- 1Department of Gynecology, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Department of Pathology, The Affiliated Hospital of Qingdao University, Qingdao, China
Objective: The purpose of this study was to identify the clinical features of endometrial cancers (EC) with microsatellite instability-high (MSI-H) and BRCA1/2 mutations (BRCAm).
Methods: We selected patients diagnosed with EC who were MSI-H from the Affiliated Hospital of Qingdao University between March 2021 and December 2024. The clinical information was collected after applying the inclusion and exclusion criteria. Based on BRCA1/2 status, we divided the patients into two groups: the MSI-H only group (M group), and the MSI-H and BRCAm group (MB group). After comparing the clinical characteristics of the two groups, Kaplan-Meier (K-M) curves were employed to analyze the distribution of progression-free survival (PFS) between them. Finally, both univariate and multivariate Cox proportional hazards regression models were utilized to assess various prognostic variables.
Results: A total of 177 patients were included in the final analysis, of whom 28 were identified as having BRCAm. The rate of laparoscopic surgery in M group was higher than that in MB group (87.25% vs. 67.86%, p-value=0.022). The lymph node metastasis rate in MB group was significantly higher than that in M group (14.29% vs. 2.01%, p-value=0.011). Additionally, a higher proportion of patients received immunotherapy in MB group (7.14% vs. 2.68%, p-value=0.011). The recurrence rates in M group and MB group were 1.34% and 17.86%, respectively (p-value=0.003). K-M analysis indicated that there was statistically significant difference in PFS between the two groups (p-value=0.006). Furthermore, we did not identify any independent risk factors that influenced prognosis in our study.
Conclusion: The co-occurrence of BRCAm and MSI-H is associated with high rates of lymph node metastasis and recurrence. However, BRCAm was not an independent prognostic factor influencing PFS of EC with MSI-H.
1 Introduction
Endometrial cancer (EC) is the most prevalent gynecologic malignancy, with a significant rise in both incidence and mortality rates (1). Since the recent integration of genomic characterization into daily clinical practice by The Cancer Genome Atlas (TCGA), EC is classified into four categories (1): polymerase epsilon (POLE) ultramutated, characterized by POLE mutations (2); microsatellite instability hypermutated (MSI-H), identified by mismatch repair deficiency (MMRd) and high microsatellite instability (3); copy-number low (CN-L), which is defined by a lack of a specific molecular profile (NSMP); and (4) copy-number high (CN-H), primarily characterized by TP53 mutations and serous-like EC (2, 3). MSI-H tumors exhibit a hypermutated phenotype and a high neoantigen load, making them particularly sensitive to immune checkpoint blockade (ICB) (4). Across various solid tumors, MSI-H status has emerged as a predictive biomarker for robust responses to PD-1/PD-L1 inhibitors (5). Recent phase II studies of neoadjuvant PD-1 blockade with dostarlimab in patients with MSI-H locally advanced rectal cancer have reported clinical complete responses in all treated individuals, allowing the omission of chemoradiotherapy and surgery in many cases and fundamentally challenging the traditional multimodal treatment paradigm (6). EC has the highest rate of MSI-H among all tumor types, with MSI-H present in 17% to 40% of EC cases (7, 8). Multiple clinical trials and real-world studies have demonstrated substantial and durable clinical benefits of ICB in patients with advanced, recurrent, or metastatic MSI-H EC (9, 10). These agents are now incorporated into major international guidelines as a standard systemic treatment option for this molecular subgroup. Therefore, MSI-H EC represents a biologically distinct and therapeutically actionable entity of significant clinical relevance. The primary cause of MSI-H is a defect in the DNA MMR genes responsible for correcting mismatched bases (7). Microsatellites are DNA segments composed of repeated sequences, typically ranging from one to six nucleotides in length (11). In cells with MMRd, these segments are susceptible to errors, leading to instability in their length and resulting in MSI-H (12). Particularly, hypermethylation of the mutL homolog 1 (MLH1) promoter (MLH1ph, 70%-75%), along with somatic mutations (15%-20%) and germline mutations (5%-10%) in MLH1, MutS homolog 2 (MSH2), MSH6, PMS1 homolog 2 (PMS2), and/or epithelial cell adhesion molecule (EPCAM), is associated with an accumulation of DNA replication errors in microsatellite regions (13, 14). Traditionally, MSI-H can be detected through immunohistochemistry (IHC) for MMR proteins absence, including MSH2, MSH6, MLH1, and PMS2, as well as through polymerase chain reaction (PCR)-based assays (11).
MSI-H EC typically exhibits a high tumor mutation burden (TMB-H), which is likely to predict a greater prevalence of homologous recombination (HR) gene variations (15, 16). HR is a multistep DNA repair process that addresses DNA double-stranded breaks (DSBs) (17). The BRCA1 and BRCA2 genes are the most altered HR genes and play crucial roles in the homologous recombination repair (HRR) pathway (18). Impaired function of the BRCA1/2 can result in a deficiency in the HRR pathway, referred to as homologous recombination deficiency (HRD) (19). Previous reports have identified a potential co-occurrence of BRCA1/2 mutations (BRCAm), which are targets for poly (ADP-ribose) polymerase inhibitors (PARPi), and MSI-H, a biomarker linked to responses to ICB (20–22). However, Sokol et al. found that since MSI-H tumors often contain thousands of mutations throughout the exome, some of these BRCAm may represent monoallelic bystander events that leave the second copy of BRCA intact, which does not lead to sensitivity to PARPi (22).
