- 1Department of Oncology, Zhuji People’s Hospital of Zhejiang Province, Zhuji, Zhejiang, China
- 2Department of Internal Medicine, School of Medicine, Shaoxing University, Shaoxing, Zhejiang, China
Despite the success of anti-BRAF therapy in melanoma, data from randomized clinical trials are lacking for targeted therapy against BRAF mutations—typically the V600E variant—in pancreatic adenocarcinoma, which is associated with a poor prognosis under traditional cytotoxic chemotherapy. Here, we report a case of an elderly patient with advanced pancreatic adenocarcinoma harboring a BRAF V600E mutation who received low-dose dabrafenib and trametinib and achieved satisfactory clinical outcomes. We describe a 78-year-old female with BRAF V600E-mutant pancreatic adenocarcinoma. The patient was diagnosed with AJCC clinical stage IV (cT3N2M1) pancreatic adenocarcinoma and she declined chemotherapy because of her advanced age. Owing to the BRAF V600E mutation, the patient was started on combined BRAF- and MEK inhibitors (dabrafenib/trametinib). CT scans showed PR on 31 December 31, 2024, and repeated CT scans showed SD on May 2025. At the time of drafting this report, the patient had achieved 8 months of PFS. This case suggests that dose-adjusted dabrafenib combined with trametinib might be a potentially effective treatment strategy for elderly patients with advanced pancreatic adenocarcinoma harboring BRAF V600E mutations.
Introduction
Pancreatic adenocarcinoma (PAC) represents 90% of all pancreatic cancers and is aggressive with a poor prognosis (1). It is estimated to become the second leading cause of cancer-related death in the United States by 2030, with a 5-year survival rate of about 10% (2, 3). Since the pancreas is located in the retroperitoneum, the disease often manifests insidiously with nonspecific symptoms. Most patients diagnosed at advanced stages are unable to undergo surgical resection (4).
The first-line chemotherapy treatments for advanced disease are AG (gemcitabine and nab-paclitaxel) and FOLFIRINOX (oxaliplatin, irinotecan, fluorouracil, and leucovorin). However, the median overall survival (OS) for these two regimens is 8.7 months (as reported by Goldstein D, et al.) and 11.1 months (as reported by Conroy T, et al.), respectively (5–7). In the phase III NAPOLI 3 trial, first-line NALIRIFOX (liposomal irinotecan, fluorouracil, leucovorin, and oxaliplatin) significantly improved median OS compared with AG (11.1 vs 9.2 months; HR 0.83, p = 0.036) in metastatic pancreatic ductal adenocarcinoma. The efficacy difference between the NALIRIFOX regimen and the FOLFIRINOX regimen remains to be elucidated (8). Due to the propensity for resistance to currently available therapies, more effective treatment strategies for patients with advanced pancreatic adenocarcinoma are needed.
BRAF mutations, typically V600E, can activate downstream kinases and culminate in uncontrolled cell growth and survival (9). The combination of dabrafenib (a BRAF inhibitor) and trametinib (a MEK inhibitor) has been approved by the FDA for treating advanced or metastatic melanoma, non-small cell lung cancer, and anaplastic thyroid cancer with BRAF V600E mutations, with reported objective response rates (ORRs) of 64%, 38%, and 56%, respectively (10–13). However, the efficacy of targeting the same genetic alteration varies across different tumors (14).
In pancreatic adenocarcinoma, about 3% of patients harbor the BRAF V600E mutations (15). Due to the rarity of these mutations, reported research on BRAF inhibitors in pancreatic adenocarcinoma is restricted primarily to case reports. Here, we add a case to the growing literature describing an elderly patient with advanced pancreatic adenocarcinoma harboring a BRAF V600E mutation who received low-dose dabrafenib combined with trametinib and achieved clinical benefit.
