Abstract
Objective:
Additional prognostic factors in patients with early-stage oral squamous cell carcinoma (OSCC) may optimize disease staging by identifying patients with high-risk constellations and facilitating risk-stratified therapies that lead to improved treatment outcome. Body mass index (BMI) is an established tool that is routinely recorded in everyday clinical practice and has demonstrated prognostic relevance in various other diseases. However, sufficient evidence regarding its impact in OSCC is lacking. The aim of this study is to evaluate the prognostic significance of pretherapeutic BMI in surgically treated OSCC patients.
Materials and methods:
This retrospective analysis included all patients with primary OSCC who underwent surgical therapy with or without the need for adjuvant therapy at Charité – Universitätsmedizin Berlin over a seven-year period. BMI was categorized based on the World Health Organization classification and correlated with clinical outcome. Overall survival (OS) and recurrence-free survival (RFS) were examined using Kaplan-Meier curves, Cox regression analysis, and log-rank test. The hazard ratios (HR) are presented together with 95% confidence intervals (CI).
Results:
A total of 394 patients (male: 257 (65.2%), female: 137 (34.8%)) with a mean age of 60.0 years were included. Of these, 25 (6.3%) met the criteria for underweight, 195 (49.2%) for normal weight, 121 (30.6%) for overweight and 55 (13.9%) for obesity. Underweight patients showed a significantly lower mean OS of 46.0 months (CI: 30.9-61.2 months) and RFS of 36.5 months (CI: 24.4-48.6 months) compared to all other BMI categories. In the multivariate Cox regression for OS, a reduced risk was observed for both normal-weight (HR: 0.32 CI: 0.11-0.90, p=0.031) and overweight patients (HR: 0.17 CI: 0.05-0.59, p=0.005) relative to those who were underweight. Regarding RFS, overweight patients demonstrated a significantly reduced risk compared to underweight individuals (HR: 0.28 CI: 0.09-0.89, p=0.031).
Conclusion:
Our results underscore the prognostic significance of pretherapeutic BMI as an independent risk factor in OSCC, particularly highlighting the need for intensified preoperative management in underweight patients due to their compromised outcomes.
Introduction
With an incidence rate of 389.485 and a mortality rate of 188.230 patients per year, oral squamous cell carcinoma (OSCC) represents a clinically significant malignancy in the global context (1). Despite considerable advancements in therapy, 5-year-survival rates remain poor at approximately 56%, underscoring the ongoing need for innovation in pretherapeutic risk stratification (2, 3).
Although there is a growing understanding of the influence of host-related factors - such as nutritional status and H-index (4, 5) - these are not yet incorporated into current clinical guidelines, including those issued by the German Society, and are only marginally addressed by the National Comprehensive Cancer Network (NCCN) (6, 7). Among routinely assessed parameters in clinical practice, body mass index (BMI) - calculated as the ratio of weight to height squared (8) - has garnered increasing attention. Given its established prognostic relevance in other malignancies, particularly lung and gastric cancer (9–11), interest in its potential role in OSCC has grown steadily.
To date, only a limited number of studies have examined the prognostic significance of BMI in OSCC, and their findings remain inconsistent (12–14). Both favorable and unfavorable prognostic associations have been reported. For example, poorer survival has been observed in underweight patients with locally advanced disease (12), while other studies report higher recurrence rates among overweight individuals (14). However, due to methodological limitations - such as inclusion of only selected subgroups (e.g., patients with locally advanced tumors (12)) - comparability across studies remains limited. Further research is therefore warranted to establish more robust and generalizable evidence.
Surgical resection, potentially followed by adjuvant radiotherapy with or without concurrent chemoradiotherapy, is recommended as the standard treatment for OSCC in current clinical guidelines (6, 7). In contrast, primary chemoradiotherapy plays a subordinate role. Postoperative difficulties with oral food intake commonly lead to malnutrition, which may be further exacerbated by the side effects of adjuvant chemotherapy or radiotherapy (15, 16). In this context, a preexisting state of cachexia may further exacerbate the problem, highlighting the need for pretherapeutic stratification of high-risk patients (4). Early identification could allow for timely implementation of supportive nutritional and therapeutic interventions (13).
Given the limited data on the prognostic impact of pretherapeutic BMI in surgically treated patients with OSCC, the question arises as to whether the integration of this parameter into routine clinical.
practice is warranted. This study aimed to validate the prognostic significance of pretherapeutic BMI in a representative cohort of surgically treated OSCC patients.
Materials and methods
Ethics statement
The Ethics Committee of the Faculty of Medicine Charité - Universitätsmedizin Berlin approved this study (EA2/028/15).
Patient cohort
This study included all patients diagnosed with OSCC, who underwent surgical treatment including complete neck dissection at the Department of Oral and Maxillofacial Surgery, Charité – Universitätsmedizin Berlin, between 2005 and 2011. Tumor staging was conducted according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging manual (17). Clinical data, including tumor stage, histopathological parameters (TNM classification and tumor grade), were collected retrospectively. Additional clinical information—such as age, gender, tumor site, treatment regimen, survival time, and recurrence—was obtained from electronic medical records. Body mass index (BMI) was categorized based on the World Health Organization (WHO) classification: underweight (BMI <18.5 kg/m²), normal weight (≥18.5 to <25 kg/m²), overweight (≥25 to <30 kg/m²) and obese (≥30 kg/m²) (8).
Overall survival (OS) was defined as the time from the date of initial diagnosis to death from any cause. Patients who experienced no event were censored at the date of last contact or at the end of the observation period (31st of May 2017), whichever occurred first, if the patient was still alive. In cases where the exact date of initial diagnosis was unavailable (e.g., external diagnoses), the date of surgical therapy was used as a surrogate. Recurrence-free survival (RFS) was defined as the time from completion of therapy to the occurrence of either a recurrence or death.
Survival analyses were conducted for the entire cohort as well as stratified by categorized BMI. Additionally, further analyses were performed according to the conventional subdivision into early stage (UICC I and II) and late stage tumors (UICC III and IV).
Statistical analysis
Data were collected using Microsoft Excel (Microsoft Corporation, Redmond, USA), and statistical analyses were conducted using IBM SPSS Statistics Version 29.0.2.0 and R Version 4.0.3 (18). Categorical variables are presented as frequencies and percentages, while continuous variables are reported as means with standard deviations (SD). Group comparisons for categorical or ordinal variables were performed using the Chi-square test or Fisher’s exact test, as appropriate.
OS and RFS were analyzed using Kaplan–Meier survival estimates, with differences assessed via the log-rank test. Univariate and multivariate Cox proportional hazards regression analyses were used to identify prognostic factors for OS and RFS. All p-values are considered exploratory and are reported without adjustment for multiple comparisons; p-values less than α = 0.05 were regarded as statistically significant. Hazard ratios (HRs) and mean survival times are presented with corresponding 95% confidence intervals (CI).
Results
Clinicopathological features
A total of 394 patients with surgically treated OSCC were included in this study, comprising 257 males (65.2%) and 137 females (34.8%). The mean age of patients was 60.0 years, with a range from 27 to 92 years. As previously described, all patients underwent neck dissection as part of their surgical treatment (unilateral: 45.4%; bilateral: 54.6%).
In our cohort, 25 patients (6.3%) were classified as underweight, 195 (49.2%) as normal weight, 121 (30.6%) as overweight and 55 (13.9%) as obese. As shown in Table 1, no statistically significant correlations were found between BMI and histopathological characteristics, including grading, T stage, N stage, or UICC stage. In contrast, BMI was significantly associated with age at the time of surgery, with underweight patients presenting at a younger age (p=0.036). Univariate analysis demonstrated a significant correlation between BMI and both smoking (Cramer-V: 0.169; p=0.016) and alcohol history (Cramer-V: 0.194; p=0.003), with a higher prevalence of these factors among underweight patients.