Emerging data indicate considerable heterogeneity within each molecular subgroup of EC. For example, within the NSMP category, specific patterns such as estrogen receptor (ER)-negative NSMP tumors have been associated with significantly worse outcomes, underscoring the need for further prognostic refinement within molecular classes (23). During routine review of molecular profiling reports at our institution, we observed that a subset of MSI-H EC harbored concomitant BRCAm. Given that MB are established biomarkers for responsiveness to ICB and PARPi, respectively, we hypothesized that MSI-H EC with co-occurring BRCAm might represent a distinct clinicopathologic subset with different treatment patterns and prognoses compared to MSI-H tumors without BRCAm. Therefore, we specifically focused on MSI-H EC and conducted an exploratory analysis of patients with or without BRCAm to generate preliminary data that may help refine prognostic assessment within this clinically important molecular subtype.
2 Materials and methods
This study was approved by the ethics committee of the Affiliated Hospital of Qingdao University (AHQU; approval number: QYFYWZLL 29592). Informed consent was acquired from the patients before the study started. All procedures performed in studies involving human participants were conducted in compliance with the ethical standards of the institutional and/or national research committee and in accordance with the 1964 Helsinki declaration and its subsequent amendments or equivalent ethical standards.
2.1 Study population selection
This retrospective study included patients diagnosed with EC who were MSI-H from AHQU between March 2021 and December 2024.
We provided information regarding genetic testing at AHQU below. (1) Genetic testing approach: Next-generation sequencing (NGS). (2) Reference genome: GRCh37/hg19. (3) Clinical database: 1000 Genomes, ExAC, gnomAD, ClinVar, BRCA Exchange InSiGHT, DBSNP, Cancer Hotspots, and COSMIC. (4) Mutation detection software: ADXHS-tBRCA-5p/ADXHS-gBRCA-CNV/ADXHS-tBPTM/ADXHS-gHRR-EN/ADXHS-tHRR-EN.
Patients were included in the final analysis if they met all of the following criteria: (1) diagnosis of EC confirmed by postoperative histopathology; (2) MSI-H status confirmed on NGS-based genetic testing and BRCAm status available; (3) initial treatment and primary surgical management for EC performed at AHQU; and (4) complete clinicopathologic, treatment, and follow-up data. Exclusion criteria were: (1) incomplete key clinical, pathological, treatment, or follow-up data; (2) patients who did not receive initial or surgical treatment for EC at AHQU; and (3) patients who were lost to follow-up.
2.2 Clinical characteristics
We divided the patients into two groups: the MSI-H only (M) and the MSI-H and BRCAm (MB) groups, based on their BRCA1/2 status. The following variables were selected: age of diagnosis; body mass index (BMI); number of pregnancies; age at menopause; serum cancer antigen 125 (CA125) level before initial treatment; serum carbohydrate antigen 199 (CA199) level before initial treatment; serum carcinoembryonic antigen (CEA) level before initial treatment; surgical modality; perform pelvic lymph node dissection or not; postoperative pathology showed whether lymph node metastasis or not; histological type; histological grade; the International Federation of Gynecology and Obstetrics (FIGO) stage; postoperative immunohistochemical expressions of MMR proteins, ER, and progesterone receptor (PR); preoperative treatment; postoperative adjuvant treatment.
At our institution, pre-treatment serum CA125, CA199, and CEA are routinely measured in patients with suspected gynecologic malignancies as part of the standard preoperative evaluation. The purposes are to screen for possible concomitant gastrointestinal or ovarian malignancies when markedly elevated values are observed and to document baseline levels for subsequent surveillance in those patients with abnormal markers. In the present study, these tumor markers were recorded only as exploratory clinicopathologic variables and were not used to determine patient eligibility or to guide treatment decisions.
All patients were followed up every 2–4 months in the first 2 years after completing primary treatment. The follow-up interval was then extended to every 6–12 months in the subsequent 3 years. The follow-up period ended in October 2025. The monitoring protocol included a clinical and gynecologic examination at each visit; measurement of serum CA125, CA199, and CEA levels only in patients whose corresponding markers were elevated at baseline; and imaging studies when indicated by symptoms, physical findings, or abnormal tumor marker levels. Recurrent EC is defined as the recurrence of EC at any site after a patient has received initial treatment and has achieved a PFS of at least six months.
Overall survival (OS) and progression-free survival (PFS) were the primary study end points. OS was calculated from the date of diagnosis to the date of death from any cause, while PFS was calculated as the period from diagnosis to disease recurrence or progression.
2.3 Statistical analysis
The results were presented as means ± standard deviation (SD) for normally distributed variables, medians (25th percentile, 75th percentile) for non-normally distributed variables, numbers (percentages) for categorical variables. Univariate differences were analyzed using independent t-tests for normally distributed variables, Mann-Whitney U tests for non-normally distributed variables, and chi-square tests for categorical variables. Kaplan-Meier (K-M) curves were used to analyze the distribution of PFS across various groups. Furthermore, the log-rank test was applied to compare the differences between the curves. To evaluate various prognostic variables, univariate and multivariate Cox proportional hazards regression models were utilized to calculate hazard ratios (HR) and 95% confidence intervals (CI). To evaluate prognostic factors for PFS, we first performed univariate Cox proportional hazards regression analyses. Variables with a p-value < 0.05 in the univariate analysis were then included in the multivariable Cox model.
All statistical analyses were performed with SPSS software (version 29.0; IBM Corp., USA). A probability (p)-value of <0.05 was considered statistically significant.
3 Results
A total of 886 patients diagnosed with EC who had genetic testing results were identified at AHQU between March 2021 and December 2024. Among these, 215 patients were classified as MSI-H. Subsequently, 27 patients were excluded due to incomplete clinical data, 9 patients did not receive initial or surgical treatment at AHQU, and 2 patients were lost to follow-up. Ultimately, 177 patients were included in the final analysis (Figure 1).