Case presentation
A 78-year-old Chinese female who initially presented with back pain was admitted to our hospital (Zhuji People’s Hospital) on September 2024. On October 2024, contrast-enhanced computed tomography (CT) images showed enlargement of the pancreatic head, dilation of the pancreatic and bile ducts, and swelling of multiple peripancreatic and retroperitoneal lymph nodes (Figure 1A). A positron emission tomography/computed tomography (PET/CT) scan indicated the possibility of a malignant tumor originating from the pancreatic head, with the enlarged lymph nodes in the peripancreatic, hepatic portal, portocaval, hepatogastric, retrocrural, retroperitoneal, bilateral iliac, and bilateral inguinal regions, consistent with metastatic disease. The CA19–9 level was 757 KIU/L (normal reference value range: 0.0–30.0 KIU/L). Her medical history included hypertension for 10 years, controlled with valsartan and felodipine. She was a non-smoker and had a height of 160 cm and a weight of 52 kg. On physical examination, neither the spleen nor the liver was palpable. No family history of cancer was noted.
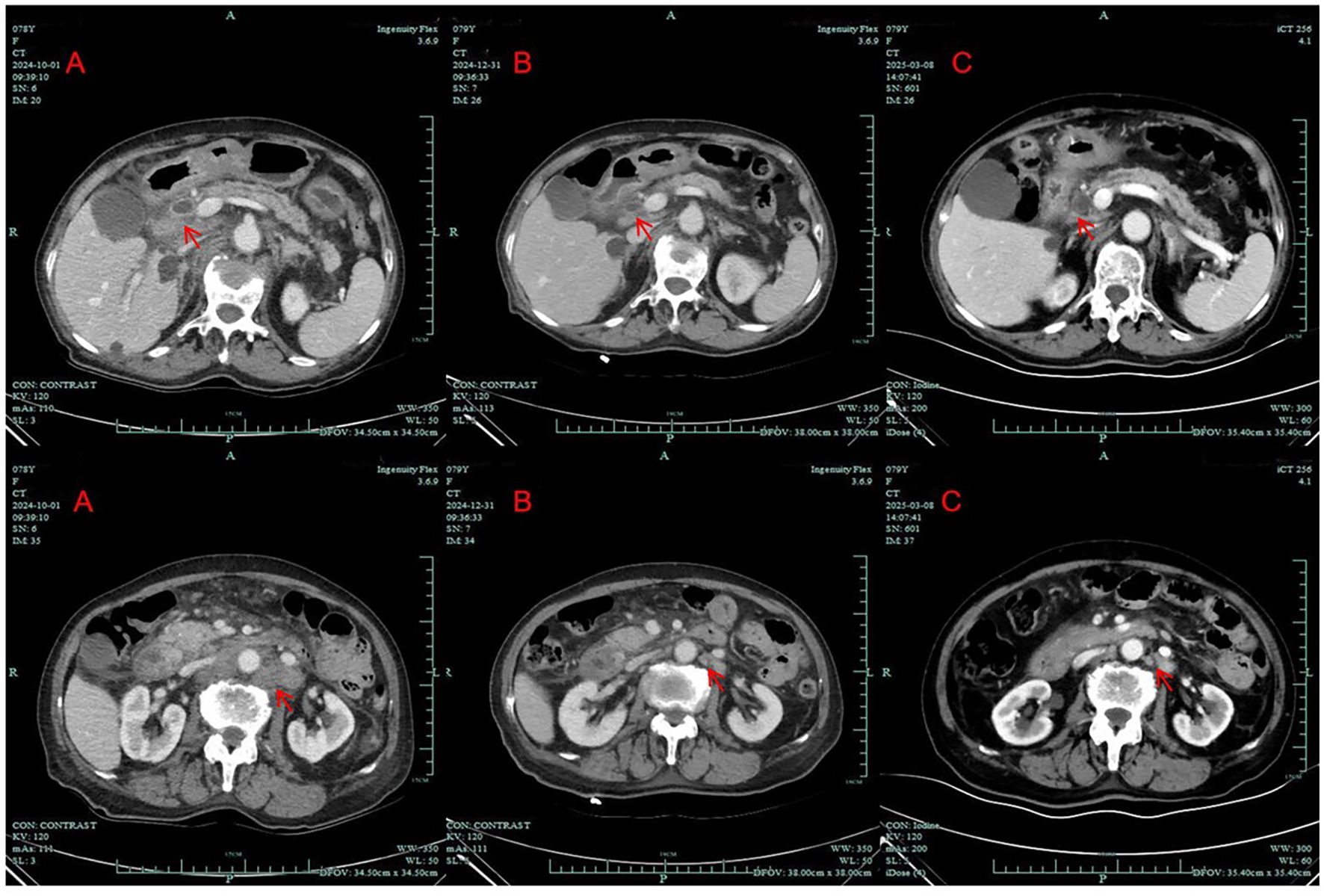
Figure 1. Computed tomography scans of the patient; (A) before treatment (October 1, 2024); (B) after 7 weeks of treatment with dabrafenib and trametinib (December 31, 2024); (C) after approximately 4 months of treatment with dabrafenib and trametinib (March 8, 2025). (arrows: tumor lesion and enlarged lymph node).