Table 1
| Total | BMI [kg/m2] | |||||
|---|---|---|---|---|---|---|
| <18.5 | 18.5 - 24.9 | 25.0 - 29.9 | ≥30 | |||
| 25 (6.3%) | 195 (49.2%) | 121 (30.6%) | 55 (13.9%) | P-value | ||
| Age | 55.0 years | 59.7 years | 61.8 years | 59.4 years | 0.036 | |
| Sex | ||||||
| Female | 137 (34.8%) | 10 (40.0%) | 72 (37.1%) | 34 (28.1%) | 21 (38.9%) | 0.314 |
| Male | 257 (65.2%) | 15 (60.0%) | 122 (62.9%) | 87 (71.9%) | 33 (61.1%) | |
| History of Smoking | ||||||
| Yes | 272 (75.1%) | 21 (91.2%) | 143 (79.9%) | 74 (67.3%) | 34 (68.0%) | 0.016 |
| No | 90 (24.9%) | 2 (8.7%) | 36 (20.1%) | 36 (32.7%) | 16 (32.0%) | |
| History of Alcohol | ||||||
| Yes | 272 (75.3%) | 22 (95.7%) | 142 (80.2%) | 71 (65.7%) | 37 (69.8%) | 0.003 |
| No | 89 (24.7%) | 1 (4.3%) | 35 (19.8%) | 37 (34.3%) | 16 (30.2%) | |
| Grading | ||||||
| G1 | 31 (8.0%) | 2 (8.0%) | 15 (7.8%) | 11 (9.3%) | 3 (5.6%) | 0.663 |
| G2 | 279 (71.7%) | 15 (60.0%) | 141 (73.4%) | 81 (68.6%) | 42 (77.8%) | |
| G3 | 79 (20.3%) | 8 (32.0%) | 36 (18.8%) | 26 (22.0%) | 9 (16.7%) | |
| pT stage | ||||||
| T1 | 174 (44.2%) | 8 (32.0%) | 87 (44.8%) | 58 (47.9%) | 21 (38.9%) | 0.304 |
| T2 | 144 (36.5%) | 13 (52.0%) | 60 (30.9%) | 46 (38.0%) | 25 (46.3%) | |
| T3 | 45 (11.4%) | 2 (8.0%) | 26 (13.4%) | 13 (10.7%) | 4 (7.4%) | |
| T4a | 30 (7.6%) | 2 (8.0%) | 20 (10.3%) | 4 (3.3%) | 4 (7.4%) | |
| T4b | 1 (0.3%) | 0 | 1 (0.5%) | 0 | 0 | |
| pN stage | ||||||
| N0 | 265 (67.3%) | 19 (76.0%) | 124 (63.9%) | 88 (72.7%) | 34 (63.0%) | 0.083 |
| N1 | 62 (15.7%) | 2 (8.0%) | 28 (14.4%) | 18 (14.9%) | 14 (25.9%) | |
| N2 | 67 (17.0%) | 4 (16.0%) | 42 (21.6%) | 15 (12.4%) | 6 (11.1%) | |
| pUICC stage | ||||||
| I | 132 (33.7%) | 6 (24.0%) | 66 (34.2%) | 44 (36.7%) | 16 (29.6%) | 0.331 |
| II | 95 (24.2%) | 9 (36.0%) | 39 (20.2%) | 32 (26.7%) | 15 (27.8%) | |
| III | 78 (19.9%) | 4 (16.0%) | 34 (17.6%) | 26 (21.7%) | 14 (25.9%) | |
| IVa | 86 (21.9%) | 6 (24.0%) | 53 (27.5%) | 18 (15.0%) | 9 (16.7%) | |
| IVb | 1 (0.3%) | 0 | 1 (0.5%) | 0 | 0 | |
| Resection Status | ||||||
| R0 | 352 (89.6%) | 23 (92.0%) | 168 (86.6%) | 110 (91.7%) | 51 (94.4%) | 0.484 |
| R1 | 34 (8.7%) | 2 (8.0%) | 19 (9.8%) | 10 (8.3%) | 3 (5.6%) | |
| R2 | 3 (0.8%) | 0 | 3 (1.5%) | 0 | 0 | |
| Rx | 4 (1.0%) | 0 | 4 (2.1%) | 0 | 0 | |
| Lymphatic Invasion | ||||||
| Yes | 31 (16.0%) | 2 (16.7%) | 15 (15.6%) | 11 (19.3%) | 3 (10.3%) | 0.761 |
| No | 163 (84.0%) | 10 (83.3%) | 81 (84.4%) | 46 (80.7%) | 26 (89.7%) | |
| Vascular Invasion | ||||||
| Yes | 5 (2.5%) | 0 | 4 (3.8%) | 1 (1.8%) | 0 | 0.560 |
| No | 192 (97.5%) | 11 (100%) | 100 (96.2%) | 56 (98.2%) | 30 (100%) | |
| Extracapsular Spread | ||||||
| Yes | 53 (13.5%) | 3 (12.5%) | 32 (16.5%) | 11 (9.1%) | 7 (13.0%) | 0.315 |
| No | 340 (86.5%) | 21 (87.5%) | 162 (83.5%) | 110 (90.9%) | 47 (87.0%) | |
| Adjuvant Radio(chemo)therapy | ||||||
| Yes | 111 (28.2%) | 6 (24.0%) | 63 (32.5%) | 29 (24.0%) | 13 (24.1%) | 0.321 |
| No | 283 (71.8%) | 19 (76.0%) | 131 (67.5%) | 92 (76.0%) | 41 (75.9%) | |
Clinicopathological characteristics of OSCC patients in relation to their pretherapeutic BMI.
Bold values indicate statistical significance.
Survival analysis according to pretherapeutic BMI
In the entire cohort OS averaged 88.78 months (95% CI: 83.5–94.1), with a median of 106.1 months (95% CI: 85.6–127.2). RFS showed a mean of 72.9 months (95% CI: 67.3–78.4) and a median of 74.5 months (95% CI: 57.8–91.3). The 5-year overall survival rate was 65%.
The Kaplan–Meier analysis revealed a mean OS of 46.0 months (CI: 30.9-61.2 months) in underweight patients, which was significantly lower than that of normal weight (89.4 months CI: 81.8-97.0 months, p<0.001), overweight (97.4 months CI: 88.4-106.5 months, p<0.001) and obese patients (83.0 months CI: 70.3-95.8 months, p<0.001) (Figure 1). The 5-year survival rates, in the order mentioned before, were 39%, 64%, 74%, and 64%, respectively.
Figure 1
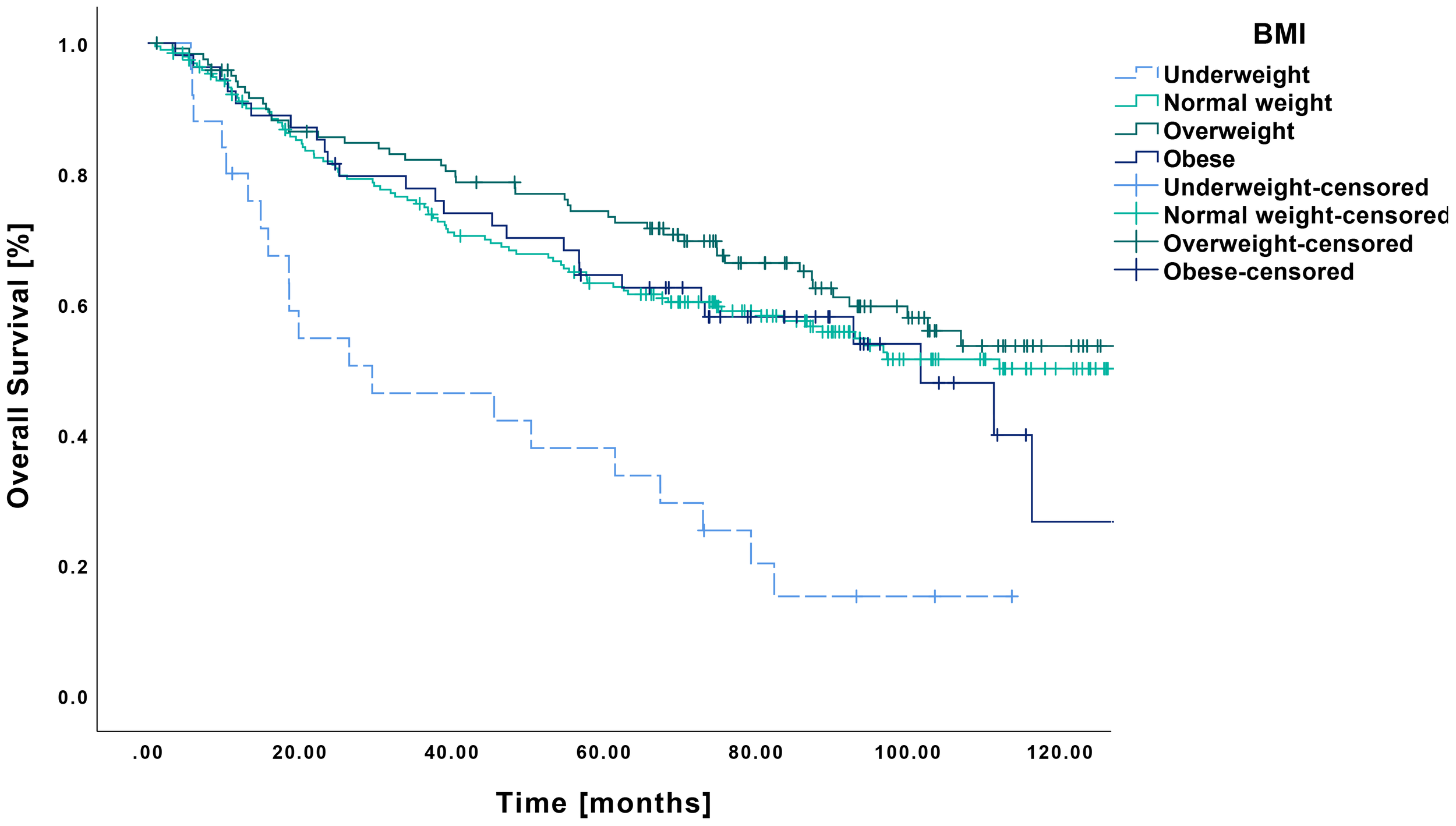
Kaplan-Meier curves showing OS in relation to pretherapeutic BMI. Patients classified as underweight (n=25) demonstrated a significantly reduced overall survival compared to those with normal weight (n=194, p<0.001), overweight (n=121, p<0.001) or obesity (n=54, p<0.001).