3.1 Characteristics of the patients
Table 1 showed the baseline characteristics of the selected patients. There were M and MB groups at 149 and 28 cases, respectively. In M group, 87.25% of patients underwent laparoscopic surgery, while 12.75% underwent open abdominal surgery. In MB group, 32.14% of patients underwent laparoscopic surgery, and 67.86% underwent open abdominal surgery. The difference between the two groups in the surgical modality was statistically significant (p-value=0.022). What’s more, the lymph node metastasis rate in MB group was significantly higher than that in M group (14.29% vs. 2.01%, p-value=0.011). In terms of postoperative adjuvant treatment, a larger number of patients in MB group received immunotherapy compared to those in M group. (7.14% (2/28) vs. 2.68% (4/149), p-value=0.011). Significantly, one patient underwent PARPi maintenance treatment following the completion of immunotherapy in our study. Additionally, the recurrence rate in MB group was 17.86%, while the recurrence rate in M group was 1.34%. This difference was statistically significant (p-value=0.003). No other significant differences were observed.
3.2 Details of BRCAm
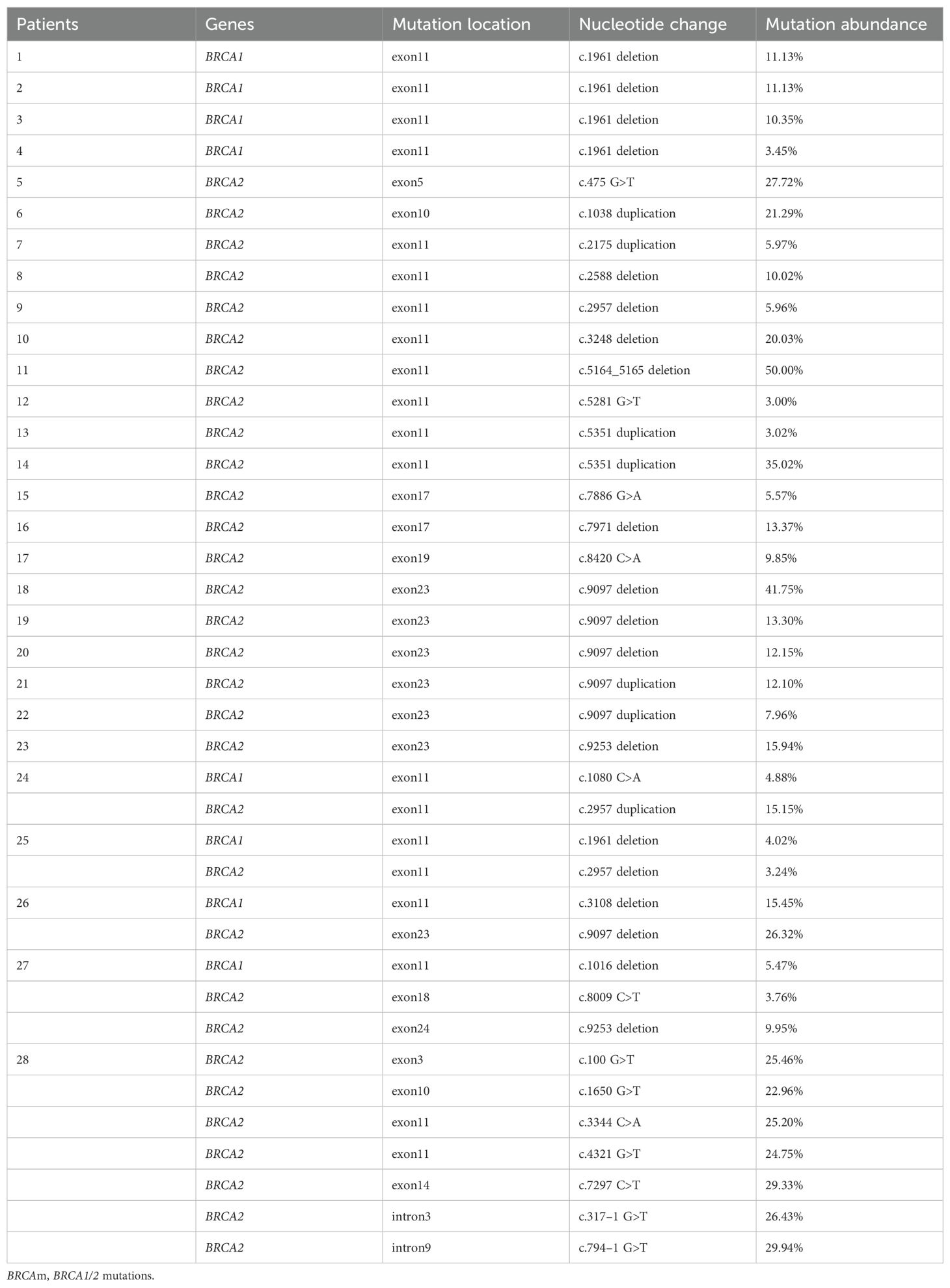
Table 2 showed the detail of BRCAm sites in this study. Four patients had a BRCA1 mutation, 19 patients had a BRCA2 mutation, and five patients had both BRCA1 and BRCA2 mutation. Most patients in this study had an exon 11 mutation. Additionally, the c.1961 deletion and the c.9097 deletion were the most common nucleotide changes in BRCA1 mutation group and BRCA2 mutation group, respectively.
3.3 Survival analysis and prognostic factors
In this study, only seven patients experienced a relapse. Of these, two patients presented with MSI-H alone, while five patients exhibited both MSI-H and BRCAm. Notably, no deaths have been reported as of October 2025 in this study. According to the K-M survival analysis presented in Figure 2, there was statistically significant difference in PFS between the two groups (p-value=0.006).
