduced muscle mass—potentially attributable to fat infiltration, immunosuppression, cachexia-associated metabolic disturbances, and protein reserve depletion—serves as an independent predictor of postoperative complications, overall survival, and disease-free survival in gastric cancer patients. However, due to heterogeneity in assessment criteria and measurement tools, only two studies demonstrated that sarcopenia did not significantly impact survival or prognosis in this population.
Conclusion: Postoperative sarcopenia exhibits a high prevalence after gastric cancer surgery and is a significant predictor of adverse clinical outcomes. This underscores the importance of prioritizing muscle mass preservation in postoperative management and integrating its assessment into preoperative risk stratification. However, the current body of evidence is limited by inconsistent diagnostic criteria and a lack of mechanistic studies. Future research should focus on establishing standardized diagnostic frameworks through multidisciplinary collaboration and developing targeted interventions to improve patient prognosis.
1 Introduction
Gastric cancer, a malignancy originating from the gastric mucosal epithelium, represents a significant global health burden. Data indicate that an estimated 19.3 million new cancer cases occurred worldwide in 2020, with gastric cancer accounting for approximately 1.09 million cases (1). Cancer-related deaths approached 10 million, including roughly 769,000 gastric cancer fatalities, underscoring its persistent status as a major public health challenge globally (2). Patients with gastric cancer frequently experience persistent digestive dysfunction due to anatomical alterations, manifesting as chronic eating difficulties, vomiting, diarrhea, and malabsorption. Sarcopenia—a syndrome characterized by progressive loss of muscle mass and function—exhibits multifactorial pathogenesis involving chronic inflammation, malnutrition, mitochondrial dysfunction, prolonged disuse, neuromuscular degeneration, and insufficient physical activity (3).
Research demonstrates that tumor-associated inflammatory metabolic dysregulation and hypercatabolic states significantly contribute to sarcopenia pathogenesis in gastric cancer, with prevalence rates ranging from 10.0% to 57.7% (4). The underlying mechanisms involve proinflammatory cytokine-mediated enhancement of proteolytic pathways, where excessive IL-6 and TNF-α in the tumor microenvironment persistently activate both ubiquitin-proteasome and autophagy-lysosomal systems, accelerating muscle protein catabolism (5). Concurrently, tumor-induced insulin resistance and dysregulated lipid metabolism compromise bioenergetic supply to muscle tissue (6), while gastrointestinal obstruction and malabsorption further exacerbate protein-energy malnutrition, establishing a self-perpetuating vicious cycle (7). Notably, aberrant myokine secretion resulting from muscle atrophy modulates critical signaling pathways, including JAK/STAT and mTOR, thereby altering the tumor microenvironment to promote cancer proliferation and metastasis (8).
Despite accumulating evidence supporting the association between gastric cancer and sarcopenia, research on their bidirectional mechanisms remains fragmented due to methodological heterogeneity, population diversity, and lack of standardized interventions, precluding comprehensive systematic synthesis. This review, therefore, aims to consolidate existing evidence by integrating findings across study designs, analyzing how population characteristics modulate association strength, evaluating comparative merits of sarcopenia assessment tools, and elucidating the clinical implications of their interplay—ultimately informing the development of integrated management strategies encompassing screening, assessment, and targeted interventions for gastric cancer patients.
2 Materials and methods
This scoping review consolidates current knowledge on the sarcopenia-gastric cancer relationship through a five-phase methodology comprising research question development, systematic literature screening, rigorous study selection, standardized data extraction, and critical evidence synthesis (9), with all results reported in strict adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines (10). The study protocol was registered on the Open Science Framework with the registration number https://doi.org/10.17605/OSF.IO/JC9VD.
2.1 Research questions
This review addresses three core research questions: 1. What is the reported prevalence range of sarcopenia among gastric cancer patients in existing studies? 2. How does sarcopenia affect survival and prognosis in gastric cancer patients? 3. What are the underlying biological mechanisms governing the bidirectional relationship between gastric cancer and sarcopenia?
2.2 Search strategy
This study conducted a systematic literature search under the guidance of a professional librarian, encompassing records from database inception to 1 July 2025, across PubMed, Web of Science, Embase, Cochrane Library, CNKI, Wanfang, and VIP databases, utilizing the key terms “gastric cancer” and “sarcopenia” as primary search parameters (Table 1).
2.3 Study selection
Literature management and screening were performed using Zotero software. Inclusion criteria comprised (1) POS framework adherence: P (Participants)—adults (≥18 years) with clinically confirmed gastric cancer and sarcopenia; O (Outcomes)—gastric cancer-related complications, survival outcomes, and prognosis; S (Study design)—empirical human studies (randomized controlled trials, cohort studies, case-control studies, cross-sectional studies); (2) no restrictions on demographic characteristics or geographical regions. Exclusion criteria included (1) non-empirical studies (e.g., reviews, editorials, theoretical articles), (2) secondary data analyses, (3) non-English literature, and (4) studies failing to report outcomes examining the gastric cancer-sarcopenia relationship.
2.4 Data extraction
This study implemented a standardized data extraction protocol whereby two researchers independently extracted literature information using predefined Excel templates, with discrepancies resolved by a third reviewer. Extracted variables included first author, publication year, study design, country, gastric cancer staging, sample size, patient age, sarcopenia diagnostic criteria, assessment metrics, gastric cancer patient outcomes, and their interrelationship.
2.5 Evidence synthesis
Data were categorized according to research context, sample characteristics, assessment tools, metrics, outcome presentations, and key findings, with this review specifically centering on elucidating the bidirectional relationship between gastric cancer and sarcopenia.
2.6 Critical appraisal of included studies
The methodological quality of included studies was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Tools (11). As all incorporated studies were cohort designs, the corresponding checklist containing 11 appraisal items was applied.