The multivariate Cox regression model, which included clinical and histopathological characteristics, identified BMI and resection status as independent prognostic factor for OS (Table 2). Specifically, patients with normal weight demonstrated a reduced risk with a HR of 0.32 (CI: 0.11-0.90, p=0.031) and overweight patients with an HR of 0.17 (CI: 0.05-0.59, p=0.005), all compared to underweight patients. In contrast, no significant risk reduction was observed in obese patients (HR 0.35 CI: 0.10-1.21, p=0.099).
Table 2
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95%-CI | P-value | HR | 95%-CI | P-value | |
| BMI | ||||||
| Underweight | 1 | 1 | ||||
| Normal weight | 0.35 | 0.22 – 0.58 | <0.001 | 0.32 | 0.11 – 0.90 | 0.031 |
| Overweight | 0.29 | 0.17 – 0.49 | <0.001 | 0.17 | 0.05 – 0.59 | 0.005 |
| Obese | 0.41 | 0.23 – 0.72 | 0.002 | 0.35 | 0.10 – 1.21 | 0.099 |
| Sex | ||||||
| male | 1 | 1 | ||||
| female | 0.93 | 0.68 – 1.27 | 0.643 | 0.89 | 0.43 – 1.80 | 0.736 |
| History of Smoking | ||||||
| Yes | 1 | 1 | ||||
| No | 0.52 | 0.33 – 0.79 | 0.002 | 0.86 | 0.39 – 1.87 | 0.699 |
| History of Alcohol | ||||||
| Yes | 1 | 1 | ||||
| No | 0.76 | 0.52 – 1.11 | 0.157 | 1.14 | 0.56 – 2.33 | 0.720 |
| Grading | ||||||
| G1 | 1 | 1 | ||||
| G2 | 1.81 | 0.95 – 3.45 | 0.073 | 3.48 | 0.46 – 26.29 | 0.227 |
| G3 | 2.89 | 1.46 – 5.75 | 0.002 | 2.19 | 0.25 – 19.50 | 0.483 |
| pT Stage | ||||||
| T1 | 1 | 1 | ||||
| T2 | 2.41 | 1.70 – 3.44 | <0.001 | 0.35 | 0.10 – 1.17 | 0.088 |
| T3 | 3.94 | 2.53 – 6.14 | <0.001 | 0.92 | 0.24 – 3.55 | 0.901 |
| T4a | 3.29 | 1.92 – 5.64 | <0.001 | 1.52 | 0.13 – 17.51 | 0.737 |
| T4b | 0 | – | 0.961 | – | – | – |
| pN Stage | ||||||
| N0 | 1 | 1 | ||||
| N1 | 1.73 | 1.16 – 2.56 | 0.007 | 0.58 | 0.13 – 1.17 | 0.473 |
| N2 | 3.03 | 2.13 – 4.31 | <0.001 | 1.67 | 0.14 – 19.72 | 0.682 |
| UICC Stage | ||||||
| I | 1 | 1 | ||||
| II | 2.41 | 1.52 – 3.80 | <0.001 | 2.95 | 0.74 – 11.74 | 0.125 |
| III | 3.37 | 2.14 – 5.31 | <0.001 | 3.80 | 0.72 – 20.24 | 0.117 |
| IVa | 4.78 | 3.09 – 7.39 | <0.001 | 1.87 | 0.13 – 27.19 | 0.646 |
| IVb | 0 | – | 0.962 | |||
| Resection Status | ||||||
| R0 | 1 | 1 | ||||
| R1 | 3.15 | 2.09 – 4.77 | <0.001 | 6.10 | 1.60 – 23.34 | 0.008 |
| R2 | 5.64 | 1.38 – 22.95 | 0.016 | – | – | <0.001 |
| Rx | 1.0 | 0.14 – 7.16 | 1.0 | 4.48 | 0.38 – 53.49 | 0.236 |
| Lymphatic Invasion | ||||||
| Yes | 1.96 | 1.14 – 3.37 | 0.015 | 2.64 | 1.0 – 6.98 | 0.051 |
| No | 1 | 1 | ||||
| Vascular Invasion | ||||||
| Yes | 1.29 | 0.41 – 4.09 | 0.668 | 0.83 | 0.10 – 7.11 | 0.870 |
| No | 1 | 1 | ||||
| Extracapsular Spread | ||||||
| Yes | 2.99 | 2.09 – 4.27 | <0.001 | 3.28 | 0.93 – 11.52 | 0.064 |
| No | 1 | 1 | ||||
| Adjuvant Radio(chemo)therapy | ||||||
| Yes | 2.21 | 1.63 – 2.99 | <0.001 | 0.55 | 0.19 – 1.54 | 0.254 |
| No | 1 | 1 | ||||
Univariate and multivariate Cox regression analysis for OS.
Bold values indicate statistical significance.
Regarding RFS, a similar pattern emerged, with underweight patients showing significantly poorer outcomes (Figure 2). Their mean RFS was 36.5 months (CI: 24.4-48.6 months), which differed significantly from that of normal weight (73.0 months CI: 65.0-81.0 months, p<0.001), overweight (78.3 months CI: 68.7-87.8 months, p<0.001) and obese patients (74.4 months, CI: 60.5-88.3 months, p<0.001).