The patient underwent left inguinal lymphadenectomy on 7 October 7, 2024, which revealed a mass in the inguinal region measuring 2.0 × 2.0 cm. Pathological examination confirmed metastatic adenocarcinoma consistent with pancreatic cancer (Figure 2). The Ki-67 index was 70%. Genetic testing identified a BRAF V600E mutation and a TP53 mutation and revealed wild-type status for ALK, BRCA1/2, PIK3CA, EGFR, ERBB2, KRAS, NRAS, and ROS1. The patient was diagnosed with clinical stage IV (cT3N2M1) pancreatic adenocarcinoma according to the American Joint Committee on Cancer 8th edition staging system.).
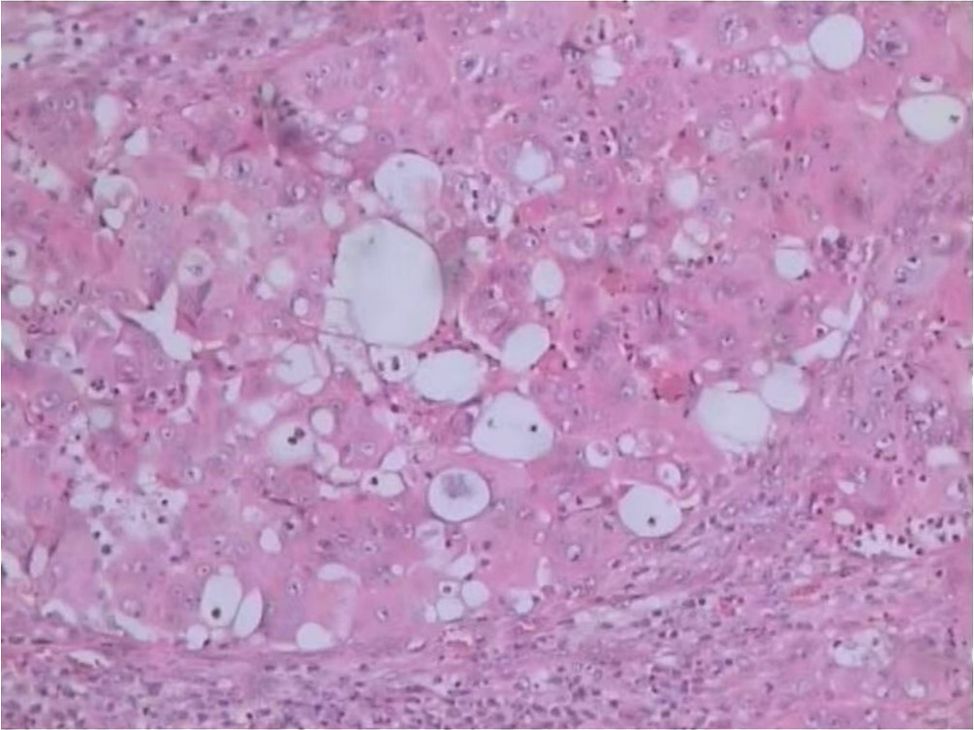
Figure 2. Histopathological image of metastatic pancreatic adenocarcinoma (hematoxylin-eosin, original magnification x100).
