- 1College of Traditional Chinese Medicine, Changchun University of Chinese Medicine, Changchun, Jilin, China
- 2Department of Integrated Chinese and Western Medicine, Jilin Cancer Hospital, Changchun, Jilin, China
Background: The present research is a meta-analysis aimed at quantifying the influence of the hemoglobin, albumin, lymphocyte, and platelet (HALP) score on the overall survival (OS) and disease-free survival (DFS) in breast cancer patients. The objective of this research is to examine the prognostic significance of the HALP score in breast cancer patients and evaluate its predictive performance for survival outcomes.
Methods: This research was conducted adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We retrieved PubMed, EMBASE, Web of Science, Cochrane Library, and Google Scholar for studies relevant to the link between the HALP score and the prognosis of breast cancer up to July 3, 2025. The quality of the studies was appraised with Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) and the Newcastle-Ottawa Scale (NOS). MetaDiSc 1.4, Stata SE 18, and Review Manager 5.4 were leveraged to examine the risk of bias and to conduct statistical analysis.
Results: Among 971 studies, nine fulfilled the inclusion criteria, encompassing a total of 4,560 individuals. An increased HALP score was linked to favorable OS (HR = 0.61, 95% CI 0.39-0.97, p < 0.05). No statistically significant association was noted between the HALP score and DFS in breast cancer patients. The area under the summary receiver operating characteristics (SROC) curve (AUC) of the HALP score for predicting pathological complete response (pCR) was 0.57.
Conclusion: Elevated HALP scores may represent a prognostic biomarker for favorable survival outcomes in breast cancer patients.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/myprospero identifier CRD420251031999.
1 Background
Breast cancer (BC) is a principal global health challenge, seriously jeopardizing women’s lives and health, and the incidence of BC is gradually increasing worldwide (1). However, substantial heterogeneity exists in the molecular expression profiles of BC. Factors such as the variations of gene expression patterns can influence the prognosis of BC (2). Despite advances in therapeutic approaches and medications, the overall survival (OS) of patients with BC remains suboptimal. Therefore, it is clinically meaningful to identify predictive factors that impact the progression and prognosis of BC. Currently, a number of immunonutritional biomarkers associated with BC have been identified, including peripheral neutrophil-to-lymphocyte ratio (NLR) (3) and prognostic nutritional index (PNI) (4).
Over recent years, the hemoglobin, albumin, lymphocyte, and platelet score (HALP) has emerged as a novel, cost-effective prognostic marker. It was initially proposed by Chen et al. in 2015 (5) to utilize the HALP score in forecasting the prognosis of gastric cancer. The score was computed as [hemoglobin (g/L) × albumin (g/L) × lymphocytes (/L)]/platelets (/L). Previously, a meta-analysis was executed to measure the influence of the HALP score on the prognosis of unselected solid tumors in 13,110 patients. The results denoted that a reduced HALP score was correlated with poorer OS (6). Some studies, however, suggested that no significant prognostic significance of the HALP score was discovered in BC (7).
Systemic inflammatory status and immune nutritional status are important indicators for assessing the prognosis of BC. The HALP score, as a comprehensive prognostic assessment tool, comprehensively reflects the patient’s systemic inflammatory level and immune nutritional status, providing a practical reference for predicting clinical prognosis and developing treatment strategies (8). It has been shown that the chronic inflammatory microenvironment can promote the occurrence and development of tumors through multiple mechanisms (9). In terms of lifestyle intervention, the study by Giosia et al. (10) shows that the Mediterranean diet pattern can significantly reduce the level of circulating inflammatory markers (such as TNF-α), which may be one of the potential mechanisms for reducing the risk of cancer.
BC-specific molecular subtypes are also key factors influencing patient prognosis. Previous studies have indicated that the progression of triple-negative BC (TNBC) is particularly closely related to the immune microenvironment (11). High levels of tumor-infiltrating lymphocytes (TILs) not only suggest that TNBC patients respond better to neoadjuvant chemotherapy but are also closely associated with improved survival outcomes. TNBC with significant TIL infiltration may be more sensitive to immunotherapy (12). The above evidence collectively suggests that the HALP score, as an integrative indicator reflecting systemic inflammatory status, may provide important references for predicting the prognosis of BC patients by assessing key pathophysiological processes related to tumor progression.
Therefore, the present research aims to quantify the prognostic utility of the HALP score for OS and DFS in BC patients via a meta-analysis. Besides, this research aims to assess whether the score can effectively enable early intervention in these patients and to better monitor and identify high-risk populations. In the meantime, the predictive performance of the score for pathological complete response (pCR) was also evaluated in individuals with BC.
2 Method
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13). The study protocol was registered in the PROSPERO database (CRD420251031999).
2.1 Search strategy
We retrieved related studies on PubMed, EMBASE, Web of Science, Cochrane Library, and Google Scholar through July 3, 2025. Search terms included “BC” and “HALP score”, as well as their combinations with Boolean operators (AND, OR, NOT). Search strategy details are delineated in Supplementary Table S1. Additionally, references of the included studies were manually screened to avoid omission.
2.2 Inclusion criteria and exclusion criteria
The inclusion criteria were comprised of (a) study design: observational studies, (b) population: BC patients, (c) with HALP scores measured as a categorical variable and comparative assessment between high and low scores, (d) with OS, DFS, and pCR as outcomes, and (e) no language restrictions. The exclusion criteria included (a) review articles, meta-analyses, or commentaries, (b) inadequate data for extraction, (c) animal or in vitro studies, (d) irrelevant research objectives, (e) non-BC populations, and (f) use of non-standard methods to calculate HALP.
2.3 Study selection
Two researchers (CJ and WB) independently screened the studies in EndNote, guided by the inclusion and exclusion criteria. Specifically, the titles and abstracts were reviewed and filtered, and then the full texts were downloaded and evaluated. In case of disagreement between the two researchers on the same study, it would be resolved by discussion with a third researcher (ZY).
2.4 Data extraction
Two researchers (CJ and WB) independently retrieved data and collected the following information with the predefined criteria: (a) study characteristics: first author, year of publication, type of study (cohort, case-control, among others), country, and sample size; (b) patients’ baseline information: age, gender, molecular typing, pathologic stage, Ki67 index, and lymph node metastasis; (c) treatment regimen: interventions and treatment strategies; (d) outcomes: HR and 95% CI for OS and DFS, and classification metrics of pCR, including true positives, false positives, true negatives, and false negatives. When the researchers held different opinions, it would be determined after consultation with a third researcher.
2.5 Quality assessment
Two researchers (CJ and WB) independently appraised the validity of the enrolled studies based on predefined criteria. Different evaluation tools were selected depending on the outcomes: cohort studies with OS as the outcome were appraised with the Newcastle-Ottawa Scale (NOS) (14). The scale encompassed three dimensions: selection, comparability, and outcome. The total score was 9 points. A score of 7–9 was considered high quality, 4–6 was considered medium quality, and less than 4 was considered low quality. Case-control studies were assessed using the corresponding version of NOS. The detailed information on the NOS scale is provided in Supplementary Table S2. Diagnostic studies with pCR as the outcome were appraised with Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) (15). It examined the risk of bias and clinical applicability in four domains: patient selection, index test, reference standard, and flow and timing. The evaluation results were analyzed on Review Manager 5.4. All evaluation results were agreed upon through cross-checking, and if differences arose, they were resolved as above.