Figure 2
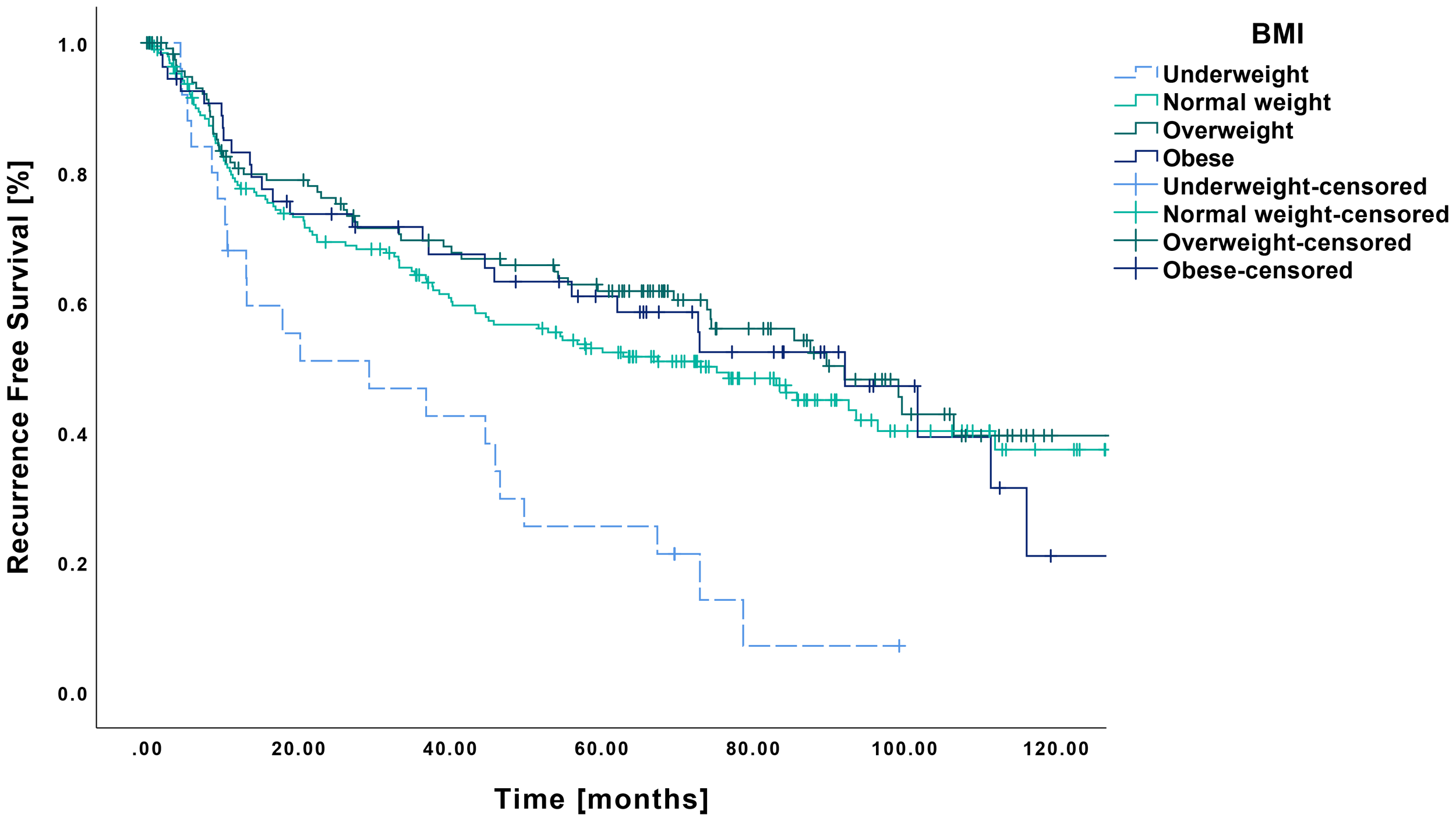
Kaplan-Meier curves showing RFS in relation to pretherapeutic BMI. RFS was significantly reduced in underweight patients (n=25) compared to those with normal weight (n=194, p<0.001), overweight (n=121, p<0.001) or obesity (n=54, p<0.001).
In the multivariate analysis, underweight and R2 resection emerged as independent risk factors for RFS. Overweight patients demonstrated a prognostic advantage over underweight patients, with a HR of 0.28 (CI: 0.09-0.89, p=0.031), whereas no significant differences were observed for normal weight (HR: 0.51 CI: 0.19-1.38, p=0.188) and obese patients (HR 0.44 CI: 0.13-1.43, p=0.170). A detailed overview is provided in Table 3.
Table 3
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95%-CI | P-value | HR | 95%-CI | P-value | |
| BMI | ||||||
| Underweight | 1 | 1 | ||||
| Normal weight | 0.44 | 0.28 – 0.71 | <0.001 | 0.51 | 0.19 – 1.38 | 0.188 |
| Overweight | 0.37 | 0.22 – 0.61 | <0.001 | 0.28 | 0.09 – 0.89 | 0.031 |
| Obese | 0.43 | 0.24 – 0.75 | 0.003 | 0.44 | 0.13 – 1.43 | 0.170 |
| Sex | ||||||
| male | 1 | 1 | ||||
| female | 1.02 | 0.76 – 1.36 | 0.889 | 0.884 | 0.42 – 2.16 | 0.793 |
| History of Smoking | ||||||
| Yes | 1 | 1 | ||||
| No | 0.55 | 0.37 – 0.80 | 0.002 | 0.88 | 0.42 – 1.86 | 0.745 |
| History of Alcohol | ||||||
| Yes | 1 | 1 | ||||
| No | 0.81 | 0.57 – 1.16 | 0.258 | 1.32 | 0.66 – 2.66 | 0.434 |
| Grading | ||||||
| G1 | 1 | 1 | ||||
| G2 | 1.97 | 1.04 – 3.75 | 0.038 | 3.23 | 0.43 – 24.25 | 0.253 |
| G3 | 3.25 | 1.64 – 6.42 | <0.001 | 3.17 | 0.37 – 26.92 | 0.290 |
| pT Stage | ||||||
| T1 | 1 | 1 | ||||
| T2 | 2.19 | 1.57 – 3.05 | <0.001 | 0.88 | 0.28 – 2.81 | 0.831 |
| T3 | 3.41 | 2.23 – 5.21 | <0.001 | 1.76 | 0.47 – 6.52 | 0.401 |
| T4a | 3.37 | 2.04 – 5.55 | <0.001 | 2.82 | 0.20 – 40.53 | 0.446 |
| T4b | 5.36 | 0.74 – 38.86 | 0.097 | – | – | – |
| pN Stage | ||||||
| N0 | 1 | 1 | ||||
| N1 | 1.58 | 1.09 – 2.31 | 0.016 | 0.84 | 0.21 – 1.3.41 | 0.812 |
| N2 | 2.53 | 1.81 – 3.54 | <0.001 | 1.27 | 0.10 – 15.81 | 0.852 |
| UICC Stage | ||||||
| I | 1 | 1 | ||||
| II | 2.10 | 1.38 – 3.21 | <0.001 | 1.60 | 0.42 – 6.02 | 0.491 |
| III | 2.87 | 1.89 – 4.38 | <0.001 | 2.17 | 0.43 – 10.90 | 0.348 |
| IVa | 3.86 | 2.58 – 5.78 | <0.001 | 1.70 | 0.10 – 29.53 | 0.715 |
| IVb | 6.45 | 0.88 – 47.21 | 0.066 | |||
| Resection Status | ||||||
| R0 | 1 | 1 | ||||
| R1 | 3.22 | 2.18 – 4.77 | <0.001 | 3.29 | 0.95 – 11.45 | 0.061 |
| R2 | 5.70 | 1.81 – 18.00 | 0.003 | 13.04 | 1.22 – 139.21 | 0.033 |
| Rx | 0.72 | 0.10 – 5.12 | 0.793 | 2.60 | 0.23 – 29.52 | 0.444 |
| Lymphatic Invasion | ||||||
| Yes | 1.86 | 1.12 – 3.11 | 0.017 | 1.93 | 0.78 – 4.77 | 0.156 |
| No | 1 | 1 | ||||
| Vascular Invasion | ||||||
| Yes | 1.66 | 0.61 – 5.52 | 0.326 | 3.51 | 0.64 – 19.22 | 0.147 |
| No | 1 | |||||
| Extracapsular Spread | ||||||
| Yes | 2.44 | 1.73-3.45 | <0.001 | 2.28 | 0.66 – 7.93 | 0.195 |
| No | 1 | 1 | ||||
| Adjuvant Radio(chemo)therapy | ||||||
| Yes | 2.05 | 1.54 – 2.74 | <0.001 | 0.60 | 0.23 – 1.59 | 0.307 |
| No | 1 | 1 | ||||
Univariate and multivariate Cox regression analysis for RFS.
Bold values indicate statistical significance.
Survival analysis according to pretherapeutic BMI and stage of disease
Additional analyses were conducted stratified by early- (UICC I and II) and late-stage disease (UICC III and IV) to investigate potential stage-specific differences in the prognostic relevance of BMI.
Early-stage OSCC (UICC I and II)
In early-stage patients (n=227), the mean OS was 102.9 months (CI: 96.6-109.1 months), with a 5-year survival rate of 77%, respectively. In this cohort, RFS averaged 87.1 months (CI: 80.0-94.2 months).
In terms of OS in relation to pretherapeutic BMI, underweight patients demonstrated lower survival compared to all other BMI categories (Figure 3). The underweight cohort showed a mean OS of 54.9 months (CI: 32.7-77.1 months), resulting in statistically significant differences when contrasted with normal weight (104.3 months CI: 95.0-113.7 months, p<0.001), overweight (103.7 months CI: 94.4-113.7 months, p<0.001) and obese patients (105.1 months CI: 90.5-119.7 months, p<0.001).