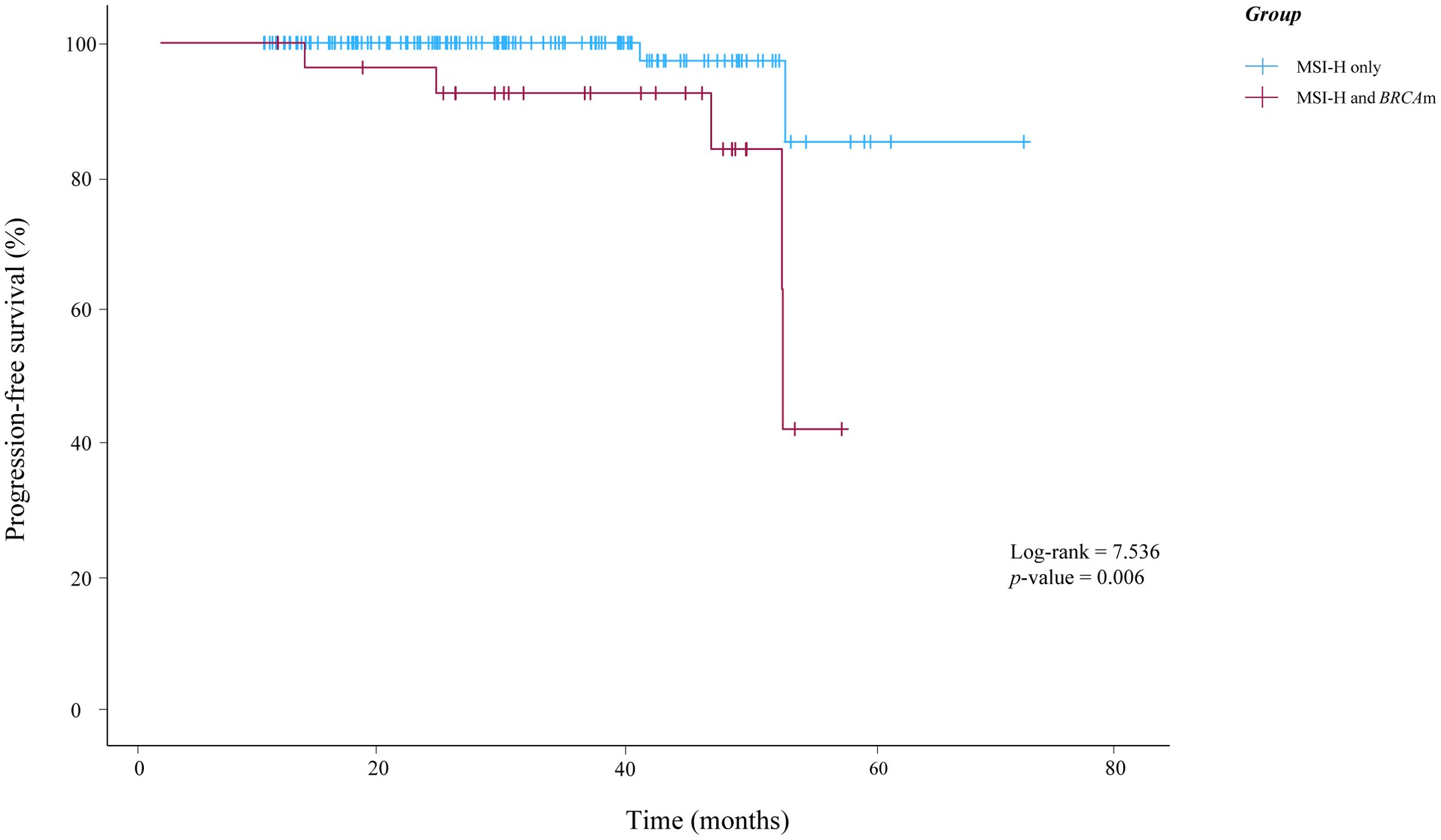
Figure 2. Kaplan-Meier curves illustrating the progression-free survival of patients in this study. BRCAm, BRCA1/2 mutations; MSI-H, microsatellite instability-high.
In the univariate analysis using the Cox proportional hazards regression model, significant prognostic factors included serum CA125 level (p-value=0.004), serum CA199 level (p-value=0.049), lymph node metastasis (p-value=0.012), preoperative treatment (p-value=0.034), and BRCAm (p-value=0.014). These five variables were therefore included in the multivariable Cox model. In the multivariable analysis adjusting simultaneously for these covariates, none of them remained statistically significant (all p-value > 0.05). The details of the univariate and multivariate Cox regression analyses were presented in Table 3.

Table 3. Univariate and multivariable Cox proportional hazard regression analysis of progression-free survival.
4 Discussion
Currently, the number of targeted cancer therapies based on genomic profiles has increased significantly, contributing to the advancement of personalized medicine (22). However, when genomic profiling reveals multiple potential therapeutic options, it creates a diagnostic dilemma in which the optimal initial choice of systemic therapy may be uncertain. Moore et al. reported a case of progressive metastatic prostate cancer characterized by the co-occurrence of MSI-H and BRCAm (24). They suggested that the ICB should be considered before PARPi for these patients because BRCAm are likely carriers of MSI-H but are unlikely to be driver molecular alterations (24). As the tumor type with the highest rate of MSI-H, we investigated whether EC exhibiting both MSI-H and BRCAm displays distinct clinical characteristics. We explored the clinical features, treatment patterns, and outcomes associated with MSI-H and BRCAm in EC.