The patient received two cycles of chemotherapy with gemcitabine plus nab-paclitaxel on October 2024 and November 2024, respectively. However, she did not complete the full course of the second chemotherapy cycle due to personal reasons. Considering her age, she and her family declined further chemotherapy. At that time, the CA19–9 level was 778 KIU/L. Because of the presence of the BRAF V600E mutation and the patient’s advanced age, she received oral low-dose dabrafenib (50 mg twice daily) and trametinib (2 mg once daily) with informed consent from November 2024.
CT imaging assessment was conducted on 31 December 31, 2024. The best response according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 was partial response (PR) (Figure 1B). The CA19–9 level decreased steadily. Repeated abdominal CT scans showed stable disease (SD) on 8 March 8, 2025 (Figure 1C). The CA19–9 level was 192.0 KIU/L at that time. Repeated CT scans still showed SD on 26 May 26, 2025.
In addition, the patient tolerated treatment with dabrafenib and trametinib well. No grade 3 or 4 treatment-related adverse events (TRAEs) were observed during the treatment period. At the time of drafting this case report, the patient had achieved 8 months of PFS (progression-free survival (PFS).
Discussion
Pancreatic adenocarcinoma is often diagnosed late at advanced stages and traditionally portends a dismal prognosis. Currently, genomic sequencing enables approaches for molecularly targeted therapies, but few effective targeted therapies have been confirmed in PAC (16). There are four known major known gene mutations in pancreatic adenocarcinoma: KRAS, TP53, CDKN2A, and SMAD4. However, none of these has been effectively targeted in clinical practice using current therapeutic regimens (17, 18).
The only targeted agent currently approved for pancreatic adenocarcinoma now is olaparib for patients harboring BRCA 1/2 mutations, but these mutations are present in only 5% of patients with pancreatic cancer (19, 20). Due to the lack of effective treatments for common mutations, we believe that the precision therapies for subsets of patients with specific genetic alterations are the key to advancing treatment strategies for advanced pancreatic adenocarcinoma.
The RAS/RAF/MEK/ERK pathway, also known as the mitogen-activated protein kinase (MAPK) pathway, is a key intracellular signaling pathway that regulates diverse cellular functions and plays a vital role in oncogenesis and the growth of transformed cells (13, 21). BRAF, a serine/threonine kinase located immediately downstream in the Ras signaling pathway, is mutated in approximately 15% of all cancers (22). When mutated, BRAF can activate downstream kinases and culminate in uncontrolled cell growth and survival (9). Most mutations arise from the substitution of valine with glutamic acid at codon 600, known as the BRAF V600E mutations. These mutations were reported by Yaman B et al. to show an inconsistency rate of up to 14.5% between primary and metastatic lesions in melanoma cases, although analogous analyses have not been conducted in pancreatic cancer (23).
BRAF V600E mutations are present in about half of melanomas and have been successfully targeted with BRAF inhibitors in melanomas, such as dabrafenib (24). It is worth noting that the combination of BRAF inhibitors and MEK inhibitors, such as trametinib—which suppresses MEK1/2 and thereby blocks downstream signaling of the MAPK pathway—has been introduced to reduce hyperproliferative cutaneous events and delay the development of acquired drug resistance during BRAF monotherapy. Thus, the combination of BRAF and MEK inhibitors has become the standard treatment for advanced melanoma (25).