Figure 3
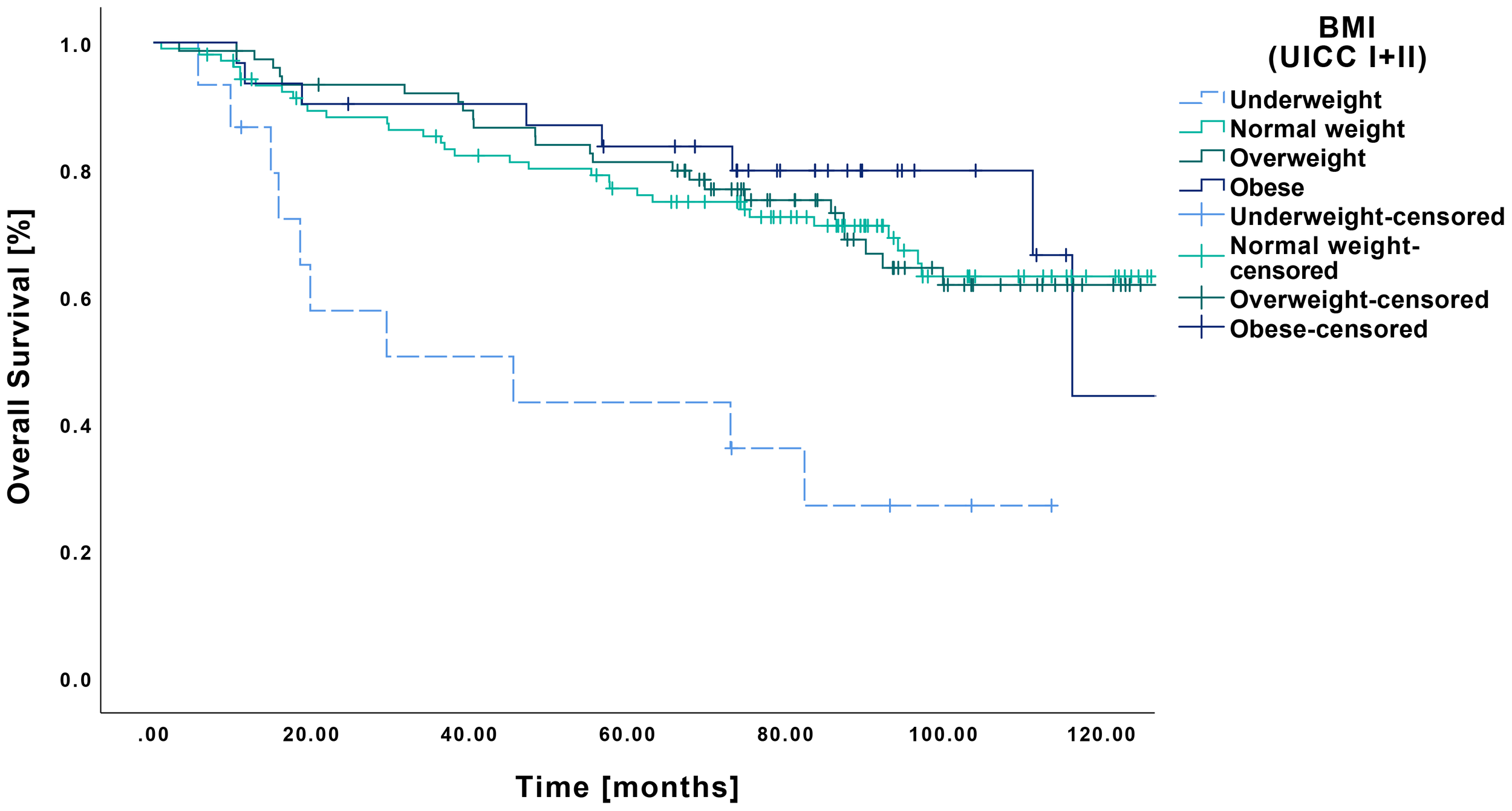
Kaplan-Meier curves showing OS of early-stage OSCC (UICC I and II) in relation to pretherapeutic BMI. Underweight patients (n=15) showed a significantly lower OS compared to normal weight (n=105, p<0.001), overweight (n=76, p<0.001), and obese patients (n=31, p<0.001).
A similar pattern was observed for RFS, with the underweight group showing inferior outcomes compared to all other BMI categories (Figure 4). Specifically, the underweight patients had a mean RFS of 40.6 months (CI: 22.9-58.3 months), which differed significantly from that of the normal weight (86.1 months CI: 75.4-96.8 months, p=0.002), overweight (91.0 months CI: 79.9-102.0 months, p<0.001) and obese groups (98.3 months CI: 81.5-115.0 months, p<0.001).
Figure 4
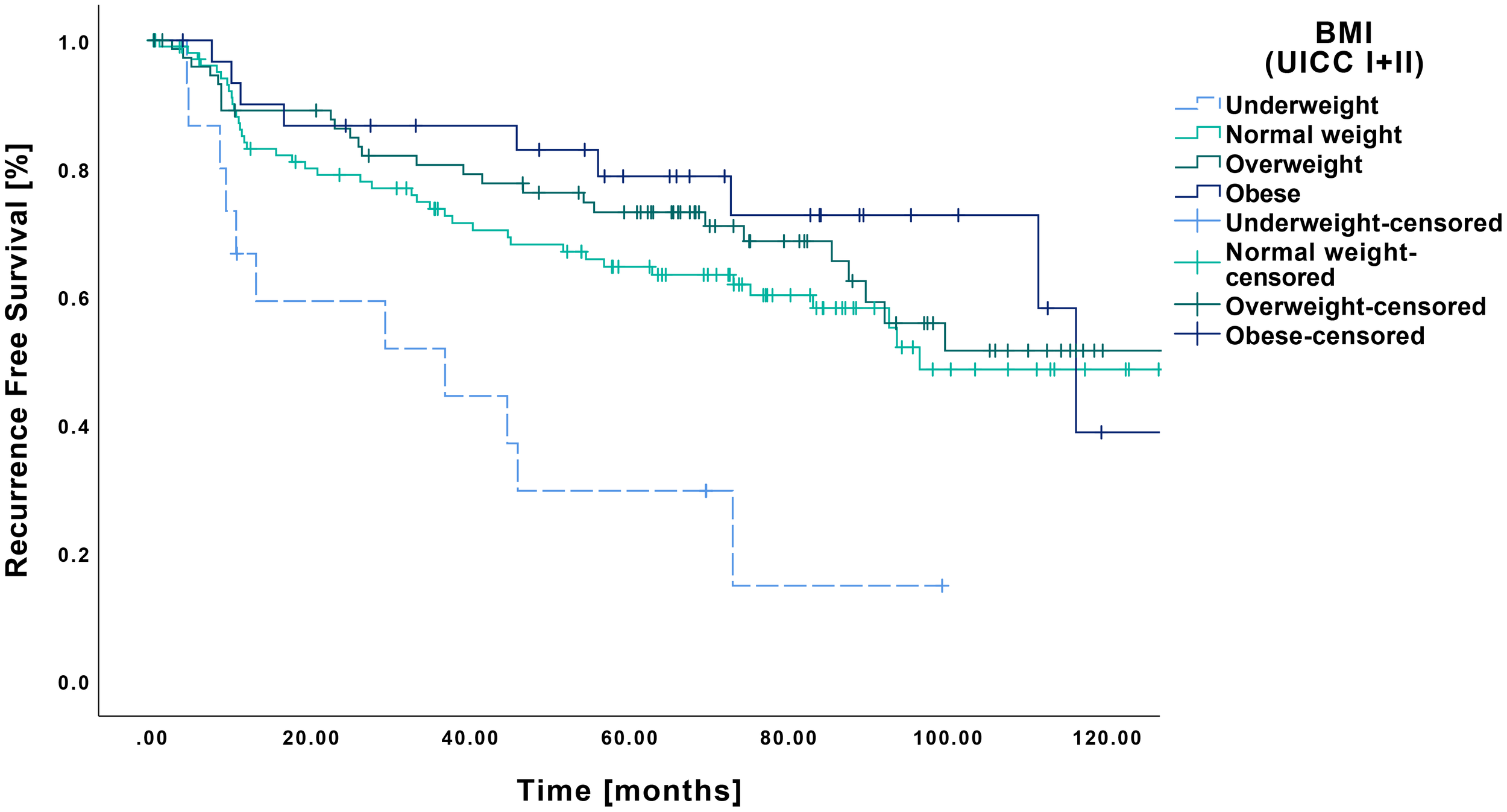
Kaplan-Meier curves showing RFS of early-stage OSCC (UICC I and II) in relation to pretherapeutic BMI. Underweight patients (n=15) demonstrated a significantly shorter RFS compared to normal weight (n=105, p=0.002), overweight (n=76, p=0<0.001) and obese patients (n=31, p<0.001).