3 Results
3.1 Search results and literature characteristics
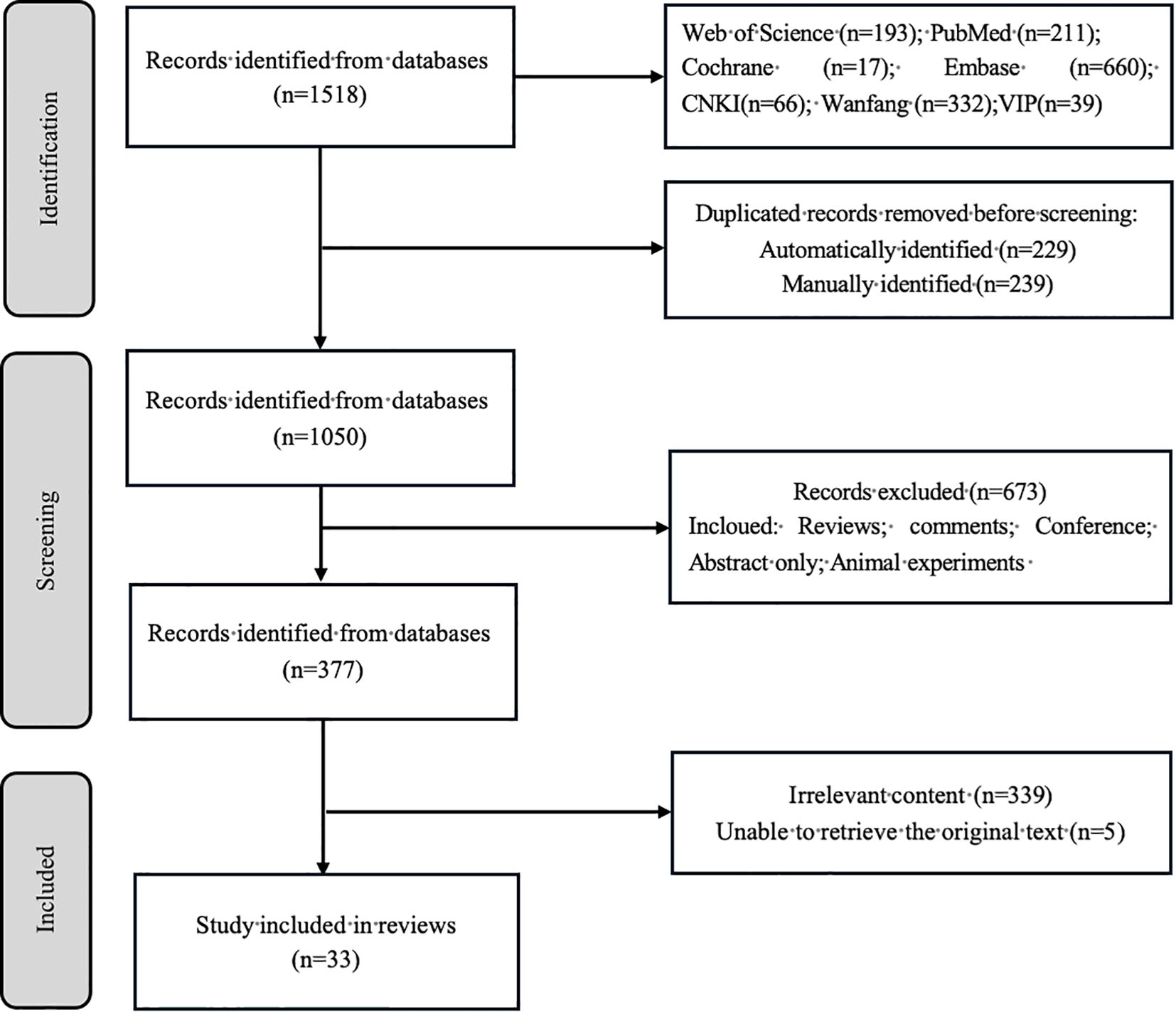
A comprehensive search identified 1,518 publications. Following deduplication (n = 468 excluded), title/abstract screening eliminated 673 records, yielding 377 articles for full-text assessment. Ultimately, 33 studies met the inclusion criteria and were incorporated into this review. The selection process is detailed in Figure 1. Quality appraisal confirmed that all eligible studies were retained for analysis (Appendix 1).
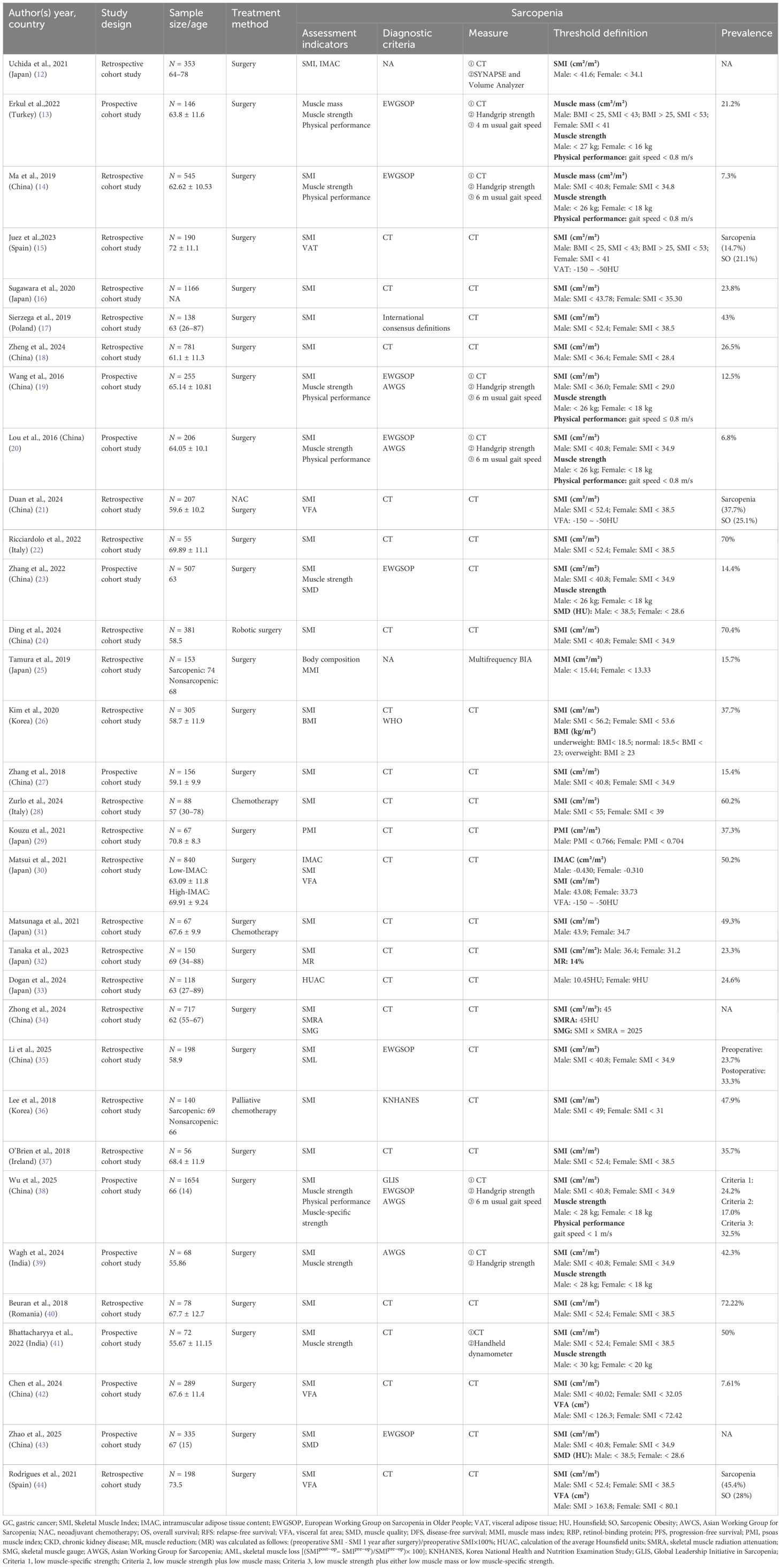
Geographically, 25 studies originated from Asian countries, including 13 from China (14, 18–21, 23, 24, 27, 34, 35, 38, 42, 43), 8 from Japan (12, 16, 25, 29–33), 2 from South Korea (26, 36), and 2 from India (39, 41). European contributions comprised eight studies: Spain (n = 2) (15, 44), Italy (n = 2) (22, 38), with single studies from Turkey (13), Poland (17), Ireland (37), and Romania (40). Publications spanned 2016–2025, encompassing 10,679 participants aged 26–89 years. All studies employed cohort designs, with 23 retrospective cohorts (12, 14–18, 21, 22, 24–26, 28–37, 40, 44) and 10 prospective cohorts (13, 19, 20, 23, 27, 38, 39, 41–43). Regarding therapeutic approaches, surgery was reported as the primary gastric cancer treatment in most studies, while only three investigations incorporated chemotherapy (28, 31, 36)—including one combining surgical and chemotherapeutic approaches (31) (Tables 2, 3).
3.2 Prevalence and assessment of sarcopenia in gastric cancer patients
This systematic review synthesizes evidence of a 6.8%–72.22% sarcopenia prevalence in gastric cancer patients, with assessment metrics including SMI (12, 14–24, 26–28, 30–32, 34–44), IMAC (12, 30), VFA (21, 30, 42, 44), VAT (15), PMI (29), BMI (26), physical performance (13, 14, 19, 20, 23, 38, 39, 41), body composition (25), SMD (23, 43), muscle strength (13, 14, 19, 20, 23, 38, 39, 41), and muscle-specific strength (38), where CT emerged as the predominant diagnostic modality implemented alongside criteria from the EWGSOP (13, 14, 19, 20, 23, 25, 38, 43), AWGS (19, 20, 38, 39), KNHANES (38), WHO (26), and international consensus definitions (17), while VAT specifically serves as a biomarker for sarcopenic obesity with thresholds at −150 to −50 HU measured through volumetric analysis (12), dynamometry (13, 14, 19, 20, 38, 39), multifrequency BIA (25), and handheld dynamometer (41), revealing significant heterogeneity in threshold definitions across instruments and inconsistent cutoffs for identical tools (Table 3).
3.3 Survival and prognosis of gastric cancer
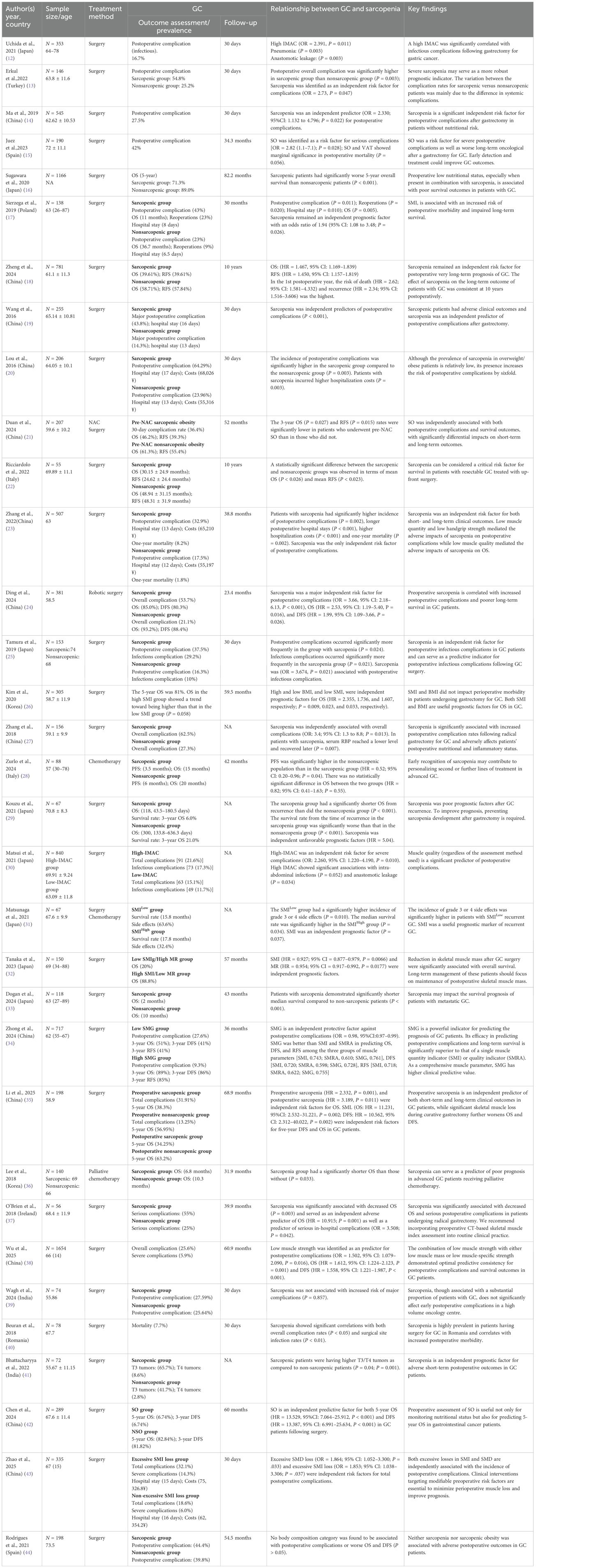
Analysis of gastric cancer outcomes in the included literature primarily focused on postoperative complications, encompassing overall complications (24, 27, 38) and major complications (24, 37); survival metrics including OS (16–18, 21, 22, 24, 26, 28, 29, 32–36, 42), DFS (24, 34, 42), and RFS (18, 21, 22, 34); as well as hospitalization duration and costs (17, 18, 20, 23, 43). Two additional studies evaluated sarcopenia’s impact on chemotherapy delays (43) and treatment-related toxicities (31) in gastric cancer patients. Evidence indicates significantly elevated overall complication rates among sarcopenic patients, with major complication rates reaching 12.9%–43.8% in this subgroup. Regarding survival outcomes, sarcopenia substantially reduced long-term survival rates and increased recurrence risk. Sarcopenic patients incurred higher hospitalization costs with prolonged hospital stays. Follow-up durations varied considerably across studies: short-term (30-day) assessments (12–14, 20, 25, 39, 40, 43), intermediate term (3–5 years) (15–17, 21, 23, 24, 26, 28, 32–38, 42, 44), and long