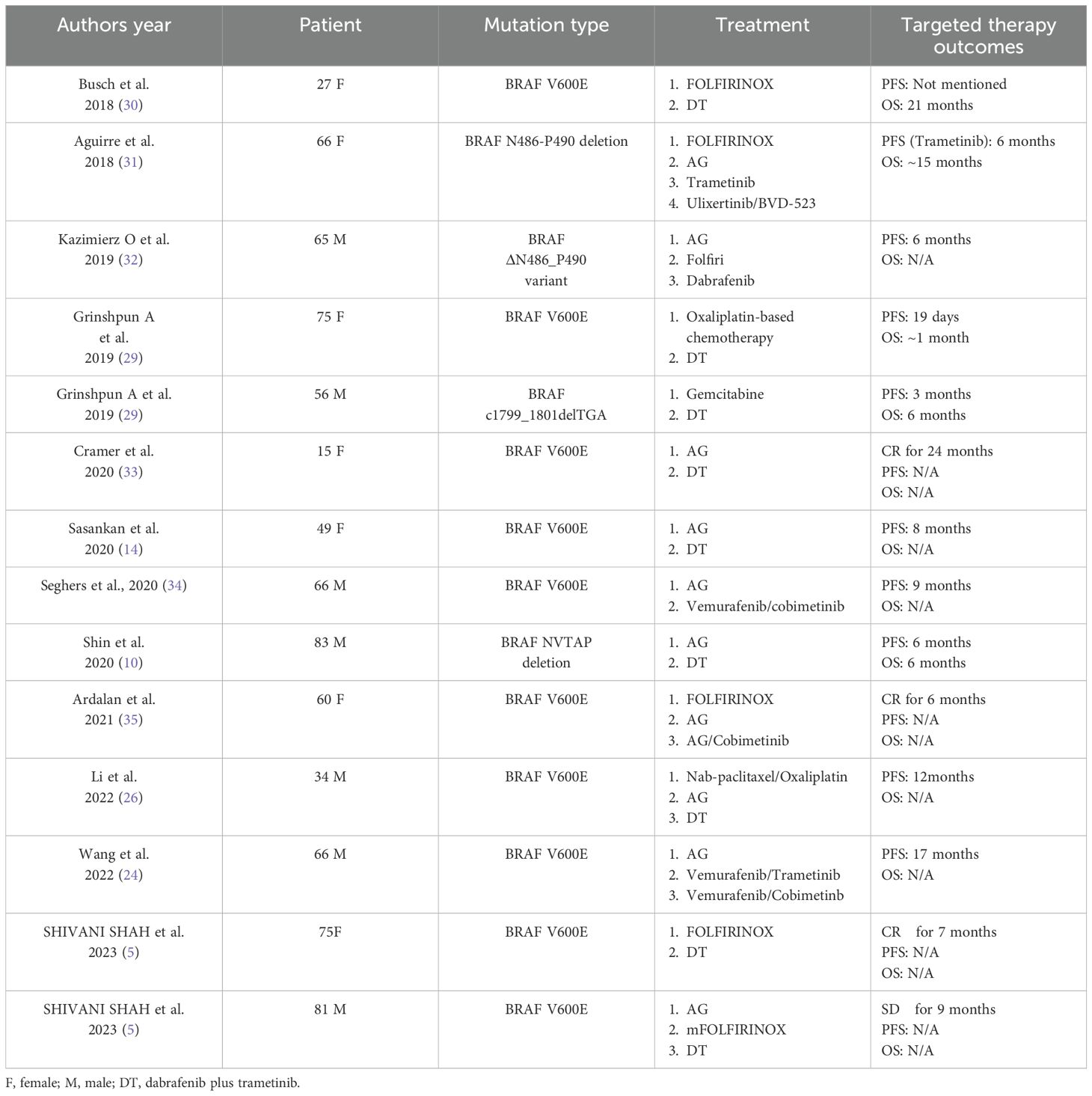
Research has shown that the BRAF V600E mutation is mutually exclusive with KRAS mutation (9). Among patients with KRAS wild-type pancreatic adenocarcinoma (representing 10% of all cases), 30% harbor BRAF mutations, accounting for 3% of all PAC. To date, no therapeutic trials have been published targeting this rare molecular subgroup. These mutations are typically associated with poor prognosis (15, 26, 27). A phase II trial (NCT04390243) is currently underway to assess the efficacy of the combination therapy with binimetinib and encorafenib in pancreatic cancer patients with with a somatic BRAF V600E mutation (28). Apart from this, reported research on BRAF inhibitors in BRAF-mutated pancreatic adenocarcinoma is limited to a handful of case reports or brief mentions within larger analyses of all non-melanoma cancers. We summarize the relevant cases of advanced pancreatic adenocarcinoma patients treated with BRAF inhibitors in Table 1. For example, Grinshpun et al. reported that a patient with advanced pancreatic adenocarcinoma and BRAF V600E mutation who received treatment with dabrafenib plus trametinib, resulting in a marked decline in CA19–9 levels; however, the patient died of an acute abdomen after only 19 days of treatment (29). Sasankan et al. reported on a 49-year-old patient with pancreatic adenocarcinoma harboring a BRAF V600E mutation who was treated with dabrafenib and trametinib as second-line therapy. The dosages of both agents were reduced due to treatment-related toxicity, including septic shock and neutropenic fever. The patient responded well for 8 months before experiencing progressive disease (PD) (14). However, due to the small number of reported cases, it remains difficult to draw definitive conclusions.