2.6 Statistical analysis
Stata SE 18 was leveraged for pooled analysis of the data from studies that reported OS and DFS outcomes, with HR and 95% CI as effect indicators. The I2 statistics were applied to quantify heterogeneity to determine whether to employ a random-effects model or a fixed-effects model. When the I2 statistic was > 50%, it denoted a high degree of heterogeneity, and thus a random-effects model was selected; otherwise, a fixed-effects model was applied. Subgroup analyses and regression analyses were executed by the countries of the study population, thus determining whether the country was a source of heterogeneity. A leave-one-out sensitivity analysis was executed to appraise the robustness of the findings. Egger’s test was employed to examine publication bias, with p < 0.05 denoting statistical significance. For studies reporting with pCR, MetaDisc 1.4 was leveraged to pool sensitivity, specificity, negative likelihood ratio (-LR), and positive likelihood ratio (+LR). The random-effects model was also employed to construct the summary receiver operating characteristic (SROC) curve and to compute the area under the curve (AUC) to investigate the diagnostic performance reported in the included studies.
3 Results
3.1 Study selection
In the present research, the database search yielded 971 studies. After deleting 24 duplicates, excluding 919 studies by the title and abstract, 19 by the full text, 9 studies were eventually included. The main reasons for exclusion included a mismatch with the scope or objective of the present study, inaccessible data, and a non-systematic review design. The detailed screening process is demonstrated in Figure 1.
3.2 Baseline characteristics
Nine studies were enrolled in this research (7, 16–23), comprising five from Turkey (7, 16, 18, 21, 23), three from China (17, 20, 22), and one derived from a United States database (19). In total, the research encompassed 4,560 patients. Three studies included male patients (7, 16, 18), amounting to nine male patients in total. Five studied female patients (17, 19–22), and one did not mention the gender of the participants. All studies were retrospective and were published in the last 5 years. Concerning molecular subtype, two studies included individuals with TNBC (20, 21). The outcome metrics in the included studies were different. Four studies reported on pCR (16–18, 23), two on OS (19, 20), one on OS and progression-free survival (PFS) (17), and two on OS and DFS (7, 21). The nine studies differed in the treatment regimens. One study enrolled those treated with surgery following neoadjuvant therapy (NAT) (17), one involved neoadjuvant chemotherapy (NAC) (18), two included post-NAC surgery (7, 22), one imposed no limitations on treatment regimen (21), one enrolled patients who were not surgical candidates and received NAC (predominantly the paclitaxel and carboplatin regimen), one enrolled patients who did not receive any preoperative chemotherapy (22), and one employed Doxorubicin/Cyclophosphamide → Docetaxel/Trastuzumab/Pertuzumab (AC-THP) combined with surgery. The details are delineated in Table 1.
3.3 Quality evaluation
Among the nine included studies, five reported (7, 19–22) with OS as the primary outcome indicator, and thus were assessed with NOS. Studies scoring 7 and above on the scale were classified as high-quality, and the five studies all scored between 7 and 8 (mean score 7.5), as shown in Table 2. Four studies (16–18, 23) reported pCR, and thus QUADAS-2 was leveraged for quality assessment in these diagnostic studies. The results denoted that the included studies had high clinical applicability and a moderate risk of bias, as shown in Figure 2.
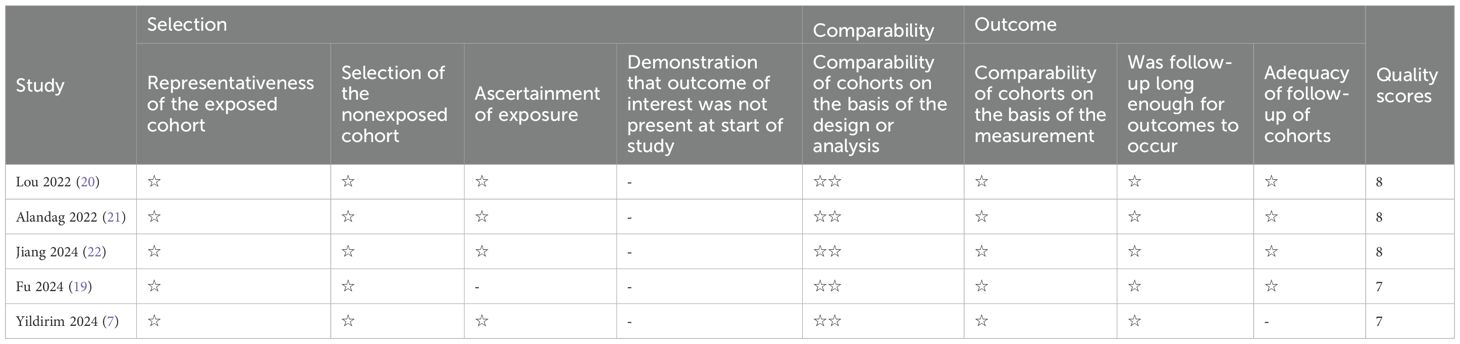
Table 2. Quality assessment with the Newcastle-Ottawa Scale for the studies with overall survival as an outcome.