term (18, 22). Regarding short-term outcomes, sarcopenia substantially increases postoperative complication risks, including infectious complications (12, 25, 30), anastomotic leakage (12, 30), and major complications (19, 24); prolongs hospital stays (17, 20, 23); and elevates healthcare costs (20, 23). Sarcopenia independently predicts reduced survival, significantly diminishing 5-year overall survival (16, 18, 42) and disease-free survival (18, 24), with particularly pronounced effects in metastatic/advanced disease (33, 36). Concurrent evidence indicates sarcopenia correlates with higher chemotherapy-related toxicities (31) and treatment delay risks (34). However, two studies reported no significant impact of sarcopenia or body composition alterations on postoperative complications or survival outcomes (39, 44) (Table 3).
3.4 Insights into the mechanism of action between gastric cancer and sarcopenia
Through a review of the included literature, we have preliminarily summarized that the core mechanisms underlying the interaction between sarcopenia and gastric cancer may encompass four key aspects. First, sarcopenia exacerbates postoperative risks in gastric cancer patients through disordered nutritional metabolism (12, 14, 16, 19). Second, inflammation and immune suppression mediate bidirectional adverse effects (12, 16, 18, 20). Third, surgical stress and tumor progression act synergistically to cause harm (13, 17, 18, 21). Finally, the fourth aspect may involve the compounded risk resulting from altered body composition, such as sarcopenic obesity (12, 15, 18, 19). Please refer to the schematic diagram of the mechanism of action for specific details (Figure 2).
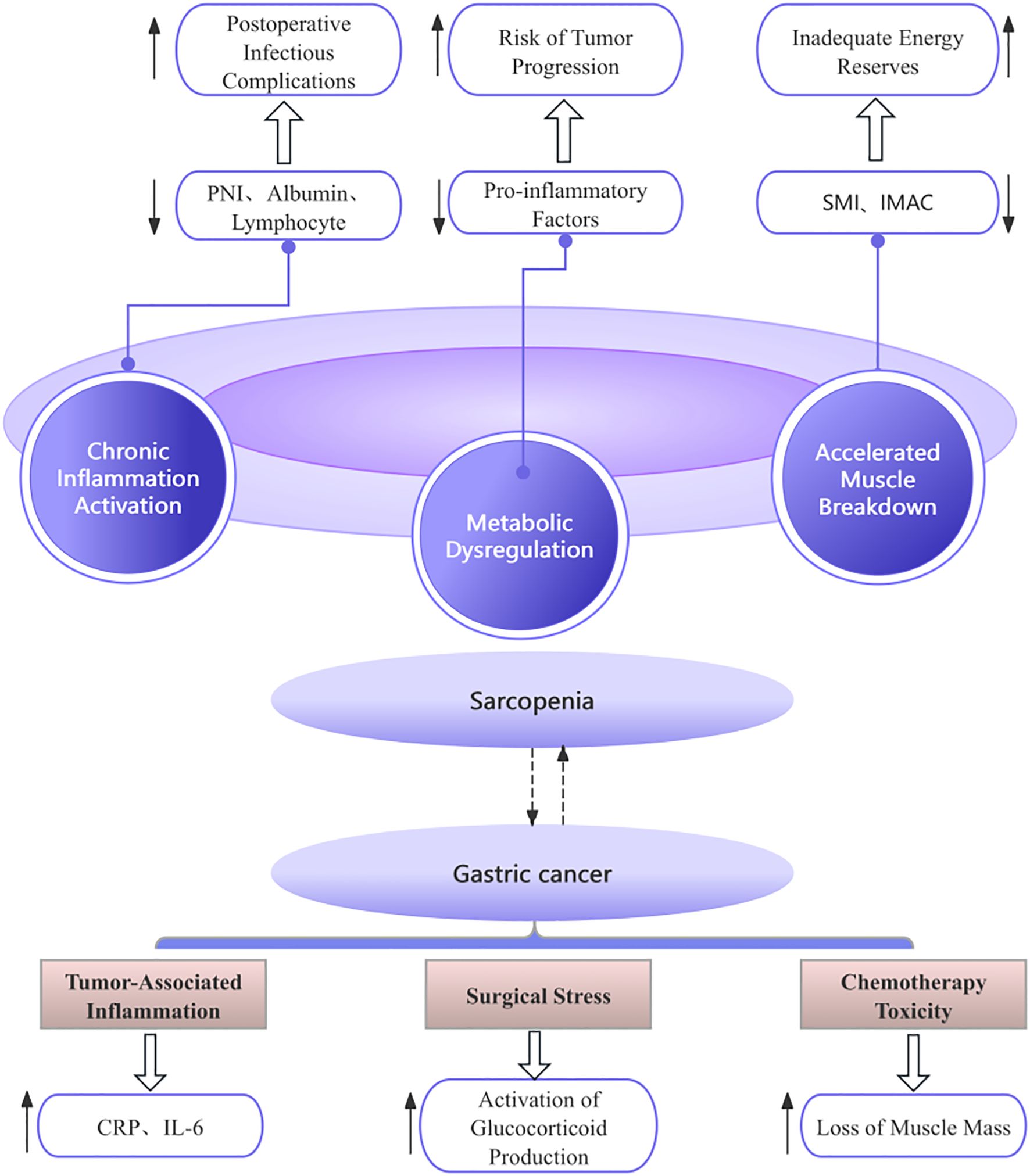
Figure 2. Diagram of the mechanism of action. PNI, Prognostic Nutritional Index; SMI, Skeletal Muscle Index; IMAC, intramuscular adipose tissue content.