In this report, we share a case of a 78-year-old patient with advanced pancreatic adenocarcinoma who refused chemotherapy due to advanced age and subsequently received low-dose dabrafenib combined with trametinib based on the presence of a BRAF V600E mutation. She tolerated the modified doses well, and repeat CT scans after 7 weeks of treatment showed PR. At the time of drafting this report, she had achieved 8 months of PFS. The patient is still being monitored for further response.
Because of her advanced age and concerns regarding adverse reactions, the patient initiated treatment with a lower dose of dabrafenib (50 mg, twice daily), which is below the reduced dosages reported in other case reports (e.g., 150 mg twice daily). The patient has demonstrated a non-inferior survival benefit compared with previously reported cases and showed favorable tolerability. In view of the successful experience of this case, we suggest that dose-adjusted dabrafenib plus trametinib might be a potentially effective treatment strategy for elderly patients with advanced pancreatic adenocarcinoma harboring BRAF V600E mutations. We aim to add a new case to the available literature with the hope of contributing to the growing discussion regarding the treatment of advanced pancreatic adenocarcinoma with BRAF mutations.
Conclusions
We report a case of an elderly patient with BRAF V600E-mutant advanced pancreatic adenocarcinoma who received low-dose dabrafenib plus trametinib and achieved satisfactory clinical outcomes. Dose-adjusted dabrafenib combined with trametinib might be a potentially effective treatment strategy for elderly patients with advanced pancreatic adenocarcinoma harboring BRAF V600E mutations, and needs to be further evaluated clinically.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Institutional Ethics Committee of Zhuji People’s Hospital (20250228; February 28, 2025). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
LL: Writing – original draft, Writing – review & editing. XZ: Writing – original draft. YG: Writing – original draft. MT: Investigation, Writing – original draft. WZ: Writing – original draft, Investigation. BC: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PAC, pancreatic adenocarcinoma; AG, gemcitabine and nab-paclitaxel; FOLFIRINOX, oxaliplatin, irinotecan, fluorouracil, and leucovorin; NALIRIFOX, liposomal irinotecan, fluorouracil, leucovorin, and oxaliplatin; OS, overall survival; CT, computed tomography; RECIST, Response Evaluation Criteria in Solid Tumors; PR, partial response; SD, stable disease; TRAEs, treatment-related adverse events; PFS, progression-free survival; PD, progressive disease; F, female; M, male; DT, dabrafenib plus trametinib.