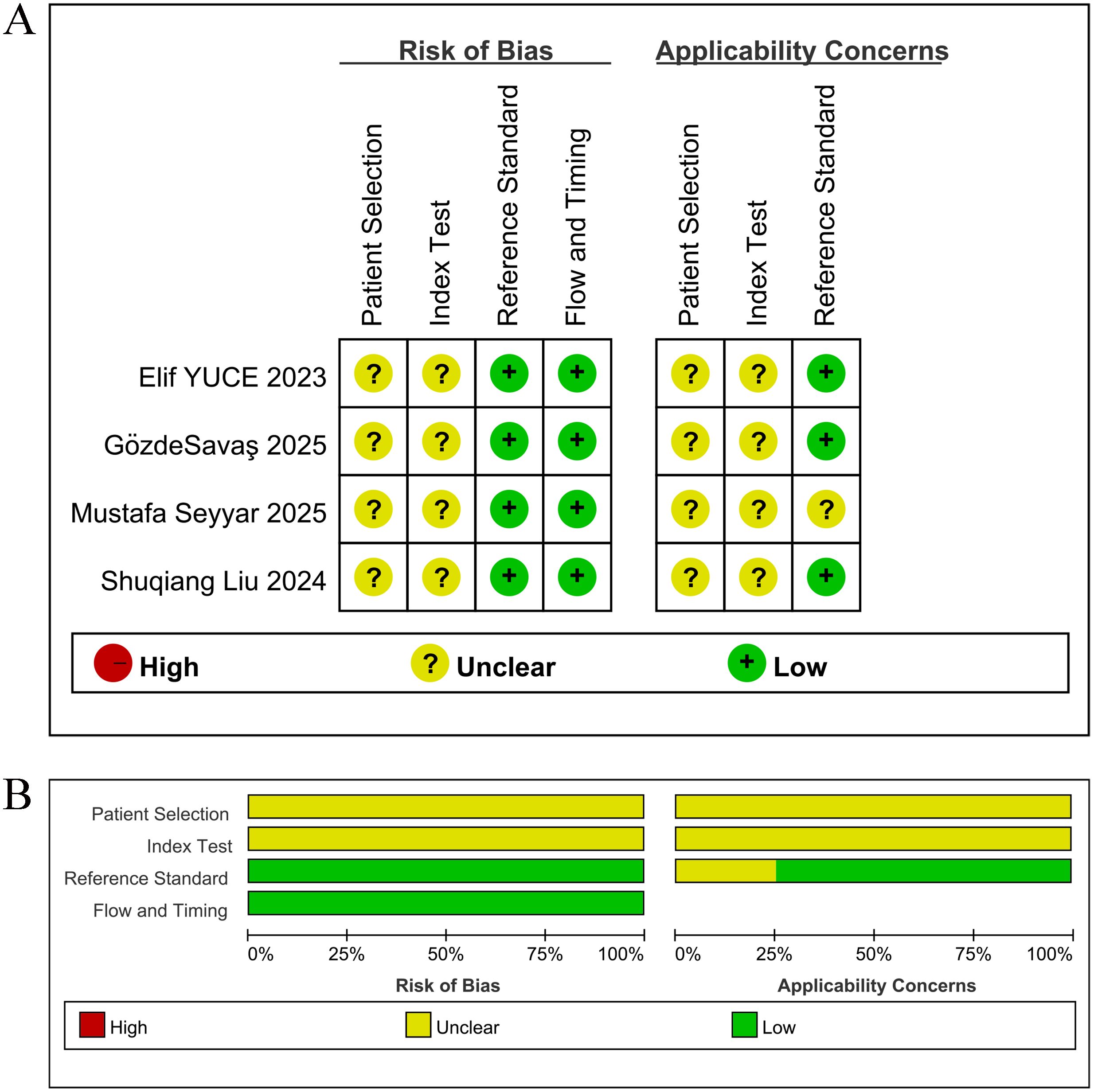
Figure 2. Risk of bias assessment, rated as low (+), medium ()? or high (-) risk per domain. (A) Risk of bias and applicability concerns for each included study; (B) Summary of the proportions of risk of bias and applicability concerns across all studies.
3.4 The link of the HALP score with survival outcomes in BC patients
Among the nine included studies, five (7, 19–22) reported OS as the primary outcome, and thus a meta-analysis of the link of the HALP score with OS was implemented utilizing data from the five studies. The results denoted that individuals with an increased HALP score before treatment had notably longer OS (HR = 0.61, 95% CI 0.39-0.97, p < 0.05), but marked heterogeneity was observed between the studies (I² = 63.2%, p > 0.05) (Figure 3). Therefore, a subgroup analysis was executed on the effect of the country of the study population on OS (Figure 4). The analysis indicated that different countries were not the source of heterogeneity. Two of the studies reported HR values for DFS (7, 21) (pooled I² =0.0%, p=0.374), and thus a fixed-effects model was leveraged (HR = 1.07, 95% CI 0.66-1.75, p>0.05). The findings indicated no statistical significance (Figure 3).
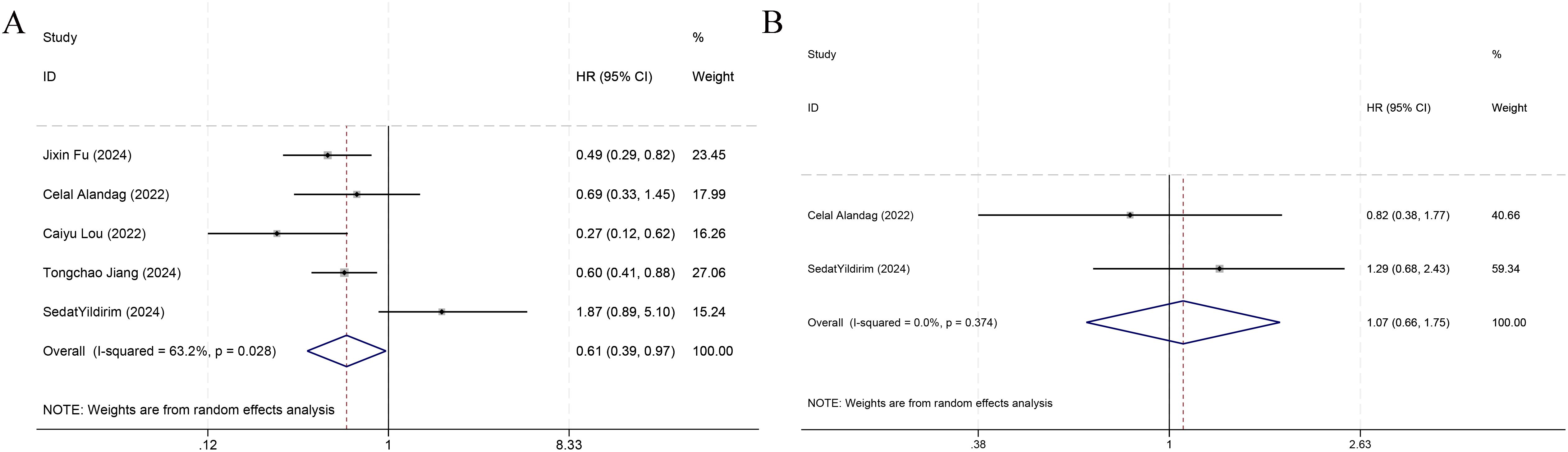
Figure 3. Forest plots demonstrating the link of HALP with overall survival (A) and disease-free survival (B).
The study of Jiang et al. (22) examined the prognostic utility of the HALP score for PFS. Via a multivariate Cox analysis, their study established that the HALP score was independently prognostic for PFS in BC patients (HR: 0.707, 95% CI 0.538-0.930, p=0.013). The study of Zhao et al. (24) examined the prognostic utility of the HALP score for recurrence-free survival (RFS) in 411 individuals with early invasive BC. The result denoted that reduced HALP scores demonstrated a significant correlation with poorer RFS (HR = 0.08, 95% CI 0.024-0.265, p < 0.0001).