4 Discussion
Gastric cancer treatment risks increase with advancing age, while the prevalence of sarcopenia is notably higher in older populations (45). Therefore, precise nutritional risk assessment and careful selection of management strategies are clinically essential for elderly patients. Multiple studies have confirmed that sarcopenia is a significant risk factor for survival following curative surgery for gastric cancer (46, 47), although the exact relationship between sarcopenia and gastric cancer remains incompletely understood. Consistent with the majority of existing evidence, this review confirms that sarcopenia significantly increases the risk of postoperative complications, reduces overall survival (OS) and disease-free survival (DFS), and is associated with prolonged hospitalization and higher medical costs (37, 43). Notably, one study reported a fivefold increase in the risk of major complications among sarcopenic patients compared to non-sarcopenic patients after adjusting for covariates (19). Li et al. (35) identified both preoperative and postoperative sarcopenia as independent risk factors for OS in gastric cancer patients (35). However, conflicting evidence exists regarding the effect of sarcopenia on OS (48). There is growing research interest in the impact of sarcopenic obesity on gastric cancer outcomes (20, 21). Due to the complexity of screening procedures, clinicians often rely excessively on BMI for nutritional assessment, which may lead to underrecognition of nutritional risks in overweight or obese patients (49).
However, several limitations persist in current research, including the absence of standardized diagnostic criteria for sarcopenia—particularly in the systematic assessment of muscle strength and physical performance—which contributes to substantial discrepancies in reported prevalence rates. Most existing studies depend on preoperative imaging for muscle mass evaluation, with CT being the most widely used modality, despite ongoing debate regarding its validity in accurately reflecting whole-body musculature (25). Bioelectrical impedance analysis (BIA), although radiation-free and cost-effective, has not been routinely incorporated into preoperative assessment protocols (50). Moreover, muscle strength evaluations such as grip dynamometry can be influenced by subjects’ volitional effort or preexisting hand pathologies, potentially affecting measurement accuracy (51). Current sarcopenia diagnosis predominantly relies on SMI cutoffs; however, conventional definitions often overlook age as a critical modifier of muscle quality. Excessive reliance on SMI alone may therefore introduce bias into research outcomes (52). The revised 2018 EWGSOP guidelines explicitly incorporated “reduced muscle quality” as a core diagnostic criterion (53), underscoring the inadequacy of muscle quantity alone in predicting gastric cancer prognosis and highlighting the necessity of incorporating comprehensive functional assessments. A recent multicenter study integrating CT imaging and clinical data from three prospective gastric cancer cohorts proposed a composite metric known as the SMG, which synergistically evaluates both muscle mass and quality and may outperform single-parameter indices (54). This approach is conceptually analogous to diamond valuation, which considers both carat weight and clarity; nevertheless, the utility of SMG remains underexplored in gastric oncology. Concurrently, IMAC has emerged as a quantifiable biomarker of muscle quality, with emerging evidence indicating that IMAC-guided prehabilitation programs may contribute to improved surgical outcomes (12).
The pathophysiological interplay between gastric cancer and sarcopenia involves complex mechanisms, with no definitive causal relationship yet established. Current evidence indicates that gastric cancer patients exhibit heightened susceptibility to sarcopenia, primarily mediated through accelerated protein catabolism, systemic inflammatory responses, metabolic dysregulation, and reduced nutritional intake—processes intrinsically linked to cancer cachexia (55). Cachexia further impairs skeletal muscle regenerative capacity (56), while the concomitant loss of muscle-derived myokines, which exert anti-inflammatory and anti-tumor effects, may facilitate cancer progression (57). The immunometabolic imbalance hypothesis posits that fat-infiltrated skeletal muscle secretes aberrant adipokines that suppress NK cell function, thereby exacerbating postoperative immunosuppression and predisposing patients to infectious complications (58). Notably, males with sarcopenic obesity demonstrate elevated perioperative risk, largely attributable to increased adipose tissue friability that compromises surgical exposure (59). Concurrently, inadequate protein reserves in sarcopenic patients impair postoperative tissue repair under hypercatabolic stress, leading to delayed wound healing and prolonged hospitalization (60, 61). Skeletal muscle serves as an amino acid reservoir that mobilizes substrates for biosynthetic defense during surgical trauma; sarcopenia-induced amino acid deficiency restricts this reparative capacity, thereby increasing infection susceptibility (62). Importantly, most available studies do not adequately evaluate post-gastrectomy dietary intake patterns, which precludes definitive attribution of muscle loss to either surgical sequelae or underlying cancer pathophysiology (29).