References
1. De Jesus VHF, Donadio MDS, de Brito ABC, and Gentilli AC. A narrative review on rare types of pancreatic cancer: should they be treated as pancreatic ductal adenocarcinomas? Ther Adv Med Oncol. (2024) 16:1758–8340. doi: 10.1177/17588359241265213
2. Rahib L, Wehner MR, Matrisian LM, and Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. (2021) 4:2574–3805. doi: 10.1001/jamanetworkopen.2021.4708
3. Wang X, ChangSheng Y, Yun L, and Xiaodan G. Pancreatic adenocarcinoma with distant soft tissue metastases: a case report and literature review. Front Surg. (2025) 12:1447865. doi: 10.3389/fsurg.2025.1447865
4. Lim JE, Chien MW, and Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. (2003) 237:74–85. doi: 10.1097/00000658-200301000-00011
5. Shivani S, Rana T, Pragnan K, and Dulabh M. Targeted therapy for BRAF V600E positive pancreatic adenocarcinoma: two case reports. Cancer Genom Proteom. (2023) 20:398–403. doi: 10.21873/cgp.20391
6. Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, et al. Nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. (2015) 107:dju413. doi: 10.1093/jnci/dju413
7. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. (2011) 364:1817–25. doi: 10.1056/NEJMoa1011923
8. Wainberg ZA, Melisi D, Macarulla T, Roberto PC, Chandana SR, Fouchardière CDL, et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial. Lancet. (2023) 402:1272–81. doi: 10.1016/S0140-6736(23)01366-1
9. Witkiewicz AK, McMillan EA, Balaji U, Baek G, and Lin WC. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. (2015) 6:6744. doi: 10.1038/ncomms7744
10. Shin JE, An HJ, Park HS, Kim H, and Shim BY. Efficacy of dabrafenib/trametinib in pancreatic ductal adenocarcinoma with BRAF NVTAP deletion: A case report. Front Oncol. (2022) 12:976450. doi: 10.3389/fonc.2022.976450
11. Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. (2015) 372:30–9. doi: 10.1056/NEJMoa1412690
12. Zhou I, Plana D, and Palmer AC. Tumor-specific activity of precision medicines in the NCI-MATCH trial. MedRxiv. (2024) 30(4):786–792. doi: 10.1101/2023.03.30.23287951
13. Subbiah V, Kreitman RJ, Wainberg ZA, Gazzah A, Lassen U, Stein A, et al. Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med. (2023) 29:1103–12. doi: 10.1038/s41591-023-02321-8
14. Sasankan S, Rebuck L, Darrah G, Harari Turquie M, and Rabinowitz I. Metastatic pancreatic cancer with BRAF and P53 mutations: case report of therapeutic response to doublet targeted therapy. Case Rep Oncol. (2020) 13:1239–43. doi: 10.1159/000510096
15. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. (2015) 373:726–36. doi: 10.1056/NEJMoa1502309
16. Qian ZR, Rubinson DA, Nowak JA, Oyarvide VM, Dunne RF, Kozak MM, et al. Association of alterations in main driver genes with outcomes of patients with resected pancreatic ductal adenocarcinoma. JAMA Oncol. (2018) 4:e173420. doi: 10.1001/jamaoncol.2017.3420
17. Herbst B and Zheng L. Precision medicine in pancreatic cancer: treating every patient as an exception. Lancet Gastroenterol Hepatol. (2019) 4:805–10. doi: 10.1016/S2468-1253(19)30175-X
18. Zhang XF, Mao T, Zhang B, Xu H, Cui JJ, Jiao F, et al. Characterization of the genomic landscape in large-scale Chinese patients with pancreatic cancer. EBioMedicine. (2022) 77:103897. doi: 10.1016/j.ebiom.2022.103897
19. Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline brca-mutated metastatic pancreatic cancer. N Engl J Med. (2019) 381:317–27. doi: 10.1056/Nejmoa1903387
20. Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. (2016) 531:47–52. doi: 10.1038/Nature16965
21. Friday B and Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. (2008) 14:342–6. doi: 10.1158/1078-0432.CCR-07-4790
22. Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, and Velculescu VE. Tumorigenesis: rAF/RAS oncogenes and mismatchrepair status. Nature. (2002) 418:934. doi: 10.1038/418934a
23. Yaman B, Kandiloğlu G, and Akalin T. BRAF-V600 mutation heterogeneity in primary and metastatic melanoma: A study with pyrosequencing and immunohistochemistry. Am J Dermatopathol. (2016) 38:113–20. doi: 10.1097/DAD.0000000000000404
24. Wang ZY, He D, Chen C, Liu XB, and Ke NW. Vemurafenib combined with trametinib significantly benefits the survival of a patient with stage IV pancreatic ductal adenocarcinoma with BRAF V600E mutation: A case report. Front Oncol. (2022) 11:801320. doi: 10.3389/fonc.2021.801320
25. Robert C, Flaherty K, Nathan P, Hersey P, Garbe C, Milhem M, et al. Five-year outcomes from A phase 3 metric study in patients with braf V600 E/K-mutant advanced or metastatic melanoma. Eur J Cancer. (2019) 109:61–9. doi: 10.1016/J.Ejca.2018.12.015
26. Li HS, Yang K, and Wang Y. Remarkable response of BRAF (V600E)-mutated metastatic pancreatic cancer to BRAF/MEK inhibition: a case report. Gastroenterol Rep. (2021) 10:2052–0034. doi: 10.1093/gastro/goab031
27. Zaanan A, Dabout V, Garinet S, Giraud D, Perkins G, Taieb J, et al. Encorafenib, binimetinib and cetuximab in BRAF V600E-mutated advanced pancreatic adenocarcinoma. ESMO Open. (2024) 9:103975. doi: 10.1016/j.esmoop.2024.103975
28. Clinical Trials.gov. Binimetinib and encorafenib for the treatment of pancreatic cancer in patients with a somatic BRAF V600E mutation . Available online at: https://clinicaltrials.gov/study/NCT04390243?term=NCT04390243&rank=1 (Accessed October 23, 2025).