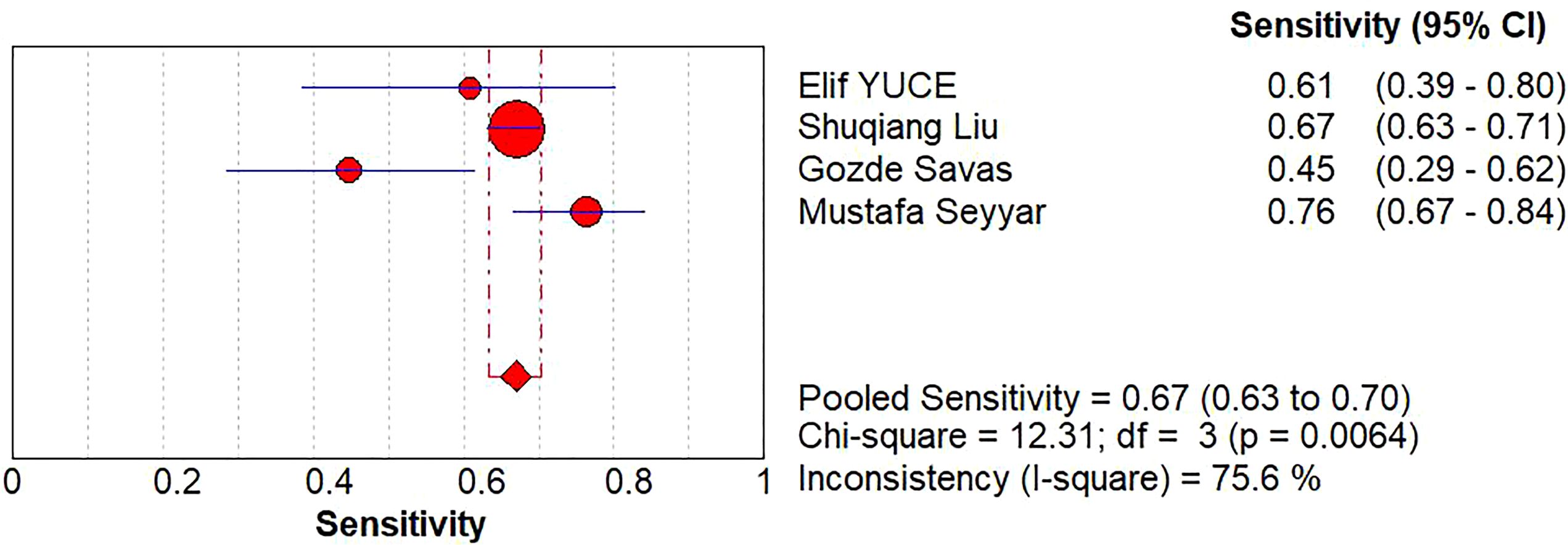
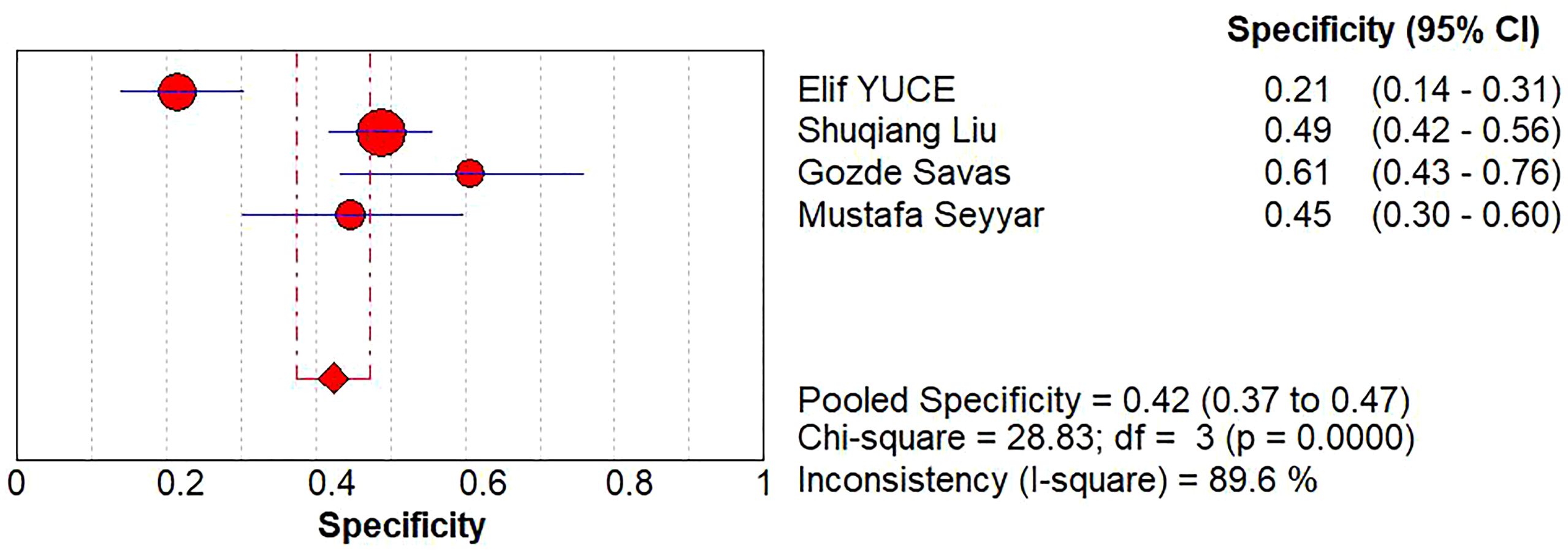
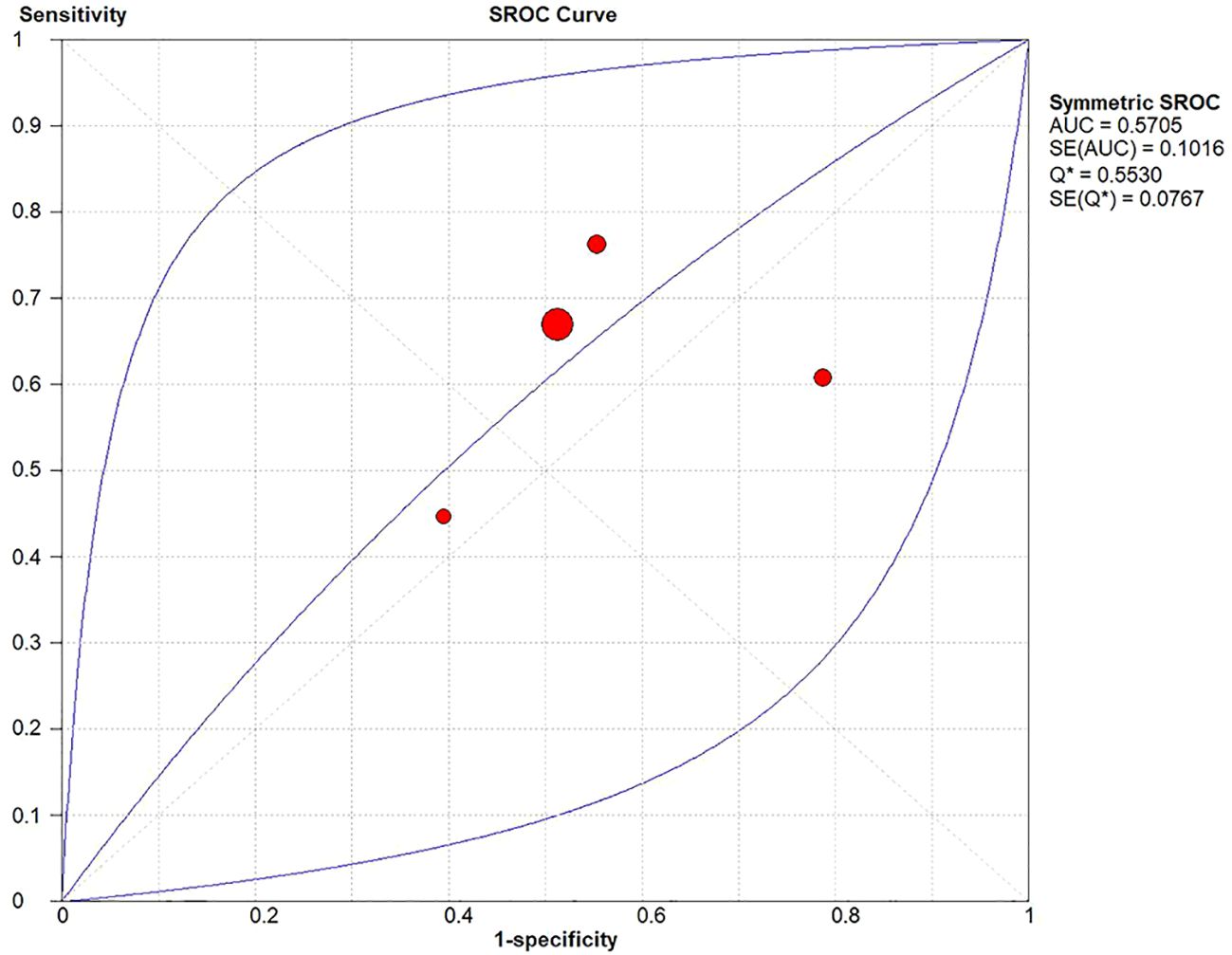
A diagnostic meta-analysis was executed on the four studies with pCR as the outcome (16–18, 23). The Spearman’s correlation coefficient was 0.4 (p=0.6>0.05), indicating no threshold effect, which allowed for a pooled analysis of sensitivity and specificity. The pooled sensitivity was 67% (95% CI: 63%-70%); specificity was 42% (95% CI: 37%-47%); +LR was 1.15 (95% CI: 0.90-1.47); -LR was 0.83 (95% CI: 0.56-1.22); and the diagnostic odds ratio (DOR) was 1.39 (95% CI: 0.73- 2.65). SROC curves were generated, and the AUC was 0.57. Forest plots of sensitivity and specificity are shown in Figures 5, 6, and SROC curves are illustrated in Figure 7.