Late-stage OSCC (UICC III and IV)
In the cohort of late-stage tumors (n=167), the mean OS was 69.1 months (CI: 61.0-77.2 months), with a RFS of approximately 53.6 months (CI: 45.7-61.4 months). The 5-year survival rate was 49%.
Regarding OS according to BMI, underweight patients showed a mean survival of 33.9 months (CI: 16.5-51.4 months), which differed significantly from normal weight (70.8 months CI: 59.7-71.8 months, p=0.006) and overweight patients (79.7 months CI: 63.1-96.3 months, p=0.002) (Figure 5). In contrast, no significant difference was observed compared to obese patients (53.4 months CI: 38.8-67.9 months, p=0.080), although a clear trend was apparent.
Figure 5
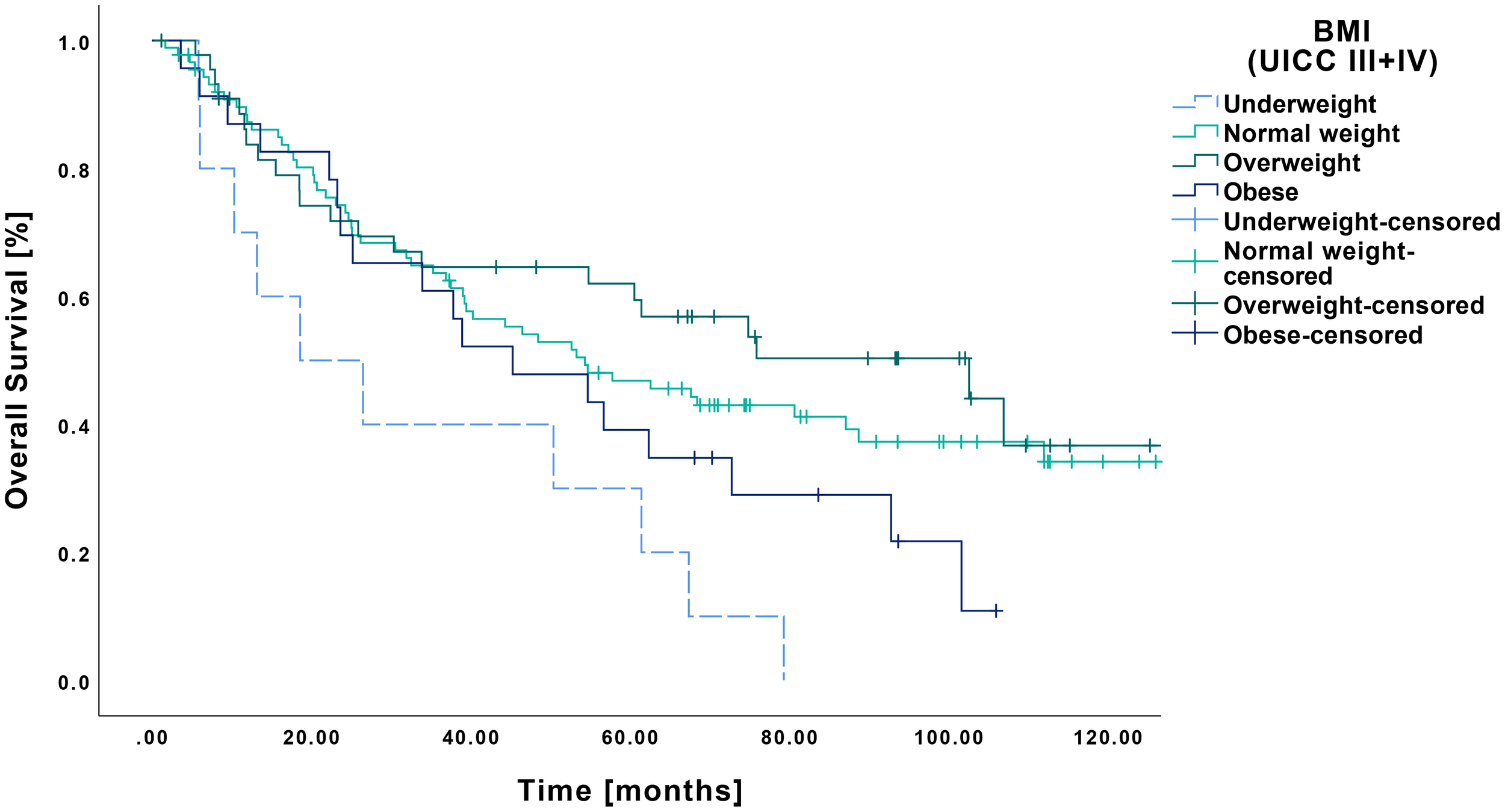
Kaplan-Meier curves showing OS of late-stage OSCC (UICC III and IV) in relation to pretherapeutic BMI. RFS was significantly reduced in underweight patients (n=10) compared to those with normal weight (n=89, p=0.006) and overweight (n=45, p=0.002), whereas no significant difference was observed when compared to obese patients (n=23, p=0.080).
RFS among underweight patients with late-stage tumors was 31.5 months (CI: 14.9-48.2 months). No significant differences were observed in comparison to normal weight (57.8 months CI: 46.5-69.0 months, p=0.073), overweight (52.6 months CI: 38.6-66.8 months, p=0.093) or obese patients (44.7 months CI: 28.4-61.0 months, p=0.247) (Figure 6).
Figure 6
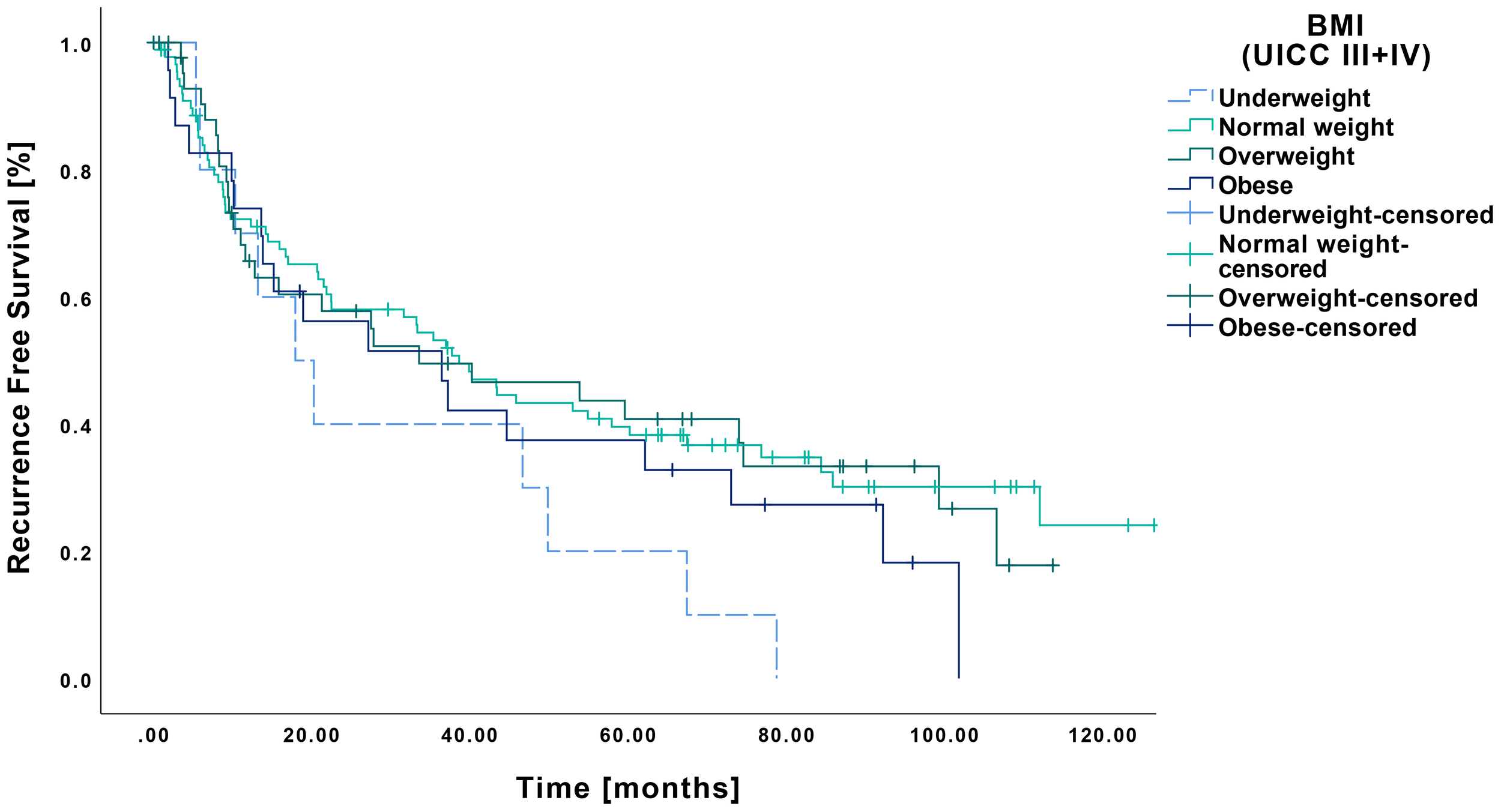
Kaplan-Meier curves showing RFS of late-stage OSCC (UICC III and IV) in relation to pretherapeutic BMI. Patients classified as underweight (n=10) demonstrated no significant differences regarding RFS compared to those with normal weight (n=89, p=0.073), overweight (n=45, p=0.093) or obesity (n=23, p=0.247).