Our genetic testing data is obtained through NGS, which provides high sensitivity and comprehensive detection capabilities (25). In this study, the molecular typing rates of EC that we observed were comparable to those reported in other studies. And we found that the median ages in these two groups were 58 and 56 years, which is younger than the average age at diagnosis of 63 years (26). The data from the Surveillance, Epidemiology, and End Results (SEER) program, which spans from 1990 to the present, indicate a consistent increase in cases among women under the age of 50. The average BMI was approximately 25 kg/m², which is classified as overweight in our study. Compared to other cancers, EC has the strongest association with obesity (27). A recent meta-analysis of 30 prospective studies found that each 5 kg/m² increase in BMI was associated with a 54% higher risk of developing EC (28). A national cohort study in Denmark demonstrated that the risk of EC is reduced, regardless of whether a pregnancy ends shortly after conception or at 40 weeks of gestation, due to biological processes occurring within the first weeks of pregnancy (29). Our study found that the median number of pregnancies was 2 and 3, respectively. The average age at menopause in our study was approximately 51 years. A recent study reported a positive association between the age at menopause and EC suggesting that late menopause increases the risk of developing EC (30). We found that the median levels of serum CA125, CA199, and CEA were normal in this study. Similarly, previous literature has reported on the role of various serum tumor markers in EC, indicating that elevations occur in only 20% to 30% of patients (31). Lymph node assessment is critically important to decide treatment in EC and most patients in our study received pelvic lymph node dissection. However, lymph node dissection carries specific surgical risks and potential complications, and in response, a sentinel lymph node strategy has been developed and refined over the past decade (32). Moreover, the majority of histological grades were classified as grade II, and the FIGO stage was IA2. This indicates that most patients with EC were in the early stages and had moderately differentiated tumors. This finding aligns with the fact that nearly 75% of patients with EC present with FIGO stage I disease (26). Particularly, the proportion of patients with stage IV was higher in MB group compared to M group, however, this difference was not statistically significant due to the small number of cases. In our study, MB group did not exhibit distinct immunohistochemical characteristics. Immunohistochemistry for MMR proteins, including MLH1, MSH2, MSH6, and PMS2, has emerged as the preferred diagnostic surrogate for MSI globally (33). MSI-H typically indicates reduced or deficient activity of the MutSα protein complex, which is responsible for the initial recognition of DNA mismatches. This reduction can occur due to mutations or inactivation of the heterodimer partners MSH2 or MSH6. Additionally, the MutLα protein complex, which subsequently nicks DNA at sites of mismatch to initiate repair, may also exhibit reduced activity due to mutations or inactivation of its heterodimer partners MLH1 or PMS2. The loss of MLH1/PMS2 or MSH2/MSH6 expression is the most commonly observed pattern in MSI-H tumors (34). Moreover, a significant proportion of all EC cases express both ER and PR (34). The immunohistochemical expressions of MMR proteins, ER, and PR in our study were consistent with previous study. Multiple prospective studies have aimed to identify women with early-stage disease who are at the highest risk of relapse and to develop effective adjuvant therapies (26). However, to date, no such strategy has demonstrated an improvement in OS. And most patients in our study did not receive neoadjuvant therapies. In our study, the clinical characteristics of MB group mentioned above did not appear to be unique.
In this study, we found that the number of patients who underwent laparoscopic surgery was higher than that of those who had open abdominal surgery. Since the adoption of laparoscopic surgery for EC, the incidence of laparoscopic procedures has gradually increased compared to open surgery. This is attributed to the fact that laparoscopic surgery offers oncologic outcomes comparable to those of open surgery, while also reducing surgical morbidity and improving the quality of life for patients (35). Laparoscopic surgery has become the standard approach in modern medical practice, offering significant advantages. In our study, the proportion of open abdominal surgeries in MB group was higher than that in M group. This discrepancy may be attributed to the fact that patients in the former group had a greater likelihood of extensive metastasis to lymph nodes and other sites, which influenced the choice of surgical methods. We interpret the observed association between MB group and a higher rate of open surgery as an indirect indication of a greater disease burden in some patients. In our study, patients in MB group more frequently exhibited lymph node metastasis and showed a numerically higher proportion of advanced FIGO stage. However, this latter difference did not reach statistical significance, likely due to the limited sample size. These factors may have influenced surgeons to prefer laparotomy over laparoscopy in selected cases to ensure thorough exploration and cytoreduction. Additionally, MB group exhibited higher rates of lymph node metastasis and recurrence. Generally, MSI-H and BRCAm typically exhibit a TMB-H and increased chromosomal fragmentation compared to MSI-H alone, which may indicate a higher likelihood of lymph node metastasis and recurrence. Preoperative treatment is reserved for patients with more advanced tumor stages or unfavorable presentations, such as bulky pelvic or para-aortic lymph nodes, suspected distant metastases, or poor general condition that makes immediate standard surgery unsafe. Therefore, receiving preoperative treatment primarily reflects a worse baseline disease status rather than the direct effect of a specific molecular subtype. Since MSI-H and BRCAm status were determined from postoperative NGS, these molecular features did not influence the decision to administer neoadjuvant therapy. Consequently, the association between preoperative treatment and prognosis observed in the univariate analysis should be interpreted as an indicator of more aggressive disease rather than evidence that preoperative therapy itself worsens outcomes in MSI-H EC. Specifically, MSI-H typically induces a robust anti-tumor immune response, characterized by the infiltration of CD8+ T cells (36). However, BRCAm not only upregulate the expression of Snail and vascular endothelial growth factor (VEGF), promoting epithelial-mesenchymal transition (EMT) and angiogenesis, but also recruit immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs), which can facilitate immune evasion by tumors (37). The 2023 National Comprehensive Cancer Network (NCCN) Guidelines for Uterine Tumors (Version 2) recommend immunotherapy as a first-line treatment for advanced (stage III-IV) and recurrent metastatic EC for the first time, based on findings from the NRG-GY018 trial and the RUBY trial (38). The use of immunotherapy in patients with MSI-H and/or BRCAm in our cohort aligns with current guideline-based practices and is not intended to be presented as a novel therapeutic strategy. The proportion of patients receiving immunotherapy was higher in MB group compared to M group. In MB group, five patients received either immunotherapy or chemotherapy in combination with immunotherapy, of which three patients experienced a recurrence (recurrence time: 4, 17, and 30 months). However, there was no recurrence among the patients who received either immunotherapy or chemotherapy in combination with immunotherapy in M group. This may be related to our above discussion that patients with MSI-H and BRCAm may have a higher TMB. Consequently, these patients are more likely to experience relapse or metastasis, leading to a higher proportion receiving immunotherapy. In our study, some advanced patients did not receive immunotherapy either because they were diagnosed with EC before 2023 or due to financial constraints. Patients in MB group exhibited a higher likelihood of recurrence, despite receiving a greater proportion of postoperative adjuvant treatment. Those who received immunotherapy, whether in combination with chemotherapy or not, were selected for individual analysis, and patients in the BRCAm group also demonstrated a higher recurrence rate.