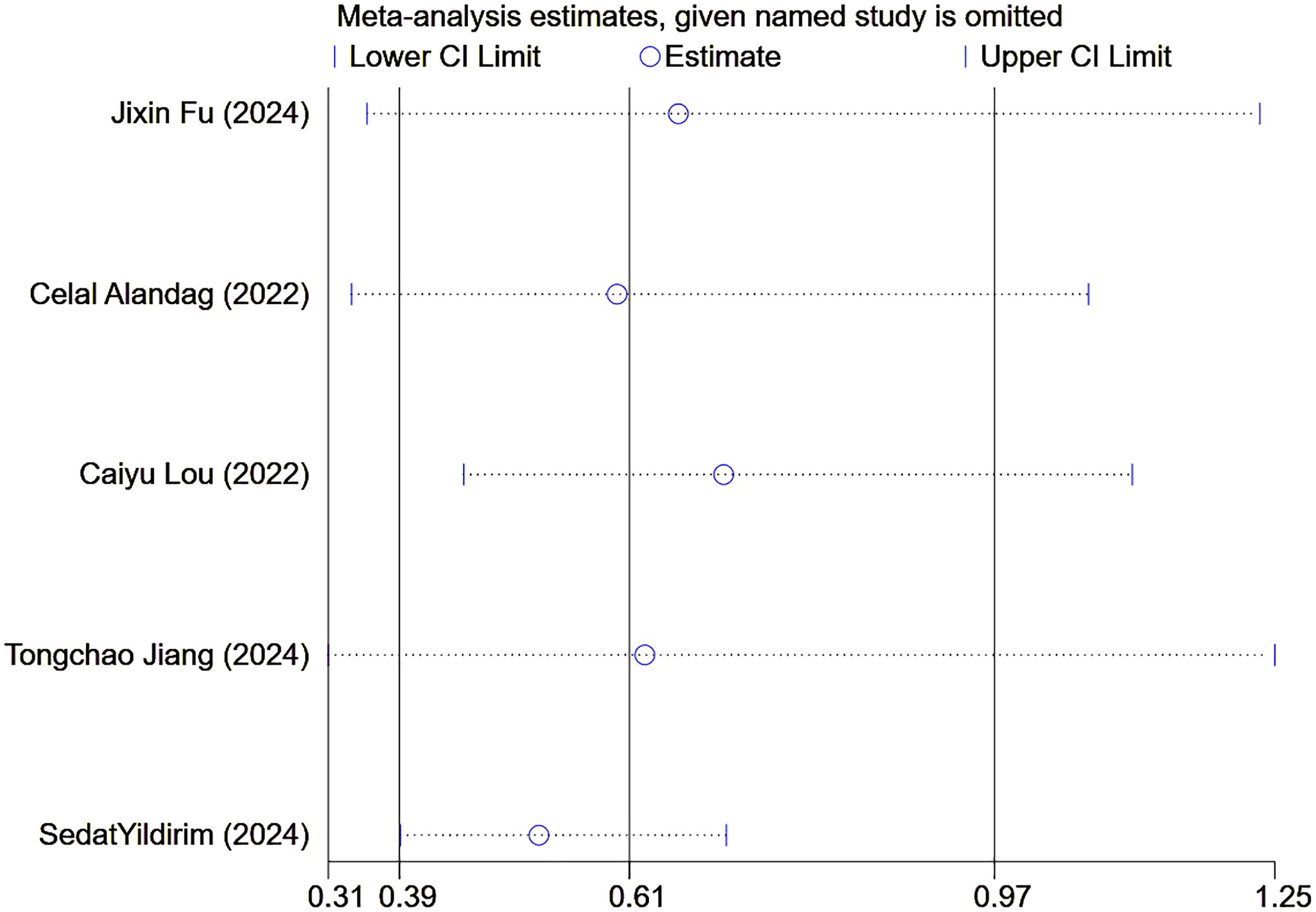
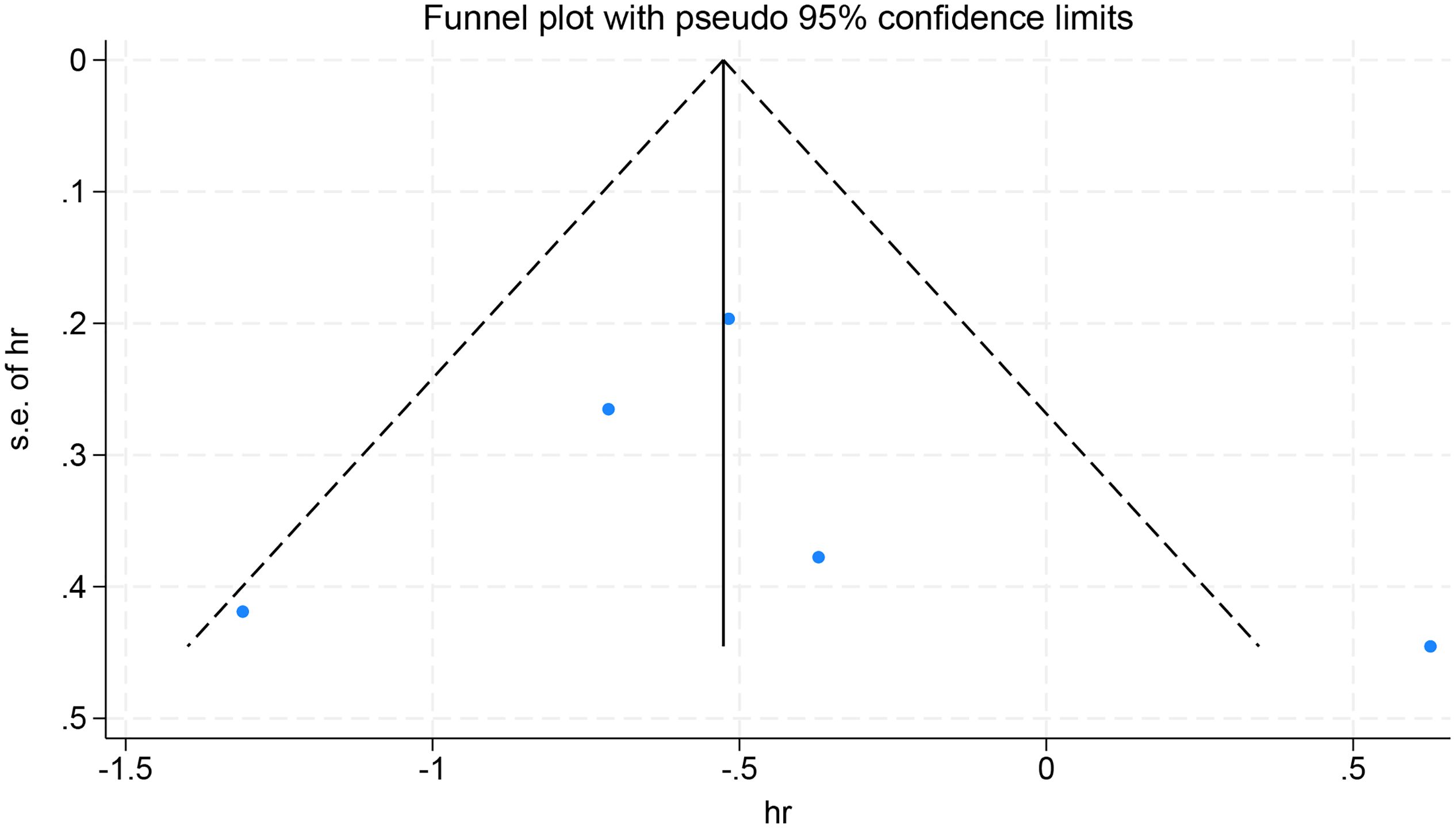
Finally, sensitivity analyses and publication bias analyses were performed on the five studies with OS as the observed outcomes. The result of the sensitivity analyses remained robust when excluding any one of the five studies (Figure 8). No significant publication was detected in the Egger’s test (p=0.385), with symmetrical scatter points in the funnel plot, as illustrated in Figure 9.
4 Discussion
Accurate prediction of survival outcomes in BC patients is critical for delivering personalized therapy, and thus, investigating novel biomarkers to predict survival outcomes becomes imperative. Multiple contemporary studies have shown that a high HALP score is correlated with favorable survival outcomes in cancer patients. However, knowledge gaps persist in the prognostic utility of the HALP score in BC patients. Therefore, we performed a meta-analysis, including nine studies and 4560 BC patients, which preliminarily aimed to examine the prognostic and diagnostic performance of the HALP score in BC. We found that an elevated HALP score predicted improved OS in BC patients (p < 0.05), but lacked predictive value for DFS and demonstrated insufficient predictive power for pCR (AUC = 0.57).
In BC patients, the present study yielded consistent results for predicting OS, PFS, and RFS with previous studies (6, 25) for OS, PFS, and RFS, but found that the HALP score had no predictive value (p > 0.05) for DFS, which differed from prior findings (6, 25). Possible reasons are listed as follows: On the one hand, it may be explained by the paucity of studies on DFS (n=2), and the pooled effect estimates may be more susceptible to random variations or extreme values, reducing the stability of the results. On the other hand, the difference in results may also stem from the heterogeneity between studies, including different molecular types and treatment regimens. Even though the results were inconsistent with some studies, owing to the limited number of included reports, it is infeasible to adequately analyze these factors. This suggests that standardized, large-scale studies need to be conducted in the future to corroborate the prognostic capacity of the HALP score for DFS outcomes in BC. The diagnostic accuracy in the four studies with pCR as the outcome is limited. First, it may be owing to the disparate molecular subtypes of BC in the studies included. Studies have reported that HER2-positive (HR-negative) BC exhibits the highest rates of breast pCR (bpCR) and nodal pCR (npCR), and HER2-negative (HR-positive) BC exhibits the lowest rates of bpCR (26). Secondly, different definitions of pCR can also influence the results. Gözde Savaş et al. defined pCR as no evidence of invasive carcinoma and ductal carcinoma in situ (11), which is slightly different from the definitions of pCR in other studies. Finally, the different treatment regimens included in the studies can likewise affect the clinical and pathologic response and thus influence our results.