This review synthesizes current evidence regarding the association between gastric cancer and sarcopenia, delineating the prevalence of sarcopenia among gastric cancer patients and evaluating its prognostic implications. While consolidating key insights, several limitations must be acknowledged. First, the predominance of retrospective study designs inherently constrains the assessment of core diagnostic parameters for sarcopenia—such as muscle strength and physical performance—which are frequently unavailable in archival datasets. Second, a geographical selection bias is evident, with studies predominantly involving East Asian populations and a notable scarcity of data from Western demographics. Furthermore, the use of heterogeneous assessment metrics across studies complicates comparative analysis. To address these issues, future efforts should focus on establishing integrated diagnostic criteria that combine artificial intelligence—enhanced imaging with validated biomarkers. Such advances would improve diagnostic accuracy and facilitate the identification of patients who may benefit from early nutritional and therapeutic interventions aimed at increasing muscle mass and improving clinical outcomes. Additionally, mechanistic studies are needed to elucidate the role of muscle density in gastric cancer progression, alongside randomized controlled trials to determine whether targeted interventions for sarcopenia significantly improve long-term survival.
5 Conclusion
This study demonstrates that sarcopenia is highly prevalent after gastric cancer surgery and serves as a significant predictor of adverse postoperative outcomes. However, its underlying mechanisms and standardized diagnostic criteria require further elucidation. Methodological variations and the lack of uniform assessment metrics across existing studies have contributed to inconsistent conclusions. Future efforts should focus on developing muscle preservation strategies and multimodal diagnostic approaches that integrate both mass and functional parameters to improve diagnostic accuracy and clinical outcomes. Moreover, prospective studies are essential to establish causal relationships between sarcopenia and gastric cancer progression, thereby facilitating evidence-based clinical pathways.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
XW: Investigation, Methodology, Writing – original draft. XS: Investigation, Methodology, Writing – original draft. YyW: Investigation, Methodology, Writing – original draft. YjW: Investigation, Methodology, Writing – original draft. JR: Supervision, Visualization, Writing – review & editing. XF: Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Ilic M and Ilic I. Epidemiology of stomach cancer. World J Gastroenterol. (2022) 28:1187–203. doi: 10.3748/wjg.v28.i12.1187
3. Argilés JM, López-Soriano FJ, and Busquets S. Mediators of cachexia in cancer patients. Nutrition. (2019) 66:11–5. doi: 10.1016/j.nut.2019.03.012
4. Xie K, He D, Zhao T, Liu T, and Tang M. Gastric cancer with sarcopenia: an area worth focusing on. Curr Treat Options Oncol. (2023) 24:1305–27. doi: 10.1007/s11864-023-01122-y
5. Ma DW, Cho Y, Jeon MJ, Kim JH, Lee IJ, Youn YH, et al. Relationship between sarcopenia and prognosis in patient with concurrent chemo-radiation therapy for esophageal cancer. Front Oncol. (2019) 9:366. doi: 10.3389/fonc.2019.00366
6. Raun SH, Knudsen JR, Han X, Jensen TE, and Sylow L. Cancer causes dysfunctional insulin signaling and glucose transport in a muscle-type-specific manner. FASEB J. (2022) 36:e22211. doi: 10.1096/fj.202101759R
7. Morton M, Patterson J, Sciuva J, Perni J, Backes F, Nagel C, et al. Malnutrition, sarcopenia, and cancer cachexia in gynecologic cancer. Gynecol Oncol. (2023) 175:142–55. doi: 10.1016/j.ygyno.2023.06.015
8. Shi X, Wang X, Yao W, Shi D, Shao X, Lu Z, et al. Mechanism insights and therapeutic intervention of tumor metastasis: latest developments and perspectives. Signal Transduct Target Ther. (2024) 9:192. doi: 10.1038/s41392-024-01885-2
9. Khalil H, Peters M, Godfrey CM, McInerney P, Soares CB, and Parker D. An evidence-based approach to scoping reviews. Worldviews Evid Based Nurs. (2016) 13:118–23. doi: 10.1111/wvn.12144
10. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
11. Porritt K, Gomersall J, and Lockwood C. JBI’s Systematic Reviews: Study selection and critical appraisal. Am J Nurs. (2014) 114:47–52. doi: 10.1097/01.NAJ.0000450430.97383.64
12. Uchida T, Sekine R, Matsuo K, Kigawa G, Umemoto T, Kijima K, et al. Association between low preoperative skeletal muscle quality and infectious complications following gastrectomy for gastric cancer. Surg Today. (2021) 51:1135–43. doi: 10.1007/s00595-020-02225-x
13. Erkul O, Cekic AB, Cansu A, Yildirim R, and Guner A. Effects of sarcopenia on postoperative outcomes in patients who underwent gastrectomy for gastric cancer. J Surg Res. (2022) 274:196–206. doi: 10.1016/j.jss.2021.12.051
14. Ma BW, Chen XY, Fan SD, Zhang FM, Huang DD, Li B, et al. Impact of sarcopenia on clinical outcomes after radical gastrectomy for patients without nutritional risk. Nutrition. (2019) 61:61–6. doi: 10.1016/j.nut.2018.10.025
15. Juez LD, Priego P, Bajawi M, Cuadrado M, Blázquez LA, Sánchez-Picot S, et al. Impact of sarcopenic obesity on long-term cancer outcomes and postoperative complications after gastrectomy for gastric cancer. J Gastrointest Surg. (2023) 27:35–46. doi: 10.1007/s11605-022-05492-w