According to previous literature, 83% of MSI-H patients are classified as TMB-H, while both MSI-H and TMB-H/microsatellite stable (MSS) patients show a higher rate of BRCAm (22). This mutation is a single allelic mutation and is categorized as a bystander effect. It does not lead to abnormal poly (ADP-ribose) polymerase function, indicating that there will be no positive treatment response to PARPi. We speculate that, compared to patients with MSI-H only, those with MSI-H and BRCAm exhibit a higher TMB, which may contribute to increased tumor metastasis and recurrence. Based on this inference, we do not routinely administer PARPi to EC with MSI-H and BRCAm to avoid exposing them to potential side effects without a corresponding likelihood of therapeutic benefit. In our study, one patient chose to undergo PARPi maintenance treatment following the completion of immunotherapy, and no disease recurrence has been observed in this individual. Further research and clinical observations are necessary to determine whether patients with MSI-H and BRCAm indeed have a higher TMB, whether this is a bystander effect, and whether such patients can derive benefits from PARPi treatment.
Exon11 was the most common mutation location in our study. Similarly, among BRCAm in ovarian cancer, exon11 is also the most common mutation location. This can be attributed to the fact that exon 11 of BRCA1/2 is particularly large, with approximately half of the mutations occurring in this region (39). We observed differences in PFS between the two groups, with patients carrying BRCAm exhibiting a shorter PFS. But we did not identify any independent risk factors that influenced prognosis in our study. However, some studies have identified independent risk factors that affect the prognosis of patients with EC. For example, the PORTEC-3 trial demonstrated that patients with stage III disease who received chemoradiation therapy followed by four cycles of carboplatin and paclitaxel chemotherapy experienced improved OS and five-year PFS rates compared to those who underwent radiation therapy alone (40, 41). Additionally, age, lymph node metastasis, histological type, histological grade, FIGO stage, and other factors are also considered to be related to the patient’s prognosis (26, 42, 43).
Some limitations also need to be considered in this study. First, because our cohort included patients treated up to 2024, the follow-up period for the most recently treated patients was relatively short, which may lead to underestimation of late recurrences and survival events. Therefore, our findings should be interpreted with caution, and longer-term follow-up and external validation are warranted. Second, a small number of patients with MSI-H EC were lost to follow-up (2/215, 0.9%) or excluded due to incomplete baseline data. If these patients had systematically worse outcomes than those who remained in the cohort, this could have introduced attrition bias and non-random missingness, potentially leading to an overestimation of PFS. Because outcome data were unavailable for these individuals, we could not fully rule out this possibility. Third, this study involved a surgery-based cohort. We included only patients who underwent primary surgical treatment at our institution and had complete clinical data. Patients with inoperable disease, poor performance status, or incomplete records were excluded from our analysis, which may introduce potential selection bias. Finally, this was a retrospective, single-center study that included only 28 patients with concurrent BRCAm. We hope our study will encourage future multi-center research with larger cohorts and longer follow-up periods to validate our findings and more reliably identify independent prognostic factors.
5 Conclusion
Compared to M group, MB group showed a higher rate of lymph node metastasis, immunotherapy, and recurrence. Although the K-M curves indicated that patients with BRCAm experienced shorter PFS compared to patients with MSI-H only, BRCAm was not identified as an independent prognostic factor influencing PFS in patients with MSI-H. In summary, the coexistence of MSI-H and BRCAm may indicate a higher TMB, which is associated with increased rates of lymph node metastasis and recurrence compared to M group. We hope that this single-center study will inspire further research that includes longer follow-up periods and a larger patient population. This will provide a more comprehensive understanding of the clinical characteristics of EC with MSI-H and BRCAm, thereby facilitating the selection of more precise and personalized treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the ethics committee of the Affiliated Hospital of Qingdao University (approval number: QYFYWZLL 29592). Informed consent was acquired from the patients before the study started.