Hemoglobin, as a key molecule for oxygen transport, directly affects the blood’s oxygen-carrying capacity. When hemoglobin levels decrease, insufficient tissue oxygen supply can induce hypoxia in the tumor microenvironment, thereby activating hypoxia-inducible factors (such as HIF-1α), enhancing tumor invasion and metastasis, promoting angiogenesis, and ultimately driving malignant progression of BC and affecting patient prognosis (27). In addition, hemoglobin concentration also reflects the patient’s nutritional status, and its level is often used to define the degree of anemia in clinical practice. The trajectory of hemoglobin levels in the early stages of BC treatment is closely related to survival outcomes, while the mortality rate of the persistent anemia group is significantly higher than that of the normal hemoglobin group in multivariate analysis (28). It is worth noting that excessive secretion of TGF-β in the tumor microenvironment can inhibit erythrocyte production through organ-specific mechanisms, thereby leading to anemia (29). Multiple studies have confirmed that low hemoglobin levels before or during treatment are important predictors of poor disease control and reduced survival. Liang et al. (30) further demonstrate that in BC patients, those with low hemoglobin levels had a significantly increased risk of tumor metastasis.
Serum albumin is another important indicator of nutritional status, and a decrease in serum albumin level may indicate malnutrition or even cachexia. Albumin is able to reduce pro-inflammatory fatty acids, combat oxidation, and maintain plasma osmotic pressure. Therefore, higher albumin levels may improve patient survival by inhibiting tumor progression (31). Basic research has further shown that albumin can regulate the activity of autocrine growth factors, thereby affecting the proliferation of MCF-7 BC cells (32). Clinical data also confirm that low serum albumin levels are negatively correlated with the survival rate of patients with BC at all stages (HR = 3.53; P = 0.0033) (33).
In terms of immune regulation, lymphocytes, as a core immune component, induce tumor cell apoptosis through cytotoxicity. Reduced lymphocyte levels weaken immune surveillance and promote tumor progression (34). For example, in TNBC, for every 10% increase in TILs, patient survival increases by 10% (35). Therefore, lymphocyte-related inflammatory markers can provide important clues for the diagnosis, treatment, and prognostic assessment of BC (36).
Meanwhile, platelets play a complex role in tumor progression. Cancer patients often have a hypercoagulable state, which increases the risk of thromboembolism. More importantly, platelets directly participate in the regulation of tumor angiogenesis and metastasis by secreting a variety of chemokines and growth factors (such as VEGF and PDGF) (37–39).