16. Sugawara K, Yamashita H, Urabe M, Okumura Y, Yagi K, Aikou S, et al. Poor nutritional status and sarcopenia influences survival outcomes in gastric carcinoma patients undergoing radical surgery. Eur J Surg Oncol. (2020) 46:1963–70. doi: 10.1016/j.ejso.2020.04.044
17. Sierzega M, Chrzan R, Wiktorowicz M, Kolodziejczyk P, and Richter P. Prognostic and predictive implications of sarcopenia in Western patients undergoing gastric resections for carcinoma of the stomach. J Surg Oncol. (2019) 120:473–82. doi: 10.1002/jso.25509
18. Zheng HL, Wei LH, Xu BB, Zheng HH, Xue Z, Chen QY, et al. Prognostic value of preoperative sarcopenia in gastric cancer: A 10-year follow-up study. Eur J Surg Oncol. (2024) 50:108004. doi: 10.1016/j.ejso.2024.108004
19. Wang SL, Zhuang CL, Huang DD, Pang WY, Lou N, Chen FF, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: A prospective study. Ann Surg Oncol. (2016) 23:556–64. doi: 10.1245/s10434-015-4887-3
20. Lou N, Chi CH, Chen XD, Zhou CJ, Wang SL, Zhuang CL, et al. Sarcopenia in overweight and obese patients is a predictive factor for postoperative complication in gastric cancer: A prospective study. Eur J Surg Oncol. (2017) 43:188–95. doi: 10.1016/j.ejso.2016.09.006
21. Duan C, Wu M, Wen X, Zhuang L, and Sun J. Sarcopenic obesity predicts short- and long-term outcomes after neoadjuvant chemotherapy and surgery for gastric cancer. Jpn J Clin Oncol. (2024) 54:975–85. doi: 10.1093/jjco/hyae080
22. Ricciardolo AA, De Ruvo N, Serra F, Prampolini F, Solaini L, Battisti S, et al. Strong impact of sarcopenia as a risk factor of survival in resected gastric cancer patients: first Italian report of a Bicentric study. Updates Surg. (2022) 74:283–93. doi: 10.1007/s13304-021-01175-4
23. Zhang FM, Zhang XZ, Zhu GL, Lv LQ, Yan XL, Wu WX, et al. Impact of sarcopenia on clinical outcomes of patients with stage I gastric cancer after radical gastrectomy: A prospective cohort study. Eur J Surg Oncol. (2022) 48:541–7. doi: 10.1016/j.ejso.2021.08.021
24. Ding P, Wu H, Li T, Wu J, Yang L, Yang J, et al. Impact of preoperative sarcopenia on postoperative complications and prognosis in patients undergoing robotic gastric cancer surgery: A propensity score matching study. Nutrition. (2024) 123:112408. doi: 10.1016/j.nut.2024.112408
25. Tamura T, Sakurai K, Nambara M, Miki Y, Toyokawa T, Kubo N, et al. Adverse effects of preoperative sarcopenia on postoperative complications of patients with gastric cancer. Anticancer Res. (2019) 39:987–92. doi: 10.21873/anticanres.13203
26. Kim EY, Jun KH, Kim SY, and Chin HM. Body mass index and skeletal muscle index are useful prognostic factors for overall survival after gastrectomy for gastric cancer: Retrospective cohort study. Med (Baltimore). (2020) 99:e23363. doi: 10.1097/MD.0000000000023363
27. Zhang Y, Wang JP, Wang XL, Tian H, Gao TT, Tang LM, et al. Computed tomography-quantified body composition predicts short-term outcomes after gastrectomy in gastric cancer. Curr Oncol. (2018) 25:e411–22. doi: 10.3747/co.25.4014
28. Zurlo V, Rosa F, Rinninella E, Pontolillo L, Beccia V, Maratta M, et al. Impact of muscle mass loss on outcomes in advanced or metastatic gastric cancer patients receiving a second-line treatment. Eur Rev Med Pharmacol Sci. (2024) 28:1575–84. doi: 10.26355/eurrev_202402_35486
29. Kouzu K, Tsujimoto H, Sugasawa H, Ishibashi Y, Itazaki Y, Tsuchiya S, et al. Impact of postoperative reduced skeletal muscle on prognosis after recurrence in gastric cancer. Mol Clin Oncol. (2021) 14:3. doi: 10.3892/mco.2020.2165
30. Matsui R, Inaki N, and Tsuji T. Impact of preoperative muscle quality on postoperative severe complications after radical gastrectomy for gastric cancer patients. Ann Gastroenterol Surg. (2021) 5:510–8. doi: 10.1002/ags3.12452
31. Matsunaga T, Satio H, Miyauchi W, Shishido Y, Miyatani K, Murakami Y, et al. Impact of skeletal muscle mass in patients with recurrent gastric cancer. World J Surg Oncol. (2021) 19:170. doi: 10.1186/s12957-021-02283-6
32. Tanaka Y, Aoyagi K, Umetani Y, Tanaka YU, Kaku H, Minami T, et al. Impact of skeletal muscle mass reduction on long-term survival after radical resection of gastric cancer. Anticancer Res. (2023) 43:3779–86. doi: 10.21873/anticanres.16563
33. Dogan O, Sahinli H, Duzkopru Y, Akdag T, and Kocanoglu A. Is sarcopenia effective on survival in patients with metastatic gastric cancer? World J Gastrointest Oncol. (2024) 16:1861–8. doi: 10.4251/wjgo.v16.i5.1861
34. Zhong Q, Huang JB, Lu J, Xue LW, Lin GT, Xie JW, et al. Predictive value of a new muscle parameter in patients with resectable gastric cancer: A pooled analysis of three prospective trials. Ann Surg Oncol. (2024) 31:3005–16. doi: 10.1245/s10434-024-14913-w
35. Li X, Ding P, Wu J, Wu H, Yang P, Guo H, et al. Preoperative sarcopenia and postoperative accelerated muscle loss negatively impact survival after resection of locally advanced gastric cancer. BMC Cancer. (2025) 25:269. doi: 10.1186/s12885-025-13674-3
36. Lee JS, Kim YS, Kim EY, and Jin W. Prognostic significance of CT-determined sarcopenia in patients with advanced gastric cancer. PloS One. (2018) 13:e0202700. doi: 10.1371/journal.pone.0202700
37. O’Brien S, Twomey M, Moloney F, Kavanagh RG, Carey BW, Power D, et al. Sarcopenia and post-operative morbidity and mortality in patients with gastric cancer. J Gastric Cancer. (2018) 18:242–52. doi: 10.5230/jgc.2018.18.e25