Author contributions
YS: Writing – original draft, Data curation, Methodology. YW: Methodology, Writing – original draft. LH: Writing – original draft, Visualization. GL: Writing – original draft, Data curation. ZC: Writing – original draft, Data curation. XX: Writing – review & editing. GY: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Galant N, Krawczyk P, Monist M, Obara A, Gajek Ł, Grenda A, et al. Molecular classification of endometrial cancer and its impact on therapy selection. Int J Mol Sci. (2024) 25:5893. doi: 10.3390/ijms25115893
2. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. (2013) 497:67–73. doi: 10.1038/nature12113
3. Léon-Castillo A. Update in the molecular classification of endometrial carcinoma. Int J Gynecol Cancer: Off J Int Gynecol Cancer Society. (2023) 33:333–42. doi: 10.1136/ijgc-2022-003772
4. Berek JS, Renz M, Kehoe S, Kumar L, and Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstetr: Off Organ Int Fed Gynaecol Obstetr. (2021) 155 Suppl 1:61–85. doi: 10.1002/ijgo.13878
5. Wang F, Chen G, Qiu M, Ma J, Mo X, Liu H, et al. Neoadjuvant treatment of IBI310 plus sintilimab in locally advanced MSI-H/dMMR colon cancer: A randomized phase 1b study. Cancer Cell. (2025) 43:1958–67.e2. doi: 10.1016/j.ccell.2025.09.004
6. Cercek A, Foote MB, Rousseau B, Smith JJ, Shia J, Sinopoli J, et al. Nonoperative management of mismatch repair-deficient tumors. N Engl J Med. (2025) 392:2297–308. doi: 10.1056/NEJMoa2404512
7. Oaknin A, Gilbert L, Tinker AV, Brown J, Mathews C, Press J, et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J Immunother Cancer. (2022) 10:e003777. doi: 10.1136/jitc-2021-003777
8. Tuninetti V, Farolfi A, Rognone C, Montanari D, De Giorgi U, and Valabrega G. Treatment strategies for advanced endometrial cancer according to molecular classification. Int J Mol Sci. (2024) 25:11448. doi: 10.3390/ijms252111448
9. Powell MA, Cibula D, O’Malley DM, Boere I, Shahin MS, Savarese A, et al. Efficacy and safety of dostarlimab in combination with chemotherapy in patients with dMMR/MSI-H primary advanced or recurrent endometrial cancer in a phase 3, randomized, placebo-controlled trial (ENGOT-EN6-NSGO/GOG-3031/RUBY). Gynecol Oncol. (2025) 192:40–9. doi: 10.1016/j.ygyno.2024.10.022
10. Turinetto M, Lombardo V, Pisano C, Musacchio L, and Pignata S. Pembrolizumab as a single agent for patients with MSI-H advanced endometrial carcinoma. Expert Rev Anticancer Ther. (2022) 22:1039–47. doi: 10.1080/14737140.2022.2126356
11. Riedinger CJ, Esnakula A, Haight PJ, Suarez AA, Chen W, Gillespie J, et al. Characterization of mismatch-repair/microsatellite instability-discordant endometrial cancers. Cancer. (2024) 130:385–99. doi: 10.1002/cncr.35030
12. Li K, Luo H, Huang L, Luo H, and Zhu X. Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int. (2020) 20:16. doi: 10.1186/s12935-019-1091-8
13. Post CCB, Stelloo E, Smit V, Ruano D, Tops CM, Vermij L, et al. Prevalence and prognosis of lynch syndrome and sporadic mismatch repair deficiency in endometrial cancer. J Natl Cancer Institute. (2021) 113:1212–20. doi: 10.1093/jnci/djab029
14. Pasanen A, Loukovaara M, and Bützow R. Clinicopathological significance of deficient DNA mismatch repair and MLH1 promoter methylation in endometrioid endometrial carcinoma. Modern Pathol: An Off J United States Can Acad Pathol Inc. (2020) 33:1443–52. doi: 10.1038/s41379-020-0501-8
15. da Costa A and Baiocchi G. Genomic profiling of platinum-resistant ovarian cancer: The road into druggable targets. Semin Cancer Biol. (2021) 77:29–41. doi: 10.1016/j.semcancer.2020.10.016
16. Gonzalez-Bosquet J, Weroha SJ, Bakkum-Gamez JN, Weaver AL, McGree ME, Dowdy SC, et al. Prognostic stratification of endometrial cancers with high microsatellite instability or no specific molecular profile. Front Oncol. (2023) 13:1105504. doi: 10.3389/fonc.2023.1105504
17. Doig KD, Fellowes AP, and Fox SB. Homologous recombination repair deficiency: an overview for pathologists. Modern Pathol: An Off J United States Can Acad Pathol Inc. (2023) 36:100049. doi: 10.1016/j.modpat.2022.100049
18. Murai J and Pommier Y. BRCAness, homologous recombination deficiencies, and synthetic lethality. Cancer Res. (2023) 83:1173–4. doi: 10.1158/0008-5472.Can-23-0628
19. Stewart MD, Merino Vega D, Arend RC, Baden JF, Barbash O, Beaubier N, et al. Homologous recombination deficiency: concepts, definitions, and assays. Oncol. (2022) 27:167–74. doi: 10.1093/oncolo/oyab053
20. Patel PS, Algouneh A, and Hakem R. Exploiting synthetic lethality to target BRCA1/2-deficient tumors: where we stand. Oncogene. (2021) 40:3001–14. doi: 10.1038/s41388-021-01744-2
21. Adashek JJ, Subbiah V, and Kurzrock R. From tissue-agnostic to N-of-one therapies: (R)Evolution of the precision paradigm. Trends Cancer. (2021) 7:15–28. doi: 10.1016/j.trecan.2020.08.009
22. Sokol ES, Jin DX, Fine A, Trabucco SE, Maund S, Frampton G, et al. PARP inhibitor insensitivity to BRCA1/2 monoallelic mutations in microsatellite instability-high cancers. JCO Precis Oncol. (2022) 6:e2100531. doi: 10.1200/po.21.00531