Discussion
The aim of this study was to explore the prognostic significance of pretherapeutic BMI in a representative cohort OSCC patients OSSC that were treated surgically with or without the need for adjuvant therapy.
In the present cohort, no significant associations were found between BMI categories and histopathological characteristics such as tumor stage, grading, lymphatic or vascular invasion. These findings are consistent with previously published cohorts, which also reported no influence of pretherapeutic BMI on histological phenotype (13, 19). In contrast, significant correlations were observed between BMI and age, smoking, and alcohol consumption in our cohort. Underweight patients tended to present at a younger age and predominantly exhibited classic risk factors such as tobacco and alcohol use. This excessive risk profile may contribute to earlier disease onset, and comorbidities associated with active smoking and alcohol abuse may explain the lower BMI observed. However, considering other studies that reported no association between BMI, age, and risk behavior (13, 19), these interpretations remain speculative.
In our overall cohort, underweight patients showed a significantly worse OS and RFS compared to all other BMI categories. Multivariate analysis revealed a reduced risk for normal-weight and overweight patients in comparison to those who were underweight.
Some studies have reported similar findings (12, 13). For example, Chang et al. observed a poorer prognosis among underweight patients in a comparable cohort of 320 surgically treated individuals (13). Based on their results, the authors proposed preoperative nutritional optimization as a potential intervention (13). However, a key limitation of their study was the BMI classification: all patients with a BMI above 25 were grouped together, with no distinction made between overweight and obese categories (13). This lack of granularity limits the comparability with other studies. Moreover, when evaluating the generalizability of the present study’s findings, the markedly high proportion of male patients (91.9%) must also be taken into consideration (13). In contrast, Wang et al. reported a higher recurrence rate and poorer prognosis among obese patients, although underweight individuals were found to experience increased rates of postoperative complications (14). Other authors, however, have proposed that BMI has no significant impact on prognosis, instead emphasizing the relevance of other host-related factors (5).
In patients with locally advanced disease, OS was significantly higher in both normal-weight and overweight individuals, while RFS showed a clear trend in favor of these groups. In a single-institution study, Ma et al. reported a prognostic advantage for overweight patients compared to those of normal weight (20). However, in contrast to the present study, their cohort included only patients treated with primary radio chemotherapy (20). The high rate of metabolic response observed among overweight and obese patients may have contributed to these findings (20). Furthermore, their study included tumors from the entire head and neck region. Given the superior response rates of oropharyngeal squamous cell carcinomas to primary radio chemotherapy (21), the comparability with the present, clearly defined OSCC cohort is limited. Another possible explanation could be the so-called obesity paradox, although its very existence remains a subject of ongoing debate (22). This theory suggests that, despite the well-established negative health effects of obesity, a survival advantage has been observed in certain chronic diseases and cancers (22). However, some authors attribute these findings, at least in part, to methodological limitations in the underlying studies (22). A potential improvement could be the inclusion of additional factors, such as body composition (23).
Zhao et al. also demonstrated an inferior prognosis in underweight patients compared to overweight and obese individuals (12). However, it is important to note the inconsistent definition of BMI categories in their study, with the threshold between overweight and obesity set at 27.5 (12). A standardized classification and subsequent pooling of data would be desirable to enable more robust evaluations in future analyses.
In contrast to the aforementioned studies, obese patients in our cohort did not demonstrate a survival advantage over underweight individuals. While this does not indicate an axiomatically inferior prognosis compared to normal-weight and overweight patients, a general disadvantage remains evident. A similar finding was reported by Iyengar et al. in their cohort of early-stage tongue cancer patients, where obese individuals had poorer outcomes compared to those with normal weight (19). One important distinction compared to other studies is the relatively low rate of adjuvantly treated patients (n=8) (19). As previously discussed, this subgroup may benefit more distinctly from treatment, potentially contributing to the improved outcomes observed in obese patients elsewhere (20).
In light of the existing latency between diagnosis and the actual surgery — as is the case, for example, with CAD/CAM planning — some authors are exploring the potential for dedicated preoperative patient preparation (24). With the increasing establishment of neoadjuvant therapeutic approaches (25, 26), this potential continues to grow. For both OSCC and other malignancies, such as esophageal carcinoma, promising data have emerged regarding this patient preparation approach referred to as prehabilitation (24, 27). For example, optimal management of diabetes mellitus and the use of immunonutrition have been associated with improved clinical outcomes, such as a reduction in wound infections (28, 29). Our data identify a new subgroup that requires dedicated preoperative optimization — regardless of whether the reduced BMI represents a surrogate marker for an underlying disease or indicates malnutrition per se. In this context, optimization of underlying comorbidities, targeted speech therapy, physiotherapy, and improvement of nutritional intake according to the underlying cause may be beneficial.
In our cohort, multivariate analysis revealed - besides the already discussed effects of BMI - a significant impact of R1- or R2 resection on OS and RFS. Several studies have previously demonstrated the negative prognostic impact of incomplete resection in OSCC (30). Current clinical guidelines recommend adjuvant therapy in the presence of such intraoperative risk factors, not least for this reason (6, 7). Some authors even suggest a direct association between resection status and the histopathological aggressiveness of the tumor, indicating that an incomplete resection may reflect an inherently more invasive disease biology (31).
Our study is based on a large, well-defined cohort of patients with OSCC who underwent curatively intended surgery followed by adjuvant therapy where indicated. Limitations of this study include its retrospective design, the use of the 7th edition of the AJCC classification system, and incomplete documentation of certain histopathological features such as perineural invasion or depth of invasion. Moreover, precise data on the cause of death were not available, thereby limiting the ability to draw causal conclusions. Additionally, the reasons for foregoing adjuvant therapy were not always provided. Given that BMI could potentially influence treatment decisions and, consequently, patient prognosis, prospective studies with precise correlation between BMI, therapeutic choices and outcomes are needed.