38. Wu GF, He CH, Xi WT, Zhai WB, Li ZZ, Zhu YC, et al. Sarcopenia defined by the global leadership initiative in sarcopenia (GLIS) consensus predicts adverse postoperative outcomes in patients undergoing radical gastrectomy for gastric cancer: analysis from a prospective cohort study. BMC Cancer. (2025) 25:679. doi: 10.1186/s12885-025-13967-7
39. Wagh MS, Balan AK, Mathew AP, Rakesh CA, Krishna J, Chandramohan K, et al. Sarcopenia in gastric cancer and its impact on early postoperative outcome. Cancer Treat Res Commun. (2024) 40:100829. doi: 10.1016/j.ctarc.2024.100829
40. Beuran M, Tache C, Ciubotaru C, Vartic M, Hostiuc S, Prodan A, et al. Sarcopenia is a predictive factor for postoperative morbidity and mortality in patients having radical gastrectomy for cancer. Chirurgia (Bucur). (2018) 113:678–86. doi: 10.21614/chirurgia.113.5.678
41. Bhattacharyya S, Devi P, Das PK, Samantara S, Kp KM, Pradhan S, et al. The analysis of surgical outcomes in operable gastric cancer patients presenting with or without sarcopenia. Indian J Surg Oncol. (2022) 13:511–5. doi: 10.1007/s13193-022-01514-w
42. Chen W, Yuan Q, Li X, Yao J, Yuan L, Chen X, et al. The role of sarcopenic obesity for the prediction of prognosis of patients with gastrointestinal cancer. Cancer Med. (2024) 13:e7452. doi: 10.1002/cam4.7452
43. Zhao H, Dong Q, Chen C, Pan L, Liu S, Cheng J, et al. Perioperative body composition changes and their clinical implications in patients with gastric cancer undergoing radical gastric cancer surgery: a prospective cohort study. J Gastrointest Surg. (2025) 29:101877. doi: 10.1016/j.gassur.2024.101877
44. Rodrigues V, Landi F, Castro S, Mast R, Rodríguez N, Gantxegi A, et al. Is sarcopenic obesity an indicator of poor prognosis in gastric cancer surgery? A cohort study in a western population. J Gastrointest Surg. (2021) 25:1388–403. doi: 10.1007/s11605-020-04716-1
45. Iannuzzi-Sucich M, Prestwood KM, and Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. (2002) 57:M772–7. doi: 10.1093/gerona/57.12.m772
46. Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. (2015) 261:1173–83. doi: 10.1097/SLA.0000000000000743
47. Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: A meta-analysis. Ann Surg. (2018) 268:58–69. doi: 10.1097/SLA.0000000000002679
48. Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Med (Baltimore). (2016) 95:e3164. doi: 10.1097/MD.0000000000003164
49. Gioulbasanis I, Martin L, Baracos VE, Thézénas S, Koinis F, and Senesse P. Nutritional assessment in overweight and obese patients with metastatic cancer: does it make sense? Ann Oncol. (2015) 26:217–21. doi: 10.1093/annonc/mdu501
50. Tinsley GM, Morales E, Forsse JS, and Grandjean PW. Impact of acute dietary manipulations on DXA and BIA body composition estimates. Med Sci Sports Exerc. (2017) 49:823–32. doi: 10.1249/MSS.0000000000001148
51. Ali NA, O’Brien JM Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, et al. Midwest Critical Care Consortium. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. (2008) 178:261–8. doi: 10.1164/rccm.200712-1829OC
52. Faulkner JA, Larkin LM, Claflin DR, and Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. (2007) 34:1091–6. doi: 10.1111/j.1440-1681.2007.04752.x
53. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
54. Weinberg MS, Shachar SS, Muss HB, Deal AM, Popuri K, Yu H, et al. Beyond sarcopenia: Characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J. (2018) 24:278–84. doi: 10.1111/tbj.12952
55. Peixoto da Silva S, Santos JMO, Costa E Silva MP, Gil da Costa RM, and Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. (2020) 11:619–35. doi: 10.1002/jcsm.12528
56. Inaba S, Hinohara A, Tachibana M, Tsujikawa K, and Fukada SI. Muscle regeneration is disrupted by cancer cachexia without loss of muscle stem cell potential. PloS One. (2018) 13:e0205467. doi: 10.1371/journal.pone.0205467
57. Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. (2013) 62:882–9. doi: 10.1136/gutjnl-2011-300776
58. Park J, Morley TS, Kim M, Clegg DJ, and Scherer PE. Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. (2014) 10:455–65. doi: 10.1038/nrendo.2014.94
59. Yoshikawa K, Shimada M, Kurita N, Iwata T, Nishioka M, Morimoto S, et al. Visceral fat area is superior to body mass index as a predictive factor for risk with laparoscopy-assisted gastrectomy for gastric cancer. Surg Endosc. (2011) 25:3825–30. doi: 10.1007/s00464-011-1798-7
60. Ebhardt HA, Degen S, Tadini V, Schilb A, Johns N, Greig CA, et al. Comprehensive proteome analysis of human skeletal muscle in cachexia and sarcopenia: a pilot study. J Cachexia Sarcopenia Muscle. (2017) 8:567–82. doi: 10.1002/jcsm.12188
61. Biolo G, Zorat F, Antonione R, and Ciocchi B. Muscle glutamine depletion in the intensive care unit. Int J Biochem Cell Biol. (2005) 37:2169–79. doi: 10.1016/j.biocel.2005.05.001
Keywords: gastric cancer, stomach neoplasm, sarcopenia, sarcopenic obesity, prognosis
Citation: Wang X, Sun X, Wu Y, Wang Y, Ren J and Fang X (2025) The association between gastric cancer and sarcopenia: a scoping review. Front. Oncol. 15:1684186. doi: 10.3389/fonc.2025.1684186
Received: 12 August 2025; Accepted: 03 October 2025;
Published: 16 October 2025.
Edited by:
Antonio Mario Scanu, University of Sassari, ItalyReviewed by:
Shaun Sabico, King Saud University, Saudi ArabiaMehmet Uluşahin, Karadeniz Technical University, Türkiye
Copyright © 2025 Wang, Sun, Wu, Wang, Ren and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuedong Fang, ZmFuZ3hkMTk2MUAxNjMuY29t
 Xue Wang1
Xue Wang1 Xuefeng Sun
Xuefeng Sun Yuanyu Wu
Yuanyu Wu Xuedong Fang
Xuedong Fang