Based on the above mechanisms, the HALP score, as an immunonutritional marker that comprehensively reflects a patient’s nutritional status, degree of anemia, and immune function, is closely related to the prognosis of BC (40). This score, by integrating multiple key indicators, provides an important basis for prognostic prediction, especially for BC patients generally with a high risk of malnutrition, and for older patients with a higher incidence of anemia, which has important clinical value (41, 42). There is a complex interaction between hemoglobin concentration and the tumor hypoxic microenvironment. On the one hand, tumor-induced oxygenation impairment can cause changes in blood rheology and increase blood viscosity. On the other hand, the decrease in hemoglobin caused by anemia may further aggravate tumor hypoxia, forming a vicious cycle (27, 43).
The HALP score represents a cost-efficient biomarker, which is dynamic and detectable. It can be repeatedly measured during treatment and rehabilitation to dynamically monitor changes in the patient’s physical condition. By integrating indicators of blood cell counts and albumin levels from routine blood tests, it can efficiently pinpoint BC patients with nutritional and immunological damage. This scoring system provides clinicians with an objective basis for assessment and helps to identify high-risk patients at an early stage. Thus, it assumes a critical role in optimizing the quality of life and extending survival duration for patients. Nevertheless, our findings revealed limited diagnostic validity of the score when using pCR as the outcome measure. Therefore, combined analysis with other biomarkers, such as NLR, should be performed in the future. The purpose is to improve the predictive performance for patient survival and prognosis and thus optimize clinical decision-making and individualized treatment.
There are several significant limitations of this study that warrant attention. First, with nine studies included, the sample size of the present research was relatively limited. This may reduce the statistical power of the current research and compromise the external validity of the findings. Second, all studies enrolled were retrospective in design. Despite the clinical feasibility of this approach, there is an unavoidable risk of selection bias and information bias, which makes it difficult to establish an exact causal relationship. Therefore, subsequent prospective multicenter studies need to be conducted for verification. In terms of population representativeness, the existing studies mainly focused on Asian populations (Turkish and Chinese patients) and did not examine populations in other regions, such as Europe and Africa. This limits the applicability of the findings on a global scale. Moreover, significant confounding bias may result from the disparities among studies in key variables such as treatment regimens, molecular types of BC (e.g., Luminal A, Luminal B, HER2-positive, TNBC) and HALP score cutoffs. This may trigger overestimation or underestimation of the effect sizes and increase inter-study heterogeneity. Notably, though the publication bias test showed no statistical significance, the limited number of included studies (n=5) may result in insufficient statistical power to fully exclude potential publication bias. Therefore, we recommend cautiously interpreting the pooled HR for OS. Future studies should focus on addressing these methodological limitations to offer a more reliable foundation for evidence-based medicine.
The present research validates that the HALP score demonstrates prognostic significance in BC, though its predictive utility for specific molecular subgroups remains unelucidated. Therefore, multicenter, large-sample, prospective studies need to be carried out in the future to substantiate our findings. The cutoff values for the HALP score varied across studies. Hence, they were standardized, and confounding bias was reduced to make the results more reliable. Current studies related to the HALP score are all retrospective in design and only obtain static data from patients at a single time point. Future studies should establish a dynamic monitoring system to achieve continuous tracking and evaluation of patients’ conditions. This will help reflect the clinical changes in patients more comprehensively and accurately.
5 Conclusion
The HALP score can efficiently forecast the OS of individuals with BC and has important value for prognostic assessment. Future prospective studies are required to validate this finding. Standardized assessment protocols are also needed to pinpoint the applicability of the HALP score in different subgroups of BC patients. Additionally, as HALP is an easily accessible prognostic indicator, the integration of HALP into existing risk prediction models may further enhance the predictive efficacy and is worth exploring in depth.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JC: Conceptualization, Writing – original draft, Writing – review & editing. BW: Methodology, Writing – review & editing. NJ: Formal Analysis, Investigation, Writing – review & editing. YZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1684940/full#supplementary-material
References
1. Xiong X, Zheng LW, Ding Y, Chen YF, Cai YW, Wang LP, et al. Breast cancer: pathogenesis and treatments. Signal Transduct Target Ther. (2025) 10:49. doi: 10.1038/s41392-024-02108-4
2. Zhang X. Molecular classification of breast cancer: relevance and challenges. Arch Pathol Lab Med. (2023) 147:46–51. doi: 10.5858/arpa.2022-0070-RA
3. Ethier JL, Desautels D, Templeton A, Shah PS, and Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. (2017) 19:2. doi: 10.1186/s13058-016-0794-1
4. Peng P, Chen L, Shen Q, Xu Z, and Ding X. Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) score for predicting outcomes of breast cancer: A systematic review and meta-analysis. Pak J Med Sci. (2023) 39:1535–41. doi: 10.12669/pjms.39.5.7781
5. Chen XL, Xue L, Wang W, Chen HN, Zhang WH, Liu K, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. (2015) 6:41370–82. doi: 10.18632/oncotarget.5629
6. Xu H, Zheng X, Ai J, and Yang L. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: A systematic review and meta-analysis of 13,110 patients. Int Immunopharmacol. (2023) 114:109496. doi: 10.1016/j.intimp.2022.109496
7. Yildirim S, Dogan A, Akdag G, Yüksel Yasar Z, Bal H, Kinikoglu O, et al. The role of laboratory indices on treatment response and survival in breast cancer receiving neoadjuvant chemotherapy. Sci Rep. (2024) 14:12123. doi: 10.1038/s41598-024-63096-7
8. Hojilla CV, Wood GA, and Khokha R. Inflammation and breast cancer: metalloproteinases as common effectors of inflammation and extracellular matrix breakdown in breast cancer. Breast Cancer Res. (2008) 10:205. doi: 10.1186/bcr1980
9. Fernandes Q, Inchakalody VP, Bedhiafi T, Mestiri S, Taib N, Uddin S, et al. Chronic inflammation and cancer; the two sides of a coin. Life Sci. (2024) 338:122390. doi: 10.1016/j.lfs.2023.122390
10. Di Giosia P, Stamerra CA, Giorgini P, Jamialahamdi T, Butler AE, and Sahebkar A. The role of nutrition in inflammaging. Ageing Res Rev. (2022) 77:101596. doi: 10.1016/j.arr.2022.101596
11. Yamanouchi K and Maeda S. The efficacy of inflammatory and immune markers for predicting the prognosis of patients with stage IV breast cancer. Acta Med Okayama. (2023) 77:37–43. doi: 10.18926/amo/64360
12. Li X, Yang J, Peng L, Sahin AA, Huo L, Ward KC, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. (2017) 161:279–87. doi: 10.1007/s10549-016-4059-6
13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
14. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Symposium Syst Reviews: Beyond Basics. (2014).