29. Grinshpun A, Zarbiv Y, Roszik J, Subbiah V, and Hubert A. Beyond KRAS: practical molecular targets in pancreatic adenocarcinoma. Case Rep Oncol. (2019) 12:7–13. doi: 10.1159/000496018
30. Busch E, Kreutzfeldt S, Agaimy A, Mechtersheimer G, Horak P, Brors B, et al. Successful BRAF/MEK inhibition in a patient with BRAF(V600E)-mutated extrapancreatic acinar cell carcinoma. Cold Spring Harb Mol Case Stud. (2020) 6:2373–873. doi: 10.1101/mcs.a005553
31. Aguirre AJ, Nowak JA, Camarda ND, Moffitt RA, Ghazani AA, Hazar-Rethinam M, et al. Real-time genomic characterization of advanced pancreatic cancer to enable precision medicine. Cancer Discov. (2018) 8:1096–111. doi: 10.1158/2159-8290.CD-18-0275
32. Wrzeszczynski KO, Rahman S, Frank MO, Arora K, Shah M, Geiger H, et al. Identification of targetable BRAFΔN486_P490 variant by whole-genome sequencing leading to dabrafenib-induced remission of a BRAF-mutant pancreatic adenocarcinoma. Cold Spring Harb Mol Case Stud. (2019) 5:2373–873. doi: 10.1101/mcs.a004424
33. Cramer S, Marcus MA, Ramkissoon S, Szabo S, and Pressey JG. Pediatric BRAF (V600E)-mutated pancreatic acinar cell carcinoma with complete and durable response to Dabrafenib and Trametinib. JCO Precis Oncol. (2020) 4:801–5. doi: 10.1200/PO.19.00343
34. Seghers AK, Cuyle PJ, and Van Cutsem E. Molecular targeting of a BRAF mutation in pancreatic ductal adenocarcinoma: case report and literature review. Target Oncol. (2020) 15:407–10. doi: 10.1007/s11523-020-00727-9
Keywords: pancreatic adenocarcinoma, BRAF V600E, case report, dabrafenib, trametinib, dose-adjusted
Citation: Liu L, Zhu X, Guo Y, Tang M, Zhou W and Chen B (2025) Dabrafenib plus trametinib in an elderly patient with BRAF V600E-mutant advanced pancreatic adenocarcinoma: A case report. Front. Oncol. 15:1687796. doi: 10.3389/fonc.2025.1687796
Received: 18 August 2025; Accepted: 10 November 2025; Revised: 27 October 2025;
Published: 24 November 2025.
Edited by:
Jeffrey Peter Townsend, Yale University, United StatesReviewed by:
Tuan Hoang, Princess Margaret Cancer Centre, CanadaMoein Rajaei, Yale University, United States
Copyright © 2025 Liu, Zhu, Guo, Tang, Zhou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baisong Chen, MTM3MDY4NTU2OTlAMTM5LmNvbQ==
 Linger Liu1
Linger Liu1 Baisong Chen
Baisong Chen