23. Vermij L, Jobsen JJ, León-Castillo A, Brinkhuis M, Roothaan S, Powell ME, et al. Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry. Br J Cancer. (2023) 128:1360–8. doi: 10.1038/s41416-023-02141-0
24. Moore C, Naraine I, and Zhang T. Complete remission following pembrolizumab in a man with mCRPC with both microsatellite instability and BRCA2 mutation. Oncol. (2024) 29:716–20. doi: 10.1093/oncolo/oyae156
25. Mandlik JS, Patil AS, and Singh S. Next-generation sequencing (NGS): platforms and applications. J Pharm Bioallied Sci. (2024) 16:S41–s5. doi: 10.4103/jpbs.jpbs_838_23
26. Lu KH and Broaddus RR. Endometrial cancer. N Engl J Med. (2020) 383:2053–64. doi: 10.1056/NEJMra1514010
27. Hazelwood E, Sanderson E, Tan VY, Ruth KS, Frayling TM, Dimou N, et al. Identifying molecular mediators of the relationship between body mass index and endometrial cancer risk: a Mendelian randomization analysis. BMC Med. (2022) 20:125. doi: 10.1186/s12916-022-02322-3
28. Aune D, Navarro Rosenblatt DA, Chan DS, Vingeliene S, Abar L, Vieira AR, et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Ann Oncol Off J Eur Soc Med Oncol. (2015) 26:1635–48. doi: 10.1093/annonc/mdv142
29. Husby A, Wohlfahrt J, and Melbye M. Pregnancy duration and endometrial cancer risk: nationwide cohort study. BMJ. (2019) 366:l4693. doi: 10.1136/bmj.l4693
30. Sun X, Zhang Y, Shen F, Liu Y, Chen GQ, Zhao M, et al. The histological type of endometrial cancer is not associated with menopause status at diagnosis. Biosci Rep. (2022) 42:BSR20212192. doi: 10.1042/bsr20212192
31. Zeng X, Zhang Z, Gao QQ, Wang YY, Yu XZ, Zhou B, et al. Clinical significance of serum interleukin-31 and interleukin-33 levels in patients of endometrial cancer: A case control study. Dis Markers. (2016) 2016:9262919. doi: 10.1155/2016/9262919
32. Van Trappen P, De Cuypere E, Claes N, and Roels S. Robotic staging of cervical cancer with simultaneous detection of primary pelvic and secondary para-aortic sentinel lymph nodes: reproducibility in a first case series. Front Surgery. (2022) 9:905083. doi: 10.3389/fsurg.2022.905083
33. Addante F, d’Amati A, Santoro A, Angelico G, Inzani F, Arciuolo D, et al. Mismatch repair deficiency as a predictive and prognostic biomarker in endometrial cancer: A review on immunohistochemistry staining patterns and clinical implications. Int J Mol Sci. (2024) 25:1056. doi: 10.3390/ijms25021056
34. Wagner VM and Backes FJ. Do not forget about hormonal therapy for recurrent endometrial cancer: A review of options, updates, and new combinations. Cancers. (2023) 15:1799. doi: 10.3390/cancers15061799
35. Kim SI, Park DC, Lee SJ, Song MJ, Kim CJ, Lee HN, et al. Survival rates of patients who undergo minimally invasive surgery for endometrial cancer with cervical involvement. Int J Med Sci. (2021) 18:2204–8. doi: 10.7150/ijms.55026
36. Narayanan S, Kawaguchi T, Peng X, Qi Q, Liu S, Yan L, et al. Tumor infiltrating lymphocytes and macrophages improve survival in microsatellite unstable colorectal cancer. Sci Rep. (2019) 9:13455. doi: 10.1038/s41598-019-49878-4
37. Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol. (2013) 31:1748–57. doi: 10.1200/jco.2012.43.1882
38. Eskander RN, Sill MW, Beffa L, Moore RG, Hope JM, Musa FB, et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. (2023) 388:2159–70. doi: 10.1056/NEJMoa2302312
39. Ichijima Y, Sin HS, and Namekawa SH. Sex chromosome inactivation in germ cells: emerging roles of DNA damage response pathways. Cell Mol Life Sci: CMLS. (2012) 69:2559–72. doi: 10.1007/s00018-012-0941-5
40. de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. (2018) 19:295–309. doi: 10.1016/s1470-2045(18)30079-2
41. de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. (2019) 20:1273–85. doi: 10.1016/s1470-2045(19)30395-x
42. Yang Y, Wu SF, and Bao W. Molecular subtypes of endometrial cancer: Implications for adjuvant treatment strategies. Int J Gynaecol Obstetr: Off Organ Int Fed Gynaecol Obstetr. (2024) 164:436–59. doi: 10.1002/ijgo.14969
Keywords: BRCA1, BRCA2, endometrial neoplasms, homologous recombination deficiency, microsatellite instability
Citation: Shan Y, Wang Y, Huang L, Li G, Cui Z, Xing X and Yin G (2025) Clinical characteristics of endometrial cancers with microsatellite instability-high and BRCA1/2 mutations. Front. Oncol. 15:1685642. doi: 10.3389/fonc.2025.1685642
Received: 14 August 2025; Accepted: 21 November 2025; Revised: 20 November 2025;
Published: 04 December 2025.
Edited by:
Stefano Restaino, Ospedale Santa Maria della Misericordia di Udine, ItalyReviewed by:
Komsun Suwannarurk, Thammasat University, ThailandOmar Hamdy, Mansoura University, Egypt
Copyright © 2025 Shan, Wang, Huang, Li, Cui, Xing and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoming Xing, eGlhb21pbmcueGluZ0BxZHUuZWR1LmNu; Guangjie Yin, eWluZ2owNTMyQDE2My5jb20=
 Yuping Shan
Yuping Shan Yan Wang1
Yan Wang1 Zhumei Cui
Zhumei Cui Xiaoming Xing
Xiaoming Xing