15. Lee J, Mulder F, Leeflang M, Wolff R, Whiting P, and Bossuyt PM. QUAPAS: an adaptation of the QUADAS-2 tool to assess prognostic accuracy studies. Ann Intern Med. (2022) 175:1010–8. doi: 10.7326/m22-0276
16. Savaş G, Günel N, and Özet A. Can pretreatment lactate dehydrogenase to albumin ratio predict pathological complete response after neoadjuvant chemotherapy in breast cancer patients? J Med Biochem. (2025) 44:339–46. doi: 10.5937/jomb0-43900
17. Liu S, Jiang C, Wu D, Zhang S, Qiao K, Yang X, et al. Development of predictive models for pathological response status in breast cancer after neoadjuvant therapy based on peripheral blood inflammatory indexes. BMC Womens Health. (2024) 24:560. doi: 10.1186/s12905-024-03400-9
18. Yuce E, Karakullukcu S, Bulbul H, Alandag C, Saygin I, and Kavgaci H. The effect of the change in hemoglobin-albumin-lymphocyte-platelet scores occurring with neoadjuvant chemotherapy on clinical and pathological responses in breast cancer. Bratisl Lek Listy. (2023) 124:59–63. doi: 10.4149/bll_2023_009
19. Fu J, Yue X, Zou Y, Zhang J, Wang X, and Zhang D. Association of hemoglobin, albumin, lymphocyte, and platelet score with risk of all-cause and cause-specific mortality among cancer survivors: NHANES 1999-2018. Front Oncol. (2024) 14:1402217. doi: 10.3389/fonc.2024.1402217
20. Lou C, Jin F, Zhao Q, and Qi H. Correlation of serum NLR, PLR and HALP with efficacy of neoadjuvant chemotherapy and prognosis of triple-negative breast cancer. Am J Transl Res. (2022) 14:3240–6.
21. Alandag C, Yilmaz M, Ucar M, Demir N, Erdis E, and Yucel B. Prognostic significance of HALP score in early stage triple-negative breast cancer. EJMI (2022) 6:409–16. doi: 10.14744/ejmi.2022.74639
22. Jiang T, Sun H, Xue S, Xu T, Xia W, Wang Y, et al. Prognostic significance of hemoglobin, albumin, lymphocyte, and platelet (HALP) score in breast cancer: a propensity score-matching study. Cancer Cell Int. (2024) 24:230. doi: 10.1186/s12935-024-03419-w
23. Seyyar M, Şancı PC, Köşeci T, Karakayalı A, Akdağ M, Temi YB, et al. HALP-H index as a prognostic biomarker for predicting pathological complete response in early-stage HER2-positive breast cancer-A multicenter retrospective cohort study. J Clin Med. (2025) 14:4431–4445. doi: 10.3390/jcm14134431
24. Zhao Z and Xu L. Prognostic significance of HALP score and combination of peripheral blood multiple indicators in patients with early breast cancer. Front Oncol. (2023) 13:1253895. doi: 10.3389/fonc.2023.1253895
25. Li J, Zheng J, Wang P, and Lv D. Prognostic significance of hemoglobin, albumin, lymphocyte and platelet score in solid tumors: a pooled study. Front Immunol. (2024) 15:1483855. doi: 10.3389/fimmu.2024.1483855
26. Chen SC, Yu CC, Chang HK, Lin YC, Lo YF, Shen SC, et al. Discrepancy of breast and axillary pathologic complete response and outcomes in different subtypes of node-positive breast cancer after neoadjuvant chemotherapy. J Cancer. (2021) 12:5365–74. doi: 10.7150/jca.62830
27. Chen X, Zhou H, and Lv J. The importance of hypoxia-related to hemoglobin concentration in breast cancer. Cell Biochem Biophys. (2024) 82:1893–906. doi: 10.1007/s12013-024-01386-7
28. Lee CL, Tsai CH, Yeh DC, Lin CS, Li YF, and Tzeng HE. Hemoglobin level trajectories in the early treatment period are related with survival outcomes in patients with breast cancer. Oncotarget. (2017) 8:1569–79. doi: 10.18632/oncotarget.13679
29. Yuan T, Jia Q, Zhu B, Chen D, and Long H. Synergistic immunotherapy targeting cancer-associated anemia: prospects of a combination strategy. Cell Commun Signal. (2023) 21:117. doi: 10.1186/s12964-023-01145-w
30. Liang RB, Yu K, Wu JL, Liu JX, Lin Q, Li B, et al. Risk factors and their diagnostic values for ocular metastases in invasive ductal carcinoma. Cancer Med. (2021) 10:824–32. doi: 10.1002/cam4.3656
31. Tang Q, Li X, and Sun CR. Predictive value of serum albumin levels on cancer survival: a prospective cohort study. Front Oncol. (2024) 14:1323192. doi: 10.3389/fonc.2024.1323192
32. Laursen I, Briand P, and Lykkesfeldt AE. Serum albumin as a modulator on growth of the human breast cancer cell line, MCF-7. Anticancer Res. (1990) 10:343–51.
33. Lis CG, Grutsch JF, Vashi PG, and Lammersfeld CA. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr. (2003) 27:10–5. doi: 10.1177/014860710302700110
34. Dunn GP, Old LJ, and Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. (2004) 21:137–48. doi: 10.1016/j.immuni.2004.07.017
35. Stanton SE and Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. (2016) 4:59. doi: 10.1186/s40425-016-0165-6
36. Qi X, Chen J, Wei S, Ni J, Song L, Jin C, et al. Prognostic significance of platelet-to-lymphocyte ratio (PLR) in patients with breast cancer treated with neoadjuvant chemotherapy: a meta-analysis. BMJ Open. (2023) 13:e074874. doi: 10.1136/bmjopen-2023-074874
37. Suzuki-Inoue K. Platelets and cancer-associated thrombosis: focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood. (2019) 134:1912–8. doi: 10.1182/blood.2019001388
38. Franco AT, Corken A, and Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. (2015) 126:582–8. doi: 10.1182/blood-2014-08-531582
39. Lazar S and Goldfinger LE. Platelets and extracellular vesicles and their cross talk with cancer. Blood. (2021) 137:3192–200. doi: 10.1182/blood.2019004119
40. Vlatka P, Marko L, Stefan M, and Dorian L. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a novel prognostic factor for patients with diffuse large B-cell lymphoma. J Cancer Res Ther. (2022) 18:725–32. doi: 10.4103/jcrt.jcrt_174_21
41. Keaver L, O’Callaghan N, O’Sullivan A, Quinn L, Loftus A, and McHugh CM. Female cancer survivors are more likely to be at high risk of malnutrition and meet the threshold for clinical importance for a number of quality of life subscales. J Hum Nutr Diet. (2021) 34:868–80. doi: 10.1111/jhn.12877
42. Madeddu C, Neri M, Sanna E, Oppi S, and Macciò A. Experimental drugs for chemotherapy- and cancer-related anemia. J Exp Pharmacol. (2021) 13:593–611. doi: 10.2147/jep.S262349
Keywords: breast cancer, HALP score, meta-analysis, systematic review, prognosis
Citation: Cai J, Wang B, Jing N and Zhang Y (2025) Prognostic value of the HALP score in breast cancer: a systematic review and meta-analysis. Front. Oncol. 15:1684940. doi: 10.3389/fonc.2025.1684940
Received: 13 August 2025; Accepted: 19 November 2025; Revised: 10 November 2025;
Published: 02 December 2025.
Edited by:
Durmuş Ayan, Niğde Ömer Halisdemir University, TürkiyeCopyright © 2025 Cai, Wang, Jing and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Zhang, emhhbmd5dWVqbGNjQDE2My5jb20=
 Jia Cai
Jia Cai Bo Wang
Bo Wang Niancai Jing2
Niancai Jing2