In summary, our results underscore the prognostic significance of pretherapeutic BMI as an independent risk factor in OSCC. Additional studies investigating the direct effect of prehabilitation on clinical outcomes in this patient subgroup are warranted.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethik-Kommission der Charité – Universitätsmedizin Berlin. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
MR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. CD: Conceptualization, Methodology, Writing – review & editing. FM: Conceptualization, Validation, Writing – review & editing. EH: Formal analysis, Validation, Writing – review & editing. SK: Supervision, Validation, Visualization, Writing – review & editing. MH: Resources, Supervision, Validation, Writing – review & editing. KN: Data curation, Methodology, Writing – review & editing. KJ: Conceptualization, Data curation, Project administration, Validation, Writing – review & editing. J-DR: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
During the preparation of this work the author(s) used Chat GPT-4 architecture to improve language style and translation. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Conflict of interest
Author KJ was employed by the company Klinikum Chemnitz gGmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the author(s) used Chat GPT-4 architecture to improve language style and translation. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Bray F Laversanne M Sung H Ferlay J Siegel RL Soerjomataram I et al . Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2
Rogers SN Brown JS Woolgar JA Lowe D Magennis P Shaw RJ et al . Survival following primary surgery for oral cancer. Oral Oncol. (2009) 45:201–11. doi: 10.1016/j.oraloncology.2008.05.008
3
Kademani D Bell RB Bagheri S Holmgren E Dierks E Potter B et al . Prognostic factors in intraoral squamous cell carcinoma: the influence of histologic grade. J Oral Maxillofac Surg. (2005) 63:1599–605. doi: 10.1016/j.joms.2005.07.011
4
Martinovic D Tokic D Puizina Mladinic E Usljebrka M Kadic S Lesin A et al . Nutritional management of patients with head and neck cancer-A comprehensive review. Nutrients. (2023) 15. doi: 10.3390/nu15081864
5
Valero C Zanoni DK Pillai A Ganly I Morris LGT Shah JP et al . Host factors independently associated with prognosis in patients with oral cavity cancer. JAMA Otolaryngol Head Neck Surg. (2020) 146:699–707. doi: 10.1001/jamaoto.2020.1019
6
Leitlinienprogramm Onkologie . (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Diagnostik und Therapie des Mundhöhlenkarzinoms, Langversion 3.0, 2021, AWMF Registernummer . Available online at: https://www.leitlinienprogramm-onkologie.de/leitlinien/mundhoehlenkarzinom/ (Accessed October 4, 2025).
7
National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology: head and neck cancers (Version 2.2025) (2025). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
8
WHO Consultation on Obesity . Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva, Switzerland: World Health Organization (1999). Available online at: https://iris.who.int/handle/10665/42330 (Accessed November 5, 2025).
9
Jiang M Fares AF Shepshelovich D Yang P Christiani D Zhang J et al . The relationship between body-mass index and overall survival in non-small cell lung cancer by sex, smoking status, and race: A pooled analysis of 20,937 International lung Cancer consortium (ILCCO) patients. Lung Cancer. (2021) 152:58–65. doi: 10.1016/j.lungcan.2020.11.029
10
Lam VK Bentzen SM Mohindra P Nichols EM Bhooshan N Vyfhuis M et al . Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. (2017) 104:52–7. doi: 10.1016/j.lungcan.2016.11.017
11
Lee JH Park B Joo J Kook MC Kim YI Lee JY et al . Body mass index and mortality in patients with gastric cancer: a large cohort study. Gastric Cancer. (2018) 21:913–24. doi: 10.1007/s10120-018-0818-x
12
Zhao TC Liang SY Ju WT Liu Y Tan YR Zhu DW et al . Normal BMI predicts the survival benefits of inductive docetaxel, cisplatin, and 5-fluorouracil in patients with locally advanced oral squamous cell carcinoma. Clin Nutr. (2020) 39:2751–8. doi: 10.1016/j.clnu.2019.11.037
13
Chang WC Yang CY Lin CS Lin CK Chen YW . Pretreatment body mass index as a prognostic predictor in patients with oral squamous cell carcinoma. Clin Oral Investig. (2020) 24:2781–8. doi: 10.1007/s00784-019-03141-2
14
Wang C Pan Y Xu Q Li B Kim K Mao M et al . Relationship between body mass index and outcomes for patients with oral squamous cell carcinoma. Oral Dis. (2019) 25:87–96. doi: 10.1111/odi.12963
15
Larsson M Hedelin B Johansson I Athlin E . Eating problems and weight loss for patients with head and neck cancer: a chart review from diagnosis until one year after treatment. Cancer Nurs. (2005) 28:425–35. doi: 10.1097/00002820-200511000-00004
16
Trotti A Bellm LA Epstein JB Frame D Fuchs HJ Gwede CK et al . Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. (2003) 66:253–62. doi: 10.1016/S0167-8140(02)00404-8
17
Edge SB Byrd DR Compton CC Fritz AG Trotti A Green FL eds. AJCC cancer staging manual. 7th ed. New York, NY: Springer (2010).
18
Core Team R . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2020). Available online at: https://www.R-project.org.
19
Iyengar NM Kochhar A Morris PG Morris LG Zhou XK Ghossein RA et al . Impact of obesity on the survival of patients with early-stage squamous cell carcinoma of the oral tongue. Cancer. (2014) 120:983–91. doi: 10.1002/cncr.28532
20
Ma SJ Khan M Chatterjee U Santhosh S Hashmi M Gill J et al . Association of body mass index with outcomes among patients with head and neck cancer treated with chemoradiotherapy. JAMA Netw Open. (2023) 6:e2320513. doi: 10.1001/jamanetworkopen.2023.20513
21
Mendenhall WM Morris CG Amdur RJ Hinerman RW Malyapa RS Werning JW et al . Definitive radiotherapy for tonsillar squamous cell carcinoma. Am J Clin Oncol. (2006) 29:290–7. doi: 10.1097/01.coc.0000209510.19360.f9
22
Banack HR Stokes A . The ‘obesity paradox’ may not be a paradox at all. Int J Obes (Lond). (2017) 41:1162–3. doi: 10.1038/ijo.2017.99
23
Donini LM Pinto A Giusti AM Lenzi A Poggiogalle E . Obesity or BMI paradox? Beneath the tip of the iceberg. Front Nutr. (2020) 7:53. doi: 10.3389/fnut.2020.00053
24
Galmiche A Saidak Z Bettoni J Ouendo M Testelin S . Therapeutic perspectives for the perioperative period in oral squamous cell carcinoma (OSCC). Front Oral Health. (2021) 2:764386. doi: 10.3389/froh.2021.764386
25
Uppaluri R Haddad RI Tao Y Le Tourneau C Lee NY Westra W et al . Neoadjuvant and adjuvant pembrolizumab in locally advanced head and neck cancer. N Engl J Med. (2025) 393:37–50. doi: 10.1056/NEJMoa2415434
26
Schoenfeld JD Hanna GJ Jo VY Rawal B Chen YH Catalano PS et al . Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: A phase 2 open-label randomized clinical trial. JAMA Oncol. (2020) 6:1563–70. doi: 10.1001/jamaoncol.2020.2955
27
González Santos S Martí Gelonch L González Jorrín N González Osinalde M Rosell Romero N . Preoperative risk assessment and prehabilitation strategies in patients undergoing an esophagectomy for cancer resections: a single center retrospective analysis and a review of the literature. Front Anesthesiol. (2024) 3. doi: 10.3389/fanes.2024.1358847
28
Lien KH Padua PFC Tay ZY Kao HK Hung SY Huang Y et al . Influence of hyperglycemia on treatment outcomes of oral cavity squamous cell carcinoma. J Oral Maxillofac Surg. (2020) 78:935–42. doi: 10.1016/j.joms.2020.01.018
29
Aeberhard C Mayer C Meyer S Mueller SA Schuetz P Stanga Z et al . Effect of preoperative immunonutrition on postoperative short-term outcomes of patients with head and neck squamous cell carcinoma. Head Neck. (2018) 40:1057–67. doi: 10.1002/hed.25072
30
Woolgar JA Rogers S West CR Errington RD Brown JS Vaughan ED . Survival and patterns of recurrence in 200 oral cancer patients treated by radical surgery and neck dissection. Oral Oncol. (1999) 35:257–65. doi: 10.1016/S1368-8375(98)00113-4
31
Sutton DN Brown JS Rogers SN Vaughan ED Woolgar JA . The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. (2003) 32:30–4. doi: 10.1054/ijom.2002.0313
Summary
Keywords
oral squamous cell carcinoma, prognostic marker, BMI, body mass index, prognosis, surgical treatment, OSCC
Citation
Richter M, Doll C, Mrosk F, Hofmann E, Koerdt S, Heiland M, Neumann K, Jöhrens K and Raguse J-D (2025) Prognostic significance of pretherapeutic body mass index in surgically treated oral squamous cell carcinoma. Front. Oncol. 15:1686528. doi: 10.3389/fonc.2025.1686528
Received
15 August 2025
Accepted
24 October 2025
Published
17 November 2025
Volume
15 - 2025
Edited by
Stefano Cavalieri, Fondazione IRCCS Istituto Nazionale dei Tumori, Italy
Reviewed by
Carla Pisani, Azienda Ospedaliero Universitaria Maggiore della Carità, Italy
Tobias Ettl, University Medical Center Regensburg, Germany
Updates
Copyright
© 2025 Richter, Doll, Mrosk, Hofmann, Koerdt, Heiland, Neumann, Jöhrens and Raguse.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maximilian Richter, maximilan.richter@charite.de
†These authors have contributed equally to this work and share last authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.